Abstract
In the management of indeterminate depth burns (IDB), common challenges include the ability to predict time to healing and regenerative potential, risk of burn wound progression, and timing of excision. Several technologies exist to aid in determination of the depth of a burn injury, yet surgeons continue to rely on the naked eye—visual assessment—as the standard of care. Newer and improved imaging technologies are closing in on the goal of inexpensive, accurate, non-invasive modalities for depth determination. Likewise, management of indeterminate depth burns is becoming more sophisticated as newer wound healing technologies continue to be developed. By describing what is meant by “indeterminate” depth burns, and their associated challenges, we hope to stimulate interest in research to develop new therapies and management strategies. The ultimate goal is to treat indeterminate burns without the need for autografts.
Keywords: Burn Depth, Skin Substitutes, Depth Determination, Scarring, Indeterminate Depth Burn
Introduction
The depth of a burn wound dictates the healing course, need for surgery, and morbidity associated with the injury.1,2 Thus, depth is one of the most important determinants of burn wound management. Indeterminate depth burns (IDB) represent a perplexing diagnostic and management challenge for clinicians. The term “indeterminate depth burn” (IDB) is typically used to describe cases of deeper partial thickness burns with an unknown healing potential; in such wounds, healing is expected to take longer than 2–3 weeks. While full thickness burns clearly require excision and grafting for wound closure, and superficial partial thickness burns usually heal with non-operative wound care, no definitive strategies exist for the management of IDB.3
In this review, we will explore the determinants of burn depth and regenerative capacity, summarize the methods of depth determination, examine the recent literature on the natural progression of burn depth, reflect on current advances in burn wound healing for management of IDB, and discuss how these technologies affect both surgical and non-surgical management of IDBs. Ultimately, the goal is to identify gaps in our knowledge and stimulate further discussion and research that will influence the way surgeons manage IDBs, with a focus on enhancing regenerative capacity, reducing scarring, and improving patient outcomes.
Burn depth and diagnostic approaches for depth determination
Burn Depth:
In the early 1950s, Jackson described two degrees of burn depth based on appearance of skin after injury – partial thickness skin loss and full thickness skin loss. He noted that the presence or absence of “sufficient living epithelial elements to resurface the area” signaled the difference between these two depths.4 Today, burn depth is designated by a structural-anatomical classification system (Table 1) ranging from superficial injury of the epidermis only, to full thickness burns extending beyond the dermis (see Figure 1). This more extensive classification system, described in the next section, is a useful guide, however, it is important to note that burns are heterogenous and the categories of depth are not always easily delineated in the clinical setting.
Table 1-.
Visual Assessment of Burn Depth
| Clinical Depth | Layer Affected | Visual Characteristics | Tactile Characteristics | Prognosis |
|---|---|---|---|---|
| Superficial (First Degree) | Epidermis only | Erythema, dry | Painful | Heals within a few days |
| Superficial Partial Thickness (Second Degree) | Epidermis and superficial dermis | Erythema, blisters, moist, weeping | Painful, blanchable with applied pressure | Heals within 3–15 days |
| Deep Partial Thickness (Second Degree) | Epidermis and deep dermis | Erythema or pale, +/− blisters, moist or dry | Painful or anesthetic, non-blanchable with applied pressure | May heal within 2–3 weeks |
| Indeterminate Depth Burn | Epidermis and deep dermis or full dermis | Erythema or pale, +/− blisters, moist or dry | Painful or anesthetic, non-blanchable with applied pressure | Healing time unknown but likely longer than 2–3 weeks |
| Full Thickness (Third Degree) | Complete epidermis and dermis destruction into subcutaneous layer. | Dry, leathery, white/brown/black in color | Non-painful, non-blanchable with applied pressure | Healing slowly from edges only, requires autograft for normal orderly healing |
Figure 1:
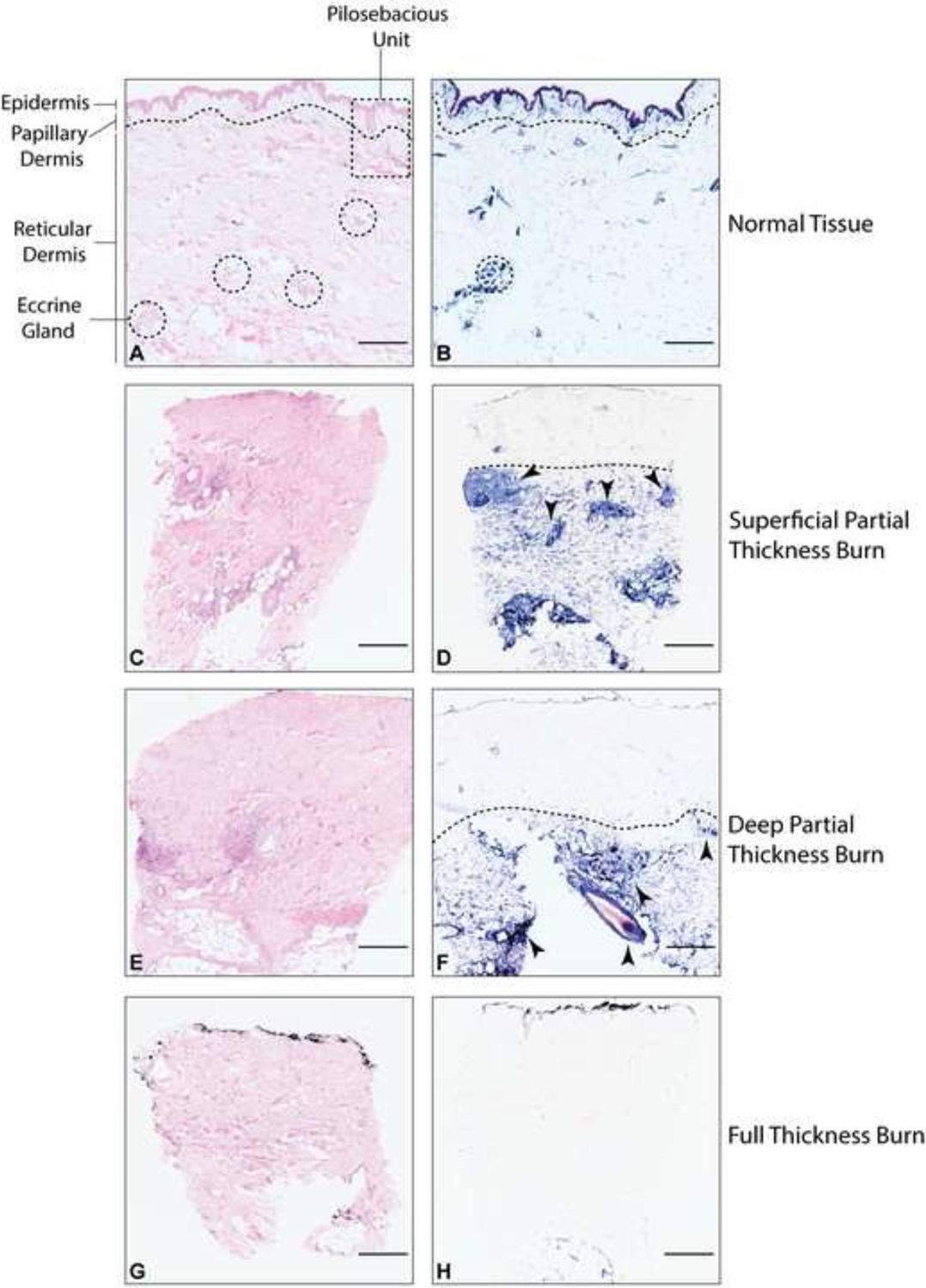
Histologic sections illustrating normal human skin, and representative biopsies from partial thickness, and full thickness burned human skin tissue. A, C, E, G are hematoxylin and eosin (H&E) stained tissue (scale bar = 500 microns). B, D, F, H are lactate dehydrogenase (LDH) stained tissue with viable cells stained blue (scale bar = 500 microns). A and B illustrate normal tissue. The dotted line represents the interface between papillary dermis and reticular dermis. The dermis contains the pilosebacous unit (PSU) (partially visible in the dotted rectangle). The PSU, composed of a hair follicle, arrector pili muscle, and associated sebaceous gland, extends from the epidermis into the dermis and represents an important regenerative niche for wound healing. Dotted circles represent eccrine gland structures, representing another important regenerative center located throughout the dermis and often deeper than the PSUs. Panels C and D represent a superficial partial thickness burn. Arrows point to regenerative centers. Panels E and F show a deep partial thickness burn with arrows pointing to regenerative centers. Panels G and H represent a full thickness burn with no visible regenerative potential.
Burn depth is influenced by both extrinsic and intrinsic factors. In thermal burns, extrinsic factors include temperature, contact time, pressure, and specific heat capacity (related to thickness) of the skin.5 Insights into the important role of patient-specific characteristics in burn wound healing have been learned from studies in the elderly; in fact, age is one of the most significant factors affecting burn wound healing.6,7 Factors common in the elderly population that contribute to the morbidity and mortality from burn wounds include thinner, less elastic skin, a delayed hypermetabolic response, comorbidities such as hyperglycemia and hyperlipidemia, weakened immune response from acquired or inherited causes, and finally, a delay in wound healing secondary to stem cell senescence.8
Depth Determination:
Historically, to classify burn depth, surgeons relied upon visual assessment of physical characteristics on the surface of the burn. This classification method underwent several iterations until 1995, when the International Society for Burn Injuries, in collaboration with the World Health Organization, published a guide to burn treatment that described four depths of burns (Table1).9–11 The four classifications (superficial, superficial partial thickness, deep partial thickness, and full thickness) were based on clinical signs as well as their associated histologic diagnosis and prognosis.12 Visual assessment is still the most commonly used method for burn depth determination; however, histological and advanced imaging methods are also available. Each method has unique strengths and weaknesses.13–16 For the purpose of this review, we will summarize three main methods of burn depth assessment.
