Abstract
Main Problem
Frailty is an established risk factor for cognitive decline and Alzheimer's disease. Few studies have examined the longitudinal relationship between frailty and cognition.
Methods
Participants of Rush Memory and Aging project (n = 625, 67.5% female, 83.2 ± 5.9 years at baseline) underwent annual clinical evaluations (average follow‐up 5.6 ± 3.7 years) followed by neuropathologic assessment after death. A frailty index was calculated from 41 health variables at each evaluation. Clinical diagnosis of MCI and/or dementia was ascertained by clinical data review (blinded to neuropathological data) after death. Age, sex, education, and neuropathological burden (10‐item index) were evaluated as covariates. Frailty trajectories were calculated using a mixed effects model.
Results
At baseline the mean frailty index = 0.24 ± 0.12 and increased at rate of 0.026 or ~1 deficit per year. At death, 27.7% of the sample had MCI, and 38.6% had dementia. Frailty trajectories were significantly steeper among those individuals who were ultimately diagnosed as clinically impaired prior to death, even after controlling for age, sex, education, and neuropathological index.
Conclusions
Findings suggest a strong link between health status (frailty index) and dementia, even after considering neuropathology. Frailty trajectories were associated with risk for MCI and dementia, underscoring the importance of addressing frailty to manage dementia risk.
Keywords: aging, Alzheimer's disease, dementia, frailty index
In this study of how changes in the degree of frailty affected the probability of a diagnosis of Alzheimer’s dementia, we highlight two key findings: (1) frailty increased at a rate of approximately one deficit per year in a sample of older adults from retirement communities in the USA; and (2) people who ultimately developed MCI or Alzheimer’s dementia became frailer more quickly than those who did not, regardless of their neuropathological burden. These results underscore the importance of addressing frailty to manage dementia risk.

1. INTRODUCTION
As our population ages, the burden of age‐related disease, including dementia, is growing. Many risk factors for dementia have been identified both in midlife and later life, 1 but it remains unclear how they interact to produce dementia in late life.
Frailty is a measure of physiologic vulnerability and can be characterized by the accumulation of health deficits over time. 2 Frailty is an established risk factor for cognitive decline and dementia. 3 , 4 , 5 Longitudinal changes in frailty have been associated with adverse health outcomes including mortality, 6 health service use, 7 disease‐specific morbidity, 8 institutionalization, and disability. 9 Few studies have examined the longitudinal relationship between frailty and cognition. 10 , 11 Those that have demonstrate that changes in frailty and cognition are correlated, 12 and a shared pathologic basis is suspected. 10 , 11 In a previous report, we have shown that frailty influences the relationship between neuropathology and clinical presentation of dementia in Alzheimer's disease. 13
Here, we use data from the Rush Memory and Aging Project, a longitudinal clinical‐pathologic cohort study to extend this work by: (1) describing longitudinal change in frailty and (2) examining how frailty trajectories are associated with sex, neuropathology, and clinical diagnosis of mild cognitive impairment (MCI) and dementia.
2. METHODS
2.1. Study design & participants
Data presented here were from the Rush Memory and Aging Project (MAP), which has been described in depth elsewhere. 14 Briefly, MAP is a clinical‐pathologic cohort study that, since its inception in 1997, has enrolled over 2100 older adults with annual clinical evaluations. This study recruited from residential facilities, senior and subsidized housing, church groups, and social service agencies in Northeastern Illinois. Participants were eligible for enrollment if they were able and willing to sign an informed consent and an Anatomical Gift Act and agreed to donate their brain, spinal cord, and other biospecimens at death. Participants also signed a repository consent that allowed their data to be repurposed for other studies. MAP was approved by an Institutional Review Board of Rush University Medical Center, Chicago, IL, USA. Data access can be requested at www.radc.rush.edu. Complete case analysis was employed and participants were included in the current analyses if they had autopsy data (so that we could adjust for neuropathology) and a valid frailty index (n = 625); there was minimal loss to follow‐up, though many participants have not yet died and therefore do not have autopsy data.
