Purpose of review
Continuous glucose monitoring (CGM) systems are Food and Drug Administration approved devices for the ambulatory setting; however, they remain investigational systems for inpatient use. This review summarizes the most recent and relevant literature on the use of continuous glucose monitoring in the hospital setting.
Recent findings
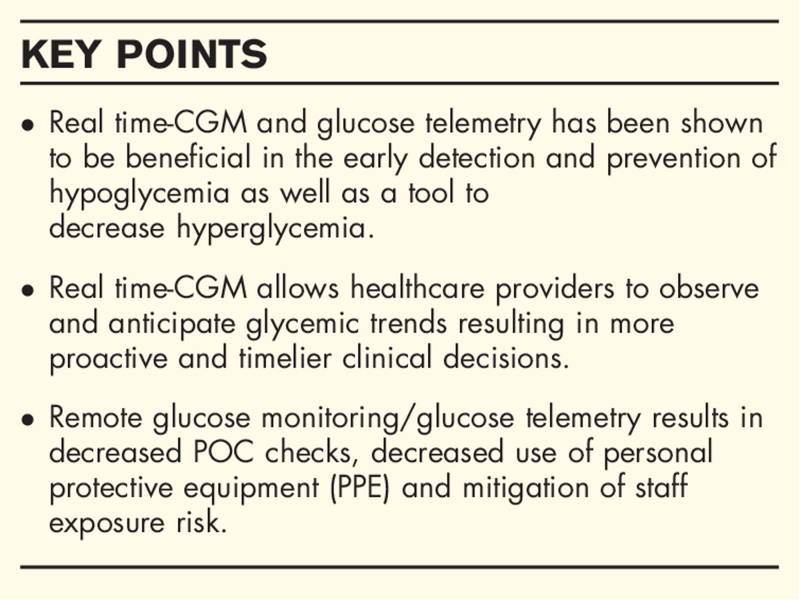
CGM provides real-time glucose data that enable healthcare professionals to make proactive and timelier clinical decisions with regards to diabetes management. CGM devices appear to be safe and accurate systems for glucose monitoring in the hospital setting. Real-time CGM systems and glucose telemetry can decrease hypoglycemia and reduce hyperglycemia in hospitalized patients with diabetes. Remote glucose monitoring decreases the need of frequent Point-of-care checks and personal protective equipment use while also mitigating staff exposure risk which is timely in the advent of the COVID-19 pandemic. Although most nursing staff have limited exposure and training on CGM technology, early studies show that CGM use in the hospital is well received by nurses.
Summary
Given the evidence in the current literature regarding CGM use in the hospital, CGM devices may be incorporated in the inpatient setting.
Keywords: CGM, continuous glucose monitoring, glucose telemetry, hospital, hypoglycemia, inpatient
INTRODUCTION
More than 10.5% of the US population have either diagnosed or undiagnosed diabetes [1]. Uncontrolled diabetes leads to microvascular and macrovascular complications resulting in the development of multiple comorbidities and eventual hospitalizations [2,3]. The high prevalence of diabetes in the inpatient setting, which accounts for over 25% of hospitalized patients, leads to increased hospital-related costs [4,5].
Among hospitalized patients, the presence of dysglycemia – defined as hyperglycemia, hypoglycemia, or significant glycemic variability- has been associated with adverse clinical outcomes [6]. Currently, bedside point-of-care glucose (POC) is the recommended method of glucose testing for hospitalized patients with diabetes [7]. Continuous glucose monitoring (CGM) systems offer a different modality of glucose monitoring compared to traditional POC, measuring glucose concentration in the interstitial fluid every few minutes. CGM systems have revolutionized outpatient glucose monitoring since its US Food and Drug Administration (FDA) approval in 1999 [8]. In the hospital, multiple studies have examined the use of CGM devices in the noncritical and critical care settings, some showing promising results [9,10]. Most of the inpatient studies performed assessed accuracy of CGM compared to POC, which is not considered standard of reference. To date, there are no studies comparing CGM glucose with those from a laboratory glucose analyzer as the reference. In addition, only a few studies examined whether CGM devices can have additional benefits compared to POC in hospitalized patients with diabetes or hyperglycemia. Thus, CGM has not yet been approved by the FDA to be utilized in the inpatient setting.
On March 11, 2020, the World Health Organization (WHO) declared Coronavirus-19 (COVID-19) a global pandemic. Patients with diabetes were found to have an increased risk for developing severe COVID-19 [11] and have a higher risk of mortality [12,13] compared to the general population [14]. To help conserve personal protective equipment (PPE) use and mitigate staff exposure in the inpatient hospital setting, the FDA issued guidance which did not object to the use of CGM devices in the inpatient setting during the pandemic [15–17]. Previous articles have reviewed the use of CGM devices in the hospital in both the noncritical care and critical care setting [9,18–21]. In this article, we present a comprehensive review focusing on studies published after the COVID-19 pandemic declaration that utilized CGM technology in the hospital.
