Abstract
There is mixed evidence on the association between headache and attention-deficit/hyperactivity disorder (ADHD), as well as headache and ADHD medications. This systematic review and meta-analysis investigated the co-occurrence of headache in children with ADHD, and the effects of ADHD medications on headache. Embase, Medline and PsycInfo were searched for population-based and clinical studies comparing the prevalence of headache in ADHD and controls through January 26, 2021. In addition, we updated the search of a previous systematic review and network meta-analysis of double-blind randomized controlled trials (RCTs) on ADHD medications on June 16, 2020. Trials of amphetamines, atomoxetine, bupropion, clonidine, guanfacine, methylphenidate, and modafinil with a placebo arm and reporting data on headache as an adverse event, were included. Thirteen epidemiological studies and 58 clinical trials were eligible for inclusion. In epidemiological studies, a significant association between headache and ADHD was found [odds ratio (OR) = 2.01, 95% confidence interval (CI) = 1.63–2.46], which remained significant when limited to studies reporting ORs adjusted for possible confounders. The pooled prevalence of headaches in children with ADHD was 26.6%. In RCTs, three ADHD medications were associated with increased headache during treatment periods, compared to placebo: atomoxetine (OR = 1.29, 95% CI = 1.06–1.56), guanfacine (OR = 1.43, 95% CI = 1.12–1.82), and methylphenidate (OR = 1.33, 95% CI = 1.09–1.63). The summarized evidence suggests that headache is common in children with ADHD, both as part of the clinical presentation as such and as a side effect of some standard medications. Monitoring and clinical management strategies of headache in ADHD, in general, and during pharmacological treatment are recommended.
Key words: ADHD medication, attention-deficit/hyperactivity disorder, headache, meta-analysis, systematic review
Introduction
Headache in childhood is common and disabling. The estimated prevalence during 1 month to lifetime is 58.4%; migraine affects 7.7% (Abu-Arafeh, Razak, Sivaraman, & Graham, 2010). Pediatric headaches have been linked to sleep problems (Dosi et al., 2013; Esposito, Parisi, Miano, & Carotenuto, 2013), emotional dysfunction (Arruda & Bigal, 2012a; Fuh et al., 2010), impaired peer relations (Karwautz et al., 1999), and poor academic performance (Arruda & Bigal, 2012b; Genizi et al., 2013). Its clinical management is challenging due to the multifactorial origin and co-existing medical and psychiatric conditions (Bellini et al., 2013; Guidetti, Faedda, & Siniatchkin, 2016; Whitehouse & Agrawal, 2017).
Attention-deficit/hyperactivity disorder (ADHD) is a neurodevelopmental condition characterized by impairing inattention, hyperactivity and impulsivity, with a worldwide prevalence of 5–7% in children and adolescents (Polanczyk, Willcutt, Salum, Kieling, & Rohde, 2014; Thomas, Sanders, Doust, Beller, & Glasziou, 2015). Youths with ADHD have been reported to frequently suffer from comorbid headaches (Akmatov, Ermakova, & Batzing, 2021; Kutuk et al., 2018). Headache can not only have a negative effect on general well-being, but it may also aggravate cognitive problems defining or being associated with ADHD, such as inattention and impaired information processing (Moutran et al., 2011; Riva et al., 2006; Waldie, Hausmann, Milne, & Poulton, 2002). As a common side effect during pharmacological treatment of ADHD (Clavenna & Bonati, 2017), the headache might also decrease compliance to medications (Ahmed & Aslani, 2013) and increase rates of treatment failure (Buitelaar et al., 2015), with negative long-term consequences for outcomes (Barkley, 2008).
Therefore, a better understanding of the association of headache and ADHD, both as comorbidity and a side effect of medication, might help optimizing clinical management. However, although an extensive body of epidemiological research and clinical trials of ADHD medications exist, the current knowledge of the occurrence of headaches in children and adolescents with ADHD remains limited in several ways. First, estimated odds ratios (ORs) for headache in ADHD v. non-ADHD populations (typically developing children and those with neurodevelopmental disorders other than ADHD) vary across studies. Therefore, pooled results from a meta-analysis could provide a more robust and precise point estimate with a higher statistical power than the information derived from individual studies. Second, previous systematic reviews investigated the risk of ADHD in individuals with headaches, not vice versa (Salem et al., 2018). Thus, aggregated data focusing on headaches in pediatric ADHD as the target population are not available. Third, reviews on the association between ADHD medications and headache in children included randomized control trials (RCTs) regardless of blindness (Cheng, Chen, Ko, & Ng, 2007; Punja et al., 2016; Ruggiero et al., 2014; Storebø et al., 2015; Wang et al., 2017), which could introduce bias. In addition, there is a lack of information on possible differences between ADHD medications.
Thus, we conducted a systematic review and meta-analysis to examine two research questions (RQ): (1) Does the prevalence of headache differ between children with and without ADHD? (2) Is the use of common medications for ADHD associated with headaches in children?
Methods
This systematic review was conducted in accordance with the PRISMA statement (Liberati et al., 2009). The protocol was preregistered with PROSPERO (CRD42020176574).
Review question 1: Does the prevalence of headache differ between children and adolescents with and without ADHD?
Eligibility criteria
Case-control and cross-sectional/longitudinal studies which recruited either community or clinical samples with a control group comparing the odds of having headaches between children and adolescents diagnosed with ADHD and controls without ADHD were eligible. The population of interest was children and adolescents (aged ⩽18 years) with a diagnosis of ADHD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM) (-III, -III-R, -IV, -IV-TR or 5) or hyperkinetic disorder (HKD) in the International Classification of Diseases and Related Health Problems (ICD) (−8, −9, −10), ascertained by either clinical assessment or validated instruments, or a positive answer to the question to parents: “Did a doctor/healthcare professional ever tell you that your child has ADHD?” The indicators for headache were either (1) A positive answer to the question to participants/parents: “Do you have (Have you ever had) headache/migraine?” “Does your child have (Has your child ever had) headache/migraine?”; or (2) a diagnosis of primary headache (migraine, tension-type headache, cluster headache, and other types) recorded in medical files/registries, or made by clinicians in a research setting. OR was the principal summary measure when comparing headaches in children and adolescents with ADHD v. the comparison group.
Search strategy
A systematic search was conducted with the assistance of two information specialists from the Karolinska Institutet University Library using the databases Embase, Medline and PsycInfo from their inception to January 26, 2021. This search was limited to studies published in English and in peer-reviewed journals. The complete search strategy is provided in eSearch strategy in the Supplement.
Study selection and coding
Two reviewers independently screened the abstracts of the retrieved articles for eligibility, and then assessed the full text of potentially relevant publications. A manual search for additional studies was performed by screening reference lists of identified pertinent review articles. Disagreements between reviewers were resolved by consensus. When no consensus could be reached, a third reviewer acted as arbitrator. Data extraction was conducted using a standardized form by two independent reviewers. Extracted information included: study type, study settings, diagnostic tools for ADHD and headache, number and demographics characteristics of participants, and information for risk of bias assessment.
Risk of bias assessment
The risk of bias was assessed independently by two reviewers using the Newcastle–Ottawa scale (NOS) (Wells et al., 2009). Methodological quality including the risk of misclassification and selection bias, comparability between cases and controls, and appropriate statistical analysis was appraised and rated in eight items. Any discrepancies in the scoring were resolved by discussion and reviewer consensus.
Review question 2: Is the use of common medications for ADHD associated with headaches in children and adolescents?
