Purpose of review
This review aims to explore the different imaging modalities, such as chest radiography (CXR), computed tomography (CT), ultrasound, PET/CT scan, and MRI to describe the main features for the evaluation of the chest in COVID-19 patients with ARDS.
Recent findings
This article includes a systematic literature search, evidencing the different chest imaging modalities used in patients with ARDS from COVID-19. Literature evidences different possible approaches going from the conventional CXR and CT to the LUS, MRI, and PET/CT.
Summary
CT is the technique with higher sensitivity and definition for studying chest in COVID-19 patients. LUS or bedside CXR are critical in patients requiring close and repeated monitoring. Moreover, LUS and CXR reduce the radiation burden and the risk of infection compared with CT. PET/CT and MRI, especially in ARDS patients, are not usually used for diagnostic or follow-up purposes.
Keywords: acute respiratory failure, chest radiography, computed tomography, coronavirus disease 2019, lung ultrasound
INTRODUCTION
Coronavirus disease 2019 (COVID-19) pandemic has affected millions of people worldwide causing a wide spectrum of clinical manifestation [1], ranging from asymptomatic forms to acute respiratory distress syndrome (ARDS) requiring mechanical ventilation [2]. An incidence of ARDS between 20 and 67% in hospital-admitted patients, and up to 100% in mechanically ventilated ones has been reported [3▪]. Severe disease is associated with male sex, advanced age, obesity, diabetes, chronic lung disease, hypertension, and sarcopenia [4]. SARS-CoV-2 has a cytopathic effect on lung AT2 cells with alveolar damage and reduction of surfactant expression that may result in diffuse alveolar–capillary barrier dysfunction and ultimately in the development of ARDS [5–7]. Given the high contagiousness of the disease and the rapid spread of the infection, healthcare systems had to face a profound reorganization with the aim for protecting healthcare workers and at the same time ensuring the best care for patients. Dedicated pathways for COVID-19 patients were created and many departments were turned into sub-intensive or ICU in order to increase the number of available beds [8]. The relative lack of resources and hospital beds in the very first period of the pandemic created the need for hospitals to establish, which patients to admit with priority, and the selection often depended on an accurate patient evaluation performed in the emergency department [9,10▪]. In this setting, chest imaging had a key role in the diagnosis, evaluation, and risk stratification of patients. As first choice imaging modality, the Fleischner Society suggested computed tomography (CT), whereas the American College of Radiology and the Society of Thoracic Radiology recommended a two-view chest radiography (CXR) [11,12]. Other authors proposed a lung ultrasound (LUS) examination [13,14].
Box 1.
no caption available
Chest imaging played a crucial role also for hospitalized patients, on one hand to monitor treatment effects in the most severe cases, on the other to assess the risk of deterioration in less critical ones.
Aim of this work is to give an overview of the different imaging techniques used for chest evaluation in COVID-19 patients with ARDS.
LITERATURE SEARCH
Two authors (S.C and L.D.M) independently conducted a systematic review using EMBASE (Elsevier) and PubMed databases to evaluate the most relevant articles on chest imaging in patients with ARDS from COVID-19. The systematic literature search was performed on 9 September 2020 and updated on 14 October 2020. Search terms used were ‘acute respiratory distress syndrome’, ‘computed tomography’, ‘ARDS’, ‘X-ray’, ‘COVID-19’, ‘chest radiography’, and ‘ultrasound’. Articles published from 1 January 2020 in English language, which included patients diagnosed with COVID-19 and ARDS, and had adequate imaging findings were included in the review. Two investigators (S.C. and L.D.M.) independently separately analyzed and evaluated all articles for eligibility. Additional articles were collected by analyzing the references of the selected studies. The total number of articles screened was 232, of which 56 were selected for this review.
CHEST X-RAY
As previously mentioned, the American College of Radiology and the Society of Thoracic Radiology have suggested the use of CXR for the diagnosis and monitoring of COVID-19 patients, although literature data report a lower sensitivity (69%) of CXR compared with CT in detecting abnormalities in the initial stages of COVID-19 [15▪,16▪]. Indeed, CXR is easily available, carries lower radiation burden and can be performed directly at the bedside, limiting the workforce needed for transporting the patients outside the wards and, most importantly, reducing the risk of contagion for patients and healthcare workers [10▪,17]. Typical findings in patients with SARS-CoV-2 infection at CXR are ground-gloss opacities (GGOs), reticular pattern, and consolidations [10▪,15▪,16▪]. According to the Fleischner Society, GGOs are areas of hazy increased lung opacity less dense than consolidation, and in such areas, pulmonary vessels images are not distinguishable. Reticular pattern consists in a sum of many small linear opacities, which appears as a net [18]. Finally, consolidations are described as areas of high attenuation that erases both vessels and airways walls margins. In the initial stages of the disease, the most frequent pattern at baseline CXRs was GGO, either isolated or associated to other patterns, with a peripheral distribution prevailingly in inferior lobes [15▪,16▪].