2.2. Measures
2.2.1. Frailty index
The frailty index (FI) is a measure of health status that reflects the extent of age‐related deficit accumulation and vulnerability to adverse health outcomes. 2 A FI was constructed from 41 items (Appendix 1) according to standard criteria. 15 Candidate variables included symptoms, signs, comorbidities, and function; variables strongly related to the outcome (i.e. cognitive variables) were excluded. The FI was calculated as:
For example, a person with 5 of the 41 potential deficits measured has an FI score of 5/41 = 0.12. Higher FI scores indicate poorer health and theoretically, the FI ranges from 0–1, with linear regression models using units of the FI of 0.01. The frailty index was calculated based on clinical data for each participant at each annual evaluation (mean follow‐up = 5.6 ± 3.7 years, range 0–17 years) and trajectories were plotted over time.
2.2.2. Clinical diagnoses
At the time of death, an experienced neurologist reviewed select clinical data and rendered a summary diagnosis; this was done blinded to all postmortem data. This process is based on the criteria of the joint working group of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association (NINCDS/ADRDA). 16 Participants were classified as having: no cognitive impairment (NCI; coded as 0), mild cognitive impairment (MCI; coded as 1), or dementia (including possible and probable Alzheimer's). For our purposes, possible or probable dementia were coded as 2 and other dementias were excluded (n = 9) as they were undetermined or diverse in origin.
2.2.3. Neuropathological index
Burden of neuropathology was quantified at autopsy using an index composed of 10 unique neuropathological features (amyloid load, neurofibrillary tangle density, TDP‐43, hippocampal sclerosis, cerebral amyloid angiopathy, gross infarcts, gross chronic infarcts, atherosclerosis, arteriolosclerosis, and presence of Lewy bodies). This index and the details of neuropathological assessment have been detailed elsewhere. 17
2.2.4. Confounders
Age, sex, education were evaluated as confounders; all were treated as time‐invariant covariates. Age was measured in years and calculated from birth to date of last cognitive assessment before death. Education was self‐reported in years. Sex was a self‐report binary variable, with female as the referent. Time in study (years) was also included as a covariate.
2.3. Statistical analyses
Descriptive statistics (t‐tests, chi‐square, and analyses of variance [ANOVA]) were used to describe the characteristics of the sample. Frailty was plotted against time (unadjusted). ANOVAs were used to evaluate unadjusted differences in baseline frailty by sex, neuropathological index groups, and clinical diagnosis.
Multilevel (also known as mixed effects) models were used to model linear within‐person change in frailty over time, as well as between‐person differences in frailty. Models were built from an empty means, fixed intercept, base model, and terms were added sequentially. Terms were conserved if they demonstrated a significant change in deviance from the previous model.
First, we modeled frailty change over time (FI ~ random intercept + time). A sensitivity analysis to determine whether frailty change over time was quadratic rather than linear was undertaken by adding a quadratic term for time. Then, we evaluated whether frailty trajectory differed as a function of key covariates including sex, neuropathological index (tertiles), and clinical diagnosis, by modeling the interaction between each covariate and time in the prediction of frailty (in separate models). Finally, we tested whether frailty trajectory differed over levels of clinical diagnosis after controlling for all other covariates, including the neuropathological index. This final mixed linear model was as follows:
Sensitivity analyses were undertaken to examine whether this was also true in a subset of participants with Alzheimer's dementia (no cognitive impairment vs. Alzheimer's dementia).
All analyses were completed using R version 3.5.2. Figures were truncated at 11 years, as less than 10% of the sample remained and estimates became unstable.
3. RESULTS
Most (67.5%) of the 625 autopsied participants were female, and the mean age at baseline was 83.2 ± 5.9 years. Participants were followed for 5.6 ± 3.7 years from baseline (range 0–17 years), by which time 33.8% remained cognitively normal, 27.7% had MCI, and 38.6% had dementia (Table 1). Higher baseline frailty was associated with being female (p < 0.0001), but not with neuropathological burden (p = 0.26) or cognitive status at death (p = 0.10).
