Summary:
Background:
Nonalcoholic steatohepatitis (NASH) is a common cause of chronic liver disease. There is a major need to understand the efficacy of different pharmacological agents for the treatment of NASH.
Aim:
To assess the relative rank-order of different pharmacological interventions in fibrosis improvement and NASH-resolution.
Methods:
A comprehensive search of several databases was conducted by an experienced librarian. We included randomized-controlled-trials (RCTs) comparing pharmacological interventions in patients with biopsy-proven NASH. The primary outcome was ≥ 1 stage improvement in fibrosis. The secondary outcome was NASH-resolution.
Results:
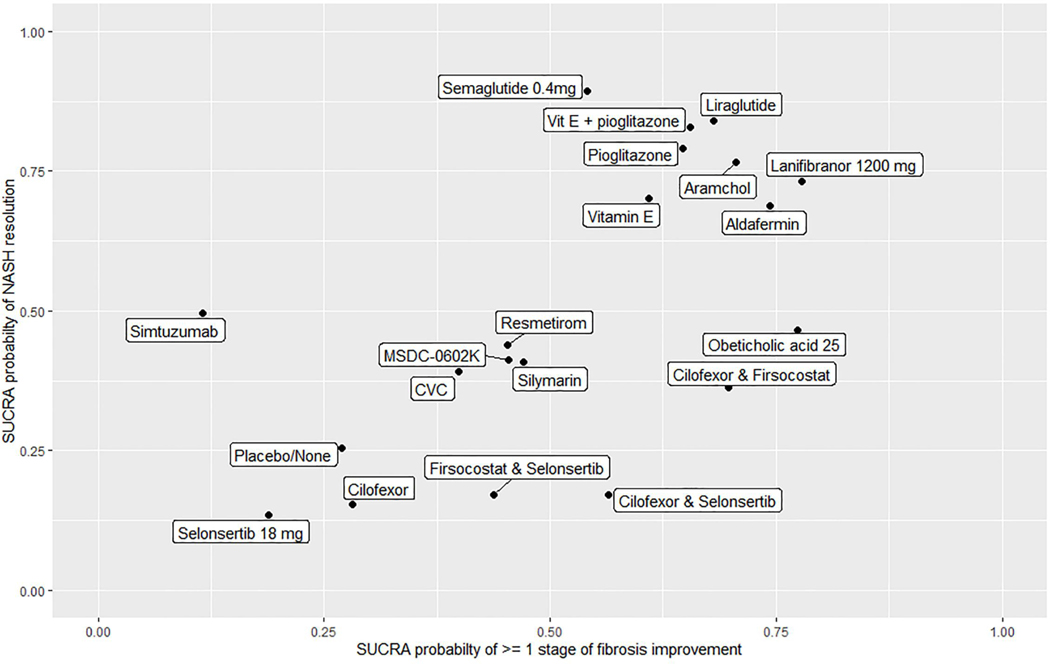
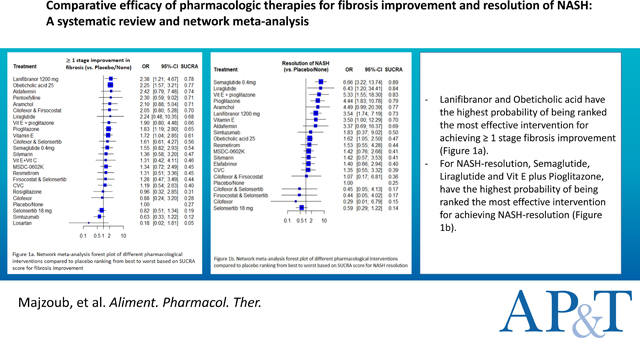
A total of 26 RCTs with 23 interventions met the eligibility criteria. Lanifibranor and Obeticholic acid had the highest probability of being ranked the most effective intervention for achieving ≥ 1 stage of fibrosis improvement (SUCRA 0.78) and (SUCRA 0.77), respectively. For NASH-resolution, Semaglutide, Liraglutide and Vit E plus Pioglitazone had the highest probability of being ranked the most effective intervention for achieving NASH-resolution (SUCRA 0.89), (SUCRA 0.84) and (SUCRA 0.83) respectively. Lanifibranor, Obeticholic acid, Pioglitazone and Vitamin E were significantly better than placebo in achieving ≥ 1 stage of fibrosis improvement. Conversely, Semaglutide, Liraglutide, Vit E plus Pioglitazone, Pioglitazone, Lanifibranor and Obeticholic acid were significantly better than placebo in achieving NASH-resolution.
Conclusion:
These data provide relative rank-order efficacy of various NASH therapies in terms of their improvements in liver fibrosis and NASH-resolution. Therapies that have been shown to improve NASH-resolution may be combined with therapies that have an anti-fibrotic effect to further boost treatment response rate in future.
Keywords: Fibrosis, end-point, NAFLD, NASH resolution, histologic response
Graphical Abstract
Introduction:
Nonalcoholic steatohepatitis (NASH) is one of the most common causes of chronic liver disease in the United States (US)1,2. NASH is a progressive liver disease and can lead to cirrhosis and hepatocellular carcinoma leading to increased liver-related morbidity and mortality3. It is the second leading indication of liver transplantation in the US1. NASH is commonly associated with obesity, insulin resistance and diabetes4. Due to rising rates of obesity and diabetes, the prevalence of NASH and NASH related cirrhosis and HCC is rising worldwide 5.
Lifestyle interventions are the current main stay of the management of NASH including dietary caloric restriction, Mediterranean diet, and increased physical activity4. Several pharmacologic therapies are in various phases of clinical development for the management of NASH and NASH related fibrosis. However, there are no food and drug administration (FDA) approved therapies for the management of NASH6.
For therapies to be deemed effective by the FDA in NASH related fibrosis, they have to demonstrate benefit in improving long-term clinical outcomes. However, as part of the subpart H approval pathway, if a pharmacologic therapy is able to demonstrate either ≥ 1 stage improvement in fibrosis stage without worsening of NASH or NASH resolution without worsening of fibrosis it is eligible to receive conditional approval pending demonstration of long-term clinical benefit.
Emerging data from Phase 2b and 3 trials suggest that treatment effect relative to placebo is small. Furthermore, there is significant heterogeneity in treatment response and certain therapies are more likely to improve fibrosis whereas other agents are more likely to lead to resolution of NASH. There is a major unmet understanding of the relative efficacy of different pharmacological agents for the treatment of NASH7. Therefore, we aimed to perform a systematic review and network meta-analysis of studies that assess the effect of different pharmacological interventions on NASH in assessing their relative rank-order in fibrosis improvement as well as NASH resolution. In-depth understanding of these two outcomes would help better synergize future combination therapeutic approaches in NASH to further improve treatment response rates.
Methods:
We performed a systematic review and network meta-analysis of studies that assess the effect of different pharmacological interventions on NASH. We reported the results according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. The study was based on a pre-established protocol ID CRD42020194405.