Visual assessment
Visual assessment of burn wounds is a simple and inexpensive method to determine depth of injury, and is enhanced by consideration of tactile information such as pain, blanching, and moisture content of the wound. Surgeons develop assessment skills, often in an apprentice-like fashion, through years of observing patterns of healing in their patients.17 However, visual assessment has some challenges. In one study, burn surgeons correctly predicted wounds they expected to heal within three weeks 89% of the time; yet surgeons were correct only 65–75% of the time when they predicted non-healing.2 Another study analyzed 951 cases of <15% total body surface area (TBSA) burns and found that in 27.5% of burns, the initial diagnosis of depth was different than the final diagnosis.18 Of these wounds, 44% were not as deep as initially assessed. In a study of indeterminate burns in 40 patients, two independent observers with experience in burn care performed clinical assessments of burn depth over the first week. Their assessments were found to be correct in 40.6%, 61.5%, 52.5%, 71.4%, and 100% of patients for assessments on days 0, 1, 3, 5 and 8 respectively, suggesting that determination of depth becomes more reliable over the course of healing.19 In this same study, superficial dermal burn depth determination by clinical assessment was overestimated in 50%, 38.5%, 57.7%, 30.8% and 0% of patients on days 0, 1, 3, 5, 8 post burn, respectively, and would have led to unnecessary grafting in one third of patients if the decision to operate had been made based on clinical judgment alone.
Intraoperatively, visual assessments made during tangential excision allows the surgeon to determine how much tissue to remove, while attempting to leave as much viable tissue as possible in the wound bed. Complicating this assessment is the use of tourniquets and infiltration of vasoconstrictor agents (e.g. epinephrine) that are often employed to decrease the amount of blood loss during excision.20 There is a fine balance between inadvertently removing viable tissue and leaving necrotic tissue in the wound bed; the latter risks autograft loss, as necrotic tissue increases inflammation and hinders wound healing. Therefore, surgeons tend to over-excise the wound, as evidenced by studies showing viable cells present in excised burn tissue.1 The consequences of overestimation in the eventual outcome of wound healing have not been evaluated in humans. However, it is clear that over-excision increases donor-site surface area for the purpose of autografting, and there are morbidities associated with the additional donor-site wounds.
Histologic assessment
The gold standard for diagnosing depth of injury in burn wounds is the histologic assessment of a full thickness wound biopsy using hematoxylin and eosin (H&E) staining. Unlike visual assessment, there is very high inter- and intra- observer reliability between histological assessments of epithelialization.21 When visual and histologic assessments were compared, there was no agreement between the observers about the depth of burn injury.
In histologic assessments, various characteristics of dermal elements in the skin tissue are evaluated to determine the depth of injury in burn tissue. A study of burn samples taken 30 minutes after injury in a porcine model revealed that the depth of injury varies depending on the different dermal elements that are assessed, including the vasculature, collagen, and epithelial-lined skin appendages such as hair follicles and eccrine glands.22 In another study, microvascular occlusion was used as a measure to determine the extent of dermal damage, and in turn the depth of burn.23 New methods continue to be developed. Data from our laboratory have shown that the use of a lactate dehydrogenase (LDH) assay as a marker for cell viability (see Figure 1) is an easier method to consistently assess tissue viability as compared with H&E stain interpretation, even when performed by a novice.24,25
While histological assessment may be superior to visual assessment, it is not a common practice due to the pain associated with performing a biopsy, the heterogeneity of the wound bed necessitating multiple biopsies within one wound, and the length of time it takes for the staining procedure. However, histologic assessment of wound depth is a useful technique in the research setting, as an objective measure of healing potential and responses to treatment.
Imaging
In light of the fact that visual assessment can be unreliable, and histology is not feasible for intraoperative depth assessment, surgeons have turned to imaging technologies to gain more objective data for burn depth assessment. Extensive reviews of imaging for burn depth assessment and healing potential have been performed elsewhere.26–29 In this section, we present some of the established and emerging advanced imaging technologies employed to measure burn depth in humans.
Indocyanine Green (ICG) Microangiography:
ICG is an amphipathic molecule that has been tested extensively for safety since the 1950s and has been used for microangiography in laparoscopic, vascular, reconstructive, and oncologic surgery.30 When ICG is injected into the bloodstream it normally binds to lipoprotein (ICG-LP), protecting the hydrophilic domain of the ICG molecule from binding the lipid bilayer of intact cell membranes, a characteristic that is important in detecting microperfusion. Video fluorescent microangiography allows for real-time dynamic assessment of tissue perfusion. In burn wounds, the decrease in blood flow related to microvascular occlusion and edema leads to a reduction in ICG fluorescence, allowing for identification of the depth of injury with high sensitivity.31 In a study of 20 patients with burn injuries of various depths, ICG microangiography correlated with visual and histological evaluation of burns. However, this study found that measurements are influenced by bandages, blood, and ointments, resulting in a decreased absorption of ICG signal by 63±36%, which ultimately leads to over- and under-estimation of burn depth.32
While ICG is mainly used to measure microvascular perfusion, a recent study revealed a novel property of ICG many hours after the microangiography signal is gone.33 This study suggested that 12 to 24 hours after IV injection, ICG-LP diffused into the tissues and the exposed hydrophobic domain of ICG was bound to the exposed phospholipids of necrotic cell membranes, thereby acting as a necrosis-avid dye. The use of this property of ICG could be harnessed to augment pre- and intra-operative determination of necrotic tissue to enhance visual assessment in surgical decision-making in burn injury.
Thermography:
Thermography is based on the premise that skin emits infrared (IR) radiation, which can be correlated with temperature. Diminished blood flow results in decreased temperature in deep dermal and full thickness burns, whereas superficial burns demonstrate increased blood flow and emit higher IR radiation. Still in use today, this technique was pioneered in the 1960s by Lawson, who was able to predict burn wound depth in dogs that correlated 90% with histological analysis, using a technique called static thermography.34 However, the results of this technique can be confounded by evaporative heat loss, variable depth of blood vessels based on location of burn and body habitus, and finally, active changes in the wound as a result of the formation of granulation tissue.29 Hackett found that evaporative heat loss can be mitigated by placement of a polyethylene barrier (cling film).35 In that study, Hackett was able to predict the depth of the burn using thermography more accurately than clinical evaluation (90% versus 60% of the time). An experimental technique offering improvements over static thermography, called active dynamic thermography (ADT), has shown some promise in pilot studies.27 ADT measures heat conduction trends over time after application of heat to the skin.36 Normal tissue dissipates heat through IR radiation and through conduction or convection to blood vessels, while burned skin can only conduct heat through IR radiation. This variation in heat conduction can be used to create a 3D image of the wound. ADT technology has potential for use with indeterminate depth burns but is still in early stages of development.
Laser Doppler Imaging (Laser Scanning):
Laser Doppler Imaging (LDI) for evaluation of burn wounds was first described in 1975 by Stern.16 Originally evolving from Doppler principles, early investigations of LDI demonstrated 70–100% accuracy in prediction of wounds that would heal within 21 days, and 93–100% accuracy in determining wounds that would fail to heal. However, it became apparent that the technique is limited by the natural evolution of burn wounds over time, the variable behavior of wound perfusion based on depth of injury, and environmental and physiologic factors. In addition, early models of LDI required physical contact between the Doppler probe and the burn, thus increasing risk for pain and infection, and decreasing the surface area that could be investigated.2 As LDI developed, the technology was refined to include scanning capabilities, eliminating the need for direct contact with skin surface and expanding the size of surface area available for evaluation. The positive predictive value of this technique was found to be as high as 98.4%, and it is currently the only FDA-approved imaging technique for burn assessment.26 A study of 28 burn patients performed by Merz et al. demonstrated the utility of LDI analysis at 24 hours post burn.37 All wounds clinically evaluated to be full thickness burns underwent excision and grafting. Wounds that were classified as superficial, deep, or indeterminate thickness were clinically followed for 3 weeks. In their analysis, the authors found LDI to have a 93.1% positive predictive value in predicting partial thickness wounds that were clinically assessed to heal in less than 3 weeks. Only 6.9% of partial thickness wounds that were predicted to heal by LDI ultimately required grafting. From the same dataset, LDI had a negative predictive value of only 69.3%. Of note, 70% of these wounds that were classified as indeterminate depth required excision and grafting within 8 days, while the remaining 30% healed without surgery.
Optical Measurement (Reflection-Optical Multispectral Imaging):
Optical measurement, the next frontier in imaging of burn wounds, obtains data from optical changes in the dermal components of denatured skin (collagen structure, blood flow, water content, etc.) by measuring absorption and scattering of light. This method provides structural and functional assessment of burn wounds.27,29 Table 2 summarizes the advantages and disadvantages of the most promising of these imaging technologies. A combination of three non-invasive imaging techniques—Photoplethysmography (PPG), Real Image, and Multispectral Imaging—has recently been employed to discriminate healthy from superficial and deep burned tissue, as well as viable wound bed.38 Features from all three imaging technologies were incorporated into a machine-learning algorithm to develop a set of imaging characteristics used to discriminate different injury depths. The combination of three technologies proved superior to each technology alone. Image acquisition is fast, and the technology has the potential for intraoperative use. One substantial limitation of this technology is that PPG relies on pulse variation, which is not feasible for intraoperative use if operating under tourniquet or with vasoconstrictive drugs. As this technology continues to be refined, combinations of imaging and machine learning algorithms may represent the future of burn depth determination.
