Abstract
Background
Indomethacin therapy for closure of patent ductus arteriosus (PDA) frequently causes oliguria and occasionally more serious renal dysfunction. Low dose dopamine has been suggested as a means for preventing this side effect.
Objectives
To determine whether dopamine therapy prevents indomethacin‐mediated deterioration in renal function in the preterm newborn infant without serious adverse effects. Subgroup analyses were planned to assess the effects of dopamine on patients given indomethacin as prophylaxis of intraventricular hemorrhage, and patients given indomethacin as treatment of PDA.
Search methods
Standard methods of the Cochrane Neonatal Review Group (CNRG) were used. We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 3, 2009), MEDLINE (1966 to 2009), EMBASE (1974 to 2009) and CINAHL (2001 to 2009). In addition, we contacted the principal investigators if necessary to ascertain required information.
Selection criteria
Randomized or quasi‐randomized studies of the effects of dopamine on urine output, glomerular filtration rate, fluid balance or incidence of renal failure, in preterm newborn infants receiving indomethacin. The comparison group should have received no dopamine.
Data collection and analysis
We used the standard methods of the Cochrane Collaboration and those of the CNRG. For categorical outcomes, we calculated typical estimates for relative risk and risk difference. For continuous outcomes the weighted mean difference (WMD) was calculated. Fixed effect models were assumed for meta‐analysis.
Main results
Three studies were found (total number randomized patients 75) that fulfilled the entry criteria for this review. All were single center trials that enrolled NICU patients receiving indomethacin for symptomatic PDA. There are no (or only partial) results for effects of dopamine on several of the primary outcomes, including death before discharge, serious intraventricular hemorrhage, cystic periventricular leukomalacia, or renal failure. There has been inadequate investigation of the effects of dopamine on cerebral perfusion or cardiac output, or GI complications, or endocrine toxicity. Dopamine improved urine output [WMD 0.68 ml/kg/hour (95% CI 0.22, 1.44)], but there was no evidence of effect on serum creatinine (WMD 2.04 micromoles/liter, CI ‐17.90, 21.97) or the incidence of oliguria (urine output < 1 ml/kg/hour) (RR 0.73, CI 0.35, 1.54). There was no evidence of effect of dopamine on the frequency of failure to close the ductus arteriosus (RR 1.11, CI 0.56, 2.19).
Authors' conclusions
There is no evidence from randomized trials to support the use of dopamine to prevent renal dysfunction in indomethacin‐treated preterm infants.
Plain language summary
Dopamine versus no treatment to prevent renal dysfunction in indomethacin‐treated preterm newborn infants
Dopamine has not been shown to prevent adverse effects of indomethacin on the kidneys of preterm babies. Indomethacin is a drug used in preterm babies to prevent brain hemorrhage or to help close off PDA (patent ductus arteriosus ‐ when a channel between the lungs and heart does not close off after birth as it should). Indomethacin often causes fluid retention and reduced flow of urine, which can sometimes cause deterioration in kidney (renal) function. The drug dopamine is sometimes used along with indomethacin to try and prevent negative impact on the kidneys. The review found there is not enough evidence from trials to show there is any value in giving dopamine to babies being treated with indomethacin.
Background
Description of the condition
Use and side effects of indomethacin in neonates:
Indomethacin has been used in preterm infants for two main indications:
treatment of patent ductus arteriosus (PDA) (Nehgme 1992; Clyman 1996);
prophylaxis of intraventricular hemorrhage (Fowlie 1997).
The two most frequent side effects of indomethacin therapy are oliguria (Barrington 1994) and decrease in cerebral blood flow (Mosca 1997). More uncommon, but serious potential side effects of indomethacin include renal failure (Cifuentes 1979), hyperkalemia, and gastrointestinal bleeding or perforation (Alpan 1985; Kuhl 1985).
Description of the intervention
Rationale for using dopamine in association with indomethacin, putative benefits and risks: If oliguria can be prevented and overall fluid status improved, then other outcomes could also be affected. Dopamine is often administered in the hope that its actions on specific dopaminergic receptors in the renal vasculature will increase renal perfusion by selectively mediating renal vasodilatation (Goldberg 1972), thus leading to increased renal blood flow, increased glomerular filtration rate and increased urine output. However, there is no reliable evidence that dopaminergic vasodilatation occurs in the neonatal mammalian or human renal circulation (Cheung 1996). Potential interactions of dopamine with indomethacin also exist. One study showed that in healthy human adults the renal vasodilation of low dose dopamine was prevented by indomethacin (Manoogian 1988). It may be that the usual physiologic cascade which follows dopaminergic stimulation in renal vascular muscle involves release of prostacyclin and, therefore, might be affected by indomethacin treatment.
