Abstract
Androgens are steroid hormones that play a critical role in brain development and sexual maturation by acting upon both androgen receptors (AR) and estrogen receptors (ERα/β) after aromatization. The contribution of estrogens from aromatized androgens in brain development and the central regulation of metabolism, reproduction, and behavior is well defined, but the role of androgens acting on AR has been unappreciated. Here, we map the sex specific expression of Ar in the adult and developing mouse brain. Postnatal days (PND) 12 and 21 were used to target a critical window of prepubertal development. Consistent with previous literature in adults, sex‐specific differences in Ar expression were most profound in the bed nucleus of the stria terminalis (BST), medial amygdala (MEA) and medial preoptic area (MPO). Ar expression was also high in these areas at PND 12 and 21 in both sexes. In addition, we describe extra‐hypothalamic and extra‐limbic areas that show moderate, consistent and similar Ar expression in both sexes at both prepubertal time points. Briefly, Ar expression was observed in olfactory areas of the cerebral cortex, the hippocampus, several thalamic nuclei, and cranial nerve nuclei involved in autonomic sensory and motor function. To further characterize forebrain populations of Ar expressing neurons and determine whether they also coexpress estrogen receptors, we examined expression of Ar, Esr1 and Esr2 in prepubertal mice in selected nuclei. We found populations of neurons in the BST, MEA and MPO that coexpress Ar, but not Esr1 or Esr2, whereas others express a combination of the three receptors. Our findings indicate that various brain areas express Ar during prepubertal development and may play an important role in female neuronal development and physiology.
Keywords: gonadal steroids, postnatal development, puberty, sex differences
The neuroanatomical distribution of androgen receptor (Ar) mRNA in male and female mice is described and characterized at two prepubertal time points that frame an active period of brain development. We expand upon previous literature to map Ar distribution in cerebral cortex, thalamus, hypothalamus, and brainstem. Additionally, we identified forebrain sites that coexpress Ar with Esr1 and/or Esr2 during development.
1. INTRODUCTION
Gonadal steroids, including androgens and estrogens, play a dominant role in the development of sex differences in the brain. During male embryonic development, expression of the Sry gene located on the Y chromosome leads to differentiation of bipotental gonads into testes, which begin secreting testosterone. 1 , 2 , 3 , 4 Embryonic testosterone is locally converted to estradiol by the enzyme P450 aromatase (CYP19A1), 5 , 6 , 7 which acts to masculinize and defeminize specific brain nuclei via estrogen receptor alpha (ERα) and estrogen receptor beta (ERβ). 8 , 9 , 10 , 11 Both effects take place during the organizational window of development, 12 , 13 , 14 when the bipotential brain is most sensitive to the organizational effect of gonadal steroids. Developing females, which lack Sry, do not develop testes or produce testosterone, and are protected from maternal estradiol by the presence of alpha‐fetoprotein in utero, and therefore differentiate toward a feminized brain. 15 , 16 As a result, several adult brain sites display gonadal steroid‐dependent sexual dimorphism. 17 , 18 , 19 , 20
During puberty, increased activity of hypothalamic gonadotropin‐releasing hormone neurons drives pituitary synthesis and release of gonadotropins, which induce gonadal steroid secretion and production of mature gametes. 21 Testosterone activates developmentally programmed brain circuits to generate male specific behaviors, whereas cyclical ovarian steroids have a similar role in females. 22 Circulating levels of androgens are higher in males during and after completion of puberty, 23 , 24 , 25 whereas very low levels of androgens are detected during the prepubertal stage in both sexes. In the hypothalamus, however, androgen receptor immunoreactivity (AR‐IR) is observed throughout postnatal development in rodents. AR‐IR is higher in male mice at postnatal day (PND) 5, but comparable at 15 days of age, when increasing numbers of female neurons show AR‐IR. 26 This is highly relevant because the prepubertal window between PND 12 and 22 accounts for the greatest differences in temporal gene expression 27 , 28 indicating that active neurodevelopmental changes occur prior to puberty and the activation of the hypothalamic‐pituitary‐gonadal axis, when circulating gonadal steroids are low, particularly in females.
The requirement of gonadal steroids and sexual dimorphism in specific brain nuclei for reproduction has been widely demonstrated, 29 , 30 but the same cannot be said of non‐reproductive sex‐dependent or sex‐associated brain responses and function. Among them, emotion, motivation, addiction and energy balance are well‐defined. 31 Notably, sex is one of the most relevant risk factors for a variety of psychiatric and neurologic disorders, most of which show clinical onset in peripubertal stages. 32 Whether this is a direct effect of developmental testosterone is not clear. In both sexes, many adult brain areas outside reproductive centers are androgen sensitive and express AR, 33 but the distribution of Ar expression in male and female brain during the prepubertal time window has been poorly defined.
In the present study, we performed a comprehensive analysis of Ar expression in the mouse brain, expanding upon previous descriptions 26 , 34 , 35 , 36 , 37 to include all main subdivisions (e.g., neocortex, thalamus, brainstem, circumventricular organs) in both sexes. In addition, we mapped the distribution of Ar expression in the developing brain, specifically at PND 12 and 21, which frame a critical window of pubertal development. 27 , 28 Finally, we evaluated whether Ar is coexpressed with Esr1 and/or Esr2 in prepubertal forebrain neurons to gain insight into the nuclei that express Ar but not genomic acting ERs. Our data provide a greater in‐depth anatomical map of reproductive and non‐reproductive sites of androgen action in the male and female mouse brain during pubertal transition.
2. METHODS
2.1. Animal ethics
All research animals were acquired, used and maintained in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals, 38 the US Public Health Service's Policy on Humane Care and Use of Laboratory Animals, and Guide for the Care and Use of Laboratory Animals, as well as federal, state and local laws. Procedures and protocols were approved by the University of Michigan Committee on Use and Care of Animals (IACUC, Animal Protocol: PRO8712).
2.2. Animals
C57BL/6J (JAX® mice, stock #000664), mice were housed in an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) accredited facility at the University of Michigan Medical School. Mice were housed under a 12:12 light/dark photocycle at 21–23°C and 30%–70% relative humidity. Mice were provided water ad libitum and were fed a phytoestrogen‐reduced diet (16% protein, 4.0% fat, 48.5% carbohydrate, Teklad 2916 irradiated global rodent diet; Envigo) or a phytoestrogen‐reduced, higher protein and fat diet (19% protein, 9% fat, 44.9% carbohydrate, Teklad 2919 irradiated global rodent diet; Envigo) for breeding and lactating females. Phytoestrogen‐reduced diets were used to avoid any effects of exogenous dietary estrogens on AR expression in experimental mice. Adult male mice were single housed at least 1 week prior to euthanasia to control for housing status, which may impact testosterone levels, 39 and androgen‐regulated AR expression. 36 , 37 Adult female mice (group housed) were euthanized during diestrus, after completing at least two estrous cycles. Cycle stage was determined by vaginal lavage with predominately leukocytes 40 and confirmed by uterine weight below 100 mg. 41
Sample size was 5–9 animals per sex and per age group (PND 12, PND 21, and adult).
2.3. Tissue preparation
Adult (PND 56–70) and PND 21 mice were deeply anesthetized with isoflurane and transcardially perfused with diethyl pyrocarbonate (DEPC)‐treated 0.1 M phosphate‐buffered saline (PBS) until liver and lungs cleared (approximately 1 min), followed by 10% neutral buffered formalin (NBF) for 10 min. Brains were dissected and postfixed for 2 h, then transferred to 20% sucrose in DEPC‐treated 0.1 M PBS overnight for cryoprotection. PND 12 mice were anesthetized with isoflurane and euthanized by decapitation. Brains were dissected and fixed in 10% NBF for 4 h, then transferred to 20% sucrose in 10% NBF for 48–72 h at 4°C. PND 12 and PND 21 brains were embedded in optimal cutting temperature (OCT) compound, frozen on dry ice and stored at −80°C. Brains from PND 12 and 21 mice were sectioned at 30 µm thickness on the frontal plane into four or five series on a cryostat (CM 3050S; Leica). Sections were directly collected onto SuperFrost Plus slides (Fisher Scientific) and stored at −20°C. Adult brains were sectioned at 30 µm thickness on the frontal plane into five series on a freezing microtome (SM 2010R; Leica). Sections were stored at −20°C in DEPC‐treated cryoprotectant.
2.4. Immunohistochemistry
AR immunoreactivity was visualized using a modified tyramide signal amplification method as described previously. 42 Brain sections were rinsed with 0.1 M PBS, incubated in 0.6% hydrogen peroxide for 30 min, rinsed with 0.1 M PBS and then blocked with 3% normal donkey serum with 0.25% TritonX‐100 for 1 h at room temperature. Sections were incubated overnight with rabbit anti‐AR antibody (dilution 1:200; AbCam [EPR1535(2)], catalog. no. ab133273; RRID: AB_11156085). A series with no primary antibody was included as a negative control (Figure 1A,B). Sections were rinsed with 0.1 M PBS and then incubated for 1 h with biotinylated donkey anti‐rabbit immunoglobulin G (dilution 1:500; Jackson ImmunoResearch Laboratories, catalog. no. 711‐065‐152; RRID: AB_2340593), followed by incubation in avidin‐biotin solution in 0.1 M PBS (dilution 1:1000; Vector Laboratories) for 1 h. Next, sections were incubated in biotinylated tyramide (dilution 1:250; Perkin Elmer) with 0.009% hydrogen peroxide for 10 min, followed by incubation with streptavidin‐conjugated AlexaFluor 594 (dilution 1:1000; Invitrogen, ThermoFisher) for 1 h. Sections were mounted onto gelatin‐coated slides and coverslipped with ProLong Gold Antifade mounting medium (Invitrogen, ThermoFisher).
FIGURE 1.
