Abstract
Since diagnostic sampling material must be considered as infectious, we evaluated whether extraction buffers of SARS-CoV-2 rapid antigen test kits may inactivate SARS-CoV-2. Of concern, seven of nine tested buffers lacked potent virucidal activity. To reduce risk of infection during assay performance, virucidal antigen extraction buffers that efficiently inactivate virus should replace the extraction buffers in these commercially available point-of-care devices.
Keywords: SARS-CoV-2 rapid antigen test, Point-of-care devices, Antigen extraction buffer, Assay safety, Virucidal buffer
1. Introduction
In late 2019, SARS-CoV-2 emerged as a human pathogen resulting in a pandemic with several million deaths worldwide [1]. Countermeasures taken include vaccination, social distancing, wearing masks, and the identification and isolation of SARS-CoV-2 infected individuals. The gold standard for SARS-CoV-2 detection are nucleic acid amplification tests which are highly sensitive but have practical limitations [2]. SARS-CoV-2 rapid antigen tests allow convenient mass testing in a cheaper and more flexible way, can be performed directly on site and deliver fast results [3]. Therefore, the use of these point-of-care devices became an indispensable strategy to contain the pandemic.
Several SARS-CoV-2 antigen tests have been approved by authorities and are commercially available [3]. Rapid antigen tests require sampling of saliva or nasopharyngeal/nasal swabs followed by extraction of the viral antigen in an antigen extraction buffer and subsequent analysis by lateral flow immunoassay. To allow subsequent assay performance, antigen extraction buffers are milder than RNA extraction buffers which are generally virus-inactivating [4]. Since the sampling material, i.e. saliva and nasopharyngeal swabs, must be considered as infectious, the WHO recommends the handling of sample material and its testing in a biological safety cabinet [5]. However, the tests are also performed at places without adequate safeguards, e.g. in the private (household) and public (school, work place, restaurants) [3]. We here evaluated whether extraction buffers of commercially available SARS-CoV-2 antigen tests inactivate SARS-CoV-2, which would minimize the risk of infection during and after assay performance.
2. Material and methods
A detailed description of materials and methods is available in the supplementary material.
3. Results
Hence, we here evaluated whether antigen extraction buffers of nine commercially available SARS-CoV-2 antigen test kits (buffers B1-B9, Table S1) inactivate SARS-CoV-2, which would minimize the risk of infection during and after assay performance. As control, a newly developed antigen extraction buffer with known virucidal activity (buffer B10, Table S1) was included. To be within the range of SARS-CoV-2 concentrations detected in patient swabs [6, 7] we used viable SARS-CoV-2 adjusted to 3.2 × 1010 RNA copies/ml. In order to mimic the physiological environment in the upper respiratory tract, SARS-CoV-2 D614G variant was mixed with a “respiratory secretion-mimicking interfering substance”, which contains albumin, mucin and yeast, following a quantitative suspension test protocol [8 – 10]. This solution was then mixed with buffers B1-B10 and incubated for 1 or 15 min, a period of time which corresponds to the most common extraction and running times of rapid antigen tests.
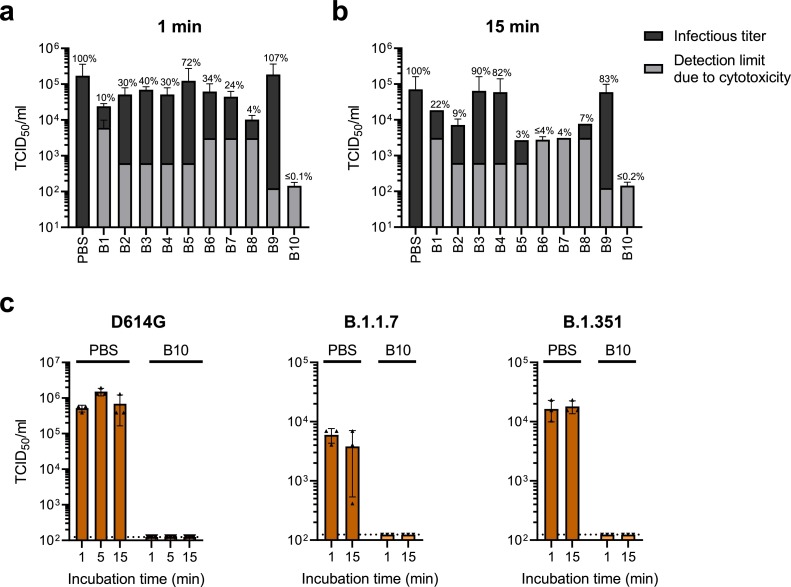
Remaining viral infectivity was then determined by 50% tissue culture infectious dose (TCID50) analysis. As shown in Fig. 1 a and 1b, most buffers did not fully inactivate SARS-CoV-2 infectivity. The PBS control demonstrated high infectivity with 1.7 × 105 TCID50/ml after 1 min incubation (Fig. 1a), and 7.1 × 104 TCID50/ml after 15 min incubation (Fig. 1b). After 1 min incubation, virucidal B10 diminished infectivity below the detection limit, which corresponds to a 99.92% reduction (Fig. 1a). After 15 min incubation, B6, B7 and B10 inactivated SARS-CoV-2 below the detection limit, yielding a 96.10% (B6), 95.62% (B7) or 99.80% (B10) reduction in infectivity, respectively. Residual infection was still detectable upon incubation with the other buffers, with B3, B4 and B9 showing almost no virucidal activity (Fig. 1b).
