Abstract
Anxious adults show changes in smell function that are consistent with a durable shift in sensitivity toward particular odorants and away from others. Little is known regarding the development of these changes, including whether they exist in youth, are stable during the transition from childhood to adolescence, and whether odorant properties (e.g. trigeminal features, hedonic valence) affect anxiety-related differences in detection. To address this, we measured smell detection thresholds to phenyl ethyl alanine (PEA), a rose-like odorant with little trigeminal properties, and guaiacol (GUA), a smoke-like odorant with high trigeminal properties. These thresholds were measured at baseline and after an acute stress challenge, the Trier Social Stress Tests, in 131 healthy youth (in 4th, 7th, and 10th grades, age 9–16 years) that reported normal to elevated levels of anxiety. At baseline, high anxious youth exhibited heightened sensitivity to GUA coupled with reduced sensitivity to PEA, as well as a further exaggeration of this bias with acute stress. Importantly, sex, age, and hedonic valence moderated the relationship between trait anxiety and sensitivity to both odorants. Smell function and its aberrations are often overlooked in the literature on biomarkers of stress and anxiety. Taken together with the extant literature, these findings suggest that greater attention is warranted to characterize potential novel olfactory therapeutic targets—across the lifespan.
Keywords: olfaction, trigeminal, development, threat, Trier Social Stress Test
Introduction
Olfaction serves a primary role in the detection and signaling of potential danger in our food, environment, and social interactions (Stevenson 2010). Sense of smell functions, in part, as a threat detector. Accordingly, heightened olfactory function during periods when increased threat detection is required, such as when perceived threat, emotional stress, or fear is high, would likely promote survival. A number of animal and human laboratory studies confirm the enhancing effects of threat on olfactory function, reporting that detection or discrimination of odorants improves with aversive conditioning (e.g. odor–shock pairings) (Fletcher and Wilson 2002; Li et al. 2008; Kass et al. 2013), and that odor identification is enhanced (Hoenen et al. 2017) and detection thresholds reduced (Pacharra et al. 2016) amidst acute psychosocial distress.
In the real world, the influence of trait or more sustained anxiety on human olfactory function are consistent with an enduring shift in odor sensitivity toward specific odorants and away from others. In posttraumatic stress disorder (PTSD), for example, both subjective and objective evidence suggests increased sensitivity toward potentially dangerous odorants (Cortese et al. 2015; Cortese et al. 2018). These particular fear–olfactory relationships are hypothesized to be driven by the close link in associative memory that exists between emotional trauma and the odors often present during those experiences (e.g. burning odors perceived while escaping from a house fire, body odors perceived during a sexual assault, etc.). Yet numerous disorders other than PTSD as well as anxiety-related personality traits—without etiology linked to specific contexts—have been associated with olfactory sensitivities as well (Croy et al. 2011; Schecklmann et al. 2013; Houghton et al. 2019). These relationships suggest that olfactory sensitivities are not necessarily grounded in associative learning. In addition, some suggest that additional factors are involved; for example, some have hypothesized that odor hedonics plays a critical role in threat-related changes in olfactory function, such that humans with anxious traits or disorders specifically process unpleasant odorants differently (La Buissonnière-Ariza et al. 2013). Other human data, as well as work in laboratory animals (Galliot et al. 2012), suggest that trigeminal properties of odorants are the key to contextual threat and anxiety-related odor sensitivities. Our own work in healthy adults showed a significant association between increased anxiety sensitivity (i.e. fear of experiencing interoceptive and cognitive symptoms of anxiety) and increased sensitivity for guaiacol (GUA), a smoke-like odorant with high trigeminal properties (Houghton et al. 2019). Along the same lines, an increased ability to detect carbon dioxide (CO2), a pure trigeminal stimulus, was demonstrated in healthy adults with elevated neuroticism, a trait marked by tendencies to experience negative emotions like fear and anxiety (Croy et al. 2011). Given that the intranasal trigeminal system functions, in part, to detect irritants and potentially harmful airborne chemicals in the environment, increased sensitivity to odorants with high trigeminal properties is consistent with numerous other safety behaviors that are enhanced in those with anxiety-related traits and disorders.
While fear and anxiety have enhancing effects on certain elements of olfactory function, evidence from our, and other, laboratories suggests that they may simultaneously relate to certain deficits in olfactory structure and function (Vasterling et al. 2000; Dileo et al. 2008; Croy et al. 2013; Cortese et al. 2015; Wilkerson et al. 2018). Combat Veterans with PTSD, compared with healthy combat Veterans, have less gray matter volume of both primary and secondary olfactory cortices (Cortese et al. 2015), impaired odor identification ability, and reduced detection sensitivity to a specific odorant called phenyl ethyl alcohol (PEA) (Wilkerson et al. 2018). Interestingly PEA, a rose-like odorant widely used to assess olfactory function, is relatively selective to the olfactory nerve/circuit, having little, if any, action on the trigeminal nerve/brain circuit (Doty et al. 1978). Thus, combining the results, fear and anxiety may have opposite relationships on the olfactory and intranasal trigeminal systems, such that sensitivity to PEA may be reduced and GUA enhanced in people with anxious traits or disorders.
As olfactory sensitivities and deficits become increasingly recognized across anxiety-related traits and disorders, a gap in knowledge exists regarding how these seemingly incongruent findings coexist. A better understanding of how the olfactory system modulates different odorants in response to stress/anxiety, and how specific odor factors such as hedonic valence and trigeminal properties influence the stress/anxiety–olfactory relationship is needed. Additionally, given that the predominance of evidence for stress/anxiety-related odor sensitivities has been obtained from adults, little is known regarding development of this relationship, including whether these stress- and anxiety-related olfactory sensitivities emerge during childhood and adolescence, or if they represent predispositional factors. Thus, this study aimed to test the hypothesis that both trait-level anxiety and the experience of acute stress associate with shifts in sensitivity toward the intranasal trigeminal “threat” system and away from the olfactory system. Hypothesis testing was achieved by determining how severity of trait anxiety and Trier Social Stress Test (TSST)-related acute stress influence the detection sensitivity of PEA and GUA, 2 odorants with different trigeminal and hedonic properties. The main study hypotheses included (i) that elevated self-reported anxiety severity would associate with increased sensitivity to GUA, the odorant with greater trigeminal properties, compared with PEA, the odorant with little to no trigeminal properties, and (ii) that the baseline shift in sensitivity toward GUA and away from PEA would be further broadened by acute stress induction, the TSST. Additionally, our novel focus on children and adolescents allowed us to explore the emergence of these stress/anxiety–olfactory interactions.
Materials and methods
Participants
Participants for the current olfactory study were recruited from the larger CHArleston Resiliency Monitoring (CHARM) study, a prospective study of anxiety risk and resilience in a large community sample (N = 360) of typically developing youth that was ongoing at the Medical University of South Carolina (MUSC). Youth enrolled into CHARM were recruited as 3rd, 6th, or 9th graders and followed for 2 years. The olfactory substudy recruited participants from CHARM at their 1-year follow-up timepoint. Thus, all participants in the present investigation were either 4th, 7th, or 10th graders at the time of testing. Exclusion criteria for the parent study (CHARM) were limited, and included (i) non-English speaking, (ii) caregiver unwilling to participate, (iii) symptoms of psychosis, (iv) developmental delay (e.g. IQ < 85), (v) cognitive impairment, and (vi) significant functional impairment that would preclude the child from successfully completing study procedures, which also included structural and functional MRI. An additional exclusion criterion was added for the olfactory substudy that included problems with nose/sense of smell (e.g. recent upper respiratory infection, chronic rhinosinusitis, polyps, head injury, etc.). In addition, participants were given the ability to opt out of the olfactory substudy but continue with other study procedures associated with CHARM. Signed written informed consent, approved by the MUSC Institutional Review Board (IRB), was obtained prior to participation.
