Abstract
Background
Literature is scarce on primary sarcopenia among Indian older adults. This study was aimed to estimate the prevalence of primary sarcopenia among older persons in India using the European Working Group on Sarcopenia in the Older People 2010 (EWGSOP) diagnostic criteria and to elucidate the factors leading to its development.
Methodology
Two hundred twenty‐seven subjects over 60 years of age attending the geriatric outpatient clinic were recruited for the study. Sarcopenia was diagnosed based on set criteria for gait speed, handgrip, and skeletal muscle mass assessment by dual‐energy x‐ray absorptiometry.
Result
The prevalence of primary sarcopenia in the study population was 39.2% (n = 89). Male patients were more sarcopenic than women, 47% (n = 72) vs 23% (n = 17). Obese subjects (body mass index > 25 kg/m2) had a lower prevalence of sarcopenia (odds ratio = 0.10; 95% confidence interval = 0.05–0.19). There was no association between sarcopenia and other postulated risk factors like low vitamin D levels, dietary protein or carbohydrate intake, or sedentary lifestyle.
Conclusion
Contrary to published data, primary sarcopenia appears to be higher among older Indians using presently available guidelines. Community studies with validated cutoffs suited for the Indian subcontinent may yield a lower prevalence of primary sarcopenia.
Keywords: dual energy x‐ray absorptiometry, European Working Group on Sarcopenia in the Older People 2010, Indian population, outpatient healthy older adults, prevalence of primary sarcopenia
This study was aimed to estimate the prevalence of primary sarcopenia among older persons in India using the European Working Group on Sarcopenia in the Older People 2010 (EWGSOP) diagnostic criteria and to elucidate the factors leading to its development. The prevalence of primary sarcopenia in this study population was 39% (n = 89). Obese subjects (body mass index > 25 kg/m2) had a lower prevalence of sarcopenia (odds ratio = 0.10; 95% confidence interval = 0.05–0.19). Contrary to published data, primary sarcopenia appears to be higher among older Indians.
![]()
1. INTRODUCTION
Sarcopenia is an age‐related muscle condition associated with a progressive, generalized loss of muscle mass and performance that has a direct impact on an older person’s functional status and mortality. 1 Sarcopenia can be broadly classified as “primary” or purely related to aging, and “secondary,” as the name suggests, is a result of an intrinsic disease process. 2 Identifying and treating the underlying cause is essential in the management of secondary sarcopenia. The prevalence of sarcopenia differs widely due to variations in diagnostic protocols, cutoffs used in the assessments, study population, and ethnicity. 3
To streamline the definition of sarcopenia, The European Working Group on Sarcopenia in Older People (EWGSOP) formulated a diagnostic algorithm for sarcopenia in 2010 for patient care and research settings. 4 The EWGSOP consensus used a combination of criteria and sarcopenia was diagnosed in the presence of low muscle mass, along with either low muscle strength or low physical performance. In 2019, the revised consensus EWGSOP2 was published with updated guidelines on the definition, diagnosis, and the assessment of the severity of sarcopenia. Low muscle strength was considered a vital characteristic of sarcopenia. The presence of low muscle quantity or quality confirms the diagnosis of sarcopenia. Subjects with reduced physical performance were considered to have severe sarcopenia. 5
Subsequently, the Asian Sarcopenia Working Group (ASWG) in 2014 published a consensus on the diagnosis of sarcopenia, which was further revised in 2019 for improved diagnostic accuracy. 6 , 7
Literature on sarcopenia among Indian older adults is scarce, both in the community and hospital settings. The diagnosis of sarcopenia among older Indian adults is challenging due to the lack of normative data on parameters from which cutoffs can be defined to assess muscle quantity, strength, and performance in the Indian setting. Using published data from the west can lead to over‐ or underestimation of the burden of sarcopenia in our country.
The main objective of this study was to estimate the prevalence of primary sarcopenia among healthy older outpatient attendees. The secondary objective was to assess the association between sarcopenia and factors, such as dietary patterns, exercise routine, vitamin D levels, and body mass index (BMI).
2. METHODS AND MATERIALS
2.1. Study design, population, and setting
This was a prospective cross‐sectional study conducted between January 2015 and January 2016 in the Department of Geriatrics, Christian Medical College, a tertiary care hospital. Consenting participants, aged 60 years and over, attending the Geriatric outpatient clinic were recruited for the study. The Institutional Review Board (IRB) of Christian Medical College approved the study protocol, IRB no. 9078, dated June 10, 2014.