Eligibility criteria
We included double-blind RCTs in either parallel or crossover design with a placebo arm and treatment period for at least 7 consecutive days. Trials without adequate randomization (defined as in Cortese et al., 2018) or washout period were excluded. The study population was children and adolescents (aged ⩾5 years and ⩽18 years) with a diagnosis of ADHD by either DSM (III, III-R, IV, IV-TR or 5) or of HKD according to the ICD (−8, −9, −10). While Cortese et al. (2018), due to the need of adhering to the transitivity assumption, included only medications as monotherapy and in tablet/capsule forms, here we allowed the intervention as ADHD medications (monotherapy or add-on to non-pharmacological treatment) in any formulation (tablet, capsule, chewable compound, liquid, transdermal) and dosing strategy, including (1) methylphenidate (including dexmethylphenidate), (2) amphetamines (including lisdexamfetamine), and (3) non-stimulants (atomoxetine, bupropion, clonidine, guanfacine, and modafinil). The comparator was the placebo arm. The outcome of interest was a headache in the safety assessment, obtained by either spontaneously report, open-ended questioning (e.g. do you have any physical discomforts?), or questionnaires/checklists for side effects. Studies published in peer-reviewed journals or available data uploaded on websites of protocol registration for clinical trials were considered.
Search strategy
We based this part of the review on a search performed for a systematic review and network meta-analysis of ADHD medications by Cortese et al. (2018), which, according to the AMSTAR 2 tool (Shea et al., 2017), we deemed to be of high quality, as all 16 items of the AMSTAR 2, including those related to the search, were scored as “Yes.” The search included a broad set of databases [PubMed, BIOSIS Previews, CINAHL, Cochrane Library, EMBASE, ERIC, MEDLINE, PsycINFO, OpenGrey, Web of Science Core Collection, ProQuest Dissertations & Theses: UK & Ireland, ProQuest Dissertations & Theses A&I, and WHO International Trials Registry Platform (CTRP) (including ClinicalTrials.gov)]. In addition, they had searched for unpublished studies or data sets by contacting drug companies and study authors. The search for the network meta-analysis by Cortese et al. (2018). is updated once per year, and the search performed on 16 June 2020 was used for the present review.
Study selection and coding
All articles receiving full-text assessment in the previous review (the full list of included and excluded studies with exclusion reasons from Cortese et al., 2018) and the additional articles found in the updated search were screened independently by two reviewers using the inclusion and exclusion criteria of the present review. Disagreements were resolved by discussion between the two review authors. Data extraction was also performed independently for the following information: publication detail, trial registration identification number, characteristics of trial participants, study design, interventions, methods of outcome measurement, and information for risk of bias assessment.
Risk of bias assessment
The revised Cochrane risk of bias tool for randomized trials (RoB 2.0) (Sterne et al., 2019) was used for assessing bias arising from the following five domains: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result. The overall risk-of-bias judgment was rated as low risk of bias, some concerns, and high risk of bias, which was the lowest rating for any of the criteria (e.g. if any domain was scored high risk of bias, the study was considered high risk of bias). The appraisal was performed independently by two reviews and disagreements were solved by consensus.
Planned methods of analysis
The metafor package (Viechtbauer, 2010) in R version 3.2.4. (RStudio Team, 2015) was used for computing pooled estimates and 95% confidence intervals (CIs). A random-effects model was adopted in data syntheses considering the sample and methodological diversity among the included epidemiological studies and clinical trials. For RQ1, the ORs of headache in children and adolescents with ADHD v. the comparison group were synthesized. In addition, a pooled prevalence estimate (the proportion of headaches in children with ADHD) was calculated. Freeman–Tukey double arcsine transformations (Freeman & Tukey, 1950) were used to stabilize the variance, and the harmonic mean of individual sample sizes (Miller, 1978) was used in the back-transformation. For RQ2, we calculated the pooled OR comparing the risk of headache among participants in the treatment arm with those in the placebo arm for each specific ADHD medication (data of safety analysis were used). The heterogeneity for each meta-analysis was estimated with the Q and I2 statistics (Higgins & Thompson, 2002). The significance of publication bias was tested using Egger's weighted regression and estimated visually based on funnel plots. Subgroup analysis based on potential covariates (e.g. maximal dosage, dosing strategy) was used to address significant heterogeneity of outcome measures across studies.
Results
Review question 1
Study selection and characteristics
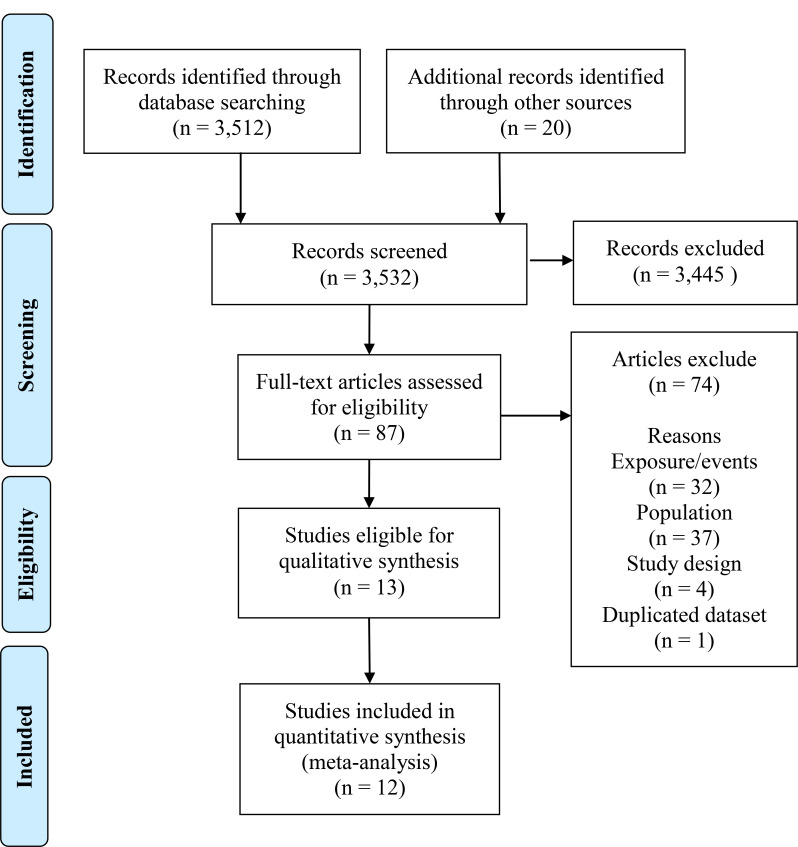
A total of 13 records were identified for inclusion in the review (eIncluded articles 1 in the Supplement). See study selection process in Fig. 1 and reasons for exclusion of references for which the full text was assessed in eExclusion reasons 1 in the Supplement. Characteristics of included articles are summarized in Table 1A and eTable 1 in the Supplement. The total sample included 267 556 children and adolescents with ADHD, identified mainly by validated instruments (n = 6). The comparison group comprised of 2 464 878 participants, all recruited from the community.
Fig. 1.
PRISMA flow chart of review process and study selection for epidemiological studies.
Table 1.
