Abstract
Antimicrobial peptides (AMPs) are a promising class of compounds being developed against multi-drug resistant bacteria. Hybridization has been reported to increase antimicrobial activity. Here, two proline-rich peptides (consP1: VRKPPYLPRPRPRPL-CONH2 and Bac5-v291: RWRRPIRRRPIRPPFWR-CONH2) were combined with two arginine-isoleucine-rich peptides (optP1: KIILRIRWR-CONH2 and optP7: KRRVRWIIW-CONH2). Proline-rich antimicrobial peptides (PrAMPs) are known to inhibit the bacterial ribosome, shown also for Bac5-v291, whereas it is hypothesized a “dirty drug” model for the arginine-isoleucine-rich peptides. That hypothesis was underpinned by transmission electron microscopy and biological small-angle X-ray scattering (BioSAXS). The strength of BioSAXS is the power to detect ultrastructural changes in millions of cells in a short time (seconds) in a high-throughput manner. This information can be used to classify antimicrobial compounds into groups according to the ultrastructural changes they inflict on bacteria and how the bacteria react towards that assault. Based on previous studies, this correlates very well with different modes of action. Due to the novelty of this approach direct identification of the target of the antimicrobial compound is not yet fully established, more research is needed. More research is needed to address this limitation. The hybrid peptides showed a stronger antimicrobial activity compared to the proline-rich peptides, except when compared to Bac5-v291 against E. coli. The increase in activity compared to the arginine-isoleucine-rich peptides was up to 6-fold, however, it was not a general increase but was dependent on the combination of peptides and bacteria. BioSAXS experiments revealed that proline-rich peptides and arginine-isoleucine-rich peptides induce very different ultrastructural changes in E. coli, whereas a hybrid peptide (hyP7B5GK) shows changes, different to both parental peptides and the untreated control. These different ultrastructural changes indicated that the mode of action of the parental peptides might be different from each other as well as from the hybrid peptide hyP7B5GK. All peptides showed very low haemolytic activity, some of them showed a 100-fold or larger therapeutic window, demonstrating the potential for further drug development.
Keywords: antimicrobial peptide, hybrid peptide, BioSAXS, multi-drug resistance, antimicrobial compound, mode of action, ultrastructural changes, TEM
Introduction
In the light of the current headlines of the COVID-19 pandemic, the problem of resistance against antibiotics is still progressing and is frequently called a silent pandemic. Especially the beginning of the COVID-19 pandemic demonstrated severely the multitude of effects of an infectious disease where no effective treatment is available. The development of microbial resistance against natural occurring or human-made antibiotics is a natural selection process and was soon discovered after the use of the first antibiotic. With the emergence of multi-drug resistant strains and a dwindling development of antibacterial drugs with new modes of action, the health care systems worldwide are under threat to face a more severe pandemic if no novel antibiotics are developed. Many operations, transplantations, and immunosuppressant therapies may not be performable any longer, leaving modern medicinal care devastated. Hence, it is important and urgent to expand the discovery and translation of new alternatives to treat bacterial infections, especially with new modes of action.
According to the world health organization (WHO, 2019), more than 700,000 people die worldwide each year because of antibiotic-resistant infections. If nothing is done about this antimicrobial-resistant crisis, it was estimated in the O’Neil Report, that the numbers will increase to 10,000,000 by 2050, (https://amr-review.org/Publications.html). In 2017 the WHO has identified a list of priority pathogens where research and development shall focus on the development of novel antimicrobials. Some pathogens that were already included in other programs were omitted in that list, for example, Mycobacterium tuberculosis. The WHO defined three groups, Priority 1 (CRITICAL), that includes carbapenem-resistant Acinetobacter baumannii, carbapenem-resistant Pseudomonas aeruginosa and carbapenem-resistant, ESBL-producing Enterobacteriaceae (including Klebsiella, E. coli, Serratia, and Proteus), Priority 2 (HIGH), that includes vancomycin-resistant Enterococcus faecium, methicillin-resistant and vancomycin-intermediate and resistant Staphylococcus aureus, clarithromycin-resistant Helicobacter pylori, fluoroquinolone-resistant Campylobacter spp. fluoroquinolone-resistant Salmonellae, and cephalosporin-resistant and fluoroquinolone-resistant Neisseria gonorrhoeae, Priority 3 (MEDIUM) penicillin-non-susceptible Streptococcus pneumoniae, ampicillin-resistant Haemophilus influenzae and fluoroquinolone-resistant Shigella spp.
Antimicrobial peptides (AMPs) are an extremely diverse group of compounds, found in all kingdoms of life that show various biological functionalities, for example, antibacterial, antifungal, antiviral, antiparasitic, anticancer, and immunomodulatory (Mahlapuu et al., 2016). Several databases are dedicated to AMPs (Pirtskhalava et al., 2016; Wang et al., 2016; Kang et al., 2019). What makes them very interesting for antimicrobial drug development is the fact that they have also numerous modes of action (Brogden, 2005), including inhibiting lipid 2, a cell-wall precursor essential for the bacteria (de Leeuw et al., 2010; Schmitt et al., 2010), blocking the synthesis of important outer membrane proteins (Carlsson et al., 1991), binding to intracellular molecules like histones, RNA, DNA (Kobayashi et al., 2000; Hale and Hancock, 2007; Cho et al., 2009; Xie et al., 2011), DNA-dependent enzymes (Marchand et al., 2006; Hilpert et al., 2010), and ribosomes (Knappe et al., 2016; Krizsan et al., 2014, 2015b; Mardirossian et al., 2014, 2018b, 2019a, 2020). Furthermore, a study investigated the effects of AMP’s on blood components, since most peptides have low bioavailability and therefore the most probable manner of administration for systemic use is intravenously (Yu et al., 2015). Consequently, the safety and efficacy of some AMPs have been investigated in clinical trials (Czaplewski et al., 2016; Greber and Dawgul, 2017).
Bac5 (43mer) is a proline-rich AMP (PrAMP) from the cathelicidin class, isolated from bovine neutrophils more than 30 years ago (Gennaro et al., 1989). Recently, fragments of Bac5 were investigated and the mode of action was described (Mardirossian et al., 2018a). Bac5 fragments inhibit bacterial protein synthesis as other PrAMPs and are most active against Gram-negative pathogens (Tokunaga et al., 2001; Mardirossian et al., 2019b). The N-terminal 1–17 amino acid fragment of Bac5, called Bac5(1–17), retained antimicrobial activity and the same overall mechanism of action (Mardirossian et al., 2019b). Bac5(1–17) was optimized using spot synthesis and variant 291 (RWRRPIRRRPIRPPFWR-CONH2) showed improved antimicrobial activity while displaying low toxicity and the same mode of action as the parental peptide (Mardirossian et al., 2019a).