Measures
Odorant detection.
Thresholds for both PEA, a rose-like scent with low trigeminal properties, and guaiacol (GUA), a smoke-like scent with high trigeminal properties, were obtained with 2 versions of the Snap and Sniff Threshold Test (Sensonics International, Haddon Heights, NJ, USA). Each contained a series of wands with decreasing concentration of odorant (PEA or GUA) that ranged from the most intense (10−2) to the least intense (10−9) concentration. In a single staircase method with forced choices, a wand containing a given concentration of odorant was presented under the nose in rapid succession with an odorless wand. Study participants made a choice as to which wand had the stronger smell. Subsequent presentation of odorant (higher or lower concentration) against odorless was dependent on a correct or incorrect response for each trial. This method was repeated until 7 reversals (up and down the staircase) were made. Detection threshold was determined by the mean of the last 4 reversals (Pierce et al. 1996; Doty 2009). Each administration of the Snap and Sniff Threshold Test took approximately 5–10 min. In addition to threshold scores, hedonic value of PEA and GUA was obtained by asking participants whether they considered the odorant “pleasant,” “unpleasant,” or “neutral.”
Multidimensional Anxiety Scale for Children, 2nd Edition.
Overall anxiety severity was determined via the Multidimensional Anxiety Scale for Children—2nd Edition (MASC-2), a 50-item self-report instrument that assessed emotional, physical, cognitive, and behavioral symptoms of anxiety (March 2013). Participants responded using a Likert scale ranging from 0 (never true about me) to 4 (often true about me). Higher scores reflected an increased severity of trait or enduring anxiety. A standardized cutoff of 65 that denotes elevated anxiety on the MASC-2 separated those that endorsed low anxiety severity (LAS) from those with high anxiety severity (HAS). Good internal consistency (a coefficient alpha of 0.92 for the self-reported total score), test–retest reliability (all correlations > 0.80; P < 0.001), and strong convergent validity with other published measures of anxiety symptoms have been previously established (March et al. 1997; March and Sullivan 1999).
Subjective stress.
Subjective stress was obtained using a 100-mm visual analog scale with the prompt “how stressed are you feeling right now?” and anchor points of 0 = “not at all” to 100 = “extremely.”
Trier Social Stressor Test
The developmentally adapted Trier Social Stressor Task (TSST) is a well-validated psychosocial stress induction paradigm used in children and adolescents to reliably activate physiological and emotional distress (Kirschbaum et al. 1993; Calhoun et al. 2014). Briefly, the TSST requires a participant to quickly (1-min) prepare and present a 3-min speech on why the individual would be a good candidate to join a reality TV show about friendship. A confederate monitored the speech, but provided no verbal or facial feedback except to prompt the participant to continue talking if the individual stopped prior to the end of the 3-min time period.
Procedure
To examine changes in odor sensitivity caused by psychosocial stress induction, thresholds for PEA and GUA were determined at baseline (prior to the TSST) and follow-up (after the TSST). Order of testing for the 2 odorants was counterbalanced, so that half of all participants were tested with PEA followed by GUA, and half were tested with GUA followed by PEA. Participants completed the MASC-2 after completing the TSST and post-TSST odor threshold measurement. Given the extensive literature in both humans and laboratory animals on the process of social buffering and the ability of a caregiver to mitigate the negative effects of stress (Nachmias et al. 1996; Sanchez et al. 2015), it is important to note that caregivers were not present during any study procedures.
Statistical analysis
SPSS Version 27 and Hayes’ PROCESS toolbox (Hayes 2013) was utilized for all analyses. Potential differences in demographics across the LAS and HAS anxiety groups were assessed with chi-square and univariate ANOVAs. A bivariate split of the data for the change in subjective stress (follow-up minus baseline) separated those who endorsed lower and higher TSST-related subjective stress. A mixed-factors ANOVA was used to determine the effects of anxiety on acute stress (TSST)-related changes in olfactory function. Within-subject factors included Odor (PEA versus GUA) and Time (baseline versus follow-up). Between-subject factors included sex, age group (4th, 7th, and 10th grades), and anxiety (LAS, HAS). Additional univariate ANOVAs and planned comparisons (paired samples t-tests or Wilcoxon signed ranks tests for categorical variables) were run separately to further explore significant main effects and interactions from the main analysis.
To judge the credibility of all identified significant differences using the “frequentist” approach, we reanalyzed planned contrasts with the corresponding Bayesian independent-samples and matched-pairs counterparts, estimating diffuse prior distributions without assuming equal variances. To quantify significance of evidence for either the Null hypothesis (H0: equal means of the samples) or the alternative hypothesis (Ha: unequal means of the samples), we calculated a Bayes factor (BF) for each comparison using Rouder’s method (Rouder et al. 2009). Commonly used thresholds to define weighted evidence of a hypothesis were used to establish evidence for Ha over H0: BF < 0.01 denoted “extreme evidence,” BF 0.01–0.033 denoted very strong evidence, BF 0.033–0.1 denoted “strong evidence,” BF 0.1–0.33 denoted “moderate evidence,” BF 0.33–1 denoted “anecdotal evidence,” and BF ≥ 1 denoted “no evidence” and H0 cannot be rejected. Evidence for the null hypothesis H0 was assessed in a similar way using the reciprocal thresholds: BF ≤ 1 (“no evidence”), BF 1–3 (“anecdotal evidence”), BF 3–10 (“moderate evidence”), BF 10–30 (“strong evidence”), BF 30–100 (“very strong evidence”), and BF > 100 (“extreme evidence”).
Although ≥65 is established for elevated anxiety on the MASC-2, our data showed that 37.4% (N = 49) of the study subjects reported scores between 55 and 75, i.e., close to the standardized cutoff value separating individuals of low and high anxiety. Therefore, to relax dichotomization bias, we also used the continuous total score on the MASC-2 as predictor for odor sensitivity. We estimated the corresponding slopes and their credibility by applying classical and Bayesian linear regression (BLR) analysis. In the latter was used the least-informative reference-prior, based only on model and available data, and the nonparametric Jeffreys–Zellner–Siow method to compute a BF. While BFs in BLR are traditionally implemented reciprocal to BFs in t-tests, we aimed to reduce confusion by defining BF values from BLR analyses in the same direction as t-test BFs, i.e. ≤1 indicated evidence for Ha (a good fit of the linear model) and >1 indicated evidence for H0 (intercept model, no linear change).
Finally, we assessed whether Sex, Age, and Odor Hedonics moderated the significant relationships shown between Anxiety Severity and Odor Detection Sensitivity (i.e. GUA at baseline and PEA and follow-up). First, for both Age and Odor Hedonics, we utilized a (simple) moderation analysis without including Sex as a second moderator. We subsequently differentiated by Sex using a moderated moderation model where Sex (potentially) moderated the moderation of either Age or Odor Hedonics. We also considered a double-moderator model where Sex and either Age or Odor Hedonics moderated the relationship between Anxiety Severity and Odor Detection Threshold independently. However, this model proved inferior to the Sex-moderated moderation model. To ensure reliable and heteroscedasticity-robust inference, we used Davidson–MacKinnon’s HC3 estimator of the regression coefficients’ covariance matrix in all moderation analyses (Hayes and Cai 2007). For visualization purposes, we fitted (multiple) regression models with no interaction (Fig. 2), age-by-anxiety (Fig. 3), and hedonics-by-anxiety (Fig. 4) interaction terms to the data and derived corresponding 95% confidence intervals. Age plots demonstrate the results of statistical tests that differentiated the youth according to “mean age minus one standard deviation” (youngest), “mean age” (middle), and “mean age plus one standard deviation” (oldest). In effect, this method split the youth also by grade level (4th, 7th, and 10th), given the tight association between age and grade. All reported P-values stemmed from 2-sided testing.
Fig. 2.