All participants had to have a comprehensive geriatric assessment and appropriate investigations. Subjects with chronic obstructive lung disease, congestive cardiac failure, chronic liver disease, chronic kidney disease, moderate to severe osteoarthritis, symptomatic peripheral neuropathy, neurodegenerative disorders (including cognitive impairment), underlying malignancies, ongoing untreated or uncontrolled medical conditions, and recurrent falls were excluded from the study, as these conditions are conventional causes of secondary sarcopenia. 5 In addition, patients who presented with conditions causing acute pain, participants hospitalized in the last 10 weeks or those admitted for more than 7 days during the previous 6 months were excluded, as their underlying illnesses could have compromised assessments. 8
2.2. Sarcopenia diagnosis and determinants
The EWGSOP 2010 diagnostic criteria was used to diagnose sarcopenia in the study subjects (Figure 1). The subjects walked at a regular pace through a 6‐meter course – he/she accelerated in the first meter; the gait speed was measured in the mid four meter zone and the subject decelerated in the final meter. The best result of the two trials were taken. Subjects with a gait speed of < 0.8 m/s were considered to have a reduced gait speed. Grip strength measurement was performed using a JAMAR Mg4800 digital handheld dynamometer, and a decreased grip strength was defined as < 30 and < 20 kg for men and women respectively. Skeletal Muscle Mass Index (SMM) was measured using Discovery A dual‐energy x‐ray absorptiometry (DXA) machine. A coefficient of variation (CV) of 3% was noted. SMM of below 7.26 kg/m2 in men and 5.5 kg/m2 in women was the cutoff used for reduced muscle mass. 4
FIGURE 1.
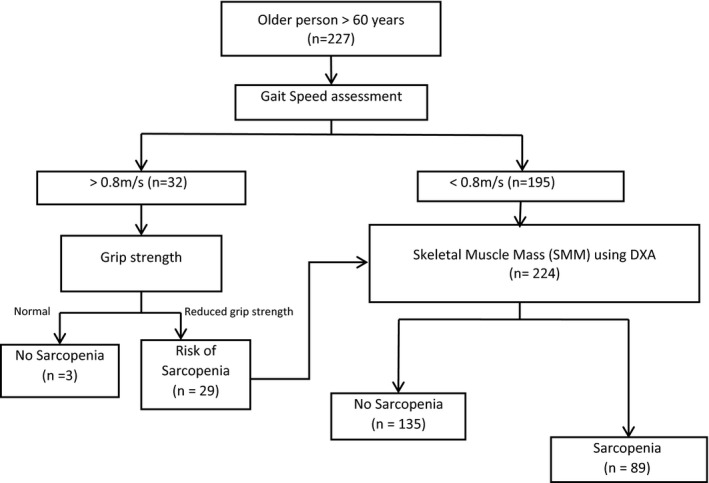
European Working Group on Sarcopenia in the Older People (EWGSOP) 2010 diagnostic protocol for sarcopenia
Other relevant data, including demographic profile, exercise routine using the International Physical Activity Questionnaire (IPAQ), 9 occupational status, socioeconomic status (as per the modified Kuppuswamy scale), BMI, and underlying comorbidities, were collected for all study participants. A nutrition expert documented a detailed dietary pattern with particular emphasis on protein intake of these individuals. Good protein intake was defined as consumption of more than 0.8 g/kg/day. Serum vitamin D levels, thyroid‐stimulating hormone (TSH), hemoglobin, creatinine, and HbA1c was estimated for all participants.
2.3. Statistical analysis
We assumed the prevalence of sarcopenia to be 20% based on a systematic review by Cruz‐Jentoft et al. 3 A sample size of 250 was calculated, for a precision of 5% and a 95% confidence level.
Data entry was done using EpiData software and analyzed using SPSS Statistics version 18. Pearson’s chi‐square test was used to analyze differences in demographic and other characteristics for categorical parameters. Independent samples t test was used for normally distributed continuous data, and the Mann–Whitney U test was used for non‐normally distributed continuous data. Evaluation and identification of risk factors of sarcopenia were analyzed using simple and multiple regression analyses. Descriptive statistics, including means and standard deviations, medians, and interquartile range, were used to summarize the various characteristics of the study participants. A P value of < 0.05 was regarded as being statistically significant.
3. RESULTS
3.1. Study population characteristics
During the study period, 11,604 patients visited the Geriatric outpatient department. Based on the exclusion criteria, 9996 (86%) patients were excluded. Of the remaining patients, only 252 (2.2%) participants could be enrolled as 658 (5.7%) did not give consent, and 698 (6.0%) could not be included, as they could not undergo skeletal muscle mass assessment. Ultimately, 227 (1.95%) subjects were included in the final analysis as six did not meet the study protocol, and SMM data were missing for 19 participants (Figure 2). We were not able to assess vitamin D levels and dietary patterns for 5 and 10 participants, respectively. However, they were included in the final calculation of the prevalence of sarcopenia in the study population.
FIGURE 2.