We have developed a novel prediction method (unpublished results) for AMPs with a low haemolytic activity that was based on our peptide library screen of 3,000 members (Mikut et al., 2016). Two arginine-isoleucine-rich peptides optP1 (KIILRIRWR) and optP7 (KRRVWIIW), were identified from this new prediction strategy, showing broad-spectrum activity. The peptide optP7 was used as a lead and selected for studying lipidation, glycosylation, cyclisation, and grafting into a cyclotide (Grimsey et al., 2020; Koehbach et al., 2021). Hybridization of different AMPs has been investigated to further improve their performance (Shang et al., 2020; Wade et al., 2018, 2019). Here we report the creation of different hybrid molecules using peptides optP1 (KIILRIRWR) and optP7 (KRRVWIIW) in combination with the Bac5(1–17) variant 291 (RWRRPIRRRPIRPPFWR-CONH2) and a consensus sequence from proline-rich antimicrobial peptides, called consP1 (VRKPPYLPRPRPRPL-CONH2). The antimicrobial activity and haemolytic activity of the parental and hybrid peptides were determined. Small-angle X-ray scattering (SAXS) measurements, performed at the BioSAXS beamline, showed differences in the ultrastructural changes of E. coli for two selected hybrid peptides compared to the parental peptides.
Materials and Methods
Peptides
Automated solid-phase peptide synthesis (SPPS) on a MultiPep RSI peptide synthesizer (Intavis, Tuebingen; Germany) was used to produce the AMPs. We applied 9-fluorenyl-methoxycarbonyl-tert-butyl (Fmoc/tBu) strategy and used N,N,N′,N′-Tetramethyl-O-(1H-benzotriazole-1-yl)uronium hexafluorophosphate (HBTU) activation. Crude peptides were analysed and purified to the homogeneity of >90% by liquid chromatography-electrospray ionisation mass spectrometry (LC-ESI-MS). For a detailed description please see (Grimsey et al., 2020). The peptide hyP7B5Cys and consP1 were purchased at Synpeptide Co., Ltd. (Shanghai, China).
Bacterial Strains
In this project the following bacterial strains were used: 1) methicillin-resistant Staphylococcus aureus (S. aureus) HO 5096 0412 (a neonatal infection isolate, isolated in Ipswich, England in 2005, obtained from Jody Lindsay (St. George’s, University of London), 2) Escherichia coli (E. coli, UB1005, F-, LAM-, gyrA37, relA1, spoT1, metB1, LAMR) used solely for transmission electron microscopy, E. coli (ATCC 25922), 3) Pseudomonas aeruginosa PAO1 obtained from Dr Robert E.W. Hancock (Department of Microbiology and Immunology, University of British Columbia).
Bacteriological Media and Culture Conditions
Mueller-Hinton broth (MHb) (Merck, Life Science UK Limited, Dorset, UK)) was prepared concurring to the manufacturer’s directions was used to grow the bacterial strains. Liquid bacterial cultures were incubated at 37°C for 18–20 h on a shaker, bacterial culture on solid media was incubated for 18–24 h at 37°C.
Minimal Inhibitory Concentration Determination
A broth microdilution assay was applied to determine the minimum inhibitory concentrations (MIC) in MHb for both, using 5 × 105 CFU/ml (MICs in Table 1) and 108 CFU/ml (BioSAXS and electron microscopy, values in Table 2). A detailed description of the method can be found in the open-source publication by von Gundlach et al. (von Gundlach et al., 2019a).
TABLE 1.
Minimum inhibitory concentrations (MIC) in µM. Data are for n = 3 with modal values reported; all values determined in Mueller-Hinton broth (MHb) using 5 × 105CFU/ml bacteria; MRSA = methicillin-resistant Staphylococcus aureus, HC50 represents the peptide concentration needed to induce haemolysis in human red blood cells at 50%. * means that all peptides are C-terminal amidated. PA stands for P. aeruginosa, n.a. for not applicable. The therapeutic window is given for MRSA only, as an example for their potential as a drug development candidate.
| Name | Description | Sequence* | MRSA | E. coli | PA | HC50 | Therapeutic window HC50/MIC(MRSA) |
|---|---|---|---|---|---|---|---|
| optP1 | Optimized 9mer | KIILRIRWR | 1.5 | 1 | 6 | 205 | 137 |
| optP7 | Optimized 9mer | KRRVRWIIW | 6 | 1.5 | 3 | >195 | >32.5 |
| consP1 | Consensus sequence derived from multiple alignment | VRKPPYLPRPRPRPL | >139 | 35 | >139 | >139 | n.a |
| hyP1CoG1 | Hybrid peptide with Gly linker, optP1 C-terminal | KIILRIRWRGGGVRKPPYLPRPRPRPL | 2.5 | 1 | 1 | >157 | >62.8 |
| hyP1CoG2 | Hybrid peptide with Gly linker, optP1 C-terminal | VRKPPYLPRPRPRPLGGGKIILRIRWR | 2.5 | 1 | 2.5 | >157 | >62.8 |
| hyP7CoG1 | Hybrid peptide with Gly linker, optP7 C-terminal | VRKPPYLPRPRPRPLGGGKRRVRWIIW | 5 | 1 | 5 | >154 | >30.8 |
| hyP7CoG2 | Hybrid peptide with Gly linker, optP7 N-terminal | KRRVRWIIWGGGVRKPPYLPRPRPRPL | 5 | 2.5 | 2.5 | >154 | >30.8 |
| Bac5-v291 | Optimized Bac5(1–17) variant 291 | RWRRPIRRRPIRPPFWR | 27 | 1.7 | 27 | >278 | >10.3 |
| hyP7B5G | Hybrid peptide with Gly linker, optP7 C-terminal | RWRRPIRRRPIRPPFWRGGGKRRVRWIIW | 2 | 2 | 2 | 102 | 51 |
| hyP7B5K | Hybrid peptide with Lys linker, optP7 C-terminal | RWRRPIRRRPIRPPFWRKKKKRRVRWIIW | 2 | 2 | 2 | 82 | 41 |
| hyP7B5GK | Hybrid peptide with Gly-Lys linker, optP7 C-terminal | RWRRPIRRRPIRPPFWRKGKGKGKRRVRWIIW | 1 | 1 | 2 | >120 | >120 |
| hyP7B5Cys | Hybrid peptide with disulfide bridge, designed to be cleaved in the cytosol, optP7 C-terminal | RWRRPIRRRPIRPPFWRKGKC-S-S-CKGKRRVRWIIW | 1 | 0.6 | 2 | 102 | >102 |
TABLE 2.
Minimum inhibitory concentrations (MIC) in µM. Data are for n = 3 with modal values reported; all values determined in Mueller-Hinton broth (MHb) using 1 × 108CFU/ml bacteria; * All peptides are C-terminal amidated.
| Name | Description | Sequence* | E. coli |
|---|---|---|---|
| optP7 | Optimized 9mer | KRRVRWIIW | 14 |
| Bac5-v291 | Optimized Bac5(1–17) variant 291 | RWRRPIRRRPIRPPFWR | 1.1 |
| hyP7B5GK | Hybrid peptide with Gly-Lys linker, optP7 C-terminal | RWRRPIRRRPIRPPFWRKGKGKGKRRVRWIIW | 4 |
| hyP7B5Cys | Hybrid peptide with disulfide bridge, designed to be cleaved in the cytosol, optP7 C-terminal | RWRRPIRRRPIRPPFWRKGKC-S-S-CKGKRRVRWIIW | 4 |
Haemolytic Activity Assessment
The protocol to determine the haemolytic activity of the peptides and HC50 values were described previously (Grimsey et al., 2020). Briefly, human blood was washed and diluted with PBS (4% v/v) and incubated with a pre-made dilution series of the peptides and incubated for 1 h at 37 C. The 100% haemolysis value was achieved by incubation with Triton X (1% final concentration). Haemoglobin was measured at 414 and 546 nm using an ELISA plate reader. HC50 values were determined using the Prism software (GraphPad).