TABLE 1.
Sample demographics (n=625)
| Age (years at baseline; mean ± SD) a | 83.1 ± 5.9 |
| Age (years at death; mean ± SD) a | 89.7 ± 6.1 |
| Sex (n, % female) | 422, 67.5 |
| Education (years at baseline, mean ± SD) a | 14.5 ± 2.9 |
| Cognitive status at time of death (n, %) | |
| Cognitively normal | 211, 33.8 |
| Mild cognitive impairment | 173, 27.7 |
| Dementia | 241, 38.6 |
| MMSE at baseline (mean ± SD, median) | 26.7 ± 4.0, 28.0 |
| MMSE at last evaluation before death (mean ± SD) a | 21.5 ± 8.7 |
| Neuropathological index at time of death (mean ± SD) a | 0.36 ± 0.17 |
| Frailty Index at baseline (mean ± SD, median) | 0.24 ± 0.12, 0.22 |
| Frailty Index at time of death (mean ± SD) a | 0.41 ± 0.18 |
| Time in study (years baseline to last evaluation before death; mean ± SD, median) | 5.6 ± 3.7, 5.0 |
Abbreviation: SD, Standard deviation.
Normally distributed.
Frailty changed significantly over time (estimate = 0.026 per 0.01 FI unit, 95% confidence interval [CI] 0.025–0.027, p < 0.0001); this means the FI increased at a rate of about one deficit per year on average (0.026*41 deficits in FI), though there was much heterogeneity in the individual trajectories (Figure 1).
FIGURE 1.
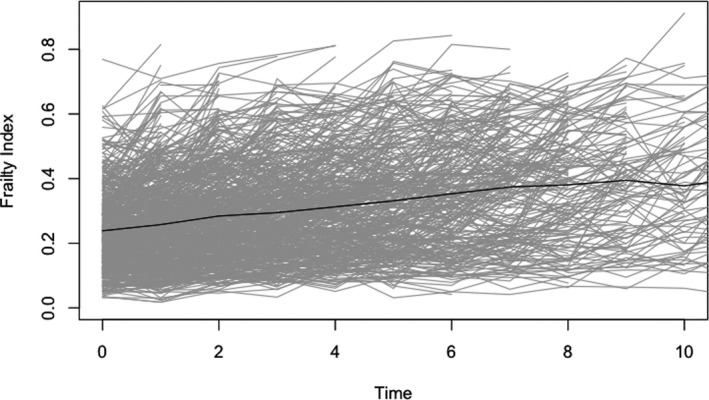
Longitudinal change in frailty as measured by the frailty index; grey lines indicate individual trajectories, black line indicates average trajectory
Prior to adjusting for covariates, frailty trajectory differed as a function of neuropathological index (time*neuropathological index interaction estimate = 0.020, 95% CI 0.013–0.026, p < 0.0001) and clinical diagnosis (time*MCI interaction estimate = 0.005, 95% CI 0.003–0.008, p < 0.0001; time*dementia interaction estimate = 0.021; 95% CI 0.018–0.023, p < 0.0001), see Figure 2. Specifically, higher neuropathological burden and worse clinical diagnosis were associated with accumulating deficits at a significantly faster rate (i.e. increasing frailty index score). Frailty trajectory did not differ by sex (sex*time interaction estimate = −0.0002, 95% CI −0.003–0.002, p = 0.84).
FIGURE 2.