Table 2-.
| Modality | Advantages | Disadvantages |
|---|---|---|
Microscopy:
|
|
|
Perfusion imaging:
|
|
|
Tomography:
|
|
|
Spectroscopy:
|
|
|
Burn progression and implications to timing of depth determination
Evaluation of burn depth is especially challenging in the early hours to days after burn injury, as the wound continues to evolve. While there is some controversy among burn surgeons regarding the idea that burn progression occurs in humans, animal models of thermal injury have demonstrated the phenomenon anywhere from 48 hours to two weeks after injury.39,40 Shortly after burn injury, the appearances of the wound can be divided into three zones corresponding to the presumed degree of tissue damage: the inner zone of coagulation (necrosis), the zone of ischemia (stasis), and the outer zone of hyperemia (Figure 2). In Jackson’s initial description of these zones, developed after detailed daily observations of 20 patients with confirmation of findings in over 1200 patients, he noted that the appearances evolve over 3 weeks. He further emphasizes that what appears in week 1 – mostly due to the vascular state of the superficial dermis – is independent of the appearance 2–3 weeks after injury, which is more indicative of repair4. Burn progression and its association with the injury zones is comprehensively reviewed by Shupp et al.,40 therefore we will only highlight the implications for the timing of depth determination here. While early excision and grafting is a standard treatment for deep partial and full thickness burns, the optimal timing of what is considered “early” is not well defined.41 In addition to the systemic response to injury, local responses such as inflammatory cell infiltration, microthromboses leading to ischemia, and oxidative stress from reperfusion play a major role in burn wound progression.42 In a murine model of burns, a study compared the inflammatory response in very early (24 hours after burn) and later (8 days after burn) injuries.41 Delayed excision resulted in a more severe cytokine and chemokine regulatory cell response compared with early excision. Together, timing of burn depth determination and timing of excision may influence burn progression potential. By understanding the wound environment in the “at risk” zone of ischemia (Figure 2) we will be able to test therapeutic approaches that can diminish or inhibit the progression of injury to necrosis, potentially decreasing the areas requiring autografting.
Figure 2:
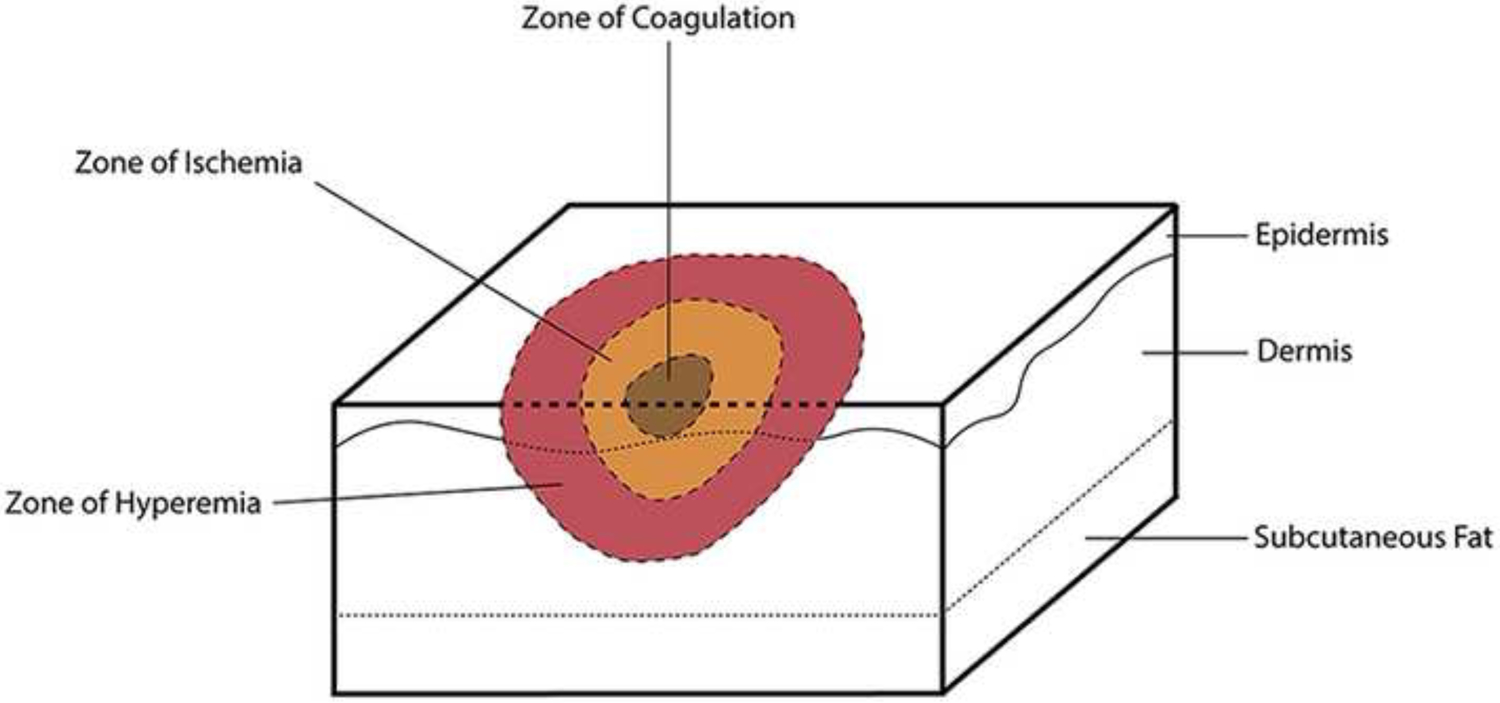
Zones of burn injury.
Management of burn wounds and implications in indeterminate depth burns
Burn treatment can be divided into two categories: operative and non-operative management. Whereas superficial burns are managed non-operatively with wound care, deeper wounds that are not expected to heal within 3 weeks benefit from excision and grafting.2 This treatment paradigm is supported by research revealing that deeper burns heal through activation of fibroblasts in the reticular dermis, which can lead to scarring.43 In addition, early excision and grafting is associated with shorter hospital stays, and less time away from work.44,45 Despite this evidence, questions regarding the exact timing of excision and grafting remain. In the next few sections we will review the evidence behind operative and non-operative management of burn injuries, including challenges associated with indeterminate depth burns.
Early excision and grafting
In 1970, Dr. Zora Janzekovic published what is perhaps the most influential case series to describe the benefits of early excision and grafting in management of full and deep partial thickness burns.45 She presented findings of wound healing from a study of more than 1600 burn patients over the course of 10 years. The main points, supported by the clinical findings, included the use of occlusive dressing for the first 3–5 days to prevent desiccation of the wound, and excision and immediate autografting between days 3 and 5 after a burn. While the concept of early excision was known at the time, this study highlighted the importance of early grafting for preventing pain and infection. Dr. Janzekovic also noted that the determination of the precise depth of injury can only occur at the time of excision. While this study was pivotal in promoting early excision and grafting in the field, the potential healing capacity of IDBs was not addressed.
Knife excision and grafting
The most common way to excise burn wounds is with a surgical knife, such as the Goulian, Blair, or Humby knives (Figure 3). One of the earliest randomized controlled trials (RCT) comparing early excision and grafting with non-operative management in indeterminate depth wounds was reported by Engrav et al. in 1983.46 In this study, 22 patients who underwent excision and grafting were compared with 25 patients who underwent non-operative management of their burns with twice daily hydrotherapy, debridement, and silver sulfadiazine cream. All patients had burns < 20% Total Body Surface Area (TBSA). IDBs were classified clinically as lacking features of full thickness but having decreased sensation and capillary refill. Patients with wounds that were deemed likely to heal within 3 weeks were excluded from the study. The study showed that patients who underwent early (day 5–7 after burn) excision and grafting had more blood loss, but shorter hospital stay, lower hospital costs and less time off work compared with those who had their wounds managed non-operatively. In addition, the severity and quantity of burn scar hypertrophy were increased in the group of patients that were treated non-operatively. Twelve of the 25 patients in the non-operative group eventually required surgery. The study concluded that early excision and grafting was the preferred method for management of IDBs. No mortality data were reported in this study.
Figure 3:

Excision of burn with Goulian knife. A) Represents a picture prior to excision of a full thickness burn. B) Indicates the first tangential excision with arrows pointing to thrombosed blood vessels.
The first meta-analysis of six RCTs evaluating the importance of early excision and grafting of burns was published in 2006 by Song et al.44 Studies included all age groups and contained data regarding mortality, blood loss, blood transfusion, wound healing time, length of stay, sepsis, operating room time, and long-term morbidity. The meta-analysis revealed that early excision and grafting (within 1–6 days after burn) was associated with increased blood transfusion requirements but overall shorter length of stay. When stratified to exclude patients with inhalation injury, early excision and grafting was associated with decreased mortality compared with initial non-operative management until eschar separation with delayed grafting. Due to the limited number of patients and heterogeneity of reported results, no conclusions could be drawn regarding duration of sepsis, wound healing time, skin graft take, and long-term morbidity.
Hydrosurgery
Determination of the amount of necrotic tissue to excise is subjective and relies on surgeon experience. In an effort to minimize the amount of viable tissue excised, excisional methods such as hydrosurgery were developed. Hydrosurgery involves using a narrow nozzle to apply a jet of saline, which fans out into a narrow wall of high velocity saline that acts as a fine scalpel.47 Through the Venturi effect, the increase of velocity through the narrow nozzle is accompanied by a loss of pressure at the nozzle tip. This loss in pressure acts as a vacuum that removes the debrided tissue. The velocity can be adjusted (10 settings) to modulate debridement, which in theory results in decreased loss of viable tissue. The depth of excision using the Versajet system® (Smith and Nephew, Andover, MA), compared to a Goulian knife on discarded abdominoplasty samples showed that Versajet® at its highest setting (10) resulted in removal of 200 uM of tissue (1/10 of dermis thickness), whereas the Goulian knife removed an average of 738 ± 86 uM (3–4/10 dermis thickness).3 However, this study is misleading for burn excision since the investigators measured the thickness of Versajet® excision using normal skin and compared this to excision of burned tissue using the Goulian knife. Burned tissue is inflamed and likely has varying thicknesses related to the inflammation.