Sample Preparation, Measurement and Data Evaluation for BioSAXS
A detailed description of the sample preparation for BioSAXS, the BioSAXS measurement and consequently the data evaluation methods can be found in the open-source publication by von Gundlach et al. (von Gundlach et al., 2019). In brief, suspensions of the bacteria after different treatments with two x MIC10^8 (40 min, 37) were injected by an autosampler (Franke et al., 2012). After multiple exposures, the background was subtracted, and the scattering curves calculated. Data evaluation was done using the toolbox Gait-CAD and SciXMiner (“Peptide Extension”) (Mikut, 2010; Mikut et al., 2017). Data were analysed using a principal component analysis (PCA) using the frequency range 0.05 nm−1 to 0.412 nm−1.
Electron Microscopy
Sample preparation for transmission electron microscopy was performed by the Image Resource Facility, St Georges University. Overnight cultures of E. coli were logarithmically grown to 108 CFU/ml. Untreated and treated with optP7 samples were incubated for specific time points. At the desired time points sample was removed, centrifuged, washed with 0.1 M PIPES buffer pH 7 (Merck Life Science UK Limited, Dorset, UK), and fixed with 2.5% glutaraldehyde in cacodylate buffer (Merck Life Science UK Limited, Dorset, UK). The samples were then washed with PIPES buffer and incubated in 2% osmium tetroxide (Taab Laboratories, Berkshire, UK). A series of ethanol washes followed to dehydrate the samples with 70, 90, and thrice with 100% ethanol for at least an hour. The samples were then washed with propylene oxide (Scientific Laboratory Supplies Ltd., Nottingham, UK) and transferred as a 1:1 mixture into resin (Agar Scientific, Chelmsford, UK) for 45 min. Then this mixture was replaced with 100% resin and placed at 60°C for 48 h. Thin sections of resin (80–100 nm) were cut using a Reichert-Jung ultracut E Microtome (Leica Microsystems, Wetzlar, Germany) and collected on copper mesh grids (Agar Scientific, Chelmsford, UK). They were then stained with 2% uranyl acetate in an aqueous solution (Taab Laboratories, Berkshire, UK) for 15 min followed by Reynolds lead citrate stain (VWR International Ltd., Leicestershire, UK) (Reynolds, 1963) for 2 min. Samples were examined using a Hitachi 7100 electron microscope (Hitachi Europe Limited, Berkshire, UK) operating at 75 kV.
Results
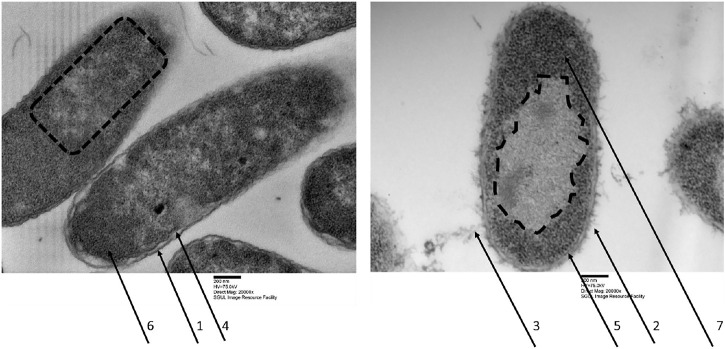
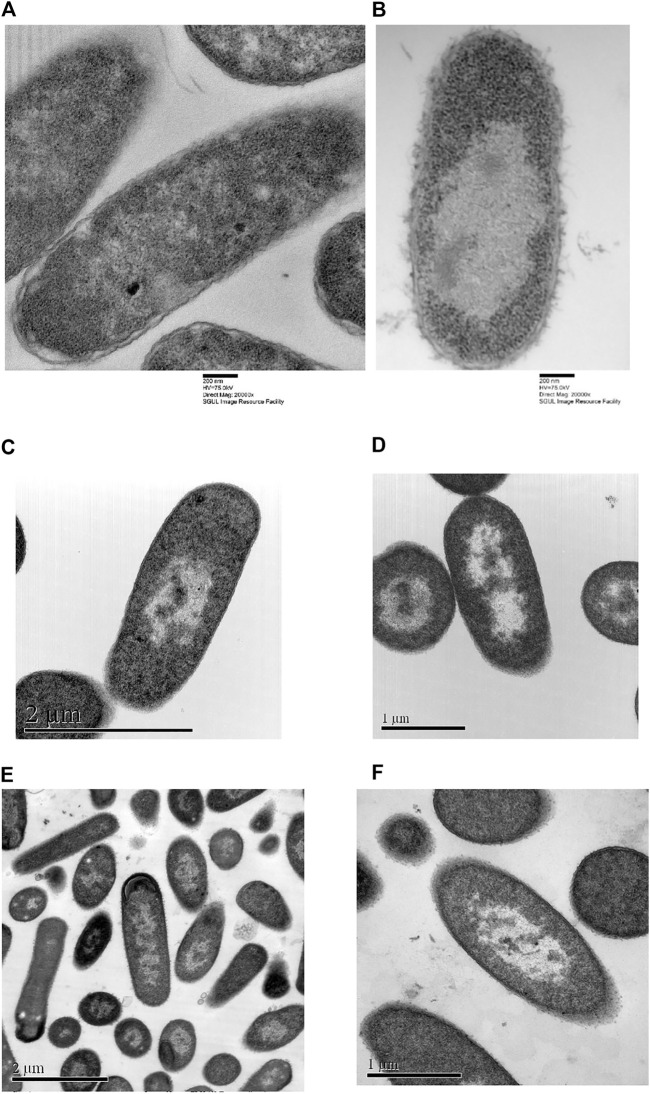
Quite early on in our drug development process, we have selected optP7 over optP1 for a slightly better MIC against P. aeruginosa. Further investigations were performed with peptide optP7 only. Firstly, we investigated E. coli cells treated with optP7 by transmission electron microscopy (TEM) to get a better understanding of the mode of action/ultrastructural changes of the peptides on E. coli, see Figure 1. In order to achieve a sufficient number of bacteria to form a pellet, a bacterial concentration of 1 × 108 CFU/ml was used. The MIC of optP7 against E. coli at 1 × 108 CFU/ml was determined at 14 μM, see Table 2. The peptide was incubated with the bacteria for 60 min at twice the MIC (28 µM). The vast majority of the cells showed no sign of lysis, see Figure 1. The outer membrane of E. coli of the untreated sample appears thick and relatively smooth (Figure 1, arrow 1), whereas for the peptide treated one, it was damaged (Figure 1, arrow 2) and blebbing was visible (Figure 1, arrow 3), where lipopolysaccharides (LPS) form small vesicles and are shed from the cell. The inner membrane for both, the untreated control (Figure 1A, arrow 4), and the peptide treated E. coli (Figure 1, arrow 5) seemed to remain intact and no spillage of the cytosol content was observed. The DNA in untreated bacterial cells comprised dispersed fibrils in the cytoplasm, see Figure 1, dotted lines. The bacterial DNA is concentrated in a ‘DNA–plasm’ called the nucleoid (Ohniwa et al., 2007). The peptide optP7 creates an enlarged and spherical nucleoid, dotted line in Figure 1., typically seen in DNA-dependent RNA polymerase inhibitors and protein synthesis inhibitors. Compared to the untreated bacteria (Figure 1, arrow 6), the peptide treated ones show more pronounced granular substructures (Figure 1, arrow 7) in the cytosol could indicate aggregation of cytosolic proteins. These substructures were observed before using small AMPs (Von Gundlach et al., 2016a, 2019).