Why it is important to do this review
Despite the potential serious side effects of indomethacin on kidney, gut and brain, indomethacin is frequently used for the medical treatment of PDA. Furosemide has been used in an attempt to prevent oliguria in indomethacin‐treated preterm infants, but in a systematic review Brion 2001 found no evidence of benefit. A systematic review of the effects of dopamine in indomethacin‐treated infants has not been reported, and is needed.
The effects of indomethacin on renal and gut blood flow might well differ depending on the blood flow to these areas prior to indomethacin, and therefore might exacerbate the reduction in renal and gastrointestinal blood flow which is related to the PDA. Therefore, we plan a subgroup analysis in patients receiving indomethacin as prophylaxis and in those receiving indomethacin as treatment of PDA.
This review updates the existing review "Dopamine versus no treatment to prevent renal dysfunction in indomethacin‐treated preterm newborn infants" published in the Cochrane Database of Systematic Reviews (Barrington 2002).
Objectives
Primary objective:
To determine whether concomitant therapy with dopamine is effective in reducing the incidence of renal dysfunction in preterm infants receiving indomethacin, without increasing cerebral injury, mortality, or the rate of failure to close the PDA.
Secondary objective:
To assess effects of dopamine on the above variables in two subgroups:
patients given indomethacin as prophylaxis of intraventricular hemorrhage;
patients given indomethacin as treatment of PDA.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi‐randomized controlled trials were considered.
Types of participants
Preterm infants, less than or equal to 36 weeks gestation at birth receiving indomethacin for either PDA closure or prophylaxis, or prophylaxis against intraventricular hemorrhage, during the first month of life.
Types of interventions
Dopamine compared to no treatment. Studies with dopamine started before, simultaneously with, or after indomethacin administration were considered acceptable.
Types of outcome measures
Primary outcomes
Mortality before discharge;
Intraventricular hemorrhage, grade three or four;
Cystic periventricular leukomalacia;
Renal failure (either oliguria, defined as a urine output less than 1 ml/kg/hour or an elevation in creatinine by more than 40 micromoles/L);
Failure to close the ductus arteriosus;
Need for surgical PDA ligation.
Secondary outcomes
Gastrointestinal bleeding;
Intestinal perforation;
Necrotizing enterocolitis;
Cerebral blood flow (measured using validated methodology, such as Near Infra‐Red Spectroscopy);
Cardiac output (measured using validated methodology, such as doppler ultrasound);
Urine output, renal function (creatinine values or fractional sodium excretion);
Low serum thyroxine, more than two SD below a reference mean for age, analyzed within 1 week after starting the study intervention.
Search methods for identification of studies
Electronic searches
The standard methods of the Cochrane Neonatal Review Group (CNRG) were used. We searched the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 3, 2001), MEDLINE (1966 to 2001) using PubMed as the search engine, EMBASE (1974 to 2001) and ). Search terms "indomethacin" and "dopamine" and "infant, newborn" were used. The search was limited to controlled clinical trials. This search was updated in November 2001.
In 2009, we updated the search as follows: The Cochrane Library, MEDLINE (search via PubMed), CINAHL and EMBASE were searched from 2001 to 2009. Search terms: indomethacin and dopamine. Limits: human, newborn infant and clinical trial. No language restrictions were applied.
Searching other resources
We searched personal files and recent abstracts of the Pediatric Academic Societies. Abstracts available on CDRom (1998 to 2001) were searched electronically; 1990 to 1998 abstracts were searched manually by looking for "dopamine" in the index.
Clinical trials registries were also searched for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp).
Data collection and analysis
The standard methods of the Cochrane Neonatal Review Group Guidelines were employed.
Selection of studies
Reports were first reviewed to determine whether there was a concurrent control group, and rejected if not. The method of assignment to control and intervention groups was then determined and if not random or quasi random, then the trial was discarded.
Data extraction and management
The review author extracted, assessed and coded all data for each study using a form that was designed specifically for this review. For each study, final data was entered into RevMan by the review author.
Assessment of risk of bias in included studies
The standard methods of the Cochrane Neonatal Review Group were employed. Each identified trial was assessed for methodological quality with respect to a) masking of allocation b) masking of intervention c) completeness of follow‐up d) masking of outcome assessment. This information is included in the Characteristics of Included Studies table.
