Purpose of review
Noninvasive respiratory support has been widely applied during the COVID-19 pandemic. We provide a narrative review on the benefits and possible harms of noninvasive respiratory support for COVID-19 respiratory failure.
Recent findings
Maintenance of spontaneous breathing by means of noninvasive respiratory support in hypoxemic patients with vigorous spontaneous effort carries the risk of patient self-induced lung injury: the benefit of averting intubation in successful patients should be balanced with the harms of a worse outcome in patients who are intubated after failing a trial of noninvasive support.
The risk of noninvasive treatment failure is greater in patients with the most severe oxygenation impairment (PaO2/FiO2 < 200 mmHg).
High-flow nasal oxygen (HFNO) is the most widely applied intervention in COVID-19 patients with hypoxemic respiratory failure. Also, noninvasive ventilation (NIV) and continuous positive airway pressure delivered with different interfaces have been used with variable success rates. A single randomized trial showed lower need for intubation in patients receiving helmet NIV with specific settings, compared to HFNO alone.
Prone positioning is recommended for moderate-to-severe acute respiratory distress syndrome patients on invasive ventilation. Awake prone position has been frequently applied in COVID-19 patients: one randomized trial showed improved oxygenation and lower intubation rate in patients receiving 6-h sessions of awake prone positioning, as compared to conventional management.
Summary
Noninvasive respiratory support and awake prone position are tools possibly capable of averting endotracheal intubation in COVID-19 patients; carefully monitoring during any treatment is warranted to avoid delays in endotracheal intubation, especially in patients with PaO2/FiO2 < 200 mmHg.
Keywords: acute respiratory failure, awake prone position, COVID-19, high flow nasal oxygen, noninvasive respiratory support
INTRODUCTION
The optimal management of hypoxemic respiratory failure is debated. Most recent guidelines suggest caution in using noninvasive respiratory support – namely high-flow nasal oxygen (HFNO), noninvasive ventilation (NIV) or continuous positive end-expiratory pressure (CPAP) – for the early treatment of hypoxemic respiratory failure due to COVID-19 [1] and non-COVID-19 etiology [2]. Early endotracheal intubation has been advocated in the early phases of the pandemic to limit the risks related to prolonged exposure of injured lungs to the potential harms of intense inspiratory efforts and high tidal volumes [3].
Box 1.
no caption available
However, intubation with invasive mechanical ventilation can lead to serious complications, including ventilator-induced lung injury, intensive care unit (ICU)-acquired pneumonia, diaphragmatic atrophy and ICU-induced delirium, with detrimental effects on clinical outcome [4,5]. During the COVID-19 pandemic, the unprecedented lack of mechanical ventilators and ICU beds led clinicians to extensively use noninvasive respiratory support for the treatment of hypoxemic respiratory failure [6–9].
Patients with hypoxemic respiratory failure often show dysregulated respiratory drive. The harmful effects of spontaneous breathing with intense inspiratory effort can result in self-induced lung injury (P-SILI). P-SILI may worsen the clinical outcome of patients who require endotracheal intubation after having received noninvasive respiratory support [10,11].
As the debate remains open, we searched MEDLINE-PubMed databases for the relevant articles (up to August 2021) assessing the physiological and clinical effects of noninvasive respiratory support in patients with acute respiratory failure of COVID-19 etiology.
In this review, we provide an overview of the available evidence regarding the use of noninvasive respiratory support in COVID-19 patients, highlighting its benefits and potential risks.
PATIENT SELF-INDUCED LUNG INJURY
In hypoxemic respiratory failure, lung injury yields altered respiratory mechanics and increased dead space; inflammation combined to biochemical stimuli induced by hypoxemia, respiratory acidosis with hypercarbia, and the ‘chemomechanical’ variations due to atelectasis and alveolar derecruitment increase patient's ventilatory demand. This results in a shift of brain homeostasis toward a lower level of PaCO2, which can cause spontaneous ventilation with high inspiratory effort, large tidal volumes and tachypnea, leading to abnormal inspiratory swings of pleural pressure and consequent increment of the transpulmonary pressure (alveolar pressure – esophageal pressure as a surrogate of the pleural pressure). As a consequence, baro-, volu- and atelec-trauma are generated. These mechanisms lead to the progression of lung injury [12▪,13▪,14–18]. Additionally, the increase in transmural pressure of lung vessels combined with their increased permeability concur to alveolar flooding and negative pressure pulmonary edema [15,19].
Under this scenario, the damaged lung can exhibit two distinct patterns: the healthy lung has a more fluid-like condition in the nondependent regions, whereas the most damaged and atelectatic regions have a solid-like pattern (the dependent regions). The solid-like regions transmit pleural pressure differently from the fluid-like regions, finally generating intra-tidal heterogeneity of transpulmonary pressure. This causes an intra-tidal shift of gas from nondependent regions of the lung to the dependent regions; this occult movement of air is called ‘pendelluft’, and can overstretch dependent lung regions independently from the size of inspired volume, increasing inflammation and regional strain [20].
Delivering high positive end-expiratory pressure (PEEP) during spontaneous breathing might render spontaneous effort noninjurious through different mechanisms: (1) it increases functional residual capacity, reducing the extension of atelectatic regions, finally decreasing the mechanical stimuli yielding the increase of respiratory drive, (2) it yields diaphragmatic uncoupling, reducing the inspiratory effort, (3) it limits the occurrence of pendelluft phenomenon by favoring a more homogeneous transmission of the pleural pressure across the lung tissue [12▪,21,22].
Although these considerations may advocate against the use of noninvasive respiratory support, it appears that patients with hypoxemic respiratory failure due to COVID-19 exhibit average lower inspiratory effort than non-COVID-19 patients with similar oxygenation impairment, possibly indicating a reduced risk of P-SILI [23].
Available data indicate that, in patients with PaO2/FiO2 > 200mmHg, noninvasive respiratory support is safe and effective. Differently, in patients with PaO2/FiO2 ≤ 200 mmHg, the best balance between the benefits and harms of maintaining spontaneous breathing with noninvasive respiratory support has yet to be identified [8,24]. When considering a noninvasive respiratory support trial, the optimal strategy should aim to limit the risk of endotracheal intubation, without increasing the risk of P-SILI: [25] this is of particular importance given the high failure rate of noninvasive respiratory support in COVID-19 hypoxemic respiratory failure when compared to non-COVID-19 patients [26▪].
HIGH-FLOW NASAL OXYGEN
HFNO is a technique that delivers high flow rates (60 L/min) of humidified and heated oxygen at adjustable FiO2 through nasal cannula. HFNO allows (1) accurate delivery of the set FiO2 by limiting dilution of inhaled gas, (2) provides carbon dioxide washout of the upper airways and reduction of physiological dead space when the flow rates is > 30 L/min (3) and variable PEEP that increases with flow rates, ultimately reducing inspiratory effort [27,28].
A randomized trial showed that HFNO might reduce intubation rate and mortality in moderate-to-severe hypoxemic respiratory failure when compared to low flow oxygen and face mask NIV [24], and the latest guidelines [2,29] suggest HFNO as the optimal first line intervention to correct hypoxemia during de novo respiratory failure.
Despite the initial concerns about the risk of viral aerosolization and transmission to healthcare workers – that can be mitigated by a surgical mask on top of high-flow nasal cannula, which also improves oxygenation [30]-HFNO has been widely applied in patients with acute respiratory failure due to COVID-19 in heterogeneous clinical scenarios, with highly variable outcomes in terms of endotracheal intubation and mortality rate [31–37] (Table 1).
Table 1.
Clinical trials of HFNO in acute hypoxemic respiratory failure of COVID-19 etiology
| Publication | PMID | Study design | Setting | Patient Population | Treatment | Intubation Rate | Mortality Rate | Main finding | Secondary findings |
| Bonnet et al.[47], 2021 | 33638752 | Retrospective multicenter study | ICU | COVID-19 AHRF At admission O2 flow rate 9 lt/min and PaO2 69 [63–82] | SOT n = 62 HFNO n = 76 | SOT 74% [95% CI 62 to 83] HFNO 51% [95% CI 40 to 62] | SOT 26% [95% CI 17 to 38] HFNO 16% [95% CI 9 to 26] | HFNO oxygen for AHRF due to COVID-19 is associated with a lower rate of invasive mechanical ventilation compared to SOT | Mortality and ICU LOS did not differ. The number of VFD was lower in the HFNO group. A ROX index higher than 4.88 and higher SAPSII were associated with IMV. |
| Chandel et al.[49], 2021 | 33328179 | Multicentered retrospective study | Mixed population | COVID-19 AHRF PaO2/FiO2 not reported ROX index after 2 h of HFNO 4.5 [3.3–6.0] | HFNO n = 272 | 40% [95% CI 34 to 46] | 17% [95% CI 13 to 21] | Prolonged usage of HFNO was not associated with worse clinical outcomes compared with shorter trials in those that ultimately required mechanical ventilation | The ROX index was sensitive for the identification of subjects who were successfully managed with HFNO and a cut off of 3.67 at 12 h was identified |
| Demoule et al.[31], 2020 | 32758000 | Retrospective study | ICU | COVID-19 AHRF HFNO: PaO2/FiO2 126 [86–189] No-HFNO: PaO2/FiO2 130 [97–195] | Matched sample: HFNO n = 137 no-HFNO n = 137 | HFNO 55% [95% CI, 46 to 63] no-HFNO 72% [95% CI, 64 to 79] | HFNO 21%, [95% CI 15 to 29] no-HFNO 22% [95% CI 16 to 30] | HFNO significantly reduces intubation and subsequent invasive mechanical ventilation compared to standard oxygen therapy, but does not affect case fatality | |
| Ehrmann et al.[128▪▪], 2021 | 34425070 | Prospective collaborative randomized controlled meta trial, | Mixed setting | COVID-19 AHRF SpO2/FiO2 awake PP 147.9 (43.9) SpO2/FiO2 standard care148.6 (43.1) | Awake PP n = 564 Standard care n = 557 All patients treated with HFNO FiO2 0.6 [0.5 – 0.8] Awake PP HFNO flow 50 l/min [40–55] Standard care HFNO flow 40 l/min [40–50] | Treatment failure Awake PP 40% [95% CI 36 to 44] Treatment failure Standard care 46% [95% CI 42 to 50] IMV Awake PP 33% [95% CI 29 to 37] IMV Standard care 40% [95% CI 36 to 44] | Awake PP 21% [95% CI 18 to 24] Standard care 24% [95% CI 20 to 27] | Awake PP reduces the proportion of patients intubated or dying within 28 days of enrolment, 223 (40%) in the awake PP group vs 257 (46%) in the standard of care, P = 0.007, relative risk reduction 0.86 [95% CI 0.75 to 0.98]. Patients that received PP for longer sessions had lower treatment failure rate. | Awake PP significantly improves blood oxygenation, respiratory rate and ROX index during PP. The benefit was maintained after supination. |
| Franco et al.[67▪▪], 2020 | 32747398 | Retrospective multicenter study | Non-ICU | COVID-19 AHRF PaO2/FiO2 138 (66) | HFNO n = 163 CPAP n = 330 PEEP 10.2 (1.6) cmH2O Helmet 149 (99%) Face mask 2 (1%) NIV n = 177 PEEP 9.5 (2.2) cmH2O Pressure Support 17.3 (3) cmH2O Helmet 15 (21%) Face mask 57 (79%) | Recieved IMV: HFNO 29% [95% CI 24 to 36] CPAP 25% [95% CI 20 to 30] NIV 28% [95% CI 22 to 35] HFNO Failure 38% [Ci 31 to 47] CPAP Failure 47% [95% CI 42 to 53] NIV Failure 53% [95% CI 46 to 60] | 30 day mortality: HFNO 16% [95% CI 11 to 22] CPAP 30% [95% CI 26 to 35] NIV 31% [95% CI 24 to 38] Difference not significant at adjusted analysis | Noninvasive respiratory support outside of ICU is feasibile, and mortality rates compare favourably with previous reports. There was no difference among the interfaces at the adjusted analysis. | Noninvasive respiratory support was associated with risk of staff contamination. |
| Gaulton et al.[87], 2020 | 32984836 | Retrospective, multicenter study | ICU | COVID-19 AHRF SpO2 < 92% with 6l/min nasal cannula Body mass index, kg/m2, mean (sd) = 35.5 (8.6) | Helmet CPAP n = 17 HFNO n = 42 PEEP 5–10 cmH2O | ETI at 7 days CPAP 18% [6 to 41] HFNO 52% [38 to 67] | Death at 7 days CPAP 6% [1 to 27] HFNO 19% [10 to 33] | Difference in the intubation rate was significant after adjustment for age. | In obese patients Helmet CPAP is effective in reducing the ETI rate. |
| Geng et al.[37], 2020 | 32295710 | Case series | Non-ICU | COVID-19 AHRF PaO2/FiO2 259.88 (58) | HFNO n = 8 | 0% [95% CI 0 to 32] | 0% [95% CI 0 to 32] | HFNO is safe and effective in mild AHRF of COVID-19 etiology | |
| Grieco et al.[70▪▪], 2021 | 33764378 | Randomized controlled multicenter trial | ICU | COVID-19 AHRF NIV PaO2/FiO2 105 [83–125] HFNO PaO2/FiO2 102 [80–124] | Helmet NIV n = 54 Continuous treatment PEEP 12 [10–12] cmH2O Pressure Support 10 [10–12] cmH2O HFNO n = 55 | Helmet NIV 30% [95% CI 19 to 43] HFNO 51% [95% CI 38 to 64] | HFNO 25% [16 to 38] Helmet NIV 24% [95% CI 15 to 37] | Helmet NIV+HFNO or HFNO alone do not affect respiratory support free days. | Helmet NIV reduces rate of ETI and increases invasive VFD at day 28. |
| Hernandez-Romieu et al.[30], 2020 | 32804790 | Retrospective study | ICU | COVID-19 AHRF PaO2/FiO2 not reported for the overall cohort. At intubation, PaO2/FiO2 148 [111–205] | HFNO n = 109 Only IMV n = 97 | 72% [95% CI 62 to 79] | HFNO 22% [95% CI 15 to 31] Only IMV 40% [95% CI 31 to 50] | A trial of noninvasive respiratory support, including HFNO, in an attempt to avoid intubation, is not associated with increased mortality. | Use of noninvasive respiratory support is not associated with worse pulmonary compliance and oxygenation, among those who eventually require mechanical ventilation. |
| Liu et al.[40▪▪], 2021 | 33573999 | Retrospective multicentre study | ICU | COVID-19 AHRF PaO2/FiO2 HFNO 116 [66–252] PaO2/FiO2 NIV 113 [68–183] | HFNO n = 366 NIV n = 286 Type and setting of NIV is not reported | HFNO 56% [95% CI 51 to 61] NIV 74% [95% CI 68 to 78] | HFNO 49% [95% CI 44 to 54] NIV 62% [95% CI 56 to 67] | The nomogram and online calculator are simple to use and able to predict the risk of failure in patients with covid-19 treated with HFNO and NIV | Age, number of comorbidities, ROX index, Glasgow coma scale score, and use of vasopressors on the first day of noninvasive respiratory support were independent risk factors for noninvasive respiratory support failure |