Many authors proposed the use of severity score indexes for the evaluation of CXR, based on the type and extension of lung alterations, and demonstrated that higher scores correlated with worse clinical conditions and outcomes. More extended consolidations on initial CXR are associated with a higher rate of hospitalization and invasive mechanical ventilation [9,16▪,19,20].
In mild COVID-19 infection, typical CXR findings consist of bilateral peripheral, multifocal GGOs. Conversely, in critical patients with acute respiratory failure and ARDS, multiple, bilateral consolidations are usually observed. Consolidations appear in most cases after 10–12 days from the onset of symptom and are frequently bilateral, preferentially localized in the lower lung fields [10▪,15▪,16▪] (Figs. 1c, 2, and 3b)
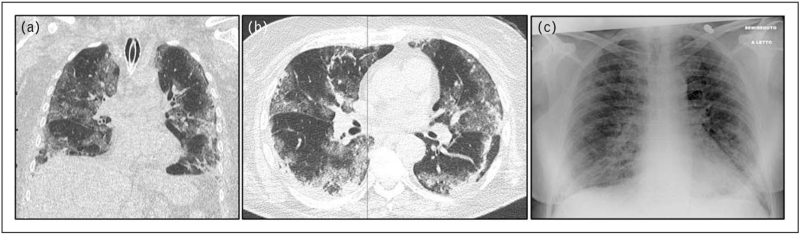
FIGURE 1.
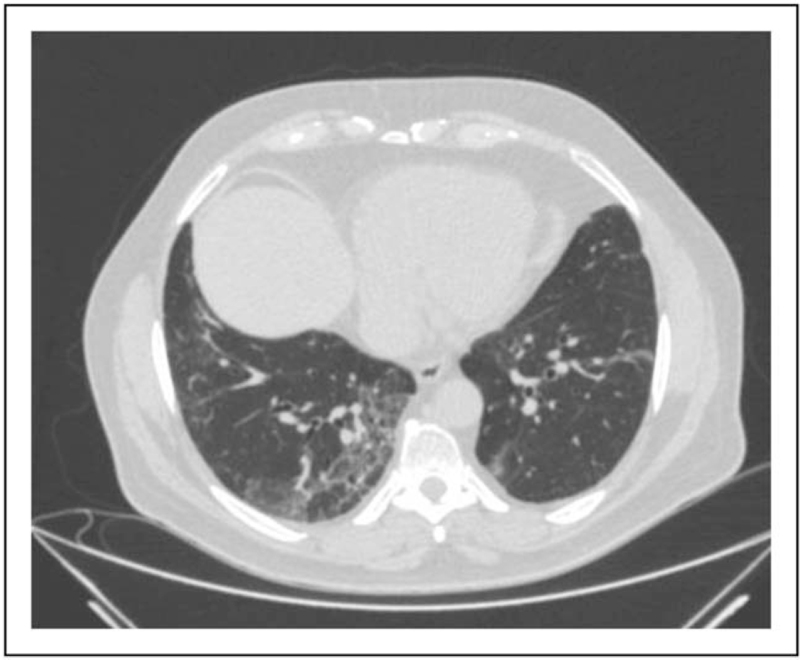
Acute respiratory failure in a 70-year-old patient. Chest imaging of a 70-year-old patient with acute respiratory failure because of COVID-19 treated with invasive mechanical ventilation (endotracheal tube in the trachea). (a) Coronal view of chest CT scan showing bilateral GGOs and consolidations with central and peripheral distribution. (b) Axial view of chest CT scan showing bilateral GGOs and consolidations with central and peripheral distribution.(c) Bedside CXR showing bilateral GGO and peripheral consolidations. COVID-19, coronavirus disease 2019; CT, computed tomography; CXR, chest radiography; GGOs, ground-gloss opacities.