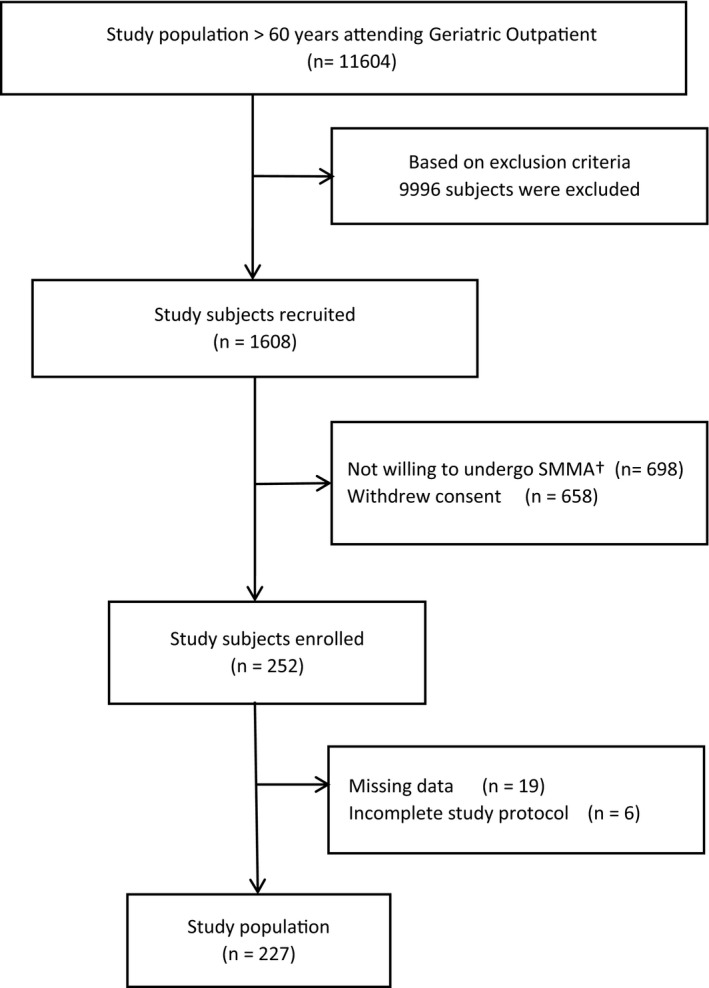
Flowchart – participant enrollment. †SMMA – Skeletal muscle mass assessment
The mean age of the subjects was 65.1 ± 4.4 years (range of 60–84 years). Around 23 (10%) of the subjects were over 70 years of age and 154 (68%) men were included in the study. Gait speed was reduced in 195 (85.9%) of the subjects. Among participants with normal gait speed (n = 32), only three had normal handgrip strength (HGS). Participants with reduced gait speed and low HGS underwent SMM assessment (Figure 1).
None of the participants belonged to the lower socioeconomic status, and only 6% were illiterate. All subjects were independent for Activities of Daily Living as per the Barthel index. The IPAQ questionnaire showed that only 18% of subjects indulged in regular physical activity, whereas none of the subjects complied with Health‐Enhancing Physical Activity (HEPA; Table 1).
TABLE 1.
Study population characteristics
| Nonsarcopenic subjects n = 138(%) | Sarcopenic subjects n = 89(%) | P value | |
|---|---|---|---|
| Age, y | 64.9 ± 4.2 | 65.7 ± 4.7 | 0.287 |
| Age category, y | |||
| 60–64 | 70 (62.5) | 42 (37.5) | 0.858 |
| 65–70 | 54 (58.7) | 38 (41.3) | |
| ≥ 70 | 14 (60.9) | 9 (39.1) | |
| Sex | |||
| Male (n = 154) | 82 (53.2) | 72 (46.8) | <0.001 |
| Female (n = 73) | 56 (76.7) | 17 (23.3) | |
| Gait speed, (m/s) | |||
| < 0.8 (n = 195) | 122 (62.6) | 73 (37.4) | 0.241 |
| > 0.8 (n = 32) | 16 (50) | 16 (50) | |
| Education | |||
| Primary education (n = 55) | 38 (69.1) | 17 (30.9) | 0.221 |
| Secondary and higher secondary education (n = 103) | 64 (62.1) | 39 (37.9) | |
| Graduation and above (n = 69) | 35 (50.7) | 34 (49.3) | |
| Social support status | |||
| Living alone (n = 11) | 9 (81.8) | 2 (18.2) | 0.208 |
| Living with spouse or children (n = 129) | 129 (59.7) | 87 (40.3) | |
| Socioeconomic status | |||
| Upper and middle class (n = 160) | 95 (59.4) | 65 (40.6) | 0.509 |
| Upper lower class (n = 67) | 40 (59.7) | 27 (40.3) | |
| Lower class (n = 0) | 0 | 0 | |
| Exercise level based on IPAQ | |||
| Inactive (n = 187) | 118 (63.1) | 69 (36.9) | 0.123 |
| Minimally active (n = 40) | 20 (50) | 20 (50) | |
| Comorbidities | |||
| No comorbidities (n = 99) | 62 (62.6) | 37 (37.4) | 0.354 |
| Diabetes (n = 76) | 45 (59.2) | 31 (40.8) | 0.837 |
| Hypertension (n = 94) | 55 (58.5) | 39 (41.5) | 0.642 |
| Smoking history | |||
| Current smoking or past smoking history (n = 42) | 23 (54.8) | 19 (45.2) | 0.387 |
| No history of smoking (n = 185) | 115 (62.2) | 70 (37.8) | |
| Alcohol consumption | |||
| Current/past consumption (n = 23) | 16 (69.6) | 7 (30.4) | 0.500 |
| No alcohol consumption (n = 204) | 122 (59.8) | 82 (40.2) | |
| BMI, kg/m2 | 26.14 ± 3.54 | 22.32 ± 3.09 | <0.001 |
| Underweight (< 18.5; n = 10) | 1 (10) | 9 (90) | <0.001 |
| Normal (18.5–24.9; n = 112) | 46 (41.1) | 66 (58.9) | |
| Overweight (> 25; n = 105) | 91 (86.7) | 14 (13.3) | |
| Laboratory parameters | |||
| Hemoglobin, g/dl | 13.2 ± 1.50 | 13.2 ± 1.46 | 0.793 |
| Vitamin D levels (N = 30–75 ng/ml) | 23.3 ± 10.3 | 27.03 ± 11.4 | 0.012 |