Fig. 1.
Virucidal activity of extraction buffers in rapid SARS-CoV-2 antigen tests. a, b) A solution of 10% SARS-CoV-2 D614G, 10% interfering substance (5% (w/v) albumin fraction V, 0.4% (w/v) mucin from bovine submaxillary glands type I-S, 5% (w/v) yeast extract) and 80% of the respective buffer B1-B10 or phosphate buffer saline (PBS) was analyzed according to quantitative suspension tests as described in DIN EN 14,476 [8,9]. After incubation for (a) 1 min or (b) 15 min, viral infectivity was quantified by TCID50 endpoint titration on Vero E6 cells. Detection limits caused by cytotoxicity of buffers are shown in gray, infectious titers in black. c) 10% virus (D614G variant or VOCs B.1.1.7 or B.1.351) was spiked into 90% pooled saliva and mixed 1:4 with B10 or PBS. After the indicated incubation times, an endpoint titration on Vero E6 cells was performed to determine TCID50/ml. Dotted lines indicate limit of detection due to cytotoxicity. a–c) Shown are means of three independent experiments ± SD.
Buffer B10 displayed highest virucidal activity but lowest cytotoxic effects (Fig. 1a, b). Thus, we further evaluated its virucidal activity against circulating SARS-CoV-2 variants of concern (VOCs). As saliva rather than nasopharyngeal swabs are proposed as novel gold standard sample for SARS-CoV-2 diagnostic [11, 12], we additionally analyzed the effect of B10 on VOCs B.1.1.7 (Alpha) or B.1.351 (Beta) in the presence of saliva. As shown in Fig. 1c, B10 inactivated the three SARS-CoV-2 strains already after one minute of incubation, with TCID50/ml values below the detection limit, which corresponds to 99.98% (D614G), 97.93% (B.1.1.7) and 99.25% (B.1.351) decrease in infectivity, respectively.
Moreover, B10 not only effectively inactivated SARS-CoV-2, but also other enveloped viruses including measles, influenza A, Zika, and herpes simplex viruses 1 and 2, whereas non-enveloped adenovirus was not affected (Figure S1). After proving the virucidal activity of B10, we evaluated its performance in actual rapid antigen tests. We replaced the provided kit buffer with B10 and performed the test with a positive and negative sample yielding the expected results (Figure S2).
4. Discussion
Collectively, our findings imply that SARS-CoV-2 is often not properly inactivated during currently available rapid antigen tests. The apparent low virucidal activity of many antigen extraction buffers is alarming because virus-contaminated sampling material and test components such as tubes and lateral flow cassettes as well as working surfaces may pose a risk of infection for those that are involved in the assay workflow. Interestingly, the British Department of Health and Social Care reported similar findings showing that most tested antigen extraction buffers do not exhibit potent virucidal activity against SARS-CoV-2 [13].
It is thus imperative that current rapid antigen tests lacking a virucidal extraction buffer are subjected to a suitable and sufficient risk assessment, and that appropriate safety precautions are taken, in particular when larger numbers of tests are carried out at the same time, e.g. during testing in schools or companies.
Since manufacturers and/or providers of rapid antigen tests do not disclose the composition of their extraction buffers, we cannot provide explanations for why some buffers inactivate virus and others do not. However, it is noteworthy that antigen extraction buffers, which effectively inactivate SARS-CoV-2 and other enveloped viral pathogens are available. Moreover, B10 allowed unchanged test performance upon replacement of the extraction buffer showing that the virus-inactivating effect of B10 does not interfere with the antibody-antigen interaction in the test cassette. Thus, ready-to-use buffers that inactivate virus and allow downstream assays, should quickly replace the non-virucidal antigen extraction buffers in available or new SARS-CoV-2 rapid antigen tests to reduce risk of infection and allow safe handling of these point-of-care devices.
Funding
This work was supported by the Federal Ministry for Economic Affairs and Energy on the basis of a decision by the German Bundestag (to J.M.). Funding agencies did not have any role in writing or decision making. C.C., T.W., L.O., L.R., and R.G. are part of the International Graduate School in Molecular Medicine Ulm.
Author contributions
C.C. and T.W. generated SARS-CoV-2 stocks, performed BSL-3 experiments and coordinated the study; L-R.O. performed HSV and ZIKV experiments; A.G. performed AdV and MeV experiments; L.R. performed IAV experiments; D.P.J.A. performed experiment with test cassettes; C.C. supported HSV and ZIKV experiments, arranged figures and drafted the manuscript; J.A.M. and R.G. advised experimental design and helped writing the manuscript; J.M. is responsible for the study, supervised all work and wrote the manuscript; all authors critically reviewed the manuscript.