Box 1.
no caption available
METHODS
Two independent reviewers (CG, EKS) conducted an electronic PubMed search to identify relevant publications published between March 11, 2020 to June 30, 2021. Multiple keywords were used including, ‘inpatient CGM’, ‘inpatient continuous glucose monitoring’, ‘hospital continuous glucose monitoring’, ‘CGM and COVID-19’, ‘inpatient continuous glucose monitoring and COVID-19’, ‘inpatient CGM and COVID-19’, ‘inpatient CGM and SARS-COV-2’, ‘intensive care unit continuous glucose monitoring’, ‘intensive care CGM’, ‘intensive care unit (ICU) continuous glucose monitoring’, ‘noncritical care CGM’, and ‘non-ICU CGM’.
We included studies that used CGM in adult, nonpregnant patients who were either observed or admitted to the hospital for medical or surgical conditions and excluded studies that were done for purely research purposes. We excluded publications that solely included the pediatric and pregnant population. Publications which used CGM combined with continuous subcutaneous infusion (’insulin pumps’) or automated insulin delivery systems (’closed loop systems’) were also excluded from this review.
Continuous glucose monitoring studies in non-COVID-19 patients
The majority of the studies conducted in non-COVID-19 patients evaluated the accuracy of CGM devices and were performed in the noncritical care setting, including a large group of medical and surgical patients (Table 1). There were only two single-center RCTs in the noncritical care setting that enrolled patients with type 2 diabetes mellitus (T2DM) evaluating glycemic outcomes with use of real-time-CGM [22▪▪,23].
Table 1.
Inpatient CGM Studies in non-COVID-19 patients
| Reference | Population | Study design | CGM used | Study aim | Results |
| Singh et al.[22▪▪] | T2DM (n = 72) Non-ICU Medicine | RCT Single center | Dexcom G6 | RT-CGM/GTS for the prevention of hypoglycemia | RT-CGM/GTS had fewer hypoglycemic events (<70 mg/dL) per patient vs. POC group (0.67 vs. 1.69, P = 0.024). |
| Fortmann et al.[23] | T2DM (n = 110) Non-ICU Medicine/Surgery | RCT Single center | Dexcom G6 | RT-CGM for management of acute hyper-/hypoglycemia | RT-CGM reduced percentage of time spent in hyperglycemia > 250 mg/dL vs. UC (27% vs. 33%, P = 0.04) |
| Davis et al.[24▪▪] | T2DM (n = 209) DM (n = 9) Non-ICU Medicine/Surgery | Pooled analysis of clinical studies Multicenter | Dexcom G6 | Accuracy study between CGM and POC | CGM had an overall MARD of 12.8% and median ARD of 10.1%. |
| Galindo et al.[30] | T2DM (n = 97) Non-ICU Medicine/Surgery | Prospective study Multicenter | Abbott FreeStyle Libre Pro | Feasibility and accuracy study between CGM and POC | CGM had an overall MARD of 14.8%. |
| Nair et al.[25] | DM (n = 10) Non-ICU Surgery | Prospective study Single center | Dexcom G6 | Peri-operative accuracy study between CGM and POC | CGM had an overall MARD of 9.4%. |
| Tripyla et al.[26] | T2DM (n = 8) T1DM (n = 2) Pancreatic diabetes (n = 5) Prediabetes (n = 5) Non-ICU Surgery | Prospective study Single center | Dexcom G6 | Peri-operative accuracy study between CGM and POC | CGM had an overall MARD of 12.7% and median ARD of 9.9%. |
| Perez-Guzman et al.[27] | No DM (n = 15) OR and CICU Surgery | Prospective study Single center | Dexcom G6 | Peri-operative accuracy study between CGM and POC | - CGM had an overall MARD of 12.9% and median ARD of 10.5%. - Intermittent signal loss during surgery (electrocautery interference). |
| Migdal et al.[28] | T1DM/T2DM (n = 49) Non-ICU Medicine/Surgery | Pooled analysis of clinical studies Multicenter | Dexcom G6 | Accuracy of CGM during radiologic procedures | For diagnostic studies using radiation (X-rays, CT scan, Angiography), CGM had an overall MARD of 13.3% preimaging and 12.7% postimaging. |
| Dillmann et al.[32] | T1DM (n = 28) T2DM (n = 25) Non-ICU Medicine | Prospective study Single center | Medtronic Guardian Connect (Enlite) | Feasibility study of glucose telemetry using Guardian Connect | - In those with T2DM and those hospitalized for acute complications, TIR significantly increased between the start of the hospitalization and end of hospitalization, from 75.7% (95%CI 48.5–84.6) to 82.2% (95%CI 63.2–91.8) [P = 0.043], and from 58.3% (95%CI 46.3–69.7) to 66.4% (95%CI 55.6–75.5) [P = 0.031], respectively. - 95% of nurses found GC to be useful while 64% reported that it saved time |
| Bichard et al.[31] | T1DM (n = 8) T2DM (n = 2) Non-ICU Medicine | Prospective study Single center | Abbot FreeStyle Libre Pro | Feasibility study between CGM and POC in the setting of DKA | Mean POC (11.1 [3.2] mmol/L, range: 4.2 to 18.9 mmol/L) of the 167 paired measurements was higher than the mean CGM level (9.2 [3.2] mmol/L, range: 2.6 to 18.0 mmol/L) however, both were highly correlated (r = 0.84, P < 0.001) |