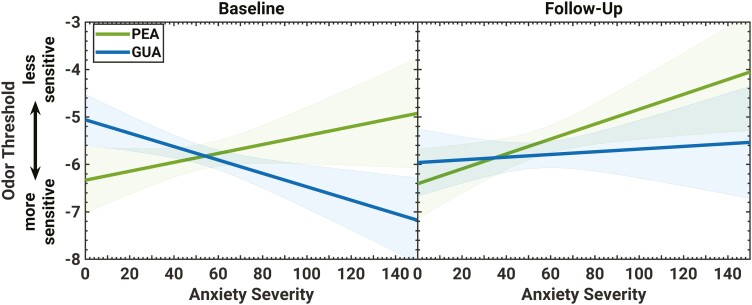
The linear relationship between odor threshold and anxiety severity measured by the MASC-2. Shaded areas indicate the 95% confidence interval (CI) of the prediction. Note that the regression lines’ CI naturally takes its minimum at the mean of anxiety severity and increases toward the scale’s margins. Adding further experimental data with comparable statistical properties, one can expect that 95% of the newly obtained regression lines will lie within the shaded area. Only the linear relationships between anxiety and GUA at baseline and anxiety and PEA at follow-up had a statistically significant slope different from zero (no change with MASC-2).
Fig. 3.
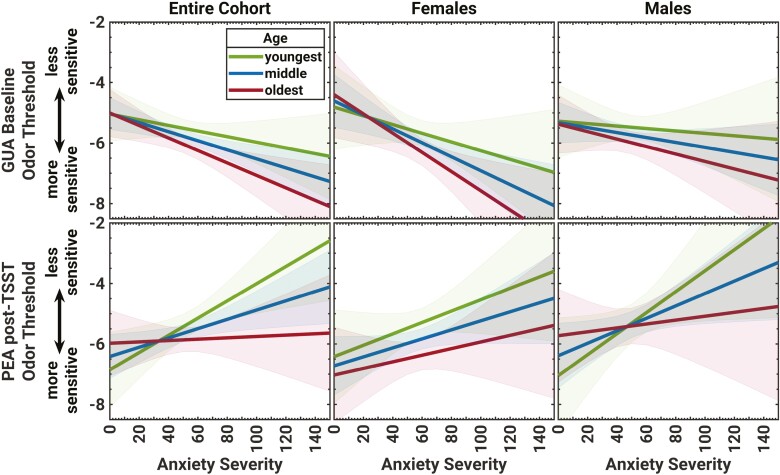
The moderation of the linear relationship between anxiety severity score (MASC-2) and baseline odor sensitivity to GUA by age (top) and post-TSST sensitivity to PEA by age (bottom). The predicted linear relationship between odor threshold and anxiety severity is separated for the 3 levels of age, as well as sex. Shaded areas indicate the 95% confidence interval (CI) of the prediction. Age and sex moderated the linear relationships between anxiety severity and odor sensitivity to GUA at baseline, with significant effects in the 7th and 10th grade females, and to PEA at follow-up, with significant effects in the youngest males (4th grade).
Fig. 4.
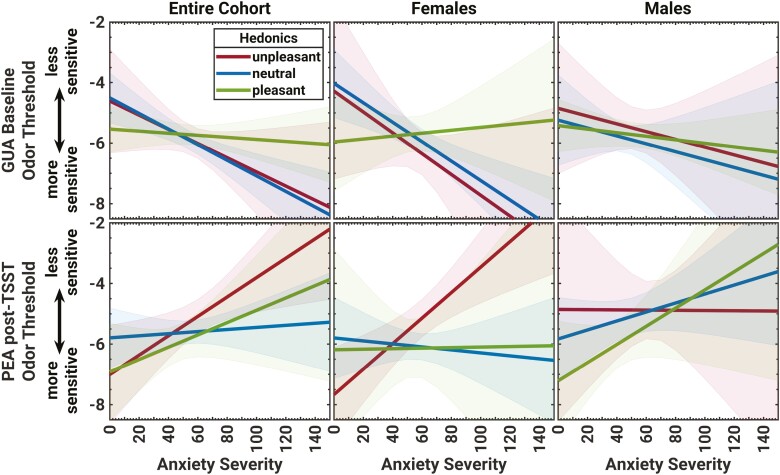
How odor hedonics moderated the linear relationship between anxiety severity (MASC-2) and odor sensitivity to GUA at baseline and PEA after the stress induction. Graphs depict the predicted linear relationship between odor threshold and anxiety severity, separately for the 3 levels of hedonics, and by sex. Shaded areas indicate the 95% confidence interval (CI) of the prediction. Hedonics and sex moderated the linear relationships between anxiety severity and odor sensitivity, with significant effects for the females that judged GUA and PEA to be neutral and unpleasant, respectively.
Results
Participant characteristics
As part of the larger CHARM study, participants underwent a full Diagnostic and Statistical Manual, 5th edition (DSM-V) (American Psychiatric Association 2013) assessment by a trained clinician. Thirteen of the youth who completed the olfactory substudy met criteria for a DSM-V disorder and were excluded from these analyses. Thus, the present CHARM subsample included 131 mentally healthy youth (68 male/63 female) with an average age of 12.4 ± 2.4 (range 9–16) years. The subsample was 64% White, 31% Black, 3% multiracial, and 2% other, with 7% identifying as Hispanic/LatinX. Sixty-six percent of the subsample was living in the home with both parents. Annual household income of the subsample broke down as follows: 15% earned <$20k/year, 18% earned $20–60k/year, and 67% earned >$60k/year. Anxiety severity measured via the MASC-2 varied greatly (M = 54.4 ± 22.1, range 3–150). Groups split by high and low anxiety severity (HAS vs. LAS) were assessed for differences in demographic and behavioral characteristics (see Table 1). While previous evidence in youth suggests that sex (Chopra et al. 2008; Monnery-Patris et al. 2009), age (Koelega 1994), and body mass index (Herz et al. 2020) can each influence smell function, there were no significant, or trending, HAS versus LAS group differences for these factors, as well as others including ethnicity, family living situation, household income, hedonic ratings for PEA or GUA, subjective stress at baseline, follow-up, or in the TSST-related change in subjective stress (all Ps > 0.1). A trending difference in the percentage of white youth in the high versus low anxiety group was noted (P = 0.08). As expected, MASC-2 total scores differed significantly between the HAS and LAS groups (P < 0.001, see Table 1).
Table 1.
Demographic characteristics of the entire sample as well as groups obtained from dichotomizing anxiety severity (LAS: MASC-2 < 65, HAS: MASC-2 ≥ 65).
| Cohort | LAS | HAS | ||||
|---|---|---|---|---|---|---|
| (N = 131) | (N = 89) | (N = 42) | χ2 or t | P | ||
| Gender–male | N (%) | 68 (51.9) | 50 (56.2) | 18 (42.9) | 2.03 | ns |
| Race–white | 82 (62.6) | 51 (57.3) | 31 (73.8) | 5.18 | 0.075 | |
| Ethnicity–non-Hispanic | 119 (90.8) | 80 (89.9) | 39 (92.8) | 0.43 | ns | |
| Home–living with both parents | 83 (63.4) | 52 (58.4) | 31 (73.8) | 2.56 | ns | |
| Household income–>$80k/yr | 78 (59.5) | 52 (58.4) | 26 (61.9) | 0.16 | ns | |
| PEA Hedonics–unpleasant | 32 (24.4) | 20 (22.5) | 12 (28.6) | 3.79 | ns | |
| GUA Hedonics–unpleasant | 23 (17.6) | 15 (16.9) | 8 (19.0) | 1.57 | ns | |
| Age (years) | mean ± SD | 12.4 ± 2.4 | 12.5 ± 2.3 | 12.4 ± 2.4 | 0.21 | ns |
| BMI | 22.4 ± 6.3 | 22.6 ± 6.6 | 21.9 ± 5.5 | 0.54 | ns | |
| MASC-2 (total score) | 54.4 ± 22.2 | 43.2 ± 13.6 | 78.1 ± 17.7 | 12.40 | <0.001 | |
| Subjective stress (baseline) | 5.1 ± 13.4 | 4.1 ± 10.2 | 7.5 ± 18.8 | 1.28 | ns | |
| Subjective stress (follow-up) | 57.4 ± 31.0 | 54.8 ± 29.9 | 63.3 ± 33.1 | 1.39 | ns | |
| Change in subjective stress | 52.3 ± 34.6 | 50.7 ± 31.6 | 55.8 ± 40.7 | 0.73 | ns |
BMI, body mass index; GUA, guaiacol; LAS, low anxiety severity; HAS, high anxiety severity; MASC-2, Multidimensional Anxiety Scale for Children, 2nd Edition; PEA, phenyl ethyl alcohol.
ns = P > 0.1.