| Creatinine (N = 0.6–1.4 mg/dl) | 0.87 ± 0.24 | 0.92 ± 0.24 | 0.177 |
| TSH (N = 0.55–4.78 m IU/ml) | 3.15 ± 2.88 | 3.52 ± 2.36 | 0.464 |
| HbA1c, % | 6.41 ± 1.34 | 6.57 ± 1.24 | 0.247 |
| Dietary pattern, 24 h | |||
| Calorie intake, Kcal | 1756 ± 379.1 | 1749.8 ± 321.4 | 0.877 |
| Protein intake, g | 53.7 ± 17.7 | 50.8 ± 15.1 | 0.229 |
| Carbohydrate intake, g | 306.9 ± 95.8 | 304.8 ± 73.7 | 0.858 |
| Fat intake, g | 36.4 ± 12.33 | 35.7 ± 10.9 | 0.625 |
Abbreviations: BMI, body mass index; IPAQ, International Physical Activity Questionnaire
Based on the Mini Nutritional Assessment (MNA), none of the participants were malnourished. According to the World Health Organization (WHO) Body Mass Index criteria, only 10 (4%) and 18 (8%) were underweight (BMI < 18.5 kg/m2) and obese (BMI > 30 kg/m2), respectively, whereas 112 (49.3%) had normal BMI and 87 (38.3%) were overweight. Hypertension was prevalent in 94 subjects (41%), whereas diabetes mellitus was seen in 76 (56%) subjects.
3.2. Primary and secondary objectives
The overall prevalence of sarcopenia was 39.2% (n = 89) using the EWGSOP 2010 consensus. A higher proportion of men were sarcopenic compared with women, 47% (n = 72) vs 23% (n = 17 P value < 0.001). On applying the SMM‐DXA EWGSOP 2018 criteria (cutoffs for men and women being < 7.0 kg/m2 and < 5.5 kg/m2, respectively), the prevalence of sarcopenia was 29% (n = 67). Using ASWG (2014 and 2019) criteria, the prevalence was 28% (n = 63; Table 2). The prevalence of sarcopenia among the male subjects declined to 32% using these cutoffs. In women, however, the prevalence declined to 18% when the ASWG criteria (2014 and 2019) were applied (Table 2).
TABLE 2.
Prevalence of sarcopenia based on different SMM – DXA cutoffs, as suggested by different guidelines
| EWGSOP 2010 a n (%) | EWGSOP 2018 b n (%) | ASWG 2014 AND 2019 c n (%) | |
|---|---|---|---|
| Prevalence of sarcopenia | 89 (39.2) | 67 (29.5) | 63 (27.8) |
| Prevalence in men | 72 (46.8) | 50 (32.5) | 50 (32.5) |
| Prevalence in women | 17 (23.3) | 17 (23.3) | 13 (17.8) |
Abbreviations: ASWG, Asian Sarcopenia Working Group; DXA, dual‐energy x‐ray absorptiometry; EWGSOP, European Working Group on Sarcopenia in the Older People; SMM, Skeletal Muscle Mass Index.
SMM (ASM/ht2) (kg/m2) cutoff by using DXA – EWGSOP 2010 – men < 7.26 and women < 5.5.
EWGSOP 2018 male < 7.0 and women < 5.5.
ASWG 2014 and 2019 – men < 7.0 women < 5.4.
Univariate analysis failed to show a significant association between sarcopenia and increasing age, educational status, socioeconomic class, social support status, comorbidities, and addictions using both EWGSOP 2010 and EWGSOP 2018 criteria. Based on the EWGSOP 2010 criteria, sarcopenia was more prevalent among men (47% vs 23%, P value < 0.001). However, this gender difference ceased to exist when EWGSOP 2018 criteria was used. (Tables 1 and 3). Sarcopenia was seen in fewer subjects with higher BMI (> 25 kg/m2) on univariate (P < 0.001) and multivariate analyses (odds ratio = 0.10; 95% confidence interval [CI] = 0.05–0.19) after adjusting for age, sex, and vitamin D levels (Table 3).
TABLE 3.