| Furushima et al.[33] | DM/UnDM (n = 40) ICU Medicine | Prospective study Single center | Abbot FreeStyle Libre | To determine the MAGE using CGM data in septic patients and to assess associations of MAGE with clinical outcomes and oxidative stress | Nonsurvivors had a higher median value of MAGE [68.8 (IQR: 39.7–97.2) mg/dL] compared to survivors [39.3 (IQR: 19.9–53.5) mg/dL], (P = 0.02). |
| Abdelhamid et al.[34] | T2DM (n = 31) Non-ICU Medicine/Surgery | Prospective study Multicenter | Dexcom G4 | Detection of hypoglycemia in ICU survivors after ICU discharge | - 12 patients (39%; 95% CI, 22–56%) experienced at least one hypoglycemic episode. - Hypoglycemia in the ICU survivors were predominantly nocturnal (40/51 hr, 78%), asymptomatic (25/29 episodes, 86%), with 5.24% ± 5.50% of total monitoring time spent in hypoglycemia. |
ARD, Absolute Relative Difference; CGM, Continuous Glucose Monitoring; CICU, Cardiac Intensive Care Unit; DKA, Diabetic Ketoacidosis; DM, Publication did not specify type of Diabetes Mellitus; GC, Guardian Connect; GTS, Glucose Telemetry System; ICU, Intensive Care Unit; IQR, Interquartile Range; MAGE, Mean amplitude of glycemic excursions; MARD, Mean Absolute Relative Difference; No DM, No history of Diabetes Mellitus; OR, Operating Room; POC, Point-of-Care Capillary Glucose Testing; RCT, Randomized Control Trial; RT, Real Time; RT-CGM, Real-Time-Continuous Glucose Monitoring; T1DM, Type 1 Diabetes Mellitus; T2DM, Type 2 Diabetes Mellitus; UC, Usual Care.
For the accuracy studies that utilized the Dexcom G6 system (Dexcom, Inc., San Diego, CA, USA), the overall mean absolute relative difference (MARD) ranged between 9.4–12.9% and the median absolute relative difference (ARD) ranged between 9.9 and 10.5% [24▪▪,25–27]. A large multicenter pooled accuracy analysis of CGM data from 218 medicine and surgery patients (n = 4067 matched glucose pairs) with diabetes revealed a MARD of 12.8% and median ARD of 10.1% [24▪▪]. Dexcom G6 system was evaluated in the perioperative setting in 10 patients with diabetes scheduled to undergo elective surgery and reported an overall MARD of 9.4% [25]. Another perioperative accuracy study which involved patients without a history of diabetes who underwent coronary artery bypass surgery showed an overall MARD of 12.9% and a median ARD of 10.5% and noted intermittent signal loss during surgery likely secondary to electrocautery interference [27]. Given the concern for possible inaccuracy during certain imaging procedures, another study assessed CGM accuracy during certain radiologic procedures (X-rays, computed tomography scan, and angiography), reporting an overall MARD of 13.3% preimaging and 12.7% postimaging [28].
In addition to evaluating accuracy, studies have been conducted examining the utility of CGM devices in the prevention of hypoglycemia and management of hyperglycemia [22▪▪,23]. Utilizing the glucose telemetry system (GTS), a randomized controlled trial (RCT) by Singh et al. enrolled 72 patients with T2DM at higher risk of inpatient hypoglycemia randomized to either real time-CGM/GTS or POC [22▪▪]. GTS utilizes Bluetooth technology and software applications wherein real time-CGM data are sent from the sensor/transmitter worn by the patient to the iPhone located in the patient's room. Thereafter, by utilizing wireless internet connectivity, the iPhone transmits the glucose data to an iPad at the nursing station [29▪]. Interim analysis results showed that the real time-CGM/GTS group had fewer hypoglycemic events (<70 mg/dL) per patient (0.67 [95% confidence interval, CI 0.34–1.30] vs. 1.69 [1.11–2.58], P = 0.024) and fewer clinically significant hypoglycemic events (<54 mg/dL) per patient (0.08 [0.03–0.26] vs. 0.75 [0.51–1.09], P = 0.003) compared with the POC group. No significant adverse events were reported. Only two subjects withdrew from the study due to minor bleeding after sensor insertion [22▪▪]. In another RCT, real time-CGM was utilized in order to improve glucose control in the hospital. An advanced practice nurse reviewed glucose data from the previous 24 h using Dexcom Clarity to make recommendations to the primary teams-hospitalists. In this study, real time-CGM reduced percentage of time spent in hyperglycemia >250 mg/dL versus usual care (27% vs. 33%, P = 0.04) [23].
Freestyle Libre Pro CGM (Abbott Diabetes Care, Alameda, CA, USA) accuracy was evaluated in 97 patients with DM. The authors reported an overall MARD of 14.8%. Mean daily glucose was significantly higher by POC compared with CGM (188.9 ± 37.3 vs. 176.1 ± 46.9 mg/dL, P = 0.001). Hypoglycemia detected by POC was significantly lower compared with CGM (<70 mg/dl 14% [n = 14] vs. 56% [n = 54]; P < 0.001) and < 54 mg/dL (4.1% [n = 4] vs. 36% [n = 35]; P < 0.001), respectively [30]. Freestyle Libre system was also used to compare POC versus CGM values for adjustment of insulin infusion rates for DKA treatment. Mean POC (11.1 [3.2] mmol/L, range: 4.2--18.9 mmol/L) of the 167 paired measurements was higher than the mean CGM glucose (9.2 [3.2] mmol/L, range: 2.6 to 18.0 mmol/L), though both were highly correlated (r = 0.84, P < 0.001) [31].