| Mellado-Artigas et al.[33], 2021 | 33573680 | Prospective observational study | ICU | COVID-19 AHRF Only IMV PaO2/FiO2 117 (51) HFNO PaO2/FiO2 121 (49) | HFNO n = 61 Only IMV n = 61 | 38% [95% CI 27 to 50] | Only IMV 21% [95% CI 13 to 33] HFNO 15% [95% CI 8 to 26] | HFNO was associated with an increase in VFDs at 28 days when compared with early IMV and with reduction in ICU length of stay. | Mortality was not different in the patients that were intubated early and in the patients that failed HFNO. |
| Montiel et al.[30], 2020 | 32990864 | Prospective observational study | ICU | COVID-19 AHRF PaO2/FiO2 83 (± 22) | HFNO n = 21 | Not reported | Not reported | A surgical mask placed on patient's face already treated by a HFNO device would offer an advantage in terms of oxygenation in COVID-19 patients admitted in ICU with severe AHRF. | The oxygenation improvement is associated with neither a clinically significant change in the PaCO2 nor subjective patient complaints. |
| Panadero et al.[44], 2020 | 32983456 | Retrospective study | Non-ICU | COVID-19 AHRF SpO2/FiO2 in HFNO success 103.0 (3.4) ROX index in HFNO success 4.0 (1.4) SpO2/FiO2 in HFNO failure 101.4 (5.1) ROX index in HFNO failure 3.7 (1.0) | HFNO n = 40 | 52% [95% CI 37 to 67] | 22% [95% CI 12 to 37] | HFNO therapy is a useful treatment in ARDS in order to avoid ETI or as a bridge therapy, and no increased mortality was observed secondary to delayed intubation | After initiating HFNO, a ROX index below 4.94 predicts the need for intubation. |
| Rosén et al.[127▪], 2021 | 34127046 | Multicenter randomized clinical trial | Non-ICU | COVID-19 AHRF Standard care n = 39 PaO2/FiO2 standard care 115 [94–130] Prone n = 36 PaO2/FiO2 prone 115 [86–130] | HFNO standard care n = 29 HFNO prone n = 31 NIV standard care n = 27 PEEP 8 [6–8] NIV prone n = 21 PEEP 7 [6–10] | Standard care group 33% [95% CI 20 to 49] Prone group 33% [95% CI 20 to 50] | Control group 8% [95% CI 3 to 20] Prone group 17% [95% CI 8 to 22] | The implemented protocol for awake PP increased duration of awake PP but did not reduce the rate of intubation in patients with AHRF due to COVID-19 compared to standard care. | Nine patients (23%) in the control group had pressure sores compared with two patients (6%) in the prone group, P = 0.03, there were no difference in the use of NIV, vasopressors, continuous renal-replacement therapy, ECMO, VFD, hospital and ICU length of stay and mortality among the two groups. |
| Suliman et al.[43], 2021 | 33471350 | Diagnostic research | Mixed population | COVID-19 AHRF At intubation PaO2/FiO2 91 [60–110] | HFNO n = 69 | 59% [95% CI 48 to 70] | Not reported | ROX index is a simple noninvasive promising tool for predicting discontinuation of high-flow oxygen therapy and could be used by clinicians in the assessment of progress and the risk of intubation in COVID-19 patients with pneumonia | The ROX index on the 1st day of admission was significantly associated with the presence of comorbidities, COVID-19 clinical classification, CT findings and intubation |
| Vega et al.[34], 2021 | 34049831 | Retrospective analysis of prospectively collected data | Non-ICU | COVID-19 AHRF SpO2/FiO2 155 [106–190] | HFNO n = 120 | 29% [95% CI 21 to 38] | 7.5% [95% CI 4 to 14] | ROX index with cut off of 5.99 may be useful in guiding clinicians in their decision to intubate patients (especially in moderate acute respiratory failure) treated outside ICU | Among the components of the index SpO2/FiO2 had greater predictive value |
| Vianello et al.[35], 2020 | 32703883 | Retrospective study | ICU | COVID-19 AHRF PaO2/FiO2 108 [52–296] | HFNO n = 28 Rescue NIV n = 9 NIV settings, interfaces, and whether CPAP is codified as NIV is not reported | HFNO failure 32% [95% CI 18 to 51] Rescue NIV failure 56% [95% CI 27 to 81] ETI 18% [95% CI 8 to 36] | 11% [95% CI 4 to 27] | HFNO can be considered an effective and safe means to improve oxygenation in less severe forms of AHRF secondary to COVID-19 not responding to conventional oxygen therapy | Severity of hypoxemia and C reactive protein level were correlated with HFNO failure |
| Wang et al.[41], 2020 | 32232685 | Retrospective study | Mixed population | COVID-19 AHRF PaO2/FiO2 209 [179–376] in success patients PaO2/FiO2 142 [130–188] in failure patients | HFNO n = 17 only IMV n = 1 first line NIV n = 9 rescue NIV n = 7 | HFNO failure and rescue NIV 41% [95% CI 22 to 64] HFNO 12% [95% CI 3 to 34] First line NIV failure 11% [2 to 42] Rescue NIV failure 29% [8 to 64] | Not reported | HFNO was the most common ventilation support for patients, and rescue NIV was often used in case of HFNO failure | Patients with lower PaO2/FiO2 were more likely to experience HFNO failure |
| Wang et al.[39], 2020 | 32267160 | Retrospective study | ICU | SpO2/FiO2 in the overall cohort 279 [157–328] | HFNO n = 35 NIV n = 34 IMV n = 100 | HFNO 66% [95% CI 49 to 79] HNFO failure 77% [95% CI 61 to 88] NIV failure 79% [95% CI 63 to 90] | HFNO 80% [95% CI 64 to 90] NIV 77% [95% CI 61 to 88] IMV 97% [95% CI 92 to 99] | Older patients with comorbidities are at increased risk of mortality. Real-time monitoring of SpO2/FiO2 and regular measurements of lymphocyte count and inflammatory markers may be essential to disease management. | A total of 128 out of 145 (88.3%) patients who developed ARDS died at or before 28 days. |
| Wendel Garcia et al.[36], 2021 | 34034782 | Retrospective subanalysis of data | ICU | COVID-19 AHRF PaO2/FiO2 123 [92, 167] | SOT n = 87 HFNO n = 87 NIV n = 87 MV n = 92 | SOT 64% [95% CI 53 to 63] HFNO 52% [95% CI 41 to 62] NIV 49% [95% CI 39 to 60] | SOT 18% [95% CI 11 to 27] HFNO 20% [95% CI 13 to 29] NIV 37% [27 to 47] | A trial of HFNO appeared to be the most balanced initial respiratory support strategy. | Compared to the other respiratory support strategies, NIV was associated with a higher overall ICU mortality P = 016 and should be avoided. |
| Xia et al.[46], 2020 | 32826432 | Retrospective multicenter study | Mixed population | COVID-19 AHRF PaO2/FiO2 available in only 12 patients: 122 (51) | HFNO n = 43 | 30% [95% CI 19 to 45] HFNO failure 47% [95% CI 33 to 61] | 32% [95% CI 20 to 48] | Early HFNO may be an effective respiratory support modality for COVID-19 patients with mild to moderate AHRF, most severe cases need IMV or NIV | Male and lower oxygenation at admission were the two strongest predictors of HFNO failure. |
| Yang W. et al.[39], 2020 | 32267160 | Retrospective study | ICU | COVID-19 AHRF SpO2/FiO2 in the overall cohort 279 [157–328] | HFNO n = 35 NIV n = 34 IMV n = 100 | HFNO 66% [95% CI 49 to 79] HNFO failure 77% [95% CI 61 to 88] NIV failure 79% [95% CI 63 to 90] | HFNO 80% [95% CI 64 to 90] NIV 77% [95% CI 61 to 88] IMV 97% [95% CI 92 to 99] | Older patients with comorbidities are at increased risk of mortality. Real-time monitoring of S/F and regular measurements of lymphocyte count and inflammatory markers may be essential to disease management. | A total of 128 out of 145 (88.3%) patients who developed ARDS died at or before 28 days. |
| Yang X. et al.[66], 2020 | 32105632 | Retrospective study | ICU | PaO2/FiO2 100 [66.6–126.7] in survivors PaO2/FiO2 62 [52–74] nonsurvivors | Overall cohort n = 52 HFNO n = 33 NIV n = 29 IMV n = 22 | The progression among the interfaces is not reported | HFNO 48% [95% CI 32 to 65] NIV 79% [95% CI 62 to 90] IMV 86% [95% CI 67 to 95] | Among 52 critically ill patients with COVID-19 infection, 32 (61.5%) patients had died at 28 days. | Older patients (>65 years) with comorbidities and ARDS are at increased risk of death. |
| Zhou et al.[37], 2020 | 32171076 | Retrospective multicenter study | Mixed Population | PaO2/FiO2 at enrollment is not reported | HFNO n = 41 NIV n = 26 IMV n = 32 NIV settings, interfaces, and whether CPAP is codified as NIV is not know | Not reported | HFNO 80% [CI66 to 90] NIV 92% [95% CI 96 to 98] IMV 97% [95% CI 84 to 99] | Older age, high SOFA score, and d-dimer greater than 1 μg/mL could help clinicians to identify patients with poor prognosis at an early stage. | Noninvasive respiratory support and invasive mechanical ventilation have high mortality rate. |
| Zucman et al.[42], 2020 | 32671470 | Retrospective study | ICU | COVID-19 AHRF FiO2 at admission 0.8 [0.6–1] Median SpO2 96% [94–98] | HFNO n = 60 | 65% [95% CI 52 to 76] | 17% [9 to 28] | Early application of NHF as first-line ventilatory support during COVID-19-related AHRF may have obviated the need for intubation in up to a third of cases. | The ROX index measured within the first 4 h after NHF initiation could be an easy-to-use marker of early ventilatory response. |
Values are displayed as means (SD) or medians [Interquartile range].
Failure was defined as either intubation, death while still on noninvasive respiratory support, or escalation to other noninvasive respiratory support to avoid endotracheal intubation. AHRF, acute hypoxemic respiratory failure; ARDS, acute respiratory distress syndrome; awake PP, awake prone position; CPAP, continuous positive end-expiratory pressure; FiO2, fraction of inspired oxygen; HFNO, high-flow nasal oxygen; ICU, intensive care unit; IQR, interquartile range; NIV, noninvasive ventilation; PaO2, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; SpO2, peripheral capillary oxygen saturation; VFD, Ventilatory Free Days.
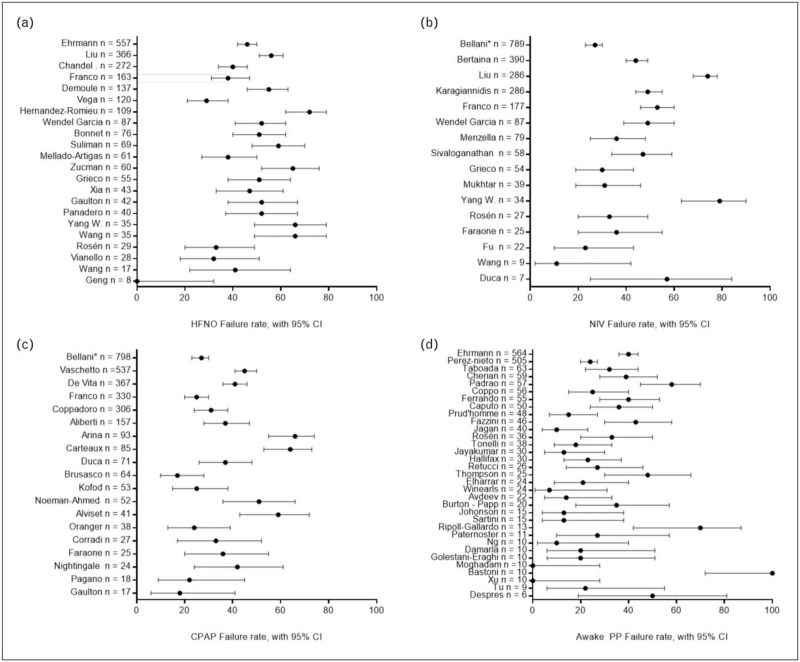
In ICU and non-ICU settings, patients with COVID-19 and PaO2/FiO2 ratio < 300 mmHg have been treated with HFNO, showing rates of endotracheal intubation and mortality rate as low as 0% in very mild patients [38] up to a failure rate as high as 80% in the most severe ones [39,40▪▪] (Fig. 1a).
FIGURE 1.
Panel reporting the failure rate [95% CI] of patients with hypoxemic respiratory failure treated with noninvasive respiratory support. Failure rate was defined as occurrence of endotracheal intubation or death. Only the patients without limitation of treatment were considered for the figure. Except from the bottom – right figure, nonrandomized studies including awake prone position were excluded from the figure, due to the possible selection bias of patients treated with conventional therapy. The studies with the bigger sample size are displayed at the top of the figure. (a) Forest plot of patients treated with HFNO in the supine position. (b) Forest plot of patients treated with NIV as first line of therapy. (c) Forest plot of patients treated with CPAP as first line of therapy. (d) Forest plot of patients treated with awake prone position, regardless of the kind of noninvasive respiratory support used. ∗It was not possible to differentiate between CPAP and NIV that were both considered as noninvasive respiratory support. CPAP, continuous positive end-expiratory pressure; HFNO, high-flow nasal oxygen; NIV, noninvasive ventilation.
Indeed, during the COVID-19 pandemic, HFNO has shown a great efficacy in patients with a PaO2/FiO2 ratio > 200 mmHg [38,41], whereas it may be associated to higher risk of failure when PaO2/FiO2 < 200 mmHg [42–45].
Overall, available data indicate that HFNO in COVID-19 patients with acute respiratory failure do not increase mortality rate and may be an effective strategy in mild-to-moderate cases to reduce the need for mechanical ventilation and critical care support [46,47].
As any other form of noninvasive respiratory support, HFNO should be applied under strict clinical monitoring to promptly detect treatment failure and reduce the risks related to delayed endotracheal intubation, P-SILI and poor prognosis. The ROX index, which is the SpO2 normalized to FiO2 times respiratory rate has been validated in the non-COVID setting [48], has been examined by several authors to be adapted to COVID-19 patients. Preliminary data show that ROX index in COVID-19 patients could be a simple marker to predict the risk of HFNO failure and could be used to prevent the delay in endotracheal intubation: however, different thresholds have been suggested, ranging from 3.67 to 5.37, and the optimal cut-off for COVID-19 patients remains to be clarified [42–44,47,49].
NONINVASIVE VENTILATION
NIV has progressively been identified in the last 20 years as valid treatment in the management of acute respiratory failure.
Specific indications are exacerbation of chronic obstructive pulmonary disease, cardiogenic pulmonary edema, pneumonia in immunocompromised patients and weaning of previously intubated stable patients with chronic obstructive pulmonary disease.
Conversely, in the treatment of hypoxemic respiratory failure, NIV use has been associated with conflicting results, and the most recent guidelines suggest caution in its application [2,29]. Clinical outcome improves when NIV allows to avoid endotracheal intubation. Differently, if intubation is needed after NIV, mortality is increased, possibly due to delayed intubation and the prolonged exposure of injured lungs to P-SILI [50].
In the recent years, various interfaces have been used, as face (or oro-nasal/full-face) mask, nasal masks, mouthpieces, nasal pillows or plugs, and helmet; each has its own peculiarities and pitfalls, that must be part of the clinician's evaluation.
NIV improves oxygenation, reduces dyspnea, inspiratory effort and work of breathing [51], and might reduce the rate of endotracheal intubation and ICU mortality rate [52▪▪]; however, in case of failure, it leads to delayed intubation, worsening clinical outcome [50].
Oro-nasal or full-face masks, when compared to the helmets, allow greater unloading of respiratory muscles [53], unless specific settings – including high PEEP, high-pressure support and low pressurization time – are used [54].
The helmet allows to deliver relatively high PEEP with minimum air leakage and good tolerability, allowing the patient to receive continuous treatments with enhanced comfort [55]; this is particularly important in the early phases of hypoxemic respiratory failure, when high PEEP seems a promising tool to mitigate the risk of P-SILI [21,56,57].
In 2016, a randomized study showed lower intubation rate and improved outcome in hypoxemic patients treated with helmet NIV vs those treated with face-mask NIV; whereas patients with helmet received a median sustained PEEP of 8 cmH2O, patients treated with face mask received a median PEEP of 5.1 cmH2O [58].
During the COVID-19 pandemic, NIV has been used both as a first-line therapy and as rescue therapy after HFNO, in patients with wide range of severity [59–65] (Table 2).
Table 2.