For the update in 2009, the risk of bias table was completed in order to address the following questions:
1. Sequence generation: Was the allocation sequence adequately generated?
2. Allocation concealment: Was allocation adequately concealed?
3. Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
4. Incomplete outcome data: Were incomplete outcome data adequately addressed?
5. Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?
6. Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias?
Measures of treatment effect
Statistical analyses was performed using Review Manager software. For categorical outcomes, estimates for relative risk and risk difference were calculated. For outcomes measured on a continuous scale, estimates for weighted mean difference were calculated. 95% confidence intervals were used.
Assessment of heterogeneity
Heterogeneity between trials was evaluated by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. A fixed effects model for meta‐analysis.
Data synthesis
The meta‐analysis was done using review Manager software (RevMan 5). For categorical outcomes, we calculated typical estimates for relative risk and risk difference and used as denominator the total number of randomized patients. 95% confidence intervals were used. For continuous outcomes the weighted mean difference was calculated. Fixed effect models were assumed for meta‐analysis. All meta‐analyses were to be done using the fixed effect model.
Subgroup analysis and investigation of heterogeneity
Planned subgroup analysis:
patients given indomethacin as prophylaxis of intraventricular hemorrhage;
patients given indomethacin as treatment of PDA.
Results
Description of studies
See tables Characteristics of Included Studies and Characteristics of Excluded Studies.
Results of the search
Our search yielded six studies, including three randomized controlled trials (Baenziger 1999; Fajardo 1992; Seri 1984). A fourth trial which has only appeared as an abstract is awaiting assessment, as it is unclear whether the treatment and control groups were contemporaneous (Cochran 1989). Two additional non‐randomized studies were excluded: Tulassay 1983 and Seri 1988.
Included studies
All three randomized studies qualifying for this review were single center trials among indomethacin‐treated NICU patients with symptomatic PDA. All were small studies, leading to a total enrollment in published randomized trials of 75 infants.
Outcomes:
Each of the trials appears to have a primary objective of investigating the effects of dopamine on the renal dysfunction associated with indomethacin therapy of a PDA. Other important clinical outcomes are not reported in any trial (mortality before discharge, intraventricular hemorrhage, periventricular leukomalacia). The method used for assessing ductal closure is not stated in any study. The methods used for assessing changes in renal function appear to be appropriate.
Subjects:
Two studies were limited to preterm infants (Fajardo 1992; Seri 1984), whereas in Baenziger 1999, although most of the patients were premature, the gestational ages extended up to 38 weeks and three days. There were no clear pre‐stated limits for Seri 1984, but all patients were less than 35 weeks gestation.
Clinical diagnosis of a PDA was followed by ultrasound confirmation in Baenziger 1999 and Fajardo 1992, together with confirmation of hemodynamic significance by a left atrial to aortic root ratio of >1.3. No echocardiography appears to have been performed in Seri 1984.
Drug doses:
The majority of patients in Baenziger 1999 and Fajardo 1992 received indomethacin at a dose of 0.2 mg/kg per dose, every 12 hours, for three doses. Fajardo 1992 varied the second and third indomethacin doses based on postnatal age at the time of starting (0.1 mg/kg for infants < two days of age, 0.25 mg/kg for infants > seven days).
In Seri 1984 two doses of indomethacin of 0.3 mg/kg were given with a 12 hour interval; this does not reflect current dosing used in the majority of NICUs.
Dopamine was administered at a dose of 4 microg/kg/min in Baenziger 1999. The dose was 2 microg/kg/min in Fajardo 1992 for all 14 infants randomly assigned to the dopamine group; when no effect was apparent, a further 10 non‐randomized infants were studied at a dose of 4 microg/kg/min. Seri 1984 studied five infants at a dose of 2 microg/kg/min and three infants at 4 microg/kg/min; the choice of dose appears to have been based on blood pressure.
Excluded studies
Two non‐randomized studies were excluded: Tulassay 1983 is a study in which the authors used historical controls. Seri 1988 is a study in which infants received dopamine on the basis of clinical condition: controls did not require dopamine, whereas patients in the treatment group received dopamine for edema, moderate oliguria, poor peripheral perfusion and/or mild systemic hypotension. Thus, this latter study is not a randomized or quasi‐randomized study.