A pilot study assessing feasibility of Guardian Connect CGM (Medtronic, Northridge, CA, USA) in patients with T2DM and those who were hospitalized for acute complications revealed time in range (TIR) significantly increased between the start of the hospitalization and end of hospitalization, from 75.7% (95% CI 48.5–84.6) to 82.2% (95% CI 63.2–91.8) [P = 0.043], and from 58.3% (95% CI 46.3–69.7) to 66.4% (95% CI 55.6–75.5) [P = 0.031], respectively. Ninety-five percentage of nurses found Guardian Connect to be useful, whereas 64% found that it saved them time [32]. Another study assessed the association of mean amplitude of glycemic excursions (MAGEs) with clinical outcomes using CGM data in patients with sepsis which showed higher median MAGE [68.8 (interquartile range, IQR:39.7–97.2) mg/dL] in nonsurvivors compared to survivors [39.3 (IQR 19.9–53.5) mg/dL] (P = 0.02). Higher MAGE was associated with higher ICU mortality rate and less ICU-free survival days [33]. A multicenter study used CGM to evaluate impact of hypoglycemia on morbidity and mortality in patients who survived their ICU stay. The authors reported that hypoglycemia in ICU survivors was predominantly nocturnal (40/51 hr, 78%), asymptomatic (25/29 episodes, 86%), with 5.24% ± 5.50% of total monitoring time spent in hypoglycemia [34].
Continuous glucose monitoring studies in COVID-19 patients
Most of the published studies in COVID-19 patients focused on feasibility and accuracy of inpatient CGM in COVID-19 patients to improve glycemic outcomes and reduce burden for healthcare professionals (Table 2). Notably, most studies were either observational studies or case reports with a small number of subjects. To date, there are no published RCTs examining CGM devices in COVID-19 patients.
Table 2.
Inpatient CGM Studies in COVID-19 patients
| Reference | Population | Study Design | CGM Used | Study Aim | Results |
| Faulds et al.[38▪▪] | T1DM (n = 2) T2DM (n = 16) No DM (n = 1) ICU Medicine | Retrospective analysis Single center | Dexcom G6 | Feasibility of RT-CGM for insulin infusion titration | - CGM had an overall MARD of 13.9 ± 7.8% (median 11.9, IQR 3.3–29.4) on day 1 and 13.5 ± 8.1% (median 10.6, IQR 9.0–15.0) on days 2 through 7. - Use of CGM resulted in 71% reduction in POC use - Negative association found between BMI and MARD (coefficient = -0.291, P = 0.007). |
| Chow et al.[42] | DM (n = 30) ICU Medicine | Retrospective study Single center | Dexcom G6 | Feasibility and accuracy study of RT-CGM and POC | - 14% reduction in mean glucose during RT-CGM management vs. pre RT-CGM management (235.7 ± 42.1 to 202.7 ± 37.6 mg/dl, P = 0.003). - Use of CGM resulted in 50% reduction in POC use - 63% of nurses reported RT-CGM helped improved clinical care while 49% reported concomitant reduction in PPE use. |
| Agarwal et al.[36] | T1DM (n = 3) T2DM (n = 6) No DM (n = 2) ICU Medicine | Prospective study Single center | Dexcom G6 | Feasibility and accuracy study between CGM and POC | - CGM had an overall MARD of 12.58% and median ARD of 6.3% - Use of CGM resulted in an estimated 60% reduction in POC use |
| Reutrakul et al.[37] | DM (n = 9) Non-ICU Medicine | Prospective study Single center | Dexcom G6 | Feasibility and accuracy study between CGM and POC | CGM had an overall MARD of 9.77% |
| Sadhu et al.[35] | T1DM (n = 1) T2DM (n = 8) Prediabetes (n = 1) Posttransplant DM (n = 1) ICU Medicine | Retrospective study Single center | Medtronic Guardian Connect Dexcom G6 | Feasibility and accuracy study between CGM and POC | - Overall MARD was 13.1% for Medtronic and 11.1% for Dexcom (P = 0.13) - Sensor insertion for both systems were easily done however the Medtronic sensor required more steps as calibration was required when compared to Dexcom. Both systems were noted have a tedious initial setup (i.e., creation of individual accounts on manufacturer's cloud-based platforms) |
| Ushigome et al. [46] | T2DM (n = 1) Non-ICU Medicine | Case report Single center | Dexcom G4 | Feasibility study of RT-CGM for insulin infusion titration | Safe and effective management of hyperglycemia using RT-CGM for insulin infusion without increasing hypoglycemia risk. |
| Davis GM et al.[44▪] | T2DM (n = 9) ICU Medicine | Prospective study Single center | Dexcom G6 | Proof of concept study utilizing hybrid CGM/POC protocol and Glucommander | - During protocol use, 75.7% of sensor glucose values > 100mg/dL were within 20% of the reference POC, with a mean number of POC tests per day of 8.24 ± 3.06 (63% reduction in POC use) - Sensor readings were lower during hypoperfusion states (PEA, shock) and with signal loss during cardiac arrest and defibrillator use. - Sensor accuracy was also impacted during therapeutic hypothermia and position changes including pronation or inadvertent sensor compression. |