Univariate and multivariate analyses with odds ratio for underlying factors based on EWGSOP 2010* and EWGSOP 2018**
| Parameters | EWGSOP 2010 a | EWGSOP 2018 b | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||||
| Groups n (%) | P value | OR (95% CI) | P value | Groups n (%) | P value | OR (95% CI) | P value | |||
| Nonsarcopenic | Sarcopenic | Nonsarcopenic | Sarcopenic | |||||||
| Age, y | ||||||||||
| 60−64 | 70 (62.5) | 42 (37.5) | 0.858 | 1.26 (0.41–3.88) | 0.69 | 83 (74.1) | 29 (25.9) | 0.475 | 1.29 (0.42–3.98) | 0.653 |
| 65–70 | 54 (58.7) | 38 (41.3) | 1.29 (0.42–3.91) | 0.66 | 61 (66.3) | 31 (33.7) | 0.99 (0.32–3.07) | 0.991 | ||
| > 70 | 14 (60.9) | 9 (39.1) | Reference | 16 (69.6) | 7 (30.4) | Reference | ||||
| Gender | ||||||||||
| Male | 82 (53.2) | 72 (46.8) | < 0.001 | 2.950 (1.41–6.16) | 0.004 | 104 (67.5) | 50 (32.5) | 0.165 | 1.29 (0.61–2.71) | 0.506 |
| Female | 56 (76.7) | 17 (23.3) | Reference | 56 (76.7) | 17 (23.3) | Reference | ||||
| Vitamin D levels, ng/ml | ||||||||||
| ≤ 20 | 50 (67.6) | 24 (32.4) | 0.146 | 1.01 (0.53 –2.26) | 0.805 | 55 (74.3) | 19 (25.7) | 0.436 | 0.92 (0.42–1.98) | 0.824 |
| > 20 | 84 (56.8) | 64 (43.2) | Reference | 101 (68.2) | 47 (31.8) | Reference | ||||
| BMI, kg/m2 | ||||||||||
| < 25 | 47 (38.5) | 75 (61.5) | < 0.001 | Reference | < 0.001 | 63 (51.6) | 59 (48.4) | < 0.001 | Reference | < 0.001 |
| ≥ 25 | 91 (86.7) | 14 (13.3) | 0.094 (0.05–0.19) | 97 (92.4) | 8 (7.6%) | 0.081 (0.034–0195) | ||||
| Protein intake per day | ||||||||||
| Reduced protein intake | 64 (58.2) | 46 (41.8) | 0.579 | 0.93 (0.49–1.76) | 0.823 | 75 (68.2) | 35 (31.8) | 0.461 | 0.95 (0.48–1.88) | 0.889 |
| Good protein intake | 67 (56.4) | 40 (37.4) | Reference | 78 (72.9) | 29 (27.1) | Reference | ||||
Statistically significant P values < 0.05 are in bold.
Abbreviations: BMI, body mass index; CI, confidence interval; DXA, dual‐energy x‐ray absorptiometry; EWGSOP, European Working Group on Sarcopenia in the Older People; OR, odds ratio; SMM, Skeletal Muscle Mass Index.
As per the study protocol.
Using the revised DXA SMM cutoffs.
Normal levels of vitamin D and consumption of a good quantity of protein daily does not protect one from sarcopenia (Table 3).
4. DISCUSSION
This is the first study of its kind which looked at the prevalence of primary sarcopenia among older Indian outpatient attendees. Although the ASWG 2014 diagnostic criteria was available when the study was conducted, we used the EWGSOP 2010 consensus, as it was the most widely used criteria for defining sarcopenia when this study commenced.
In our study, nearly a third of the study population had primary sarcopenia using EWGSOP 2018 SMM‐DXA cutoffs. Published community prevalence data for sarcopenia varied from 1% to 29%. 3 An Indian study published in 2019 among outpatients found that 53% had sarcopenia using ASWG 2014 criteria. 10 However, it is noteworthy that most of the research on sarcopenia has included subjects with secondary sarcopenia. A meta‐analysis published in 2020 also concluded that sarcopenia is highly prevalent among older adults with chronic disease conditions. 11 However, a recent outpatient study done in Thailand among older adults using ASWG criteria reported a 10% prevalence of primary sarcopenia. 12
EWGSOP 2010 and 2018 reference cutoffs for assessing muscle strength, muscle quantity/quality, and physical performance are derived mainly from Western population studies and may not be suitable for use in older Indian adults. For example, in this study of apparently healthy older individuals, almost 86% were found to have reduced gait speed, suggesting that the gait speed cutoff used may not accurately reflect poor physical performance. In another Indian outpatient based study, a lower cutoff for gait speed of 0.6 m/s was suggested, which may have been more appropriate in our context. 13 A study done by Marwaha et al 14 among 1045 healthy Indian women using DXA suggested that a cutoff < 5.11 kg/m2 (< 20th centile) to diagnose reduced SMM in Indian women was appropriate. This is lower than EWGSOP and ASWG cutoffs. Therefore, normative data for deriving the cutoffs of sarcopenia determinants are needed to diagnose sarcopenia in different geographic populations accurately.