Risk of bias in included studies
The three trials are of moderate quality. All results presented are from patients randomized to dopamine or no dopamine. Only Fajardo 1992 blinded the intervention. The studies are all very small and fail to report some important clinical outcomes. Baenziger 1999 randomized 15 patients to control, but only reports urine output and fractional sodium excretion from 10 of these infants, apparently because of one death and the use of furosemide in the other four infants. Fajardo 1992 does not describe the randomization process well, but the intervention was masked and it could perhaps be assumed that the allocation was also. Seri 1984 used one of two different doses of dopamine depending on the systolic blood pressure.
Effects of interventions
Dopamine vs. no treatment in indomethacin‐treated infants with PDA (COMPARISON 1):
There are no (or only partial) results for several important clinical outcomes, including the following primary outcome measures: death before discharge, serious intraventricular hemorrhage, periventricular leukomalacia, or renal failure. There has been inadequate investigation of the effects of dopamine for this indication on the following secondary outcomes: cerebral blood flow, cardiac output, GI complications, or endocrine toxicity.
Renal function (Outcomes 1.1 to 1.4):
There are data for the three secondary outcomes describing aspects of renal function.
Dopamine was associated with a minor increase in urine output [WMD 0.68 ml/kg/hour (95% CI 0.22, 1.14) n = 69] (Outcome 1.1). There is no evidence of effect of dopamine on serum creatinine [(WMD 2.04 micromoles/liter (95% CI ‐17.90, 21.97) n = 59] (Outcome 1.2). There is no demonstrated effect on fractional sodium excretion [WMD 0.47% (95% CI ‐0.74, 1.68) n = 69] (Outcome 1.3). The incidence of oliguria (urine output < 1 ml/kg/hour) in Baenziger 1999 was not shown to be affected by dopamine administration (RR 0.73, CI 0.35, 1.54) (Outcome 1.4). Clinically important degrees of renal impairment are not reported in any of the studies.
Failure to close the ductus arteiosus (Outcome 1.5):
Three studies reported on the effect of dopamine on ductal closure in preterm infants receiving indomethacin. There was no evidence of effect of dopamine on the frequency of failure to close the ductus arteriosus (typical RR 1.11, CI 0.56, 2.19).
Discussion
Despite there having been three randomized controlled trials, only 75 babies have been randomized in total. The power of the individual studies, or of this systematic review, to detect effects on clinical outcomes is therefore limited.
The mechanism of oliguria caused by indomethacin is not well understood. Other cyclooxygenase inhibitors appear to cause less oliguria (Bergamo 1989), and in newborn infants these other agents have less effect on renal blood flow than does indomethacin (Pezzati 1999). Differential effects of various cyclooxygenase inhibitors on the kidney may depend on the ratio of their activity on the two cyclooxygenase isozymes, COX‐1, which preferentially mediates renal side effects (Vane 1998), and COX‐2, which preferentially mediates the antiinflammatory response (Smith 1995). Indomethacin is more active against COX‐1 than ibuprofen, which may explain the increased frequency and severity of renal side effects. In newborn piglets indomethacin administration affects a number of regional circulations (renal, gastrointestinal and cerebral) more than other cyclooxygenase inhibitors (Chemtob 1991; Speziale 1999); actions of indomethacin other than cyclo‐oxygenase inhibition may be in part responsible, such as the propensity for causing an increase in concentrations for lipoxygenase products. The majority of infants who receive indomethacin have some decrease in urine flow, and the best predictor of severe oliguria is the pre‐indomethacin urine output (Barrington 1994). Most often oliguria is self limited and of little significance. However major complications do occasionally occur (Barrington 1994), and hyponatremia, hyperkalemia, and fluid retention requiring adjustments of fluid therapy are fairly common. Rarely, renal failure may occur. Fluid retention could possibly lead to worse pulmonary outcomes. Studies of agents to prevent oliguria are thus warranted. Although dopamine in this review did cause a slight increase in urine output, this result was largely due to one study with very small sample size (Seri 1984).
As noted above there is little evidence that dopamine improves either renal perfusion or renal function in the newborn. Indeed the role of dopamine for this indication has recently been called into question for adult patients (Thompson 1994; McCrory 1997), and recent systematic reviews of the effects of low dose dopamine on renal function in the critically ill adult (Kellum 2001) and the critically ill infant and child (Prins 2001) also show no evidence of effect .