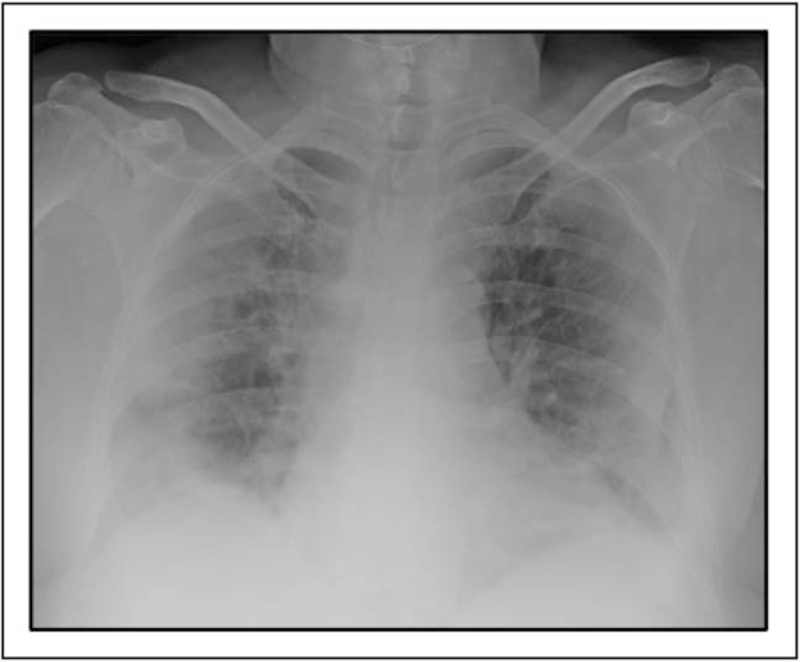
FIGURE 2.
Acute respiratory failure in a 60-year-old patient. Bedside CXR of a 60-year-old patient with acute respiratory failure because of COVID-19, showing bilateral peripheral confluent consolidations. COVID-19, coronavirus disease 2019; CXR, chest radiography.
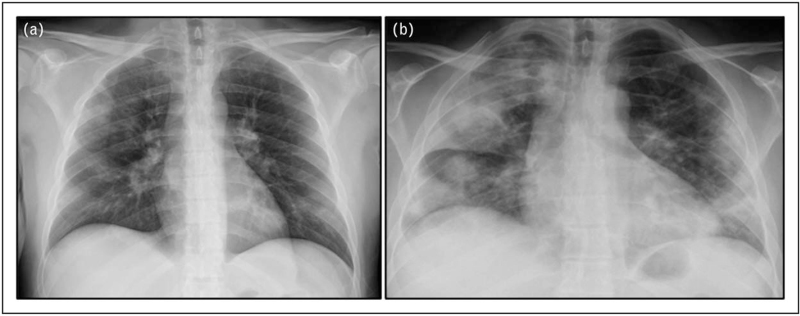
FIGURE 3.
The evolution of chest radiography findings in a 40-year-old patient with coronavirus disease 2019. Bedside CXRs of a 40-year-old patient with COVID-19. (a) Baseline CXR showing bilateral GGOs with a peripheral predominant distribution in medium-lower lobes. (b) CXR at day 5 showing bilateral, multifocal consolidations, more evident in the right lung. CXR, chest radiography; GGOs, ground-gloss opacities.
In patients with ARDS undergoing invasive mechanical ventilation, the execution of multiple bedside CXRs is a very useful tool to monitor the disease progression and treatment response. In patients with progressive disease, serial CXRs show a progressive extension of parenchymal consolidations, reaching the ‘white lung’ pattern, with bilateral confluent consolidations, in more critical cases [10▪,21] (Fig. 3a and b).
Interestingly, Artificial Intelligence models have been applied to CXRs with the aim of improving diagnostic accuracy and lowering the need of human workforce during the pandemic [9,22].
COMPUTED TOMOGRAPHY
CT is the gold-standard for the evaluation of pathological chest finding in COVID-19 patients [23].
When compared with CXR, CT is a longer and more expensive examination, characterized by a higher radiation burden, requires a higher workload and carries a higher risk of contagion spread but is much more sensitive in the early stage of the disease [20,23].
In the early phases of the pandemic characterized by a rapid increase in the number of patients, some authors advocated the use of CT scan as an aid for early diagnosis as CT findings can forerun the onset of symptoms and can help to reduce the rate of false-negative nucleic acid tests [6,17,24,25]. The most frequent findings at CT are GGOs, consolidations, reticular interlobular septal thickening, pleural thickening, air bronchograms, bronchus distortion, and crazy paving [6,26,27] (Figs. 4 and 5).
FIGURE 4.