Two RCTs (adult and pediatric populations) comparing hydrosurgery to conventional knife methods found no difference in healing time, postoperative pain, or contractures.48,49 Adequate debridement was achieved with both techniques. Operating times in the adult study were longer for large burns using hydrosurgery, but shorter when hydrosurgery was used for difficult areas such as genitals, hands, and feet.48 In the pediatric study, the investigators also found a significantly higher amount of dermal preservation with hydrosurgery.49 However, the increase in the amount of viable dermis left behind was not associated with any functional outcomes such as decreased healing time or contracture rates. This may be a reflection on the highly operator-dependent nature of hydrosurgery.
In summary, hydrosurgery has demonstrated utility as an adjunct to conventional therapy, especially in hard to excise areas, but is not versatile enough to replace knife excision completely, especially for larger and deeper burns. In combination with improved identification of necrotic tissue, the precision of hydrosurgery may be useful in surgical excision of indeterminate depth burns.
Enzymatic debridement
In an effort to preserve healthy dermis, investigators have turned to biologic and chemical processes to aid the debridement of necrotic tissue. Whereas the technologies discussed so far are based on interventions at the tissue level, enzymatic debridement removes damaged tissue at the molecular level. This technique has been used for hundreds of years.50 Acids, enzymes of plant origin, and proteolytic enzymes of bacterial origin have been reported in the literature.50 Here we present two examples of current enzymatic treatments that are in use today. Because the enzymes are removing the necrotic tissue and leaving behind the viable tissue, selective debridement would reduce the need to define the depth at the time of treatment. For a more in-depth discussion of other enzymatic debridement methods, refer to an excellent review of the subject by Edmondson et al.50
Bromelain-based enzymatic agents:
NexoBrid® (EDNX; MediWound, Yavne, Israel) is a bromelain-based enzymatic debridement agent that has been under development for 30 years.51 In the literature it is also referred to as debriding gel dressing (DGD). This agent was approved in the European Union in 2012, and remains under investigation in the United States.52 It is a mixture of purified proteolytic proteins with bromelain, a derivative of pineapple stems. DGD acts only on dead tissue and cannot penetrate viable dermis, thus providing selective and safe debridement. In an RCT, NexoBrid® was shown to reduce the time to complete debridement, the need for surgery, the area of burn excised, and the need for autografting, without changes in scar quality or complication rate.53 A recent European consensus statement has also endorsed DGD for the management of thermal burns up to 15% TBSA in adults, including management of circumferential burns, as DGD reduces the incidence of compartment syndrome based on recent evidence.54 Some drawbacks of DGD are severe pain after application, which may be mitigated by analgesics, and difficulty maintaining occlusive dressings on wounds in areas such as the perineum. NexoBrid® is in Phase III clinical trials in the U.S. More RCTs are needed to establish its efficacy and feasibility before it is broadly accepted.52,55
Collagenase:
Collagenase ointment generally refers to a collagenase proteolytic mixture derived from Clostridium histolyticum, although collagenase mixtures derived from other bacterial sources such as Streptomyces also exist.56,57 Because collagenase is specific to collagen, a protein comprising up to 75% of the dermis, it is considered a ‘selective’ debriding agent.58 A recent systematic review identified three high-quality studies using collagenase for enzymatic debridement of partial thickness wounds.59 In two studies, collagenase was compared with silver sulfadiazine for management of partial thickness burns, and was found to be equivalent to silver sulfadiazine in time to wound healing, time to clean wound bed, and need for grafting after 10 days.58,59 There have been no studies comparing collagenase to bromelain-based agents for selective debridement.
Adjuncts and alternatives to autografting
A period of “watchful waiting” may be beneficial, with minimal added risk to the patient with IDB, by providing the possibility to achieve wound healing over time without autografting. This concept differs from the delayed grafting after eschar separation that was shown to increase mortality, as described below. Rather, IDBs may possess a potential regenerative capacity that is not clearly apparent early after injury. Therefore, a period of observation along with the use of adjuncts to enhance autologous wound healing may be possible. We present several options that may enhance the watchful waiting strategy and avoid or reduce the need for autografting and associated donor site morbidity.
Biological tissue substitutes
While an autograft represents the best replacement of lost epidermis and dermis, there is significant morbidity associated with autograft donor sites. Additionally, in very large TBSA burns there may be insufficient donor sites elsewhere on the patient’s body for complete early wound closure. For these reasons, allografts, xenografts, and several biological tissue substitutes have been considered for use as temporary wound coverage until autografting is possible, in healing of deep partial thickness burns, or in conjunction with widely meshed autografts.60 Attributes of these substitutes include temporary barrier function to prevent fluid loss and infection, as well as physical and biological properties that provide a scaffold to stimulate new dermis growth and enhance re-epithelialization. Here we present examples of skin substitute technologies that are currently used in practice or are under clinical investigation.
Allograft/Xenograft:
Cadaveric skin (allograft) can provide temporary coverage until the wound is autografted, or can be used as a biologic dressing over IDB burns to enhance the wound healing environment.61,62 Prior to placement of the allograft, the wound requires excision of necrotic tissue prior to autografting or will need to be debrided if using the allograft as a biologic dressing. This debridement can be accomplished using a curved metal instrument (Norsen-Belmed Inc. Mchenry, IL) that has a blunt edge which allows for more aggressive debridement of adherent necrotic tissue. While allografts are useful as a short-term covering when autograft donor is unavailable, as a test graft when there are concerns about the readiness of the wound bed to accept an autograft, or as a biologic dressing to improve the wound healing environment, they are costly and have the potential risk of transmitting pathogens.
When allografts are unavailable or cost-prohibitive, xenografts (skin from a donor of a different species) can be used as an alternative. The most commonly used xenograft source in the United States is porcine, however other sources include a variety of animals such as sheep, frogs, and fish.63,64 Much like autografts, xenografts also provide temporary wound coverage; they do not fully revascularize prior to rejection, and should be viewed as a biologic dressing rather than a permanent substitute.65 They also have the potential to provoke an antigenic response, transmit disease, and may pose ethical and religious considerations that limit their use. Additionally, in order for the allo- or xenograft to adhere, the wound requires excision down to viable tissue. Excision using currently available methods increases the risk that viable dermal cells, which could contribute to autologous wound healing, will be removed in the process. The use of allo- or xenograft in combination with enzymatic debridement (discussed earlier) is a potential way to enhance wound healing without the need for autografting.
Placental membrane:
The placental membrane, composed of the amnion and chorion layers, is a reservoir of growth factors and cytokines that promote wound healing and prevent fibrosis, infection, and scarring.66 These membranes have the advantage of being immune-privileged, therefore they do not elicit an inflammatory immune response. Cryopreserved or dehydrated human placental products allow for long-term storage and avoid the potential bioburden associated with fresh placental membranes.65 While these products have historically been used for treatment of diabetic and venous stasis ulcers, recent data suggest some success in the treatment of burns.67 Placental membranes can be placed directly on debrided superficial partial thickness burns, for example after using a Norsen as described above, to promote re-epithelization. Alternatively, the placental membrane can be used in a staged technique, followed by split thickness skin grafts (STSG).67 At our institution, we have anecdotal experience using dehydrated placental membranes on IDB to facilitate more rapid healing than with standard daily wound care (Figure 4). Additionally, the inflammation associated with prolonged wound healing anecdotally appeared to subside with placental membrane treatment. Advantages of the placental membranes include easy adherence and off-the-shelf availability. However, these membranes are currently more costly per square centimeter than allografts – ten times more at our institution; they can also be difficult to handle, and degrade quickly in the wound bed.66
Figure 4:
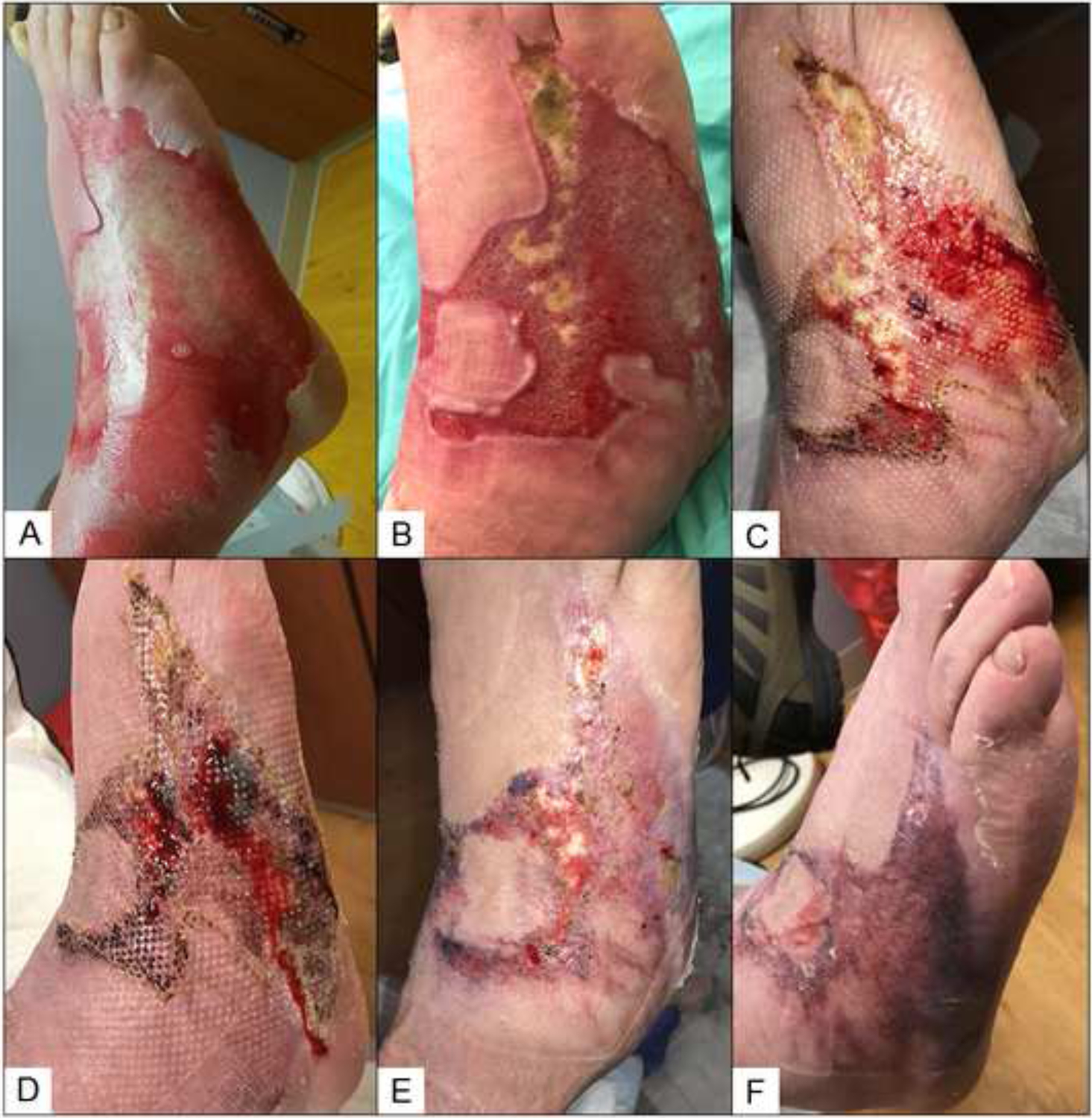
Management of an indeterminate depth burn using placental membrane after hydrosurgery excision in a patient who was a poor autograft candidate due to comorbidities. A) Full thickness burn on the day of injury, B) 15 days post burn (PB), postoperative day (POD) 0, C) 21 days PB POD6, D) 28 days PB, POD13, E) 35 days PB, POD 27, F) 49 days PB, POD 41.