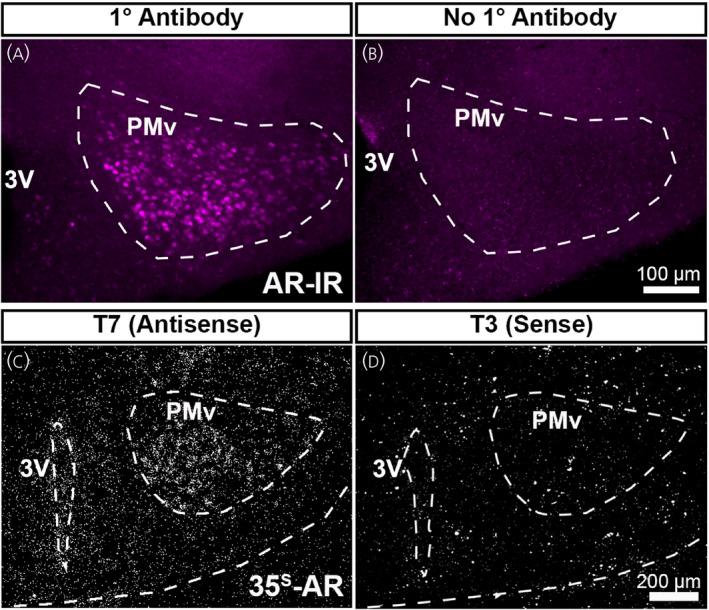
Validation of androgen receptor (AR) immunohistochemistry and Ar in situ hybridization probe. (A, B) Fluorescent images showing AR‐immunoreactivity (AR‐IR) in the adult female mouse brain (postnatal day [PND] 56–70). AR‐IR was observed in sections incubated in primary antibody (A), but not in sections without primary antibody (B). (C, D) Darkfield images showing silver grain deposition corresponding to Ar hybridization signal in adjacent sections from the same brain (PND 12 male mouse). A signal was observed in sections hybridized with an antisense probe (C), but not with a sense probe (D). PMv, ventral premammillary nucleus; 3V, third ventricle. Scale bar = 100 µm (A, B), 200 µm (C, D)
2.5. In situ hybridization (ISH)
Adult brain sections were mounted onto Superfrost Plus slides (Fisher Scientific) in DEPC‐treated 0.1 m PBS, air dried overnight at room temperature and stored at −20°C. For pretreatment, slides were thawed at room temperature for 15–20 min and then fixed with 10% NBF for 15 min. Slides were rinsed with DEPC‐treated PBS, then dehydrated with increasing concentrations of ethanol and cleared with xylene. Slides were rehydrated, boiled in sodium citrate (0.01 M sodium citrate, pH 6.0 in DEPC‐H2O) in a microwave for 10 min, dehydrated and air dried for 30 min at room temperature.
To generate a 35S‐labelled Ar cRNA riboprobe, a cDNA template was first generated by reverse transcriptase‐polymerase chain reaction (PCR) amplification using cDNA obtained from whole mouse hypothalamic RNA (TRIzol Reagent; Ambion, Life Technologies) and the primer pairs: Forward 5′‐CAACCAGATTCCTTTGCTGCC‐3′ and Reverse 5′‐GAGCTTGGTGAGCTGGTAGAA‐3′ (NCBI accession number NM_013476.4; Mus musculus androgen receptor [Ar], mRNA, target region 3042–3551, product length 510 bp). Linear template PCR products were gel purified in accordance with the manufacturer's protocol (QIAquick Gel Extraction Kit, 28706; Qiagen). To generate an antisense cRNA 35S‐Ar riboprobe by in vitro transcription, the linear template was incubated with 35S‐UTP (UTPαS; Perkin Elmer) and T7 RNA polymerase in accordance with the manufacturer's protocol (Promega). A control sense cRNA 35S‐Ar riboprobe was generated with T3 RNA polymerase using the same protocol (Figure 1C,D). Riboprobes were diluted to 10 × 106 cpm mL–1 in hybridization buffer (50% formamide, 10 mm Tris‐HCl, pH 8.0 [Invitrogen], 5 mg of tRNA, 10 mM dithiothreitol, 10% dextran sulfate, 0.3 M NaCl, 1 mm ethylenediaminetetraacetic acid, 1 × Denhardt's solution, 0.1% sodium dodecyl sulfate, 0.1% sodium thiosulfate). Hybridization solution was applied to slides, which were coverslipped and incubated overnight at 57°C. The following morning, slides were treated with RNAse A (Roche Applied Bioscience) for 30 min and then treated with a series of high stringency washes in sodium chloride‐sodium citrate buffer. Slides were dehydrated, air dried and then placed into an X‐ray film cassette with Biomax MR film (Carestream) for 1–2 days. Slides were dipped in NTB autoradiographic emulsion (Kodak, VWR), dried and stored at 4°C in foil‐wrapped slide boxes for 5 days per 1 day of film exposure. Slides were developed with GBX (Carestream Dental) developer and fixer, then dehydrated with graded ethanol, cleared with xylene and coverslipped with DPX mounting media (Electron Microscopy Sciences).
To generate neuroanatomical references, slides with adjacent sections of PND 12 and 21 male and female brains were dipped in 0.25% thionin for 45 s, quickly rinsed in water, dehydrated in increasing concentration of ethanol and cleared in xylene. Slides were coverslipped with DPX mounting media.
2.6. Fluorescent ISH
For fluorescent ISH, PND 12 and PND 21 mice were deeply anesthetized with isoflurane and euthanized by decapitation. Brains were rapidly removed, embedded in OCT compound, frozen on dry ice and stored at −80°C. Brains were sectioned at 16 µm thickness on the frontal plane into five series on a cryostat (CM 3050S; Leica). Sections were directly collected onto SuperFrost Excell slides (Fisher Scientific) and stored at −80°C. Tissue sections were fixed in 10% NBF for 15 min and then dehydrated with graded ethanol. An RNAscope™® Multiplex Fluorescent Assay v2 (Advanced Cell Diagnostics [ACD]) kit was used for blocking, hybridization and amplification steps, in accordance with the manufacturer's instructions. Briefly, endogenous peroxidase activity was blocked with H2O2 for 10 min, washed in DEPC‐treated water and then sections were gently digested with Protease IV for 30 min at room temperature. Sections were then incubated with probes targeting M. musculus Ar (ACD catalog. no. 316991‐C2, NCBI Accession no. NM_013476.3, target region: 1432–2422), Esr1 (ACD catalog. no. 478201‐C3, NCBI Accession no. NM_007956.5, target region: 678–1723), and Esr2 (ACD catalog. no. 316121, NCBI Accession no. NM_207707, target region: 424–1875) for 2 h at 40°C. Following hybridization, probes were labelled via tyramide signal amplification with fluorescent dyes (Opal520, OpalCy3 or OpalCy5; Akoya Biosciences). Sections were counterstained with DAPI (4′,6‐diamidino‐2‐phenylindole), then coverslipped with Prolong Gold antifade mounting media (ThermoFisher). The probes have been validated by ACD, but we performed an additional control by analyzing the previously described distribution of all three genes, as well as our own ISH using radioisotopes.
2.7. Microscopy and image acquisition
Digital images were acquired using an Axio Imager M2 (Carl Zeiss) with a digital camera (AxioCam, Zeiss) using Zen Pro 2 (Zeiss). Digital images of fluorescent ISH were acquired using a Nikon A1si inverted confocal microscope and Nikon Elements at the University of Michigan BRCF Microscopy Core. Photomicrographs of films were acquired using a SteREO Discovery.V8 stereomicroscope with a digital camera (AxioCam, Carl Zeiss), using the same magnification, illumination and exposure time for each image. Dark field photomicrographs for silver grains (hybridization signal) were acquired using the same illumination and exposure time for each section, at 10× magnification.
2.8. Images
Adobe Photoshop (Adobe Creative Cloud) was used to prepare digital images, including adjusting resolution to 300 dpi, adjustment of image size, addition of annotation and labels, conversion to greyscale, unsharp mask, and levels. Uniform adjustments were made to every image. Mouse brain coordinates were estimated from Paxinos and Franklin's Mouse Brain in Stereotaxic Coordinates atlas. 43 Abbreviations are based on the Allen Mouse Brain Atlas (PND 56; Coronal Reference Atlas, Allen Institute for Brain Science, Allen Mouse Brain Atlas; http://mouse.brain‐map.org/static/atlas).
2.9. Data analysis
Estimation of a hybridization signal was obtained by analysis of integrated optical density (IOD) using ImageJ (NIH; http://rsb.info.nih.gov/ij) as described previously. 44 , 45 Briefly, IOD values were calculated as the total IOD of a constant region of interest after subtracting background intensity. Quantification of Ar silver grain IOD was performed in one 30‐µm thick section, on one hemisphere of each animal (n = 5–9 per group), at approximately the same rostrocaudal level. Qualitative analysis was subjective based on relative expression (e.g., highest expression = ++++; lowest expression = +) and was performed by two independent evaluators. Co‐expression of Ar, Esr1 and Esr2 in male and female PND 12 and PND 21 mice was evaluated in one 16‐μm thick section, on one hemisphere of each animal. As a result of the punctate nature of the fluorescent signal and lack of definition of cellular borders, the quantification reflects only an estimation of co‐expression relative to total number of Ar expressing cells. Only forebrain sites with clear expression of all three genes were quantified.
2.10. Statistical analysis
Data are reported as the mean ± SEM. Analysis was performed using Prism, version 8 (GraphPad Software Inc.). Normal distribution of data was analyzed using the Shapiro–Wilk test (significance alpha 0.05). An unpaired t test with Welch's correction was used for normally distributed data and a Mann–Whitney non‐parametric test was used for non‐normally distributed data, to analyze IOD. Exact values are reported. p < 0.05 was considered statistically significant.
3. RESULTS
3.1. Distribution of Ar mRNA in adult mouse brain
Ar mRNA expression was visualized using ISH histochemistry. The hybridization signal on autoradiographic film was evaluated in male and female brain sections (n = 5–9 per sex) (Figure 2A). Adult brains were systematically examined and compared with published data as an initial control. 26 , 36 , 37 Analysis of AR‐IR was also performed as a control for areas that had not been fully described in previous publications (n = 3–4 per sex) (Figure 3).
FIGURE 2.
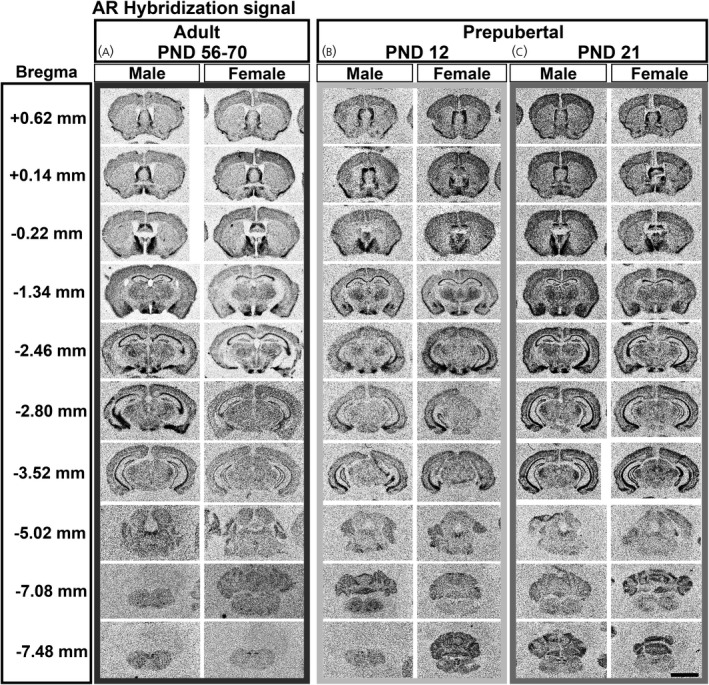
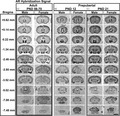
Ar mRNA hybridization signal expression in male and female prepubertal and adult brain. Images from scanned autoradiographic film of adult, postnatal day [PND] 56–70 (A), and prepubertal, PND 12 (B) and PND 21 (C), male and female mouse brain. Select coronal sections are shown in rostral to caudal order. Darker signal indicates higher expression of Ar mRNA. Approximate distance from bregma (left column) derived from adult mouse brain (Paxinos and Franklin atlas). Scale bar = 4000 µm
FIGURE 3.