Muscle mass and strength have been shown to peak between the second and third decade in White subjects. 15 However, a community study concluded that peak muscle mass and strength was achieved only in the third and fourth decades of life in Indians, and the mean muscle mass and muscle strength were reduced compared to White subjects. 16 Thus, ethnicity and race have a role to play in the pathophysiology of sarcopenia. Variations in demographic profile, nutritional status, chronic disease conditions, lifestyle, diet, and cultural practices have an influence on peak skeletal muscle mass and the rate of muscle loss and function, thus leading to a wide variation in the observed prevalence of sarcopenia across different population groups. 4 , 5 , 17 , 18 , 19 , 20
In our study, we found that men were more sarcopenic than women, as per the EWGSOP 2010 consensus (P < 0.001). Nevertheless, when the current cutoffs were applied, it failed to show an association between gender and the prevalence of sarcopenia (Table 3). An Indian hospital‐based study published in 2020 which looked at computerized tomogram assessment of sarcopenia showed men were eight times more likely to be sarcopenic than women. 21 Similar findings were reported from a Chinese community study (19.2% men vs 8.6% women). 22 Possible reasons include the rapid decline in muscle mass due to age‐dependent reduction in testosterone and insulin‐like growth factor‐1 levels in men. 23
Sarcopenia was seen in fewer patients who were overweight, as compared with those who were said to be normal or underweight, as per the WHO criteria for body mass. This is consistent with recent data reported from Asian populations. 24 , 25 A high BMI may not always imply increased fat, but may indicate improved lean skeletal mass to fat ratio. Therefore, it may be prudent to measure adiposity when BMI is elevated, in the presence of sarcopenia, to diagnose sarcopenic obesity. 24
Similar to an Indian study done by Anbalagan et al among young diabetics, the daily dietary protein intake did not differ significantly between sarcopenic and healthy subjects (P = 0.229). 26 Nevertheless, many cohort studies had shown a reduction in the rate of decline in muscle mass with aging when protein supplementation was provided to older adults. 27 , 28 Prior and ongoing consumption of good quality protein is essential to maintain muscle bulk and prevent loss of muscle function. 29 , 30 , 31 Indian diets are predominantly cereal‐based and possess relatively poor‐quality protein when compared with legumes, meat, milk, or milk‐based products. One of the reasons for an increase in the burden of sarcopenia in India is probably due to an insufficient quantity and quality of protein intake at a younger age, leading to a lower baseline muscle mass. 32
Exercise levels did not correlate with sarcopenia in this study (P = 0.12). Previous studies have shown that regular moderate to vigorous physical activity can prevent and aid in treating sarcopenia. 33 , 34 However, in this study, only a small number of subjects maintained an active lifestyle. Hence, we feel that more extensive studies are required to ascertain the relation between exercise levels and sarcopenia.
In this study, low vitamin D levels did not contribute to the development of sarcopenia (P = 0.146). Although this vitamin has an established biological effect on skeletal muscle physiology, previous experimental studies have produced conflicting results regarding vitamin D treatment or supplementation in the prevention or treatment of sarcopenia. 35 , 36 , 37
4.1. Limitations
This was a relatively small study. Second, this was a hospital‐based study, and the results may not be applicable to the general population. However, it is pertinent to note that the participants were active community‐dwelling adults. Third, the study population did not include subjects from lower socioeconomic or lower literacy groups. This was a selection bias, as this hospital is a private hospital where people have to pay for services.
5. CONCLUSION
There is an urgent need for comprehensive community‐based studies to obtain normative data to accurately address the burden of sarcopenia among older Indians. Based on available cutoffs, primary sarcopenia appears to be common among older Indian adults, especially in men and individuals with lower BMI. Larger studies are required to assess the role of vitamin D and exercise levels on the prevention of sarcopenia.
CONFLICT OF INTEREST
Nothing to disclose.
AUTHOR CONTRIBUTIONS
Rahman, Viggeswarpu, and Paul contributed to the study conception and design. Material preparation, data collection, and analysis were performed by Rahman and Yadav. The first draft of the manuscript was written by Wilson and all authors commented on subsequent versions of the manuscript. All authors read and approved the final manuscript.
CONSENT TO PARTICIPATE
Obtained from each of the study participants at the time of enrollment.
CONSENT FOR PUBLICATION
Obtained from each of the study participants at the time of enrollment.
6. ACKNOWLEDGEMENTS
We thank the Departments of Endocrinology, Clinical Dietetic Services and Clinical Biochemistry of Christian Medical College and Hospital, Vellore for the assistance provided during the study.