Eligibility Criteria:
Studies were included in our systematic review if they met the following criteria : (i) they were randomized controlled trials (RCTs) phase (II, III or, IV); (ii) enrolled patients with biopsy-proven NASH; (iii) compared one or more of established or potentially beneficial therapies for NASH based on American Association for the Study of Liver Diseases (AASLD) guidelines4 to each other or to placebo; (iv) had a follow up duration of at least 6 months; and (v) reported the primary outcome (biopsy-proven ≥ 1 stage improvement in fibrosis) and/or the secondary outcome (biopsy-proven NASH resolution defined as lobular inflammation 0–1 and ballooning 0).
Studies were excluded if they were: (i) observational studies; (ii) trials of lifestyle interventions; (iii) trials with follow up duration < 6 months; (iv) trials of futile therapy based on AASLD guidelines (e.g., metformin, omega-3 fatty acids, statins, etc.); or (v) enrolling < 40 patients.
Search Strategy:
We updated the search of our previous systematic review by Singh et al. 2015 8. A comprehensive search of several databases from 2014 to June 23rd, 2020 was conducted by an experienced medical librarian. The databases included Ovid MEDLINE(R) and Epub Ahead of Print, In-Process & Other Non-Indexed Citations, and Daily, Ovid EMBASE, Ovid Cochrane Central Register of Controlled Trials, Ovid Cochrane Database of Systematic Reviews, Web of science and Scopus. (The actual search strategy is available in the supplementary).
Study Selection Process:
Two independent investigators (A.M.M., T.N.) reviewed the titles and abstracts of all citations identified by the search. Full-text manuscripts were retrieved for the included abstracts and were subsequently screened for eligibility by two independent investigators (A.M.M., T.N.). Disagreements at this level were resolved by consensus and a third reviewer if needed.
Data extraction and risk of bias assessment:
Data extraction was done individually by (A.M.M., T.N., A.B. or N.M.). Extracted data were reviewed again by (A.M.M). A standardized form was used to extract data about the characteristics of: (i) included studies (first author, year of publication, geographical location and duration of follow up); (ii) patients (age, gender, BMI, diabetes); (iii) NAFLD (NAFLD activity score (NAS), ALT,AST); (iv) intervention(s) and comparison(s)(dosing and schedule of the agent and concomitant non-pharmacological interventions); and (v) outcomes (number of patients who achieved at least 1 stage improvement in fibrosis as a primary outcome and number of patients with NASH resolution as a secondary outcome). Patients with no follow up biopsy were deemed to be treatment failure. Risk of bias in the included studies was assessed using the Cochrane risk of bias assessment tool in which studies were deemed to be at low, high, or unclear risk9.
Data Synthesis and Statistical Analysis
For each comparison, odds ratios (OR) and 95% confidence intervals (95% CI) were meta-analyzed using the DerSimonian–Liard random-effects model10. We assessed statistical heterogeneity using the I2 statistic, with values greater than 50% suggesting substantial heterogeneity11. Publication bias was assessed by evaluating small study effects suggested by funnel plot asymmetry12. Next, we performed a frequentist network meta-analysis based on a random-effects consistency model following a multivariate meta-regression approach as described by Schwarzer et al13, and Rücker et al14,15 using R (R Core Team, 2020) and STATA v.16.0 (College Station, TX). The frequentist approach provides a point estimate from the network along with 95% CI from the frequency distribution of the estimate. We evaluated coherence in the networks by using the back-calculation method to split direct and indirect evidence16. We calculated the relative ranking of the interventions for achieving the primary and the secondary outcome as their surface under the cumulative ranking (SUCRA). SUCRA values range between 0 when a treatment is certainly the worst, and 1 when a treatment is certainly the best17, as such, higher scores correspond to higher ranking for achieving ≥ 1 stage improvement in fibrosis and/or NASH resolution
Certainty of Evidence
We followed the GRADE approach to rate the certainty of evidence of estimates derived from direct meta-analysis17,18,19. In this approach, direct evidence from RCTs starts at high certainty and can be rated down, based on risk of bias, indirectness, imprecision, inconsistency (or heterogeneity), and/or publication bias, to levels of moderate, low, and very low.
We followed the Confidence in Network Meta-Analysis (CINeMA) approach to rate the certainty of evidence of estimates derived from network meta-analysis, which is an adaptation of the GRADE approach18. CINeMA approach covers 6 domains: within-study bias (referring to the impact of risk of bias in the included studies), reporting bias (referring to publication and other reporting bias), indirectness, imprecision, heterogeneity, and incoherence. The reviewer’s input is required at the study level for within-study bias and indirectness. Then, applying user-defined rules, judgments are made as (no concerns, some concerns, or major concerns) to each domain. Judgments across domains can be summarized to obtain 4 levels of certainty for each relative treatment effect, corresponding to the usual GRADE assessments of very low, low, moderate, or high20,21.
Results:
4820 titles and abstracts were identified using the search strategy; 26 RCTs met our inclusion criteria (Figure S1 in the supplementary figures demonstrates the study selection process through a flow chart). Table 1 in the supplements summarizes the characteristics of the included RCTs.
Overall, these 26 trials had 5129 patients and 23 interventions. Four of the included studies were single center22–25; all the others were multicenter. Follow up duration ranged between 24 – 104 weeks. Seventeen of the included studies were two-arm trials comparing active agent with placebo22–39. Six studies included multiple arms to compare one active agent in different doses with placebo40–45. Two studies had three arms comparing two different interventions and placebo46,47. One study compared 6 distinctive interventions and placebo in a seven-arm trial48.
Table 2 in the supplements describes the baseline characteristics of patients included in the studies. The mean age of the patients ranged from 47 to 63 and from 45 to 59 in the intervention group and placebo group, respectively. Two studies included only nondiabetic patients26,47; on the other hand, three studies included only diabetic or prediabetics patients28,44,46. The mean NAFLD activity score at baseline ranged from (3 to 5.7).
Overall, Studies were judged to be at low risk of bias. Some of the studies were funded by pharmaceutical companies; which we explicitly reported as a study characteristics, but not in the risk of bias assessment, which is based on Cochrane risk of bias assessment tool9.
Table3 in the supplements summarizes the risk of bias assessment for the included studies.
Primary outcome: ≥ 1 stage improvement in fibrosis
Twenty-three different interventions were studied for this outcome (Figure 1). Four individual studies showed statistically significant improvement for ≥ 1 stage fibrosis25,34,37,45. Phase 2b NATIVE trial45 showed that Lanifibranor was superior to placebo (OR 2.38; 95% CI, 1.21–4.67).