Supplementary material
The supplementary table and figures are available in the supplementary material.
Declaration of Competing Interests
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Authors C.C., T.W. and J.M. declare conflicts of interest. C.C. has an employment contract with DRG Instruments, the manufacturer of B5. T.W. has an employment contract with CANDOR Bioscience, the manufacturer of B10. J.M. and CANDOR Bioscience are part of a research consortium that develops advanced SARS-CoV-2 diagnostics.
Acknowledgements
This work was supported by the Federal Ministry for Economic Affairs and Energy on the basis of a decision by the German Bundestag (to J.M.). Funding agencies did not have any role in writing or decision making. C.C., T.W., L.O., L.R., and R.G. are part of the International Graduate School in Molecular Medicine Ulm.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jcv.2021.105062.
Appendix. Supplementary materials
References
- 1.John Hopkins University & Medicine . 2021. COVID-19 Dashboard By the Center for Systems Science and Engineering (CSEE) At Johns Hopkins University (JHU) https://coronavirus.jhu.edu/map.html (accessed May 20, 2021) [Google Scholar]
- 2.Kevadiya B.D., Machhi J., Herskovitz J., Oleynikov M.D., Blomberg W.R., Bajwa N., Soni D., Das S., Hasan M., Patel M., Senan A.M., Gorantla S., McMillan J., Edagwa B., Eisenberg R., Gurumurthy C.B., Reid S.P.M., Punyadeera C., Chang L., Gendelman H.E. Diagnostics for SARS-CoV-2 infections. Nat. Mater. 2021;20:593–605. doi: 10.1038/s41563-020-00906-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Toptan T., Eckermann L., Pfeiffer A.E., Hoehl S., Ciesek S., Drosten C., Corman V.M. Evaluation of a SARS-CoV-2 rapid antigen test: potential to help reduce community spread? J. Clin. Virol. 2021;135 doi: 10.1016/j.jcv.2020.104713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Welch S.R., Davies K.A., Buczkowski H., Hettiarachchi N., Green N., Arnold U., Jones M., Hannah M.J., Evans R., Burton C., Burton J.E., Guiver M., Cane P.A., Woodford N., Bruce C.B., Roberts A.D.G., Killip M.J. Analysis of inactivation of SARS-CoV-2 by specimen transport media, nucleic acid extraction reagents, detergents, and fixatives. J. Clin. Microbiol. 2020;58:1–13. doi: 10.1128/JCM.01713-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization (2020), Laboratory biosafety guidance related to coronavirus disease (COVID-19), (2020).
- 6.Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-.L., Peiris M., Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.T.C. Jones, G. Biele, B. Mühlemann, T. Veith, J. Schneider, J. Beheim-Schwarzbach, T. Bleicker, J. Tesch, M.L. Schmidt, L.E. Sander, F. Kurth, P. Menzel, R. Schwarzer, M. Zuchowski, J. Hofmann, A. Krumbholz, A. Stein, A. Edelmann, V.M. Corman, C. Drosten, Estimating infectiousness throughout SARS-CoV-2 infection course, Science (80-.). 373 (2021) eabi5273. https://doi.org/10.1126/science.abi5273. [DOI] [PMC free article] [PubMed]
- 8.Eggers M., Terletskaia-Ladwig E., Enders M. How effective is hand washing against influenza virus? Hyg. + Medizin. 2009;34:492–498. [Google Scholar]
- 9.Chemical disinfectants and antiseptics, Quantitative suspension test for the evaluation of virucidal activity in the medical area. Test method and requirements (Phase 2/Step 1). German version. EN 14476:2019-10., n.d. https://doi.org/10.31030/2874698 (2019).
- 10.Meister T.L., Brüggemann Y., Todt D., Conzelmann C., Müller J.A., Groß R., Münch J., Krawczyk A., Steinmann J., Steinmann J., Pfaender S., Steinmann E. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome Coronavirus 2. J. Infect. Dis. 2020;222:1289–1292. doi: 10.1093/infdis/jiaa471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan S.H., Allicock O., Armstrong-Hough M., Wyllie A.L. Saliva as a gold-standard sample for SARS-CoV-2 detection. Lancet. Respir. Med. 2021;2600:19–21. doi: 10.1016/S2213-2600(21)00178-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Teo A.K.J., Choudhury Y., Tan I.B., Cher C.Y., Chew S.H., Wan Z.Y., Cheng L.T.E., Oon L.L.E., Tan M.H., Chan K.S., Hsu L.Y. Saliva is more sensitive than nasopharyngeal or nasal swabs for diagnosis of asymptomatic and mild COVID-19 infection. Sci. Rep. 2021;11:3134. doi: 10.1038/s41598-021-82787-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Public Health England, SARS-CoV-2 inactivation testing: interim report, (2021). https://www.gov.uk/government/publications/covid-19-phe-laboratory-assessments-of-inactivation-methods (accessed May 21, 2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.