Clinical trials of NIV in acute hypoxemic respiratory failure of COVID-19 etiology
| Publication | PMID | Study design | Setting | Patient Population | Treatment | Intubation Rate | Mortality Rate | Main finding | Secondary findings |
| Bellani et al.[69▪], 2021 | 33395553 | Single day observational study | Ward | COVID-19 AHRF PaO2/FiO2 172 (102) | NIV + CPAP n = 798 215 (27%) patients with limitations of treatment Helmet was used for 617 patients, face mask for 248 Noninvasive respiratory support initiated 1 [0–4] days after hospital admission PEEP was 10.8 (2.6), ranging from 2 to 20 | Noninvasive respiratory support failure 38% [95% CI 34 to 41] in the overall cohort Noninvasive respiratory support failure 27% [23 to 30] in patients with no limitations of treatment cohort Noninvasive respiratory support failure 67% [61 to 73] in patients with limitations of treatment cohort | Overall mortality was 25% [95% CI 22 to 28] | Noninvasive respiratory support outside the ICU is feasible and approximately 10% of COVID-19 patients present in the hospital were treated with noninvasive respiratory support, with a predominant use of helmet CPAP. | Overall rate of success was > 60% in the overall cohort and 73% in patients with no limitations of treatment. |
| Bertaina et al.[59], 2021 | 33727235 | Observational prospective registry | Mixed setting | COVID-19 AHRF 51% of patients had SpO2 < 92% on room air | NIV n = 390 NIV settings, interfaces, and whether CPAP is codified as NIV is not know | NIV failure 44% [95% CI 40 to 49] Received ETI 16% [95% CI 13 to 20] | Overall cohort 38% [95% CI 33 to 43] Among intubated patients 58% [95% CI 46 to 70%] | NIV may have a significant role in supporting patients with COVID-19-related respiratory failure. It effectively supported and prevented the need for intubation of more than half of those treated. Those failing had a very poor in-hospital survival rate. | After adjustment, age, hypertension, room air SpO2 at presentation, lymphocytopenia, in-hospital use of antibiotics were independently associated with NIV failure. |
| Burns et al.[78], 2020 | 32624494 | Retrospective study | Non-ICU | COVID-19 AHRF SpO2 < 94% in Venturi Mask 40% | CPAP n = 23 BIPAP n = 5 BIPAP settings: max PEEP = 10.2 (2.9) cmH2O max Pinsp = 22.4 (6) cmH2O CPAP settings: Max PEEP = 12.7 (2.1) cmH2O | Not reported | BIPAP 40% [95% CI 12 to 77] CPAP 52% [33 to 71] | Ward based noninvasive respiratory is a good treatment option, with a mortality around 50%. | The only statistically significant difference between survivors and nonsurvivors was the presence of ‘classical’ imaging appearances, P = 0.034. |
| Duca et al.[60], 2020 | 32766538 | Retrospective study | Non-ICU | COVID-19 AHRF CPAP PaO2/FiO2 131 [97–190] NIV PaO2/FiO2 87 [53–120] IMV at arrival PaO2/FiO2 76 [60–177] | CPAP n = 71 Helmet CPAP, PEEP = 15 [12–18] cmH2O NIV n = 7 NIV, PEEP = 16 [12–20] cmH2O IMV at arrival = 7 IMV at arrival, PEEP = 18 [10–18] cmH2O | CPAP intubation rate 37% [95% CI 26 to 48] NIV intubation rate 0% [95% CI 0 to 35] CPAP failure 92% [95% CI 83 to 96] NIV failure 57% [95% CI 25 to 84] | CPAP 76% [95% CI 65 to 84] NIV 57% [95% CI 25 to 84] IMV at arrival 100% [95% CI 65 to 100] | In case of limited resources, the use of early CPAP or NIV in the ward or in the emergency department could be a valid strategy. | CPAP failure occurred in a high percentage of patients. |
| Faraone et al.[61], 2020 | 33222116 | Retrospective study | Non-ICU | COVID-19 AHRF PaO2/FiO2 130 (65) 25 (50%) patients had patients with limitations of treatment | NIV n = 25 CPAP n = 25 Interface: full face or oro-nasal mask Duration of treatment in the overall cohort: 187 (181) hours PEEP started at 5 cmH2O, up to 12 cmH2O IPAP set at 15cmH2O, up to 20–25 cmH2O | Patients with no limitations of treatment: 36% [95% CI 20 to 55] CPAP failure 44% [95% CI 27 to 63] NIV failure 68% [95% CI 48 to 83] | Patients with limitations of treatment 88% [95% CI 70 to 96] Patients with no limitations of treatment 12% [95% CI 4 to 30] | Noninvasive respiratory was useful in avoiding intubation in patients with no limitations of treatment. | The rate of infection among healthcare workers was low. |
| Franco et al.[67▪▪], 2020 | 32747398 | Retrospective multicenter study | Non-ICU | COVID-19 AHRF PaO2/FiO2 138 (66) | HFNO n = 163 CPAP n = 330 PEEP 10.2 (1.6) cmH2O Helmet 149 (99%) Face mask 2 (1%) NIV n = 177 PEEP 9.5 (2.2) cmH2O Pressure Support 17.3 (3) cmH2O Helmet 15 (21%) Face mask 57 (79%) | Recieved IMV: HFNO 29% [95% CI 24 to 36] CPAP 25% [95% CI 20 to 30] NIV 28% [95% CI 22 to 35] HFNO Failure 38% [Ci 31 to 47] CPAP Failure 47% [95% CI 42 to 53] NIV Failure 53% [95% CI 46 to 60] | 30 day mortality: HFNO 16% [95% CI 11 to 22] CPAP 30% [95% CI 26 to 35] NIV 31% [95% CI 24 to 38] Difference not significant at adjusted analysis | Noninvasive respiratory support outside of ICU is feasibile, and mortality rates compare favourably with previous reports. There was no difference among the interfaces at the adjusted analysis. | Noninvasive respiratory support was associated with risk of staff contamination. |
| Fu et al.[62], 2021 | 34109190 | Retrospective study | Mixed population | COVID-19 AHRF NIV as initial therapy PaO2/FiO2 174.4 [158.0–208.7] NIV as rescue therapy PaO2/FiO2 179.27 [165.9–224.1] | NIV as initial therapy n = 22 NIV as rescue therapy n = 17 PEEP in NIV success: 6 [6–7] PEEP in NIV failure: 6 [6–6.3] Pressure Support in NIV success: 7 [6–7] Pressure Support in NIV failure: 6 [6–6.3] | NIV as initial therapy 23% [95% CI 10 to 43] NIV as rescue therapy 65% [95% CI 41 to 83] | NIV initial therapy 5% [95% CI 8 to 22] NIV as rescue therapy 12% [95% CI 3 to 34] | Close attention should be paid to patients with PaO2/FiO2 < 200 mmHg after 1–2 h of NIV. | Using NIV as rescue therapy after HFNO failure is associated with higher risk of IOT and detrimental outcomes. |
| Grieco et al.[70▪▪], 2021 | 33764378 | Randomized controlled multicenter trial | ICU | COVID-19 AHRF NIV PaO2/FiO2 105 [83–125] HFNO PaO2/FiO2 102 [80–124] | Helmet NIV n = 54 Continuous treatment PEEP 12 [10–12] cmH2O Pressure Support 10 [10–12] cmH2O HFNO n = 55 | Helmet NIV 30% [95% CI 19 to 43] HFNO 51% [95% CI 38 to 64] | HFNO 25% [16 to 38] Helmet NIV 24% [95% CI 15 to 37] | Helmet NIV + HFNO or HFNO alone do not affect respiratory support free days. | Helmet NIV reduces rate of ETI and increases invasive VFD at day 28. |
| Hua et al.[63], 2020 | 32546258 | Retrospective, multicenter study | ICU | COVID-19 AHRF PaO2/FiO2 not reported | SOT n = 204 IMV n = 113 NIV n = 152 NIV settings, interfaces, and whether CPAP is codified as NIV is not reported | Not reported | SOT 6% [95% CI 4 to 11] IMV 92% [95% CI 86 to 96] NIV 41% [95% CI 33 to 49] | Patients who were invasively ventilated exhibited pessimistic outcome. | |
| Karagiannidis et al.[68▪▪], 2020 | 32735842 | Retrospective, nation-wide study | Mixed population | COVID-19 AHRF PaO2/FiO2 not reported | NIV n = 286 IMV only n = 1318 NIV settings, interfaces, and whether CPAP is codified as NIV is not reported | NIV failure 49% [95% CI 44 to 55] | NIV 51% [95% CI 45 to 56] IMV only 53% [95% CI 50 to 55] | In the German health-care system, in which hospital capacities have not been overwhelmed by the COVID-19 pandemic, mortality has been high for patients receiving mechanical ventilation. | Mortality in patients aged 80 or older was 72%. |
| Liu et al.[40▪▪], 2021 | 33573999 | Retrospective multicentre study | ICU | COVID-19 AHRF PaO2/FiO2 HFNO 116 [66–252] PaO2/FiO2 NIV 113 [68–183] | HFNO n = 366 NIV n = 286 NIV settings, interfaces, and whether CPAP is codified are NIV is not reported | HFNO 56% [95% CI 51 to 61] NIV 74% 95% CI [68 to 78] | HFNO 49% [95% CI 44 to 54] NIV 62% [95% CI 56 to 67] | The nomogram and online calculator are simple to use and able to predict the risk of failure in patients with covid-19 treated with HFNO and NIV. | Age, number of comorbidities, ROX index, Glasgow coma scale score, and use of vasopressors on the first day of noninvasive respiratory support were independent risk factors for noninvasive respiratory support failure. |
| Menzella et al.[64], 2021 | 33728822 | Retrospective cohort study | Non-ICU | COVID-19 AHRF PaO2/FiO2 120.1 (41.6) | NIV n = 79 PEEP: 9.46 (2.37) cmH2O IPAP: 17.7 (2.2) cmH2O | ETI rate after the exclusion of patients with limitations of treatment and 2 sudden deaths 36% [95% CI 25 to 48] NIV failure in the overall cohort 52% [95% CI 41 to 63] | Mortality in the 20 intubated patients was 43% [95% CI 25 to 63] 18 (23%) patients had patients with limitations of treatment 2 (3%) patients died of sudden death | NIV was effective in almost half of the patients. | At a multivariate Cox regression model only SOFA score at admission was significantly associated with the risk of failure. |
| Mukhtar et al.[65], 2020 | 32736030 | Retrospective study | ICU | COVID-19 AHRF PaO2/FiO2 in NIV success 170 [112–224] PaO2/FiO2 in NIV failure 175 [118–205] | NIV n = 39 NIV settings, interfaces, and whether CPAP or HNFO are codified as NIV is not reported | Need for ETI 23% [13 to 38] NIV failure 31% [95% CI 19 to 46] | 26% [15 to 41] | The use of NIV was successful in 77% of patients | |
| Rosén et al.[127▪], 2021 | 34127046 | Multicenter randomized clinical trial | Non-ICU | COVID-19 AHRF Standard care n = 39 PaO2/FiO2 standard care 115 [94–130] Prone n = 36 PaO2/FiO2 prone 115 [86–130] | HFNO standard care n = 29 HFNO prone n = 31 NIV standard care n = 27 PEEP 8 [6–8] NIV prone n = 21 PEEP 7 [6–10] | Standard care group 33% [95% CI 20 to 49] Prone group 33% [95% CI 20 to 50] | Control group 8% [95% CI 3 to 20] Prone group 17% [95% CI 8 to 22] | The implemented protocol for awake PP increased duration of awake PP but did not reduce the rate of intubation in patients with AHRF due to COVID-19 compared to standard care. | Nine patients (23%) in the control group had pressure sores compared with two patients (6%) in the prone group, P = 0.03, there were no difference in the use of NIV, vasopressors, continuous renal-replacement therapy, ECMO, VFD, hospital and ICU length of stay and mortality among the two groups. |
| Sivaloganathan et al.[75], 2020 | 32811662 | Retrospective Study | Mixed population | COVID-19 AHRF Worst PaO2/FiO2 ratio: NIV only: 127.5 [107–153] NIV + MV: 104.26 [96–126] IMV only: 115 [92–134] NIV – limitations of treatment: 75 [61–104] | NIV only n = 31 NIV + MV n = 27 IMV only n = 21 NIV–limitations of treatment n = 24 NIV settings, interfaces, and whether CPAP is codified as NIV is not reported | Patients with no limitations of treatment: 47% [95% CI 34 to 59] | Patients with no limitations of treatment: 5% [95% CI 2 to 14] Patients with limitations of treatment: 83% [95% CI 64 to 93] | NIV is safe and has low failure and mortality rate especially in patients with no limitations of treatment. | The only variable associated with risk of intubation was admission SOFA |
| Vianello et al.[35], 2020 | 32703883 | Retrospective study | ICU | COVID-19 AHRF PaO2/FiO2 108 [52–296] | HFNO n = 28 Rescue NIV n = 9 NIV settings, interfaces, and whether CPAP is codified as NIV is not reported | HFNO failure 32% [95% CI 18 to 51] Rescue NIV failure 56% [95% CI 27 to 81] ETI 18% [95% CI 8 to 36] | 11% [95% CI 4 to 27] | HFNO can be considered an effective and safe means to improve oxygenation in less severe forms of AHRF secondary to COVID-19 not responding to conventional oxygen therapy | Severity of hypoxemia and C reactive protein level were correlated with HFNO failure |
| Wang et al.[41], 2020 | 32232685 | Retrospective study | Mixed population | COVID-19 AHRF PaO2/FiO2 209 [179–376] in success patients PaO2/FiO2 142 [130–188] in failure patients | HFNO n = 17 only IMV n = 1 first line NIV n = 9 rescue NIV n = 7 | HFNO failure and rescue NIV 41% [95% CI 22 to 64] HFNO 12% [95% CI 3 to 34] First line NIV failure 11% [2 to 42] Rescue NIV failure 29% [8 to 64] | Not reported | HFNO was the most common ventilation support for patients, and rescue NIV was often used in case of HFNO failure | Patients with lower PaO2/FiO2 were more likely to experience HFNO failure |
| Wendel Garcia et al.[36], 2021 | 34034782 | Retrospective subanalysis of data | ICU | COVID-19 AHRF PaO2/FiO2 123 [92, 167] | SOT n = 87 HFNO n = 87 NIV n = 87 MV n = 92 | SOT 64% [95% CI 53 to 63] HFNO 52% [95% CI 41 to 62] NIV 49% [95% CI 39 to 60] | SOT 18% [95% CI 11 to 27] HFNO 20% [95% CI 13 to 29] NIV 37% [27 to 47] | A trial of HFNO appeared to be the most balanced initial respiratory support strategy. | Compared to the other respiratory support strategies, NIV was associated with a higher overall ICU mortality P = 016 and should be avoided. |
| Yang W. et al.[37], 2020 | 32267160 | Retrospective study | ICU | COVID-19 AHRF SpO2/FiO2 in the overall cohort 279 [157–328] | HFNO n = 35 NIV n = 34 IMV n = 100 | HFNO 66% [95% CI 49 to 79] HNFO failure 77% [95% CI 61 to 88] NIV failure 79% [95% CI 63 to 90] | HFNO 80% [95% CI 64 to 90] NIV 77% [95% CI 61 to 88] IMV 97% [95% CI 92 to 99] | Older patients with comorbidities are at increased risk of mortality. Real-time monitoring of S/F and regular measurements of lymphocyte count and inflammatory markers may be essential to disease management. | A total of 128 out of 145 (88.3%) patients who developed ARDS died at or before 28 days. |
| Yang X. et al.[66], 2020 | 32105632 | Retrospective study | ICU | COVID-19 AHRF PaO2/FiO2 100 [66.6–126.7] in survivors PaO2/FiO2 62 [52–74] nonsurvivors | Overall cohort n = 52 HFNO n = 33 NIV n = 29 IMV n = 22 | The progression among the interfaces is not reported | Mortality at 28 days HFNO 48% [95% CI 32 to 65] NIV 79% [95% CI 62 to 90] IMV 86% [95% CI 67 to 95] | Among 52 critically ill patients with SARS-CoV-2 infection, 32 (61·5%) patients had died at 28 days. | Older patients (>65 years) with comorbidities and ARDS are at increased risk of death. |
| Zhou et al.[37], 2020 | 32171076 | Retrospective multicenter study | Mixed Population | PaO2/FiO2 at enrollment is not reported | HFNO n = 41 NIV n = 26 IMV n = 32 NIV settings, interfaces, and whether CPAP is codified as NIV is not know | Not reported | HFNO 80% [CI66 to 90] NIV 92% [95% CI 96 to 98] IMV 97% [95% CI 84 to 99] | Older age, high SOFA score, and d-dimer greater than 1 μg/mL could help clinicians to identify patients with poor prognosis at an early stage. | Noninvasive respiratory support and invasive mechanical ventilation have high mortality rate. |
Values are displayed as means (SD) or medians [Interquartile range].
Failure was defined as either intubation, death while still on noninvasive respiratory support, or escalation to other noninvasive respiratory support to avoid endotracheal intubation. AHRF, acute hypoxemic respiratory failure; ARDS, acute respiratory distress syndrome; awake PP, awake prone position; CPAP, continuous positive end-expiratory pressure; FiO2, fraction of inspired oxygen; HFNO, high-flow nasal oxygen; ICU, intensive care unit; IQR, interquartile range; NIV, noninvasive ventilation; PaO2, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; SpO2, peripheral capillary oxygen saturation; VFD, Ventilatory Free Days.
NIV showed variable success rates during the pandemic, possible due to the heterogeneous interfaces, settings and protocols applied; Wang et al. report a failure rate of 11% in mild-to-moderate patients when NIV is used as first-line therapy [39], but in the most severe patients it can be as high as 80% [40▪▪,66]; to date, the largest observational studies have found consistent failure rates, ranging between 40% and 50% [67▪▪,68▪▪,69▪] (Fig. 1b).