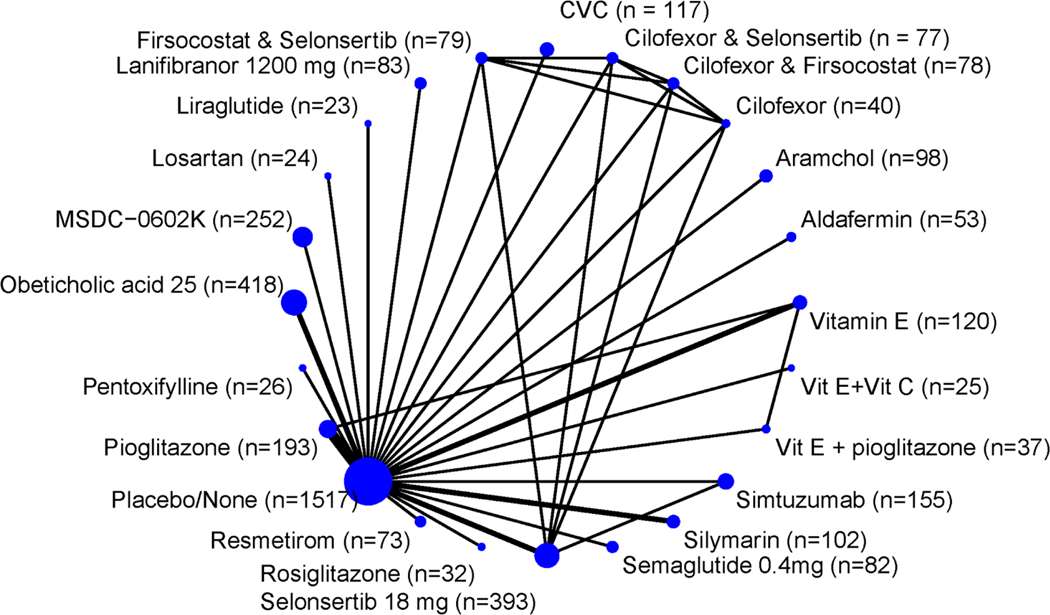
Figure 1.
Network of the included studies with the available direct comparisons for the primary outcome. The size of the nodes and the thickness of the edges are weighted according to the number of patients and the number of studies evaluating each treatment, respectively.
Two studies compared Obeticholic acid versus placebo; Neuschwander-Tetri et al. 201534 showed that Obeticholic acid was superior to placebo (OR 2.30; 95% CI, 1.22–4.35) and Younossi et al. 201937 also showed that Obeticholic acid was superior to placebo (OR 2.22; 95% CI, 1.44–3.42).
Wah Kheong et al. 201725 showed that Silymarin was superior to placebo (OR 4.54; 95% CI, 1.18–17.43).
There was no statistically significant difference between placebo and the other 20 studied agents in the other trials. (Figure S2 with all direct comparisons for fibrosis improvement is provided in the supplementary figures)
Direct Meta-analysis
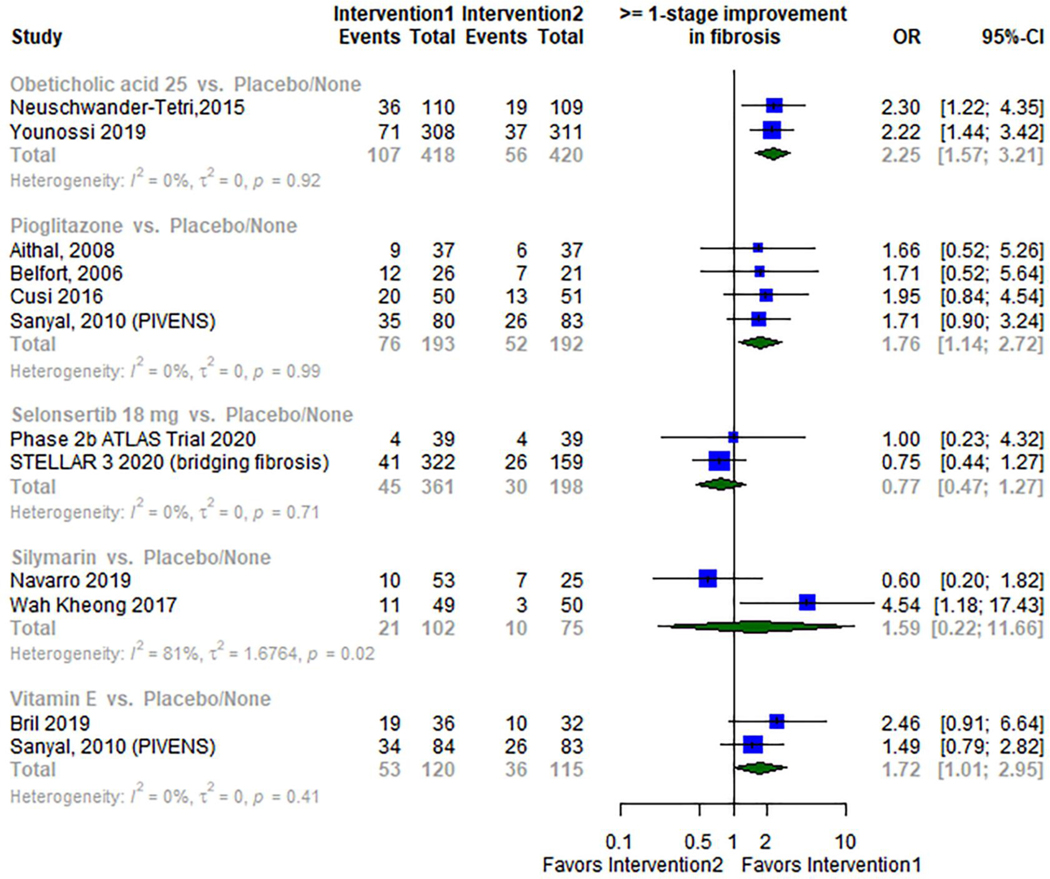
When compared to placebo, the odds of achieving at least 1 stage improvement of fibrosis were statistically significantly higher in patients receiving Obeticholic acid (OR 2.25; 95% CI: 1.57–3.21; I2 0%; two RCTs34,37 with 438 patients), Pioglitazone (OR 1.76; 95% CI: 1.14–2.72; I2 0%; four RCTs22,26,28,47 with 385 patients), and vitamin E (OR 1.72; 95% CI: 1.01–2.95; I2 0%; two RCTs46,47 with 235 patients). In contrast, Silymarin and Selonsertib were not associated with a statistically significant improvement in fibrosis when compared with placebo (OR 1.59; 95% CI: 0.22–11.66; I2 81%; two RCTs25,33 with 177 patients) and (OR 0.77; 95% CI: 0.47–1.27; I2 0%; two RCTs31,42 with 559 patients) respectively. Figure 2
Figure 2.
Meta-analysis forest plots of different pharmacological interventions compared to placebo for the primary outcome
Network Meta-analysis:
Compared to placebo, Lanifibranor, Obeticholic acid, Pioglitazone and Vitamin E were statistically significantly better in achieving ≥ 1 stage of fibrosis improvement (OR 2.38; 95% CI: 1.21–4.67), (OR 2.25; 95% CI 1.57–3.21), (OR 1.83; 95% CI 1.19 – 2.80) and (OR 1.72; 95% CI 1.04– 2.85) respectively; Other interventions did not demonstrate superiority against placebo. (Supporting file that describes the direct and indirect comparisons of all the studied agents against each other for fibrosis improvement is available in the supplementary).