Summary of characteristics of the included studies
| A. Review question 1. Characteristics of epidemiological studies (n = 13) | |||
| Country (n) | Brazil (2), Canada (1), Germany (1), Sweden (2), Turkey (1), USA (6) | ||
| Type of study (n) | Case-control (3), Cross-sectional (10) | ||
| Study temporality (n) | All cross-sectional | ||
| Settings of ADHD recruitment (n) | Clinical (2), Community (11) | ||
| ADHD diagnostic assessment (n) | ICD-9 (1), ICD-10 (1), DSM-III (1), DSM-IV (2), DSM-5 (1), A-TAC (1), SNAP-IV (2), SDQ (3), CBCL (1), Conner-10 items (1), parent-report questionnaire (2) | ||
| Type of headache (n) | Headache/migraine (8), Migraine (5), Tension Headache (3) | ||
| Diagnostic indicator for headache (n) | Clinical diagnosis (1), medical records/registry (2), parent report (10), self-report (1) | ||
| Survey period (n) | 1 year (7), 4 years (1), lifetime (5) | ||
| B. Review question 2. Characteristics of clinical trials (n = 58) | |||
| Amphetamine (n = 10) | Country (n) | USA (7), Japan (1), sample from 10 European countries (1), sample from USA, Canada, and European countries (1) | |
| Study design (n) | All double-blind; all parallel design; all naturalistic setting; all monotherapy | ||
| Treatment duration (n) | 3w (2), 4w (5), 6w (1), 7w (1), 8w (1) | ||
| Method for identifying headache (n) | Parent report questionnaire (1), spontaneously report (1), open-ended question (1), NR (7) | ||
| Participants discontinued due to headache (n) | Reported cases (1), no cases (6), NR (3) | ||
| Total participants in the safety analysis | Treatment group: 1780, placebo group: 892 | ||
| Pooled rate of headache during treatment | Treatment group: 14.8%, placebo group: 13.1% | ||
| Atomoxetine (n = 22) | Country (n) | USA (10), Germany (2), Australia (1), Italy (1), Japan (1), Russia (1), Spain (1), Sweden (1), Taiwan (1), the Netherlands (1), sample from Europe and Australia (1), sample from USA, Europe, and Canada (1) | |
| Study design (n) | All double-blind; parallel (21), crossover (1); all natralistic setting; all monotherapy | ||
| Treatment duration (n) | 6w (6), 8w (7), 9w (2), 10w (2), 12w (3), 13w (1), 18w (1) | ||
| Method for identifying headache (n) | Parent report questionnaire (1), Spontaneously report (2), open-ended question (12), NR (7) | ||
| Other remarks (n) | All participants stimulant-naïve (2), all participants comorbid with ASD (1), all participants comorbid with an anxiety disorder (1), all participants comorbid with ODD or CD (3), all participants comorbid with MDD (1), all participants comorbid with tics (1) | ||
| Participants discontinued due to headache (n) | Reported cases (4), no cases (11), NR (7) | ||
| Total participants in the safety analysis | Treatment group: 2387, placebo group: 1470 | ||
| Pooled rate of headache during treatment | Treatment group: 16.3%, placebo group: 12.7% | ||
| Bupropion (n = 1) | Country | USA | |
| Study design | Quadruple blind, parallel design; natralistic setting; add-on to CBT | ||
| Treatment duration | 16w | ||
| Method for identifying headache | NR | ||
| Other remarks | all participants comorbid with SUD | ||
| Participants discontinued due to headache | NR | ||
| Total participants in the safety analysis | Treatment group: 53, placebo group: 52 | ||
| Rate of headache during treatment | Treatment group: 22.6%, placebo group: 26.9% | ||
| Clonidine (n = 1) | Country | USA | |
| Study design | Double-blind, parallel design; natralistic setting; monotherapy | ||
| Treatment duration | 16w | ||
| Method for identifying headache | Spontaneously report | ||
| Participants discontinued due to headache | 0 | ||
| Total participants in the safety analysis | Treatment group: 31, placebo group: 30 | ||
| Rate of headache during treatment | Treatment group: 16.1%, placebo group: 10.0% | ||
| Guanfacine (n = 8) | Country (n) | USA (6), sample from USA and Canada (1), sample from USA, Canada and Europe (1) | |
| Study design (n) | All double-blind; all parallel design; natralistic setting (7), laboratory classroom study (1); all monotherapy | ||
| Treatment duration (n) | 5w (1), 6w (1), 8w (2), 9w (2), 13w (2) | ||
| Method for identifying headache (n) | Spontaneously report (1), NR (7) | ||
| Participants discontinued due to headache (n) | Reported cases (2), no cases (3), NR (3) | ||
| Total participants in the safety analysis | Treatment group: 1275, placebo group: 681 | ||
| Pooled rate of headache during treatment | Treatment group: 23.0%, placebo group: 17.3% | ||
| Methylphenidate (n = 17) | Country (n) | USA (15), 10 European countries (1), sample from USA, Canada, Germany, Hungary, Sweden (1) | |
| Study design (n) | All double blind; parallel (15), crossover (2); natralistic setting (16), classroom setting (1); monotherapy (16), add-on to CBT (1) | ||
| Route (n) | Oral (16), transdermal (1) | ||
| Drug release profile (n) | Immediate-release (3), extended-release (7), OROS (6) | ||
| Treatment duration (n) | 1w (2), 3w(3), 4w(3), 5w (1), 6w (2), 7w (3), 8w (1), 12w (1), 16w(1) | ||
| Method for identifying headache (n) | Spontaneously report (5), parent report questionnaire (3), open-ended question (1), NR (9) | ||
| Other remarks (n) | All participants females (1), All participants comorbid with SUD (1), using both parent-report questionnaires and spontaneously report by participants to identify headache (1) | ||
| Participants discontinued due to headache (n) | Reported cases (2), no cases (10), NR (5) | ||
| Total participants in the safety analysis | Treatment group: 2077, placebo group: 1294 | ||
| Pooled rate of headache during treatment | Treatment group: 16.6%, placebo group: 12.5% | ||
| Modafinil (n = 6) | Country (n) | USA (5), Iran (1) | |
| Study design (n) | All double-blind; all parallel design; all natralistic setting; all monotherapy | ||
| Treatment duration (n) | 4w (1), 6w (2), 7w (1), 9w (2) | ||
| Method for identifying headache (n) | Spontaneously report (3), parent report questionnaire (1), physician-administered questionnaire (1), NR (1) | ||
| Participants discontinued due to headache (n) | Reported cases (2), no cases (2), NR (2) | ||
| Total participants in the safety analysis | Treatment group: 520, placebo group: 298 | ||
| Pooled rate of headache during treatment | Treatment group: 16.7%, placebo group: 14.3% | ||
ICD, International Classification of Diseases and Related Health Problems; DSM, Diagnostic and Statistical Manual of Mental Disorders; A-TAC, Autism-Tics, ADHD and other Comorbidities; SNAP-IV, Swanson, Nolan, and Pelham Rating Scale; SDQ, Strengths and Difficulties Questionnaire; CBCL, Child Behavior Checklist; NR, not reported; ASD, autism spectrum disorder; ODD, oppositional defiant disorder; CD, conduct disorder; MDD, major depressive disorder; SUD, substance use disorder; CBT, cognitive behavior therapy.