Lastly, a randomized controlled trial compared the efficacy of continuous helmet NIV vs HFNO alone in COVID-19 patients affected by moderate-to-severe hypoxemia. In this study, despite the lack of a significant difference on the primary outcome (median days free of respiratory support at 28 days, helmet group 20 [interquartile range (IQR), 0–25] vs HFNO group 18 [IQR, 0–22]), the authors reported a significant difference in the intubation rate (30% in the helmet group vs 51% in the HFNO group; P = 0.03), with no difference in mortality [70▪▪].
NIV can be a powerful instrument as optimal settings and adequate interface are provided, but careful selection and strict clinical monitoring of patients are mandatory to reduce the risk of delayed intubation.
CONTINUOUS POSITIVE AIRWAY PRESSURE
The use of CPAP in hypoxemic respiratory failure has been proposed more than 20 years ago, but a randomized controlled trial failed to prove its efficacy in reducing the intubation rate in patients with hypoxemic respiratory failure of other etiologies [71].
Nevertheless, in the subsequent years, Helmet CPAP has become increasingly used to increase blood oxygenation and reduce the risk of intubation in patients with moderate-to-severe hypoxemia compared with standard oxygen [72,73].
Traditionally CPAP is provided with a device able to provide high flow rates of fresh gas flow (inlet port) and an adjustable PEEP valve (outlet port), being highly cost-effective in the emergency context and easily used outside the ICU.
CPAP has been adopted to increase blood oxygenation and to avoid endotracheal intubation and as ceiling of treatment in patients with limitation of care: in patients who were not candidate for receiving invasive mechanical ventilation, CPAP has been used as a rescue therapy, with mortality ranging from 0% to 90% [67▪▪,74▪,75–85] (Table 3).
Table 3.
Clinical trials of CPAP in acute hypoxemic respiratory failure of COVID-19 etiology
| Publication | PMID | Study design | Setting | Patient Population | Treatment | Intubation Rate | Mortality Rate | Main finding | Secondary findings |
| Aliberti et al.[81], 2020 | 32747395 | Observational prospective multicenter cohort study | High dependency unit | COVID-19 AHRF PaO2/FiO2 142 [97–203] 65 patients with limitations of treatment 92 patients with no limitations of treatment | Helmet CPAP n = 157 PEEP 10.8 (2.3) cmH2O 4 cases discontinued CPAP for intolerance | Overall population, CPAP failure 45% [37 to 52] Patients with no limitations of treatment, CPAP failure 37% [95% CI 28 to 47] | Overall cohort 29% [95% CI 22 to 36] Patients with limitations of treatment 55% [95% CI 43 to 67] Patients with no limitations of treatment 10% [95% CI 5 to 18] | Helmet CPAP is feasible in the high dependency unit and is associated with a failure rate < 40% in patients with no limitations of treatment with moderate to severe AHRF. | CPAP failure was associated with the severity of pneumonia on admission and higher baseline values of interleukin-6. |
| Alviset et al.[82], 2020 | 33052968 | Retrospective study | Mixed setting | COVID-19 AHRF SpO2 < 90% oxygen therapy 15lt/min with nonrebreather face mask | Face Mask CPAP n = 41 PEEP 5–10 cmH2O 2 cases discontinued CPAP for intolerance | 59% [95% CI 43 to 72] | 29% [18 to 44] | CPAP is feasible outside of the ICU | The intubation rate was lower than 60%, with a mortality rate less than 1/3. |
| Arina et al.[79], 2020 | 33196858 | Retrospective study | ICU | COVID-19 AHRF PaO2/FiO2 97 [75–135] | CPAP n = 93 CPAP settings are not provided The exact number of patients with limitations of treatment is not provided | Failure in the overall cohort was 66% [55 to 74] 47 (51%) of patients were intubated, while 14 (15%) had CPAP as ceiling of treatment | 43% [33 to 53] | At a multivariate model C-reactive protein and NT-proBMP had sensitivity of 0.75 [95% CI 0.62 to 0.86] and specificity of 0.83 [95% 0.61–0.95]. | After 6 h of treatment in patients of the CPAP success group a PaO2/FiO2 raise of 77% was observed, while a raise of only 38% in patients that failed CPAP. |
| Bellani et al.[69▪], 2021 | 33395553 | Single day observational study | Ward | COVID-19 AHRF PaO2/FiO2 172 (102) | NIV + CPAP n = 798 215 (27%) patients with limitations of treatment Helmet was used for 617 patients, face mask for 248 Noninvasive respiratory support initiated 1 [0–4] days after hospital admission PEEP was 10.8 (2.6), ranging from 2 to 20 | Noninvasive respiratory support failure 38% [95% CI 34 to 41] in the overall cohort Noninvasive respiratory support failure 27% [23 to 30] in patients with no limitations of treatment cohort Noninvasive respiratory support failure 67% [61 to 73] in patients with limitations of treatment cohort | Overall mortality was 25% [95% CI 22 to 28] | Noninvasive respiratory support outside the ICU is feasible and approximately 10% of COVID-19 patients present in the hospital were treated with noninvasive respiratory support, with a predominant use of helmet CPAP. | Overall rate of success was > 60% in the overall cohort and 73% in patients with no limitations of treatment. |
| Brusasco et al.[89], 2021 | 33033151 | Retrospective multicenter study | Non-ICU | COVID-19 AHRF PaO2/FiO2 119 [99–153] | CPAP n = 64 PEEP 10 cmH2O in all patients | CPAP failure 17% [10 to 28] ETI 11% [5 to 21] | Overall mortality 14% [95% CI 8 to 25] Died on CPAP 6% [95% CI 2 to 15] Died on IMV 8% [95% CI 4 to 17] | CPAP was feasible in patients with moderate to severe AHRF | At univariate analysis CPAP failure correlated with sex, hypertension, diabetes, COPD, three or more comorbidities and lung weight, but at multivariate analysis only hypertension remained significant (OR 7.33, 95% CI 1.5 to 34, P = 0.012). |
| Burns et al.[78], 2020 | 32624494 | Retrospective study | Non-ICU | COVID-19 AHRF SpO2 < 94% in Venturi Mask 40% | CPAP n = 23 BIPAP n = 5 BIPAP settings: max PEEP = 10.2 (2.9) cmH2O max Pinsp = 22.4 (6) cmH2O CPAP settings: Max PEEP = 12.7 (2.1) cmH2O | Not reported | BIPAP 40% [95% CI 12 to 77] CPAP 52% [33 to 71] | Ward based noninvasive respiratory support is a good treatment option, with a mortality around 50%. | The only statistically significant difference between survivors and nonsurvivors was the presence of ‘classical’ imaging appearances, P = 0.034. |
| Carteaux et al.[81], 2021 | 33655452 | Retrospective study | Intermediate Care Unit and ICU | COVID-19 AHRF PaO2/FiO2 160 [115–258] | CPAP n = 85 Interface: oro-nasal mask CPAP was designed with a Boussignac valve protected by a filter, and free flow oxygen rate of 15 l/min [15–15] | Predefined criteria for intubation were present. 64% [95% CI 53 to 73] | 27% [95% CI 19 to 37] | Adding a filter to the Boussignac valve does not affect the delivered pressure but may variably increase the resistive load depending on the filter used. | Clinical assessment suggests that CPAP designed with a Boussignac valve and a filter is a frugal solution to provide a ventilatory support and improve oxygenation during a massive COVID-19 outbreak. |
| Coppadoro et al.[77], 2021 | 33627169 | Retrospective multicenter study | Non-ICU | COVID-19 AHRF PaO2/FiO2 103 [79–176] | CPAP n = 306 Patients with no limitations of treatment n = 176 Patients with limitations of treatment n = 130 PEEP 10 [7–10] cmH2O Helmet CPAP was delivered for 21 h/day, for the first 48 h, and from day 3 to 5 for 19 h/day | CPAP failure overall cohort 48% [95% CI 42 to 54] CPAP failure in patients with no limitations of treatment 31% [24 to 38] | Hospital mortality in patients with no limitations of treatment 12% [95% CI 8 to 18] Hospital mortality in patients with limitations of treatment 72% [95% CI 64 to 79] | Treatment of COVID-19 AHRF outside the ICU is feasible with Helmet CPAP, with a mortality rate of 12%. It was also used in patients with limitations of treatment, improving survival in almost 1/3 of cases. | CPAP failure was independently associated with C-reactive protein, time to oxygen mask failure, lower PaO2/FiO2 during CPAP and number of comorbidities. |
| Corradi et al.[77], 2020 | 33197604 | Single-center pilot study | ICU | COVID-19 AHRF PaO2/FiO2 195 [168–246] | Helmet CPAP n = 27 PEEP = 10 cmH2O | Predefined criteria for ETI 33% [95% CI 17 to 52] | 11% [95% CI 4 to 28] | CPAP failure was significantly associated with diaphragmatic thickening fraction at multivariate analysis, the best threshold was 21.4% | |
| De vita et al.[80], 2021 | 33500220 | Retrospective multicenter study | High Intensity Unit | COVID-19 AHRF PaO2/FiO2 success 120 [75–160] PaO2/FiO2 failure 103 [60–152] | CPAP n = 367 Helmet was applied in 281 (77%) patients and face mask in 71 (19%) patients. Values from 15 patients were missing. Initial PEEP was 10–12 cmH2O, to be increased up to 15cmH2O | Predefined criteria for intubation 41% [95% CI 36 to 46] | Not reported | In patients treated with CPAP, age, LDH and percentage change in PaO2/FiO2 after starting are predictors of intubation. | The use of CPAP avoided IMV in more than half of the patients. |
| Duca et al.[60], 2020 | 32766538 | Retrospective study | Non-ICU | COVID-19 AHRF CPAP PaO2/FiO2 131 [97–190] NIV PaO2/FiO2 87 [53–120] IMV at arrival PaO2/FiO2 76 [60–177] | CPAP n = 71 Helmet CPAP, PEEP = 15 [12–18] cmH2O NIV n = 7 NIV, PEEP = 16 [12–20] cmH2O IMV at arrival = 7 IMV at arrival, PEEP = 18 [10–18] cmH2O | CPAP intubation rate 37% [95% CI 26 to 48] NIV intubation rate 0% [95% CI 0 to 35] CPAP failure 92% [95% CI 83 to 96] NIV failure 57% [95% CI 25 to 84] | CPAP 76% [95% CI 65 to 84] NIV 57% [95% CI 25 to 84] IMV at arrival 100% [95% CI 65 to 100] | In case of limited resources, the use of early CPAP or NIV in the ward or in the emergency department could be a valid strategy. | CPAP failure occurred in a high percentage of patients. |
| Faraone et al.[61], 2020 | 33222116 | Retrospective study | Non-ICU | COVID-19 AHRF PaO2/FiO2 130 (65) 25 (50%) patients had patients with limitations of treatment | NIV n = 25 CPAP n = 25 Interface: full face or oro-nasal mask Duration of treatment in the overall cohort: 187 (181) hours PEEP started at 5 cmH2O, up to 12 cmH2O IPAP set at 15cmH2O, up to 20–25 cmH2O | Patients with no limitations of treatment: 36% [95% CI 20 to 55] CPAP failure 44% [95% CI 27 to 63] NIV failure 68% [95% CI 48 to 83] | Patients with limitations of treatment 88% [95% CI 70 to 96] Patients with no limitations of treatment 12% [95% CI 4 to 30] | Noninvasive respiratory was useful in avoiding intubation in patients with no limitations of treatment. | The rate of infection among healthcare workers was low. |
| Franco et al.[67▪▪], 2020 | 32747398 | Retrospective multicenter study | Non-ICU | COVID-19 AHRF PaO2/FiO2 138 (66) | HFNO n = 163 CPAP n = 330 PEEP 10.2 (1.6) cmH2O Helmet 149 (99%) Face mask 2 (1%) NIV n = 177 PEEP 9.5 (2.2) cmH2O Pressure Support 17.3 (3) cmH2O Helmet 15 (21%) Face mask 57 (79%) | Recieved IMV: HFNO 29% [95% CI 24 to 36] CPAP 25% [95% CI 20 to 30] NIV 28% [95% CI 22 to 35] HFNO Failure 38% [Ci 31 to 47] CPAP Failure 47% [95% CI 42 to 53] NIV Failure 53% [95% CI 46 to 60] | 30 day mortality: HFNO 16% [95% CI 11 to 22] CPAP 30% [95% CI 26 to 35] NIV 31% [95% CI 24 to 38] Difference not significant at adjusted analysis | Noninvasive respiratory support outside of ICU is feasibile, and mortality rates compare favourably with previous reports. There was no difference among the interfaces at the adjusted analysis. | Noninvasive respiratory support was associated with risk of staff contamination. |
| Gaulton et al.[87], 2020 | 32984836 | Retrospective, multicenter study | ICU | COVID-19 AHRF SpO2 < 92% with 6l/min nasal cannula Body mass index, kg/m2, mean (sd) = 35.5 (8.6) | Helmet CPAP n = 17 HFNO n = 42 PEEP 5–10 cmH2O | ETI at 7 days CPAP 18% [6 to 41] HFNO 52% [38 to 67] | Death at 7 days CPAP 6% [1 to 27] HFNO 19% [10 to 33] | Difference in the intubation rate was significant after adjustment for age. | In obese patients Helmet CPAP is effective in reducing the ETI rate. |
| Kofod et al.[84], 2021 | 33889343 | Retrospective study | Non-ICU | COVID-19 AHRF PaO2/FiO2 101 (36) Patients with no limitations of treatment n = 27 Patients with limitations of treatment n = 26 | CPAP n = 53 Interface: Face Mask 30 patients received CPAP between 18 and 24 h a day. PEEP 10.5 [10–12] cmH2O | CPAP failure overall cohort 72% [49 to 87] CPAP patients with no limitations of treatment 25% [95% CI 15 to 38] | Overall mortality 58% [45 to 71] CPAP limitations of treatment 92% [95% CI 76 to 98] CPAP patients with no limitations of treatment 13% [7 to 25] | CPAP seems to have positive effect on oxygenation and respiratory rate in most patients with severe respiratory failure caused by COVID-19, but the prognosis for especially elderly patients with high oxygen requirement and with a ceiling of treatment in the ward is poor. | A positive and significant (P = 0.002) immediate response of CPAP was seen on respiratory rate, decreased from 28.6 (7.6) to 26.9 (6.2), and SpO2, increased from 90.7 (3.5) to 92.7 (3.2) with a decrease of oxygen flow rate from 27.4 (13.3) to 23.3 (10.7). |
| Nightingale et al.[85], 2020 | 32624495 | Retrospective study | Non-ICU | COVID-19 AHRF PaO2/FiO2 122 [97–175] | CPAP n = 24 Interface: face mask PEEP 8.75 [7.5–10] cmH2O | CPAP failure 42% [95% CI 24 to 61] | 21% [9 to 40] | Over half of patients (58%) avoided mechanical ventilation and a total of 19 out of 24 (79%) were discharged | There have been no cases of COVID-19 among nursing staff who looked after this cohort of patients. |
| Noeman-Ahmed et al.[86], 2020 | 33140491 | Retrospective study | Acute Respiratory Care Unit | COVID-19 AHRF PaO2/FiO2 123.15 (59.56) Patients with no limitations of treatment n = 41 Patients with limitations of treatment n = 11 | CPAP n = 52 Interface: full-face mask Starting PEEP 10 cmH2O titrated to 12.5 cmH2O or 15 cmH2O if SpO2 is ≤ 94% with a FiO2 of 60%. | Patients with no limitations of treatment CPAP failure 51% [95% CI 36 to 66] | Patients with no limitations of treatment 20% [95%CI 10 to 34] 20% Patients with limitations of treatment 91% [95% CI 62 to 98] | CPAP success rate in the overall cohort was 40% with a mortality of 23%. | Predictors of success were: SpO2/FiO2, PaO2/FiO2, respiratory rate, neutrophil to lymphocyte ratio. |
| Oranger et al.[86], 2020 | 32430410 | Retrospective study with short term historical control | Non-ICU | COVID-19 AHRF O2 > 6 l/min to maintain SpO2 ≥ 92% | case CPAP n = 38 control SOT n = 14 Interface: face mask with high end domiciliary ventilator PEEP 10 (adjusted between 8 and 12) cmH2O CPAP was delivered for 8 [4–11] hours per day | Day 7 follow-up control SOT failure 54% [95% CI 33 to 79] case CPAP 24% [95% CI 13 to 39] | Day 7 follow-up control SOT 21% [95% CI 8 to 48] case CPAP 0 [95% CI 0 to 9%] | CPAP is feasible in deteriorating COVID-19 patients managed in a pulmonology unit. | None of the CPAP patients had to be intubated under cardiac arrest or high emergency conditions. |
| Pagano et al.[91], 2020 | 32629100 | Observational prospective study | Non-ICU | COVID-19 AHRF PaO2/FiO2 152 (82) | CPAP n = 18 Interface: Helmet PEEP 10 cmH2O FiO2 titrated to SpO2 > 93%. | CPAP ETI rate 22% [95% CI 9 to 45] The number of patients with no limitations of treatment and patients with limitations of treatment is not clearly defined. | Overall mortality 61% [95% CI 39 to 80] | Eleven patients died (61%), 4 among the responders (defined as patients with an improve of PaO2/FiO2 of at least 15% after 1 h of CPAP) and 7 in nonresponders | Among responders 5 (27.7%) patients showed improvement in lung ultrasound score. |
| Vaschetto et al.[74▪], 2021 | 33527074 | Retrospective multicenter study | Non-ICU | COVID-19 AHRF PaO2/FiO2 108 [71–157] Patients with no limitations of treatment n = 397 Patients with limitations of treatment n = 140 | CPAP n = 537 Interface: Helmet n = 399 (74%) Face mask n = 123 (23%) Both n = 15 (3%) PEEP 10 [10–12] cmH2O | Patients with no limitations of treatment 45% [95% CI 41 to 50] | Overall mortality 34% [95% CI 30 to 38] Patients with no limitations of treatment group mortality 21% [95% CI 17 to 25] Limitations of treatment mortality 73% [95% CI 65 to 79] | CPAP is feasible outside the ICU, with overall in-hospital mortality similar to that reported in other studies. Mortality is closely related to the therapeutic goal, patients having limitations of treatment being affected by much higher mortality. | Intubation delay represents a risk factor for mortality (hazard ratio 1.093, 95% CI 1.010–1.184). |
Values are displayed as means (SD) or medians [Interquartile range].