FIGURE 1.
Transmission electron micrographs of Escherichia coli, left untreated samples, right treated with optP7 for 60 min, 20,000 times magnification. The dotted lines indicate the nucleoid, arrows one and two show the outer membrane, three shows blebbing of the outer membrane and release of LPS, four and five show intact inner membrane, six and seven show granular structures in the cytosol.
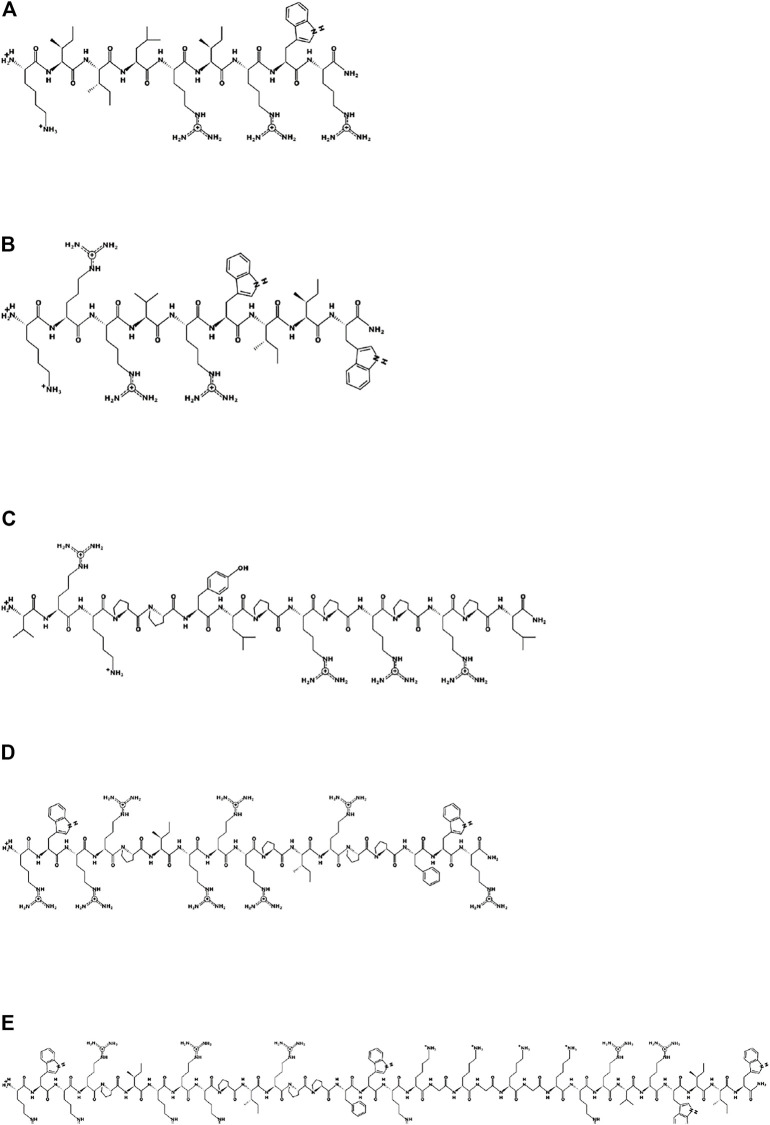
Based on the two arginine-isoleucine-rich peptides optP1 and optP7, hybrid peptides were created by merging with two proline-rich peptides. One PrAMP was the optimized Bac5(1–17) variant 291 (Mardirossian et al., 2019a). The second one was created by a multiple sequence alignment of PrAMPs. From the alignment, a consensus sequence was derived (consP1: VRKPPYLPRPRPRPL-CONH2), see Figure 2. Various linkers were tested to combine these peptides, see Table 1.
FIGURE 2.
Schematic representation of the parent peptides and one hybrid peptide using the program PEPDRAW (http://pepdraw.com/). (A) optP1, KIILRIRWR-CONH2, (B) optP7, KRRVRWIIW-CONH2, (C) consP1, VRKPPYLPRPRPRPL-CONH2, (D) Bac5-v291, RWRRPIRRRPIRPPFWR-CONH2, (E) hyP7B5GK, RWRRPIRRRPIRPPFWRKGKGKGKRRVRWIIW-CONH2.
All peptides were tested against the Gram-negative bacteria Pseudomonas aeruginosa PA01 and Escherichia coli UB1005, and the Gram-positive methicillin-resistant bacteria Staphylococcus aureus HO 5096 0412 (MRSA). The peptides optP1 and optP7 showed strong activity against all tested bacteria, see Table 1. The peptide consP1 showed only activity against E. coli and Bac5-v291 showed a medium activity against MRSA and P. aeruginosa, and strong activity against E. coli. The first four hybrid peptides (hyP1CoG1/G2 and hyP7CoG1/G2) are combinations of optP1 and optP7 with consP1. We wanted to investigate whether the order of the peptides within the hybrid molecule plays a role concerning their antimicrobial and/or their hemolytic activity, see Table 1. Therefore the same flexible linker (GGG) was used in all four peptides to be able to compare the effect of the order of peptides. The combination of consP1 and optP1 resulted in hyP1CoG1 and hyP1CoG2. Both hybrid peptides showed a strongly increased activity against all bacteria when compared to consP1. When compared to optP1 they showed a decrease in activity against MRSA. The antimicrobial activity against E. coli remains unchanged in comparison to optP1. In contrast, hyP1CoG1 shows a 6-fold improvement in antimicrobial activity against P. aeruginosa and hyP1CoG2 shows a 2.4-fold improvement compared to optP1. The combination consP1 and optP7 resulted in the hybrid peptides of hyP7CoG1 and hyP7CoG2, see Table 1. Both peptides demonstrate a strong improvement in activity when compared to consP1. When compared to optP7, nearby the same antimicrobial activity against MRSA was determined. The peptide hyP7CoG1shows a similar MIC against E. coli, hyP7CoG2 shows a 1.7-fold decrease compared to optP7. Activity against P. aeruginosa dropped 1.7-fold for hyP7CoG1and was very similar for hyP7CoG2 when compared to optP7. In addition, the order of peptides within the hybrid molecule seems not to be important for their antimicrobial or hemolytic activity. Based on that observation, the order of peptides in the next set of hybrid peptides was not changed (first Bac5-v291 followed by optP7). In this set, we investigated four different linkers. The first was the GGG-linker for flexibility (hyP7B5G), the second a KKK-linker to improve charge (hyP7B5K), the third a GKGKG-linker to combine flexibility and charge (hyP7B5GK). The fourth linker was similar to the third, however, the hybridisation was achieved by a cysteine bridge (hyP7B5Cys). The cysteine bridge is expected to be cleaved in the reducing environment of the bacterial cytosol and both peptides could then act independently once inside the bacterial cytosol. Where the use of the third and fourth linker show a slightly higher antimicrobial activity, the third linker (GKGKG) provides the least toxicity combined with strong antimicrobial activity.