Subjective stress was significantly increased by the TSST
A Time-by-Sex-by-Age-by-Anxiety repeated-measures mixed ANOVA indicated a significant main effect of Time (F(1,106) = 199.42, P < 0.001, ηp2 = 0.65), but no other main effects or interactions on subjective stress. Further analysis confirmed that the TSST caused a significant, with extreme evidence, increase in subjective stress in the overall cohort (t(118) = 16.41, P < 0.001; BF < 0.001), as well as in each of the groups (LAS: t(80) = 14.44, P < 0.001, BF < 0.001 and HAS: t(36) = 8.34, P < 0.001, BF < 0.001). Table 1 shows the subjective stress results for the entire study cohort and split across the LAS and HAS groups, showing no significant, or trending, group differences (all Ps > 0.1, all BFs > 2.5).
Detection sensitivity to GUA and PEA in the overall cohort
An Odor-by-Time-by-Subjective Stress mixed ANOVA demonstrated a significant Odor-by-Time (F(1,117) = 5.38, P = 0.02, ηp2 = .04), but no other significant, or trending, main or interactive effects (all P > 0.1). The Odor-by-Time interaction in the overall cohort seemed to be driven by a significant pre- to post-TSST reduction in sensitivity to PEA (Baseline: MPEA = −5.82 ± 1.51, range −9.0 to −2.1, Follow-up: MPEA = −5.55 ± 1.63, range −8.9 to −2.0; t(130) = 2.15, P = 0.03). However, the corresponding Bayesian approach and BF of 1.51 suggested anecdotal evidence for the null hypothesis, meaning that the significance of this contrast was questionable. No differences were noted for GUA (Baseline: MGUA = −5.83 ± 1.20, range −9.0 to −2.8; Follow-up: MGUA = −5.81 ± 1.53, range −9.0 to −2.0; t(130) = 0.15, P = 0.88; BF = 14.28). Thus, the overall cohort showed negligible effects on detection sensitivity, including no effects of the TSST on sensitivity to either PEA or GUA. Notably, this was the case even when accounting for higher and lower subjective distress secondary to the TSST.
Anxiety severity had significant associations with detection sensitivity to GUA and PEA
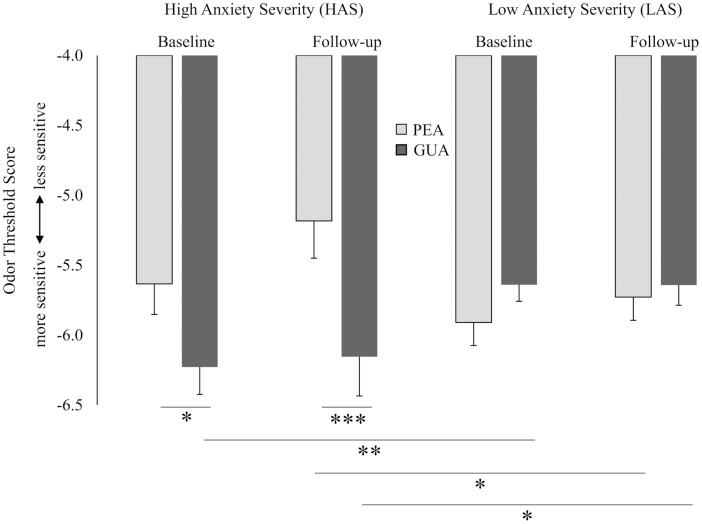
An Odor-by-Time-by-Anxiety mixed ANOVA indicated a significant Odor-by-Anxiety interaction (collapsed across Time; F(1,129) = 10.72, P = 0.001, ηp2 = 0.08), as well as significant Odor-by-Anxiety interactions at both time points (baseline: F(1,129) = 5.77, P = 0.02, ηp2 = 0.04; follow-up: F(1,129) = 10.48, P = 0.002, ηp2 = 0.08). These results suggested that anxiety-related odor sensitivity was specific to the odorants. Further analysis with paired samples t-tests indicated that the HAS group demonstrated a trending, but with anecdotal evidence for the null hypothesis, heightened sensitivity to GUA compared with PEA at baseline (t(41) = 1.98, P = 0.06; BF = 1.34). After acute stress induction (i.e. TSST), however, a significant and extremely evident difference in sensitivity between the odorants was noted (t(41) = 4.08, P < 0.001; BF = 0.009; see Fig. 1). In contrast, the LAS group did not show differences in sensitivity between PEA and GUA at baseline or follow-up (t(88) = 1.34, P = 0.19, BF = 4.99; t(88) = 0.44, P = 0.66, BF = 10.86, respectively). Additional analyses also confirmed group differences between HAS and LAS, with a significant and moderately evident HAS-related heightened sensitivity to GUA at the baseline (t(129) = 2.69, P = 0.008; BF = 0.24), and a trending, but with anecdotal evidence for the null hypothesis, reduction in sensitivity to PEA at the follow-up time point (t(129) = 1.80, P = 0.07; BF = 1.52; see Fig. 1).
Fig. 1.
Youth with higher compared with lower MASC-2-determined anxiety severity (HAS vs. LAS) demonstrated significantly increased sensitivity for GUA, the smoke-like odorant with high trigeminal properties, compared with sensitivity for PEA, the rose-like, relatively pure olfactory odorant at both pre- and post-TSST. The LAS youth did not demonstrate differential sensitivity to the odorants at either time point. Error bars represent standard error of the mean. ∗P ≤ 0.1, ∗∗P < 0.05, ∗∗∗P < 0.01 (all 2-tailed test of significance).
Although an established cutoff score on the MASC-2 was used to differentiate subjects with low and elevated anxiety (i.e. LAS: MASC-2 < 65, HAS: MASC-2 ≥ 65), our child and adolescent cohort did not show a pronounced bivariate separation in scores. Therefore, linear regression models to quantify the direct effects of anxiety severity on sensitivity to PEA and GUA at baseline and follow-up were also used. Consistent with the group results, a significant and moderately evident relationship between anxiety severity and sensitivity to GUA at baseline was noted (B = −0.01, R2 = 0.07, F(1,129) = 9.45, P = 0.003; BF = 0.16). For every 10-point increase in MASC-2 score, sensitivity to GUA was enhanced (i.e. odor threshold score decreased) by 0.14 half-log steps (see Fig. 2, left). For PEA, results showed a significant relationship between anxiety severity and odor sensitivity at the follow-up time point (B = 0.02, R2 = 0.05, F(1,129) = 6.14, P = 0.02; BF = 0.79, anecdotal evidence for Ha). For each 10-point increase in MASC-2 score, threshold score for PEA increased (sensitivity decreased) by 0.16 half-log steps (see Fig. 2, right).