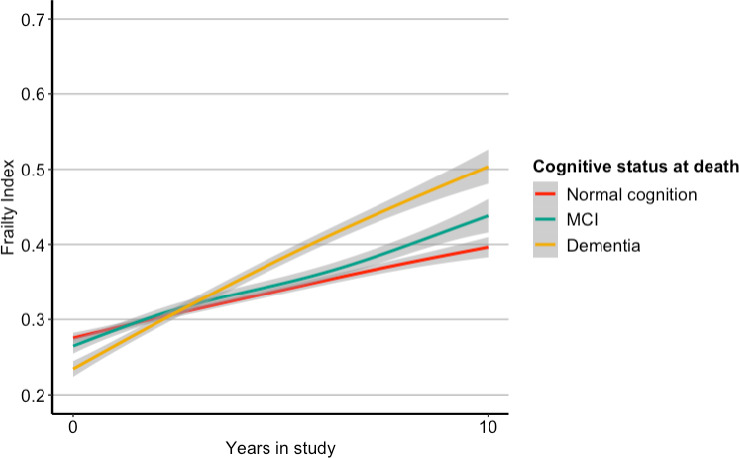
Frailty over time (years) stratified by cognitive status (adjusted)
Frailty trajectories remained significantly different over levels of clinical diagnosis after controlling for relevant covariates (age, sex, education, time in study, and neuropathological index), indicating that frailty increased over time at a faster rate in those with worsening clinical diagnosis; Table 2. Specifically, people with no cognitive impairment at death show an average increase of 0.019 FI units/year (corresponding to 0.78 additional deficits/year), people with mild cognitive impairment at death show an average increase of 0.024 FI units/year (corresponding to 0.98 additional deficits/year), and people with dementia at death show an average increase of 0.039 FI units/year (corresponding to 1.60 additional deficits/year). Age at death and the neuropathological index were found not to contribute significantly to the model; age at death was dropped from the final model, but the neuropathological index was conserved for conceptual reasons.
TABLE 2.
Mixed effects model for outcome of frailty
| Covariates | Estimate | 95% Confidence interval – lower limit | 95% Confidence interval – upper limit | p value |
|---|---|---|---|---|
| Time (years since baseline) | 0.019 | 0.017 | 0.020 | <0.0001 |
| Sex (female) | −0.057 | −0.078 | −0.036 | 0.001 |
| Education (years) | −0.002 | −0.005 | 0.002 | 0.310 |
| Time in Study | −0.018 | −0.021 | −0.015 | <0.001 |
| Clinical diagnosis | ||||
| MCI a vs. NCI b | 0.018 | −0.008 | 0.043 | 0.170 |
| Dementia vs. NCI | 0.032 | −0.006 | 0.058 | 0.015 |
| Neuropathological Index (per 0.01) | 0.022 | −0.042 | 0.086 | 0.510 |
| Time*Clinical diagnosis | ||||
| Time*MCI | 0.005 | 0.003 | 0.008 | <0.0001 |
| Time*Dementia | 0.020 | 0.018 | 0.023 | <0.0001 |
In this study of how changes in the degree of frailty affected the probability of a diagnosis of Alzheimer's dementia, we highlight two key findings: (1) frailty increased at a rate of approximately one deficit per year in a sample of older adults from retirement communities in the USA; and (2) people who ultimately developed MCI or Alzheimer's dementia became frailer more quickly than those who did not, regardless of their neuropathological burden. These results underscore the importance of addressing frailty to manage dementia risk.
Mild cognitive impairment.
No cognitive impairment.
A sensitivity analysis examined whether a quadratic model improved fit, but it did not lead to a significant change in deviance from the linear model, and the quadratic term for time was not significant. We also tested the relationships with a binary outcome variable of no cognitive impairment vs. Alzheimer's dementia and results did not change significantly.
4. DISCUSSION
In this study of how changes in the degree of frailty affected the probability of a diagnosis of Alzheimer's dementia, we highlight two key findings: (1) frailty increased at a rate of approximately one deficit per year in a sample of older adults from retirement communities in the USA; and (2) people who ultimately developed MCI or Alzheimer's dementia became frailer more quickly than those who did not, regardless of their neuropathological burden.