All the hybrid peptides in this set were 13.5-fold more active against P. aeruginosa when compared to Bac5-v291 and showed very similar MIC values when compared to optP7. Peptides hyP7B5G, hyP7B5K, and hyP7B5GK show also very similar MIC values when compared to Bac5-v291 and optP7. The exception is peptide hyP7B5Cys, which showed a 2.5-fold increase in activity compared to optP7 and a 2.8-fold increase compared to Bac5-v291. All the peptides in this set are more active against MRSA when compared to both, Bac5-v291 and optP7. Peptides hyP7B5G, hyP7B5K showed a 13.5-fold improvement, peptides hyP7B5GK and hyP7B5Cys a 27-fold improvement compared to Bac5-v291. Similarly, peptides hyP7B5G, hyP7B5K showed a 3-fold improvement, peptides hyP7B5GK and hyP7B5Cys a 6-fold improvement compared to Bac5-v291. All peptides showed very low haemolytic activity (Table 1). The peptides with the highest haemolytic activity still showed at least a 10-fold therapeutic window, some of them 100-fold or larger.
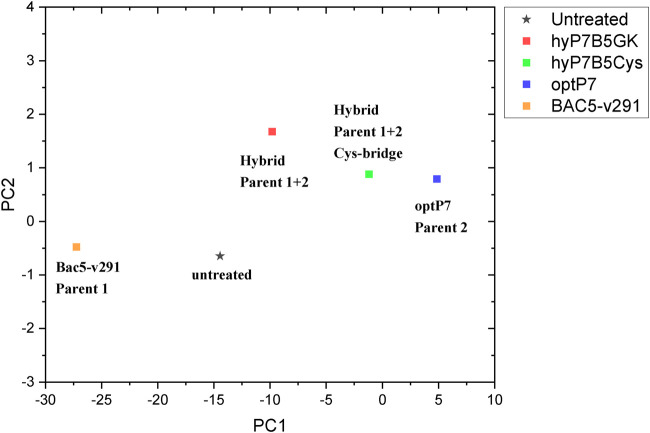
Small-angle X-ray scattering for biological samples (BioSAXS) on the P12 beamline can be used to measure ultrastructural changes within the bacteria in the range between 2 and 157 nm, from which we used the range from 20 to 120 nm for analysis of the bacteria after treatment with the antimicrobial peptides. In recent years we used this method to classify the mode of action of various antibiotics and antimicrobial peptides against E. coli and MRSA (Von Gundlach et al., 2016a; Von Gundlach et al., 2016b; Von Gundlach A. R. et al., 2019). Here we used BioSAXS to investigate the ultrastructural changes of E. coli due to the exposition of the selected parental peptides optP7, Bac5-v291, and the hybrid peptides hyP7B5GK and hyP7B5Cys. BioSAXS measurements were performed using bacteria at 1 × 108 CFU/ml to have high enough bacteria densities for the scattering experiments. The MICs for the selected peptides against E. coli at 1 × 108 CFU/ml were determined (Table 2). BioSAXS measurements were performed at 2x MIC. The corresponding scattering curves of the peptide treated bacteria are presented in Figure 3. To quantify the subtle changes between the different treatments we used a PCA analysis using SciXMiner and the “Peptide Extension” tool (Mikut, 2010; Mikut et al., 2017) (Figure 4). In particular, the two first principal components PC1 and PC2 were used for representation as they provide a suitable and sensitive indicator for structural changes caused by antibiotic treatment (Von Gundlach et al., 2016a; Von Gundlach et al., 2016b; von Gundlach A. R. et al., 2019). The two-dimensional representation of PC1 and PC2 allows to compare the peptide treated bacteria with each other and to the untreated controls. SAXS curves of treated bacteria with similar principal components showed similar internal structures while stronger structural alterations occur if points appear in separated positions in the plot. However, at the current state of knowledge using this method for the investigation of bacteria, we are not yet able to pinpoint what structural changes occur within the bacteria. That will need further research. The proline-rich peptide Bac5-v291 separated very well in the PCA from the short peptide optP7. This shows that the ultrastructural changes induced by these two peptides are very different. This is in agreement with our hypothesis that these two peptides have a different mode of action. The hybrid peptide hyP7B5GK is in between the parent peptides on PC1 and also shows an increase in PC2. The other hybrid peptide hyP7B5Cys is closer to the parent optP7. This hybrid peptide is designed to fall apart in the cytosol due to the reducing environment and it seems that optP7 in this case, drives the ultrastructural changes.
FIGURE 3.
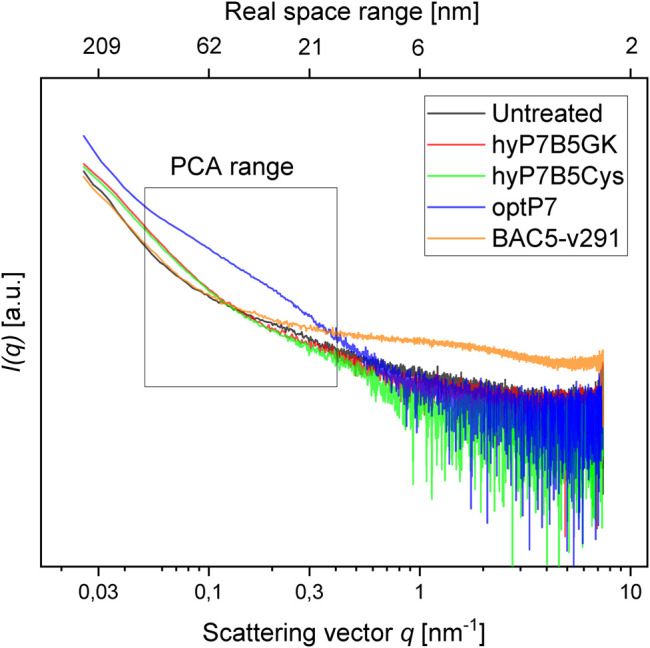
Scattering data from untreated E. coli, and treated with four different peptides after 60 min, as measured at the P12 BioSAXS beamline at PETRA III (Hamburg, Germany). The principal component analysis (PCA) was calculated using data within the box.
FIGURE 4.
Principal component analysis (PCA) graph, obtained from SAXS curves of E. coli, untreated and treated with four different peptides.
Discussion
Antimicrobial resistance is a silent pandemic that needs urgent solutions in order to be able to provide appropriate health care. Antimicrobial peptides are a very broad and diverse class of compounds that can kill multi-drug resistant bacteria and therefore have the potential to become the next generation of antibiotics. Based on the success of the hybridization strategy reported by others, for example (Fox et al., 2012; Xu et al., 2014; Wade et al., 2018, 2019; Shang et al., 2020), we hypothesized that hybrid molecules from two different classes of AMPs, which we have identified in the past as potential antimicrobial drug candidates, may lead to a further improvement of antimicrobial activity. Thus we combined the excellent activity of proline-rich and arginine-isoleucine-rich AMPs. Proline-rich AMPs (PrAMPs), like Bac5, have no lytic mode of action but enter the cytosol by the membrane transporter SbmA and to a small proportion by the MdtM complex (Mattiuzzo et al., 2007; Krizsan et al., 2015a). PrAMPs inhibit protein synthesis by targeting the bacterial ribosome (Krizsan et al., 2014; Mardirossian et al., 2020). The mode of action of the two short (9-13mer) arginine-isoleucine-rich AMPs optP1 and optP7 used in this study is not yet determined. Our current theory is that these two peptides possess a rather “dirty drug” modality by stressing the cell wall by replacing Ca2+ ions, depolarizing the cell membrane, entering the cell by passive transport and binding ATP and other negatively-charged molecules like DNA and RNA and hence interact and inhibit various processes in the cell (Hilpert et al., 2006, 2010; Von Gundlach et al., 2016a, 2019). TEM experiments revealed that the peptides induced outer membrane stress and created an enlarged and spherical nucleoid with some small denser foci, typically seen in DNA-dependent RNA polymerase inhibitors and protein synthesis inhibitors. In addition, a non-lytic mode of action was observed.