Age, sex, and hedonic valence moderated the relationship between anxiety and sensitivity to GUA at baseline
The simple moderation model for Age in the relationship between Anxiety and GUA Sensitivity at baseline was significant (full model: F(3,127) = 4.79, P = 0.003, R2 = 0.13). Although systematic moderation was not demonstrated, i.e. no interaction between Age and Anxiety Severity (F(1,127) = .86, P = 0.36, R2chg. = 0.01), a significantly stronger sensitivity-enhancing effect of Anxiety on GUA Sensitivity in the 7th and 10th graders compared with the 4th graders was observed (at 12.4 years: t(127) = 2.66, P = 0.009, B = −0.02; at 14.8 years: t(127) = 2.40, P = 0.02, B = −0.02; see Fig. 3, top-left). The Sex-moderated moderation by Age model was also significant (full model: F(7,123) = 2.45, P = 0.02, R2 = 0.16), with no Anxiety-by-Sex, or Age-by-Sex moderation effects noted (all Ps > 0.7). Further analysis indicated that the previously reported stronger effect of Anxiety on GUA Sensitivity in 7th and 10th graders was driven by the females (at 12.4 years: t(123) = 2.93, P = 0.004, B = −0.023; at 14.8 years: t(123) = 2.12, P = 0.04, B = 0.03; see Fig. 3, top-middle and top-right).
The simple moderation model for GUA Hedonics in the relationship between Anxiety and GUA Sensitivity at baseline was significant (F(5,125) = 3.16, P = 0.01, R2 = 0.12), with a significant Anxiety-by-GUA Hedonics interaction, i.e. moderation effect (F(2,125) = 3.46, P = 0.034, R2chg. = 0.04). The conditional effect of Anxiety on GUA Sensitivity was observed in the youth who rated GUA to be neutral (t(125) = 3.58, P < 0.001, B = −0.03; see Fig. 4, top-left). The Sex-moderated moderation by GUA Hedonics model was also significant (full model: F(11,119) = 2.04, P = 0.03, R2 = 0.15), but did not indicate systematic Anxiety-by-Sex, GUA Hedonics-by-Sex, or Anxiety-by-GUA Hedonics-by-Sex interactions (all Ps > 0.5). Nevertheless, evaluating the conditional effects of the focal predictor Anxiety on GUA Sensitivity at baseline indicated that the moderating effect of GUA Hedonics was mainly driven by females who rated GUA to be neutral (t(125) = 4.04, P < 0.001, B = −0.03; see Fig. 4, top-middle and top-right).
Age, sex, and hedonic valence moderated the relationship between anxiety and sensitivity to PEA at follow-up
The simple moderation model for Age in the relationship between Anxiety and post-TSST PEA Sensitivity was significant (full model: F(3,127) = 3.86, P = 0.01, R2 = 0.09). Although systematic moderation was not demonstrated, i.e. no interaction between Age-by-Anxiety Severity (F(1,127) = 2.52, P = 0.12, R2chg. = 0.02), there was a significantly stronger blunting effect of Anxiety on PEA Sensitivity at follow-up in the younger youth (t(127) = 2.54, P = 0.01, B = 0.03; see Fig. 3, bottom-left). A subsequent Sex-moderated moderation analysis was also significant (full model: F(7,123) = 2.94, P = 0.007, R2 = 0.15), but with no Anxiety-by-Sex, or Age-by-Sex moderation effects (all Ps > 0.1), and revealed that reduced post-TSST PEA Sensitivity was driven by the young males (t(123) = 2.15, P = 0.03, B = 0.04; see Fig. 3, bottom-middle and bottom-right).
The simple moderation model for PEA Hedonics in the relationship between Anxiety and post-TSST PEA Sensitivity was significant (full model: F(5,125) = 3.33, P = 0.007, R2 = 0.10), with a strong Anxiety main effect (t(125) = 3.16, P = 0.002, B = 0.03), but no significant Anxiety-by-PEA-Hedonics interaction (F(2,125) = 1.25, P = 0.29, R2chg. = 0.03). The conditional effect of Anxiety on post-TSST PEA Sensitivity was observed in the youth who rated PEA to be unpleasant (t(125) = 3.16, P = 0.002, B = 0.03) compared with those who rated PEA to be neutral or pleasant; see Fig. 4, bottom-left. A significant Sex-moderated PEA-Hedonics-moderation model (full model: F(11,119) = 3.59, P < 0.001, R2 = 0.18) showed a significant Sex main effect (t(119) = 2.09, P = 0.04, B = −2.81), a significant Anxiety-by-Sex interaction, i.e. a significant moderation of the moderator PEA Hedonics-by-Sex (t(119) = 2.25, P = 0.03, B = 0.04), and a significant Anxiety-by-PEA-Hedonics-by-Sex interaction for the unpleasant vs. neutral contrast (t(119) = 2.14, P = 0.03, B = −0.06); the Anxiety-by-PEA Hedonics interaction was significant for Females (F(2,119) = 5.31, P = 0.006), but not Males (P > .1). Further examining the conditional effects of Anxiety on post-TSST PEA Sensitivity revealed that the significant Sex effect was mainly driven by the females who rated PEA to be unpleasant (t(119) = 4.55, P < 0.001, B = 0.04); see Fig. 4, bottom-middle and bottom-right.
Discussion
The current study examined the relationship between anxiety and olfactory function in youth by determining effects of trait anxiety on specific odor detection sensitivity at baseline and after an acute stress challenge. Results confirmed our predictions that anxiety has significant, and specific, effects on odor function. Furthermore, these findings show that acute stress-related changes in olfactory function could be easily obscured by failing to consider important variables, including trait anxiety, odorant properties, sex, and age.
Our main goal was to determine whether an anxiety–odor detection sensitivity relationship exists in children and adolescents and whether this association was based more in the trigeminal than the hedonic properties of the odorants. Results showed that temperamentally or sustained elevated anxiety was associated with a baseline and a TSST acute stress-related shift in specific odor sensitivity. As expected, and consistent with our previous results in adults (Houghton et al. 2019), the youth who endorsed elevated trait anxiety symptoms were more sensitive to GUA, the smoke-like odorant with high trigeminal properties, compared with PEA, the rose-like odorant with relatively low trigeminal properties. Moreover, this baseline disparity in sensitivity to GUA compared with PEA was further increased after the TSST acute stress challenge. In contrast to those with elevated anxiety, youth with low anxiety showed none of these association or differences in smell sensitivity.
In line with an attentional bias toward potential danger that is often demonstrated in people with anxiety- and fear-related disorders, trait anxiety may lead to a shift in processing from olfactory to trigeminal (a pain-related sensory pathway) with the purpose to selectively maintain vigilance toward potential danger signaling odors. In turn, affected individuals may lose sensitivity to odorants with low trigeminal properties, while at the same time becoming more sensitive to the potential danger-related aspects of odorants that possess greater trigeminal properties (Cortese et al. 2015). Given the significantly greater trigeminal properties of GUA (Doty et al. 1978), compared with PEA (Cometto-Muñiz and Cain 1990), the present findings are consistent with that notion. In fact, we have found that odors with high activation of the intranasal trigeminal pathway (e.g. smoke, household cleaning supplies, perfume, etc.) are often endorsed as problematic by adults with anxiety disorders. Many of these odors produce the classic intranasal trigeminal effect, a physical sensation in the nose such as stinging or heat that can be irritating or even painful. We hypothesize that this physical sensation in the nose may be sensitized in disorders that involve chronically elevated anxiety, and is likely a key factor in whether an anxiety-based shift in sensitivity exists. Also consistent with this hypothesis, and strengthening the idea that the anxiety–odor sensitivity relationship is driven mainly by the intranasal trigeminal and not the olfactory system per se, are neuroimaging results for burning odor elicited increased activation in somatosensory cortex, but not piriform (1° olfactory) cortex, in combat-related PTSD (Cortese et al. 2018).