Failure was defined as either intubation, death while still on noninvasive respiratory support, or escalation to other noninvasive respiratory support to avoid endotracheal intubation. AHRF, acute hypoxemic respiratory failure; ARDS, acute respiratory distress syndrome; awake PP, awake prone position; CPAP, continuous positive end-expiratory pressure; FiO2, fraction of inspired oxygen; HFNO, high-flow nasal oxygen; ICU, intensive care unit; IQR, interquartile range; NIV, noninvasive ventilation; PaO2, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure; SAPS, Simplified Acute Physiology Score; SOFA Sequential Organ Failure Assessment; SpO2, peripheral capillary oxygen saturation; VFD, Ventilatory Free Days.
In patients where escalation to invasive mechanical ventilation was appropriate, CPAP has been used with encouraging results: largest trials showed a failure rate of the technique ranging between 20% and 40%, mostly depending on hypoxemia severity and patients’ overall clinical condition [67▪▪,69▪,80].
When compared to other noninvasive respiratory support strategies or to standard oxygen, early case-series showed a trend to a reduction in the intubation rate in patients treated with CPAP [86,87], but the largest observational trial to date [67▪▪] showed no difference in intubation and mortality rates (Fig. 1c).
Nevertheless, CPAP is a powerful instrument that can be safely used outside of the ICU, with good success in the less severe patients, especially when the Helmet interface is used. However, the increase in the PaO2/FiO2 ratio induced by PEEP might generate a false sense of security, possibly causing delays in the decision to intubate the patient: the clinician should pay close attention to the change of physiological variables over time [77,88] and, when available, should consider evaluating diaphragm thickening fraction [89,90] and lung ultrasound [91] to enhance early detection of treatment failure.
NEW STRATEGIES: AWAKE PRONE POSITIONING
In moderate-to-severe acute respiratory distress syndrome patients receiving invasive mechanical ventilation, prone positioning improves oxygenation, reduces ventilator-induced lung injury, finally reducing mortality [92▪▪,93,94▪▪].
In the midst of the pandemic, awake prone positioning was initially used on the most severe patients that required noninvasive respiratory support as a rescue strategy to avoid intubation, both in the ICU and in the non-ICU setting.
Prone positioning in spontaneously breathing patients improves oxygenation and lowers inspiratory effort [94▪▪] and respiratory rate, but the improvement is often transient, and only a minority of patients show sustained benefit after resupination [95–123] (Table 4).
Table 4.
Clinical trials of awake prone position in acute hypoxemic respiratory failure of COVID-19 etiology
| Publication | PMID | Study design | Setting | Patient Population | Treatment | Intubation Rate | Mortality Rate | Main finding | Secondary findings |
| Avdeev et al.[101], 2021 | 33494771 | Prospective multicenter observational study | Non-ICU | COVID-19 AHRF Responders PaO2/FiO2 136 [118–172] Non-Responders PaO2/FiO2 138 [113–177] | Awake PP n = 22 CPAP n = 16 STO n = 6 | 14% [95% CI 5 to 33] | 9% [95% CI 3 to 28] | Response to awake PP depends on localization of aeration loss, and lung ultrasound can predict it. | Sixteen (73%) patients improved oxygenation with awake PP, 3 patients (14%) improved dyspnea in 15 min, 12 (54%) improved dyspnea at 3 h. Responders (patient with a decrease in lung ultrasound score with PP) had shorter disease duration. |
| Bastoni et al.[102], 2020 | 32748797 | Prospective observational study | Non-ICU | COVID-19 AHRF PaO2/FiO2 68 (5) | Attempted awake PP n = 10 Awake PP failed in 4 (40%) patients. Helmet NIV or helmet CPAP, PEEP ranging from 10 to 20 cmH2O | 100% [95% CI 72 to 100] | Not available | Awake PP was feasible in 4 out of 10 patients | Awake PP improved the PaO2/FiO2 to 97 (8) but there was no difference in lung ultrasound |
| Burton-Papp et al.[103], 2020 | 33110499 | Retrospective study | ICU | COVID-19 AHRF PaO2/FiO2 123 (28) | Attempted awake PP n = 20 CPAP n = 4 CPAP: PEEP 10 [8–10] cmH2O BIPAP and CPAP n = 16 EPAP 10 [10–10] cmH2O IPAP 15 [14–16] cmH2O Median duration of each cycle 3 (IQR 2) hours Number of cycles 5 (IQR 6.3) per patients Time spent prone 18% (IQR 31) | 35% [95% CI 18 to 57] | 0% [95% CI 0 to 16] ECMO 10% [3 to 30] | In patients with moderate AHRF treated with CPAP or NIV awake PP can improve oxygenation without relevant adverse events. | Patients that needed ETI did not had a significant improvement during prone position in PaO2/FiO2 [5, 95% CI -9 to 20] vs nonintubated [41, 95% CI 29 to 53]. |
| Caputo et al.[104], 2020 | 32320506 | Pilot study | Non-ICU | COVID-19 AHRF SpO2 with supplemental oxygen was 82% [72–85] | Awake PP n = 50 | 36% [95% CI 24 to 50] | Awake early prone position in the emergency department demonstrated improved oxygen saturation in COVID-19 patients. | ||
| Cherian et al.[95], 2021 | 33845325 | Retrospective study | Non-ICU | COVID-19 AHRF Patients that required IMV PaO2/FiO2 100 [95–155] Patients that did not required IMV PaO2/FiO2 206 [100–293] | Awake PP n = 59 PP for at least 3 h/day. HFNO n = 52 NIV n = 20 | IMV in the overall cohort 39% [95% CI 28 to 52] | 32% [95% CI 22 to 45] | Awake PP can be safely performed with improvement in oxygenation. However, its institution may be beneficial only in patients with mild to moderate AHRF. | To avoid the risk of delayed intubation ROX index, improvement in PaO2/FiO2, reduction of LDH and D-Dimer should be monitored. |
| Coppo et al.[96], 2020 | 32569585 | Prospective, feasibility study | Respiratory High Dependency Unit | COVID-19 AHRF PaO2/FiO2 standard care 180.5 (76.6) | Attempted awake PP n = 56 PP was maintained for 3 [3–4] hours in 47 patients (84%) Helmet CPAP n = 44 (79%) PEEP 8.3 (2.3) Reservoir mask 9 (16%) Venturi Mask 3 (5%) | 25% [95% CI 15 to 40] | 11% [95% CI 5 to 23] | PP was feasible and effective in rapidly ameliorating blood oxygenation in awake patients with COVID-19-related pneumonia requiring oxygen supplementation. The effect was maintained after resupination in half of the patients. | PaO2/FiO2 improvement in PP 104.9 [95% CI 70.9 to 134], PaO2/FiO2 not improved after resupination 12.3 [95% CI -10.9 to 35.5]. Only 23 (50%) of patients (responders) mainted the improvement, but it was was not significant. LDH and C-reactive protein were higher in responders. |
| Damarla et al.[105], 2020 | 32551807 | Retrospective study | Mixed setting | COVID-19 AHRF Median oxygen requirement was 40% to achieve SpO2 94% (91 to 95) | Awake PP n = 10 HFNO n = 4 Nasal cannula n = 5 Room air n = 1 | 20% [95% CI 6 to 51] | 0% [95% CI 0 to 28] | PP is potentially a low-cost, easily implemented, and scalable intervention, particularly in low- and middle-income countries. | After 1h of PP SpO2 improved to 98% [97–99] and respiratory rate was reduced to 22 [18–25] from 31 [28–39]. |
| Despres et al.[106], 2020 | 32456663 | Case series | ICU | COVID-19 AHRF PaO2/FiO2 183 (144 to 212) | Awake PP n = 6 A total of 9 PP sessions were performed. HFNO in 4 sessions. SOT in 5 sessions. | 50% [95% CI 19 to 81] | Not reported | Considering these observations, PP combined with either HFNO or SOT could be proposed in spontaneously breathing, severe Covid-19 patients. | The proportion of patients with PaO2/FiO2 ratio improvement after PP appeared to be higher with HFNO compared to conventional oxygen therapy. |
| Ehrmann et al.[128▪▪], 2021 | 34425070 | Prospective collaborative randomized controlled meta trial, | Mixed setting | COVID-19 AHRF SpO2/FiO2 awake PP 147.9 (43.9) SpO2/FiO2 standard care148.6 (43.1) | Awake PP n = 564 Standard care n = 557 All patients treated with HFNO FiO2 0.6 [0.5 – 0.8] Awake PP HFNO flow 50 l/min [40–55] Standard care HFNO flow 40 l/min [40–50] | Treatment failure Awake PP 40% [95% CI 36 to 44] Treatment failure Standard care 46% [95% CI 42 to 50] IMV Awake PP 33% [95% CI 29 to 37] IMV Standard care 40% [95% CI 36 to 44] | Awake PP 21% [95% CI 18 to 24] Standard care 24% [95% CI 20 to 27] | Awake PP reduces the proportion of patients intubated or dying within 28 days of enrolment, 223 (40%) in the awake PP group vs 257 (46%) in the standard of care, P = 0.007, relative risk reduction 0.86 [95% CI 0.75 to 0.98]. Patients that received PP for longer sessions had lower treatment failure rate. | Awake PP significantly improves blood oxygenation, respiratory rate and ROX index during PP. The benefit was maintained after supination. |
| Elharrar et al.[97], 2020 | 32412581 | Prospective before after | ICU | COVID-19 AHRF O2 supplement < 4l/min in 16 (67%) patients O2 supplement ≥ 4l/min in 8 (33%) patients PaO2 78 (14) | Attempted awake PP n = 24 PP was maintained for: less than 1 h n = 4 (17%) for 1 to 3 h n = 15 (63%) for more than 3 h n = 5 (21%) | Follow up to 10 days 21% [95% CI 9 to 40] | Not reported | Responders (increased PaO2 > 20% from standard care) n = 6 [25%, 95% CI 12–45]; 3 patients were persistent responders. | 63% of patients were able to prone for more than 3 h, and 42% reported back pain. |
| Fazzini et al.[107], 2021 | https://doi.org/10.1177/1751143721996542 | Prospective observational | Non-ICU | COVID-19 AHRF PaO2/FiO2 115 (43) | Awake PP n = 46 12 (26%) patients could not tolerate PP for > 1 h. 34 (74%) awake PP for 5 h per session, 1 to 6 sessions daily. Interface: HFNO, CPAP, face mask oxygen; HFNO was the most used. | Overall cohort 43% [95% CI 30 to 58] Awake PP for > 1 h 29% [95% CI 17 to 46] Awake PP for < 1 h 83% [95% CI 55 to 95] | Overall cohort 30% [95% CI 19 to 45] Awake PP for > 1 h 26% [95% CI 15 to 43] Awake PP for < 1 h 42% [95% CI 19 to 68] | Patients that were pronated for more than 1 h had less need for endotracheal intubation than patients that were proned for less than 1 h. | PaO2/FiO2 standard care 115 (43) vs 148 (70); Respiratory rate standard care 34 (7) vs 25 (7); Prone position > 1 h less need for ICU than prone position < 1hour (41% vs 83%). |
| Ferrando et al.[108], 2020 | 33023669 | Prospective, multicenter, adjusted observational study | ICU | COVID-19 AHRF PaO2/FiO2, HFNO 111 [83–144] PaO2/FiO2, HFNO + PP 125 [99–187] | Overall cohort n = 199 HFNO n = 144 HFNO + PP n = 55 Patients in group HFNO + PP underwent pronation for at least 16h/die | HFNO 42% [34 to 50%] HFNO + PP 40% [95% CI 28 to 53] | HFNO 10% [95% CI 6 to 16] HFNO + PP 11% [95% CI 5 to 22] | The combined approach of HFNO and PP did not decrease the risk of endotracheal intubation | Patients treated with HFNO + awake PP showed a trend for delay in intubation compared to HFNO alone [median 1 (interquartile range, IQR 1.0–2.5) vs 2 IQR 1.0–3.0] days (P = 0.055), but Awake PP did not affect 28-day mortality (P = 0.92). |
| Golestani-Eraghi et al.[109], 2020 | 32473503 | Prospective, observational | ICU | COVID-19 AHRF PaO2/FiO2 < 150 | Awake PP n = 10 Helmet NIV (settings are not reported) Mean PP duration was 9 h | 20% [95% CI 6 to 51] | 20% [95% CI 6 to 51] | Authors report low intubation rate and high compliance to the intervention, suggesting that PP might be a useful tool to increase blood oxygenation in patients with moderate to severe AHRF related to COVID-19. | Improvement in oxygenation after PP: standard care PaO2 46.34 (5.2) vs PP PaO2 62.5 (4.6). |
| Hallifax et al.[110], 2020 | 32928787 | Retrospective study | Respiratory High Dependency Unit | COVID-19 AHRF FiO2 ≤ 60% n = 22 (46%) FiO2 > 60% n = 26 (54%) The proportion between patients with no limitations of treatment and patients with limitations of treatment is not specified. | Overall cohort n = 48 Attempt awake PP n = 30 Successfull proning defined as at least 2 h twice a day for 2 consecutive days. Full proning n = 11 Semiproning n = 17 Refused n = 2 CPAP only n = 22 HFNO + CPAP n = 26 CPAP PEEP ranging from 6–8 cmH2O | 23% [95% CI 13 to 37] | Patients with limitations of treatment 54% [95% CI 40 to 67] 6% [95% CI 2 to 19] died on IMV 4% [95% CI 1 to 14] still on IMV | Data from this cohort of patients managed on respiratory high dependency unit show that CPAP and awake proning are possible in a selected population of COVID-19. | Increasing age and the inability to awake prone were the only independent predictors of COVID-19 mortality. |
| Jagan et al.[111], 2020 | 33063033 | Retrospective study | Non-ICU | COVID-19 AHRF PaO2/FiO2 not reported | Overall cohort n = 105 Standard care n = 65 Self-proning n = 40 Self-proning was defined as a time spent PP greater or equal to 1 h, for at least 5 times/day, and for at least 1 h overnight. Interface not specified | Standard care 27% [95% CI 18 to 40] Self-proning 10% [95% CI 4 to 23] | Standard care 25% [95% CI 16 to 36] Self-proning 0% [95% CI 0 to 9] | Awake self-proning was well tolerated, with good compliance in 38% of patients and was associated with lower intubation rates, that remained significant after adjustment for SOFA and APACHE II. | The difference in mortality was significant at the univariate analysis but become nonsignificant after adjustment for SOFA and APACHE II. |
| Jayakumar et al.[125], 2021 | 33949237 | Multicenter, randomized, controlled feasibility trial with 3 parallel groups | Non-ICU | COVID-19 AHRF Standard care PaO2/FiO2 185.6 (126) Awake PP PaO2/FiO2 201 (119) Discharged against medical advice: 2 patients in each group. | Overall cohort n = 60 Standard care group n = 30 PP group n = 30 Encouraged to PP for at least 6 h/day. Median duration of PP per session was 2 h. 3 progressive interfaces: low flow oxygen (nonrebreather face mask) -> HFNO -> NIV (oronasal interface) | Standard care 13% [95% CI 5 to 30] Awake PP 13% [95% CI 5 to 30] | Standard care 7% [95% CI 2 to 21] Awake PP 10% [3 to 26] | In the prone group, 43% (13 out of 30) of patients were able to self-prone for 6 or more hours a day. 70% of the patients in the prone group were able to lie prone for 4 h a day. In the standard care group, 47% (14 out of 30) were completely supine and 53% spent some hours in the prone position, but none exceeded 6 h. | PaO2/FiO2 after 2 h was not different in the two groups: Standard care 171.7 (100.6) vs awake PP 198 (87.6), P = 0.3. There were no adverse events in both groups. |