Changes in intestinal blood flow parallel those in renal blood flow (Mosca 1997); they may mediate an increased risk for gastrointestinal bleeding, perforation (Kuhl 1985), and necrotizing enterocolitis which has been demonstrated in some studies. Indomethacin may reduce both cerebral blood flow and cerebral oxygenation in preterm infants with PDA (Mosca 1997). Indomethacin also increase bleeding time (Corazza 1984). All of these side effects should be taken into account when considering indomethacin therapy in the preterm infant with a PDA.
Dopamine has uncertain effects on the cerebral circulation. Thus, the effects of combined dopamine and indomethacin therapy on cerebral perfusion in newborn infants warrants study. Dopamine is an important neurotransmitter. Although systemic infusion of dopamine largely does not cross the blood brain barrier and does not affect the majority of CNS dopamine receptors, such therapy has been shown to have other effects. Dopamine receptors in the anterior pituitary and the hypothalamus are functionally outside of the blood brain barrier (Van den Berghe 1996). It appears that systemic dopamine infusion suppresses pituitary function and administration of dopamine therefore might worsen the apparent hypothyroid state that is common in preterm infants and is statistically associated with poorer developmental outcome (Van Wassenaer 1997; Van Wassenaer 1999). Dopamine may also decrease growth hormone and prolactin secretion. Dopamine receptors in the carotid body are also affected by systemic dopamine infusion, and respiratory depression may result. Thus, adequate evaluation of the efficacy and of the potential toxicities of dopamine in clinical usage is necessary.
The currently available data do not support the use of dopamine at any dose to protect renal function during indomethacin therapy. The use of dopamine for this indication in preterm infants is not supported by the published studies.
Authors' conclusions
Implications for practice.
There is no evidence from randomized trials to support the use of dopamine to prevent renal dysfunction in indomethacin‐treated preterm infants.
Implications for research.
If further studies are to be performed, major clinical outcomes should be addressed and enough patients should be enrolled to ensure that serious complications of therapy can be detected.
What's new
| Date | Event | Description |
|---|---|---|
| 28 October 2009 | New search has been performed | This review updates the existing review "Dopamine versus no treatment to prevent renal dysfunction in indomethacin‐treated preterm newborn infants" published in the Cochrane Database of Systematic Reviews, Issue 3, 2002 (Barrington 2002). Updated search found no new trials. No changes to conclusions. |
History
Protocol first published: Issue 3, 2002 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 15 September 2008 | Amended | Converted to new review format. |
Acknowledgements
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Dopamine vs no treatment in indomethacin‐treated infants with PDA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Urine output (mL/kg/hour) | 3 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.68 [0.22, 1.14] |
| 2 Serum creatinine concentration (micromoles/liter) | 2 | 59 | Mean Difference (IV, Fixed, 95% CI) | 2.04 [‐17.90, 21.97] |
| 3 Fractional sodium excretion (%) | 3 | 69 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [‐0.74, 1.68] |
| 4 Oliguria (urine output < 1 ml/kg/hour) | 1 | 33 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.35, 1.54] |
| 5 Failure to close the ductus arteriosus | 3 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.56, 2.19] |
1.1. Analysis.
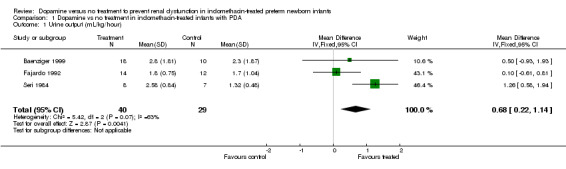
Comparison 1 Dopamine vs no treatment in indomethacin‐treated infants with PDA, Outcome 1 Urine output (mL/kg/hour).
1.2. Analysis.
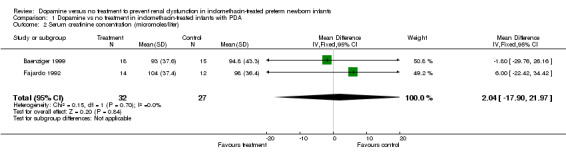
Comparison 1 Dopamine vs no treatment in indomethacin‐treated infants with PDA, Outcome 2 Serum creatinine concentration (micromoles/liter).
1.3. Analysis.
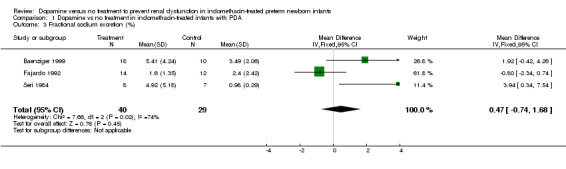
Comparison 1 Dopamine vs no treatment in indomethacin‐treated infants with PDA, Outcome 3 Fractional sodium excretion (%).