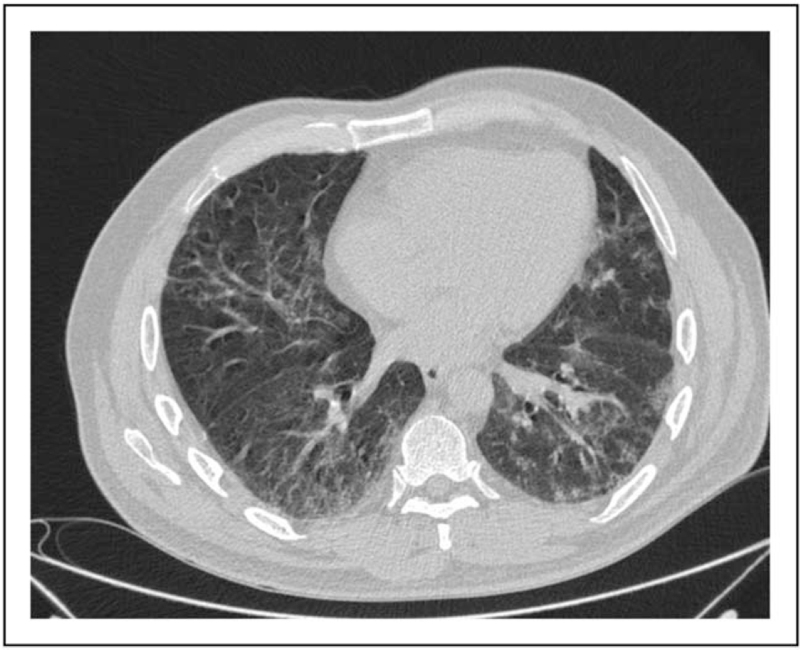
Reticular thickening, bronchiectasis, and pseudonodular opacities. CT of the chest showing diffuse inter-intralobular reticular thickening with concomitant thickened wall bronchiectasis. Millimetric pseudonodular opacities of inflammat.ory type are observed in the peripheral region in the left lower lobe. CT, computed tomography.
FIGURE 5.
Reticular thickening, bronchiectasis, and ground-gloss opacity. CT of the chest showing inter-intralobular reticular thickening in the right lobe with concomitant thickened wall bronchiectasis bilaterally. Right lower lobe shows GGO-type parenchyma opacity in submantellar location. CT, computed tomography; GGO, ground-gloss opacity.
Pleural, pericardial effusion, and lymphadenopathy are usually absent [28]. Opacities are peripheral and subpleural, typically have a peribronchovascular distribution and are more represented posteriorly in the lungs [27]. In the very early stages of the disease, these changes might be limited and show a unilateral distribution but they rapidly become bilateral [26]. Halo sign, defined as the presence of a GGO around a solid nodule, is present in a quarter of patients. Additionally, in some patients, a reversed-halo sign, defined a soft tissue density around a central GGO, has been described [29]. In addition, both bronchial wall thickening and air bronchograms are detected [17] (Fig. 1a and b and Fig. 4). In regions with high prevalence of SARS-CoV-2 infection, the sensitivity of chest CT for the diagnosis of COVID-19 reaches 97%, and this examination is important for the identification of other causes of respiratory failure [17,25]. As an example, bacterial pneumonia is characterized at CT scans by patchy shadows distributed along the bronchi or by large lobe consolidation with air bronchogram [17].
CT findings of viral pneumonia are similar to those of COVID-19; however, the latter is characterized by GGO and reticular interlobular septal thickening, which are preferentially peripheral, and pleural effusion and lymphadenopathy are less likely [29].
The Coronavirus disease 2019 Reporting and data System CT (CO-RADS) has been proposed to classify chest CT findings into 5 grades with 1 being unlikely to represent COVID and 5 being typical for the disease [30].
CT is a fundamental tool in the assessment of disease progression and severity. Pan et al. summarized in four CT-based stages the course of the disease [1,24,31]. The early-stage or stage 1 (from 0 to 4 days after symptoms onset) is characterized by subpleural unilateral or bilateral GGO distributed in the lower lobes. In the second or progressive stage (from 5 to 9 days from symptoms onset), GGO are found in a diffuse fashion and septal thickening becomes more obvious, leading to the so-called ‘crazy-paving’ pattern; moreover, consolidations can be seen. Stage 3, the peak stage (from 9 to 13 days after symptoms onset), is the most radiologically severe, characterized by diffuse parenchymal pathological changes, with dense consolidations becoming prevalent. Stage 4 is the absorption stage (from the 14th day after symptom onset), in which consolidations are slowly absorbed [31–33].
Dual Energy CT has demonstrated to be able to recognize small pulmonary embolism, not detectable with conventional CT angiography and to identify parenchymal perfusion deficits in COVID-19 patients [34].
Henkel et al. correlated CT patterns of ARDS in COVID-19 patients and postmortem pathologic lung features. In all patients, diffuse alveolar damage and capillary congestion was seen on autopsy, whereas microthrombi and superimposed acute bronchopneumonia were observed in 38 and 24% of the cases, respectively but no histopathologic pattern was associated to a specific CT pattern [35]. However, areas of GGOs reflected capillary dilatation, congestion, interstitial edema, and acute exudative diffuse alveolar damage, while consolidations reflected microthrombosis and leukocytoclastic vasculitis [35]. Bronchial wall thickening and consolidation reflected bacterial superinfection and bronchopneumonia [35].