| Shehav-Zaltman et al.[40] | T1DM (n = 1) T2DM (n = 3) | Case series Single center | Medtronic Guardian Connect (Enlite) | Feasibility study of glucose telemetry using Guardian Connect | - Mean daily glucose measurements decreased from 3.75 ± 0.86 to 1.94 ± 0.31 with CGM use (P = 0.005). - Main challenges include training alternating teams with the calibration procedures and cost from weekly replacement of sensors. |
| Chow et al. [47] | No DM (n = 1) | Case report | Dexcom G6 | Feasibility study of RT-CGM for PN-induced hyperglycemia | RT-CGM found to facilitate timely adjustments to insulin infusion in order to achieve target glucose levels. |
| Garelli et al. [48] | T2DM (n = 3) COVID-19 induced DM (Pediatric) (n = 1) Posttransplant DM (Pediatric) (n = 1) ICU Medicine | Case series Single center | Dexcom G6 | Feasibility study of a multipatient, multisite, multi-CGM sensor monitoring platform | - Developed a multipatient platform (Insumate) for simultaneous remote glucose monitoring. - All patients showed improvement in TIR from 12.8% up to 51.65% |
| Gomez et al.[43] | T2DM (n = 44) No DM (n = 16) Non-ICU & ICU Medicine | Prospective study Single center | Abbot FreeStyle Libre | Examination of glycemic control metrics using CGM | - No differences between the values of TIR, TAR, TBR, CV, or GMI in patients with the composite outcome (ICU admission, ARDS, AKI) - In a subgroup analysis for patients with hyperglycemia without diabetes, higher TAR > 180 mg/dL was seen in patients with AKI (18 vs. 1%, P = 0.01), and in those with the composite outcome (22.5% vs. 16%, P = 0.04) |
| Longo et al.[39] | T2DM (n = 27) LADA (n = 1) Non-ICU and ICU Medicine | Prospective study Single center | Dexcom G6 | Feasibility and accuracy study between CGM, POC, and Lab reference. | - CGM had an overall MARD of 13.2% (12.1% for ICU and 14% for non-ICU). - CGM glucose values showed higher accuracy when compared to glucose Lab reference (MARD 10.9%) than to POC (MARD 13.9%). |
| Shen et al.[45] | DM (n = 35) Non-ICU Medicine | Prospective study Single center | Abbot Freestyle Libre | Determine the threshold of glycemia and its association with the outcomes of COVID-19 | - Patients with composite adverse outcomes (admission to ICU, need for mechanical ventilation, or morbidity with critical illness) had significantly higher TBR (P = < 0.01) than those without composite adverse outcomes. - Mean glucose level was significantly higher in patients with composite adverse outcomes than those without (174 ± 49.0 vs.144 ± 21.2 mg/dL, P < 0.01). |
AKI, Acute Kidney Injury; ARD, Absolute Relative Difference; BMI, Body Mass Index; CGM, Continuous Glucose Monitoring; CICU, Cardiac Intensive Care Unit; CV, Coefficient of Variation; DKA, Diabetic Ketoacidosis; DM, Publication did not specify type of Diabetes Mellitus; GMI, Glucose Management Indicator; ICU, Intensive Care Unit; LADA, Latent Autoimmune Diabetes in Adulthood; MAGE, Mean amplitude of glycemic excursions; MARD, Mean Absolute Relative Difference; No DM, No History of Diabetes Mellitus; OR, Operating room; PEA, Pulseless Electrical Activity; PN, Parenteral Nutrition; POC, Point-of-Care Capillary Glucose Testing; PPE, Personal Protective Equipment; RCT, Randomized Control Trial; RT-CGM, Real-Time-Continuous Glucose Monitoring; T1DM, Type 1 Diabetes Mellitus; T2DM, Type 2 Diabetes Mellitus; TAR, Time Above Range; TIR, Time In Range.
Studies reporting accuracy of CGM predominantly utilized Dexcom G6 CGM reporting overall MARD ranging between 9.77% and 14% [35–39]. There were only two studies [35,40] that utilized the Guardian Connect (Enlite) CGM with only one study reporting the overall MARD of 13.1% [35]. An approach to overcome concerns about CGM accuracy was suggested by Faulds et al. where a hybrid CGM and POC glucose monitoring protocol to guide insulin adjustments was implemented. Nursing leadership inserted CGMs in patients requiring intravenous insulin in the critical care setting. POC glucose were used to adjust insulin rates as needed but also validate the CGM sensor. Once initial validation was achieved, CGM data alone was used for clinical decision making and insulin titration with continuation of POC to maintain validation. After ICU transfer, CGM was used alone without POC [41]. Authors enrolled a total of 19 medical ICU patients requiring intravenous insulin managed by this protocol showing feasibility of this hybrid approach. Overall MARD was 13.9% ± 7.8% on day 1 and 13.5% ± 8.1% on days 2 through 7 with a 71% reduction in POC use [38▪▪].