Dermal substitutes:
Several dermal substitutes are available for use on burn patients, such as Integra® (Integra LifeSciences, Plainsboro, NJ) and NovoSorb™ Biodegradable Temporizing Matrix (BTM; PolyNovo, Victoria, Australia). These products can be mono- or multi-layered, and serve the purpose of dermal reconstruction and wound coverage until the wound heals or until autograft can be applied. These dermal substitutes usually require full thickness dermal excision and are beyond the scope of this review of indeterminate depth burns. One example of a dermal substitute that does not require full thickness dermal excision is Fetal Bovine Collagen (FBC). FBC, such as Primatrix® (TEI Biosciences, Boston, MA), is a newer technology for the management of burns. This product is intended for the management of deep and full thickness burns. Much like Integra®, it is composed of dermal scaffold to support the growth of an STSG. The dermal scaffold is rich in type III collagen, which is one of the components of early healing. FBC has been shown to result in 80% graft take of complex traumatic surgical wounds with subsequent STSG placement.68 Unlike Integra®, It has been shown to result in wound healing without STSG in a patient with mixed full and partial thickness burn.69 Of note, the burn wound in this study was assessed clinically as a full thickness burn, however subsequent biopsies revealed that there was a mixture of full and partial thickness burns in the wound bed. This case study is an example of an IDB that was treated as a full thickness burn, and serves to illustrate the difficulty encountered by physicians with respect to depth determination.
Delayed excision and grafting
Historically, non-operative treatment of burns increased patient morbidity and mortality.45,70 While results of some retrospective studies and RCTs.13,44–46 clearly show the benefit of early excision and grafting, especially in large TBSA burns, some clinicians have raised doubts about early excision and grafting in IDBs. In a letter to the editor of the journal Burns in 2012, M.J. Hop from the Dutch Association of Burn Centres issued a call for more evidence of the efficacy of early versus delayed excision and grafting.71 Hop pointed to the differing opinions of British and Dutch burn surgeons on the subject. The Dutch surgeons argued that most evidence in support of early excision and grafting came from studies done in the 70s and 80s, and that burn care has progressed to the point that delayed excision and grafting may have decreased morbidity due to less overzealous excision. There is some evidence in a recent porcine model that this may be true.72 More studies in humans are necessary to support delayed excision and grafting.
Emerging technologies
With advances in technology and an improved understanding of wound healing, options for wound coverage are becoming more sophisticated. An ideal skin substitute--whether synthetic, allogeneic, or from the patient’s own cells--would reliably take the place of autografting. We will focus our discussion on two promising products, one recently approved, and one in the late stages of clinical trials, as well as several other technologies in earlier phases of translational research.
Cultured Epidermal Autografts:
Autologous cell transplantation using cultured epithelial autograft (CEA) has been around since the 1980s. CEA (Epicel® Vericel, Cambridge, Massachusetts) is a sheet of keratinocytes 2–8 cell layers thick requiring a full thickness skin biopsy from the patient and subsequent culture expansion over 3 weeks. This technology is fragile, difficult to apply, susceptible to infection, costly, and has variable take rates.73 Modifications to the recommended use of CEA include the use in a sandwich technique over widely meshed grafts or micrograft to overcome some of the reported disadvantages, and highlight the need for dermal elements for enhanced wound healing.74 The most robust evidence for the use of CEA comes from an 18-year long study of 88 patients treated for large burns (mean 58.5%) at Indiana University.75 This group reported graft take rates of approximately 72%. However, the group stressed the importance of adequate preparation of the wound bed, as well as the importance of early excision (within 2–3 days) of the burn. It is important to note that the group used cadaver dermis or 1:6 meshed STSG before application of CEA.
ReCell®:
ReCell® (Avita Medical, Cambridge, United Kingdom), a recently FDA-approved device indicated for the treatment of partial and full thickness burns, involves fractionating the patients cells from a small donor site into a suspension that is sprayed on the wound. This modification avoids the costly, time-consuming culturing process that is necessary for CEA.76 The cell suspension contains 1.7 × 106/cm2 cells and is composed of 65% keratinocytes, 30% fibroblasts and 3.5% melanocytes. The technique can be used in the OR and does not require specialized equipment in addition to the ReCell® system.73 In a recent multicenter RCT, the ReCell® system was compared to 2:1 meshed STSG for the management of deep partial thickness burns—defined as a wound that contained viable dermis throughout the wound base.77,78 The study found that there was no difference in healing, pain, subject satisfaction or scarring outcomes at 4 weeks. The ReCell® system had the advantage of a smaller harvested donor site (2cm2 for ReCell® vs 110cm2 for autograft) with less postoperative pain and improved patient satisfaction due to a smaller donor site. The presence of melanocytes in the cell suspension also had a favorable impact on final pigmentation compared with other systems.78 When compared with meshed autograft, ReCell® had a slightly more favorable final pigmentation, and was similar to unmeshed graft with respect to final pigmentation.78 Disadvantages of the system include high cost and loss of cells during spraying.65
StrataGraft®:
StrataGraft® (Stratatech-Mallinkcrodt, Madison, WI) is a stratified bilayer skin substitute that has shown great promise, earning an “orphan product” designation from the FDA in 2012 and “Regenerative Medicine Advanced Therapy” designation in 2017.65,79 It is composed of a dermal layer containing human fibroblasts and a fully stratified, biologically active epidermis derived from a human keratinocyte progenitor cell line.80 This product is intended for coverage of excised burns as a substitute for autografting. In the initial safety clinical trial comparing StrataGraft® to allograft in full thickness burns before autografting, StrataGraft® was found to be safe, well tolerated, and did not induce an immunologic response.81,82 A safety and efficacy multicenter clinical trial was completed in adult patients, comparing StrataGraft® with autograft in deep partial thickness burns—defined as wounds containing intact dermal elements; results have not yet been published.83 A Phase III clinical trial of StrataGraft® on deep partial thickness burns is currently enrolling, with an estimated completion date of December 2019, according to clinicaltrials.gov.83
Stem cells:
The dermal substitutes and bi-layered or multicellular substitutes discussed so far contain fully differentiated cells. In general, dermal substitutes have poor potential for revascularization and epithelialization.84 Some contain fibroblasts, which induce stem cell activation and differentiation in the wound bed through the release of cytokines, but results have been variable.84 In order to produce a skin substitute with a dermal component that is more similar to human dermis, advances in tissue engineering aim to include stem cells in the dermal layer of skin substitutes, such as the recent integration of skin-derived mesenchymal stem cells (MSCs) with Integra®.85 Potential stem cell sources include bone marrow, adipose tissue, and burn eschar.85–87 Stem cells contribute to wound healing by differentiation into skin cells, secretion of cytokines that assist in repair, and modulation of the immune response to prevent wound deterioration.84 Bone marrow-derived MSCs have been studied in humans for treatment of burns, and have shown some promise in decreasing scarring and promoting angiogenesis.84,86,88,89 Nevertheless, their utility is limited by the difficulty of obtaining a sufficient yield of stem cells from bone marrow aspirates, and the effects of bone marrow suppression seen during stress states. Alternatively, adipose cells are abundant and have similarly demonstrated potential for skin regeneration.84,87 However, there are no RCTs to date to support their use. The biggest concern plaguing stem cell therapies is the danger of tumor formation.84,90 Further studies are needed to establish the safety and feasibility of stem cell therapeutics for burn care.
Three-dimensional printing of skin:
The prospect of three-dimensional bioprinting of skin as an alternative to autografting is promising; it would allow the use of human-derived building blocks to reconstruct all components of the skin.91 Several technologies for printing tissue exist using inkjet, extrusion, laser assisted, in situ, and dynamic optical projection stereolithography (DOPsL) printers.92,93 These methods have variable printing speeds, resolution, and cell viability. DOPsL printers are the fastest of these technologies, with the highest cell viability.92 Bioink, the material used for bioprinting, is currently a mixture of extracellular matrix and cells (keratinocytes and fibroblasts). However, embryonic and adipose-derived stem cells have been reported as bioink material with post-print cell viability of 94%.94 Although in its infancy, this exciting technology holds much promise.