The frequent involvement of the pulmonary circulation in COVID-19, with diffuse pathophysiologic drivers of hypoxemia, capillary microthrombosis, and thromboembolism [4,35], has been extensively described. At CT scan, vascular alterations are seen as vascular thickening, pulmonary artery enlargement, and vascular congestion [36,37].
Finally, CT plays an important role in monitoring ARDS progression and severity [6,38]. Wu et al. performed a semi-quantitative analysis of pulmonary CT findings and proposed a score for the prediction of severe/critical ARDS, based on the quantification of pulmonary involvement. This study highlights that CT findings like consolidation, crazy-paving pattern, linear opacities, bronchial wall thickening, high CT score, and extrapulmonary lesions were characteristic of severe/critical ARDS [39,40].
LUNG ULTRASOUND
LUS is a noninvasive, well tolerated, feasible, repeatable, radiation-free, and low-cost imaging technique [41].
In recent years, it has been widely used for the study of lungs through the evaluation and quantification of B-lines, consolidations, and pleural alterations. B-lines are defined as hyperechoic artefacts originating perpendicularly from the pleura, reaching the bottom of the image and moving concomitantly with lung movements [42]. These artefacts are a sign of loss of lung ventilation and interstitial alterations, corresponding to the GGOs observed at CT and CXR [42,43]. Several studies suggest a LUS evaluation of COVID-19 patients for its repeatability and safety [13,14]. LUS can be easily performed at the bedside, and this may be particularly useful in mechanically ventilated patients or in patients on extracorporeal membrane oxygenation, who might be difficult to move to the radiology department [44].
There are multiple approaches for the execution and evaluation of LUS, and different scores have been proposed, all based on the subdivision of the lungs into sections and on the semiquantitative analysis of the findings in each section [43]. Li et al. demonstrated a prognostic role of a LUS score, with patients presenting with a higher score having worse outcomes [45]. In ARDS patients, typical LUS findings include a high number of B-lines, consolidations, and reduced lung sliding [41,46]. The presence of such findings is indeed a marker of critical respiratory conditions that frequently require invasive ventilation [46].
LUS can be used to monitor ARDS evolution and the effects of mechanical ventilation as LUS scores are related to lung aeration. For example, Conway et al.[44] suggested the use of LUS scores in mechanically ventilated COVID-19 patients for adjusting the positive end-expiratory pressure (PEEP) and recruitment maneuvers [47,48].
In ICU patients, other important applications of LUS are the recognition of complications, like pneumothorax, the guiding of invasive procedures, and the assessment of the correct position of the endotracheal tube [47,48].
Of note, all authors have emphasized the importance of an accurate training of the operator, which is frequently a nonradiologist before performing LUS [49–51].
Data regarding diagnostic performance of LUS demonstrate high accuracy (93.3%), sensitivity (100.0%), and specificity (92.9%) for severe lung lesions, confirming its usefulness in the diagnosis and monitoring of critical COVID-19 patients [17].
PET/COMPUTED TOMOGRAPHY
Patients with ARDS because of COVID-19 have peripheral GGOs and lung consolidation with a high inflammatory activity that is reflected by a high 18F-FDG uptake [17].
Quin et al. described 18F-FDG avid foci of pathological radiotracer uptake in lung parenchyma and in regional lymph nodes in a series of COVID-19 patients who underwent occasional PET/CT examinations. They stated that PET/CT findings were attributable to SARS-CoV-2 tropism as there were no inflammatory lesions in other body segments [52].
F-FDG PET/CT may also yield further data on ongoing inflammatory pathological alterations: areas with higher 18F-FDG uptake may correspond with higher erythrocyte sedimentation rates and take longer to recover [53].
The drawbacks of PET/CT are the long acquisition time, the high radiation burden, and the higher cost when compared with CXR or CT. For these reasons, it cannot be considered as a diagnostic tool alone but rather as a completion in combination with other modalities [54].
MRI
MRI chest findings in COVID-19 patients are mainly ‘incidental’, as MRI is usually performed for other indications [53].
Findings as GGOs, consolidation, reticulation, and reverse halo sign have been described by Torkian et al.