| Johonson et al.[126], 2021 | 33596394 | Pragmatic randomized controlled trial | Non-ICU | COVID-19 AHRF FiO2 at admission 21 [21–29], SpO2 at admission 94% [90–96]. Requiring supplemental O2 at admission: 11 (36.7%) patients. | Overall cohort n = 30 Standard care n = 15 Awake PP n = 15 Self-driven protocol. Only 6/15 (40%) patients were observed in PP in the first 72 h. Cumulative time spent in PP was 2.4% of total time (1.6 [95% CI 0.2 to 3.1] hours). | Standard care 7% [95% CI 12 to 30] Awake PP 13% [95% CI 4 to 38] | Standard care 0% [95% CI 0 to 20] Awake PP 13% [95% CI 4 to 38] | Patient-directed PP is not feasible in spontaneously breathing, nonintubated patients hospitalized with COVID-19. | No improvements in oxygenation were observed at 72 or 48 h. |
| Moghadam et al.[112], 2020 | 32427179 | Prospective observational study | Non-ICU | COVID-19 AHRF Mean SpO2 at arrival 85.6% | Awake PP n = 10 | 0% [95% CI 0 to 28] | 0% [95% CI 0 to 28] | SpO2 improved from 85.6% to 95.9% after awake PP. 40% of patients reported improved dyspnea. | |
| Ng et al.[113], 2020 | 32457195 | Case series | Non-ICU | COVID-19 AHRF Median room air SpO2 at arrival 91% (91 to 94) Oxygen supplementation at arrival 2l/min [2–3] | Awake PP n = 10 Cumulative median time in PP 21 h. Three (10%) patients were transferred to ICU: 1 (10%) HFNO 1 (10%) Venturi Mask 1 (10%) IMV | 10% [95% CI 2 to 40] | 10% [95% CI 2 to 40] | Awake PP can be a low-risk, low-cost maneuver which can help patients with COVID-19 pneumonia delay or reduce the need for intensive care. | Three out of 10 patients were transferred to ICU, and one died. |
| Padrao et al.[114], 2020 | 33107664 | Retroscpetive study | Non-ICU | COVID-19 AHRF Standard care SpO2 92.5% [90–94] O2 flow rate 6 l/min [5–10] SpO2/FiO2 < 235 in 56 (53%) patients Awake PP SpO2 92% [88–93] O2 flow rate 7 l/min [5–13.5] SpO2/FiO2 < 235 in 36 (63%) patients SpO2/FiO2 196 [128–254] | Overall cohort n = 166 Standard care n = 109 Awake PP n = 57 Nasal cannula 44% Venturi mask 10% Nonrebreather face mask 46% First session awake PP duration: < 1h (6%) 1–2h (14%) 2–3h (12%) 3–4h (10%) > 4h (58%) | Awake PP 58% [95% CI 45 to 70] Standard care 49% [95% CI 39 to 58] | Awake PP 11% [95% CI 5 to 21] Standard care 20% [95% CI 14 to 29] | Awake PP was not associated with a reduction of need of intubation, both at univariate or multivariate analysis. | Awake PP led to improvement in ROX index (from 5.7 [3.9–7.7] to 7.7 [5.4–11], SpO2/FiO2 (from 196 [128–254] to 224 [159–307]) and respiratory rate (from 34 [30–38] to 29 [26–32]) |
| Paternoster et al.[98], 2020 | 33067029 | Case series | High Dependency Unit | COVID-19 AHRF PaO2/FiO2 107.5 (21) | Awake PP n = 11 Awake PP cycles of 13 (1.2) hours Helmet CPAP PEEP 9.6 (1.74) cmH2O Seven (63.6%) patients needed dexmedetomidine during awake PP | 27% [95% CI 10 to 57] | 18 [95% CI 5 to 48] | In conclusion, helmet CPAP in prone position for COVID-19 severely hypoxemic acute respiratory failure resulted feasible and without complications; the infusion of dexmedetomidine to improve patients’ compliance to pronation was well tolerate. | PaO2/FiO2 at enrollment 107 (20) PaO2/FiO2 after 24 h 214.6 (73) PaO2/FiO2 after 48 h 224.6 (86.6) PaO2/FiO2 after 72 h 244.4 (106.2) P < 0.001 Respiratory rate at enrollment 27 (4) Respiratory rate after 24 h 24 (5) Respiratory rate after 48 h 22 (4) Respiratory rate after 72 h 20 (5) P < 0.001 |
| Perez-nieto et al.[124▪▪], 2021 | 34266942 | Retrospectivemulticenter study | Non-ICU | COVID-19 AHRF SpO2/FiO2 189.5 (81.6) | Awake PP n = 505 Standard care n = 322 Awake PP group was prones for at least 2 consecutive hours. Awake PP Nasal Cannula 50% HFNO 12.1% Nonrebreather face mask 37.6% Standard care Nasal Cannula 46.3% HFNO 6.8% Nonrebreather face mask 46.9% | Awake PP 24% [95% CI 20 to 27] Standard care 40% [95% CI 35 to 46] | Awake PP 20% [95% CI 17 to 24] Standard care 38% [33 to 43] | Awake PP reduces the risk for endotracheal intubation and for mortality. The reduction of risk remained significant at multivariate, and after propensity score match. | Main risk factors associated with endotracheal intubation were age, SpO2/FiO2 < 100, and the use of nonrebreather mask. |
| Prud’homme et al.[115], 2021 | 33516704 | Retrospective multicenter matched cohort study | Non-ICU | COVID-19 AHRF SpO2/FiO2 standard care 299 (45) SpO2/FiO2 awake PP 279 (84) | Awake PP n = 48 Standard care n = 48 Oxygen supplementation strategy: first line oxygen therapy, escalation to HFNO, escalation to NIV, escalation to IMV. 32 (67%) patients underwent PP from 3 to 8 h/day 16 (32%) patients underwent PP for > 8 h/day | Follow up to day 14 Awake PP 15% [95% CI 7 to 27] Standard care 17% [95% CI 9 to 30] | Follow up to day 14 Awake PP 8% [95% CI 3 to 20] Standard care 12% [6 to 25] | Awake PP reduced the need of upgrading oxygen delivery method at day 14 (15 (31.2%)) compared with conventional treatment (25 (52.1%)), P = 0.038 with a hazard ratio of 2.03 [95% CI, 1.07–3.86; P = 0.003]. | Awake PP did not decrease the need for endotracheal intubation or the mortality rate. |
| Retucci et al.[99], 2020 | 32679237 | Pilot prospective observational study | High Dependency Unit | COVID-19 AHRF PaO2/FiO2 143 [97–204] | Awake PP n = 26 Helmet CPAP Prone or lateral position lasted 1h Helmet CPAP PaO2/FiO2 180 [155–218] 39 sessions of attempted PP: 12 prone and 27 lateral positioning. | 27% [95% CI 14 to 46] | 8% [95% CI 2 to 24] | The target was an alveolar-arterial gradient decrease of more than 20% before and after PP. Of the 12 sessions of PP 33.3% of trials succeeded, 41.7% decrease in alveolar-arterial gradient of < 20% from supine position, and 25% failed. Of the 39 sessions of lateral positioning: 8% succeeded, 52% decrease in alveolar-arterial gradient < 20% from supine position, 40% failed. | Respiratory rate: standard care 23.7 (4.7), during PP 23.1 (4.5), after resupination 23.6 (4.7). PaO2/FiO2 182.9 (43), during awake PP 220 (64.5), after resupination 179.3 (43.9). |
| Ripoll-Gallardo et al.[116], 2020 | 32713387 | Retrospective case series | Non-ICU | COVID-19 AHRF PaO2/FiO2, 115 (13) | Awake PP n = 13 Interface: Helmet CPAP PEEP 10 cmH2O | 70% [95% CI 42 to 87] | 54% [95% CI 29 to 77] | Patients with moderate to severe AHRF have a high intubation rate, despite the treatment with CPAP and awake PP. | PaO2/FiO2, improved before and after PP (P = 0.003), but there was no difference in respiratory rate before and after. |
| Rosén et al.[127▪], 2021 | 34127046 | Multicenter randomized clinical trial | Non-ICU | COVID-19 AHRF Standard care n = 39 PaO2/FiO2 standard care 115 [94–130] Prone n = 36 PaO2/FiO2 prone 115 [86–130] | HFNO standard care n = 29 HFNO prone n = 31 NIV standard care n = 27 PEEP 8 [6–8] NIV prone n = 21 PEEP 7 [6–10] | Standard care group 33% [95% CI 20 to 49] Prone group 33% [95% CI 20 to 50] | Control group 8% [95% CI 3 to 20] Prone group 17% [95% CI 8 to 22] | The implemented protocol for awake PP increased duration of awake PP but did not reduce the rate of intubation in patients with AHRF due to COVID-19 compared to standard care. | Nine patients (23%) in the control group had pressure sores compared with two patients (6%) in the prone group, P = 0.03, there were no difference in the use of NIV, vasopressors, continuous renal-replacement therapy, ECMO, VFD, hospital and ICU length of stay and mortality among the two groups. |
| Sartini et al.[117], 2020 | 32412606 | Cross sectional | Non-ICU | COVID-19 AHRF PaO2/FiO2 157 (43) | Awake PP n = 15 Interface: NIV, PEEP 10 cmH2O Number of PP cycles 2 [1–3], for a total duration of 3 [1–6] hours | Follow-up 14 days Awake PP failure 13% [95% CI 4 to 38] | Follow-up 14 days 7% [95% CI 1 to 30] | All patients had improvement in PaO2/FiO2 during PP, and 12 (80%) had maintained PaO2/FiO2 improvement after resupination. | All patients had a reduction in respiratory rate during PP, that was maintained after resupination. |
| Taboada et al.[118], 2021 | 33839432 | Prospective observational study | ICU | COVID-19 AHRF PaO2/FiO2 93 [72–108] | Awake PP n = 63 Treated with HFNO and dexmedetomidine For a median of 4 [2.5–8] sessions, each lasting 36 [24–72] hours. | HFNO and awake PP failure 32% [95% CI 22 to 44] | 11% [95% CI 5 to 21] | In patients with mild to moderate AHRF treatment with a combination of dexmedetomidine, HFNO and long periods of PP led to a mortality rate of 11%. | Application of HFNO, awake PP and dexmedetomidine was relatively safe, as bradycardia (<40 bpm) during DEX infusion was observed in 5 patients (7.9%). |
| Thompson et al.[119], 2020 | 32584946 | Prospective observational study | Intermediate Care Unit, | COVID-19 AHRF SpO2 ≤ 93% in nasal cannula 6 l/min or 15 l/min via nonrebreather face mask) | Awake PP n = 25 At least 1 session lasting at least 1hour | 48% [95% CI 30 to 66] | 12% [95% CI 4 to 30] | During awake PP the range of improvement in SpO2 was 1% to 34% (median [SE], 7% [1.2%]; 95% CI, 4.6%-9.4%). | An SpO2 of 95% or greater after 1 h of PP was associated with a lower rate of intubation. |
| Tonelli et al.[120], 2021 | 33824084 | Retrospectivemulticenter study | Respiratory ICU | COVID-19 AHRF PaO2/FiO2 overall cohort 149 [78–232] PaO2/FiO2 awake PP 141 [73–223] PaO2/FiO2 standard care 153 [84–232]; P = 0.03 | Awake PP n = 38 Standard care n = 76 Awake PP encouraged for at least 3 h; number of daily PP sessions: 1 to 4 Interface: HFNO 69 (61%) Helmet CPAP 25 (22%) CPAP 8 cmH2O and then adjusted Oro-nasal NIV 19% (17) PEEP 8 cmH2O and then adjusted Pressure support 10 cmH2O and then adjusted | Awake PP 18% [95% CI 9 to 33] Standard care 39% [95% CI 29 to 51] | Awake PP 13% [95% CI 6 to 27] Standard care 22% [95% CI 14 to 33] | Awake PP in awake and spontaneously breathing Covid-19 patients is feasible and can significantly reduce intubation rate (HR = 0.59 95% CI [0.3 to 0.94], P = 0.03 after adjustment) especially in those patients undergoing HFNO (HR = 0.34 95% CI [0.12 to 0.84], P = 0.04). | Awake PP increased the respiratory support free days (standard care 15 [2–22] vs 20 [2–24] P = 0.03) and decreased the respiratory ICU (standard care 15 [3–26] vs 10 [3–21] P = 0.02) and the hospital length of stay (standard care 24 [3–45] vs 20 [3–41], P = 0.03). |
| Tu et al.[121], 2020 | 32566624 | Pilot retrospective study | ICU | COVID-19 AHRF PaO2/FiO2 lower 150 | Awake PP n = 9 Treated with HFNO Awake PP sessions applied for 5 [3–8] times, with a duration 2 [1–4] hours. | 22% [95% CI 6 to 55] | Not reported | Awake PP could improve blood oxygenation and potentially avoid endotracheal intubation. | Mean PaO2 increased from 69 (10) to 108 (14) with awake PP (P < 0.00) and PaCO2 decreased from 47 (7) to 39 (5) (P = 0.007). |
| Winearls et al.[100], 2020 | 32895247 | Retrospective Study | Non-ICU | COVID-19 AHRF PaO2/FiO2 112 [106–194] Patients with no limitations of treatment n = 14 Patients with limitations of treatment n = 10 | Attempted awake PP n = 24 Treated with CPAP, maximum PEEP 12 [12–15] cmH2O Interface not specified 2 patients did not tolerate Awake PP 12 patients received full awake PP, 10 patients semiprone lateral position The mean (SD) time of awake PP was 8 (5) hours in first day. | Patients with no limitations of treatment 7% [95% CI 1 to 31] | Patients with limitations of treatment 40% [95% CI 17 to 69] | Awake PP alongside CPAP significantly increased both the ROX index and the PaO2/FiO2 from baseline values (ROX index: 7.0 (2.5) baseline vs 11.4 (3.7) CPAP + PP, P < 0.0001; PaO2/FiO2 143 (73) mm Hg baseline vs 252 (87) mm Hg CPAP + PP, P < 0.01). | No difference in respiratory rate at any timepoint was found. |
| Xu et al.[122], 2020 | 32448330 | Retrospective multicenter study | ICU | COVID-19 AHRF PaO2/FiO2 157 (46) | Awake PP n = 10 Target time was > 16 h/day | 0% [95% CI 0 to 28] | 0% [95% CI 0 to 28] | Early awake PP combined with HFNO therapy could be used safely and effectively in young, fit, severe COVID-19 patients, and it may reduce the conversion to critical illness and the need for tracheal intubation. | After awake PP PaO2/FiO2 and increased significantly, and respiratory alkalosis decreased significantly. |
| Zang et al.[123], 2020 | 32699915 | Prospective observational study | ICU | COVID-19 AHRF Patients with severe hypoxia | Awake PP n = 23 Standard care n = 37 | Not reported | Awake PP 43% [95% CI 26 to 63] Standard care 76% [60 to 87], P < 0.001 | Early awake PP might reduce hypoxia and improve mortality. | In the awake PP group SpO2 increased from 91% (1.5) to 95.5 (1.7), P < 0.01, respiratory rate decreased from 28.2 (3) to 24.9 (1.8), P < 0.01, and ROX index increased from 3.3 (0.5) to 4 (0.5), P < 0.01. |
Values are displayed as means (SD) or medians [Interquartile range].
Failure was defined as either intubation, death while still on noninvasive respiratory support, or escalation to other noninvasive respiratory support to avoid endotracheal intubation. AHRF, acute hypoxemic respiratory failure; ARDS, acute respiratory distress syndrome; awake PP, awake prone position; CPAP, continuous positive end-expiratory pressure; FiO2, fraction of inspired oxygen; HFNO, high-flow nasal oxygen; ICU, intensive care unit; IQR, interquartile range; NIV, noninvasive ventilation; PaO2, partial pressure of arterial oxygen; PEEP, positive end-expiratory pressure; SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; SpO2, peripheral capillary oxygen saturation; VFD, Ventilatory Free Days.