Our results are consistent with other longitudinal reports linking frailty and cognition. 10 , 11 , 18 The only other study that to our knowledge has examined change in frailty with cognition and neuropathology found that baseline frailty predicted both future frailty and cognitive decline. 10 That study was from the same cohort but used a different measure of frailty. Here, we find that baseline frailty did not differ by clinical diagnosis, but frailty worsened more quickly in those who eventually developed MCI than in those with no cognitive impairment and quicker still in those who developed dementia. That study 10 also demonstrated a correlation between change in cognition and physical frailty, not just on average, but within individuals. Moreover, the association remained after controlling for five common brain pathologies (macroinfarcts, microinfarcts, Lewy bodies, AD pathology, and nigral neuronal loss). Pathologies were examined individually, and AD pathology, macroinfarcts, and nigral neuronal loss demonstrated additive risk for both frailty and cognitive decline. This is consistent with our results showing that level of pathology was associated with increased slope of frailty change.
An important difference between this study and ours is the measurement of frailty: the prior report operationalized frailty using a modified frailty phenotype—a composite measure of four impairments including grip strength, timed walk, body composition, and fatigue. By contrast, we operationalized frailty as deficit accumulation using the frailty index—an index of 41 health variables that reflect overall health state. We chose to use the frailty index to overcome some challenges of employing the frailty phenotype in observational data: the grading is crude, there are frequently floor effects in healthy samples and ceiling effects or much missing data due to performance‐based measures in impaired aging samples, and the modifications can limit generalizability. 19
Interestingly, our analysis demonstrated that longitudinal changes in frailty were not significantly associated with neuropathology after controlling for possible confounders. Other reports have linked common dementia‐related pathologies with frailty 20 , 21 and hypothesized that these common pathologies are a shared mechanism between cognitive decline and frailty, 10 though our data do not support this conclusion. It is possible that frailty influences the expression of dementia by reducing the threshold of pathology necessary to give rise to cognitive impairment. 13 Whether this reflects a single mechanism in all patients with cognitive impairment, differing single mechanisms in individual patients, or an accumulation of age‐related decrements—the combinations of which vary between patients—is not clear. Other reports using the Rush data that indicate multiple pathologies are common in late‐life dementia and that dementia itself may represent a form of pre‐terminal decline, would seem to make single uniting mechanisms less likely. In this regard, variability in responses to treatment may prove to be informative. 22
Taken together, results from this study as well as previous work suggest frailty is an important and modifiable risk factor for dementia. Work is ongoing to determine the best way to prevent and treat frailty, with a focus on multimodal interventions that address various factors including diet, exercise, sleep, and comorbidities. 23 While individual interventions may be useful, it is likely that population‐level public health approaches that target environmental drivers of health will be the most effective. 24
Our results should be interpreted with caution. Our sample was not population‐representative and due to the recruitment strategies may over‐represent frailty and dementia. Further, it would be ideal to be able to measure changes in neuropathological burden over time, but since pathological confirmation can only be completed after death, this limits our ability to make causal inferences. We excluded other dementias from analysis as there were few (n = 9) and of diverse origin. While some of the variables included in the frailty index may be theoretically related to cognition, previous work found that excluding all functional items as well as potential dementia confounders did not change the predictive value of the frailty index for dementia. 13 Future work with larger sample sizes should investigate whether these relationships hold across dementia types. Further, smaller vascular pathology should be considered in future investigations to explain additional variance.
Despite these limitations, this study has several strengths. The quality of the data and long follow‐up period makes this data unique as a clinical‐pathologic cohort. Mixed effects analyses allowed us to model person‐specific intercepts improving the validity of the model. Alzheimer's disease research has been criticized for the over‐emphasis on amyloid and tau pathology. Here, we were able to model mixed pathology, which is common in dementia. 25 , 26 , 27
Overall, results of this study suggest a strong link between frailty and Alzheimer's dementia, even after considering degree of neuropathology. Frailty trajectories over an average period of 6 years in older adults predicted dementia risk, underscoring the importance of frailty intervention and management in later life. Frailty and cognition are known to be highly related and may even share a pathologic basis, and the bidirectional mechanisms that explain how they influence each other motivate future inquiries.