Inhibitors of burn progression:
An alternative way to enhance wound healing is to inhibit the injury from progression to a deeper wound. The zone of ischemia in a burn wound has long been recognized as a target for halting burn progression.42 Potential mediators of burn progression in the zone of ischemia include thrombosis of vessels; vasoconstriction mediated by cytokines such as thromboxane A2, IL-6, and TNF- alpha; reactive oxygen species that develop from tissue hypoxia; and inflammatory cell infiltration.95 Inhibition of these targets has been shown in animal studies to lead to halting of burn progression in the zone of ischemia.95 However, our limited understanding of the wound microenvironment in the early phases after thermal injury in humans has limited the development of molecular targets for human use. This is an important area of research that could provide insights into novel therapeutic targets for indeterminate depth burns in the future.
Long term sequelae
Scar quality is a crucial outcome measure for burn patients, as abnormal or hypertrophic scarring in burn patients can result in long-term pain, pruritis, and functional restrictions.43 Microscopically, hypertrophic scars are characterized by aberrations of fibroblast action, collagen imbalance, and extracellular matrix abnormalities, ultimately resulting in the clinically observed manifestations of the scar. Scar quality can be clinically assessed by multiple objective measures, including color, thickness, relief, pliability, pain, and pruritis.96 One prospective cohort study of 141 patients evaluated scar quality between patients with various depth burns.96 In a clinical trial by the same group, Laser Doppler Imaging (LDI) was used to categorize patients into either low, intermediate, or high healing potential. Surgeons then used these results to decide on surgical intervention, no surgery, or delayed decision for surgery.97 Patients included in the trial were followed up 19 months after the burn, and scar quality was objectively assessed.96 Investigators found no difference in scar quality for intermediate and high healing potential burns, while low healing potential burns demonstrated significantly poorer scar quality. Darker skin and multiple operative debridements during the acute phase of injury were found to be independent predictors of poor scar quality. Important for the treatment of indeterminate depth burns, this study also noted that early versus late surgical intervention did not impact scar quality; and there was a trend toward faster healing in intermediate healing potential wounds that were treated non-operatively. These findings differ from some historical precedents and lend support to a “wait and see” approach.45
While development of scarring is undesirable, it can be managed with therapy and compression, as well as new operative techniques. Laser therapy of traumatic and burn scars has been shown to positively impact patient outcomes.98 Expert opinion indicates that traumatic scars benefit the most when treated with ablative fractional lasers, as compared to PDL, non-ablative fractional, and ND:YAG lasers.98 Patient-reported measures of pain, pruritis, color, and mobility show improvement following serial treatments with ablative fractional laser.98 Additionally, there may be a synergistic role for PDL plus ablative fractional lasers for improvement in pigmentation, appearance, and mobility.98
More recent studies investigating fat grafting for management of hypertrophic scarring following trauma and burns suggest a potential role for this modality in scar management. A prospective cohort study evaluated hypertrophic scars of 80 burn patients following fat transfer, with follow-up over 1 year after completion of grafting. Based on immunohistochemistry analyses, the study found significant improvement in scar appearance, normalization of fibroblasts, and collagen.99 Additional non-operative management modalities of hypertrophic scars that have been reported in the literature include topical cell division, and contraction inhibitors such as imiquimod, calcium channel blockers, tacrolimus, 5-FU, and bleomycin.43 Other treatment modalities under investigation target cellular signaling molecules such as interferon alpha, IL-10, TGF-beta, and CXCR4, which have been implicated in scar formation and fibrosis.100,101
Ultimately, in order to optimize management recommendations for indeterminate depth burns, rigorous outcomes studies evaluating long-term scar results, cost of various treatment options, and overall morbidity associated with non-operative versus operative management are necessary. Understanding the treatment-associated outcomes with burns of various depths, including IDB, will greatly improve clinical decision making earlier in the course of healing.
Conclusions
IDBs continue to represent a diagnostic and management dilemma for the burn surgeon. Visual assessment of burn depth is imprecise. Several imaging modalities exist for burn depth determination; of these, Laser Doppler Imaging is the most widely adopted.15 Newer modalities, such as confocal microscopy, tomography and spectroscopy have shown some promise, but they are not yet ready for clinical application. Indeterminate depth burns have the potential either to progress to full thickness injury or, given the right wound environment, to heal without autografting. Individual factors related to the wound microenvironment, which may contribute to burn progression and depth, remain unknown. Here we highlight gaps in our understanding of the relationship between burn injury depth and healing capacity in indeterminate depth burns. We also explore multiple avenues of research that focus on addressing the challenges associated with management of indeterminate depth burns, as well as wound coverage options. With continued research towards harnessing the regenerative potential of IDBs, it may be possible to reduce or eliminate the need for autografting in these burns in the future.
Abbreviations:
- ADT
Active Dynamic Thermography
- H&E
Hematoxilin and Eosin
- ICG
Indocyanine Green
- BTM
Biodegradable Temporizing Matrix
- CEA
Cultured Epithelial Autografts
- DGD
Debriding Gel Dressing
- DOPL
Dynamic Optical Projection Stereolithography
- FBC
Fetal Bovine Collagen
- IDB
Indeterminate Depth Burn
- IR
Infrared
- LDH
Lactate Dehydrogenase
- LDI
Laser Doppler Imaging
- LP
Lipoprotein
- PPG
Photoplethysmography
- PSU
Pilosebaceous unit
- MSC
Mesenchymal Stem Cells
- STSG
Split Thickness Skin Graft
- TBSA
Total Body Surface Area
Footnotes
Disclosure: The authors report no proprietary or commercial interest in any product mentioned or concept discussed in the article. Dr. Gibson is a site principal investigator for Stratagraft clinical trials and previously served on the speakers bureau for Stratatech-Mallinkrodt.
References
- 1.Gurfinkel R, Rosenberg L, Cohen S, et al. Histological assessment of tangentially excised burn eschars. Can J Plast Surg. 2010;18(3):e33–36. [PMC free article] [PubMed] [Google Scholar]
- 2.Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P. Assessment of burn depth and burn wound healing potential. Burns. 2008;34(6):761–769. [DOI] [PubMed] [Google Scholar]
- 3.Tenenhaus M, Bhavsar D, Rennekampff HO. Treatment of deep partial thickness and indeterminate depth facial burn wounds with water-jet debridement and a biosynthetic dressing. Injury. 2007;38 Suppl 5:S39–45. [DOI] [PubMed] [Google Scholar]
- 4.JACKSON DM. [The diagnosis of the depth of burning]. Br J Surg. 1953;40(164):588–596. [DOI] [PubMed] [Google Scholar]
- 5.Orgill DP, Solari MG, Barlow MS, O’Connor NE. A finite-element model predicts thermal damage in cutaneous contact burns. J Burn Care Rehabil. 1998;19(3):203–209. [DOI] [PubMed] [Google Scholar]
- 6.Jeschke MG, Pinto R, Costford SR, Amini-Nik S. Threshold age and burn size associated with poor outcomes in the elderly after burn injury. Burns. 2016;42(2):276–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wearn C, Hardwicke J, Kitsios A, Siddons V, Nightingale P, Moiemen N. Outcomes of burns in the elderly: revised estimates from the Birmingham Burn Centre. Burns. 2015;41(6):1161–1168. [DOI] [PubMed] [Google Scholar]
- 8.Jeschke MG, Patsouris D, Stanojcic M, et al. Pathophysiologic Response to Burns in the Elderly. EBioMedicine. 2015;2(10):1536–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson DM. In search of an acceptable burn classification. Br J Plast Surg. 1970;23(3):219–226. [DOI] [PubMed] [Google Scholar]
- 10.Jackson DM. A historical review of the use of local physical signs in burns. Br J Plast Surg. 1970;23(3):211–218. [DOI] [PubMed] [Google Scholar]