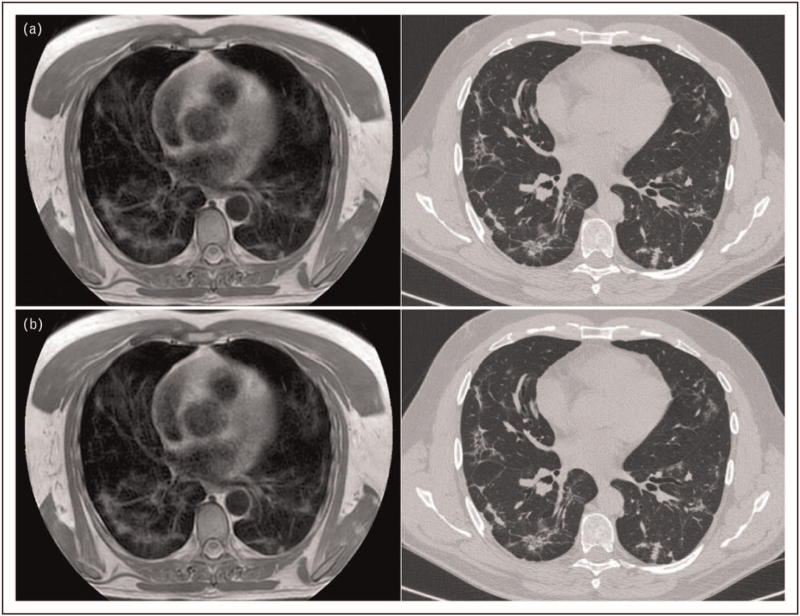
Yang et al. showed a high concordance between CT and MRI in the detection of characteristically COVID-19 findings, including GGOs and consolidation. (Fig. 6 a and b). However, fair-to-moderate agreement was observed for the evaluation of secondary findings, such as pseudocavities, crazy paving pattern, and air bronchograms [55,56].
FIGURE 6.
Reticular thickening pattern, bronchiectasis, and pleural thickening. MRI (a) and CT (b) images of the chest, showing a diffuse pattern of inter-intralobular reticular thickening with contextual thickened walled bronchiectasis. Minimal signs of pleural thickening in the right lower lobe, in the posterior region. CT, computed tomography.
A good sequence to detect MRI COVID-19 alterations are T2-weighted turbo spin-echo turbo inversion recovery magnitude (T2W TSE-TIRM) as lesions appear brighter than in the other sequences [55]. Another useful sequence is T2 PROPELLER that is obtained with respiratory gating triggered by the expiration phase of the respiratory cycle and were completed with MIP reconstruction (10 mm of slice thickness).
Moreover, MRI could provide an alternative for high-risk patients, such as pregnant women and pediatric patients who should stave off radiation exposure.
Finally, it has been proposed that MRI could play a role in the follow-up of COVID patients.
CONCLUSION
Chest CT scan is the imaging technique with higher sensitivity and reliability. In critically ill patients, who require close and repeated monitoring, the use of LUS or bedside CXR, although of lower sensitivity, can reduce the radiation dose exposure and the risk of infection spread when compared with CT. Other modalities, like PET/CT and MRI, especially in ARDS patients, are not routinely used for diagnostic or follow-up purposes, even though in the latter setting, MRI could indeed have a role [11,16▪,51,53,55].
Acknowledgements
C.G., G.M.R., and C.L. contributed for imaging selection.
The content of this manuscript does not necessarily reflect the views, policies, or opinions of the National Institutes of Health (NIH) nor the U.S. Department of Health and Human Services. Opinions expressed are those of the authors, not necessarily the NIH.
Financial support and sponsorship
B.J.W. receives funding support through the Intramural Targeted ANti-COVID-19 Program of the NIAID.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Lin S, Kantor R, Clark E. Coronavirus disease. Clin Geriatr Med 2021; 37:509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angileri S, Petrillo M, Di Meglio L, et al. Adverse events in coronavirus disease patients management: a pictorial essay. J Clin Imaging Sci 2020; 10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3▪.Grasselli G, Tonetti T, Protti A, et al. collaborators. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med 2020; 8:1201–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]; This works describes the pathophysiology of ARDS in COVID-19 patients.