Results of individual studies and syntheses of results
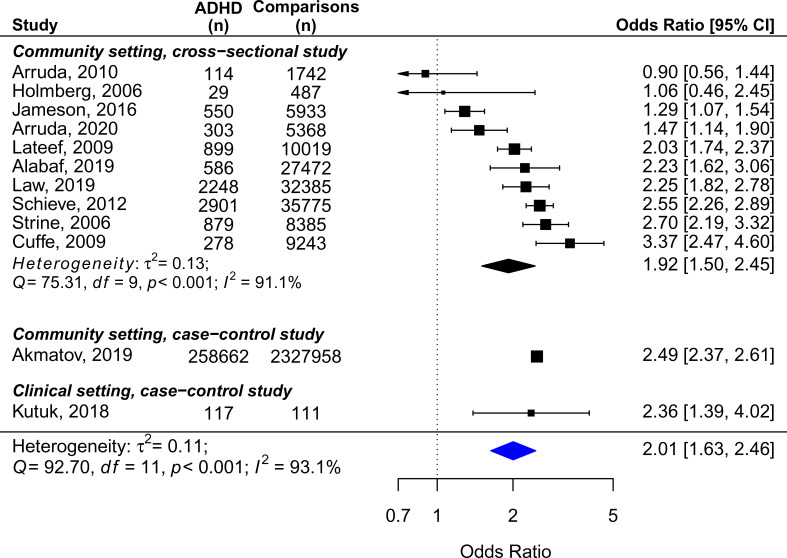
Figure 2 displays the crude ORs of the included studies and the pooled estimates (2.01, 95% CI = 1.63 − 2.46, n = 12), as well as the results of subgroup analysis for the community studies with cross-sectional design. One small outlier study with an OR of 14.79 (Kaplan, McNicol, Conte, & Moghadam, 1987), which had recruited only preschool children in clinical settings, was excluded from the meta-analysis, owing to susceptibility of selection bias. In addition, the sample size of Akmatov et al. (2021) was much larger compared to other studies. We decided to include this article for the similar OR with other studies, and the high study quality according to the risk of bias assessment. Results were heterogeneous across studies when considering all types of headache, but most studies (n = 10) suggested higher rates of headache in ADHD than in non-ADHD controls (range of crude OR 0.90–3.37). Regarding the relationships between diagnostic assessments and ORs, three studies used clinical assessment for both ADHD and headache (Akmatov et al., 2021; Kutuk et al., 2018; Law, Palermo, Zhou, & Groenewald, 2019), and the ORs were 2.49, 2.36, and 2.25. Two studies used a single question to identify both ADHD and headache (Lateef et al., 2009; Schieve et al., 2012), and the ORs were 2.03 and 2.55. Other studies which used mixed diagnostic indicators reported ORs ranging from 0.90 to 3.37. Due to the heterogeneity of findings (Q = 92.70, p < 0.001; I2 = 93.1%), we also conducted subgroup analysis for the studies with adjusted OR, the studies measuring migraine, and those measuring tension headache (Table 2). The synthesized results indicated that studies that controlled for age, sex, race, and other socioeconomic status variables found a significant association between ADHD and overall headache [n = 4, pooled OR (95% CI) = 1.98 (1.60–2.45)]. However, heterogeneity was still high among the studies measuring migraine (Q = 10.38, p = 0.035; I2 = 67.6%), and no difference in the risk of tension headache was found between ADHD and controls. The pooled prevalence of overall headache in children with ADHD over a period of 1 year to lifetime is shown in eFigure 1 [n = 11; one study did not provide the data of prevalence; pooled proportion (95% CI) = 26.6 (14.2–41.3) %], with significant heterogeneity detected.
Fig. 2.
Unadjusted ORs expressing the association between headache and ADHD in children and adolescents.
Table 2.
Summary of results of pooled ORs about the association between ADHD and headache in the main and in the subgroup meta-analyses
| Type of analysis | Studies (n) | Participants (n) | OR | 95% CI | p | Heterogeneity | Egger's test | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| τ2 | Q | p | I2 (%) | t | p | |||||||
| Review question 1 | ADHD | Control | ||||||||||
| Overall crude OR | 12 | 267 556 | 2 464 878 | 2.01 | 1.63–2.46 | <0.001 | 0.11 | 92.70 | <0.001 | 93.1 | −1.79 | 0.104 |
| Adjusted ORa | 4 | 5229 | 60 112 | 1.98 | 1.60–2.45 | <0.001 | 0.33 | 7.81 | 0.050 | 64.7 | −0.31 | 0.789 |
| Migraine | 5 | 259 782 | 2 362 651 | 2.22 | 1.76–2.79 | <0.001 | 0.04 | 10.38 | 0.035 | 67.6 | −0.94 | 0.418 |
| Tension headache | 3 | 534 | 7221 | 0.80 | 0.57–1.12 | 0.196 | 0.03 | 3.38 | 0.185 | 36.5 | 1.07 | 0.478 |
| Review question 2 | Treatment | Placebo | ||||||||||
| Amphetamine | 10 | 1780 | 892 | 1.05 | 0.80–1.38 | 0.723 | 0.04 | 11.26 | 0.258 | 21.53 | 2.10 | 0.069 |
| Atomoxetine | 22 | 2387 | 1470 | 1.29 | 1.06–1.56 | 0.010 | 0 | 18.90 | 0.528 | 0 | 1.27 | 0.221 |
| Maximum dosage < 1.5 mg/kg | 9 | 902 | 620 | 1.53 | 1.01–2.30 | 0.043 | 0.11 | 12.76 | 0.240 | 28.7 | 1.37 | 0.214 |
| Maximum dosage ⩾ 1.5 mg/kg | 13 | 1485 | 850 | 1.22 | 0.96–1.54 | 0.105 | 0 | 7.88 | 0.724 | 0 | 0.16 | 0.878 |
| Forced titration | 11 | 1368 | 708 | 1.33 | 1.01–1.75 | 0.041 | 0.02 | 13.26 | 0.209 | 8.46 | −0.11 | 0.918 |
| Flexible/optimized dose | 11 | 1019 | 762 | 1.24 | 0.93–1.65 | 0.148 | 0 | 5.51 | 0.788 | 0 | 2.66 | 0.029 |
| Bupropion | 1 | 53 | 52 | 0.79 | 0.33–1.93 | 0.613 | ||||||
| Clonidine | 1 | 31 | 30 | 1.73 | 0.38–7.99 | 0.482 | ||||||
| Guanfacine | 8 | 1275 | 681 | 1.43 | 1.12–1.82 | 0.005 | 0 | 4.17 | 0.760 | 0 | 2.05 | 0.086 |
| Methylphenidate | 17 | 2077 | 1294 | 1.33 | 1.09–1.63 | 0.005 | 0 | 10.96 | 0.812 | 0 | −0.58 | 0.570 |
| Monotherapy with oral formation | 15 | 1781 | 1070 | 1.41 | 1.12–1.78 | 0.003 | 0 | 9.81 | 0.776 | 0 | −1.22 | 0.245 |
| OROS/extended release | 13 | 1793 | 1132 | 1.37 | 1.11–1.70 | 0.003 | 0 | 7.74 | 0.805 | 0 | 0.57 | 0.578 |
| Immediate release | 3 | 139 | 90 | 1.00 | 0.33–3.00 | 0.999 | 0.28 | 2.38 | 0.305 | 27.3 | −2.64 | 0.231 |
| Modafinil | 6 | 520 | 298 | 1.24 | 0.73–2.13 | 0.429 | 0.18 | 7.61 | 0.179 | 42.3 | 0.59 | 0.589 |
OROS, osmotic-release oral system.
Adjusted variables: Jameson et al. (2016), adjusted for age, sex, race, and parental education; Lateef et al. (2009), adjusted for sex, age, race, and poverty index ratio; Schieve et al. (2012), adjusted for age, sex, race, and maternal education; Strine, Okoro, McGuire, & Balluz. (2006), adjusted for age, sex, race, parental structure, poverty status, type of health care coverage, and number of comorbid conditions.