Based on SUCRA score, Lanifibranor and Obeticholic acid had the highest probability of being ranked the most effective intervention for achieving ≥ 1 stage fibrosis improvement (SUCRA 0.78) and (SUCRA 0.77), respectively. Oppositely, Losartan, Simtuzumab and Selonsertib had the lowest probability of being ranked the most effective intervention (SUCRA 0.05), (SUCRA 0.12) and (SUCRA 0.19), respectively. Figure 3 (Supporting file and figure S3 that describe ranking probabilities for fibrosis improvement are available in the supplementary).
Figure 3.
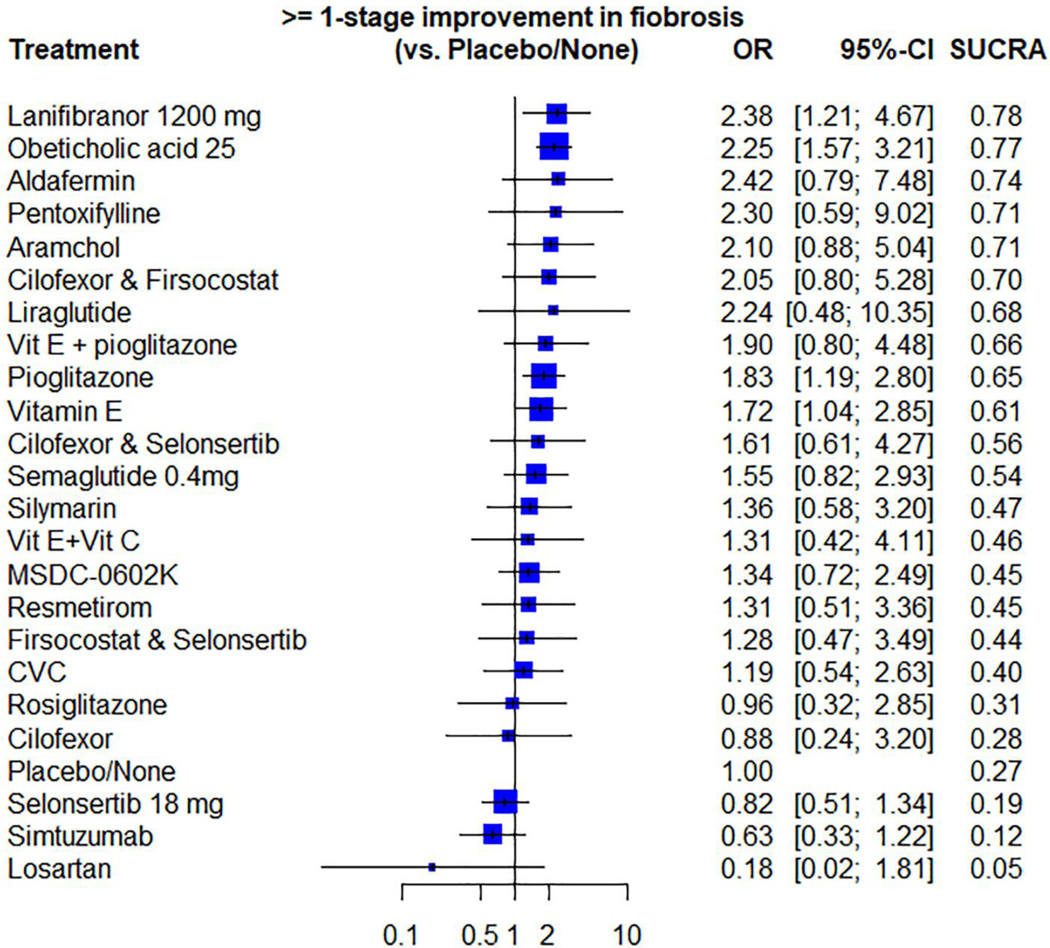
Network meta-analysis forest plot of different pharmacological interventions compared to placebo ranking from best to worst based on SUCRA score for the primary outcome
Secondary outcome: NASH resolution
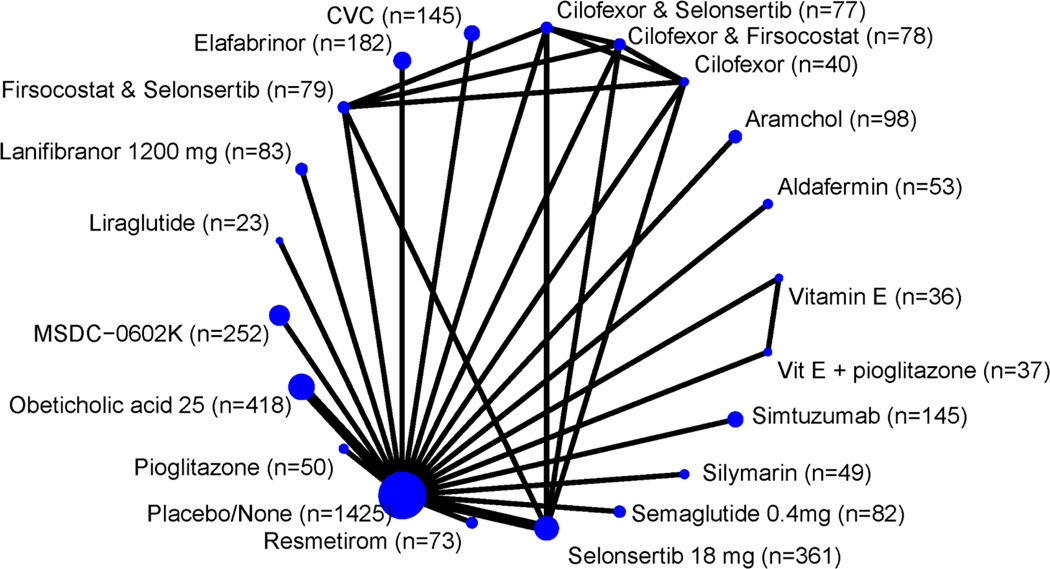
Twenty different interventions were studied for this outcome (Figure 4). Five studies had statistically significant difference when comparing placebo versus other agents22,27,35,45,46. Newsome et al. 202035 showed that Semaglutide was superior to placebo (OR 6.66; 95% CI: 3.22–13.74). When comparing Liraglutide to placebo, Armstrong et al. 201627 showed that Liraglutide was superior to placebo (OR 6.43; 95% CI: 1.20–34.41). Vitamin E plus Pioglitazone was also superior to placebo in Brial, 2019 trial46 (OR 5.33; 95% CI: 1.55–18.30). Cusi et al. 201622 compared Pioglitazone versus placebo and showed that Pioglitazone was superior (OR 4.44; 95% CI: 1.83–10.78). Finally, Lanifibranor was superior to placebo in NATIVE, 2020 trial45 (OR 3.54; 95% CI: 1.74–7.19). There was no statistically significant difference between placebo and the other 15 agents in the remaining studies. (Figure S4 with all direct comparisons for NASH resolution is provided in the supplementary figures)
Figure 4.