CONFLICTS OF INTEREST
Melissa Andrew reports grants from GSK, Pfizer, and Sanofi unrelated to the current work. David Bennett reports grants from the National Institutes of Health (NIH). Kenneth Rockwood reports personal fees from Lundbeck for attending an advisory board meeting in 2017. Kenneth Rockwood is President and Chief Science Officer of DGI Clinical, which in the last 5 years has contracts with pharma and device manufacturers (Baxter, Baxalta, Shire, Hollister, Nutricia, Roche, Otsuka) on individualized outcome measurement. Otherwise, any personal fees are for invited guest lectures and academic symposia, received directly from event organizers, chiefly for presentations on frailty. He is Associate Director of the Canadian Consortium on Neurodegeneration in Aging, which is funded by the Canadian Institutes of Health Research, and with additional funding from the Alzheimer Society of Canada and several other charities, as well as, in its first phase (2013–2018), from Pfizer Canada and Sanofi Canada. He receives career support from the Dalhousie Medical Research Foundation as the Kathryn Allen Weldon Professor of Alzheimer Research, and research support from the Canadian Institutes of Health Research, the Nova Scotia Health Research Foundation, the Capital Health Research Fund, and the Fountain Family Innovation Fund of the Nova Scotia Health Authority Foundation.
AUTHOR CONTRIBUTIONS
LW and KR conceived the study. LW, OT, JG, DW contributed to the design of the analyses. LW wrote the first draft. All authors and revised all drafts and contributed to the interpretation of analyses.
ACKNOWLEDGEMENTS
Lindsay Wallace is supported by a doctoral fellowship from the Canadian Institutes of Health Research (CIHR). Melissa Andrew’s work on frailty and dementia is part of a Canadian Consortium on Neurodegeneration in Aging (CCNA) investigation into how multi‐morbidity modifies the risk of dementia and the patterns of disease expression (Team 14). The CCNA receives funding from the Canadian Institutes of Health Research (CNA‐137794) and partner organizations. Kenneth Rockwood’s work on frailty and cognition is supported by CIHR PJT‐156114 and by the Dalhousie Medical Research Foundation Kathryn Allen Weldon Chair of Alzheimer Disease Research. The MAP is supported by NIH grant R01AG17917.
APPENDIX 1.
Items Included in the Frailty Index (n = 41)
|
|
Wallace LMK, Theou O, Godin J, et al. 10‐year frailty trajectory is associated with Alzheimer’s dementia after considering neuropathological burden. Aging Med. 2021;4:250–256. doi: 10.1002/agm2.12187
REFERENCES
- 1. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673‐2734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 2. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. 2013;381(9868):752‐762. doi: 10.1016/S0140-6736(12)62167-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buchman AS, Boyle PA, Wilson RS, Tang Y, Bennett DA. Frailty is associated with incident alzheimer’s disease and cognitive decline in the elderly. Psychos Med. 2007;69(5):483‐489. doi: 10.1097/psy.0b013e318068de1d [DOI] [PubMed] [Google Scholar]
- 4. Robertson DA, Savva GM, Kenny RA. Frailty and cognitive impairment—A review of the evidence and causal mechanisms. Ageing Res Rev. 2013;12(4):840‐851. doi: 10.1016/j.arr.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 5. Searle SD, Rockwood K. Frailty and the risk of cognitive impairment. Alzheimers Res Ther. 2015;7(1):54. doi: 10.1186/s13195-015-0140-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stow D, Matthews FE, Hanratty B. Frailty trajectories to identify end of life: a longitudinal population‐based study. BMC Med. 2018;16(1):171. doi: 10.1186/s12916-018-1148-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rockwood K, Song X, Mitnitski A. Changes in relative fitness and frailty across the adult lifespan: evidence from the Canadian National Population Health Survey. Can Med Assoc J. 2011;183(8):E487‐E494. doi: 10.1503/cmaj.101271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wallace LMK, Theou O, Kirkland SA, et al. Accumulation of Non‐Traditional Risk Factors for Coronary Heart Disease Is Associated with Incident Coronary Heart Disease Hospitalization and Death. PLoS One. 2014;9(3): doi: 10.1371/journal.pone.0090475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liu Z, Han L, Gahbauer EA, Allore HG, Gill TM. Joint trajectories of cognition and frailty and associated burden of patient‐reported outcomes. J Am Med Dir Assoc. 