Rahman R, Wilson BP, Paul TV, Yadav B, Kango Gopal G, Viggeswarpu S. Prevalence and factors contributing to primary sarcopenia in relatively healthy older Indians attending the outpatient department in a tertiary care hospital: A cross‐sectional study. Aging Med. 2021;4:257–265. doi: 10.1002/agm2.12186
Funding information
The project was funded by Christian Medical College Vellore Internal Research Grant
REFERENCES
- 1. Morley JE, Baumgartner RN, Roubenoff R, Mayer J, Nair KS. Sarcopenia. J Lab Clin Med. 2001;137(4):231‐243. doi: 10.1067/mlc.2001.113504 [DOI] [PubMed] [Google Scholar]
- 2. Santilli V, Bernetti A, Mangone M, Paoloni M. Clinical definition of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):177‐180. [PMC free article] [PubMed] [Google Scholar]
- 3. Cruz‐Jentoft AJ, Landi F, Schneider SM, et al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748‐759. 10.1093/ageing/afu115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412‐423. doi: 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cruz‐Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16‐31. doi: 10.1093/ageing/afy169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen L‐K, Liu L‐K, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian Working Group for Sarcopenia. J Am Med Dir Assoc. 2014;15(2):95‐101. doi: 10.1016/j.jamda.2013.11.025 [DOI] [PubMed] [Google Scholar]
- 7. Chen L‐K, Woo J, Assantachai P, et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J Am Med Direct Assoc. 2020;21(3):300‐307.e2. doi: 10.1016/j.jamda.2019.12.012 [DOI] [PubMed] [Google Scholar]
- 8. Welch C, Hassan‐Smith ZK, Greig CA, Lord JM, Jackson TA. Acute sarcopenia secondary to hospitalisation ‐ an emerging condition affecting older adults. Aging Dis. 2018;9(1):151‐164. doi: 10.14336/AD.2017.0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lee PH, Macfarlane DJ, Lam TH, Stewart SM. Validity of the International Physical Activity Questionnaire Short Form (IPAQ‐SF): a systematic review. Int J Behav Nutr Phys Act. 2011;8:115. doi: 10.1186/1479-5868-8-115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Singhal S, Dewangan GC, Bansal R, et al. Sarcopenia and its Association with Geriatric Syndromes and Quality of Life in Older Indian Outpatients ‐ A Cross‐sectional Pilot Observational Study. J Indian Acad Geriatr. 2019;15(2):66. doi: 10.35262/jiag.v15i2.66-74 [DOI] [Google Scholar]
- 11. Pacifico J, Geerlings MAJ, Reijnierse EM, Phassouliotis C, Lim WK, Maier AB. Prevalence of sarcopenia as a comorbid disease: a systematic review and meta‐analysis. Exp Gerontol. 2020;131:110801. doi: 10.1016/j.exger.2019.110801 [DOI] [PubMed] [Google Scholar]
- 12. Therakomen V, Petchlorlian A, Lakananurak N. Prevalence and risk factors of primary sarcopenia in community‐dwelling outpatient elderly: a cross‐sectional study. Sci Rep. 2020;10(1):19551. doi: 10.1038/s41598-020-75250-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gunasekaran V, Banerjee J, Dwivedi SN, Upadhyay AD, Chatterjee P, Dey AB. Normal gait speed, grip strength and thirty seconds chair stand test among older Indians. Arch Gerontol Geriatr. 2016;67:171‐178. doi: 10.1016/j.archger.2016.08.003 [DOI] [PubMed] [Google Scholar]
- 14. Marwaha RK, Garg MK, Bhadra K, Mithal A, Tandon N. Assessment of lean (muscle) mass and its distribution by dual energy X‐ray absorptiometry in healthy Indian females. Arch Osteoporos. 2014;9:186. doi: 10.1007/s11657-014-0186-z [DOI] [PubMed] [Google Scholar]
- 15. Metter EJ, Lynch N, Conwit R, Lindle R, Tobin J, Hurley B. Muscle quality and age: cross‐sectional and longitudinal comparisons. J Gerontol. 1999;54(5):B207‐B218. doi: 10.1093/gerona/54.5.B207 [DOI] [PubMed] [Google Scholar]
- 16. Pal R, Aggarwal A, Singh T, et al. Diagnostic cut‐offs, prevalence, and biochemical predictors of sarcopenia in healthy Indian adults: The Sarcopenia‐Chandigarh Urban Bone Epidemiological Study (Sarco‐CUBES). Eur Geriatr Med. 2020;11(5):725‐736. doi: 10.1007/s41999-020-00332-z [DOI] [PubMed] [Google Scholar]
- 17. Jeng C, Zhao L‐J, Wu K, Zhou Y, Chen T, Deng H‐W. Race and socioeconomic effect on sarcopenia and sarcopenic obesity in the Louisiana Osteoporosis Study (LOS). JCSM Clin Rep. 2018;3(2):e00027. [PMC free article] [PubMed] [Google Scholar]