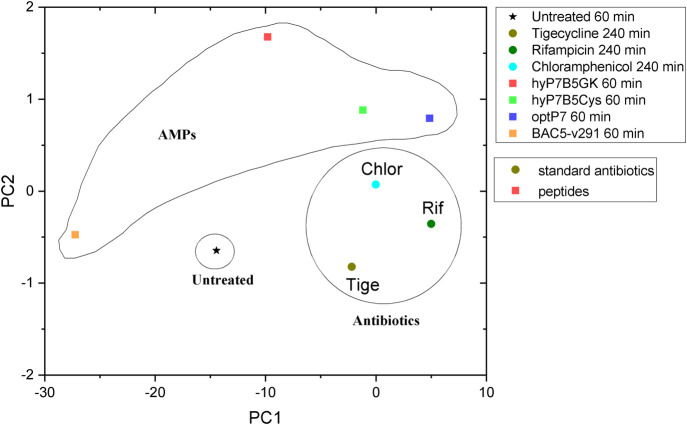
To discuss the obtained results in the context of conventional antibiotics, compared the peptides to three antibiotics, tigecycline (bacteriostatic activity by binding to the 30S ribosomal subunit of the bacterial ribosome), chloramphenicol (bacteriostatic activity by binding to the 30S ribosomal subunit of the bacterial ribosome), and rifampicin (bactericidal activity by inhibiting the DNA-dependent RNA polymerase activity), see Figure 5. This data was measured at the same beamtime session as the peptide set. As the antibiotics have slower kill kinetics than the AMPs, 240 min incubation time was used. The concentration of the standard antibiotics (tigecycline, MIC 4 μg/ml, chloramphenicol MIC 8 μg/ml, rifampicin MIC 15 μg/ml) was 3x their MIC against E. coli. The PCA showed that all peptides induced ultrastructural changes were distinctively different from conventional antibiotics. The PCA values of Bac5-v291 and hyP7B5GK deviate strongly from the untreated controls and the standard antibiotics. The PCA values of peptides hyP7B5Cys and optP7 are nearest to chloramphenicol.
FIGURE 5.
Comparison between principal component analysis (PCA) of the BioSAXS curves of E. coli treated with different AMPs and antibiotics.
Comparison between TEM pictures of E. coli treated with optP7, chloramphenicol, and tetracycline are shown in Figure 6. Very similar to the PCA of the BioSAXS data, the changes in E. coli treated with opP7 are distinctively different from the changes caused by the two antibiotics. They also show similarities, especially the enlarged and central nucleoid, and therefore explains well the closeness seen in the PCA. While TEM can only provide “snapshots” of all bacteria, BioSAXS reveals internal morphological changes averaged across hundreds of millions of bacteria, thus providing an unbiased representation of the treatment effects.
FIGURE 6.
Transmission electron micrographs of Escherichia coli. (A) Untreated samples, (B) treated with optP7 for 60 min, at 32 μg/ml, (C) and (D) treated with chloramphenicol for 240 min, 60 μg/ml, (E) and (F) treated with tetracycline for 240 min, 30 μg/ml.
All hybrid peptides showed a stronger activity against E. coli compared to the proline-rich ones, with Bac5-v291 as the only exception. The increase in activity compared to the arginine-isoleucine-rich peptides was up to 6-fold and dependent on the combination of peptides and bacteria. We have observed a very similar pattern while studying lipidation, glycosylation, cyclisation, and grafting into a cyclotide (Grimsey et al., 2020; Koehbach et al., 2021). All these modifications did not lead to a general improvement and were therefore not a “one fits all strategy”. A very important positive result was the high HC50 values of all peptides. Some of them showed a larger than 100fold therapeutic window (HC50/MIC). It demonstrates the potential of such compounds to be developed further into drug candidates against multi-drug resistant bacteria.
Interestingly, the hybrid peptide hyP7B5GK shows very different ultrastructural changes as both parents, indicating that both modes of action are creating ultrastructural changes at the same time and/or a novel mode of action occurred. In contrast, the hybrid molecule that is designed to fall apart in the bacterial cytosol by the reduction potential of that environment is closer to the parental peptide optP7, indicating a dominant contribution of the optP7 compound.
In conclusion, the creation of hybrid peptides leads in some cases to compounds with improved activity, which depends also on the target bacteria. Both parent peptides show unique effects on the ultrastructure of E. coli and the hybrid peptide hyP7B5GK shows yet another pattern of ultrastructural change, as measured by BioSAXS.
Acknowledgments
We acknowledge the use of the Image Resource Facility, St George’s University. We thank Thomas Bruckdorfer from Iris Biotechnology for the discussion and recommendations of creating a peptide that will be cleaved in the cytosol. We acknowledge the team of P12 BioSAXS beamline at EMBL, Hamburg for excellent support. KH thanks the Universe/life for the opportunity to continue on and despite the odds to be able to keep researching.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
KH conceptualization, resources (arginine-isoleucine-rich peptides, assay development), data analysis, supervision, funding acquisition, writing—original draft preparation, writing—review and editing; JG investigation, data curation (peptide synthesis and purification, MICs, haemolytic assay, TEM pictures); CR investigation, data curation (MICs, BioSAXS data analysis); NS investigation, data curation (MICs, BioSAXS sample preparation); PL-P; investigation, data curation (peptide synthesis and purification); VG resources (BioSAXS); AR VG, investigation, data curation (TEM pictures); PM, resource (BioSAXS), MS resource (proline-rich peptides), writing—review and editing; RM resource (BioSAXS data analysis), funding acquisition; AR resources (BioSAXS), supervision, funding acquisition, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by an Institute of Infection and Immunity start-up grant (KH). RM was funded by the BIFTM program of the Helmholtz Association. Funding by BMBF (BMBF 05K19PC2) is acknowledged (AR). We acknowledge the support of the Open Access Publishing Fund of the Karlsruhe Institute of Technology.