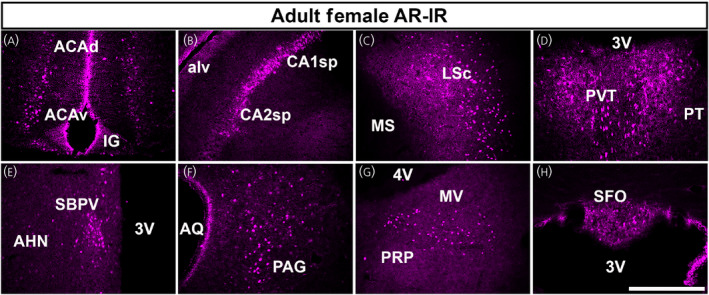
Androgen receptor (AR) immunoreactivity (AR‐IR) in adult mouse brain. (A–H) Fluorescen images showing AR‐IR in the adult female mouse brain (postnatal day [PND] 56–70). AR‐IR was observed in almost all areas where we observed Ar mRNA. Selected areas from (A) cerebral cortex (dorsal and ventral anterior cingulate area, ACAd, ACAv), (B) hippocampal formation (pyramidal layer of fields CA1 and CA2), (C) cerebral nuclei (lateral septal nucleus, caudodorsal, LSc), (D) thalamus (paraventricular nucleus of the thalamus, PVT), (E) hypothalamus (subparaventricular zone, SBPV), (F) midbrain (periaqueductal gray, PAG), (G) pons/medulla (medial vestibular nucleus, MV) and (H) circumventricular organs (subfornical organ, SFO) are shown. AHN, anterior hypothalamic nucleus; alv, alveus; AQ, cerebral aqueduct; IG, induseum griseum; MS, medial septal nucleus; PRP, nucleus prepositus; PT, parataenial nucleus; 3V, third ventricle; 4V, fourth ventricle. Scale bar = 100 µm
Patterns of hybridization signal were similar between sexes in several subdivisions of the cerebral cortex, including the motor (MO), piriform (PIR) and anterior cingulate (ACA) (Table 1). In the hippocampal formation, highest expression was observed in fields CA1 and CA2 (Bregma −1.34 through −3.52 mm) (Figure 2A) and lowest expression in the entorhinal area (ENT). As previously described for AR‐IR, 35 , 36 , 37 several cortical subplate and cerebral nuclei displayed apparent sex differences. The lateral septal nucleus (caudodorsal and rostroventral subdivisions, LSc and LSr) (Bregma +0.62, +0.14, −0.22 mm) (Figure 2A), bed nucleus of the stria terminalis (principal, BSTpr) (Bregma −0.22 mm) (Figure 2A), posterodorsal medial amygdalar nucleus (MEApd) (Bregma −1.34 mm) (Figure 2A) and posterior amygdala (PA) (Bregma −2.46 mm) (Figure 2A) showed higher Ar mRNA levels in males. The cortical amygdalar area (COA) displayed high Ar mRNA in both sexes (Table 1).
TABLE 1.
Qualitative expression of Ar mRNA distribution by nuclei in postnatal and adult mouse brain
| Brain areas and nuclei | Adult | Prepubertal | ||||
|---|---|---|---|---|---|---|
| PND 56–70 | PND 12 | PND 21 | ||||
| Male | Female | Male | Female | Male | Female | |
| Cerebral cortex | ||||||
| Motor (MO) | +/− | +/− | +/− | +/− | +/− | +/− |
| Olfactory nucleus, anterior (AON) | − | − | ++ | ++ | ++ | ++ |
| Taenia tecta (TT) | + | +/− | + | + | + | + |
| Piriform (PIR) | + | + | + | + | + | + |
| Cingulate, anterior (ACA) | + | + | + | + | + | + |
| Endopiriform (EP) | +/− | +/− | +/− | +/− | + | +/− |
| Hippocampal formation | ||||||
| Induseum griseum (IG) | + | + | + | + | + | + |
| Field CA1 (CA1) | +++ | +++ | +++ | +++ | +++ | +++ |
| Field CA2 (CA2) | +++ | +++ | +++ | +++ | +++ | +++ |
| Field CA3 (CA3) | + | + | + | + | + | + |
| Dentate gyrus (DG) | + | + | +/− | +/− | + | + |
| Entorhinal area (ENT) | +/− | +/− | + | + | +/− | +/− |
| Presubiculum/subiculum (PRE/SUB) | + | + | + | + | + | + |
| Cortical subplate and cerebral nuclei | ||||||
| Septohippocampal nucleus (SH) | + | + | + | + | + | + |
| Lateral septal nucleus, caudodorsal (LSc) | +++ | + | + | + | + | + |
| Lateral septal nucleus, rostroventral (LSr) | +++ | + | + | + | + | + |
| Bed nucleus of stria terminalis, principal nucleus (BSTpr) | ++++ | +++ | + | + | +++ | +++ |
| Cortical amygdalar area (COA) | +++ | +++ | + | + | +++ | +++ |
| Medial amygdalar nucleus, posterodorsal (MEApd) | +++ | + | + | + | +++ | +++ |
| Posterior amygdala (PA) | +++ | + | +++ | + | +++ | + |
| Thalamus and subthalamus | ||||||
| Ventral posterior complex of the thalamus (VP) | + | + | + | + | + | + |
| Paraventricular nucleus of the thalamus (PVT) | + | + | + | + | + | + |
| Nucleus of reuniens (RE) | +/− | +/− | + | + | ++ | + |
| Subthalamic/parasubthalamic, caudal (STN/PSTN) | ++ | + | + | + | ++ | ++ |
| Medial geniculate complex (MG) | + | + | + | + | ++ | + |
| Hypothalamus | ||||||
| Medial preoptic area, anterior (MPOa) | + | + | + | + | + | + |
| Medial preoptic area, posterior (MPOp) | ++++ | ++++ | ++++ | ++++ | ++++ | ++++ |
| Suprachiasmatic nucleus (SCH) | ++ | + | +/− | +/− | + | +/− |
| Paraventricular hypothalamic nucleus (PVH) | +/− | +/− | − | − | − | − |
| Periventricular hypothalamic nucleus (PV) | + | +/− | − | − | − | − |
| Subparaventricular zone (SBPV) | + | + | ++ | ++ | + | + |
| Lateral hypothalamic area (LHA) | +/− | +/− | − | − | − | − |
| Arcuate hypothalamic nucleus (ARH) | ++ | + | +/− | +/− | + | + |
| Ventromedial hypothalamic nucleus, ventrolateral (VMHvl) | + | ++ | + | + | ++ | + |
| Tuberal nucleus (TU) | + | + | − | − | + | + |
| Dorsomedial nucleus of the hypothalamus (DMH) | + | − | − | − | +/− | − |
| Dorsal premammillary nucleus (PMd) | + | + | ++ | ++ | ++ | ++ |
| Ventral premammillary nucleus (PMv) | ++++ | ++++ | +++ | +++ | +++ | +++ |
| Supramammillary nucleus (SUM) | + | +/− | + | + | + | +/− |
| Midbrain | ||||||
| Periaqueductal gray, ventrolateral (PAGvl) | +/− | +/− | +/− | +/− | + | + |
| Ventral tegmental area (VTA) | +/− | − | − | − | − | − |
| Red nucleus (RN) | +/− | − | − | − | +/− | −− |
| Dorsal raphe nucleus (DR) | +/− | +/− | +/− | +/− | + | + |
| Pons and medulla | ||||||
| Pontine reticular nucleus (PRN) | +/− | +/− | +/− | +/− | +/− | +/− |
| Superior olivary complex (SOC) | − | − | + | + | + | + |
| Principal sensory nucleus of the trigeminal (PSV) | − | − | + | +/− | + | − |
| Parabrachial nucleus (PB) | +/− | +/− | − | − | − | − |
| Dorsal tegmental nucleus (DTN) | + | + | + | + | + | + |
| Facial motor nucleus (VII) | + | +/− | + | + | + | + |
| Cochlear nuclei (CN) | +/− | − | + | + | + | + |
| Vestibular nucleus (VNC) | +/− | − | + | + | + | + |
| Nucleus ambiguus (AMB) | +/− | +/− | ++ | ++ | ++ | ++ |
| Hypoglossal nucleus (XII) | + | + | + | + | ++ | ++ |
| Nucleus of the solitary tract (NTS) | +/− | +/− | +/− | +/− | +/− | +/− |
| Dorsal motor nucleus of the vagus nerve (DMX) | + | + | + | + | ++ | ++ |
| Circumventricular organs | ||||||
| Subfornical organ (SFO) | +/− | + | + | + | +/− | +/− |
| Area postrema (AP) | +/− | +/− | +/− | +/− | + | + |
−, +/−, +, ++, +++, and ++++ represent not detected, very low, low, moderate, high, and very high expression of silver grain deposits corresponding to Ar mRNA. The Allen Mouse Brain Atlas was used as a reference for names, abbreviations, and location of nuclei.
Abbreviation: PND, postnatal day.
The thalamus and subthalamus contained a low to moderate Ar hybridization signal. Conspicuous expression was observed in the paraventricular (PVT), medial geniculate (MG) and subthalamic nuclei (STN) in both sexes (Bregma −1.34 to −3.52) (Figure 2A and Table 1).
Hypothalamic AR‐IR expression is fairly well characterized in adult mice, and the Ar hybridization signal was consistent with previous descriptions. 26 , 34 , 35 , 37 In brief, highest expression was seen in the medial preoptic area (MPO) (Bregma +0.14 mm) (Figure 2A), arcuate nucleus (ARH), ventrolateral subdivision of the ventromedial hypothalamic nucleus (VMHvl) (Bregma −1.34 mm) (Figure 2A) and ventral premammillary nucleus (PMv) (Bregma −2.46 mm) (Figure 2A). Sex differences were apparent in the suprachiasmatic nucleus (SCH) and ARH, with expression higher in male mice. A higher Ar hybridization signal was also apparent in the periventricular (PV) and dorsomedial (DMH) nuclei of the hypothalamus in males. The tuberal nucleus (TU) displayed low Ar expression, and the paraventricular hypothalamic nucleus (PVH) displayed very low expression in both sexes (Table 1). The supramammillary nucleus (SUM) was observed to have an apparent sex difference, with females exhibiting very low expression, and males with higher but still low Ar mRNA (Table 1).
In the midbrain, Ar mRNA was low in both sexes, and mainly observed in the periaqueductal gray (ventrolateral column, PAGvl) and dorsal raphe nucleus (DR) (Bregma −3.52 and −5.02 mm) (Figure 2A). Very low expression was also observed in the ventral tegmental area (VTA) and red nucleus (RN) (Table 1).