Risk of bias across studies
Data on the risk of bias of each study are presented in eTable 1 in the Supplement. The most frequent sources of risk of bias were inadequate ascertainment of ADHD and headache, as well as the lack of non-response rate in both ADHD and non-ADHD groups. Regarding the comparability of cases and controls, most studies were cross-sectional studies without control groups matched for demographic characteristics. The heterogeneity across studies became non-significant in the adjusted subgroup analysis. This underscores the need for appropriate statistical methods to address the influence of possible confounders on the estimates. In addition, most studies did not include information on the treatment status of ADHD, which might influence the prevalence of headaches.
The results of Egger's test indicated no evidence of publication bias (t = − 1.79, p = 0.104). Nevertheless, some estimates lying on the top of the funnel plot but outside the triangular region suggest sample selection variance across studies (eFigure 2 in the Supplement), which could arise from the diversity of methods for identifying participants with ADHD.
Review question 2
Study selection and characteristics
The update search by Cortese et al. (2018) retrieved 194 additional RCTs. A total of 58 trials met the eligibility criteria for the present systematic review. See all the related articles of the included trials and the exclusion reasons in eIncluded articles 2 and eExclusion reasons 2 in the Supplement.
The main characteristics of the included trials are displayed in Table 1B and eTable 2 in the Supplement. The eligible trials involved a total of 8254 participants who were allocated to the treatment groups and included in the safety analysis, and 4087 participants in the control groups assessed in the same way.
Risk of bias across studies
The majority of included trials (n = 41, 70.7%) were rated as overall low risk of bias regarding the outcome of headache, 16 trials (27.6%) with some concerns, and 1 trial (1.7%) as high risk of bias (eTable 2 in the Supplement).
Results of individual studies, syntheses of results, and publication bias
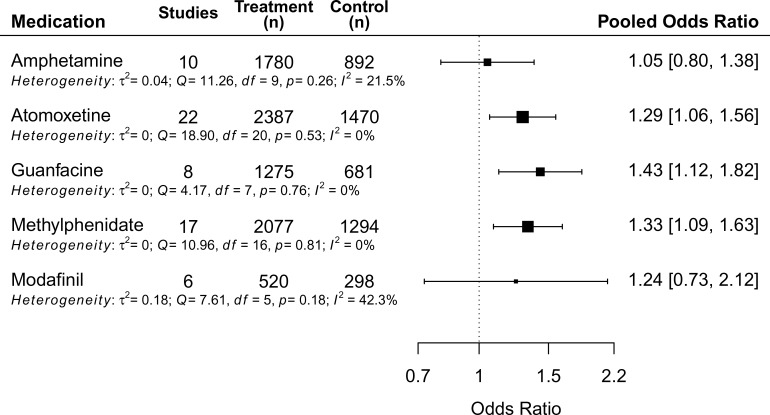
Except for bupropion and clonidine (one eligible study for each was identified by our search), the pooled OR on the risk of headache between treatment and control groups for five different ADHD medications are summarized in Fig. 3. The forest plots and funnel plots of each medication are presented in the eFigure 3–13 in the Supplement. The pooled prevalence of headache for the treatment and placebo groups across studies for each medication ranged from 14.8% (amphetamine) to 23.0% (guanfacine) in the treatment groups, and from 12.2% (methylphenidate) to 17.3% (guanfacine) in the control groups (Table 1B, eFigure 14–15). eFigure 16 displays the pooled prevalence of headache across different age groups in placebo arms (children 12.6%, adolescents 16.5%). The results of subgroup analyses are presented in Table 2. The results for each active medication (in alphabetic order) are detailed below.
Fig. 3.
Forest plot of risk of headache during treatment periods compared with placebo as reference.
Amphetamine
None of the included nine trials reported a significant difference in risk of headache between treatment and control arms [eFigure 3, pooled OR (95% CI): 1.05 (0.80 − 1.83)]. No evidence of heterogeneity was found in the meta-analysis, although the CI is wide compared to the synthesized results of other ADHD medications.
Atomoxetine
Among the total 22 trials (21 datasets), one trial reported a higher risk of headache in participants treated with atomoxetine, while others showed no difference between treatment and control groups. The synthesized results indicated a significant association between atomoxetine and headache in children with ADHD [pooled OR (95% CI): 1.29 (1.06 − 1.56)]. Two types of subgroup analyses were performed. The effects of dosage showed paradoxical findings, in which the association remained significant in trials with smaller dosage (p = 0.043), but did not survive in those with larger (maximum dosage cutoff value 1.5 mg/kg/day, Table 2 and eFigure 5). However, forced titration was more commonly used in trials with smaller dosages (55.6% v. 41.7%), and the association was also found in trials using forced titration (p = 0.041), but not in those using flexible/optimized dose (Table 2 and eFigure 6). Therefore, it should be considered that the paradoxical effect of dosage could be driven by the dosing strategy. Despite that the sample in this meta-analysis included participants with a diverse range of comorbidities, all estimates except one fitted within the triangular region of the funnel plot, indicating small effects of sampling variation on the pooled estimate.
Bupropion
One trial comparing the effectiveness of bupropion add-on to CBT in the treatment of adolescents with comorbid ADHD and substance use disorders was included. No significant difference in the risk of headache during the intervention period was found between the treatment and placebo group [OR (95% CI): 0.79 (0.33 − 1.93)].
Clonidine
The only included trial investigated the efficacy and safety profiles of clonidine and methylphenidate among children with ADHD. There was no significant difference in the risk of headache between the group treated with clonidine and placebo [OR (95% CI): 1.73 (0.38 − 7.99)].
Guanfacine
None of the eight trials in the meta-analysis reported a difference in the risk of headache between treatment and placebo groups. However, the pooled estimates indicated that the odds of headache in the treatment group were 1.43 times higher than in the placebo group [pooled OR (95% CI): 1.43 (1.12 − 1.82)].
Methylphenidate
Seventeen trials were included in the meta-analysis. None of them found a significant association between methylphenidate treatment and headache. Nevertheless, an increased risk of headache with methylphenidate was indicated by the pooled estimates [pooled OR (95% CI): 1.33 (1.09 − 1.63)], and also in the subgroup analysis on trials using an oral formation with monotherapy design [n = 15, pooled OR (95% CI): 1.41 (1.12 − 1.78)]. When considering the duration of drug action, long-acting formation [the osmotic-release oral system (OROS) and extended release] was significantly associated with a higher risk of headache with the treatment of methylphenidate compared to controls (Table 2). The pooled estimate of the three trials with immediate-release methylphenidate showed no significant difference between treatment and controls, but the CI was wide [pooled OR (95% CI): 1.00 (0.33–3.00)].
Modafinil
Of the six trials included in this review, none reported a significant difference in risk of headache between treatment and placebo arms. The results of the meta-analysis did not find a significant association between modafinil and headache [pooled OR (95% CI): 1.24 (0.73 − 2.13)]. However, the CI was wide due to the relatively small sample size.
Discussion
This is the first systematic review and meta-analysis on the association between headache and ADHD, and the effects of ADHD medications in childhood. Results indicate that in pediatric ADHD there is a doubled risk compared to those without ADHD to have a headache, with a pooled prevalence of 26.6%. Despite the heterogeneity across studies in the meta-analysis of crude data, the association survived when pooling ORs adjusted for possible confounders, including age, sex, and socioeconomic status (Bigal & Lipton, 2006). Moreover, in short-term RCTs of ADHD medications, increased risks of headache were found for atomoxetine, guanfacine, and methylphenidate treatments compared to placebos. However, no statistically significant associations of headache with amphetamine, bupropion, clonidine, and modafinil were found; yet, the precision of these estimates was limited by the number of studies, sample sizes, and methods for measuring headache. In summary, at this stage, it is premature to draw conclusions about the difference between medication in its effects on headaches.