Network of the included studies with the available direct comparisons for the secondary outcome. The size of the nodes and the thickness of the edges are weighted according to the number of patients and the number of studies evaluating each treatment, respectively.
Direct Meta-analysis
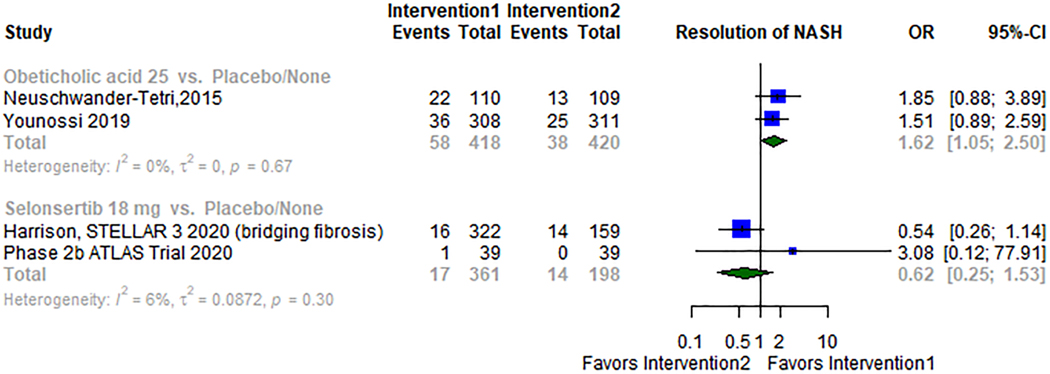
Two studies34,37 compared Obeticholic acid versus placebo; a total of 838 patients were included in the analysis. Obeticholic acid was superior to placebo (OR 1.62; 95% CI: 1.05–2.50; I2 0%) Figure 5. Selonsertib was studied in two different trials31,42 with 559 patients, and it showed no statistically significant difference when compared to placebo (OR 0.62; 95% CI 0.25–1.53; I2 6%) Figure 5.
Figure 5.
Meta-analysis forest plots of different pharmacological interventions compared to placebo for the secondary outcome.
Network Meta-analysis
Compared to placebo, Semaglutide, Liraglutide, Vit E plus Pioglitazone, Pioglitazone, Lanifibranor and Obeticholic acid were statistically significantly better in achieving NASH resolution (OR 6.66; 95% CI 3.22–13.74), (OR 6.43; 95% CI 1.20–34.41), (OR 5.33; 95% CI 1.55–18.30), (OR 4.44; 95% CI 1.83–10.78), (OR 3.54; 95% CI 1.74–7.19) and (OR 1.62; 95% CI 1.05–2.50), respectively. (Supporting file that describe the direct and indirect comparisons of all the studied agents against each other for NASH resolution is provided in the supplementary).
Semaglutide, Liraglutide, Vit E plus Pioglitazone had the highest probability of being ranked the most effective intervention for achieving NASH resolution (SUCRA 0.89), (SUCRA 0.84) and (SUCRA 0.83) respectively. On the contrary, Selonsertib, Cilofexor, Firsocostat plus Selonsertib, Cilofexor plus Selonsertib had the lowest probability of ranking the most effective intervention. (SUCRA 0.14), (SUCRA 0.15), (SUCRA 0.17) and (SUCRA 0.17) respectively. Figure 6.
Figure 6.
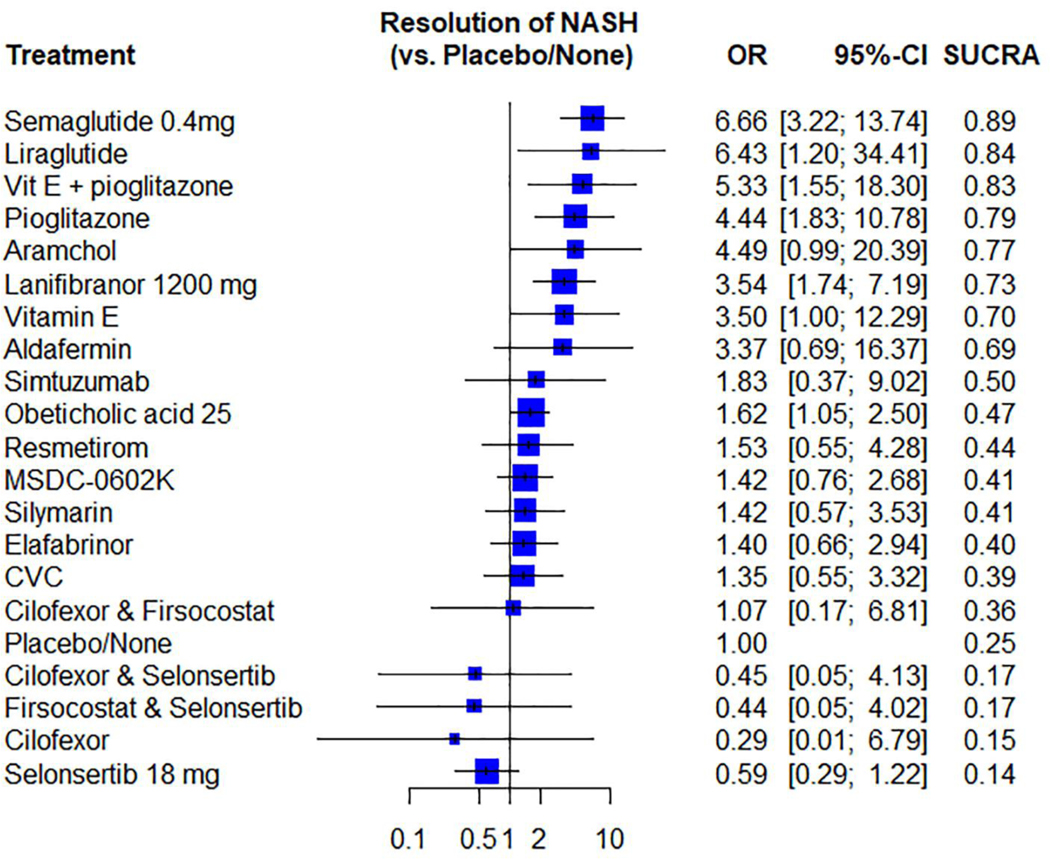
Network meta-analysis forest plot of different pharmacological interventions compared to placebo ranking from best to worst based on SUCRA score for the secondary outcome.
(Supporting file and figure S5 that describe ranking probabilities for NASH resolution are available in the supplementary).
Publication Bias and Network coherence
We were not able to assess publication bias because of the small number of studies in each comparison. There was no significant difference (incoherence) between direct and indirect estimates when both were available. The two methods had overlapping CIs for all interventions in both primary and secondary outcomes. (Two Figures S6, S7 presenting the direct and indirect comparisons for both outcomes and two files describing the back-calculation method for the coherence test for both outcomes are provided in the supplementary material)
Certainty of Evidence
For the outcome of fibrosis Improvement, compared to placebo both Obeticholic acid and Pioglitazone had a better outcome which was supported by high certainty of evidence, whereas Vitamin E had a better outcome which was supported by moderate certainty of evidence.