- 4.Schiaffino S, Albano D, Cozzi A, et al. CT-derived chest muscle metrics for outcome prediction in patients with COVID-19. Radiology 2021; 300:E328–E336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Wang Y, Zhang Y, et al. A Quantitative and Radiomics approach to monitoring ARDS in COVID-19 patients based on chest CT: a retrospective cohort study. Int J Med Sci 2020; 17:1773–1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D’Agnillo F, Walters K-A, Xiao Y, et al. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci Transl Med 2021; eabj7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragnoli B, Malerba M. Focus on the potential role of lung ultrasound in COVID-19 pandemic: what more to do? Int J Environ Res Public Health 2020; 17:8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrichiello A, Angileri SA, Ierardi AM, et al. Bedside vascular access procedures for COVID-19 patients. J Vasc Access 2020; 22:654–657. [DOI] [PubMed] [Google Scholar]
- 9.Esposito A, Casiraghi E, Chiaraviglio F, et al. Artificial intelligence in predicting clinical outcome in COVID-19 patients from clinical, biochemical and a qualitative chest X-ray scoring system. Reports Med Imaging 2021; 14:27–39. [Google Scholar]
- 10▪.Vespro V, Andrisani MC, Fusco S, et al. Chest X-ray findings in a large cohort of 1117 patients with SARS-CoV-2 infection: a multicenter study during COVID-19 outbreak in Italy. Intern Emerg Med 2021; 16:1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work is interesting for the clear description of findings in COVID-19 patients CXRs.
- 11.Rubin GD, Ryerson CJ, Haramati LB, et al. The role of chest imaging in patient management during the COVID-19 pandemic. Chest 2020; 158:106–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. ACR Recommendations for the use of chest radiography and computed tomography (CT) for suspected COVID-19 infection j American College of Radiology. Available at: https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed date14/10/2021. [Google Scholar]
- 13.Buonsenso D, Davide P, Chiaretti A. COVID-19 outbreak: less stethoscope, more ultrasound. Lancet Respir Med 2020; 8:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soldati G, Smargiassi A, Inchingolo R, et al. Is there a role for lung ultrasound during the COVID-19 pandemic? J Ultrasound Med 2020; 39:1459–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15▪.Wong HYF, Lam HYS, Fong AH-T, et al. Frequency and distribution of chest radiographic findings in patients positive for COVID-19. Radiology 2020; 296:E72–E78. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work describes the most frequent fndings in COVID-19 patients.
- 16▪.Toussie D, Voutsinas N, Finkelstein M, et al. Clinical and chest radiography features determine patient outcomes in young and middle-aged adults with COVID-19. Radiology 2020; 297:E197–E206. [DOI] [PMC free article] [PubMed] [Google Scholar]; This work makes a correlation between the baseline CXR pattern with the outcomes of the patients
- 17.Jiang ZZ, He C, Wang DQ, et al. The role of imaging techniques in management of COVID-19 in China: from diagnosis to monitoring and follow-up. Med Sci Monit 2020; 26:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hansell DM, Bankier AA, Mcloud TC, et al. Fleischner Society: glossary of terms for thoracic imaging. Radiology 2008; 246:697–722. [DOI] [PubMed] [Google Scholar]
- 19.Borghesi A, Zigliani A, Golemi S, et al. Chest X-ray severity index as a predictor of in-hospital mortality in coronavirus disease 2019: a study of 302 patients from Italy. Int J Infect Dis 2020; 96:291–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monaco CG, Zaottini F, Schiaffino S, et al. Chest x-ray severity score in COVID-19 patients on emergency department admission: a two-centre study. Eur Radiol Exp 2020; 4:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobi A, Chung M, Bernheim A, Eber C. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imaging 2020; 64:35–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blain M, Kassin MT, Varble N, et al. Determination of disease severity in COVID-19 patients using deep learning in chest X-ray images. Diagnostic Interv Radiol 2021; 27:20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zu ZY, Jiang MDi, Xu PP, et al. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology 2019; 296:E15–E25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Agricola E, Beneduce A, Esposito A, et al. Heart and lung multimodality imaging in COVID-19. JACC Cardiovasc Imaging 2020; 13:1792–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang W, Sirajuddin A, Zhang X, et al. The role of imaging in 2019 novel coronavirus pneumonia (COVID-19). Eur Radiol 2020; 30:4874–4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Solomon JJ, Heyman B, Ko JP, et al. CT of post-acute lung complications of COVID-19. Radiology 2021; 211396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojha V, Mani A, Pandey NN, et al. CT in coronavirus disease 2019 (COVID-19): a systematic review of chest CT findings in 4410 adult patients. Eur Radiol 2020; 30:6129–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou S, Zhu T, Wang Y, Xia LM. Imaging features and evolution on CT in 100 COVID-19 pneumonia patients in Wuhan. China Eur Radiol 2020; 30:5446–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Prokop M, van Everdingen W, van Rees Vellinga T, et al. CO-RADS: a categorical CT assessment scheme for patients suspected of having COVID-19—definition and evaluation. Thorac Imaging 2020; 296:E97–E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan F, Ye T, Sun P, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology 2020; 295:715–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranieri VM, Rubenfeld GD, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA 2012; 307:2526–2533. [DOI] [PubMed] [Google Scholar]