The effect size of the pooled estimate for atomoxetine was relatively small, and there was an effect of dosing strategy but not dose-related effects on headache. However, the finding may reflect a long-term tolerability profile of atomoxetine, in which headache is the most frequent treatment-emergent adverse event, reported by more than 50% of pediatric patients with 3–4 years treatment duration (Donnelly et al., 2009). For guanfacine, headache has been found to be amongst the most common reasons for discontinuation, although the most common adverse effects reported are sedation and somnolence, in part explained by its affinity to α2A adrenergic receptors (Bello, 2015). Methylphenidate is the most widely prescribed ADHD medication among children and adolescents owing to its overall efficacy and safety (Cortese et al., 2018), but has also been found to be associated with headache in a previous meta-analysis of 37 trials (Storebø et al., 2015). Moreover, long-acting methylphenidate (OROS/extended-release), which is thought with the advantage of better adherence v. immediate-release formulation (Adler & Nierenberg, 2010), was also associated with a headache when analyzed separately.
Most trials included in this systematic review were underpowered to detect side effects. This makes it difficult to draw conclusions from single trials, especially since headache naturally co-occurs with ADHD. The majority of included trials did not report an increased risk of headache. Still, a significant risk was observed for several agents when looking at the totality of trials. This might explain why headache, despite being among the most common treatment-related adverse events of ADHD medication (Clavenna & Bonati, 2017), has received limited attention in previous research and clinical practice guidelines, compared to sleep disturbance, growth delay, loss of appetite, and cardiovascular risks (Cortese et al., 2013; Graham et al., 2011). Our results highlight that clinicians should be aware of the risk of headache during treatment with ADHD medications, in order to improve adherence and avoid unnecessary harm.
Headache phenotypes in pediatric ADHD have not been discussed in detail in the literature previously. Our results suggest an increased risk of migraine in children with ADHD, but not a risk of tension-type headache, in line with the findings from a previous meta-analysis (Salem et al., 2018). Nevertheless, the classification of childhood headaches is still debated. The main reason is that headache phenotypes often change over time (Brna, Dooley, Gordon, & Dewan, 2005), and different headache diagnoses may represent a continuum rather than discrete entities (Turner et al., 2015). Postulated pathophysiological links between ADHD and headache include malfunction of the dopaminergic system and brain iron deficiency, as well as shared genetic pathways (Kutuk et al., 2018; Parisi et al., 2014). In addition, the association between ADHD and headache could be mediated by internalizing symptoms and sleep disorders, where the common dysfunction of sleep–wake and arousal system might lead to alteration of the pain processing (Guidetti et al., 2016; Pan & Yeh, 2017; Parisi et al., 2014). Unfortunately, there is currently no evidence-based guidance available regarding headache management in children with ADHD, neither regarding the effects of prophylaxis of migraine using valproate, amitriptyline and flunarizine on ADHD symptoms (Villa, Agessi, Moutran, Gabbai, & Carvalho Dde, 2016), nor for strategies of medications hierarchies for those with ADHD who also suffer from headache.
Several gaps in the literature were identified. For example, no eligible longitudinal studies aiming to disentangle the temporal relationship between the emerging of attention problems and headache symptoms were identified. In addition, only a limited number of studies recruited clinical samples of children with ADHD, which might experience headache profiles different from community samples. Further, most of the included epidemiological studies did not obtain the participants' history of pharmacological treatment. Finally, studies were not adjusted for other risk factors of headache, such as family history, medical conditions, and psychiatric comorbidity (Bellini et al., 2013; Lateef & Merikangas, 2017).
The results of this systematic review should be considered in the context of several limitations. First, the heterogeneity of outcomes across the included epidemiological studies diminishes the confidence of pooled ORs. This could partly reflect a bias of misclassification introduced by the inconsistent indicators used for identifying ADHD. Therefore, our confidence in the pooled proportion of headaches in ADHD children is restricted. Second, the majority of the included clinical trials did not detect treatment-related headaches in a standardized way, such as the Side Effects Rating Scale for stimulants (Barkley & Murphy, 2005). On the contrary, a physical examination, which always includes an open-ended question “do you have any physical discomforts?”, was the most commonly adopted method in the trials to obtain information. However, the sensitivity to headache identification by one open-ended item might be limited, compared to a well-designed instrument that collects the elicited responses from the participants in a consistent manner (Takemura et al., 2005). In addition, specific diagnoses of headaches were generally not provided. Third, the summary measure of headache used in clinical trials is the cumulative incidence rate. Since headache was detected at different visits during the study period, we were unable to analyze the relationship between time of exposure to drug and risk of headache for each medication. Fourth, we could not include studies lacking relevant data, and the prerequisite of data availability could have introduced publication bias. Fifth, the study samples in clinical trials could be highly selected. In addition to the inclusion and exclusion criteria, only voluntary participants who could comply with the protocol and had rather a good family support would be eligible to enter the trials. Furthermore, there is no data available on children with subclinical ADHD variants, who are often also in need of support and some sort of clinical action (Kirova et al., 2019). Thus, the external validity of the results for both ADHD diagnosis and symptoms of ADHD should be considered due to the possible overall limited representativeness of the samples.
In conclusion, findings show that children and adolescents with ADHD are more likely to experience headaches than their peers. Clinicians should be aware that headache is a common comorbidity of pediatric ADHD, but also a frequent side effect of pharmacological treatments. To enable practitioners to provide better care for children with comorbid ADHD and headaches, clinical guidelines supported by empirical evidence are desirable.
Acknowledgements
The authors thank Magdalena Svanberg, Emma-Lotta Säätelä, and Narcisa Hannerz for their assistance with the literature search.
Financial support
Dr Pan is supported by Tri-Service General Hospital, the Ministry of National Defense, Taiwan (R.O.C), and the Swedish Research Council.
Supplementary material
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291721004141.