In the pons and medulla, low Ar mRNA expression was observed in the dorsal tegmental (DTN), facial motor (VII), hypoglossal (XII) and dorsal motor nucleus of the vagus nerve (DMX) in both sexes (Bregma −5.02 mm) (Figure 2A). A very low hybridization signal was observed in the parabrachial nucleus (PB) and pontine reticular nucleus (PRN) (Table 1). Ar mRNA was also detected in the nucleus ambiguus (AMB) (Bregma −7.08 mm) (Figure 2A) and nucleus of the solitary tract (NTS) (Bregma −7.48 mm) (Figure 1A) of both sexes, whereas the cochlear (CN) and vestibular nuclei (VNC) displayed a very low signal in males, but not in females (Table 1).
In circumventricular organs, we observed a very low to low Ar hybridization signal in the subfornical organ (SFO) and area postrema (AP) of both sexes (Table 1).
3.2. AR‐immunoreactivity in adult mouse brain
In the adult brain, our findings thoroughly replicate previous reports by different groups. 26 , 34 , 36 , 37 In brief, high AR‐IR was observed in the BSTpr, MPO, VMHvl, PMv and MEApd. In addition, and in agreement with Ar mRNA distribution, we found a moderate to low AR‐IR in the PIR, ACA (Figure 3A), CA1 and CA2 (Figure 3B), septohippocampal nucleus (SH), LSc (Figure 3C), PVT (Figure 3D), subparaventricular zone (SBPV) (Figure 3E), PA, PAGvl (Figure 3F), DTN (laterodorsal), and many nuclei of the cranial nerves, including the principal sensory nucleus of the trigeminal nerve (PSV), VII and medial vestibular nucleus (MV, Figure 3G). Scattered AR‐IR was also observed in the SFO (Figure 3H) and AP.
3.3. Prepubertal distribution of Ar mRNA
Ar mRNA expression was analyzed in two developmental prepubertal stages, PND 12 and 21 (n = 5–9 per sex per age) (Figure 2B,C). In the cerebral cortex, both male and female mice at PND 12 and PND 21 showed consistent and similar expression between sexes (Table 1). The anterior olfactory nucleus (AON) displayed a moderate Ar hybridization signal, whereas the taenia tecta (TT), PIR, and ACA displayed a low Ar hybridization signal (Figure 4A–D). The endopiriform (EP) and MO showed a very low Ar hybridization signal in PND 12 and PND 21 mice (Table 1).
FIGURE 4.
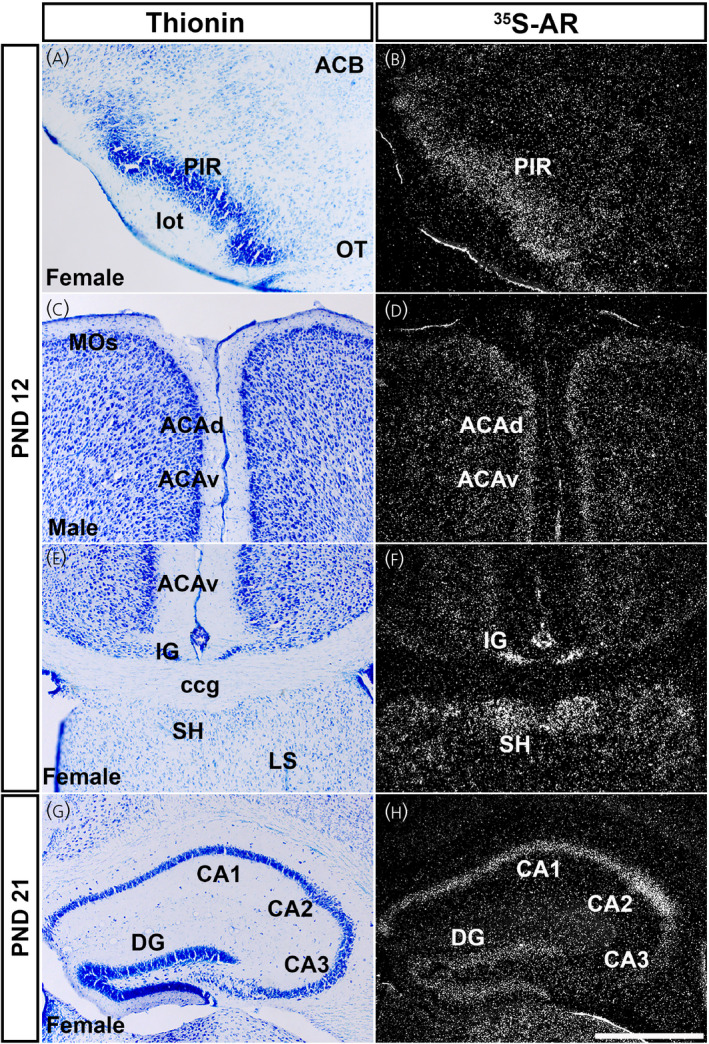
Ar mRNA expression in cerebral cortex in prepubertal male and female mice. Images showing thionin staining for neuroanatomical reference (left), silver grains corresponding to Ar mRNA (right). Low Ar expression was observed in the piriform area (PIR) (A, B), dorsal and ventral anterior cingulate area (ACAd and ACAv) (C, D), induseum griseum and septohippocampal nucleus (IG and SH) (E, F), and CA3, with high expression in field CA1 and CA2 (G, H). ACB, nucleus accumbens; ccg, genu of corpus callosum; DG, dentate gyrus; lot, lateral olfactory tract; LS, lateral septal nucleus; MOs, secondary motor area; OT, olfactory tubercle. PND, postnatal day. Scale bar = 200 µm
In the hippocampal formation, expression level of Ar mRNA in prepubertal mice was similar to that observed in adults. Briefly, expression of Ar mRNA in both male and female mice was detected in the induseum griseum (IG) (Figure 4E,F), CA1 and CA2 (Figure 4G,H), and presubiculum/subiculum (PRE/SUB). Higher expression was observed in CA1 and CA2, whereas lower expression was found in Field CA3 (CA3) (Table 1). In the dentate gyrus (DG), lower expression was observed in PND 12 male and female mice. The ENT displayed moderate expression at PND 12 in males and females, but expression decreased by PND 21.
Cortical subplate and cerebral nuclei also exhibited a consistent Ar hybridization signal in prepubertal mice in the SH (Figure 4E,F) and PA. The LSc displayed moderate expression at PND 12 (Figure 5A–C) and PND 21 (Figure 5D–F). The Ar hybridization signal was not different between sexes at PND 12 or PND 21 in the LSc (Figure 5C,F). The BSTpr showed similar levels between sexes at PND 12 and PND 21 between sexes (Figure 5G–L). The MEApd showed a similar pattern of Ar expression in between sexes at PND 12 and PND 21 (Table 1). Ar mRNA in the COA was also similar between sexes, with low expression at PND 12, increasing to high expression by PND 21 (Table 1).
FIGURE 5.
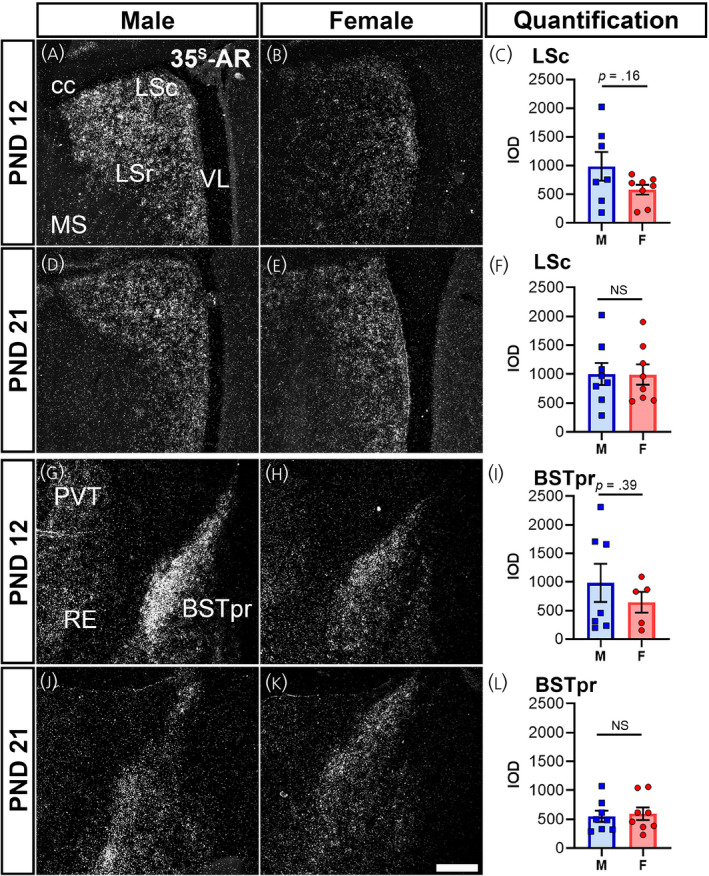
Ar mRNA expression in cerebral nuclei of male and female prepubertal mice. Silver grain deposition corresponding to Ar mRNA hybridization signal in prepubertal postnatal day (PND) 12 (A, B, G, H) and PND 21 (D, E, J, K) male (M) (A, D, G, J) and female (F) (B, E, H, K) mice. (A–F) Lateral septal nucleus, caudodorsal (LSc) and (G–L) bed nucleus of the stria terminalis, principal nucleus (BSTpr). Bar graphs showing the mean ± SEM integrated optical density (IOD) of silver grains (C, F, I, L). IOD was analyzed by a t test with Welch's correction for LSc male vs. female PND 12 (p = .16, n = 7–8 per sex), PND 21 (p = .96, n = 8 per sex), BST male vs. female PND 12 (p = .39, n = 5–7 per sex), and BST male vs. female PND 21 (p = .75, n = 8 per sex). Abbreviations: cc, corpus callosum; LSr, lateral septal nucleus, rostral (rostroventral); MS, medial septal nucleus; PVT, paraventricular nucleus of the thalamus; RE, nucleus of reuniens; VL, lateral ventricle. NS, not significant. Scale bar = 200 µm
In thalamic nuclei, moderate to high levels of Ar hybridization signal were observed in the PVT (Figure 6A,B), nucleus of reuniens (RE) (Figure 6C,D), ventral posterior complex nuclei (VP) (Figure 6E,F), STN (Figure 6G,H) and MG (Table 1). No difference between sexes and prepubertal ages was apparent.
FIGURE 6.