Both Obeticholic acid and Pioglitazone had a better outcome when compared to Selonsertib and Simtuzumab with a high certainty of evidence. Vitamin E has a better outcome when compared to Selonsertib and Simtuzumab with moderate certainty of evidence. Both Semaglutide and Vit E plus Pioglitazone have a better outcome when compared with Simtuzumab with a moderate certainty of evidence.
Other comparisons were supported by low to very low certainty of evidence because of severe imprecision. For the outcome of Nash resolution, all comparisons were supported by low to very low certainty of evidence because of major concerns for imprecision and heterogeneity (Two files showing the certainty of evidence for the whole comparisons and for the available direct comparisons can be found in the supplementary)
Discussion:
In this updated systematic review and network meta-analysis, we combined direct and indirect evidence from twenty-six RCTs with a total of 5129 patients and 23 interventions to estimate the relative efficacy of different pharmacological interventions in achieving ≥ 1 stage improvement in fibrosis and/or NASH resolution. We were able to make some key observations: (1) Lanifibranor, Obeticholic acid, Pioglitazone and Vitamin E were significantly better than placebo in achieving ≥ 1 stage of fibrosis improvement (2) Lanifibranor and Obeticholic acid had the highest probability of being ranked the most effective intervention for achieving ≥ 1 stage fibrosis improvement (3) Semaglutide, Liraglutide, Vit E plus Pioglitazone, Pioglitazone, Lanifibranor and Obeticholic acid were significantly better than placebo in achieving NASH resolution.(4) Semaglutide, Liraglutide and Vit E plus Pioglitazone had the highest probability of being ranked the most effective intervention for achieving NASH resolution (Figure 7).
Figure 7.
SUCRAs for NASH resolution and at least 1 stage fibrosis improvement.
Current AASLD guidelines4 recommend the use of vitamin E in nondiabetic adults with biopsy-proven NASH and the use of pioglitazone in both diabetic and non-diabetic adults with biopsy-proven NASH. AASLD recommends against using Metformin, UCDA, or Omega 3-fatty acids as a specific treatment for NASH. Regarding GLP-1 agonists and OCA, AASLD states that it is premature to consider them to specifically treat NASH.
Our network meta-analysis suggests that several mechanisms may provide therapeutic benefit in NASH. Certain classes of agents (such as Obeticholic acid, an FXR agonist) may have predominantly anti-fibrotic efficacy and others (such as Semaglutide, a GLP-1 analogue) may have greater efficacy in improving NASH resolution. The relative benefits of these therapies in fibrosis regression and NASH resolution will inform future choices for combination therapeutic approaches. Future, larger studies are warranted to validate these results.
The strengths of our analyses include the comprehensive assessment of the relative efficacy of twenty-three pharmacological agents for both fibrosis improvement and NASH resolution in 5129 patients. The coherent results from direct and indirect comparisons give us more confidence in our observations. We used CINeMA approach to assess the certainty of evidence for this network meta-analysis, and GRADE approach for the direct meta-analysis which benefit in generating guidelines in the future.
The study has limitations. First, there was small number of direct (head-to -head) comparative studies. Second, there is always concern about heterogeneity in any meta-analysis which can take place as differences among trials in study design, patient demographics, interventions and comparisons, outcome assessment; and this may limit the comparability of trials19,49. Third, estimates of ranking probabilities can be misleading as they are highly sensitive to both the number of studies per comparisons and the overall network configuration. An unequal number of studies per comparison may result in biased estimates of treatment rank probabilities for every network considered50;this can be seen with Aramchol in our study. Aramchol has a SUCRA: 0.77 reflecting high probability of being ranked one of the most effective intervention for achieving NASH resolution. However, it failed to achieve statistically significant better outcome when compared to placebo for NASH resolution. This biased estimate can be relatively solved by not focusing only on the summary estimates and ranking probabilities; rather taking into consideration the certainty of evidence for each comparison.
Finally, our study combined phase II and III trials in the comparison. On one hand this was an advantage as we were able to do comprehensive assessment of all potential pharmacological therapies considered for NASH. On the other hand, some of the early trials results may not be subsequently replicated in a larger sample size. A notable example is Harrison, 202030. The study did not meet primary endpoint of fibrosis improvement by >1 stage with no worsening of NASH versus placebo and the sponsor does not plan to pursue a phase III clinical trial.
Implications for Clinical Research
These results provide new supportive evidence on the use of Pioglitazone as suggested by the current AASLD Practice Guidance. Furthermore, there is moderate certainty evidence to support the use of Vitamin E in NASH. Given the data from both the FLINT trial34 and the REGENERATE trial37 there is high certainty evidence in support of efficacy of Obeticholic acid in improving NASH related fibrosis. Given only one trial data on Lanifibranor use, the evidence supporting its efficacy is low certainty and would require further validation by a larger Phase 3 trial. These data also support the regulatory requirement to have at least 2 histology-based trial to develop moderate-high certainty evidence of efficacy before widespread clinical use. This network is a major advance compared to our previous meta-analysis conducted by Singh et al. 2015 8; However, in that review a moderate certainty evidence supporting the use of Pentoxifylline for fibrosis improvement was observed. The evidence in our network meta-analysis for Pentoxifylline compared with placebo was downgraded to low in certainty.
In conclusion, we observed that several candidate agents in Phase 2b and a Phase 3 trial have been shown to improve histological features of NASH. In general, monotherapies, even when they improve histologic features of NASH, have been shown to have a small treatment effect delta relative to placebo. Larger comparative RCTs are warranted to further establish the comparative efficacy of different interventions for NASH in demonstrating ≥ 1 stage improvement in fibrosis and/or NASH resolution. Therapies that have been shown to improve NASH resolution may be combined with therapies that have an anti-fibrotic effect to further boost treatment response rate in future.
Supplementary Material
Acknowledgements:
Authorship Statement: Guarantor of the article: Rohit Loomba. Specific Author Contributions: Study concept and design: Abdul Majzoub, Rohit Loomba. Acquisition, analysis or interpretation of the data: Abdul Majzoub, Tarek Nayfeh, Abbey Barnard, Nagambika, Shravan Dave, Siddharth Singh, M. Hassan Murad, Rohit Loomba. Drafting of the manuscript: Abdul Majzoub, Rohit Loomba. Critical revision of manuscript for important intellectual content: Abdul Majzoub, Tarek Nayfeh, Abbey Barnard, Nagambika, Shravan Dave, Siddharth Singh, M. Hassan Murad, Rohit Loomba. Statistical analysis: Tarek Nayfeh, Abdul Majzoub. Study supervision: Rohit Loomba. All authors approved the final version of the manuscript.