Sensor insertion was done by a trained nurse, nurse practitioner or healthcare professional in most of the studies [35–37,38▪▪,39,42,43]. For the studies using Dexcom G6 system, glucose data was transmitted to Dexcom receivers [36,42], Android phones [38▪▪] or both iPad and iPhones [35] and were placed close to the patient's room. These receiving devices were within 10–20 feet from the transmitter to ensure Bluetooth transmission. This direct view of glucose data from outside the patients’ rooms allowed the healthcare team to monitor glucose data without entering the patients’ rooms. Other studies used remote glucose monitoring with the GTS [37,39,44▪] or Medtronic Guardian Connect System using the web-based CareLink software accessed through minimized browser windows [40].
Another study described the use of a CGM/POC hybrid protocol that combined glucose telemetry with Glucommander, a computer-based algorithm that provides adjustments of intravenous insulin delivery. This proof-of-concept study reported a mean time below range (TBR) (<70 mg/dL) was 0.6 ± 0.9%, TIR (70–180 mg/dL) of 71.4 ± 13.9%, time between 180–250 mg/dL of 19.8 ± 97%, and time above range (TAR) (>250 mg/dL) of 7.5 ± 7.3%. During protocol use, 75.7% of sensor glucose values >100 mg/dL were within 20% of the reference POC, with a mean number of 8.24 ± 3.06 POC per day (63% reduction). Sensor readings were lower in states of hypoperfusion (i.e., pulseless electrical activity, shock, and therapeutic hypothermia) with signal loss occurring during cardiac arrest and defibrillator use. Sensor accuracy was also impacted by position changes including pronation or inadvertent sensor compression [44▪].
As CGM is FDA approved and used predominantly in the outpatient setting, most nursing staff had limited exposure and training on CGM technology. Chow et al. assessed nursing staff perception and acceptance of real time-CGM revealing 63% of participating nurses viewed real time-CGM as a helpful tool improving clinical care for patients with diabetes and COVID-19. Additionally, 49% of nursing staff reported real time-CGM reduced PPE use [42]. Other studies noted reduction in POC ranging between 50 and 63% [36,42,44▪]. While implementation of a hybrid CGM and POC protocol by Faulds et al. was well received by nursing staff, there was unanimous consensus amongst them that the protocol was excessively lengthy [38▪▪]. Nursing staff also commented components for CGM use (i.e., receiver, phone, cords) and training materials were crowding their workstations.
Gomez et al. used Freestyle Libre CGM to examine the association of glycemic control metrics with clinical outcomes in the ICU and non-ICU setting [43]. No differences were seen in TIR, TAR, TBR, coefficient of variation or glucose management indicator in patients with or without admission to ICU, ARDS or AKI, or with the composite metric of these complications. In a subgroup analysis for patients with hyperglycemia without known history of diabetes, higher TAR>180 mg/dL was seen in patients with AKI (18 vs. 1%, P = 0.01), and in those with the composite outcome (22.5% vs. 16%, P = 0.04). Freestyle Libre CGM was also used to evaluate the association of glycemia with outcomes related to COVID-19 infection. Patients with the composite adverse outcomes (ICU admission, need for mechanical ventilation, or morbidity with critical illness) had significantly higher TBR (4.43 ± 11.4% vs. 0.54 ± 0.65%) [P < 0.01] and higher mean glucose values (174 ± 49.0 vs.144 ± 21.2 mg/dL, P < 0.01) than those without composite adverse outcomes [45].
CONCLUSION
The use of inpatient CGM confers numerous benefits with minimal risks. With the COVID-19 pandemic, the importance of remote glucose monitoring is highlighted now more than ever. Real time-CGM has been shown to be useful in the early detection and prevention of hypoglycemia as well as a tool to decrease hyperglycemia. Real time-CGM provides glycemic trends that may be used to enable more proactive and timelier decisions for diabetes management to reduce clinically significant events such as hypoglycemia or hyperglycemia. CGM devices also decreases the need for healthcare workers to enter the patients’ rooms and reduces frequent POC checks which can be uncomfortable and painful for patients and increases nursing workload. Furthermore, CGM use has led to a reduction in PPE use and significantly mitigates the risk of exposure for healthcare staff.
Prior to the advent of POC testing, serum blood glucose (SBG) samples were drawn and brought to the central lab for analysis. Although SBG is more accurate, this was a cumbersome and time-consuming process that at times, can result in delays in patient care. In the last few decades, glucometers became the standard of care in the inpatient setting mainly due to their convenience. Similarly, we believe that CGM devices will eventually be utilized in the hospital as standard of care as they provide a more robust and enriched data set of glycemic values than glucometers, helping clinicians and nurses to safely manage patients with diabetes without increasing workload. In conclusion, although CGM devices are currently seen as novel systems, it is only a matter of time when CGM systems will be approved for use in the inpatient setting.
Acknowledgements
None.
Financial support and sponsorship
This work was supported in part by the VA MERIT award (#1I01CX001825) from the United States (U.S.) Department of Veterans Affairs Clinical Sciences Research and Development Service.
Conflicts of interest
CG, LGS and MS declare that they have no conflict of interest. EKS has received CGM supplies from DEXCOM (to University of Maryland and Baltimore VA Medical Center) for the conduction of inpatient clinical trials.