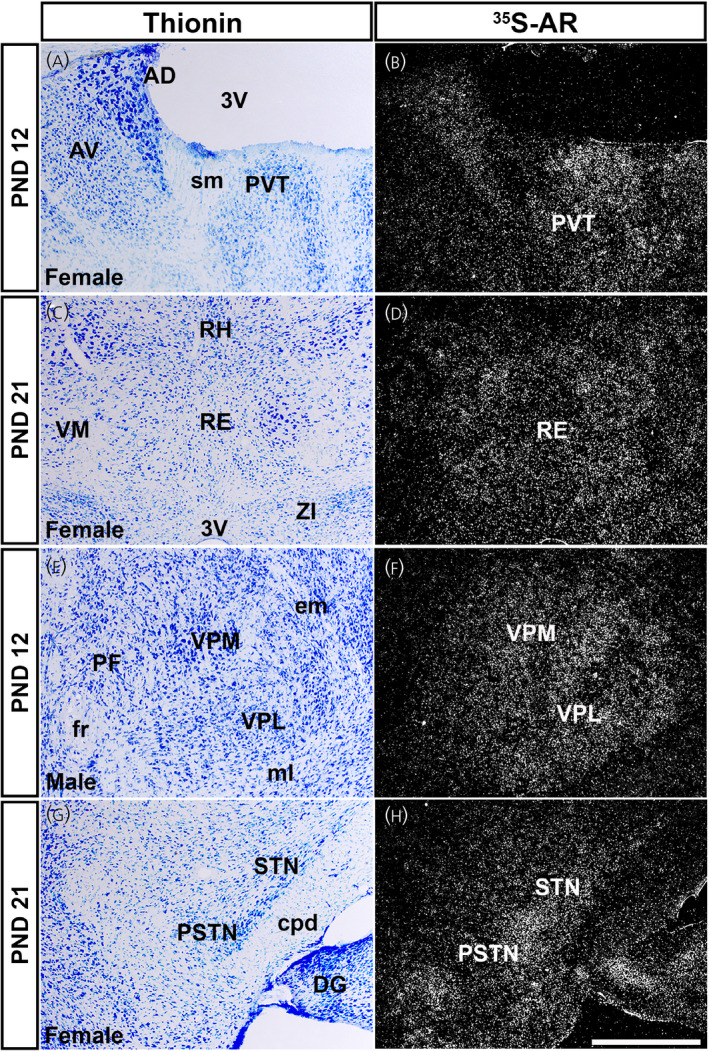
Ar mRNA expression in thalamic nuclei of male and female prepubertal mice. Images showing thionin staining for neuroanatomical reference (left), silver grains corresponding to Ar mRNA (right). (A, B) Low silver grain deposition in the paraventricular nucleus of the thalamus (PVT), with (C, D) low to moderate deposition in the nucleus of reuniens (RE), (E, F) ventral posterolateral and posteromedial nuclei of the thalamus (VPL and VPM), and (G, H) subthalamic and parasubthalamic nuclei (STN and PSTN). AD, anterodorsal nucleus of the thalamus; AV, anteroventral nucleus of the thalamus; cpd, cerebral peduncle; DG, dentate gyrus; em, external medullary lamina of the thalamus; fr, fasciculus retroflexus; ml, medial lemniscus; PF, parafascicular nucleus; RH, rhomboid nucleus; sm, stria medullaris; VM, ventral medial nucleus of the thalamus; ZI, zona incerta; 3V, third ventricle. PND, postnatal day. Scale bar = 200 µm
In the hypothalamus, the MPO and anteroventral periventricular nucleus showed similar levels of Ar in both sexes at PND 12 and PND 21 (Table 1). The SCH had similar levels of Ar at PND 12 in both sexes (Figure 7A–C,E–G); however, expression increased in male mice at PND 21 (Figure 7G). The SBPV, although apparently higher in males, was not significantly different when comparing sexes at both prepubertal ages (Figure 7D,H). The PMv showed no difference between sexes at PND 12 or PND 21 (Figure 7I–K,M–O). In the dorsal premammillary nucleus (PMd), Ar mRNA levels were low to moderate, and no difference between sexes or ages was observed (Figure 7L,P). The SUM displayed low Ar mRNA expression in both sexes at PND 12, as well as low expression in males and very low expression in females at PND 21 (Table 1). The TU exhibited no detectable Ar hybridization signal at PND 12, but a low signal was detected at PND 21 (Table 1). The PVH had no detectable Ar hybridization signal at either PND 12 or 21 (Table 1).
FIGURE 7.
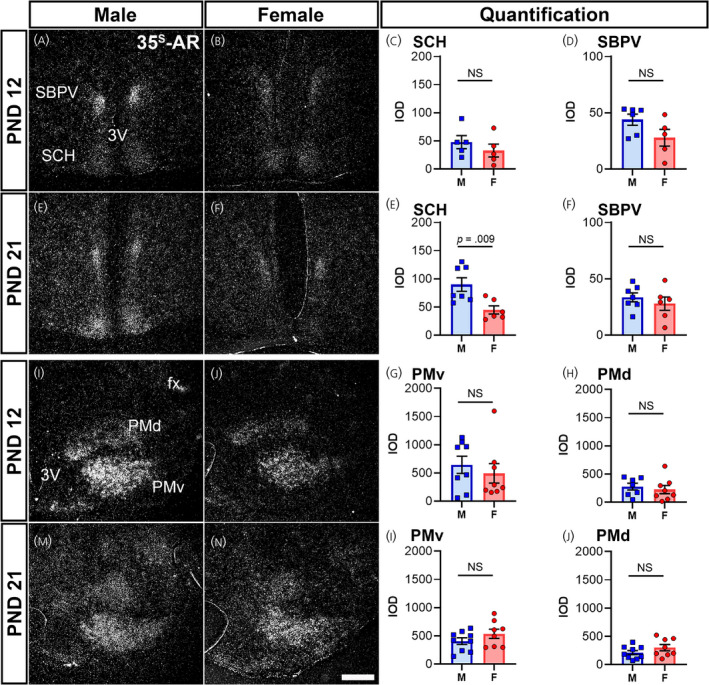
Ar mRNA expression in hypothalamic nuclei of male and female prepubertal mice. Silver grain deposition corresponding to Ar mRNA hybridization signal in prepubertal postnatal day (PND) 12 (A, B, I, J) and PND 21 (E, F, M, N) male (M) (A, E, I, M) and female (F) (B, F, J, N) mice. (A–H) Suprachiasmatic nucleus (SCH) and subparaventricular zone (SBPV), and (I–P) dorsal and ventral premammillary nuclei (PMd and PMv). Note the higher expression of Ar in the SCH of males at PND 21 (E). Bar graphs showing the mean ± SEM integrated optical density (IOD) of silver grains (C, D, G, H, K, L, O, P). IOD was analyzed by a t test with Welch's correction for SCH male vs. female PND 12 (p = .38, n = 5 per sex), SCH male vs. female PND 21 (p = .009, n = 6–7 per sex), SBPV male vs. female PND 21 (p = .45, n = 6–7 per sex), PMv male vs. female PND 21 (p = .21, n = 8–9 per sex), and PMd male vs. female PND 12 (p = .58, n = 7–8 per sex) and PND 21 (p = .19, n = 8–9 per sex), and a Mann–Whitney non‐parametric test for SBPV male vs. female PND 12 (p = .12, n = 6 per sex), and PMv male vs. female PND 12 (p = .57, n = 8 per sex). fx, fornix; 3V, third ventricle. NS, not significant. Scale bar = 200 µm
In the midbrain, expression of Ar hybridization signal was low to very low. The PAG showed low expression, which was consistent between sexes, particularly in the caudal ventrolateral column (PAGvl) (Figure 8A,B and Table 1). The DR also showed a low to very low level of Ar mRNA expression in PND 12 and PND 21 mice (Table 1).
FIGURE 8.
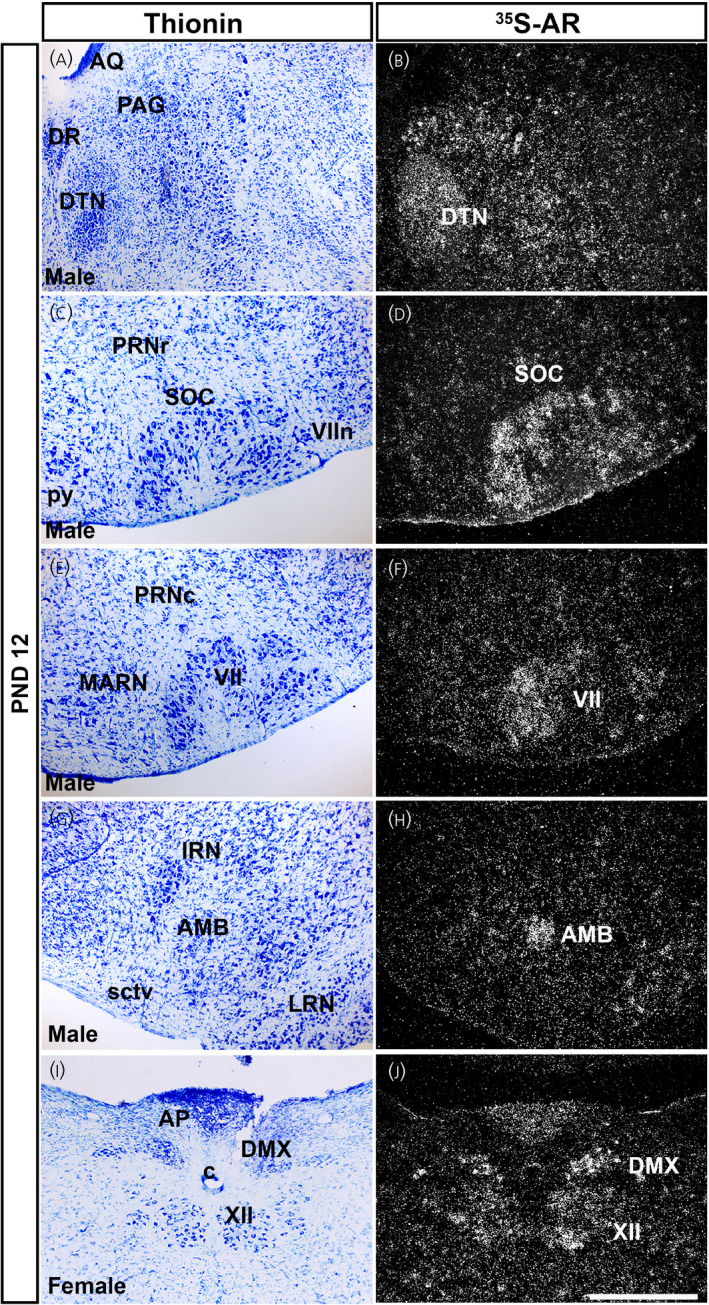
Ar mRNA expression in brainstem nuclei of prepubertal male and female mice. Images showing thionin staining for neuroanatomical reference (left), silver grains corresponding to Ar mRNA (right). (A, B) Very low to low silver grain deposition in the periaqueductal gray (PAG) and low deposition in the dorsal tegmental nucleus (DTN). (C, D) Low expression in the superior olivary complex (SOC) and (E, F) facial motor nucleus (VII). (G, H) Moderate expression in the nucleus ambiguus (AMB). (I, J) Low to moderate expression in the dorsal motor nucleus of the vagus nerve (DMX) and hypoglossal nucleus (XII). AP, area postrema; AQ, cerebral aqueduct; c, central canal of the spinal cord/medulla; DR, dorsal nucleus raphe; IRN, intermediate reticular nucleus; LRN, lateral reticular nucleus; MARN, magnocellular reticular nucleus; PRNc, pontine reticular nucleus, caudal part; PRNr, pontine reticular nucleus; py, pyramid; sctv, ventral spinocerebellar tract; VIIn, facial nerve. PND, postnatal day. Scale bar = 200 µm
In the pons and medulla, the DTN showed consistent, moderate expression in both sexes and in both prepubertal ages (Figure 8A,B and Table 1). Low Ar mRNA expression was detected in the superior olivary complex (SOC) (Figure 8C,D), VII (Figure 8E,F), VNC and CN (Table 1) in both prepubertal stages of both sexes. Low to moderate Ar expression was observed in the AMB (Figure 8G,H), DMX and XII (Figure 8I,J) in males and females. Very low to low levels of Ar mRNA were detected in the PRN and PSV (Table 1).