Funding: RL receives funding support from NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (U01DK061734, R01DK106419, P30DK120515, R01DK121378, R01DK124318), NHLBI (P01HL147835), and DOD PRCRP (W81XWH-18-2-0026). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Statement of Interests:
Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- BMI
body mass index
- HDL
high density lipoprotein
- LDL
low density lipoprotein
- NAFLD
nonalcoholic fatty liver disease
- NAS
NAFLD Activity Score
- NASH
nonalcoholic steatohepatitis
- NASH CRN
NASH Clinical Research Network
- OR
Odds Ratio
- CI
Confidence Interval
- AASLD
American Association For The Study Of Liver Diseases
- UCDA
Ursodeoxycholic acid
- GLP-1
Glucagon-like peptide 1
- OCA
Obeticholic acid
- HSC
Hepatic stellate cell
- PPAR
Peroxisome proliferator-activated receptor
Footnotes
Conflict of interests: Dr. Rohit Loomba serves as a consultant or advisory board member for Arrowhead Pharmaceuticals, AstraZeneca, Bird Rock Bio, Boehringer Ingelheim, Bristol-Myer Squibb, Celgene, Cirius, CohBar, Conatus, Eli Lilly, Galmed, Gemphire, Gilead, Glympse bio, GNI, GRI Bio, Intercept, Ionis, Janssen Inc., Merck, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Pfizer, Prometheus, Sanofi, Siemens, and Viking Therapeutics. In addition, his institution has received grant support from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly and Company, Galectin Therapeutics, Galmed Pharmaceuticals, GE, Genfit, Gilead, Intercept, Grail, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, NuSirt, Pfizer, pH Pharma, Prometheus, and Siemens. He is also co-founder of Liponexus, Inc.
References
- 1.Younossi ZM, Stepanova M, Ong J, et al. Nonalcoholic Steatohepatitis Is the Most Rapidly Increasing Indication for Liver Transplantation in the United States. Clin Gastroenterol Hepatol 2020. doi: 10.1016/j.cgh.2020.05.064 [published Online First: 2020/06/13] [DOI] [PubMed] [Google Scholar]
- 2.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67(1):123–33. doi: 10.1002/hep.29466 [published Online First: 2017/08/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017;65(5):1557–65. doi: 10.1002/hep.29085 [published Online First: 2017/01/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67(1):328–57. doi: 10.1002/hep.29367 [published Online First: 2017/07/18] [DOI] [PubMed] [Google Scholar]
- 5.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2020. doi: 10.1038/s41575-020-00381-6 [published Online First: 2020/12/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pearlman M, Loomba R. State of the art: treatment of nonalcoholic steatohepatitis. Curr Opin Gastroenterol 2014;30(3):223–37. doi: 10.1097/MOG.0000000000000060 [published Online First: 2014/04/11] [DOI] [PubMed] [Google Scholar]
- 7.Dufour JF, Caussy C, Loomba R. Combination therapy for non-alcoholic steatohepatitis: rationale, opportunities and challenges. Gut 2020;69(10):1877–84. doi: 10.1136/gutjnl-2019-319104 [published Online First: 2020/05/10] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Singh S, Khera R, Allen AM, et al. Comparative effectiveness of pharmacological interventions for nonalcoholic steatohepatitis: A systematic review and network meta-analysis. Hepatology 2015;62(5):1417–32. doi: 10.1002/hep.27999 [published Online First: 2015/07/21] [DOI] [PubMed] [Google Scholar]
- 9.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011;343:d5928. doi: 10.1136/bmj.d5928 [published Online First: 2011/10/20] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557–60. doi: 10.1136/bmj.327.7414.557 [published Online First: 2003/09/06] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JA, Sutton AJ, Ioannidis JP, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. doi: 10.1136/bmj.d4002 [published Online First: 2011/07/26] [DOI] [PubMed] [Google Scholar]
- 13.Schwarzer G, Carpenter JR, Rücker G. Meta-analysis with R: Springer; 2015. [Google Scholar]
- 14.Rucker G. Network meta-analysis, electrical networks and graph theory. Res Synth Methods 2012;3(4):312–24. doi: 10.1002/jrsm.1058 [published Online First: 2012/12/01] [DOI] [PubMed] [Google Scholar]
- 15.Rücker G, Schwarzer G, Krahn U, et al. Package ‘netmeta’. Network Meta-Analysis using Frequentist Methods (Version 07–0) 2015 [Google Scholar]
- 16.Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29(7–8):932–44. doi: 10.1002/sim.3767 [published Online First: 2010/03/10] [DOI] [PubMed] [Google Scholar]
- 17.Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64(2):163–71. doi: 10.1016/j.jclinepi.2010.03.016 [published Online First: 2010/08/07] [DOI] [PubMed] [Google Scholar]
- 18.Guyatt G, Oxman AD, Sultan S, et al. GRADE guidelines: 11. Making an overall rating of confidence in effect estimates for a single outcome and for all outcomes. J Clin Epidemiol 2013;66(2):151–7. doi: 10.1016/j.jclinepi.2012.01.006 [published Online First: 2012/05/01] [DOI] [PubMed] [Google Scholar]
- 19.Puhan MA, Schunemann HJ, Murad MH, et al. A GRADE Working Group approach for rating the quality of treatment effect estimates from network meta-analysis. BMJ 2014;349:g5630. doi: 10.1136/bmj.g5630 [published Online First: 2014/09/26] [DOI] [PubMed] [Google Scholar]
- 20.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, et al. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med 2020;17(4):e1003082. doi: 10.1371/journal.pmed.1003082 [published Online First: 2020/04/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papakonstantinou T, Nikolakopoulou A, Higgins JPT, et al. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Systematic Reviews 2020;16(1):e1080. doi: 10.1002/cl2.1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cusi K, Orsak B, Bril F, et al. Long-Term Pioglitazone Treatment for Patients With Nonalcoholic Steatohepatitis and Prediabetes or Type 2 Diabetes Mellitus: A Randomized Trial. Ann Intern Med 2016;165(5):305–15. doi: 10.7326/M15-1774 [DOI] [PubMed] [Google Scholar]
- 23.Harrison SA, Torgerson S, Hayashi P, et al. Vitamin E and vitamin C treatment improves fibrosis in patients with nonalcoholic steatohepatitis. Am J Gastroenterol 2003;98(11):2485–90. doi: 10.1111/j.1572-0241.2003.08699.x [published Online First: 2003/11/26] [DOI] [PubMed] [Google Scholar]
- 24.Ratziu V, Giral P, Jacqueminet S, et al. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology 2008;135(1):100–10. doi: 10.1053/j.gastro.2008.03.078 [published Online First: 2008/05/28] [DOI] [PubMed] [Google Scholar]