- 18. Garatachea N, Lucía A. Genes and the ageing muscle: a review on genetic association studies. Age (Dordr). 2013;35(1):207‐233. doi: 10.1007/s11357-011-9327-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kalyani RR, Corriere M, Ferrucci L. Age‐related and disease‐related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol. 2014;2(10):819‐829. doi: 10.1016/S2213-8587(14)70034-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Silva AM, Shen W, Heo M, et al. Ethnicity‐Related skeletal muscle differences across the lifespan. Am J Hum Biol. 2010;22(1):76‐82. doi: 10.1002/ajhb.20956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sreepriya PR, Pillai SS, Nair ANKK, Rahul A, Pillai S, Nair ATS. Prevalence and associated factors of sarcopenia among patients underwent abdominal CT scan in Tertiary Care Hospital of South India. J Frailty Sarcopenia Falls. 2020;5(3):79‐85. doi: 10.22540/JFSF-05-079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Du Y, Wang X, Xie H, et al. Sex differences in the prevalence and adverse outcomes of sarcopenia and sarcopenic obesity in community dwelling elderly in East China using the AWGS criteria. BMC Endocr Disord. 2019;19(1):109. doi: 10.1186/s12902-019-0432-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yamada M, Nishiguchi S, Fukutani N, et al. Prevalence of sarcopenia in community‐dwelling Japanese older adults. J Am Med Dir Assoc. 2013;14(12):911‐915. doi: 10.1016/j.jamda.2013.08.015 [DOI] [PubMed] [Google Scholar]
- 24. Pang BWJ, Wee S‐L, Lau LK, et al. Prevalence and Associated Factors of Sarcopenia in Singaporean Adults—The Yishun Study. J Am Med Dir Assoc. 2021;22(4):885.e1‐885.e10. doi: 10.1016/j.jamda.2020.05.029 [DOI] [PubMed] [Google Scholar]
- 25. Tey SL, Chew STH, How CH, et al. Factors associated with muscle mass in community‐dwelling older people in Singapore: findings from the SHIELD study. PLoS One. 2019;14(10):e0223222. doi: 10.1371/journal.pone.0223222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anbalagan VP, Venkataraman V, Pradeepa R, Deepa M, Anjana RM, Mohan V. The prevalence of presarcopenia in Asian Indian individuals with and without type 2 diabetes. Diabetes Technol Ther. 2013;15(9):768‐775. doi: 10.1089/dia.2013.0068 [DOI] [PubMed] [Google Scholar]
- 27. Houston DK, Nicklas BJ, Ding J, et al. Dietary protein intake is associated with lean mass change in older, community‐dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87(1):150‐155. doi: 10.1093/ajcn/87.1.150 [DOI] [PubMed] [Google Scholar]
- 28. Scott D, Blizzard L, Fell J, Giles G, Jones G. Associations between dietary nutrient intake and muscle mass and strength in community‐dwelling older adults: the Tasmanian Older Adult Cohort Study. J Am Geriatr Soc. 2010;58(11):2129‐2134. doi: 10.1111/j.1532-5415.2010.03147.x [DOI] [PubMed] [Google Scholar]
- 29. Yanai H. Nutrition for Sarcopenia. J Clin Med Res. 2015;7(12):926‐931. doi: 10.14740/jocmr2361w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bauer J, Biolo G, Cederholm T, et al. Evidence‐based recommendations for optimal dietary protein intake in older people: a position paper from the PROT‐AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542‐559. doi: 10.1016/j.jamda.2013.05.021 [DOI] [PubMed] [Google Scholar]
- 31. Montiel‐Rojas D, Nilsson A, Santoro A, et al. Fighting Sarcopenia in Ageing European Adults: the importance of the amount and source of dietary proteins. Nutrients. 2020;12(12):3601. doi: 10.3390/nu12123601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Minocha S, Thomas T, Kurpad AV. Dietary protein and the health‐nutrition‐agriculture connection in India. J Nutr. 2017;147(7):1243‐1250. doi: 10.3945/jn.116.243980 [DOI] [PubMed] [Google Scholar]
- 33. Sánchez‐Sánchez JL, Mañas A, García‐García FJ, et al. Sedentary behaviour, physical activity, and sarcopenia among older adults in the TSHA: isotemporal substitution model. J Cachexia Sarcopenia Muscle. 2019;10(1):188‐198. doi: 10.1002/jcsm.12369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mijnarends DM, Koster A, Schols JMGA, et al. Physical activity and incidence of sarcopenia: the population‐based AGES—Reykjavik Study. Age Ageing. 2016;45(5):614‐620. doi: 10.1093/ageing/afw090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Remelli F, Vitali A, Zurlo A, Volpato S. Vitamin D deficiency and sarcopenia in older persons. Nutrients. 2019;11(12):2861. doi: 10.3390/nu11122861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Uchitomi R, Oyabu M, Kamei Y. Vitamin D and sarcopenia: potential of vitamin D supplementation in sarcopenia prevention and treatment. Nutrients. 2020;12(10):3189. doi: 10.3390/nu12103189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shea MK, Fielding RA, Dawson‐Hughes B. The effect of vitamin D supplementation on lower‐extremity power and function in older adults: a randomized controlled trial. Am J Clin Nutr. 2019;109(2):369‐379. doi: 10.1093/ajcn/nqy290 [DOI] [PubMed] [Google Scholar]