1.4. Analysis.

Comparison 1 Dopamine vs no treatment in indomethacin‐treated infants with PDA, Outcome 4 Oliguria (urine output < 1 ml/kg/hour).
1.5. Analysis.
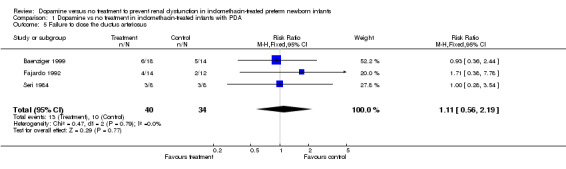
Comparison 1 Dopamine vs no treatment in indomethacin‐treated infants with PDA, Outcome 5 Failure to close the ductus arteriosus.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baenziger 1999.
| Methods | Single centre randomized trial. Masking of allocation: not stated. Masking of intervention: no. Masking of outcome assessment: no. Completeness of outcome assessment: one control patient died and not analyzed. | |
| Participants | 33 newborn infants with symptomatic PDA. 18 dopamine infants and 15 controls. | |
| Interventions | Dopamine commenced at 4 microg/kg/min 2 hours before first dose of indomethacin, which was administered at 0.2 mg/kg iv every 12 hours for 3 doses. | |
| Outcomes | Failure to close the ductus arteriosus, indices of renal function, blood pressure | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Adequate sequence generation: not stated. |
| Allocation concealment? | Unclear risk | Masking of allocation: not stated. |
| Blinding? All outcomes | High risk | Masking of intervention: no. Masking of outcome assessment: no. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Completeness of outcome assessment: one control patient died and not analyzed. |
Fajardo 1992.
| Methods | Single centre randomized trial. Masking of allocation: not stated. Masking of intervention: yes, the low dose dopamine and no dopamine groups were masked. Masking of outcome assessment: not clear. Completeness of outcome assement: yes. | |
| Participants | 26 preterm (<36 weeks gestation) infants with symptomatic PDA, hemodynamically significant by echocardiogram, were randomly allocated, 14 to the dopamine group and 12 to the control group. An additional group of 10 non randomized dopamine infants received a higher dosage of 5 microg/kg/min after the initial dose did not show an effect. (these non‐randomized infants were not included in this review). | |
| Interventions | Dopamine was commenced at 2 microg/kg/min, (n=14), 6 hours before the first dose of indomethacin. The latter was administered every 12 hours for 3 doses ranging from 0.1 to 0.25 mg/kg depending on postnatal age. | |
| Outcomes | Failure to close the ductus arteriosus, indices of renal function. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Adequate sequence generation: not stated. |
| Allocation concealment? | Unclear risk | Masking of allocation: not stated. |
| Blinding? All outcomes | Unclear risk | Masking of intervention: yes, the low dose dopamine and no dopamine groups were masked. Masking of outcome assessment: not clear. |
| Incomplete outcome data addressed? All outcomes | Low risk | Completeness of outcome assement: yes. |
Seri 1984.
| Methods | Single centre randomized trial. Masking of allocation: probably yes (envelopes with random assignment to group 1 or 2). Masking of intervention: no. Masking of outcome assessment: no. Completeness of outcome assessment: no: One patient in the control group developed intraventricular bleeding and irreversible hypotensive shock and was not analyzed. | |
| Participants | 16 preterm infants (28 to 34 weeks), 8 dopamine treated and 8 controls. | |
| Interventions | Dopamine was used at either 2 microg/kg/min or 4 microg/kg/min, commenced 2 minutes before indomethacin which was administered at 0.3 mg/kg every 12 hours for 2 doses. | |
| Outcomes | Failure to close the ductus arteriosus, various indices of renal function | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Masking of allocation: probably yes (envelopes with random assignment to group 1 or 2). |
| Allocation concealment? | Low risk | Masking of allocation: probably yes (envelopes with random assignment to group 1 or 2). |
| Blinding? All outcomes | High risk | Masking of intervention: no. Masking of outcome assessment: no. |
| Incomplete outcome data addressed? All outcomes | High risk | Completeness of outcome assessment: no: One patient in the control group developed intraventricular bleeding and irreversible hypotensive shock and was not analyzed. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Seri 1988 | Infants received dopamine on the basis of clinical condition. Controls did not require dopamine, whereas patients in the treatment group received dopamine for edema, moderate oliguria, poor peripheral perfusion and/or mild systemic hypotension. Thus, this study is not a randomized or quasi‐randomized study. |
| Tulassay 1983 | The authors used historical controls. |
Characteristics of studies awaiting assessment [ordered by study ID]
Cochran 1989.