click here to view supplementary material
Conflict of interest
Dr Häge received conference support, speaker's fee and/or served in an advisory role for Shire/Takeda and Lily. He was involved as an investigator in clinical trials by Shire, Janssen-Cilag, Otsuka, Sunovion, Servier, Lundbeck, Takeda, Nuvelution, Gedeon Richter, and Emalex. The present work is unrelated to the above relationships. Dr Buitelaar has been in the past 3 years a consultant to / member of the advisory board of / and/or speaker for Takeda/Shire, Roche, Medice, Angelini, Janssen, and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents, royalties. Dr Banaschewski served in an advisory or consultancy role for Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Shire, and Infectopharm. He received conference support or speaker's fee from Lilly, Medice, and Shire. He received royalties from Hogrefe, Kohlhammer, CIP Medien, Oxford University Press; the present work is unrelated to these relationships. Dr Cortese declares reimbursement for travel and accommodation expenses from the Association for Child and Adolescent Central Health (ACAMH) in relation to lectures delivered for ACAMH, Canadian AADHD Alliance Resource (CADDRA), British Association of Psychopharmacology (BAP), and from Healthcare Convention for educational activity on ADHD. Dr Coghill has in the last 5 years acted as an author, consultant or lecturer for Shire/Takeda, Medice and Servier. He receives royalties from Oxford University Press. Dr Bölte discloses that he has in the last 5 years acted as an author, consultant or lecturer for Medice, and Roche. He receives royalties for textbooks and diagnostic tools from Hogrefe, Kohlhammer and UTB publishers. The authors declare no other potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abu-Arafeh, I., Razak, S., Sivaraman, B., & Graham, C. (2010). Prevalence of headache and migraine in children and adolescents: A systematic review of population-based studies. Developmental Medicine and Child Neurology, 52(12), 1088–1097. [DOI] [PubMed] [Google Scholar]
- Adler, L. D., & Nierenberg, A. A. (2010). Review of medication adherence in children and adults with ADHD. Postgraduate Medicine, 122(1), 184–191. [DOI] [PubMed] [Google Scholar]
- Ahmed, R., & Aslani, P. (2013). Attention-deficit/hyperactivity disorder: An update on medication adherence and persistence in children, adolescents and adults. Expert Review of Pharmacoeconomics & Outcomes Research, 13(6), 791–815. [DOI] [PubMed] [Google Scholar]
- Akmatov, M. K., Ermakova, T., & Batzing, J. (2021). Psychiatric and nonpsychiatric comorbidities among children with ADHD: An exploratory analysis of nationwide claims data in Germany. Journal of Attention Disorders, 25(6), 874–884. [DOI] [PubMed] [Google Scholar]
- Arruda, M. A., & Bigal, M. E. (2012a). Behavioral and emotional symptoms and primary headaches in children: A population-based study. Cephalalgia, 32(15), 1093–1100. [DOI] [PubMed] [Google Scholar]
- Arruda, M. A., & Bigal, M. E. (2012b). Migraine and migraine subtypes in preadolescent children: Association with school performance. Neurology, 79(18), 1881–1888. [DOI] [PubMed] [Google Scholar]
- Barkley, R. A. (2008). Global issues related to the impact of untreated attention-deficit/hyperactivity disorder from childhood to young adulthood. Postgraduate Medicine, 120(3), 48–59. [DOI] [PubMed] [Google Scholar]
- Barkley, R. A., & Murphy, K. R. (2005). Attention-Deficit hyperactivity disorder third edition: A clinical workbook. New York: The Guilford Press. [Google Scholar]
- Bellini, B., Arruda, M., Cescut, A., Saulle, C., Persico, A., Carotenuto, M., … Guidetti, V. (2013). Headache and comorbidity in children and adolescents. The Journal of Headache and Pain, 14(1), 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello, N. T. (2015). Clinical utility of guanfacine extended-release in the treatment of ADHD in children and adolescents. Patient Preference and Adherence, 9, 877–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal, M. E., & Lipton, R. B. (2006). Migraine at all ages. Current Pain and Headache Reports, 10(3), 207–213. [DOI] [PubMed] [Google Scholar]
- Brna, P., Dooley, J., Gordon, K., & Dewan, T. (2005). The prognosis of childhood headache: A 20-year follow-up. Archives of Pediatrics & Adolescent Medicine, 159(12), 1157–1160. [DOI] [PubMed] [Google Scholar]
- Buitelaar, J., Asherson, P., Soutullo, C., Colla, M., Adams, D. H., Tanaka, Y., … Upadhyaya, H. (2015). Differences in maintenance of response upon discontinuation across medication treatments in attention-deficit/hyperactivity disorder. European Neuropsychopharmacology, 25(10), 1611–1621. [DOI] [PubMed] [Google Scholar]
- Cheng, J. Y., Chen, R. Y., Ko, J. S., & Ng, E. M. (2007). Efficacy and safety of atomoxetine for attention-deficit/hyperactivity disorder in children and adolescents-meta-analysis and meta-regression analysis. Psychopharmacology, 194(2), 197–209. [DOI] [PubMed] [Google Scholar]
- Clavenna, A., & Bonati, M. (2017). Pediatric pharmacoepidemiology − safety and effectiveness of medicines for ADHD. Expert Opinion on Drug Safety, 16(12), 1335–1345. [DOI] [PubMed] [Google Scholar]
- Cortese, S., Adamo, N., Del Giovane, C., Mohr-Jensen, C., Hayes, A. J., Carucci, S., … Cipriani, A. (2018). Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: A systematic review and network meta-analysis. The Lancet Psychiatry, 5(9), 727–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese, S., Holtmann, M., Banaschewski, T., Buitelaar, J., Coghill, D., Danckaerts, M., … Sergeant, J. (2013). Practitioner review: Current best practice in the management of adverse events during treatment with ADHD medications in children and adolescents. Journal of Child Psychology and Psychiatry, 54(3), 227–246. [DOI] [PubMed] [Google Scholar]
- Donnelly, C., Bangs, M., Trzepacz, P., Jin, L., Zhang, S., Witte, M. M., … Spencer, T. J. (2009). Safety and tolerability of atomoxetine over 3 to 4 years in children and adolescents with ADHD. Journal of the American Academy of Child and Adolescent Psychiatry, 48(2), 176–185. [DOI] [PubMed] [Google Scholar]
- Dosi, C., Riccioni, A., Della Corte, M., Novelli, L., Ferri, R., & Bruni, O. (2013). Comorbidities of sleep disorders in childhood and adolescence: Focus on migraine. Nature and Science of Sleep, 5, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito, M., Parisi, P., Miano, S., & Carotenuto, M. (2013). Migraine and periodic limb movement disorders in sleep in children: A preliminary case-control study. The Journal of Headache and Pain, 14(1), 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman, M. F., & Tukey, J. W. (1950). Transformations related to the angular and the square root. The Annals of Mathematical Statistics, 21(4), 607–611. [Google Scholar]
- Fuh, J. L., Wang, S. J., Lu, S. R., Liao, Y. C., Chen, S. P., & Yang, C. Y. (2010). Headache disability among adolescents: A student population-based study. Headache, 50(2), 210–218. [DOI] [PubMed] [Google Scholar]
- Genizi, J., Gordon, S., Kerem, N. C., Srugo, I., Shahar, E., & Ravid, S. (2013). Primary headaches, attention deficit disorder and learning disabilities in children and adolescents. The Journal of Headache and Pain, 14(1), 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham, J., Banaschewski, T., Buitelaar, J., Coghill, D., Danckaerts, M., Dittmann, R. W., … Taylor, E. (2011). European guidelines on managing adverse effects of medication for ADHD. European Child & Adolescent Psychiatry, 20(1), 17–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidetti, V., Faedda, N., & Siniatchkin, M. (2016). Migraine in childhood: Biobehavioural or psychosomatic disorder? The Journal of Headache and Pain, 17(1), 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins, J. P. T., & Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21(11), 1539–1558. [DOI] [PubMed] [Google Scholar]
- Jameson, N. D., Sheppard, B. K., Lateef, T. M., Vande Voort, J. L., He, J. P., & Merikangas, K. R. (2016). Medical comorbidity of attention-deficit/hyperactivity disorder in US adolescents. Journal of Child Neurology, 31(11), 1282–1289. [DOI] [PubMed] [Google Scholar]