Conflict of Interest
Author P.M.L.-P. was employed by TiKa Diagnostics Ltd (London, UK). KH is a founder and director of TiKa Diagnostics Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Brogden K. A. (2005). Antimicrobial Peptides: Pore Formers or Metabolic Inhibitors in Bacteria? Nat. Rev. Microbiol. 3, 238–250. 10.1038/nrmicro1098 [DOI] [PubMed] [Google Scholar]
- Cabrera J. E., Cagliero C., Quan S., Squires C. L., Jin D. J. (2009). Active Transcription of rRNA Operons Condenses the Nucleoid in Escherichia coli: Examining the Effect of Transcription on Nucleoid Structure in the Absence of Transertion. J. Bacteriol. 191, 4180–4185. 10.1128/JB.01707-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson A., Engström P., Palva E. T., Bennich H. (1991). Attacin, an Antibacterial Protein from Hyalophora Cecropia, Inhibits Synthesis of Outer Membrane Proteins in Escherichia coli by Interfering with Omp Gene Transcription. Infect. Immun. 59, 3040–3045. 10.1128/IAI.59.9.3040-3045.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J. H., Sung B. H., Kim S. C. (2009). Buforins: Histone H2A-Derived Antimicrobial Peptides from Toad Stomach. Biochim. Biophys. Acta 1788, 1564–1569. 10.1016/J.BBAMEM.2008.10.025 [DOI] [PubMed] [Google Scholar]
- Czaplewski L., Bax R., Clokie M., Dawson M., Fairhead H., Fischetti V. A., et al. (2016). Alternatives to Antibiotics-A Pipeline Portfolio Review. Lancet Infect. Dis. 16, 239–251. 10.1016/S1473-3099(15)00466-1 [DOI] [PubMed] [Google Scholar]
- de Leeuw E., Li C., Zeng P., Li C., Diepeveen-de Buin M., Lu W. Y., et al. (2010). Functional Interaction of Human Neutrophil Peptide-1 with the Cell wall Precursor Lipid II. FEBS Lett. 584, 1543–1548. 10.1016/j.febslet.2010.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. A., Thwaite J. E., Ulaeto D. O., Atkins T. P., Atkins H. S. (2012). Design and Characterization of Novel Hybrid Antimicrobial Peptides Based on Cecropin A, LL-37 and Magainin II. Peptides 33, 197–205. 10.1016/j.peptides.2012.01.013 [DOI] [PubMed] [Google Scholar]
- Franke D., Kikhney A. G., Svergun D. I. (2012). Automated Acquisition and Analysis of Small Angle X-ray Scattering Data. Nucl. Instr. Methods Phys. Res. Section A: Acc. Spectrometers, Detectors Associated Equipment 689, 52–59. 10.1016/J.NIMA.2012.06.008 [DOI] [Google Scholar]
- Gennaro R., Skerlavaj B., Romeo D. (1989). Purification, Composition, and Activity of Two Bactenecins, Antibacterial Peptides of Bovine Neutrophils. Infect. Immun. 57, 3142–3146. 10.1128/IAI.57.10.3142-3146.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greber K. E., Dawgul M. (2017). Antimicrobial Peptides under Clinical Trials. Curr. Top. Med. Chem. 17, 620–628. 10.2174/1568026616666160713143331 [DOI] [PubMed] [Google Scholar]
- Grimsey E., Collis D. W. P., Mikut R., Hilpert K. (20201862). The Effect of Lipidation and Glycosylation on Short Cationic Antimicrobial Peptides. Biochim. Biophys. Acta (BBA) - Biomembranes 1862, 183195. 10.1016/j.bbamem.2020.183195 [DOI] [PubMed] [Google Scholar]
- Hale J. D., Hancock R. E. (2007). Alternative Mechanisms of Action of Cationic Antimicrobial Peptides on Bacteria. Expert Rev. Anti. Infect. Ther. 5, 951–959. 10.1586/14787210.5.6.951 [DOI] [PubMed] [Google Scholar]
- Hilpert K., Elliott M. R., Volkmer-Engert R., Henklein P., Donini O., Zhou Q., et al. (2006). Sequence Requirements and an Optimization Strategy for Short Antimicrobial Peptides. Chem. Biol. 13, 1101–1107. 10.1016/j.chembiol.2006.08.014 [DOI] [PubMed] [Google Scholar]
- Hilpert K., McLeod B., Yu J., Elliott M. R., Rautenbach M., Ruden S., et al. (2010). Short Cationic Antimicrobial Peptides Interact with ATP. Antimicrob. Agents Chemother. 54, 4480–4483. 10.1128/AAC.01664-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin D. J., Cagliero C., Zhou Y. N. (2013). Role of RNA Polymerase and Transcription in the Organization of the Bacterial Nucleoid. Chem. Rev. 113, 8662–8682. 10.1021/cr4001429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X., Dong F., Shi C., Liu S., Sun J., Chen J., et al. (2019). DRAMP 2.0, an Updated Data Repository of Antimicrobial Peptides. Sci. Data 6, 148. 10.1038/s41597-019-0154-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knappe D., Goldbach T., Hatfield M., Palermo N., Weinert S., Sträter N., et al. (2016). Proline-rich Antimicrobial Peptides Optimized for Binding to Escherichia coli Chaperone DnaK. Protein Pept. Lett. 23, 1061–1071. 10.2174/0929866523666160719124712 [DOI] [PubMed] [Google Scholar]
- Kobayashi S., Takeshima K., Park C. B., Kim S. C., Matsuzaki K. (2000). Interactions of the Novel Antimicrobial Peptide Buforin 2 with Lipid Bilayers: Proline as a Translocation Promoting Factor. Biochemistry 39, 8648–8654. 10.1021/bi0004549 [DOI] [PubMed] [Google Scholar]
- Koehbach J., Gani J., Hilpert K., Craik D. J. (2021). Comparison of a Short Linear Antimicrobial Peptide with its Disulfide-Cyclized and Cyclotide-Grafted Variants against Clinically Relevant Pathogens. Microorganisms 9, 1249. 10.3390/MICROORGANISMS9061249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizsan A., Knappe D., Hoffmann R. (2015a). Influence of the yjiL-mdtM Gene Cluster on the Antibacterial Activity of Proline-Rich Antimicrobial Peptides Overcoming Escherichia coli Resistance Induced by the Missing SbmA Transporter System. Antimicrob. Agents Chemother. 59, 5992–5998. 10.1128/AAC.01307-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizsan A., Prahl C., Goldbach T., Knappe D., Hoffmann R. (2015b). Short Proline-Rich Antimicrobial Peptides Inhibit Either the Bacterial 70S Ribosome or the Assembly of its Large 50S Subunit. ChemBioChem 16, 2304–2308. 10.1002/cbic.201500375 [DOI] [PubMed] [Google Scholar]
- Krizsan A., Volke D., Weinert S., Sträter N., Knappe D., Hoffmann R. (2014). Insect-Derived Proline-Rich Antimicrobial Peptides Kill Bacteria by Inhibiting Bacterial Protein Translation at the 70 S Ribosome. Angew. Chem. Int. Ed. 53, 12236–12239. 10.1002/anie.201407145 [DOI] [PubMed] [Google Scholar]
- Mahlapuu M., Håkansson J., Ringstad L., Björn C. (2016). Antimicrobial Peptides: An Emerging Category of Therapeutic Agents. Front. Cell. Infect. Microbiol. 6, 194. 10.3389/fcimb.2016.00194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand C., Krajewski K., Lee H. F., Antony S., Johnson A. A., Amin R., et al. (2006). Covalent Binding of the Natural Antimicrobial Peptide Indolicidin to DNA Abasic Sites. Nucleic Acids Res. 34, 5157–5165. 10.1093/nar/gkl667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardirossian M., Barrière Q., Timchenko T., Müller C., Pacor S., Mergaert P., et al. (2018a). Fragments of the Nonlytic Proline-Rich Antimicrobial Peptide Bac5 Kill Escherichia coli Cells by Inhibiting Protein Synthesis. Antimicrob. Agents Chemother. 62, e00534. 10.1128/AAC.00534-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardirossian M., Grzela R., Giglione C., Meinnel T., Gennaro R., Mergaert P., et al. (2014). The Host Antimicrobial Peptide Bac71-35 Binds to Bacterial Ribosomal Proteins and Inhibits Protein Synthesis. Chem. Biol. 21, 1639–1647. 10.1016/J.CHEMBIOL.2014.10.009 [DOI] [PubMed] [Google Scholar]