- 11.Cope O THE TREATMENT OF THE SURFACE BURNS. Ann Surg. 1943;117(6):885–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Latarjet J A simple guide to burn treatment. International Society for Burn Injuries in collaboration with the World Health Organization. Burns. 1995;21(3):221–225. [DOI] [PubMed] [Google Scholar]
- 13.Heimbach D, Engrav L, Grube B, Marvin J. Burn depth: a review. World J Surg. 1992;16(1):10–15. [DOI] [PubMed] [Google Scholar]
- 14.Heimbach DM, Afromowitz MA, Engrav LH, Marvin JA, Perry B. Burn depth estimation--man or machine. J Trauma. 1984;24(5):373–378. [PubMed] [Google Scholar]
- 15.Israel JS, Greenhalgh DG, Gibson AL. Variations in Burn Excision and Grafting: A Survey of the American Burn Association. J Burn Care Res. 2017;38(1):e125–e132. [DOI] [PubMed] [Google Scholar]
- 16.Jaskille AD, Ramella-Roman JC, Shupp JW, Jordan MH, Jeng JC. Critical review of burn depth assessment techniques: part II. Review of laser doppler technology. J Burn Care Res. 2010;31(1):151–157. [DOI] [PubMed] [Google Scholar]
- 17.Bloemen MC, van Zuijlen PP, Middelkoop E. Reliability of subjective wound assessment. Burns. 2011;37(4):566–571. [DOI] [PubMed] [Google Scholar]
- 18.Hlava P, Moserová J, Königová R. Validity of clinical assessment of the depth of a thermal injury. Acta Chir Plast. 1983;25(4):202–208. [PubMed] [Google Scholar]
- 19.Hoeksema H, Van de Sijpe K, Tondu T, et al. Accuracy of early burn depth assessment by laser Doppler imaging on different days post burn. Burns. 2009;35(1):36–45. [DOI] [PubMed] [Google Scholar]
- 20.Karim AS, Yan A, Ocotl E, et al. Discordance between histologic and visual assessment of tissue viability in excised burn wound tissue. Wound Repair Regen. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singer AJ, Hirth D, McClain SA, Clark RA. Lack of agreement between gross visual and histological assessment of burn reepithelialization in a porcine burn model. J Burn Care Res. 2012;33(2):286–290. [DOI] [PubMed] [Google Scholar]
- 22.Singer AJ, Berruti L, Thode HC, McClain SA. Standardized burn model using a multiparametric histologic analysis of burn depth. Acad Emerg Med. 2000;7(1):1–6. [DOI] [PubMed] [Google Scholar]
- 23.Watts AM, Tyler MP, Perry ME, Roberts AH, McGrouther DA. Burn depth and its histological measurement. Burns. 2001;27(2):154–160. [DOI] [PubMed] [Google Scholar]
- 24.Gibson ALF, Shatadal S. A simple and improved method to determine cell viability in burn-injured tissue. Journal of Surgical Research. 2017;215:83–87. [DOI] [PubMed] [Google Scholar]
- 25.Gibson ALF, Bennett DD, Taylor LJ. Improving the histologic characterization of burn depth. J Cutan Pathol. 2017;44(12):998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khatib M, Jabir S, Fitzgerald O’Connor E, Philp B. A systematic review of the evolution of laser Doppler techniques in burn depth assessment. Plast Surg Int. 2014;2014:621792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paul DW, Ghassemi P, Ramella-Roman JC, et al. Noninvasive imaging technologies for cutaneous wound assessment: A review. Wound Repair Regen. 2015;23(2):149–162. [DOI] [PubMed] [Google Scholar]
- 28.Thatcher JE, Li W, Rodriguez-Vaqueiro Y, et al. Multispectral and Photoplethysmography Optical Imaging Techniques Identify Important Tissue Characteristics in an Animal Model of Tangential Burn Excision. J Burn Care Res. 2016;37(1):38–52. [DOI] [PubMed] [Google Scholar]
- 29.Thatcher JE, Squiers JJ, Kanick SC, et al. Imaging Techniques for Clinical Burn Assessment with a Focus on Multispectral Imaging. Adv Wound Care (New Rochelle). 2016;5(8):360–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodard ED, Friedlander B, Lesher RJ, Font W, Kinsey R, Hearne FT. Outbreak of hypersensitivity pneumonitis in an industrial setting. JAMA. 1988;259(13):1965–1969. [PubMed] [Google Scholar]
- 31.Kamolz LP, Andel H, Haslik W, et al. Indocyanine green video angiographies help to identify burns requiring operation. Burns. 2003;29(8):785–791. [DOI] [PubMed] [Google Scholar]
- 32.Haslik W, Kamolz LP, Andel H, Winter W, Meissl G, Frey M. The influence of dressings and ointments on the qualitative and quantitative evaluation of burn wounds by ICG video-angiography: an experimental setup. Burns. 2004;30(3):232–235. [DOI] [PubMed] [Google Scholar]
- 33.Fang C, Wang K, Zeng CT, et al. Illuminating necrosis: From mechanistic exploration to preclinical application using fluorescence molecular imaging with indocyanine green. Sci Rep-Uk. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lawson RN, Wlodek GD, Webster DR. Thermographic assessment of burns and frostbite. Can Med Assoc J. 1961;84:1129–1131. [PMC free article] [PubMed] [Google Scholar]
- 35.Hackett ME. The use of thermography in the assessment of depth of burn and blood supply of flaps, with preliminary reports on its use in Dupuytren’s contracture and treatment of varicose ulcers. Br J Plast Surg. 1974;27(4):311–317. [DOI] [PubMed] [Google Scholar]
- 36.Prindeze NJ, Fathi P, Mino MJ, et al. Examination of the Early Diagnostic Applicability of Active Dynamic Thermography for Burn Wound Depth Assessment and Concept Analysis. J Burn Care Res. 2015;36(6):626–635. [DOI] [PubMed] [Google Scholar]
- 37.Merz KM, Pfau M, Blumenstock G, Tenenhaus M, Schaller HE, Rennekampff HO. Cutaneous microcirculatory assessment of the burn wound is associated with depth of injury and predicts healing time. Burns. 2010;36(4):477–482. [DOI] [PubMed] [Google Scholar]
- 38.Juan Heredia-Juesas JET, Yang Lu, Squiers John J., King Darlene, Fan Wensheng, DiMaio J. Michael, and Martinez-Lorenzo Jose A.. Burn-injured tissue detection for debridement surgery through the combination of non-invasive optical imaging techniques. Biomedical Optics Express. 2018;9(4):1809–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Papp A, Kiraly K, Härmä M, Lahtinen T, Uusaro A, Alhava E. The progression of burn depth in experimental burns: a histological and methodological study. Burns. 2004;30(7):684–690. [DOI] [PubMed] [Google Scholar]
- 40.Shupp JW, Nasabzadeh TJ, Rosenthal DS, Jordan MH, Fidler P, Jeng JC. A review of the local pathophysiologic bases of burn wound progression. J Burn Care Res. 2010;31(6):849–873. [DOI] [PubMed] [Google Scholar]
- 41.Fear VS, Poh WP, Valvis S, et al. Timing of excision after a non-severe burn has a significant impact on the subsequent immune response in a murine model. Burns. 2016;42(4):815–824. [DOI] [PubMed] [Google Scholar]
- 42.Salibian AA, Rosario ATD, Severo LAM, et al. Current concepts on burn wound conversion-A review of recent advances in understanding the secondary progressions of burns. Burns. 2016;42(5):1025–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zuo KJ, Medina A, Tredget EE. Important Developments in Burn Care. Plast Reconstr Surg. 2017;139(1):120e–138e. [DOI] [PubMed] [Google Scholar]
- 44.Ong YS, Samuel M, Song C. Meta-analysis of early excision of burns. Burns. 2006;32(2):145–150. [DOI] [PubMed] [Google Scholar]
- 45.Janzekovic Z A New Concept in the Early Excision and Immediate Grafting of Burns. Journal of Trauma. 1970;10(12):1103–1108. [PubMed] [Google Scholar]
- 46.Engrav LH, Heimbach DM, Reus JL, Harnar TJ, Marvin JA. Early excision and grafting vs. nonoperative treatment of burns of indeterminant depth: a randomized prospective study. J Trauma. 1983;23(11):1001–1004. [DOI] [PubMed] [Google Scholar]
- 47.Cubison TC, Pape SA, Jeffery SL. Dermal preservation using the Versajet hydrosurgery system for debridement of paediatric burns. Burns. 2006;32(6):714–720. [DOI] [PubMed] [Google Scholar]
- 48.Gravante G, Delogu D, Esposito G, Montone A. Versajet hydrosurgery versus classic escharectomy for burn debridment: a prospective randomized trial. J Burn Care Res. 2007;28(5):720–724. [DOI] [PubMed] [Google Scholar]
- 49.Hyland EJ, D’Cruz R, Menon S, et al. Prospective, randomised controlled trial comparing Versajet hydrosurgery and conventional debridement of partial thickness paediatric burns. Burns. 2015;41(4):700–707. [DOI] [PubMed] [Google Scholar]
- 50.Edmondson SJ, Ali Jumabhoy I, Murray A. Time to start putting down the knife: A systematic review of burns excision tools of randomised and non-randomised trials. Burns. 2018. [DOI] [PubMed] [Google Scholar]
- 51.Rosenberg L, Shoham Y, Krieger Y, et al. Minimally invasive burn care: a review of seven clinical studies of rapid and selective debridement using a bromelain-based debriding enzyme (Nexobrid(R)). Ann Burns Fire Disasters. 2015;28(4):264–274. [PMC free article] [PubMed] [Google Scholar]
- 52.MediWound. A Study to Evaluate the Efficacy and Safety of NexoBrid in Subjects With Thermal Burns. https://clinicaltrials.gov/ct2/show/NCT02148705. Published 2014. Accessed.