- Kaplan, B. J., McNicol, J., Conte, R. A., & Moghadam, H. K. (1987). Physical signs and symptoms in preschool-age hyperactive and normal children. Journal of Developmental and Behavioral Pediatrics, 8(6), 305–310. [PubMed] [Google Scholar]
- Karwautz, A., Wöber, C., Lang, T., Böck, A., Wagner-Ennsgraber, C., Vesely, C., … Wöber-Bingöl, C. (1999). Psychosocial factors in children and adolescents with migraine and tension-type headache: A controlled study and review of the literature. Cephalalgia, 19(1), 32–43. [DOI] [PubMed] [Google Scholar]
- Kirova, A. M., Kelberman, C., Storch, B., DiSalvo, M., Woodworth, K. Y., Faraone, S. V., & Biederman, J. (2019). Are subsyndromal manifestations of attention deficit hyperactivity disorder morbid in children? A systematic qualitative review of the literature with meta-analysis. Psychiatry Research, 274, 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutuk, M. O., Tufan, A. E., Guler, G., Yalin, O. O., Altintas, E., Bag, H. G., … Ozge, A. (2018). Migraine and associated comorbidities are three times more frequent in children with ADHD and their mothers. Brain & Development, 40(10), 857–864. [DOI] [PubMed] [Google Scholar]
- Lateef, T. M., & Merikangas, K. R. (2017). Epidemiology of headache in children and adolescents. In Guidetti V., Arruda M. A., & Ozge A. (Eds.), Headache and comorbidities in childhood and adolescence (pp. 1–10). Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Lateef, T. M., Merikangas, K. R., He, J., Kalaydjian, A., Khoromi, S., Knight, E., & Nelson, K. B. (2009). Headache in a national sample of American children: Prevalence and comorbidity. Journal of Child Neurology, 24(5), 536–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law, E. F., Palermo, T. M., Zhou, C., & Groenewald, C. B. (2019). Economic impact of headache and psychiatric comorbidities on healthcare expenditures among children in the United States: A retrospective cross-sectional study. Headache, 59(9), 1504–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P. A., … Moher, D. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ (Clinical Research ed.), 339, b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, J. J. (1978). The inverse of the Freeman – Tukey double arcsine transformation. The American Statistician, 32(4), 138. [Google Scholar]
- Moutran, A. R., Villa, T. R., Diaz, L. A., Noffs, M. H., Pinto, M. M., Gabbai, A. A., & Carvalho Dde, S. (2011). Migraine and cognition in children: A controlled study. Arquivos de neuro-psiquiatria, 69(2A), 192–195. [DOI] [PubMed] [Google Scholar]
- Pan, P. Y., & Yeh, C. B. (2017). Impact of depressive/anxiety symptoms on the quality of life of adolescents with ADHD: A community-based 1-year prospective follow-up study. European Child & Adolescent Psychiatry, 26(6), 659–667. [DOI] [PubMed] [Google Scholar]
- Parisi, P., Verrotti, A., Paolino, M. C., Ferretti, A., Raucci, U., Moavero, R., … Curatolo, P. (2014). Headache and attention deficit and hyperactivity disorder in children: Common condition with complex relation and disabling consequences. Epilepsy & Behavior, 32, 72–75. [DOI] [PubMed] [Google Scholar]
- Polanczyk, G. V., Willcutt, E. G., Salum, G. A., Kieling, C., & Rohde, L. A. (2014). ADHD prevalence estimates across three decades: An updated systematic review and meta-regression analysis. International Journal of Epidemiology, 43(2), 434–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punja, S., Shamseer, L., Hartling, L., Urichuk, L., Vandermeer, B., Nikles, J., & Vohra, S. (2016). Amphetamines for attention deficit hyperactivity disorder (ADHD) in children and adolescents. The Cochrane Database of Systematic Reviews, 2, Cd009996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva, D., Aggio, F., Vago, C., Nichelli, F., Andreucci, E., Paruta, N., … Bulgheroni, S. (2006). Cognitive and behavioural effects of migraine in childhood and adolescence. Cephalalgia, 26(5), 596–603. [DOI] [PubMed] [Google Scholar]
- Ruggiero, S., Clavenna, A., Reale, L., Capuano, A., Rossi, F., & Bonati, M. (2014). Guanfacine for attention deficit and hyperactivity disorder in pediatrics: A systematic review and meta-analysis. European Neuropsychopharmacology, 24(10), 1578–1590. [DOI] [PubMed] [Google Scholar]
- Salem, H., Vivas, D., Cao, F., Kazimi, I. F., Teixeira, A. L., & Zeni, C. P. (2018). ADHD is associated with migraine: A systematic review and meta-analysis. European Child & Adolescent Psychiatry, 27(3), 267–277. [DOI] [PubMed] [Google Scholar]
- Schieve, L. A., Gonzalez, V., Boulet, S. L., Visser, S. N., Rice, C. E., Van Naarden Braun, K., & Boyle, C. A. (2012). Concurrent medical conditions and health care use and needs among children with learning and behavioral developmental disabilities, National Health Interview Survey, 2006–2010. Research in Developmental Disabilities, 33(2), 467–476. [DOI] [PubMed] [Google Scholar]
- Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., … Henry, D. A. (2017). AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ (Clinical Research ed.), 358, j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., … Higgins, J. P. T. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ (Clinical Research ed.), 366, l4898. [DOI] [PubMed] [Google Scholar]
- Storebø, O. J., Ramstad, E., Krogh, H. B., Nilausen, T. D., Skoog, M., Holmskov, M., … Gluud, C. (2015). Methylphenidate for children and adolescents with attention deficit hyperactivity disorder (ADHD). The Cochrane Database of Systematic Reviews (11), Cd009885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strine, T. W., Okoro, C. A., Mcguire, L. C., & Balluz, L. S. (2006). The associations among childhood headaches, emotional and behavioral difficulties, and health care use. Pediatrics, 117(5), 1728–1735. [DOI] [PubMed] [Google Scholar]
- Takemura, Y., Sakurai, Y., Yokoya, S., Otaki, J., Matsuoka, T., Ban, N., … Tsuda, T. (2005). Open-ended questions: Are they really beneficial for gathering medical information from patients? The Tohoku Journal of Experimental Medicine, 206(2), 151–154. [DOI] [PubMed] [Google Scholar]
- RStudio Team. (2015). RStudio: Integrated Development for R. RStudio, Inc. Boston, MA. http://www.rstudio.com/ [Google Scholar]
- Thomas, R., Sanders, S., Doust, J., Beller, E., & Glasziou, P. (2015). Prevalence of attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Pediatrics, 135(4), e994–1001. [DOI] [PubMed] [Google Scholar]
- Turner, D. P., Smitherman, T. A., Black, A. K., Penzien, D. B., Porter, J. A., Lofland, K. R., & Houle, T. T. (2015). Are migraine and tension-type headache diagnostic types or points on a severity continuum? An exploration of the latent taxometric structure of headache. Pain, 156(7), 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viechtbauer, W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 48. [Google Scholar]
- Villa, T. R., Agessi, L. M., Moutran, A. R., Gabbai, A. A., & Carvalho Dde, S. (2016). Visual attention in children with migraine: The importance of prophylaxis. Journal of Child Neurology, 31(5), 569–572. [DOI] [PubMed] [Google Scholar]
- Waldie, K. E., Hausmann, M., Milne, B. J., & Poulton, R. (2002). Migraine and cognitive function: A life-course study. Neurology, 59(6), 904–908. [DOI] [PubMed] [Google Scholar]
- Wang, S. M., Han, C., Lee, S. J., Jun, T. Y., Patkar, A. A., Masand, P. S., & Pae, C. U. (2017). Modafinil for the treatment of attention-deficit/hyperactivity disorder: A meta-analysis. Journal of Psychiatric Research, 84, 292–300. [DOI] [PubMed] [Google Scholar]
- Wells, G. A., Shea, B., O'Connell, D., Peterson, J., Welch, V., Losos, M., & Tugwell, P. (2009) The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. Retrieved from http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Whitehouse, W. P., & Agrawal, S. (2017). Management of children and young people with headache. Archives of Disease in Childhood. Education and Practice Edition, 102(2), 58–65. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit https://doi.org/10.1017/S0033291721004141.
click here to view supplementary material