- 33.Zeng Z, Xiang M, Guan H, et al. Early fibroproliferative signs on high-resolution CT are associated with mortality in COVID-19 pneumonia patients with ARDS: a retrospective study. Ther Adv Chronic Dis 2021; 12: 2040622320982171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aydin S, Kantarci M, Karavas E, et al. Lung perfusion changes in COVID-19 pneumonia: a dual energy computerized tomography study. Br J Radiol 2021; 94:20201380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henkel M, Weikert T, Marston K, et al. Lethal COVID-19: radiologic-pathologic correlation of the lungs. Radiol Cardiothorac Imaging 2020; 2:e200406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schiaffino S, Codari M, Cozzi A, et al. Machine learning to predict in-hospital mortality in COVID-19 patients using computed tomography-derived pulmonary and vascular features. J Pers Med 2021; 11:501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schiaffino S, Giacomazzi F, Esseridou A, et al. Pulmonary thromboembolism in coronavirus disease 2019 patients undergoing thromboprophylaxis. Medicine (Baltimore) 2021; 100:e24002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ierardi AM, Angileri SA, Arrichiello A, et al. Pulmonary embolism in COVID-19: ventilation and perfusion computed tomography. IDCases 2020; 21:e00805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chang YC, Yu CJ, Chang SC, et al. Pulmonary sequelae in convalescent patients after severe acute respiratory syndrome: evaluation with thin-section CT. Radiology 2005; 236:1067–1075. [DOI] [PubMed] [Google Scholar]
- 40.Li K, Wu J, Wu F, et al. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 2020; 55:327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Volpicelli G, Gargani L. Sonographic signs and patterns of COVID-19 pneumonia. Ultrasound J 2020; 12:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Soldati G, Demi M, Smargiassi A, et al. The role of ultrasound lung artifacts in the diagnosis of respiratory diseases. Expert Rev Respir Med 2019; 13:163–172. [DOI] [PubMed] [Google Scholar]
- 43.Volpicelli G, Lamorte A, Villén T. What's new in lung ultrasound during the COVID-19 pandemic. Intensive Care Med 2020; 46:1445–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Conway H, Gary L, Zochios V. Personalizing invasive mechanical ventilation strategies in coronavirus disease 2019 (COVID-19)? Associated lung injury: the utility of lung ultrasound. J Cardiothorac Vasc Anesth 2020; 34:2571–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ji L, Cao C, Gao Y, et al. Prognostic value of bedside lung ultrasound score in patients with COVID-19. Crit Care 2020; 24:700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dargent A, Chatelain E, Kreitmann L, et al. COVID-LUS study group. Lung ultrasound score to monitor COVID-19 pneumonia progression in patients with ARDS. PLoS One 2020; 15:e0236312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mongodi S, Orlando A, Arisi E, et al. Lung ultrasound in patients with acute respiratory failure reduces conventional imaging and healthcare provider exposure to COVID-19. J Ultrasound Med Biol 2020; 46:2090–2093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mojoli F, Mongodi S, Orlando A, et al. COVID-19 Pavia Crisis Unit. Our recommendations for acute management of COVID-19. Crit Care 2020; 24:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cho YJ, Song KH, Lee Y, et al. Lung ultrasound for early diagnosis and severity assessment of pneumonia in patients with coronavirus disease. Korean J Intern Med 2020; 35:771–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tung-Chen Y. Lung ultrasound in the monitoring of COVID-19 infection. Clin Med (London) 2020; 20:E62–E65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Allinovi M, Parise A, Giacalone M, et al. Lung ultrasound may support diagnosis and monitoring of COVID-19 pneumonia. Ultrasound Med Biol 2020; 46:2908–2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Qin C, Liu F, Yen TC, Lan X. 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging 2020; 47:1281–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fields BKK, Demirjian NL, Dadgar H, Gholamrezanezhad A. Imaging of COVID-19: CT, MRI, and PET. Semin Nucl Med 2021; 51:312–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rafiee F, Keshavarz P, Katal S, et al. Coronavirus disease 2019 (COVID-19) in molecular imaging: a systematic review of incidental detection of SARS-CoV-2 pneumonia on PET studies. Semin Nucl Med 2021; 51:178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Torkian P, Rajebi H, Zamani T, et al. Magnetic resonance imaging features of coronavirus disease 2019 (COVID-19) pneumonia: the first preliminary case series. Clin Imaging 2021; 69:261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yang S, Zhang Y, Shen J, et al. Clinical potential of UTE-MRI for assessing COVID-19: patient- and lesion-based comparative analysis. J Magn Reson Imaging 2020; 52:397–406. [DOI] [PMC free article] [PubMed] [Google Scholar]