Disclaimer: The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.U.S. Dept of Health and Human Services, Prevention CfDCa, Prevention CfDCa. National Diabetes Statistics Report. 2020. [Google Scholar]
- 2.American Diabetes A. 10. Cardiovascular disease and risk management: standards of medical care in diabetes. Diabetes Care 2020; 43:S111–S134. [DOI] [PubMed] [Google Scholar]
- 3.American Diabetes A. 11. Microvascular complications and foot care: standards of medical care in diabetes. Diabetes Care 2020; 43:S135–S151. [DOI] [PubMed] [Google Scholar]
- 4.Umpierrez GE, Isaacs SD, Bazargan N, et al. Hyperglycemia: an independent marker of in-hospital mortality in patients with undiagnosed diabetes. J Clin Endocrinol Metab 2002; 87:978–982. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes A. Economic costs of diabetes in the U.S in 2017. Diabetes Care 2018; 41:917–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clement S, Braithwaite SS, Magee MF, et al. Management of diabetes and hyperglycemia in hospitals. Diabetes Care 2004; 27:553–591. [DOI] [PubMed] [Google Scholar]
- 7.American Diabetes A. 15. Diabetes care in the hospital: standards of medical care in diabetes. Diabetes Care 2020; 43:S193–S202. [DOI] [PubMed] [Google Scholar]
- 8.Hirsch IB. Introduction: History of Glucose Monitoring. 2018 Aug. In: Role of Continueous Glucose Monitoring in Diabetes Treatment. Arlington (VA): American Diabetes Association; 2018 Aug. PMID: 34251770. [PubMed] [Google Scholar]
- 9.Wang M, Singh LG, Spanakis EK. Advancing the use of CGM devices in a non-ICU setting. J Diabetes Sci Technol 2019; 13:674–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satyarengga M, Siddiqui T, Spanakis EK. Designing the glucose telemetry for hospital management: from bedside to the nursing station. Curr Diab Rep 2018; 18:87. [DOI] [PubMed] [Google Scholar]
- 11.Sardu C, Gargiulo G, Esposito G, et al. Impact of diabetes mellitus on clinical outcomes in patients affected by Covid-19. Cardiovasc Diabetol 2020; 19:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spanakis EK, Yoo A, Ajayi ON, et al. Excess mortality in COVID-19-positive versus COVID-19-negative inpatients with diabetes: a nationwide study. Diabetes Care 2021; 44:e169–e170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epidemiology Working Group for Ncip Epidemic Response CCfDC, Prevention. [The epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) in China]. Zhonghua Liu Xing Bing Xue Za Zhi 2020; 41:145–151. [DOI] [PubMed] [Google Scholar]
- 15.Administration USFaD. Coronavirus (COVID-19) Update: FDA allows expanded use of devices to monitor patients’ vital signs remotely 2020 [Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-allows-expanded-use-devices-monitor-patients-vital-signs-remotely. [Google Scholar]
- 16.Dexcom. [Fact Sheet for Healthcare Providers: Use of Dexcom Continuous Glucose Monitoring Systems During the COVID-19 Pandemic]. Available from: https://www.dexcom.com/hospitalfacts. [Google Scholar]
- 17.Abbott's Freestyle Libre 14 Day System Now Available in U.S. For Hospitalized Patients With Diabetes During COVID-19 Pandemic [press release]. 2020. [Google Scholar]
- 18.Levitt DL, Silver KD, Spanakis EK. Inpatient continuous glucose monitoring and glycemic outcomes. J Diabetes Sci Technol 2017; 11:1028–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klonoff DC, Ahn D, Drincic A. Continuous glucose monitoring: a review of the technology and clinical use. Diabetes Res Clin Pract 2017; 133:178–192. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Guzman MC, Shang T, Zhang JY, et al. Continuous glucose monitoring in the hospital. Endocrinol Metab (Seoul) 2021; 36:240–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galindo RJ, Umpierrez GE, Rushakoff RJ, et al. Continuous glucose monitors and automated insulin dosing systems in the hospital consensus guideline. J Diabetes Sci Technol 2020; 14:1035–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22▪▪.Singh LG, Satyarengga M, Marcano I, et al. Reducing inpatient hypoglycemia in the general wards using real-time continuous glucose monitoring: the glucose telemetry system, a randomized clinical trial. Diabetes Care 2020; 43:2736–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a RCT that demonstrated real time-CGM/glucose telemetry system as a tool to prevent and reduce hypoglycemia in the general wards.
- 23.Fortmann AL, Spierling Bagsic SR, Talavera L, et al. Glucose as the fifth vital sign: a randomized controlled trial of continuous glucose monitoring in a non-icu hospital setting. Diabetes Care 2020; 43:2873–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24▪▪.Davis GM, Spanakis EK, Migdal AL, et al. Accuracy of dexcom G6 continuous glucose monitoring in non-critically Ill hospitalized patients with diabetes. Diabetes Care 2021; 44:1641–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the largest multicenter study that assessed the accuracy of CGM in the inpatient noncritical care setting.