- 25.Wah Kheong C, Nik Mustapha NR, Mahadeva S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clin Gastroenterol Hepatol 2017;15(12):1940–49.e8. doi: 10.1016/j.cgh.2017.04.016 [DOI] [PubMed] [Google Scholar]
- 26.Aithal GP, Thomas JA, Kaye PV, et al. Randomized, placebo-controlled trial of pioglitazone in nondiabetic subjects with nonalcoholic steatohepatitis. Gastroenterology 2008;135(4):1176–84. doi: 10.1053/j.gastro.2008.06.047 [published Online First: 2008/08/23] [DOI] [PubMed] [Google Scholar]
- 27.Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non-alcoholic steatohepatitis (LEAN): a multicentre, double-blind, randomised, placebo-controlled phase 2 study. Lancet 2016;387(10019):679–90. doi: 10.1016/S0140-6736(15)00803-X [DOI] [PubMed] [Google Scholar]
- 28.Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med 2006;355(22):2297–307. doi: 10.1056/NEJMoa060326 [published Online First: 2006/12/01] [DOI] [PubMed] [Google Scholar]
- 29.Harrison SA, Bashir MR, Guy CD, et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2019;394(10213):2012–24. doi: 10.1016/S0140-6736(19)32517-6 [DOI] [PubMed] [Google Scholar]
- 30.Harrison SA, Neff G, Guy CD, et al. Efficacy and Safety of Aldafermin, an Engineered FGF19 Analog, in a Randomized, Double-Blind, Placebo-Controlled Trial of Patients With Nonalcoholic Steatohepatitis. Gastroenterology 2021;160(1):219–31 e1. doi: 10.1053/j.gastro.2020.08.004 [published Online First: 2020/08/12] [DOI] [PubMed] [Google Scholar]
- 31.Loomba R, Lawitz E, Mantry PS, et al. The ASK1 inhibitor selonsertib in patients with nonalcoholic steatohepatitis: A randomized, phase 2 trial. Hepatology 2018;67(2):549–59. doi: 10.1002/hep.29514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McPherson S, Wilkinson N, Tiniakos D, et al. A randomised controlled trial of losartan as an anti-fibrotic agent in non-alcoholic steatohepatitis. PLoS ONE 2017;12(4):e0175717. doi: 10.1371/journal.pone.0175717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Navarro VJ, Belle SH, D’Amato M, et al. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: A randomized, double-blind, placebo controlled trial. PLoS ONE 2019;14(9):e0221683. doi: 10.1371/journal.pone.0221683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet 2015;385(9972):956–65. doi: 10.1016/S0140-6736(14)61933-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newsome PN, Buchholtz K, Cusi K, et al. A Placebo-Controlled Trial of Subcutaneous Semaglutide in Nonalcoholic Steatohepatitis. N Engl J Med 2020. doi: 10.1056/NEJMoa2028395 [published Online First: 2020/11/14] [DOI] [PubMed] [Google Scholar]
- 36.Ratziu V, Sanyal A, Harrison SA, et al. Cenicriviroc Treatment for Adults with Nonalcoholic Steatohepatitis and Fibrosis: Final Analysis of the Phase 2b CENTAUR Study. Hepatology 2020;13:13. doi: 10.1002/hep.31108 [DOI] [PubMed] [Google Scholar]
- 37.Younossi ZM, Ratziu V, Loomba R, et al. Obeticholic acid for the treatment of non-alcoholic steatohepatitis: interim analysis from a multicentre, randomised, placebo-controlled phase 3 trial. Lancet 2019;394(10215):2184–96. doi: 10.1016/S0140-6736(19)33041-7 [DOI] [PubMed] [Google Scholar]
- 38.Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology 2011;54(5):1610–9. doi: 10.1002/hep.24544 [published Online First: 2011/07/13] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Friedman SL, Ratziu V, Harrison SA, et al. A randomized, placebo-controlled trial of cenicriviroc for treatment of nonalcoholic steatohepatitis with fibrosis. Hepatology 2018;67(5):1754–67. doi: 10.1002/hep.29477 [published Online First: 2017/08/24] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrison SA, Abdelmalek MF, Caldwell S, et al. Simtuzumab Is Ineffective for Patients With Bridging Fibrosis or Compensated Cirrhosis Caused by Nonalcoholic Steatohepatitis. Gastroenterology 2018;155(4):1140–53. doi: 10.1053/j.gastro.2018.07.006 [DOI] [PubMed] [Google Scholar]
- 41.Harrison SA, Alkhouri N, Davison BA, et al. Insulin sensitizer MSDC-0602K in non-alcoholic steatohepatitis: A randomized, double-blind, placebo-controlled phase IIb study. Journal of Hepatology 2020;72(4):613–26. doi: 10.1016/j.jhep.2019.10.023 [DOI] [PubMed] [Google Scholar]
- 42.Harrison SA, Wong VWS, Okanoue T, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. Journal of Hepatology 2020. doi: 10.1016/j.jhep.2020.02.027 [DOI] [PubMed] [Google Scholar]
- 43.Ratziu V, Harrison SA, Francque S, et al. Elafibranor, an Agonist of the Peroxisome Proliferator-Activated Receptor-alpha and -delta, Induces Resolution of Nonalcoholic Steatohepatitis Without Fibrosis Worsening. Gastroenterology 2016;150(5):1147–59.e5. doi: 10.1053/j.gastro.2016.01.038 [DOI] [PubMed] [Google Scholar]
- 44.Ratziu V, Ladron-De-Guevara L, Safadi R, et al. One-year results of the global phase 2b randomized placebo-controlled arrest trial of aramchol, a stearoyl CoA desaturase inhibitor, in patients with nash. Hepatology 2018;68 (1 Supplement 1):1448A–49A.29604231 [Google Scholar]
- 45.Sven MF, Pierre B, Manal FA, et al. A randomised, double-blind, placebo-controlled, multi-centre, dose-range, proof-of-concept, 24-week treatment study of lanifibranor in adult subjects with non-alcoholic steatohepatitis: Design of the NATIVE study. Contemp Clin Trials 2020;98:106170. doi: 10.1016/j.cct.2020.106170 [published Online First: 2020/10/11] [DOI] [PubMed] [Google Scholar]
- 46.Bril F, Biernacki DM, Kalavalapalli S, et al. Role of Vitamin E for Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes: A Randomized Controlled Trial. Diabetes Care 2019;42(8):1481–88. doi: 10.2337/dc19-0167 [DOI] [PubMed] [Google Scholar]
- 47.Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med 2010;362(18):1675–85. doi: 10.1056/NEJMoa0907929 [published Online First: 2010/04/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Loomba R, Noureddin M, Kowdley KV, et al. Combination therapies including cilofexor and firsocostat for bridging fibrosis and cirrhosis due to NASH. Hepatology 2020. doi: 10.1002/hep.31622 [published Online First: 2020/11/11] [DOI] [PubMed] [Google Scholar]
- 49.Cipriani A, Higgins JP, Geddes JR, et al. Conceptual and technical challenges in network meta-analysis. Ann Intern Med 2013;159(2):130–7. doi: 10.7326/0003-4819-159-2-201307160-00008 [published Online First: 2013/07/17] [DOI] [PubMed] [Google Scholar]
- 50.Kibret T, Richer D, Beyene J. Bias in identification of the best treatment in a Bayesian network meta-analysis for binary outcome: a simulation study. Clin Epidemiol 2014;6:451–60. doi: 10.2147/CLEP.S69660 [published Online First: 2014/12/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.