| Methods | Not known |
| Participants | Not known |
| Interventions | Not known |
| Outcomes | Not known |
| Notes |
Contributions of authors
Keith Barrington (KB) and Luc Brion (LB) wrote the original review. The 2009 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne and Roger Soll) and approved by KB.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Baenziger 1999 {published data only}
- Baenziger O, Waldvogel K, Ghelfi D, Arbenz U, Fanconi S. Can dopamine prevent the renal side effects of indomethacin? A prospective randomized clinical study. Klinische Padiatrie 1999;211:438‐41. [DOI] [PubMed] [Google Scholar]
Fajardo 1992 {published data only}
- Fajardo CA, Whyte RK, Steele BT. Effect of dopamine on failure of indomethacin to close the patent ductus arteriosus [see comments]. Journal of Pediatrics 1992;121:771‐5. [DOI] [PubMed] [Google Scholar]
Seri 1984 {published data only}
- Seri I, Tulassay T, Kiszel J, Csomor S. The use of dopamine for the prevention of the renal side effects of indomethacin in premature infants with patent ductus arteriosus. International Journal of Pediatric Nephrology 1984;5:209‐14. [PubMed] [Google Scholar]
References to studies excluded from this review
Seri 1988 {published data only}
- Seri I, Hajdu J, Kiszel J, Tulassay T, Aperia A. Effect of low‐dose dopamine infusion on urinary prostaglandin E2 excretion in sick, preterm infants. European Journal of Pediatrics 1988;147:616‐20. [DOI] [PubMed] [Google Scholar]
Tulassay 1983 {published data only}
- Tulassay T, Seri I, Machay T, Kiszel J, Varga J, Csomor S. Effects of dopamine on renal functions in premature neonates with respiratory distress syndrome. International Journal of Pediatric Nephrology 1983;4:19‐23. [PubMed] [Google Scholar]
References to studies awaiting assessment
Cochran 1989 {published data only}
Additional references
Alpan 1985
- Alpan G, Eyal F, Vinograd I, Udassin R, Amir G, Mogle P, Glick B. Localized intestinal perforations after enteral administration of indomethacin in premature infants. Journal of Pediatrics 1985;106:277‐81. [DOI] [PubMed] [Google Scholar]
Barrington 1994
- Barrington KJ, Fox M. Predicting oliguria following indomethacin for treatment of patent ductus arteriosus. American Journal of Perinatology 1994;11:220‐2. [DOI] [PubMed] [Google Scholar]
Bergamo 1989
- Bergamo RR, Cominelli F, Kopple JD, Zipser RD. Comparative acute effects of aspirin, diflunisal, ibuprofen and indomethacin on renal function in healthy man. American Journal of Nephrology 1989;9:460‐3. [DOI] [PubMed] [Google Scholar]
Brion 2001
- Brion LP, Campbell DE. Furosemide for symptomatic patent ductus arteriosus in indomethacin‐treated infants (Cochrane Review). Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD001148] [DOI] [PubMed] [Google Scholar]
Chemtob 1991
- Chemtob S, Beharry K, Barna T, Varma DR, Aranda JV. Differences in the effects in the newborn piglet of various nonsteroidal antiinflammatory drugs on cerebral blood flow but not on cerebrovascular prostaglandins. Pediatric Research 1991;30:106‐11. [DOI] [PubMed] [Google Scholar]
Cheung 1996
- Cheung PY, Barrington KJ. Renal dopamine receptors: mechanisms of action and developmental aspects. Cardiovascular Research 1996;31:2‐6. [PubMed] [Google Scholar]
Cifuentes 1979
- Cifuentes RF, Olley PM, Balfe JW, Radde IC, Soldin SJ. Indomethacin and renal function in premature infants with persistent patent ductus arteriosus. J Pediatr 1979;95:583‐7. [DOI] [PubMed] [Google Scholar]
Clyman 1996
- Clyman RI. Recommendations for the postnatal use of indomethacin: an analysis of four separate treatment strategies. Journal of Pediatrics 1996;128:601‐7. [DOI] [PubMed] [Google Scholar]
Corazza 1984