Several pilot studies have investigated whether it could improve patient-centered outcomes, but results are conflicting (Fig. 1d). The largest retrospective, multicenter, observational study showed a reduction in the intubation rate in patients who received awake prone position for at least 2 consecutive hours, compared to patients who did not receive this intervention [124▪▪]. Three small, randomized feasibility trials available showed no benefit on endotracheal intubation rate and on mortality [125,126,127▪]. In the trial designed by Rosén et al.[127▪], the intervention group should have undergone prone positioning for at least 16 h per day, and the control group was allowed both the supine and the prone position, albeit the last one was not actively encouraged; this yielded relevant incidence of cross-over in the control group that, combined with relatively low time spent in prone position in the intervention group (median [IQR] time in prone positioning was 3.4 h [1.8–8.4] vs 9 [4.4–10.6] respectively), resolved in no difference in intubation rate, and the trial was stopped early for futility.
Lately, a prospective, randomized, collaborative meta-trial compared awake prone position and conventional in patients treated with HFNO with acute respiratory failure of COVID-19 etiology. The authors found a reduction in the proportion of patients intubated or dying within 28 days from enrolment (40% in the awake prone position group vs 46% in the standard of care group, P = 0.007) [128▪▪]. Interestingly, average duration of prone positioning sessions was 6 h, and longer sessions were associated with treatment success at 28 days: this finding should be interpreted as exploratory.
Awake prone position appears as a cost-effective technique that can improve the blood oxygenation and reduce the respiratory rate [129], and a recent randomized controlled trial support its routine use in patients with respiratory failure of COVID-19 etiology treated with HFNO [128▪▪].
CONCLUSION
Currently, noninvasive respiratory support is a safe option in patients with a PaO2/FiO2 ≥ 200 mmHg; in patients PaO2/FiO2 < 200 mmHg most recent randomized controlled trials suggest HFNO or helmet NIV as the most promising techniques for a noninvasive respiratory support trial [24,51,56]. In the COVID-19 pandemic, awake prone position, which has a robust physiological rationale, has been widely applied with benefits on oxygenation and a possible reduction in the rate of endotracheal intubation [87]. During any treatment, careful clinical monitoring remains mandatory not to delay the intubation and protective ventilation, especially in patients with PaO2/FiO2 < 200 mmHg.
Acknowledgements
None.
Disclosures: D.L.G. reports grants from the Italian Society of Anesthesia, Analgesia, and Intensive Care Medicine (SIAARTI), from the European Society of Intensive Care Medicine (ESICM), and GE Healthcare and travel expenses from Maquet, Getinge, and Air Liquide outside of the submitted work. M.A. reported receiving personal fees from Maquet, Chiesi, and Air Liquide and grants from GE Healthcare outside of the submitted work. The other authors declare nothing to disclose.
Financial support and sponsorship
None.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.World Heal Organ, WHO Headquarters. Clinical management Clinical management Living guidance COVID-19. 2021; https://www.who.int/publications/i/item/WHO-2019-nCoV-clinical-2021-1 [Google Scholar]
- 2.Rochwerg B, Brochard L, Elliott MW, et al. Official ERS/ATS clinical practice guidelines: noninvasive ventilation for acute respiratory failure. Eur Respir J 2017; 50:1602426.doi: 10.1183/13993003.02426-2016. [DOI] [PubMed] [Google Scholar]
- 3.Gattinoni L, Chiumello D, Caironi P, et al. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med 2020; 46:1099–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobin MJ, Laghi F, Jubran A. Caution about early intubation and mechanical ventilation in COVID-19. Ann Intensive Care 2020; 10:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Botta M, Tsonas AM, Pillay J, et al. Ventilation management and clinical outcomes in invasively ventilated patients with COVID-19 (PRoVENT-COVID): a national, multicentre, observational cohort study. Lancet Respir Med 2020; 19:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vitacca M, Nava S, Santus P, Harari S. Early consensus management for non-ICU acute respiratory failure SARS-CoV-2 emergency in Italy: from ward to trenches. Eur Respir J 2020; 55:2000632.doi: 10.1183/13993003.00632-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cutuli SL, Grieco DL, Menga LS, et al. Noninvasive ventilation and high-flow oxygen therapy for severe community-acquired pneumonia. Curr Opin Infect Dis 2021; 34:142–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grieco DL, Maggiore SM, Roca O, et al. Noninvasive ventilatory support and high-flow nasal oxygen as first-line treatment of acute hypoxemic respiratory failure and ARDS. Intensive Care Med 2021; 47:851–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dar M, Swamy L, Gavin D, Theodore A. Mechanical-ventilation supply and options for the COVID-19 Pandemic. Leveraging all available resources for a limited resource in a crisis. Ann Am Thorac Soc 2021; 18:408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grieco D, Menga L, Eleuteri D, Antoneli M. Patient self-inflicted lung injury: implications for acute hypoxemic respiratory failure and ARDS patients on noninvasive support. Minerva Anestesiol 2019; 85:1014–1023. [DOI] [PubMed] [Google Scholar]
- 11.Battaglini D, Robba C, Ball L, et al. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: a narrative review. Br J Anaesth 2021; 10.1016/j.bja.2021.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12▪.Spinelli E, Mauri T, Beitler JR, et al. Respiratory drive in the acute respiratory distress syndrome: pathophysiology, monitoring, and therapeutic interventions. Intensive Care Med 2020; 46:606–618. [DOI] [PMC free article] [PubMed] [Google Scholar]; Interesting review summarizing the patophysiology of respiratory drive in acute hypoxemic respiratory failure.
- 13▪.Vaporidi K, Akoumianaki E, Telias I, et al. Respiratory drive in critically ill patients pathophysiology and clinical implications. Am J Respir Crit Care Med 2020; 201:20–32. [DOI] [PubMed] [Google Scholar]; Interesting review summarizing the patophysiology of respiratory drive in acute hypoxemic respiratory failure.
- 14.Dangers L, Montlahuc C, Kouatchet A, et al. Dyspnoea in patients receiving noninvasive ventilation for acute respiratory failure: prevalence, risk factors and prognostic impact. Eur Respir J 2018; 52:1702637. [DOI] [PubMed] [Google Scholar]
- 15.Caironi P, Cressoni M, Chiumello D, et al. Lung opening and closing during ventilation of acute respiratory distress syndrome. Am J Respir Crit Care Med 2010; 181:578–586. [DOI] [PubMed] [Google Scholar]
- 16.Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med 2017. [DOI] [PubMed] [Google Scholar]
- 17.Yoshida T, Uchiyama A, Matsuura N, et al. Spontaneous breathing during lung-protective ventilation in an experimental acute lung injury model: high transpulmonary pressure associated with strong spontaneous breathing effort may worsen lung injury. Crit Care Med 2012; 40:1578–1585. [DOI] [PubMed] [Google Scholar]
- 18.Menga LS, Grieco DL, Rosà T, et al. Dyspnea and clinical outcome in critically ill patients receiving noninvasive support for COVID-19 respiratory failure: posthoc analysis of a randomized clinical trial. ERJ Open Res 2021; 7:00418–02021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhattacharya M, Kallet RH, Ware LB, Matthay MA. Negative-pressure pulmonary edema. Chest 2016; 150:927–933. [DOI] [PubMed] [Google Scholar]
- 20.Yoshida T, Torsani V, Gomes S, et al. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am J Respir Crit Care Med 2013; 188:1420–1427. [DOI] [PubMed] [Google Scholar]
- 21.Morais CCA, Koyama Y, Yoshida T, et al. High positive end-expiratory pressure renders spontaneous effort noninjurious. Am J Respir Crit Care Med 2018; 197:1285–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoshida T, Roldan R, Beraldo MA, et al. Spontaneous effort during mechanical ventilation: maximal injury with less positive end-expiratory pressure. Crit Care Med 2016. 1–11. 10.1097/CCM.0000000000001649 [DOI] [PubMed] [Google Scholar]
- 23.Tonelli R, Busani S, Tabbì L, et al. Inspiratory effort and lung mechanics in spontaneously breathing patients with acute respiratory failure due to COVID-19: a matched control study. Am J Respir Crit Care Med 2021. 1–15. 10.1164/rccm.202104-1029LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frat J-P, Thille AW, Mercat A, et al. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med 2015; 372:2185–2196. [DOI] [PubMed] [Google Scholar]
- 25.Goligher EC, Dres M, Patel BK, et al. Lung and diaphragm-protective ventilation. Am J Respir Crit Care Med 2020; 10.1164/rccm.202003-0655CP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26▪.Menga LS, Cese LD, Bongiovanni F, et al. High failure rate of noninvasive oxygenation strategies in critically ill subjects with acute hypoxemic respiratory failure due to COVID-19. Respir Care 2021; 10.4187/respcare.08622 [DOI] [PubMed] [Google Scholar]; Prospective observational study showing that COVID-19 patients treated with noninvasive respiratory support have higher risk of failure than matched patients with respiratory failure of other etiology.
- 27.Mauri T, Turrini C, Eronia N, et al. Physiologic effects of high-flow nasal cannula in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2017; 195:1207–1215. [DOI] [PubMed] [Google Scholar]
- 28.Basile MC, Mauri T, Spinelli E, et al. Nasal high flow higher than 60 L/min in patients with acute hypoxemic respiratory failure: a physiological study. Crit Care 2020; 24:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alhazzani W, Møller MH, Arabi YM, et al. Surviving Sepsis Campaign: guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Berlin Heidelberg: Springer; 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montiel V, Robert A, Robert A, et al. Surgical mask on top of high-flow nasal cannula improves oxygenation in critically ill COVID-19 patients with hypoxemic respiratory failure. Ann Intensive Care 2020; 10.1186/s13613-020-00744-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demoule A, Baron AV, Darmon M, et al. High-flow nasal cannula in critically Ill patients with severe COVID-19. Am J Respir Crit Care Med 2020; 202:1039–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hernandez-Romieu AC, Adelman MW, Hockstein MA, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med 2020. E1045–E1053. 10.1097/CCM.0000000000004600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mellado-Artigas R, Ferreyro BL, Angriman F, et al. High-flow nasal oxygen in patients with COVID-19-associated acute respiratory failure. Crit Care 2021. 25. 10.1186/s13054-021-03469-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vega ML, Dongilli R, Olaizola G, et al. COVID-19 pneumonia and ROX index: time to set a new threshold for patients admitted outside the ICU. Pulmonology 2021; 14:337–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vianello A, Arcaro G, Molena B, et al. High-flow nasal cannula oxygen therapy to treat patients with hypoxemic acute respiratory failure consequent to SARS-CoV-2 infection. Thorax 2020; 75:998–1000. [DOI] [PubMed] [Google Scholar]
- 36.Wendel Garcia PD, Aguirre-Bermeo H, Buehler PK, et al. Implications of early respiratory support strategies on disease progression in critical COVID-19: a matched subanalysis of the prospective RISC-19-ICU cohort. Crit Care 2021; 25:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geng S, Mei Q, Zhu C, et al. High flow nasal cannula is a good treatment option for COVID-19. Hear Lung 2020; 49:444–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med 2020; 201:1430–1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40▪▪.Liu L, Xie J, Wu W, et al. A simple nomogram for predicting failure of noninvasive respiratory strategies in adults with COVID-19: a retrospective multicentre study. Lancet Digit Heal 2021; 3:e166–e174. [DOI] [PMC free article] [PubMed] [Google Scholar]; Well designed study that provides the reader a nomogram and an online calculator to predict the risk of noninvasive respiratory support failure in COVID-19 patients.
- 41.Wang K, Zhao W, Li J, et al. The experience of high-flow nasal cannula in hospitalized patients with 2019 novel coronavirus-infected pneumonia in two hospitals of Chongqing, China. Ann Intensive Care 2020; 10:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zucman N, Mullaert J, Roux D, et al. Prediction of outcome of nasal high flow use during COVID-19-related acute hypoxemic respiratory failure. Intensive Care Med 2020; 46:1924–1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suliman LA, Abdelgawad TT, Farrag NS, Abdelwahab HW. Validity of ROX index in prediction of risk of intubation in patients with COVID-19 pneumonia. Adv Respir Med 2021; 89:1–7. [DOI] [PubMed] [Google Scholar]
- 44.Panadero C, Abad-Fernández A, Rio-Ramirez MT, et al. High-flow nasal cannula for acute respiratory distress syndrome (ARDS) due to COVID-19. Multidiscip Respir Med 2020. 15. 10.4081/mrm.2020.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu X, Liu X, Xu Y, et al. Ventilatory ratio in hypercapnic mechanically ventilated patients with COVID-19-associated acute respiratory distress syndrome. Am J Respir Crit Care Med 2020; 201:1297–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia J, Zhang Y, Ni L, et al. High-flow nasal oxygen in coronavirus disease 2019 patients with acute hypoxemic respiratory failure: a multicenter, retrospective cohort study. Crit Care Med 2020. E1079–E1086. 10.1097/CCM.0000000000004558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bonnet N, Martin O, Boubaya M, et al. High flow nasal oxygen therapy to avoid invasive mechanical ventilation in SARS-CoV-2 pneumonia: a retrospective study. Ann Intensive Care 2021; 11:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roca O, Caralt B, Messika J, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high flow therapy. Am J Respir Crit Care Med 2018. [DOI] [PubMed] [Google Scholar]
- 49.Chandel A, Patolia S, Brown AW, et al. High-flow nasal cannula therapy in COVID-19: using the ROX index to predict success. Respir Care 2021; 66:909–919. [DOI] [PubMed] [Google Scholar]
- 50.Bellani G, Laffey JG, Pham T, et al. Noninvasive ventilation of patients with acute respiratory distress syndrome. Insights from the LUNG SAFE Study. Am J Respir Crit Care Med 2017; 195:67–77. [DOI] [PubMed] [Google Scholar]
- 51.Grieco DL, Menga LS, Raggi V, et al. Physiological comparison of high-flow nasal cannula and helmet noninvasive ventilation in acute hypoxemic respiratory failure. Am J Respir Crit Care Med 2019. [DOI] [PubMed] [Google Scholar]
- 52▪▪.Ferreyro BL, Angriman F, Munshi L, et al. Association of noninvasive oxygenation strategies with all-cause mortality in adults with acute hypoxemic respiratory failure: a systematic review and meta-analysis. JAMA 2020; 324:57–67. [DOI] [PMC free article] [PubMed] [Google Scholar]; Systematic review and meta-analysis summarizing the potential harms and benefits of noninvasive respiratory support in patients affected by acute hypoxemic respiratory failure.
- 53.Racca F, Appendini L, Gregoretti C, et al. Effectiveness of mask and helmet interfaces to deliver noninvasive ventilation in a human model of resistive breathing. J Appl Physiol 2005; 99:1262–1271. [DOI] [PubMed] [Google Scholar]
- 54.Vargas F, Thille A, Lyazidi A, et al. Helmet with specific settings versus facemask for noninvasive ventilation. Crit Care Med 2009; 37:1921–1928. [DOI] [PubMed] [Google Scholar]
- 55.Antonelli M, Conti G, Pelosi P, et al. New treatment of acute hypoxemic respiratory failure: noninvasive pressure support ventilation delivered by helmet—a pilot controlled trial. Crit Care Med 2002; 30:602–608. [DOI] [PubMed] [Google Scholar]
- 56.Mauri T, Bellani G, Grasselli G, et al. Patient-ventilator interaction in ARDS patients with extremely low compliance undergoing ECMO: a novel approach based on diaphragm electrical activity. Intensive Care Med 2013; 39:282–291. [DOI] [PubMed] [Google Scholar]
- 57.Yoshida T, Fujino Y, Amato MBP, Kavanagh BP. Fifty years of research in ARDS. Spontaneous breathing during mechanical ventilation. risks, mechanisms, and management. Am J Respir Crit Care Med 2017; 195:985–992. [DOI] [PubMed] [Google Scholar]
- 58.Patel BK, Wolfe KS, Pohlman AS, et al. Effect of noninvasive ventilation delivered by helmet vs face mask on the rate of endotracheal intubation in patients with acute respiratory distress syndrome. JAMA 2016; 315:2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bertaina M, Nuñez-Gil IJ, Franchin L, et al. Noninvasive ventilation for SARS-CoV-2 acute respiratory failure: a subanalysis from the HOPE COVID-19 registry. Emerg Med J 2021; 38:359–365. [DOI] [PubMed] [Google Scholar]
- 60.Duca A, Memaj I, Zanardi F, et al. Severity of respiratory failure and outcome of patients needing a ventilatory support in the Emergency Department during Italian novel coronavirus SARS-CoV2 outbreak: Preliminary data on the role of Helmet CPAP and Non-Invasive Positive Pressure Ventilati. EClinicalMedicine 2020; 24:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Faraone A, Beltrame C, Crociani A, et al. Effectiveness and safety of noninvasive positive pressure ventilation in the treatment of COVID-19-associated acute hypoxemic respiratory failure: a single center, non-ICU setting experience. Intern Emerg Med 2021; 16:1183–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu Y, Guan L, Wu W, et al. Noninvasive ventilation in patients with COVID-19-related acute hypoxemic respiratory failure: a retrospective cohort study. Front Med 2021; 8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hua J, Qian C, Luo Z, et al. Invasive mechanical ventilation in COVID-19 patient management: the experience with 469 patients in Wuhan. Crit Care 2020. 24. 10.1186/s13054-020-03044-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Menzella F, Barbieri C, Fontana M, et al. Effectiveness of noninvasive ventilation in COVID-19 related-acute respiratory distress syndrome. Clin Respir J 2021; 15:779–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukhtar A, Lotfy A, Hasanin A, et al. Outcome of noninvasive ventilation in COVID-19 critically ill patients: a retrospective observational study. Anaesth Crit Care Pain Med 2020; 39:579–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020; 8:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67▪▪.Franco C, Facciolongo N, Tonelli R, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J 2020. 56. 10.1183/13993003.02130-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]; Relatively large observational study reporting the failure rate and the mortality rate of patients treated with noninvasive respiratory support outside the ICU during the pandemic.