In circumventricular organs, Ar mRNA expression was low to very low in the SFO and AP of both sexes at PND 12 and PND 21 (Table 1).
3.4. Ar mRNA expression overlaps with Esr1 and/or Esr2 in specific forebrain nuclei of prepubertal mice
We further mapped forebrain sites expressing Ar, Esr1 and Esr2. Because this prepubertal window shows high activity of gene transcription, 27 , 28 we focused on sex steroid receptors with well‐defined genomic actions. We examined co‐expression of Ar with Esr1 and/or Esr2 also as a result of their well described role in masculinization of the male brain during development, 46 , 47 , 48 as well as the major role that they play in female pubertal development. 49 Patterns of Ar hybridization signal using a commercial probe (ACD) for fluorescent ISH were similar to our 35S‐Ar riboprobe. Anatomical distribution of Esr1 and Esr2 was consistent with previous reports in adults. 9 , 26 , 50 , 51 Briefly, we observed a heterogenous mix of subsets of cells expressing either all three receptors, a combination of two, or only Ar, Esr1 or Esr2 in the BSTpr, MEApd and MPO.
In the BSTpr (Figure 9A–D) of PND 12 males, approximately 20% of Ar positive cells co‐expressed Esr1, 8% co‐expressed Esr2 and 3% expressed all three transcripts. The BSTpr of PND 12 females had 6% co‐expression of Ar and Esr1, 12% Ar and Esr2, and approximately 1% co‐expression of all three transcripts. At PND 21, there was an increase in the approximate co‐expression of Ar with Esr1 and Esr2 in both sexes. PND 21 males and females showed approximately 93% co‐expression of Ar and Esr1. PND 21 males had approximately 98% co‐expression of Ar and Esr2, with approximately 90% of Ar positive cells expressing all three transcripts in the BSTpr. PND 21 females, however, displayed approximately 50% co‐expression between Ar and Esr1, as well as both Esr1 and Esr2.
FIGURE 9.
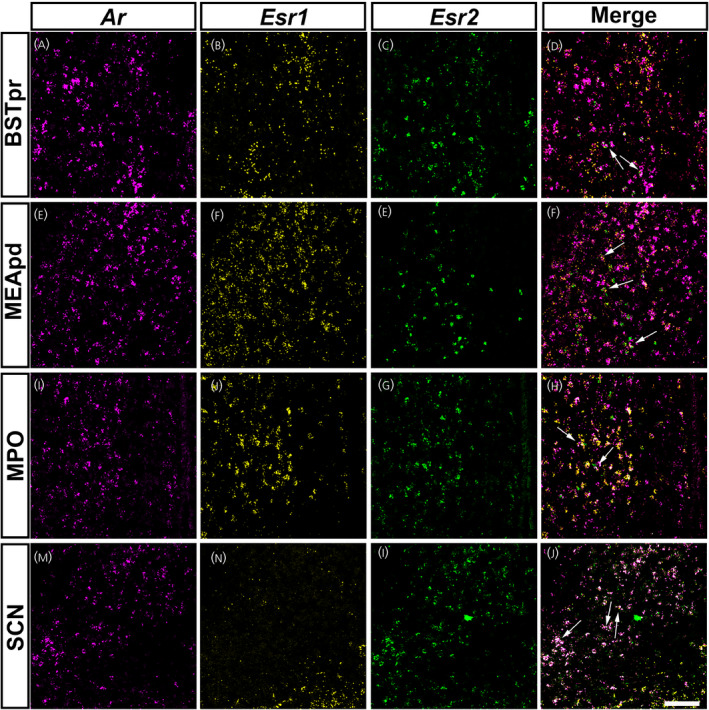
Ar mRNA expression overlaps with Esr1 and Esr2 in specific forebrain nuclei of prepubertal mice. (A–P) Images showing fluorescent in situ hybridization signal for Ar (magenta, A, E, I, M), Esr1 (yellow, B, F, J, N) and Esr2 (green, C, G, K, O). Merge of all three channels shown in (D), (H), (L) and (P). Areas with Ar and Esr1 and/or Esr2 co‐expression include the bed nucleus of the stria terminalis, principal nucleus (BSTpr) (A–D), medial amygdalar nucleus, posterodorsal (MEApd) (E–H), medial preoptic area (MPO) (I–L) and suprachiasmatic nucleus (SCH) (M–P). Arrows show dual or triple‐labeled neurons. Images shown are from postnatal day (PND) 12 female (BSTpr, MEApd, MPO) and male (SCH) mice. Scale bar = 100 µm
In the MEApd (Figure 9E–H) of PND 12 males, overlap of Ar with Esr1 was abundant (~92%), but co‐expression of Ar with only Esr2, or both Esr1 and Esr2 was more limited (~5%). PND 12 females displayed lower co‐expression compared to males in the MEApd, with approximately 30% co‐expressing Ar and Esr1, 5% co‐expressing Ar and Esr2, and only 2% expressing all three transcripts. At PND 21, both sexes displayed high co‐expression of Ar and Esr1 (~90%). PND 21 males had lower co‐expression between Ar and Esr2 and all three transcripts (~15%) compared to PND 21 females (~35% between Ar and Esr2, and all three transcripts).
The MPO (Figure 9I–L) displayed a higher percentage of overlap between Ar and Esr1 in males compared to females. In PND 12 males, approximately 80% of Ar expressing cells co‐expressed Esr1 compared to approximately 45% in PND 12 females. At PND 21, males displayed approximately 92% of co‐expression between Ar and Esr1, whereas approximately 80% of Ar positive neurons in females co‐expressed Esr1. Co‐expression between Ar and Esr2, and Ar with both Esr1 and Esr2, was much lower in both sexes at both developmental time points. Co‐expression between Ar and Esr2, and all three transcripts, at PND 12 was approximately 10% in males and 5% in females. At PND 21 in both sexes, co‐expression was between 2% and 3% between Ar and Esr2, and all three transcripts.
In the SCH (Figure 9M–P), very little Esr1 expression was observed, and therefore Ar neurons co‐expressing Esr1 were rare. Co‐expression between Ar and Esr1 was approximately 15% in males. Additional experiments will be necessary to define the specific subsets of neurons and their role in postnatal development in each brain nucleus expressing all three receptors.
4. DISCUSSION
In the present study, we describe the expression of Ar mRNA in the brain of adult and two prepubertal time points of male and female mice. We show that, at PND 12 and 21, before the activation of the hypothalamic‐pituitary‐gonadal axis, many brain nuclei express high levels of Ar in both sexes. Additionally, we highlight specific forebrain nuclei and subpopulations of Ar expressing neurons that co‐express Esr1 and/or Esr2. We focused on the genomic actions of sex steroid receptors as a result of their well described role in masculinization of the male brain during prenatal development, 46 , 47 as well as in female pubertal development and fertility. 49 , 52 Further studies will be necessary to evaluate the co‐expression of the three nuclear receptors in the entire brain and determine whether the identified brain sites express alternative estrogen receptors (e.g., G protein‐coupled ER), or the enzyme aromatase during this window of prepubertal development.
Systematic characterization of AR expression during prepubertal development is essential for understanding how androgens can shape brain organization and activation of neural circuits. Although circulating androgens are low in the prepubertal period, we show that Ar is highly expressed in many areas of the brain in both sexes during this time window. The exact role of AR in brain development in general, and in specific neuronal subpopulations, is not well described. It has been demonstrated that gonadal hormones during puberty can further organize and refine neural circuits. 53 , 54 During puberty, pruning and remodeling of synapses, morphology, density and sexual dimorphism of dendritic spines occurs throughout the brain. In many brain sites, this fine remodeling is orchestrated by gonadal hormones, particularly androgens. 55 , 56 , 57 , 58 , 59 Thus, increased Ar expression during the prepubertal window in both sexes plays a key role in the continuous developmental process towards the adult brain. Sex differences in circulating steroids during pubertal transition would ultimately determine the circuitry, morphology and neurochemical fate of the subpopulations of neurons.
Sex differences in Ar expression are most apparent in areas related to male sexual behavior and reproduction, including the well characterized BSTpr, MPO, MEApd and LS. 26 , 35 , 60 , 61 However, during development, Ar expression was similar at PND 12 between males and females in those sites, but apparently higher in males at PND 21, in agreement with previous reports showing greater hypothalamic AR‐IR in prepubertal male mice. 26 Specifically, we found higher Ar expression in the SCH of PND 21 males. In adults, SCH AR expression is key for circadian regulation and sex differences in locomotor activity. Orchidectomy feminizes night patterns of activity in males, and androgen replacement restores male‐specific patterns of activity. 62 , 63 Our findings suggest these differences are established during pubertal development around PND 21. The role of Esr2 and co‐expression with Ar is not yet known.
Although at lower levels, Ar is still prevalent in the female brain and is expressed in a multitude of different brain nuclei. The role that AR plays in the prepubertal and adult female brain is not fully understood, but models of female androgen excess demonstrate that prenatal and prepubertal androgen exposure has the potential to heavily impact female physiology. For example, polycystic ovary syndrome (PCOS) is partly characterized by female androgen excess, and can significantly impact fertility, body weight and insulin sensitivity. 64 , 65 , 66 PCOS‐like features can be replicated in mice, with one prepubertal model inducing androgen excess beginning at PND 19 67 and another at PND 21. 68 This peripubertal androgenization model induces changes upon multiple tissues, including the brain, eliciting well described effects on reproduction and metabolism. 69 , 70 The exact brain sites associated with the consequences of hyperandrogenism in females have not been fully determined. Defining selective and non‐selective brain sites responsive to androgens is an important first step for a better mechanistic understanding of the pathological origins of diseases of androgen excess.