While exploratory, the present results pertaining to age and sex suggest a developmental course for anxiety-related odor sensitivities, as age and sex moderated the relationship between anxiety severity and both baseline sensitivity to GUA and post-TSST sensitivity to PEA. Specifically, only the older, 7th and 10th grade, females with elevated trait anxiety demonstrated increased sensitivity to GUA at baseline. In fact, there was no relationship between anxiety and sensitivity to GUA in the male participants or in the youngest participants (4th grade), regardless of sex. In direct contrast to the results for GUA, it was the youngest male participants who showed the greatest association between anxiety and blunted sensitivity to PEA after the TSST acute stress challenge. Although we can only speculate on the mechanisms that underlie the moderating effects of age and sex on anxiety-related sensitivity to odors, current evidence suggests that they are likely more dependent on the biopsychosocial changes associated with puberty and stress and anxiety, than with general development of the sensory systems. In fact, the olfactory and intranasal trigeminal systems are fully developed prior to birth (Frie et al. 2018) and general functioning in youth over the age of 7 is comparable to that of adults (Hummel et al. 2007). On the other hand, adolescence (and puberty) is a critical time in development when interpersonal stressors become more prevalent (Arnett 1999), when significant shifts in the body’s stress response system (hypothalamic–pituitary–adrenal [HPA] axis) take place (Romeo 2010), and when a disparity (female > male) in clinical stress and anxiety emerges (Lewinsohn et al. 1998). Puberty is marked also by dramatic increases in sex hormones that have direct influence on the response of the HPA axis to chronic stress (Williamson et al. 2005; Romeo 2010). Given generally earlier pubertal development of females compared with males (Marshall and Tanner 1969), our results in the older females versus younger males are consistent with a relationship between anxiety-related sensitivity to odors and the hormonal changes associated with puberty and HPA axis function (Gur and Gur 2016). With additional evidence indicating that odor sensitivity can be modulated by a number of different hormones present in the naso-oropharynx (Martin et al. 2009), including circulating gonadal hormones and stress-related glucocorticoids (Kass et al. 2017; Meunier et al. 2020), our findings have particular relevance, but also underscore an unmet need, regarding the study of sex hormones and HPA axis function in relation to anxiety-based differences in smell sensitivity during this critical stage of development.
The results of the TSST acute stress challenge were somewhat different to what we expected. Instead of the TSST exacerbating an already increased sensitivity to GUA in anxious youth, results indicated that sensitivity to PEA was reduced in response to the acute stressor. While this effect was not predicted explicitly, the TSST-related reduction in sensitivity to PEA was consistent with our general hypothesis, that a further increase in disparity/shift in detection of GUA relative to PEA would be demonstrated in response to acute stress. Selective processing of smell, wherein detection is heightened for certain stimuli and at the same time reduced for others has been described as a function of odor-conditioned fear/threat (Åhs et al. 2013; Parma et al. 2015), but also by combat veterans with PTSD whose increased sensitivity to burning-related odors coincided with decreased sensitivity to many others, an effect that may not have been driven by learned associations (Cortese et al. 2015). While these findings demonstrate the ability of the olfactory and intranasal trigeminal systems to simultaneously shift processing toward behaviorally relevant stimuli and away from other less relevant stimuli, the mechanisms by which stress and anxiety influence more chronic shifts toward some, but not other, odors are not fully understood. Recent evidence (Taylor et al. 2020) that humans may be hard-wired to innately fear certain odors, as well as the role of cortisol in driving these fears, requires future study as a possible mechanism underlying the chronic anxiety–olfactory relationship. Interestingly, the relationship between anxiety and reduced sensitivity to PEA after the TSST seemed to be driven by the youngest males. This result, together with the findings for GUA, are consistent with a developing interactive process between the intranasal trigeminal and olfactory system that maintains sensitivity toward potentially harmful odorants across childhood development. That is, while the ability to directly upregulate intranasal trigeminal processing as a function of increased trait anxiety may be possible in older children, anxiety-related sensitivity to potentially harmful odorants may not be directly modulated by the intranasal trigeminal system in response to stress in younger children. Thus, in the case of younger children, maintenance or initiation of this trigeminal-olfactory shift can still occur through a reduced sensitivity to odorants with low-trigeminal properties (e.g. PEA).
Results for the hedonic valence of PEA and GUA and how it related to odor sensitivity demonstrated a much more complicated relationship than a simple anxiety-related increase in sensitivity to unpleasant odors. In fact, the youth who perceived PEA to be unpleasant showed the most TSST-related blunted sensitivity, while those who rated GUA to be neutral demonstrated the greatest trait anxiety-related baseline increase in sensitivity. Moreover, these effects of odor hedonics were demonstrated by the female, but not male, participants. Consistent with prior studies reporting that the perception of odor hedonics (i.e. preferences/aversions) is influenced by affective experience (Herz et al. 2004) including acute anxiety induction (Krusemark et al. 2013), the present results indicate a role for odor hedonic valence in anxiety-related odor sensitivity. However, the full extent of this role has yet to be fully examined.
One of the main limitations of this study was the use of just 2 odorants, GUA and PEA. To fully assess the role of odor properties in the anxiety–odor sensitivity relationship, a number of odorants with different trigeminal properties that range across hedonic valence are needed. Studies using numerous odorants are more challenging however, requiring careful consideration of factors including odor habituation and carry-over effects that could impact the accurate assessment of smell thresholds. Similarly, the present study utilized one type of acute stressor, the TSST. Given that different stressors, and stress in a social context in particular, can produce distinct neurobehavioral outcomes (Raineki et al. 2019), it is possible that the olfactory effects demonstrated in the present study related specifically to the social context of the TSST, and not to all types of stress. Thus, specificity of the stress-related effects and whether similar changes in odor sensitivity are produced by other stressor types is unclear. Further study with other stressors, including nonpsychosocial stressors, would be necessary to answer this question. Another limitation related to our inability to assess how puberty status may have influenced the anxiety-related shifts in smell function. While the recruitment strategy and longitudinal design of the parent study (i.e. CHARM) will enable the rigorous assessment of important developmental transitions and milestones, including pubertal status, the smaller size and cross-sectional nature of the present olfactory substudy did not allow for a meaningful analysis of puberty and was therefore not included in this report. And finally, an additional limitation relates to the measure of subjective stress utilized in the present study to confirm successful stress induction via the TSST. Although significantly increased in response to the acute stressor in the overall cohort as well as in both anxiety groups, subjective stress did not demonstrate a causal association with the post-TSST change in PEA. However, we as well as others have repeatedly observed discordance between objective threat-related responding and subjective distress and this paradoxical or discordant pattern is exacerbated as a function of increased trait anxiety (McTeague et al. 2010; McTeague and Lang 2012). Moreover, the fact that subjective stress and blunted PEA sensitivity did not directly relate to one another does not preclude the possibility of an additional variable, perhaps a biological factor like cortisol, driving the TSST-related change in olfactory function.
The current study has implications for vulnerability to and early identification of anxiety and stress-related psychopathology in youth. If future study confirms our preliminary findings of a developmental shift in anxiety-related olfactory and intranasal trigeminal function and that this shift is predictive of future problems, then specific odor sensitivities may have utility in predicting the potential emergence of clinical anxiety or stress-related disorders in this age group. The present results have implications for the treatment of individuals with anxiety and stress-related disorders as well. While anxiety and stress-related disorders are heterogeneous conditions, many with these diagnoses experience chronic hyperarousal as well as sensitivity to certain odors (Houghton et al. 2020). Perhaps exposure to odors with higher trigeminal action could result in reduced physiological hyper-reactivity to such odors and thus lower apprehension and avoidance behaviors. Although the current study was not conducted in those with PTSD, our results may extend to that population, in particular, for whom trauma-related odors are often potent triggers of re-experiencing (Cortese et al. 2015; Daniels and Vermetten 2016). With these individuals, odor exposure training, akin to the well-established effects of in vivo exposure, could be an effective adjunct or stand-alone treatment (Herz 2021). Our findings may also have particular relevance for multiple chemical sensitivity (MCS), a disorder highly comorbid with anxiety (Sparks et al. 1994; Bornschein et al. 2002) that is characterized by heightened sensitivity and intolerance of certain, often highly trigeminal, odors including environmental pollutants, aromatic products, and cleaning supplies (Cullen 1987; Sparks et al. 1994). Although MCS is not often diagnosed in children and adolescents (Andersson et al. 2008; Lalana Josa et al. 2021), the prevalence rate for self-reported sensitivity to odors was nearly 16% in a random sample of Swedish adolescents (Andersson et al. 2008). Importantly, behavioral therapies using exposure to odorants in the treatment of MCS have also been documented (Guglielmi et al. 1994).