- 68▪▪.Karagiannidis C, Mostert C, Hentschker C, et al. Case characteristics, resource use, and outcomes of 10 021 patients with COVID-19 admitted to 920 German hospitals: an observational study. Lancet Respir Med 2020; 8:853–862. [DOI] [PMC free article] [PubMed] [Google Scholar]; Nation-wide observational study reporting the outcomes of 10021 patientes with COVID-19.
- 69▪.Bellani G, Grasselli G, Cecconi M, et al. Noninvasive ventilatory support of patients with COVID-19 outside the intensive care units (WARd-COVID). Ann Am Thorac Soc 2021; 18:1020–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]; One of the largest observational study describing failure rate and mortality of patients treated with noninvasive respiratory support outside of the ICU.
- 70▪▪.Grieco DL, Menga LS, Cesarano M, et al. Effect of helmet noninvasive ventilation vs high-flow nasal oxygen on days free of respiratory support in patients with COVID-19 and moderate to severe hypoxemic respiratory failure: The HENIVOT Randomized Clinical Trial. JAMA 2021; 10.1001/jama.2021.4682 [DOI] [PMC free article] [PubMed] [Google Scholar]; The first randomized controlled trial comparing Helmet NIV and HFNO in patients with COVID-19 hypoxemic respiratory failure. Despite the negative primary outcome (no difference in respiratory support free days) there the Helmet NIV significantly reduced the rate of endotracheal intubation.
- 71.Delclaux C, L’Her E, Alberti C, et al. Treatment of acute hypoxemic nonhypercapnic respiratory insufficiency with continuous positive airway pressure delivered by a face mask: a randomized controlled trial. JAMA 2000; 284:2352–2360. [DOI] [PubMed] [Google Scholar]
- 72.2014; Brambilla AM, Aliberti S, Prina E, et al. Helmet CPAP vs. oxygen therapy in severe hypoxemic respiratory failure due to pneumonia. Intensive Care Med. 40:942–949. [DOI] [PubMed] [Google Scholar]
- 73.Cosentini R, Brambilla AM, Aliberti S, et al. Helmet continuous positive airway pressure vs oxygen therapy to improve oxygenation in community-acquired pneumonia: a randomized, controlled trial. Chest 2010; 138:114–120. [DOI] [PubMed] [Google Scholar]
- 74▪.Vaschetto R, Barone-Adesi F, Racca F, et al. Outcomes of COVID-19 patients treated with continuous positive airway pressure outside the intensive care unit. ERJ Open Res 2021; 7:00541–02020. [DOI] [PMC free article] [PubMed] [Google Scholar]; This relatively big retrospective observational study shows that delayed intubation in patients treated with CPAP might increase mortality.
- 75.Sivaloganathan AA, Nasim-Mohi M, Brown MM, et al. Noninvasive ventilation for COVID-19-associated acute hypoxaemic respiratory failure: experience from a single centre. Br J Anaesth 2020. 1–3. 10.1016/j.bja.2020.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Di Domenico SL, Coen D, Bergamaschi M, et al. Clinical characteristics and respiratory support of 310 COVID-19 patients, diagnosed at the emergency room: a single-center retrospective study. Intern Emerg Med 2021; 16:1051–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Coppadoro A, Benini A, Fruscio R, et al. Helmet CPAP to treat hypoxic pneumonia outside the ICU: an observational study during the COVID-19 outbreak. Crit Care 2021; 25:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burns GP, Lane ND, Tedd HM, et al. Improved survival following ward-based noninvasive pressure support for severe hypoxia in a cohort of frail patients with COVID-19: retrospective analysis from a UK teaching hospital. BMJ Open Respir Res 2020; 7:e000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arina P, Baso B, Moro V, et al. Discriminating between CPAP success and failure in COVID-19 patients with severe respiratory failure. Intensive Care Med 2021; 47:237–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.De Vita N, Scotti L, Cammarota G, et al. Predictors of intubation in COVID-19 patients treated with out-of-ICU continuous positive airway pressure. Pulmonology 2021. 19–21. 10.1016/j.pulmoe.2020.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Aliberti S, Radovanovic D, Billi F, et al. Helmet CPAP treatment in patients with COVID-19 pneumonia: a multicentre cohort study. Eur Respir J 2020. 56. 10.1183/13993003.01935-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Alviset S, Riller Q, Aboab J, et al. Continuous Positive Airway Pressure (CPAP) face-mask ventilation is an easy and cheap option to manage a massive influx of patients presenting acute respiratory failure during the SARS-CoV-2 outbreak: A retrospective cohort study. PLoS One 2020; 15:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carteaux G, Pons M, Morin F, et al. Continuous positive airway pressure for respiratory support during COVID-19 pandemic: a frugal approach from bench to bedside. Ann Intensive Care 2021. 11. 10.1186/s13613-021-00828-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kofod LM, Nielsen Jeschke K, Kristensen MT, et al. COVID-19 and acute respiratory failure treated with CPAP. Eur Clin Respir J 2021. 8. 10.1080/20018525.2021.1910191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nightingale R, Nwosu N, Kutubudin F, et al. Is continuous positive airway pressure (CPAP) a new standard of care for type 1 respiratory failure in COVID-19 patients? A retrospective observational study of a dedicated COVID-19 CPAP service. BMJ Open Respir Res 2020; 7:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oranger M, Gonzalez-Bermejo J, Dacosta-Noble P, et al. Continuous positive airway pressure to avoid intubation in SARS-CoV-2 pneumonia: a two-period retrospective case-control study. Eur Respir J 2020; 56:8–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gaulton TG, Bellani G, Foti G, et al. Early clinical experience in using helmet continuous positive airway pressure and high-flow nasal cannula in overweight and obese patients with acute hypoxemic respiratory failure from coronavirus disease 2019. Crit Care Explor 2020; 2:e0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Noeman-Ahmed Y, Gokaraju S, Powrie DJ, et al. Predictors of CPAP outcome in hospitalized COVID-19 patients. Respirology 2020; 25:1316–1319. [DOI] [PubMed] [Google Scholar]
- 89.Brusasco C, Corradi F, Di Domenico A, et al. Continuous positive airway pressure in COVID-19 patients with moderate-Tosevere respiratory failure. Eur Respir J 2021; 57:10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mercurio G, D’Arrigo S, Moroni R, et al. Diaphragm thickening fraction predicts noninvasive ventilation outcome: a preliminary physiological study. Crit Care 2021; 25:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pagano A, Porta G, Bosso G, et al. Noninvasive CPAP in mild and moderate ARDS secondary to SARS-CoV-2. Respir Physiol Neurobiol 2020; 280:103489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92▪▪.Katira BH, Osada K, Engelberts D, et al. Positive end-expiratory pressure, pleural pressure, and regional compliance during pronation: an experimental study. Am J Respir Crit Care Med 2021. 1–48. 10.1164/rccm.202007-2957oc [DOI] [PubMed] [Google Scholar]; Physiological study unreveling the mechanism of lung protection during pronation.
- 93.Guérin C, Reignier J, Richard JC, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 2013; 368:2159–2168. [DOI] [PubMed] [Google Scholar]
- 94▪▪.Yoshida T, Tanaka A, Roldan R, et al. Prone position reduces spontaneous inspiratory effort in patients with acute respiratory distress syndrome: a bicenter study. Am J Respir Crit Care Med 2021; 203:1437–1440. [DOI] [PubMed] [Google Scholar]; Physiological study unreveling the mechanism of lung protection during pronation.
- 95.Cherian SV, Li C, Roche B, et al. Predictive factors for success of awake proning in hypoxemic respiratory failure secondary to COVID-19: a retrospective cohort study. Respir Med 2021; 181:106379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coppo A, Bellani G, Winterton D, et al. Feasibility and physiological effects of prone positioning in nonintubated patients with acute respiratory failure due to COVID-19 (PRON-COVID): a prospective cohort study. Lancet Respir Med 2020; 10.1016/S2213-2600(20)30268-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Elharrar X, Trigui Y, Dols AM, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA 2020; 323:2336–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Paternoster G, Sartini C, Pennacchio E, et al. Awake pronation with helmet continuous positive airway pressure for COVID-19 acute respiratory distress syndrome patients outside the ICU: a case series. Med Intensiva 2020. 19–21. 10.1016/j.medin.2020.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Retucci M, Aliberti S, Ceruti C, et al. Prone and lateral positioning in spontaneously breathing patients With COVID-19 pneumonia undergoing noninvasive helmet CPAP treatment. Chest 2020; 158:2431–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Winearls S, Swingwood EL, Hardaker CL, et al. Early conscious prone positioning in patients with COVID-19 receiving continuous positive airway pressure: a retrospective analysis. BMJ Open Respir Res 2020; 7:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Avdeev SN, Nekludova GV, Trushenko NV, et al. Lung ultrasound can predict response to the prone position in awake nonintubated patients with COVID-19 associated acute respiratory distress syndrome. Crit Care 2021; 25:4–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bastoni D, Poggiali E, Vercelli A, et al. Prone positioning in patients treated with noninvasive ventilation for COVID-19 pneumonia in an Italian emergency department. Emerg Med J 2020; 37:565–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Burton-Papp HC, Jackson AIR, Beecham R, et al. Conscious prone positioning during noninvasive ventilation in COVID-19 patients: experience from a single centre. F1000Research 2020; 9:859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Caputo ND, Strayer RJ, Levitan R. Early self-proning in awake, nonintubated patients in the emergency department: a single ED's experience during the COVID-19 pandemic. Acad Emerg Med 2020; 27:375–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Damarla M, Zaeh S, Niedermeyer S, et al. Prone positioning of nonintubated patients with COVID-19. Am J Respir Crit Care Med 2020; 202:604–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Despres C, Brunin Y, Berthier F, et al. Prone positioning combined with high-flow nasal or conventional oxygen therapy in severe Covid-19 patients. Crit Care 2020; 24:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fazzini B, Fowler AJ, Zolfaghari P. Effectiveness of prone position in spontaneously breathing patients with COVID-19: A prospective cohort study. J Intensive Care Soc 2021. 175114372199654. 10.1177/1751143721996542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ferrando C, Mellado-Artigas R, Gea A, et al. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: a multicenter, adjusted cohort study. Crit Care 2020; 24:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Golestani-Eraghi M, Mahmoodpoor A. Early application of prone position for management of Covid-19 patients. J Clin Anesth 2020; 66:337–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hallifax RJ, Porter BM, Elder PJ, et al. Successful awake proning is associated with improved clinical outcomes in patients with COVID-19: single-centre high-dependency unit experience. BMJ Open Respir Res 2020; 7:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jagan N, Morrow LE, Walters RW, et al. The POSITIONED Study: prone positioning in nonventilated coronavirus disease 2019 patients—a retrospective analysis. Crit Care Explor 2020; 2:e0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Moghadam VD, Shafiee H, Ghorbani M, Heidarifar R. Prone positioning in management of COVID-19 hospitalized patients. Braz J AnesthesiolV 70 2020. 188–190. 10.1016/j.bjane.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ng Z, Tay WC, Benjamin Ho CH. Awake prone positioning for nonintubated oxygen dependent COVID-19 pneumonia patients. Eur Respir J 2020. 56. 10.1183/13993003.01198-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Padrão EMH, Valente FS, Besen BAMP, et al. Awake prone positioning in COVID-19 hypoxemic respiratory failure: exploratory findings in a single-center retrospective cohort study. Acad Emerg Med 2020; 27:1249–1259. [DOI] [PubMed] [Google Scholar]
- 115.Prud’homme E, Trigui Y, Elharrar X, et al. Effect of prone positioning on the respiratory support of nonintubated patients with COVID-19 and acute hypoxemic respiratory failure: a retrospective matching cohort study. Chest 2021; 160:85–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ripoll-Gallardo A, Grillenzoni L, Bollon J, et al. Prone positioning in non-intubated patients with COVID-19 outside of the intensive care unit: more evidence needed. Disaster Med Public Health Prep 2020; 14:E22–E24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sartini C, Tresoldi M, Scarpellini P, et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA 2020; 323:2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Taboada M, Baluja A, Santos L, Dos, et al. Effectiveness of dexmedetomidine combined with high flow nasal oxygen and long periods of awake prone positioning in moderate or severe COVID-19 pneumonia. J Clin Anesth 2021; 72:337–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Thompson AE, Ranard BL, Wei Y, Jelic S. Prone positioning in awake, nonintubated patients with COVID-19 hypoxemic respiratory failure. JAMA Intern Med 2020; 180:1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tonelli R, Pisani L, Tabbì L, et al. Early awake proning in critical and severe COVID-19 patients undergoing noninvasive respiratory support: a retrospective multicenter cohort study. Pulmonology 2021; 110:697–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tu G-W, Liao Y-X, Li Q-Y, et al. Prone positioning in high-flow nasal cannula for COVID-19 patients with severe hypoxemia: a pilot study. Ann Transl Med 2020; 8:598–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xu Q, Wang T, Qin X, et al. Early awake prone position combined with high-flow nasal oxygen therapy in severe COVID-19: a case series. Crit Care 2020; 24:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zang X, Wang Q, Zhou H, et al. Efficacy of early prone position for COVID-19 patients with severe hypoxia: a single-center prospective cohort study. Intensive Care Med 2020; 46:1927–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124▪▪.Perez-Nieto OR, Escarraman-Martinez D, Guerrero-Gutierrez MA, et al. Awake prone positioning and oxygen therapy in patients with COVID-19: The APRONOX study. Eur Respir J 2021. 2100265. 10.1183/13993003.00265-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]; In this relatively big observational study the use of awake prone position reduced intubation and mortality rate at univariate, at multivariate and after propensity score matched analysis.
- 125.Jayakumar D, Ramachandran DNBP, Rabindrarajan DNBE, et al. Standard care versus awake prone position in adult nonintubated patients with acute hypoxemic respiratory failure secondary to COVID-19 Infection—a multicenter feasibility randomized controlled trial. J Intensive Care Med 2021; 36:918–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Johnson SA, Horton DJ, Fuller MJ, et al. Patient-directed prone positioning in awake patients with COVID-19 requiring hospitalization (PAPR). Ann Am Thorac Soc 2021; 18:1424–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127▪.Rosén J, von Oelreich E, Fors D, et al. Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: the PROFLO multicenter randomized clinical trial. Crit Care 2021; 25:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]; Randomized controlled trial comparing patients with acute hypoxemic respiratory failure treated with awake prone position, to patients treated with the standard of care. Due to cross-over and poor adherence to protocol no difference was detected among the two groups.
- 128▪▪.Ehrmann S, Li J, Ibarra-Estrada M, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: a randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med 2021. 2600. 10.1016/s2213-2600(21)00356-8 [DOI] [PMC free article] [PubMed] [Google Scholar]; In this randomized controlled trial awake prone position reduced the incidende of treatment failure in patients with hypoxemic respiratory failure of COVID-19 etiology treated with HFNO.
- 129.Pavlov I, He H, McNicholas B, et al. Awake prone positioning in nonintubated patients with acute hypoxemic respiratory failure due to COVID-19: A systematic review of proportional outcomes comparing observational studies with and without awake prone positioning in the setting of COVID-19. Respir Care 2021. 09191. 10.4187/respcare.09191 [DOI] [Google Scholar]