- Mardirossian M., Sola R., Beckert B., Valencic E., Collis D. W. P., Borišek J., et al. (2020). Peptide Inhibitors of Bacterial Protein Synthesis with Broad Spectrum and SbmA-independent Bactericidal Activity against Clinical Pathogens. J. Med. Chem. 63, 9590–9602. 10.1021/acs.jmedchem.0c00665 [DOI] [PubMed] [Google Scholar]
- Mardirossian M., Sola R., Degasperi M., Scocchi M. (2019b). Search for Shorter Portions of the Proline-Rich Antimicrobial Peptide Fragment Bac5(1-25) that Retain Antimicrobial Activity by Blocking Protein Synthesis. ChemMedChem 14, 343–348. 10.1002/cmdc.201800734 [DOI] [PubMed] [Google Scholar]
- Mardirossian M., Pérébaskine N., Benincasa M., Gambato S., Hofmann S., Huter P., et al. (2018b). The Dolphin Proline-Rich Antimicrobial Peptide Tur1A Inhibits Protein Synthesis by Targeting the Bacterial Ribosome. Cell Chem. Biol. 25, 530–539. 10.1016/j.chembiol.2018.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardirossian M., Sola R., Beckert B., Collis D. W. P., Di Stasi A., Armas F., et al. (2019a). Proline-rich Peptides with Improved Antimicrobial Activity against E. coli, K. pneumoniae and A. Baumannii. ChemMedChem 14, 2025–2033. 10.1002/cmdc.201900465R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiuzzo M., Bandiera A., Gennaro R., Benincasa M., Pacor S., Antcheva N., et al. (2007). Role of the Escherichia coli SbmA in the Antimicrobial Activity of Proline-Rich Peptides. Mol. Microbiol. 66, 151–163. 10.1111/J.1365-2958.2007.05903.X [DOI] [PubMed] [Google Scholar]
- Mikut R. (2010). Computer-based Analysis, Visualization, and Interpretation of Antimicrobial Peptide Activities. Methods Mol. Biol. 618, 287–299. 10.1007/978-1-60761-594-1_18 [DOI] [PubMed] [Google Scholar]
- Mikut R., Bartschat A., Doneit W., González∼Ordiano J. A., Schott B., Stegmaier J., et al. (2017). The MATLAB Toolbox SciXMiner: User’s Manual and Programmer’s Guide. arXiv:1704.03298, 1–189. [Google Scholar]
- Mikut R., Ruden S., Reischl M., Breitling F., Volkmer R., Hilpert K. (20161858). Improving Short Antimicrobial Peptides Despite Elusive Rules for Activity. Biochim. Biophys. Acta (BBA) - Biomembranes 1858, 1024–1033. 10.1016/j.bbamem.2015.12.013 [DOI] [PubMed] [Google Scholar]
- Ohniwa R. L., Morikawa K., Takeshita S. L., Kim J., Ohta T., Wada C., et al. (2007). Transcription-coupled Nucleoid Architecture in Bacteria. Genes Cells 12, 1141–1152. 10.1111/j.1365-2443.2007.01125.x [DOI] [PubMed] [Google Scholar]
- Pirtskhalava M., Gabrielian A., Cruz P., Griggs H. L., Squires R. B., Hurt D. E., et al. (2016). DBAASP v.2: An Enhanced Database of Structure and Antimicrobial/cytotoxic Activity of Natural and Synthetic Peptides. Nucleic Acids Res. 44, 6503–D1112. 10.1093/nar/gkv1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt P., Wilmes M., Pugnière M., Aumelas A., Bachère E., Sahl H. G., et al. (2010). Insight into Invertebrate Defensin Mechanism of Action: Oyster Defensins Inhibit Peptidoglycan Biosynthesis by Binding to Lipid II. J. Biol. Chem. 285, 29208–29216. 10.1074/jbc.M110.143388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang L., Li J., Song C., Nina Z., Li Q., Chou S., et al. (2020). Hybrid Antimicrobial Peptide Targeting Staphylococcus aureus and Displaying Anti-infective Activity in a Murine Model. Front. Microbiol. 11, 1767. 10.3389/fmicb.2020.01767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokunaga Y., Niidome T., Hatakeyama T., Aoyagi H. (2001). Antibacterial Activity of Bactenecin 5 Fragments and Their Interaction with Phospholipid Membranes. J. Pept. Sci. 7, 297–304. 10.1002/psc.317 [DOI] [PubMed] [Google Scholar]
- von Gundlach A., Ashby M. P., Gani J., Lopez-Perez P. M., Cookson A. R., Ann Huws S., et al. (2019). BioSAXS Measurements Reveal that Two Antimicrobial Peptides Induce Similar Molecular Changes in Gram-Negative and Gram-Positive Bacteria. Front. Pharmacol. 10, 1127. 10.3389/fphar.2019.01127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Gundlach A. R., Garamus V. M., Willey T. M., Ilavsky J., Hilpert K., Rosenhahn A. (2016b). Use of Small-Angle X-ray Scattering to Resolve Intracellular Structure Changes of Escherichia coli Cells Induced by Antibiotic Treatment. J. Appl. Crystallogr. 49, 2210–2216. 10.1107/S1600576716018562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Gundlach A. R., Garamus V. M., Gorniak T., Davies H. A., Reischl M., Mikut R., et al. (2016a). Small Angle X-ray Scattering as a High-Throughput Method to Classify Antimicrobial Modes of Action. Biochim. Biophys. Acta (BBA) - Biomembranes 1858, 918–925. 10.1016/j.bbamem.2015.12.022 [DOI] [PubMed] [Google Scholar]
- Wade H. M., Darling L. E. O., Elmore D. E. (2019). Hybrids Made from Antimicrobial Peptides with Different Mechanisms of Action Show Enhanced Membrane Permeabilization. Biochim. Biophys. Acta Biomembr 1861, 182980. 10.1016/j.bbamem.2019.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade H. M., Darling L. E. O., Elmore D. E. (2018). Systematic Analysis of Hybrid Antimicrobial Peptides. Biophysical J. 114, 453a. 10.1016/j.bpj.2017.11.2503 [DOI] [Google Scholar]
- Wang G., Li X., Wang Z. (2016). APD3: the Antimicrobial Peptide Database as a Tool for Research and Education. Nucleic Acids Res. 44, D1087–D1093. 10.1093/nar/gkv1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Fleming E., Chen J. L., Elmore D. E. (2011). Effect of Proline Position on the Antimicrobial Mechanism of Buforin II. Peptides 32, 677–682. 10.1016/j.peptides.2011.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W., Zhu X., Tan T., Li W., Shan A. (2014). Design of Embedded-Hybrid Antimicrobial Peptides with Enhanced Cell Selectivity and Anti-biofilm Activity. PLoS One 9, e98935. 10.1371/journal.pone.0098935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K., Lai B. F., Gani J., Mikut R., Hilpert K., Kizhakkedathu J. N. (2015). Interaction of Blood Components with Cathelicidins and Their Modified Versions. Biomaterials 69, 201–211. 10.1016/j.biomaterials.2015.08.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.