- Corazza MS, Davis RF, Merritt TA, Bejar R, Cvetnic W. Prolonged bleeding time in preterm infants receiving indomethacin for patent ductus arteriosus. Journal of Pediatrics 1984;105:292‐6. [DOI] [PubMed] [Google Scholar]
Fowlie 1997
- Fowlie PW. Intravenous indomethacin for preventing mortality and morbidity in very low birth weight infants. Cochrane Database of Systematic Reviews 1997, Issue 3. [DOI: 10.1002/14651858.CD000174] [DOI] [PubMed] [Google Scholar]
Goldberg 1972
- Goldberg LI. Cardiovascular and renal actions of dopamine: potential clinical applications. Pharmacological Reviews 1972;24:1‐29. [PubMed] [Google Scholar]
Kellum 2001
- Kellum JA, M Decker J. Use of dopamine in acute renal failure: a meta‐analysis. Critical Care Medicine 2001;29:1526‐31. [DOI] [PubMed] [Google Scholar]
Kuhl 1985
- Kuhl G, Wille L, Bolkenius M, Seyberth HW. Intestinal perforation associated with indomethacin treatment in premature infants. European Journal of Pediatrics 1985;143:213‐6. [DOI] [PubMed] [Google Scholar]
Manoogian 1988
- Manoogian C, Nadler J, Ehrlich L, Horton R. The renal vasodilating effect of dopamine is mediated by calcium flux and prostacyclin release in man. Journal of Clinical Endocrinology and Metabolism 1988;66:678‐83. [DOI] [PubMed] [Google Scholar]
McCrory 1997
- McCrory C, Cunningham J. Low‐dose dopamine: will there ever be a scientific rationale?. British Journal of Anaesthesia 1997;78:350‐1. [DOI] [PubMed] [Google Scholar]
Mosca 1997
- Mosca F, Bray M, Lattanzio M, Fumagalli M, Tosetto C. Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. Journal of Pediatrics 1997;131:549‐54. [DOI] [PubMed] [Google Scholar]
Nehgme 1992
- Nehgme RA, O'Connor TZ, Lister G, Bracken MB. Patent Ductus Arteriosus. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992. [Google Scholar]
Pezzati 1999
- Pezzati M, Vangi V, Biagiotti R, Bertini G, Cianciulli D, Rubaltelli FF. Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. Journal of Pediatrics 1999;135:733‐8. [DOI] [PubMed] [Google Scholar]
Prins 2001
- Prins I, Plotz FB, Uiterwaal CS, Vught HJ. Low‐dose dopamine in neonatal and pediatric intensive care: a systematic review. Intensive Care Med 2001;27:206‐10. [DOI] [PubMed] [Google Scholar]
Smith 1995
- Smith WL, DeWitt DL. Biochemistry of prostaglandin endoperoxide H synthase‐1 and synthase‐2 and their differential susceptibility to nonsteroidal anti‐inflammatory drugs. Seminars in Nephrology 1995;15:179‐94. [PubMed] [Google Scholar]
Speziale 1999
- Speziale MV, Allen RG, Henderson CR, Barrington KJ, Finer NN. Effects of ibuprofen and indomethacin on the regional circulation in newborn piglets. Biology of the Neonate 1999;76:242‐52. [DOI] [PubMed] [Google Scholar]
Thompson 1994
- Thompson BT, Cockrill BA. Renal‐dose dopamine: A siren song?. Lancet 1994;344:7‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Van den Berghe 1996
- Berghe G, Zegher F. Anterior pituitary function during critical illness and dopamine treatment. Critical Care Medicine 1996;24:1580‐90. [DOI] [PubMed] [Google Scholar]
Van Wassenaer 1997
- Wassenaer AG, Kok JH, Dekker FW, Vijlder JJ. Thyroid function in very preterm infants: Influences of gestational age and disease. Pediatr Res 1997;42:604‐9. [DOI] [PubMed] [Google Scholar]
Van Wassenaer 1999
- Wassenaer AG, Kok JH, Briet JM, Pijning AM, deVijlder JJM. Thyroid function in very preterm newborns: Possible implications. Thyroid 1999;9:85‐91. [DOI] [PubMed] [Google Scholar]
Vane 1998
- Vane JR, Botting RM. Mechanism of action of anti‐inflammatory drugs. Advances in Experimental Medicine and Biology 1997;433:131‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Barrington 2002
- Barrington KJ, Brion LP. versus no treatment to prevent renal dysfunction in indomethacin‐treated preterm newborn infants. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003213] [DOI] [PMC free article] [PubMed] [Google Scholar]