Brain AR expression and distribution have been previously characterized in the rat, 61 , 71 hamster, 72 musk shrew 73 and monkey. 74 These findings, however, are not directly translatable to the mouse because of species differences. For example, AR is abundant in the dorsomedial VMH (VMHdm) of adult male rats, 20 , 61 , 71 but much less so in adult male mice. A direct comparative analysis will be necessary, but the VMHdm is highly associated with glucose homeostasis and metabolic control in mice. 75 , 76 Whether AR in VMHdm neurons has similar metabolic effects in the mouse versus rat requires further investigation. It is important to be aware of species differences, particularly in the mouse, which is a frequently used model organism in studies using genetic and molecular tools. Furthermore, because the number of neurons necessary for a specific function may not be determined a priori, moderate and low AR expression in extra‐hypothalamic and extra‐limbic areas is not irrelevant or less important. Consistent with this concept, Ar expression in cranial nerve nuclei of both sexes during pre‐pubertal development is particularly interesting. Many nuclei along the olfactory and auditory pathways express AR, but studies exploring the role of androgenic signaling in cranial nerve nuclei have been limited. 77 , 78 Androgens promote neuronal survival and axon regeneration in cranial nerve motor nuclei in male and female rats. 79 , 80 In the spinal cord, however, androgens acting on AR protect against motor neuron death in the spinal nucleus of the bulbocavernosus during postnatal development in males, resulting in a male‐biased sex difference in cell number and morphology. 81 , 82 It remains to be determined why circulating androgens induce sexual dimorphism in some areas of the brain, but not others, and why specific cell populations respond to androgens, rather than estrogens, to promote neuronal survival, and whether these events occur before puberty when androgens are low but AR expression is present in many nuclei of both sexes. The answers to these questions may require a closer look into the regulation of AR signaling complexity, including the role of alternative ligands and ligand‐independent signaling properties. 83
Although the present study examines the distribution of AR in male and female prepubertal mice, we have not systemically mapped estrogen receptors in the same experimental groups. Instead, we have examined the expression of two estrogen receptors (ERα/β) in select nuclei that express Ar during development. It is highly possible however, that subpopulations of cells in these areas only transiently express AR, ERα and/or ERβ during development as observed in the forebrain. 84 , 85 Furthermore, AR and ERs can interact with each other to modulate transactivation or signaling activity. 86 For example, ERβ can down‐regulate AR in the ventral prostate, 87 whereas AR can either inhibit or support ERα activity in breast cancer cells. 88 , 89 Additional studies will be necessary to define the specific time points of prepubertal development during which subpopulations of neurons are engaged by selective gonadal steroids, as well as the interaction of different steroid receptor pathways.
Gonadal hormones are not the only factors that contribute to sex differences in the brain. Sex chromosome genes, autosome genes for which expression is mediated by sex‐steroid receptors, epigenetics, environmental factors and exposures, factors that regulate the sensitivity of a brain region to sex steroids, and brain immune cells all contribute to brain sex differentiation. 90 Yet, it is clear that androgens play a very important, arguably dominant role in sex differentiation in rodents via AR or ERs, and their unknown role in female brain remains to be fully determined. In the attempt to decrease this gap, we focused our analysis on both sexes. The findings of the present study indicate that, in various brain areas, androgens, in addition to neuroestrogens or circulating estrogens, may also contribute to female neuronal development and physiology. Our findings also highlight the need for greater investigation of the variety of actions of androgens throughout the male and female brain, particularly during prepubertal development. Future studies targeting specific brain sites are warranted.
CONFLICT OF INTERESTS
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Alexandra Louise Cara: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing. Emily Lynn Henson: Data curation; Methodology; Validation; Writing – review & editing. Bethany Genelle Beekly: Data curation. Carol Elias: Conceptualization; Data curation; Funding acquisition; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – review & editing.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1111/jne.13063.
ACKNOWLEDGMENTS
We thank Susan Allen for expert technical assistance. This research was supported by funding from National Institute of Health (NIH) Grants R01HD069702 and R01HD096324 (CFE). AC was supported by NIH T32HD079342.
Cara AL, Henson EL, Beekly BG, Elias CF. Distribution of androgen receptor mRNA in the prepubertal male and female mouse brain. J Neuroendocrinol. 2021;33:e13063. doi: 10.1111/jne.13063
DATA AVAILABILITY
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Koopman P, Münsterberg A, Capel B, Vivian N, Lovell‐Badge R. Expression of a candidate sex‐determining gene during mouse testis differentiation. Nature. 1990;348(6300):450‐452. [DOI] [PubMed] [Google Scholar]
- 2. Sinclair AH, Berta P, Palmer MS, et al. A gene from the human sex‐determining region encodes a protein with homology to a conserved DNA‐binding motif. Nature. 1990;346(6281):240‐244. [DOI] [PubMed] [Google Scholar]
- 3. Rhoda J, Corbier P, Roffi J. Gonadal steroid concentrations in serum and hypothalamus of the rat at birth: aromatization of testosterone to 17β‐estradiol. Endocrinology. 1984;114(5):1754‐1760. [DOI] [PubMed] [Google Scholar]
- 4. Weisz J, Ward IL. Plasma testosterone and progesterone titers of pregnant rats, their male and female fetuses, and neonatal offspring. Endocrinology. 1980;106(1):306‐316. [DOI] [PubMed] [Google Scholar]
- 5. Naftolin F, MacLusky N. Aromatization hypothesis revisited. In: Serio M, ed. Sexual Differentiation: Basic and Clinical Aspects. Serono symposia publications from Raven Press 11. Raven Press; 1984:79‐91. [Google Scholar]
- 6. McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9(3):249‐263. [DOI] [PubMed] [Google Scholar]
- 7. Naftolin F, Ryan KJ, Davies IJ, et al. The formation of estrogens by central neuroendocrine tissues. In: Greep RO, ed. Proceedings of the 1974 Laurentian Hormone Conference, Vol. 31. Academic Press; 1975:295‐319. [DOI] [PubMed] [Google Scholar]
- 8. McCarthy MM, Schlenker EH, Pfaff DW. Enduring consequences of neonatal treatment with antisense oligodeoxynucleotides to estrogen receptor messenger ribonucleic acid on sexual differentiation of rat brain. Endocrinology. 1993;133(2):433‐439. [DOI] [PubMed] [Google Scholar]
- 9. Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor‐alpha and ‐beta mRNA in the rat central nervous system. Journal of Comparative Neurology. 1997;388(4):507‐525. [DOI] [PubMed] [Google Scholar]
- 10. Ogawa S, Chan J, Chester AE, Gustafsson J‐Å, Korach KS, Pfaff DW. Survival of reproductive behaviors in estrogen receptor β gene‐deficient (βERKO) male and female mice. Proc Natl Acad Sci USA. 1999;96(22):12887‐12892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor α gene. Horm Behav. 1997;32(3):176‐183. [DOI] [PubMed] [Google Scholar]
- 12. Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7(1):413‐442. [DOI] [PubMed] [Google Scholar]
- 13. Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: a reanalysis. Horm Behav. 1985;19(4):469‐498. [DOI] [PubMed] [Google Scholar]
- 14. Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65(3):369‐382. [DOI] [PubMed] [Google Scholar]
- 15. Keller M, Pawluski JL, Brock O, Douhard Q, Bakker J. The alpha‐fetoprotein knock‐out mouse model suggests that parental behavior is sexually differentiated under the influence of prenatal estradiol. Horm Behav. 2010;57(4‐5):434‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vannier B, Raynaud JP. Effect of estrogen plasma binding on sexual differentiation of the rat fetus. Mol Cell Endocrinol. 1975;3(5):323‐337. [DOI] [PubMed] [Google Scholar]
- 17. Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579(2):321‐326. [DOI] [PubMed] [Google Scholar]
- 18. Lisciotto CA, Morrell JI. Sex differences in the distribution and projections of testosterone target neurons in the medial preoptic area and the bed nucleus of the stria terminalis of rats. Horm Behav. 1994;28(4):492‐502. [DOI] [PubMed] [Google Scholar]
- 19. Madeira MD, Ferreira‐Silva L, Paula‐Barbosa MM. Influence of sex and estrus cycle on the sexual dimorphisms of the hypothalamic ventromedial nucleus: stereological evaluation and Golgi study. J Comp Neurol. 2001;432(3):329‐345. [DOI] [PubMed] [Google Scholar]
- 20. Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51(2):195‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Abreu AP, Kaiser UB. Pubertal development and regulation. Lancet Diabetes Endocrinol. 2016;4(3):254‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bakker J, Baum MJ. Role for estradiol in female‐typical brain and behavioral sexual differentiation. Front Neuroendocrinol. 2008;29(1):1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nilsson ME, Vandenput L, Tivesten Å, et al. Measurement of a comprehensive sex steroid profile in rodent serum by high‐sensitive gas chromatography‐tandem mass spectrometry. Endocrinology. 2015;156(7):2492‐2502. [DOI] [PubMed] [Google Scholar]
- 24. Gupta D, Attanasio A, Raaf S. Plasma estrogen and androgen concentrations in children during adolescence. J Clin Endocrinol Metab. 1975;40(4):636‐643. [DOI] [PubMed] [Google Scholar]
- 25. Kushnir MM, Blamires T, Rockwood AL, et al. Liquid chromatography‐tandem mass spectrometry assay for androstenedione, dehydroepiandrosterone, and testosterone with pediatric and adult reference intervals. Clin Chem. 2010;56(7):1138‐1147. [DOI] [PubMed] [Google Scholar]
- 26. Brock O, De Mees C, Bakker J. Hypothalamic expression of oestrogen receptor alpha and androgen receptor is sex‐, age‐ and region‐dependent in mice. J Neuroendocrinol. 2015;27(4):264‐276. [DOI] [PubMed] [Google Scholar]
- 27. Hou H, Uusküla‐Reimand L, Makarem M, et al. Gene expression profiling of puberty‐associated genes reveals abundant tissue and sex‐specific changes across postnatal development. Hum Mol Genet. 2017;26(18):3585‐3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Han X, Burger LL, Garcia‐Galiano D, et al. Hypothalamic and cell‐specific transcriptomes unravel a dynamic neuropil remodeling in leptin‐induced and typical pubertal transition in female mice. iScience. 2020;23(10):101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gorski RA. Sexual differentiation of the brain. Hosp Pract. 1978;13(10):55‐62. [DOI] [PubMed] [Google Scholar]
- 30. Tsukahara S, Morishita M. Sexually dimorphic formation of the preoptic area and the bed nucleus of the stria terminalis by neuroestrogens. Front Neurosci. 2020;14:797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Marrocco J, McEwen BS. Sex in the brain: hormones and sex differences. Dialogues Clin Neurosci. 2016;18(4):373‐383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Altemus M, Sarvaiya N, Neill EC. Sex differences in anxiety and depression clinical perspectives. Front Neuroendocrinol. 2014;35(3):320‐330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wood RI, Newman SW. Androgen and estrogen receptors coexist within individual neurons in the brain of the Syrian hamster. Neuroendocrinology. 1995;62(5):487‐497. [DOI] [PubMed] [Google Scholar]