In conclusion, the present findings in youth add to the growing literature that elevated trait anxiety associates with a chronic shift in smell processing characterized by increased sensitivity to some odors and decreased sensitivity to others. Results indicate also that this shift can be further exacerbated by acute stress and that trigeminal properties are a potentially important factor. Exploratory analyses suggest a developmental course for the anxiety–olfactory relationship. With a dearth of knowledge in this important area however, additional study is required to confirm developmental trajectories and mechanisms for anxiety-related shifts in smell function and if they predict the emergence of clinical anxiety- and stress-related disorders.
Funding
Funding for this study was partially supported by grant R01MH112209 (PI: Danielson) from the National Institute of Mental Health (NIMH), NIH. Views expressed in this article do not necessarily reflect those of the NIMH/NIH.
Conflict of interest
All authors declare that they have no conflicts of interest.
References
- Åhs F, Miller SS, Gordon AR, Lundström JN.. 2013. Aversive learning increases sensory detection sensitivity. Biol Psychol. 92(2):135–141. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . 2013. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington (DC): American Psychiatric Association. [Google Scholar]
- Andersson L, Johansson A, Millqvist E, Nordin S, Bende M.. 2008. Prevalence and risk factors for chemical sensitivity and sensory hyperreactivity in teenagers. Int J Hyg Environ Health. 211(5–6):690–697. [DOI] [PubMed] [Google Scholar]
- Arnett JJ. 1999. Adolescent storm and stress, reconsidered. Am Psychol. 54(5):317–326. [DOI] [PubMed] [Google Scholar]
- Bornschein S, Hausteiner C, Zilker T, Förstl H.. 2002. Psychiatric and somatic disorders and multiple chemical sensitivity (MCS) in 264 ‘environmental patients’. Psychol Med. 32(8):1387–1394. [DOI] [PubMed] [Google Scholar]
- Calhoun CD, Helms SW, Heilbron N, Rudolph KD, Hastings PD, Prinstein MJ.. 2014. Relational victimization, friendship, and adolescents’ hypothalamic-pituitary-adrenal axis responses to an in vivo social stressor. Dev Psychopathol. 26(3):605–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra A, Baur A, Hummel T.. 2008. Thresholds and chemosensory event-related potentials to malodors before, during, and after puberty: differences related to sex and age. Neuroimage. 40(3):1257–1263. [DOI] [PubMed] [Google Scholar]
- Cometto-Muñiz JE, Cain WS.. 1990. Thresholds for odor and nasal pungency. Physiol Behav. 48(5):719–725. [DOI] [PubMed] [Google Scholar]
- Cortese BM, Leslie K, Uhde TW.. 2015. Differential odor sensitivity in PTSD: implications for treatment and future research. J Affect Disord. 179:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese BM, McConnell PA, Froeliger B, Leslie K, Uhde TW.. 2015. Burning odor-elicited anxiety in OEF/OIF combat veterans: inverse relationship to gray matter volume in olfactory cortex. J Psychiatr Res. 70:58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortese BM, Schumann AY, Howell AN, McConnell PA, Yang QX, Uhde TW.. 2018. Preliminary evidence for differential olfactory and trigeminal processing in combat veterans with and without PTSD. Neuroimage Clin. 17:378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croy I, Negoias S, Symmank A, Schellong J, Joraschky P, Hummel T.. 2013. Reduced olfactory bulb volume in adults with a history of childhood maltreatment. Chem Senses. 38(8):679–684. [DOI] [PubMed] [Google Scholar]
- Croy I, Springborn M, Lötsch J, Johnston AN, Hummel T.. 2011. Agreeable smellers and sensitive neurotics–correlations among personality traits and sensory thresholds. PLoS One. 6(4):e18701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen MR. 1987. Multiple chemical sensitivities: summary and directions for future investigators. Occup Med. 2(4):801–804. [PubMed] [Google Scholar]
- Daniels JK, Vermetten E.. 2016. Odor-induced recall of emotional memories in PTSD—review and new paradigm for research. Exp Neurol. 284(Pt B): 168–180. [DOI] [PubMed] [Google Scholar]
- Dileo JF, Brewer WJ, Hopwood M, Anderson V, Creamer M.. 2008. Olfactory identification dysfunction, aggression and impulsivity in war veterans with post-traumatic stress disorder. Psychol Med. 38(4):523–531. [DOI] [PubMed] [Google Scholar]
- Doty RL. 2009. The smell threshold test administration manual. 2nd ed. Haddon Heights (NJ): Sensonics, Inc. [Google Scholar]
- Doty RL, Brugger WE, Jurs PC, Orndorff MA, Snyder PJ, Lowry LD.. 1978. Intranasal trigeminal stimulation from odorous volatiles: psychometric responses from anosmic and normal humans. Physiol Behav. 20(2):175–185. [DOI] [PubMed] [Google Scholar]
- Fletcher ML, Wilson DA.. 2002. Experience modifies olfactory acuity: acetylcholine-dependent learning decreases behavioral generalization between similar odorants. J Neurosci. 22(2):RC201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frie J, Bartocci M, Lagercrantz H, Kuhn P.. 2018. Cortical responses to alien odors in newborns: an fNIRS Study. Cereb Cortex. 28(9):3229–3240. [DOI] [PubMed] [Google Scholar]
- Galliot E, Laurent L, Hacquemand R, Pourié G, Millot JL.. 2012. Fear-like behavioral responses in mice in different odorant environments: trigeminal versus olfactory mediation under low doses. Behav Processes. 90(2):161–166. [DOI] [PubMed] [Google Scholar]
- Guglielmi RS, Cox DJ, Spyker DA.. 1994. Behavioral treatment of phobic avoidance in multiple chemical sensitivity. J Behav Ther Exp Psychiatry. 25(3):197–209. [DOI] [PubMed] [Google Scholar]
- Gur RE, Gur RC.. 2016. Sex differences in brain and behavior in adolescence: findings from the Philadelphia Neurodevelopmental Cohort. Neurosci Biobehav Rev. 70:159–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. 2013. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. New York (NY): Guilford Press. p. xvii, 507. [Google Scholar]
- Hayes AF, Cai L.. 2007. Using heteroskedasticity-consistent standard error estimators in OLS regression: an introduction and software implementation. Behav Res Methods. 39(4):709–722. [DOI] [PubMed] [Google Scholar]
- Herz S. 2021. Olfactory virtual reality: a new frontier in the treatment and prevention of posttraumatic stress disorder. Brain Sci. 11(8):1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herz RS, Beland SL, Hellerstein M.. 2004. Changing odor hedonic perception through emotional associations in humans. Int J Comp Psychol. 17(4):315–338. [Google Scholar]
- Herz RS, Van Reen E, Gredvig-Ardito CA, Carskadon MA.. 2020. Insights into smell and taste sensitivity in normal weight and overweight-obese adolescents. Physiol Behav. 221:112897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoenen M, Wolf OT, Pause BM.. 2017. The impact of stress on odor perception. Perception. 46(3–4):366–376. [DOI] [PubMed] [Google Scholar]
- Houghton DC, Howard SL, Uhde TW, Paquet C, Schlosser RJ, Cortese BM.. 2019. Odor sensitivity impairment: a behavioral marker of psychological distress? CNS Spectr. 24(4):404–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houghton DC, Stein DJ, Cortese BM.. 2020. Review: exteroceptive sensory abnormalities in childhood and adolescent anxiety and obsessive-compulsive disorder: a critical review. J Am Acad Child Adolesc Psychiatry. 59(1):78–87. [DOI] [PubMed] [Google Scholar]