- 53.Rosenberg L, Krieger Y, Bogdanov-Berezovski A, Silberstein E, Shoham Y, Singer AJ. A novel rapid and selective enzymatic debridement agent for burn wound management: a multi-center RCT. Burns. 2014;40(3):466–474. [DOI] [PubMed] [Google Scholar]
- 54.Krieger Y, Bogdanov-Berezovsky A, Gurfinkel R, Silberstein E, Sagi A, Rosenberg L. Efficacy of enzymatic debridement of deeply burned hands. Burns. 2012;38(1):108–112. [DOI] [PubMed] [Google Scholar]
- 55.Loo YL, Goh BKL, Jeffery S. An Overview of the Use of Bromelain-Based Enzymatic Debridement (Nexobrid®) in Deep Partial and Full Thickness Burns: Appraising the Evidence. J Burn Care Res. 2018;39(6):932–938. [DOI] [PubMed] [Google Scholar]
- 56.Van Wart HE. Chapter 126 - Clostridium Collagenases A2 - Rawlings, Neil D. In: Salvesen G, ed. Handbook of Proteolytic Enzymes. Academic Press; 2013:607–611. [Google Scholar]
- 57.Zacharevskij E, Baranauskas G, Varkalys K, Rimdeika R, Kubilius D. Comparison of non-surgical methods for the treatment of deep partial thickness skin burns of the hand. Burns. 2018;44(2):445–452. [DOI] [PubMed] [Google Scholar]
- 58.Ostlie DJ, Juang D, Aguayo P, et al. Topical silver sulfadiazine vs collagenase ointment for the treatment of partial thickness burns in children: a prospective randomized trial. J Pediatr Surg. 2012;47(6):1204–1207. [DOI] [PubMed] [Google Scholar]
- 59.Patry J, Blanchette V. Enzymatic debridement with collagenase in wounds and ulcers: a systematic review and meta-analysis. Int Wound J. 2017;14(6):1055–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pham C, Greenwood J, Cleland H, Woodruff P, Maddern G. Bioengineered skin substitutes for the management of burns: a systematic review. Burns. 2007;33(8):946–957. [DOI] [PubMed] [Google Scholar]
- 61.Saffle JR. Closure of the excised burn wound: temporary skin substitutes. Clin Plast Surg. 2009;36(4):627–641. [DOI] [PubMed] [Google Scholar]
- 62.Hermans MH. Porcine xenografts vs. (cryopreserved) allografts in the management of partial thickness burns: is there a clinical difference? Burns. 2014;40(3):408–415. [DOI] [PubMed] [Google Scholar]
- 63.Sheridan RL, Tompkins RG. Skin substitutes in burns. Burns. 1999;25(2):97–103. [DOI] [PubMed] [Google Scholar]
- 64.Hu Z, Yang P, Zhou C, Li S, Hong P. Marine Collagen Peptides from the Skin of Nile Tilapia (Oreochromis niloticus): Characterization and Wound Healing Evaluation. Mar Drugs. 2017;15(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haddad AG, Giatsidis G, Orgill DP, Halvorson EG. Skin Substitutes and Bioscaffolds: Temporary and Permanent Coverage. Clin Plast Surg. 2017;44(3):627–634. [DOI] [PubMed] [Google Scholar]
- 66.Fairbairn NG, Randolph MA, Redmond RW. The clinical applications of human amnion in plastic surgery. J Plast Reconstr Aesthet Surg. 2014;67(5):662–675. [DOI] [PubMed] [Google Scholar]
- 67.Reilly DA, Hickey S, Glat P, Lineaweaver WC, Goverman J. Clinical Experience: Using Dehydrated Human Amnion/Chorion Membrane Allografts for Acute and Reconstructive Burn Care. Ann Plast Surg. 2017;78(2 Suppl 1):S19–S26. [DOI] [PubMed] [Google Scholar]
- 68.Hayn E Successful treatment of complex traumatic and surgical wounds with a foetal bovine dermal matrix. Int Wound J. 2014;11(6):675–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Strong AL, Bennett DK, Spreen EB, Adhvaryu DV, Littleton JC, Mencer EJ. Fetal Bovine Collagen Matrix in the Treatment of a Full Thickness Burn Wound: A Case Report With Long-Term Follow-Up. J Burn Care Res. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Burke JF, Bondoc CC, Quinby WC. Primary burn excision and immediate grafting: a method shortening illness. J Trauma. 1974;14(5):389–395. [DOI] [PubMed] [Google Scholar]
- 71.Hop MJ, Hoogewerf CJ, van Baar ME, van der Vlies CH, Middelkoop E. A call for evidence: timing of surgery in burns. Burns. 2012;38(4):617–618. [DOI] [PubMed] [Google Scholar]
- 72.Toussaint J, Chung WT, Mc Clain S, Raut V, Singer AJ. Optimal Timing for Early Excision in a Deep Partial Thickness Porcine Burn Model. J Burn Care Res. 2017;38(1):e352–e358. [DOI] [PubMed] [Google Scholar]
- 73.Tenenhaus M, Rennekampff HO. Surgical advances in burn and reconstructive plastic surgery: new and emerging technologies. Clin Plast Surg. 2012;39(4):435–443. [DOI] [PubMed] [Google Scholar]
- 74.Chua AWC, Khoo YC, Truong TTH, Woo E, Tan BK, Chong SJ. From skin allograft coverage to allograft-micrograft sandwich method: A retrospective review of severe burn patients who received conjunctive application of cultured epithelial autografts. Burns. 2018;44(5):1302–1307. [DOI] [PubMed] [Google Scholar]
- 75.Sood R, Roggy D, Zieger M, et al. Cultured epithelial autografts for coverage of large burn wounds in eighty-eight patients: the Indiana University experience. J Burn Care Res. 2010;31(4):559–568. [DOI] [PubMed] [Google Scholar]
- 76.Wood FM, Giles N, Stevenson A, Rea S, Fear M. Characterisation of the cell suspension harvested from the dermal epidermal junction using a ReCell(R) kit. Burns. 2012;38(1):44–51. [DOI] [PubMed] [Google Scholar]
- 77.Gravante G, Di Fede MC, Araco A, et al. A randomized trial comparing ReCell system of epidermal cells delivery versus classic skin grafts for the treatment of deep partial thickness burns. Burns. 2007;33(8):966–972. [DOI] [PubMed] [Google Scholar]
- 78.Holmes Iv JH, Molnar JA, Carter JE, et al. A Comparative Study of the ReCell® Device and Autologous Spit-Thickness Meshed Skin Graft in the Treatment of Acute Burn Injuries. J Burn Care Res. 2018;39(5):694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pharmaceuticals M. U.S. FDA Designates Mallinckrodt’s StrataGraft® as Regenerative Medicine Advanced Therapy. Cisiion prnewswire. https://www.prnewswire.com/news-releases/us-fda-designates-mallinckrodts-stratagraft-as-regenerative-medicine-advanced-therapy-300489200.html. Published 2017. Accessed 2018.
- 80.Allen-Hoffmann BL, Schlosser SJ, Ivarie CA, Sattler CA, Meisner LF, O’Connor SL. Normal growth and differentiation in a spontaneously immortalized near-diploid human keratinocyte cell line, NIKS. J Invest Dermatol. 2000;114(3):444–455. [DOI] [PubMed] [Google Scholar]
- 81.Centanni JM, Straseski JA, Wicks A, et al. StrataGraft skin substitute is well-tolerated and is not acutely immunogenic in patients with traumatic wounds: results from a prospective, randomized, controlled dose escalation trial. Ann Surg. 2011;253(4):672–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schurr MJ, Foster KN, Centanni JM, et al. Phase I/II clinical evaluation of StrataGraft: a consistent, pathogen-free human skin substitute. J Trauma. 2009;66(3):866–873; discussion 873–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stratatech. StrataGraft® Skin Tissue in the Promotion of Autologous Skin Regeneration of Complex Skin Defects Due to Thermal Burns That Contain Intact Dermal Elements. https://clinicaltrials.gov/ct2/show/NCT03005106?term=stratagraft&rank=1. Accessed 11/07/2018, 2018.
- 84.Cheng JZ, Farrokhi A, Ghahary A, Jalili RB. Therapeutic Use of Stem Cells in Treatment of Burn Injuries. J Burn Care Res. 2018;39(2):175–182. [DOI] [PubMed] [Google Scholar]
- 85.Jeremias TaS, Machado RG, Visoni SB, Pereima MJ, Leonardi DF, Trentin AG. Dermal substitutes support the growth of human skin-derived mesenchymal stromal cells: potential tool for skin regeneration. PLoS One. 2014;9(2):e89542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.van der Veen VC, Vlig M, van Milligen FJ, de Vries SI, Middelkoop E, Ulrich MM. Stem cells in burn eschar. Cell Transplant. 2012;21(5):933–942. [DOI] [PubMed] [Google Scholar]
- 87.Ozturk S, Karagoz H. Experimental stem cell therapies on burn wound: do source, dose, timing and method matter? Burns. 2015;41(6):1133–1139. [DOI] [PubMed] [Google Scholar]
- 88.Rasulov MF, Vasilchenkov AV, Onishchenko NA, et al. First experience of the use bone marrow mesenchymal stem cells for the treatment of a patient with deep skin burns. Bull Exp Biol Med. 2005;139(1):141–144. [DOI] [PubMed] [Google Scholar]
- 89.Burd A, Ahmed K, Lam S, Ayyappan T, Huang L. Stem cell strategies in burns care. Burns. 2007;33(3):282–291. [DOI] [PubMed] [Google Scholar]
- 90.Polyak K, Weinberg RA. Transitions between epithelial and mesenchymal states: acquisition of malignant and stem cell traits. Nat Rev Cancer. 2009;9(4):265–273. [DOI] [PubMed] [Google Scholar]
- 91.Patra S, Young V. A Review of 3D Printing Techniques and the Future in Biofabrication of Bioprinted Tissue. Cell Biochem Biophys. 2016;74(2):93–98. [DOI] [PubMed] [Google Scholar]
- 92.He P, Zhao J, Zhang J, et al. Bioprinting of skin constructs for wound healing. Burns Trauma. 2018;6:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Binder KW ZW, Aboushwareb T, Dice D, Atala A, Yoo JJ. In situ bioprinting of the skin for burns. Journal of the Americal College of Surgeons. 2010;211(3):S76. [Google Scholar]
- 94.Tasoglu S, Demirci U. Bioprinting for stem cell research. Trends Biotechnol. 2013;31(1):10–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rose LF, Chan RK. The Burn Wound Microenvironment. Adv Wound Care (New Rochelle). 2016;5(3):106–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Goei H, van der Vlies CH, Hop MJ, et al. Long-term scar quality in burns with three distinct healing potentials: A multicenter prospective cohort study. Wound Repair Regen. 2016;24(4):721–730. [DOI] [PubMed] [Google Scholar]
- 97.Hop MJ, Stekelenburg CM, Hiddingh J, et al. Cost-Effectiveness of Laser Doppler Imaging in Burn Care in The Netherlands: A Randomized Controlled Trial. Plast Reconstr Surg. 2016;137(1):166e–176e. [DOI] [PubMed] [Google Scholar]
- 98.Anderson RR, Donelan MB, Hivnor C, et al. Laser treatment of traumatic scars with an emphasis on ablative fractional laser resurfacing: consensus report. JAMA Dermatol. 2014;150(2):187–193. [DOI] [PubMed] [Google Scholar]
- 99.Xu X, Lai L, Zhang X, et al. Autologous chyle fat grafting for the treatment of hypertrophic scars and scar-related conditions. Stem Cell Res Ther. 2018;9(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tredget EE, Levi B, Donelan MB. Biology and principles of scar management and burn reconstruction. Surg Clin North Am. 2014;94(4):793–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ding J, Ma Z, Liu H, et al. The therapeutic potential of a C-X-C chemokine receptor type 4 (CXCR-4) antagonist on hypertrophic scarring in vivo. Wound Repair Regen. 2014;22(5):622–630. [DOI] [PubMed] [Google Scholar]