- 34. Jahan MR, Kokubu K, Islam MDN, et al. Species differences in androgen receptor expression in the medial preoptic and anterior hypothalamic areas of adult male and female rodents. Neuroscience. 2015;284:943‐961. [DOI] [PubMed] [Google Scholar]
- 35. Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43(3):313‐319. [DOI] [PubMed] [Google Scholar]
- 36. Lu SF, McKenna SE, Cologer‐Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139(4):1594‐1601. [DOI] [PubMed] [Google Scholar]
- 37. Apostolinas S, Rajendren G, Dobrjansky A, Gibson MJ. Androgen receptor immunoreactivity in specific neural regions in normal and hypogonadal male mice: effect of androgens. Brain Res. 1999;817(1‐2):19‐24. [DOI] [PubMed] [Google Scholar]
- 38. Council NR. Guide for the Care and Use of Laboratory Animals. 8th ed. The National Academies Press; 2011. [PubMed] [Google Scholar]
- 39. Oyegbile TO, Marler CA. Winning fights elevates testosterone levels in California mice and enhances future ability to win fights. Horm Behav. 2005;48(3):259‐267. [DOI] [PubMed] [Google Scholar]
- 40. Caligioni CS. Assessing reproductive status/stages in mice. Curr Protoc Neurosci. 2009;48:Appendix‐4I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silveira MA, Wagenmaker ER, Burger LL, DeFazio RA, Moenter SM. GnRH neuron activity and pituitary response in estradiol‐induced vs proestrous luteinizing hormone surges in female mice. Endocrinology. 2016;158(2):356‐366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Low KL, Ma C, Soma KK. Tyramide signal amplification permits immunohistochemical analyses of androgen receptors in the rat prefrontal cortex. J Histochem Cytochem. 2017;65(5):295‐308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Paxinos G, Franklin KBJ. Paxinos and Franklin's the mouse brain in stereotaxic coordinates; Elsevier Academic Press; 2019. [Google Scholar]
- 44. Rodrigues BC, Cavalcante JC, Elias CF. Expression of cocaine‐ and amphetamine‐regulated transcript in the rat forebrain during postnatal development. Neuroscience. 2011;195:201‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cavalcante JC, Bittencourt JC, Elias CF. Female odors stimulate CART neurons in the ventral premammillary nucleus of male rats. Physiol Behav. 2006;88(1):160‐166. [DOI] [PubMed] [Google Scholar]
- 46. Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav. 1997;31(3):232‐243. [DOI] [PubMed] [Google Scholar]
- 47. Ogawa S, Chester AE, Hewitt SC, et al. Abolition of male sexual behaviors in mice lacking estrogen receptors alpha and beta (alpha beta ERKO). Proc Natl Acad Sci USA. 2000;97(26):14737‐14741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wu MV, Manoli DS, Fraser EJ, et al. Estrogen masculinizes neural pathways and sex‐specific behaviors. Cell. 2009;139(1):61‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mayer C, Acosta‐Martinez M, Dubois SL, et al. Timing and completion of puberty in female mice depend on estrogen receptor alpha‐signaling in kisspeptin neurons. Proc Natl Acad Sci USA. 2010;107(52):22693‐22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Merchenthaler I, Lane MV, Numan S, Dellovade TL. Distribution of estrogen receptor α and β in the mouse central nervous system: in vivo autoradiographic and immunocytochemical analyses. J Comp Neurol. 2004;473(2):270‐291. [DOI] [PubMed] [Google Scholar]
- 51. Saito K, He Y, Yan X, et al. Visualizing estrogen receptor‐α‐expressing neurons using a new ERα‐ZsGreen reporter mouse line. Metabolism. 2016;65(4):522‐532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O. Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proc Natl Acad Sci USA. 1993;90(23):11162‐11166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrinol. 2005;26(3‐4):163‐174. [DOI] [PubMed] [Google Scholar]
- 54. Schulz KM, Zehr JL, Salas‐Ramirez KY, Sisk CL. Testosterone programs adult social behavior before and during, but not after, adolescence. Endocrinology. 2009;150(8):3690‐3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Frankfurt M, Gould E, Woolley CS, McEwen BS. Gonadal steroids modify dendritic spine density in ventromedial hypothalamic neurons: a Golgi study in the adult rat. Neuroendocrinology. 1990;51(5):530‐535. [DOI] [PubMed] [Google Scholar]
- 56. Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J Neurosci. 1990;10(4):1286‐1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hatanaka Y, Hojo Y, Mukai H, et al. Rapid increase of spines by dihydrotestosterone and testosterone in hippocampal neurons: dependence on synaptic androgen receptor and kinase networks. Brain Res. 2015;1621:121‐132. [DOI] [PubMed] [Google Scholar]
- 58. Leranth C, Petnehazy O, MacLusky NJ. Gonadal hormones affect spine synaptic density in the CA1 hippocampal subfield of male rats. J Neurosci. 2003;23(5):1588‐1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Leranth C, Hajszan T, MacLusky NJ. Androgens increase spine synapse density in the CA1 hippocampal subfield of ovariectomized female rats. J Neurosci. 2004;24(2):495‐499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Juntti SA, Tollkuhn J, Wu MV, et al. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron. 2010;66(2):260‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. McAbee MD, DonCarlos LL. Ontogeny of region‐specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology. 1998;139(4):1738‐1745. [DOI] [PubMed] [Google Scholar]
- 62. Iwahana E, Karatsoreos I, Shibata S, Silver R. Gonadectomy reveals sex differences in circadian rhythms and suprachiasmatic nucleus androgen receptors in mice. Horm Behav. 2008;53(3):422‐430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Karatsoreos IN, Wang A, Sasanian J, Silver R. A role for androgens in regulating circadian behavior and the suprachiasmatic nucleus. Endocrinology. 2007;148(11):5487‐5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Huang A, Brennan K, Azziz R. Prevalence of hyperandrogenemia in the polycystic ovary syndrome diagnosed by the National Institutes of Health 1990 criteria. Fertil Steril. 2010;93(6):1938‐1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sanchez‐Garrido MA, Tena‐Sempere M. Metabolic dysfunction in polycystic ovary syndrome: pathogenic role of androgen excess and potential therapeutic strategies. Mol Metab. 2020;35:100937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dumesic DA, Oberfield SE, Stener‐Victorin E, Marshall JC, Laven JS, Legro RS. Scientific statement on the diagnostic criteria, epidemiology, pathophysiology, and molecular genetics of polycystic ovary syndrome. Endocr Rev. 2015;36(5):487‐525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van Houten EL, Kramer P, McLuskey A, Karels B, Themmen AP, Visser JA. Reproductive and metabolic phenotype of a mouse model of PCOS. Endocrinology. 2012;153(6):2861‐2869. [DOI] [PubMed] [Google Scholar]
- 68. Caldwell ASL, Middleton LJ, Jimenez M, et al. Characterization of Reproductive, Metabolic, and Endocrine Features of Polycystic Ovary Syndrome in Female Hyperandrogenic Mouse Models. Endocrinology. 2014;155(8):3146–3159. 10.1210/en.2014-1196 [DOI] [PubMed] [Google Scholar]
- 69. Aflatounian A, Edwards MC, Rodriguez Paris V, et al. Androgen signaling pathways driving reproductive and metabolic phenotypes in a PCOS mouse model. The Journal of Endocrinology. 2020;245(3):381‐395. [DOI] [PubMed] [Google Scholar]
- 70. Caldwell ASL, Edwards MC, Desai R, et al. Neuroendocrine androgen action is a key extraovarian mediator in the development of polycystic ovary syndrome. Proc Natl Acad Sci USA. 2017;114(16):E3334‐E3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA‐containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76‐95. [DOI] [PubMed] [Google Scholar]
- 72. Wood RI, Newman SW. Androgen receptor immunoreactivity in the male and female Syrian hamster brain. J Neurobiol. 1999;39(3):359‐370. [DOI] [PubMed] [Google Scholar]
- 73. Veney SL, Rissman EF. Immunolocalization of androgen receptors and aromatase enzyme in the adult musk shrew brain. Neuroendocrinology. 2000;72(1):29‐36. [DOI] [PubMed] [Google Scholar]
- 74. Roselli CE, Klosterman S, Resko JA. Anatomic relationships between aromatase and androgen receptor mRNA expression in the hypothalamus and amygdala of adult male cynomolgus monkeys. J Comp Neurol. 2001;439(2):208‐223. [DOI] [PubMed] [Google Scholar]
- 75. Flak JN, Goforth PB, Dell’Orco J, et al. Ventromedial hypothalamic nucleus neuronal subset regulates blood glucose independently of insulin. J Clin Invest. 2020;130(6):2943‐2952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Castorena CM, Caron A, Michael NJ, et al. CB1Rs in VMH neurons regulate glucose homeostasis but not body weight. Am J Physiol Endocrinol Metab. 2021;321(1):E146‐E155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Yu WH, McGinnis MY. Androgen receptors in cranial nerve motor nuclei of male and female rats. J Neurobiol. 2001;46(1):1‐10. [DOI] [PubMed] [Google Scholar]
- 78. Sar M, Stumpf W. Androgen concentration in motor neurons of cranial nerves and spinal cord. Science. 1977;197(4298):77‐79. [DOI] [PubMed] [Google Scholar]
- 79. Yu WH. Administration of testosterone attenuates neuronal loss following axotomy in the brain‐stem motor nuclei of female rats. J Neurosci. 1989;9(11):3908‐3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Amy Yu W‐H. Effect of testosterone on the regeneration of the hypoglossal nerve in rats. Exp Neurol. 1982;77(1):129‐141. [DOI] [PubMed] [Google Scholar]
- 81. Nordeen E, Nordeen K, Sengelaub D, Arnold A. Androgens prevent normally occurring cell death in a sexually dimorphic spinal nucleus. Science. 1985;229(4714):671‐673. [DOI] [PubMed] [Google Scholar]
- 82. Breedlove SM, Arnold AP. Sexually dimorphic motor nucleus in the rat lumbar spinal cord: response to adult hormone manipulation, absence in androgen‐insensitive rats. Brain Res. 1981;225(2):297‐307. [DOI] [PubMed] [Google Scholar]
- 83. Davey RA, Grossmann M. Androgen receptor structure, function and biology: from bench to bedside. Clin Biochem Rev. 2016;37(1):3‐15. [PMC free article] [PubMed] [Google Scholar]
- 84. Zuloaga DG, Zuloaga KL, Hinds LR, Carbone DL, Handa RJ. Estrogen receptor β expression in the mouse forebrain: age and sex differences. J Comp Neurol. 2014;522(2):358‐371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Sugiyama N, Andersson S, Lathe R, et al. Spatiotemporal dynamics of the expression of estrogen receptors in the postnatal mouse brain. Mol Psychiatry. 2009;14(2):223‐232. [DOI] [PubMed] [Google Scholar]
- 86. Panet‐Raymond V, Gottlieb B, Beitel LK, Pinsky L, Trifiro MA. Interactions between androgen and estrogen receptors and the effects on their transactivational properties. Mol Cell Endocrinol. 2000;167(1‐2):139‐150. [DOI] [PubMed] [Google Scholar]
- 87. Wu W‐F, Maneix L, Insunza J, et al. Estrogen receptor β, a regulator of androgen receptor signaling in the mouse ventral prostate. Proc Natl Acad Sci USA. 2017;114(19):E3816‐E3822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Peters AA, Buchanan G, Ricciardelli C, et al. Androgen receptor inhibits estrogen receptor‐alpha activity and is prognostic in breast cancer. Cancer Res. 2009;69(15):6131‐6140. [DOI] [PubMed] [Google Scholar]
- 89. D'Amato NC, Gordon MA, Babbs B, et al. Cooperative dynamics of AR and ER activity in breast cancer. Mol Cancer Res. 2016;14(11):1054‐1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14(6):677‐683. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