- 25.Nair BG, Dellinger EP, Flum DR, et al. A pilot study of the feasibility and accuracy of inpatient continuous glucose monitoring. Diabetes Care 2020; 43:e168–e169. [DOI] [PubMed] [Google Scholar]
- 26.Tripyla A, Herzig D, Joachim D, et al. Performance of a factory-calibrated, real-time continuous glucose monitoring system during elective abdominal surgery. Diabetes Obes Metab 2020; 22:1678–1682. [DOI] [PubMed] [Google Scholar]
- 27.Perez-Guzman MC, Duggan E, Gibanica S, et al. Continuous glucose monitoring in the operating room and cardiac intensive care unit. Diabetes Care 2021; 44:e50–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Migdal AL, Spanakis EK, Galindo RJ, et al. Accuracy and precision of continuous glucose monitoring in hospitalized patients undergoing radiology procedures. J Diabetes Sci Technol 2020; 14:1135–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29▪.Spanakis EK, Levitt DL, Siddiqui T, et al. The effect of continuous glucose monitoring in preventing inpatient hypoglycemia in general wards: the glucose telemetry system. J Diabetes Sci Technol 2018; 12:20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a pilot study that wirelessly transmitted glucose data to the nursing station, creating a remote contiuous glucose monitoring system otherwise known as the ‘glucose telemetry system’ which is a system akin to the cardiac telemetry.
- 30.Galindo RJ, Migdal AL, Davis GM, et al. Comparison of the freestyle libre pro flash continuous glucose monitoring (cgm) system and point-of-care capillary glucose testing in hospitalized patients with type 2 diabetes treated with basal-bolus insulin regimen. Diabetes Care 2020; 43:2730–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bichard LK, Rushworth RL, Torpy DJ. Flash glucose monitoring compared to capillary glucose levels in patients with diabetic ketoacidosis: potential clinical applications. Endocr Pract 2021; 27:813–818. [DOI] [PubMed] [Google Scholar]
- 32.Dillmann C, Amoura L, Fall Mostaine F, et al. Feasibility of real-time continuous glucose monitoring telemetry system in an inpatient diabetes unit: a pilot study. J Diabetes Sci Technol 2021; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Furushima N, Egi M, Obata N, et al. Mean amplitude of glycemic excursions in septic patients and its association with outcomes: A prospective observational study using continuous glucose monitoring. J Crit Care 2021; 63:218–222. [DOI] [PubMed] [Google Scholar]
- 34.Ali Abdelhamid Y, Bernjak A, Phillips LK, et al. Nocturnal hypoglycemia in patients with diabetes discharged from ICUs: a prospective two-center cohort study. Crit Care Med 2021; 49:636–649. [DOI] [PubMed] [Google Scholar]
- 35.Sadhu AR, Serrano IA, Xu J, et al. Continuous glucose monitoring in critically ill patients with covid-19: results of an emergent pilot study. J Diabetes Sci Technol 2020; 14:1065–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Agarwal S, Mathew J, Davis GM, et al. Continuous glucose monitoring in the intensive care unit during the COVID-19 pandemic. Diabetes Care 2021; 44:847–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reutrakul S, Genco M, Salinas H, et al. Feasibility of inpatient continuous glucose monitoring during the COVID-19 pandemic: early experience. Diabetes Care 2020; 43:e137–e138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38▪▪.Faulds ER, Boutsicaris A, Sumner L, et al. Use of continuous glucose monitor in critically ill COVID-19 patients requiring insulin infusion: an observational study. J Clin Endocrinol Metab 2021; 106:e4007–e4016. [DOI] [PubMed] [Google Scholar]; This is a study that describes the use of a hybrid CGM/POC protocol in patients initially requiring insulin infusion at the ICU.
- 39.Longo RR, Elias H, Khan M, Seley JJ. Use and accuracy of inpatient CGM during the COVID-19 pandemic: an observational study of general medicine and ICU patients. J Diabetes Sci Technol 2021; [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shehav-Zaltzman G, Segal G, Konvalina N, Tirosh A. Remote glucose monitoring of hospitalized, quarantined patients with diabetes and COVID-19. Diabetes Care 2020; 43:e75–e76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faulds ER, Jones L, McNett M, et al. Facilitators and barriers to nursing implementation of continuous glucose monitoring (CGM) in critically ill patients with COVID-19. Endocr Pract 2021; 27:354–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chow KW, Kelly DJ, Rieff MC, et al. Outcomes and healthcare provider perceptions of real-time continuous glucose monitoring (rtCGM) in patients with diabetes and COVID-19 admitted to the ICU. J Diabetes Sci Technol 2021; 15:607–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomez AM, Henao DC, Munoz OM, et al. Glycemic control metrics using flash glucose monitoring and hospital complications in patients with COVID-19. Diabetes Metab Syndr 2021; 15:499–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪.Davis GM, Faulds E, Walker T, et al. Remote continuous glucose monitoring with a computerized insulin infusion protocol for critically ill patients in a COVID-19 medical ICU: proof of concept. Diabetes Care 2021; 44:1055–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]; A pilot study that utilized a hybrid CGM/POC protocol that combined glucose telemetry with Glucommander which is a computerized decision support system for continuous insulin infusion and integrated a validation system for sensor glucose values into the electronic health record.
- 45.Shen Y, Fan X, Zhang L, et al. Thresholds of glycemia and the outcomes of COVID-19 complicated with diabetes: a retrospective exploratory study using continuous glucose monitoring. Diabetes Care 2021; 44:976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]