- Hummel T, Roudnitzky N, Kempter W, Laing DG.. 2007. Intranasal trigeminal function in children. Dev Med Child Neurol. 49(11):849–853. [DOI] [PubMed] [Google Scholar]
- Kass MD, Czarnecki LA, Moberly AH, McGann JP.. 2017. Differences in peripheral sensory input to the olfactory bulb between male and female mice. Sci Rep. 7:45851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass MD, Rosenthal MC, Pottackal J, McGann JP.. 2013. Fear learning enhances neural responses to threat-predictive sensory stimuli. Science. 342(6164):1389–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH.. 1993. The ‘Trier Social Stress Test’—a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 28(1–2):76–81. [DOI] [PubMed] [Google Scholar]
- Koelega HS. 1994. Prepubescent children may have specific deficits in olfactory sensitivity. Percept Mot Skills. 78(1):191–199. [DOI] [PubMed] [Google Scholar]
- Krusemark EA, Novak LR, Gitelman DR, Li W.. 2013. When the sense of smell meets emotion: anxiety-state-dependent olfactory processing and neural circuitry adaptation. J Neurosci. 33(39):15324–15332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Buissonnière-Ariza V, Lepore F, Kojok KM, Frasnelli J.. 2013. Increased odor detection speed in highly anxious healthy adults. Chem Senses. 38(7):577–584. [DOI] [PubMed] [Google Scholar]
- Lalana Josa MP, Galindo Lalana E, Bamala Cuartero C.. 2021. Chemical sensitivity in children. Med Clin (Barc). 156(8):408–409. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM, Gotlib IH, Lewinsohn M, Seeley JR, Allen NB.. 1998. Gender differences in anxiety disorders and anxiety symptoms in adolescents. J Abnorm Psychol. 107(1):109–117. [DOI] [PubMed] [Google Scholar]
- Li W, Howard JD, Parrish TB, Gottfried JA.. 2008. Aversive learning enhances perceptual and cortical discrimination of indiscriminable odor cues. Science. 319(5871):1842–1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- March JS. 2013. Multidimensional Anxiety Scale for Children. 2nd ed. Toronto (Ontario): Multi-Health Systems. [Google Scholar]
- March JS, Parker JD, Sullivan K, Stallings P, Conners CK.. 1997. The Multidimensional Anxiety Scale for Children (MASC): factor structure, reliability, and validity. J Am Acad Child Adolesc Psychiatry. 36(4):554–565. [DOI] [PubMed] [Google Scholar]
- March JS, Sullivan K.. 1999. Test–retest reliability of the multidimensional anxiety scale for children. J Anxiety Disord. 13(4):349–358. [DOI] [PubMed] [Google Scholar]
- Marshall WA, Tanner JM.. 1969. Variations in pattern of pubertal changes in girls. Arch Dis Child. 44(235):291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin B, Maudsley S, White CM, Egan JM.. 2009. Hormones in the naso-oropharynx: endocrine modulation of taste and smell. Trends Endocrinol Metab. 20(4):163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ.. 2012. The anxiety spectrum and the reflex physiology of defense: from circumscribed fear to broad distress. Depress Anxiety. 29(4):264–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM.. 2010. Aversive imagery in posttraumatic stress disorder: trauma recurrence, comorbidity, and physiological reactivity. Biol Psychiatry. 67(4):346–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier N, Raynaud A, Le Bourhis M, Grébert D, Dewaele A, Acquistapace A, Bombail V.. 2020. The olfactory mucosa, first actor of olfactory detection, is sensitive to glucocorticoid hormone. Eur J Neurosci. 51(6):1403–1418. [DOI] [PubMed] [Google Scholar]
- Monnery-Patris S, Rouby C, Nicklaus S, Issanchou S.. 2009. Development of olfactory ability in children: sensitivity and identification. Dev Psychobiol. 51(3):268–276. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K.. 1996. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Dev. 67(2):508–522. [PubMed] [Google Scholar]
- Pacharra M, Schäper M, Kleinbeck S, Blaszkewicz M, Wolf OT, van Thriel C.. 2016. Stress lowers the detection threshold for foul-smelling 2-mercaptoethanol. Stress. 19(1):18–27. [DOI] [PubMed] [Google Scholar]
- Parma V, Ferraro S, Miller SS, Åhs F, Lundström JN.. 2015. Enhancement of odor sensitivity following repeated odor and visual fear conditioning. Chem Senses. 40(7):497–506. [DOI] [PubMed] [Google Scholar]
- Pierce JD Jr, Doty RL, Amoore JE.. 1996. Analysis of position of trial sequence and type of diluent on the detection threshold for phenyl ethyl alcohol using a single staircase method. Percept Mot Skills. 82(2):451–458. [DOI] [PubMed] [Google Scholar]
- Raineki C, Opendak M, Sarro E, Showler A, Bui K, McEwen BS, Wilson DA, Sullivan RM.. 2019. During infant maltreatment, stress targets hippocampus, but stress with mother present targets amygdala and social behavior. Proc Natl Acad Sci USA. 116(45):22821–22832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo RD. 2010. Adolescence: a central event in shaping stress reactivity. Dev Psychobiol. 52(3):244–253. [DOI] [PubMed] [Google Scholar]
- Rouder JN, Speckman PL, Sun D, Morey RD, Iverson G.. 2009. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon Bull Rev. 16(2):225–237. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, McCormack KM, Howell BR.. 2015. Social buffering of stress responses in nonhuman primates: maternal regulation of the development of emotional regulatory brain circuits. Soc Neurosci. 10(5):512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schecklmann M, Schwenck C, Taurines R, Freitag C, Warnke A, Gerlach M, Romanos M.. 2013. A systematic review on olfaction in child and adolescent psychiatric disorders. J Neural Transm (Vienna). 120(1):121–130. [DOI] [PubMed] [Google Scholar]
- Sparks PJ, Daniell W, Black DW, Kipen HM, Altman LC, Simon GE, Terr AI.. 1994. Multiple chemical sensitivity syndrome: a clinical perspective. I. Case definition, theories of pathogenesis, and research needs. J Occup Med. 36(7):718–730. [PubMed] [Google Scholar]
- Stevenson RJ. 2010. An initial evaluation of the functions of human olfaction. Chem Senses. 35(1):3–20. [DOI] [PubMed] [Google Scholar]
- Taylor JE, Lau H, Seymour B, Nakae A, Sumioka H, Kawato M, Koizumi A.. 2020. Corrigendum: an evolutionarily threat-relevant odor strengthens human fear memory. Front Neurosci. 14:638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasterling JJ, Brailey K, Sutker PB.. 2000. Olfactory identification in combat-related posttraumatic stress disorder. J Trauma Stress. 13(2):241–253. [DOI] [PubMed] [Google Scholar]
- Wilkerson AK, Uhde TW, Leslie K, Freeman WC, LaRowe SD, Schumann A, Cortese BM.. 2018. Paradoxical olfactory function in combat veterans: the role of PTSD and odor factors. Mil Psychol. 30(2):120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williamson M, Bingham B, Viau V.. 2005. Central organization of androgen-sensitive pathways to the hypothalamic-pituitary-adrenal axis: implications for individual differences in responses to homeostatic threat and predisposition to disease. Prog Neuropsychopharmacol Biol Psychiatry. 29(8):1239–1248. [DOI] [PubMed] [Google Scholar]