2018;19(4):304‐309. doi: 10.1016/j.jamda.2017.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buchman AS, Yu L, Wilson RS, Boyle PA, Schneider JA, Bennett DA. Brain Pathology Contributes to Simultaneous Change in Physical Frailty and Cognition in Old Age. J Gerontol A Biol Sci Med Sci. 2014;69(12):1536‐1544. doi: 10.1093/gerona/glu117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Armstrong JJ, Godin J, Launer LJ, et al. Changes in Frailty Predict Changes in Cognition in Older Men: The Honolulu‐Asia Aging Study. J Alzheimers Dis JAD. 2016;53(3):1003‐1013. doi: 10.3233/JAD-151172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chu NM, Bandeen‐Roche K, Tian J, et al. Hierarchical development of frailty and cognitive impairment: clues into etiological pathways. J Gerontol A Biol Sci Med Sci. 2019;74(11):1761‐1770. doi: 10.1093/gerona/glz134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross‐sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush memory and aging project. Curr Alzheimer Res. 2012;9(6):646‐663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr. 2008;8(1):24. doi: 10.1186/1471-2318-8-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease Report of the NINCDS‐ADRDA Work Group* under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939. doi: 10.1212/WNL.34.7.939 [DOI] [PubMed] [Google Scholar]
- 17. Wallace L, Theou O, Darvesh S, et al. Neuropathological burden and the degree of frailty in relation to global cognition and dementia. Neurol Rev;95(24):e3269–e3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Armstrong JJ, Mitnitski A, Andrew MK, Launer LJ, White LR, Rockwood K. Cumulative impact of health deficits, social vulnerabilities, and protective factors on cognitive dynamics in late life: a multistate modeling approach. Alzheimers Res Ther. 2015;7(1):38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Theou O, Cann L, Blodgett J, Wallace LMK, Brothers TD, Rockwood K. Modifications to the frailty phenotype criteria: Systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing, and Retirement in Europe. Ageing Res Rev. 2015;21:78‐94. doi: 10.1016/j.arr.2015.04.001 [DOI] [PubMed] [Google Scholar]
- 20. Hogan DB, Maxwell CJ, Afilalo J, et al. A scoping review of frailty and acute care in middle‐aged and older individuals with recommendations for future research. Can Geriatr J. 2017;20(1):22–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Buchman AS, Yu L, Wilson RS, Schneider JA, Bennett DA. Association of brain pathology with the progression of frailty in older adults. Neurology. 2013;80(22):2055‐2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Senolytic Therapy to Modulate Progression of Alzheimer’s Disease ‐ Full Text View ‐ ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT04063124. Accessed January 30, 2020.
- 23. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385(9984):2255–2263. doi: 10.1016/S0140-6736(15)60461-5 [DOI] [PubMed] [Google Scholar]
- 24. Marteau TM, Rutter H, Marmot M. Changing behaviour: an essential component of tackling health inequalities. BMJ. 2021;372:n332. doi: 10.1136/bmj.n332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, Bennett DA. Person‐specific contribution of neuropathologies to cognitive loss in old age: Neuropathologies and Cognition. Ann Neurol. 2018;83(1):74‐83. doi: 10.1002/ana.25123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boyle PA, Yang J, Yu L, et al. Varied effects of age‐related neuropathologies on the trajectory of late life cognitive decline. Brain J Neurol. 2017;140(3):804‐812. doi: 10.1093/brain/aww341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wilson RS, Leurgans SE, Boyle PA, Schneider JA, Bennett DA. Neurodegenerative basis of age‐related cognitive decline (e–Pub ahead of print)(CME). Neurology. 2010;75(12):1070‐1078. doi: 10.1212/WNL.0b013e3181f39adc [DOI] [PMC free article] [PubMed] [Google Scholar]


