Abstract
OBJECTIVE:
To understand the relationship between cardiovascular disease (CVD) risk and frailty among men (MLWH) and women living with HIV (WLWH), or at risk for HIV.
DESIGN:
We considered 10-year coronary heart disease and atherosclerotic CVD risk by Framingham risk score (FRS, 2001 National Cholesterol Education Program Adult Treatment Program III) and Pooled Cohort Equations (PCE, 2013 American College of Cardiology/American Heart Association) in relation to the Fried Frailty Phenotype (FFP) in the Multicenter AIDS Cohort Study (MACS) and Women’s Interagency HIV Study (WIHS).
METHODS:
FFP was ascertained in MACS from 2004–2019 and in WIHS from 2005–2006 and 2011–2019. FFP score ≥3/5 components defined frailty. Repeated measures logistic regression (both cohorts) and Cox proportional hazards regression (MACS) were performed, controlled for education, income, cholesterol medication and hepatitis C virus serostatus, and among MLWH and WLWH, CD4 cell count/mL, antiretroviral therapy and HIV viral load.
RESULTS:
There were 5,554 participants (1,265 HIV seronegative [SN]/1,396 MLWH;768 SN/1,924 WLWH) included. Among men, high-risk FRS was associated with increased risk of incident frailty among SN (adjusted hazard ratio(aHR))=2.12, 95%CI:1.22–3.69) and MLWH (aHR=2.19, 95%CI:1.33–3.61). Similar associations were seen with high-risk PCE and incident frailty among SN (aHR=1.88, 95%CI:1.48–2.39) and MLWH (aHR=1.59, 95%CI:1.26–2.00). Among women, high-risk PCE was associated with frailty in SN (adjusted odds ratio(aOR)=1.43, 95%CI:1.02–2.00) and WLWH (aOR=1.36, 95%CI:1.08–1.71); however high-risk FRS was not (SN:aOR=1.03, 95%CI:0.30–3.49;WLWH:aOR=0.86, 95%CI:0.23–3.20).
CONCLUSIONS:
Higher CVD risk was associated with increased frailty regardless of HIV serostatus among men and women. These findings may inform clinical practices of screening for frailty.
Keywords: HIV, cardiovascular risk, frailty, men, women, cohort
INTRODUCTION
Frailty is characterized by physical weakness and a marked decline of physiologic reserve.[1] Higher prevalence of frailty has been reported among people living with human immunodeficiency virus (PLWH) compared to adults without HIV infection in the United States(US),[2–4] Europe,[5] China,[6] and Africa,[7] suggesting that PLWH worldwide are at increased risk for frailty. Risk factors common to cardiovascular disease (CVD) and frailty, such as older age, presence of comorbidities, smoking, physical inactivity, and poor nutrition exist, yet the relationship between CVD and frailty in PLWH is not well established.[8, 9] Cross-sectional research in the Multicenter AIDS Cohort Study (MACS) demonstrated that the Fried Frailty Phenotype (FFP) was associated with subclinical coronary atherosclerosis among HIV seronegative (SN) men, but not among men living with HIV (MLWH).[10] Cross-sectional data in the Women’s Interagency HIV Study (WIHS) showed that hypertension and cigarette smoking were associated with the FFP during middle age.[3] Some groups have suggested that frailty reflects the interaction between multiple morbidities and disability, and hence CVD contributes to frailty.[11] It may also be argued that frailty is a biologic syndrome distinct from either co-morbidities or disability. Notably, in the Cardiovascular Health Study, 7% of those who were frail according to FFP criteria had no chronic diseases.[1] Among persons without HIV, prevalence of frailty – defined by the FFP, the Frailty Index[12] or other frailty measures – was greater in those with versus without CVD.[13–16] Moreover, among persons without CVD, risk factors for CVD were associated with frailty.[15, 16]
CVD risk factors are used in clinical practice as part of CVD event risk prediction and to guide therapeutic decision making in adults, including PLWH.[17–23] However, traditional CVD risk prediction tools underperform among PLWH, and differ by sociodemographic and race/ethnicity factors.[18, 19, 22] The 2001 National Cholesterol Education Program Adult Treatment Program III (ATP-III)[24] and the 2013 American College of Cardiology/American Heart Association (ACC/AHA)[25] guidelines, with corresponding risk scores, have been widely used for primary prevention of CVD. These risk scores have important differences, however, since they were released a decade apart, there was an expansion of statin eligibility in the 2013 ACC/AHA guidelines compared to the 2001 ATP-III.[26] The ATP-III recommendations for primary prevention are based on the Framingham risk score (FRS) for coronary heart disease (CHD). The ATP-III FRS includes the components: sex, age, total cholesterol, high-density lipoprotein (HDL) cholesterol, smoking status, systolic blood pressure (SBP) and antihypertensive medication use.[24] The ACC/AHA recommendations are based on pooled cohort equations (PCE) for atherosclerotic cardiovascular disease (ASCVD) with components of race (White/African American) and diabetes, in addition to the components included in ATP-III FRS.[25]
Our objective was to consider ATP-III FRS and ACC/AHA PCE risk scores in association with frailty, defined using the FFP, among men and women enrolled in the MACS and WIHS. Understanding these associations could be important because the FFP requires assessment of physical performance (grip strength and walking speed) and may be ascertained less often than CVD risk in clinical practice. Notably, this paper is among the first to consider CVD risk profiles as a predictor of frailty among two large longitudinal cohorts of men and women living with or at risk for HIV who have been followed at 14 clinical sites across the US.
METHODS
Study Populations
The MACS and WIHS (now known as the MACS/WIHS Combined Cohort Study[27]) were prospective multi-center cohorts of US men and women living with or without HIV. Eligibility criteria, study protocols and follow-up procedures for the MACS and WIHS have been previously described.[28–32] Briefly, MACS participants were recruited at 4 sites across 3 time periods starting in 1984, and WIHS participants were recruited at 10 sites over 4 time periods starting in 1994. Data for both cohorts were collected using structured in-person interviews and standardized physical and laboratory assessments, with study visits occurring every six months. As part of a detailed medication history, participants self-reported all prescribed medications consumed, even transiently, since their last study visit. Institutional review boards at the respective clinical research centers approved the MACS and WIHS study protocols, and all participants provided written informed consent.
Participant selection and inclusion criteria
MACS and WIHS participant visits were selected for inclusion in these analyses if components of the FFP and ATP-III FRS were available at any baseline when measures were conducted among participants aged ≥20 years (Supplementary Figure 1). The ATP-III FRS is valid for adults age 20–79 years.[24] The subset of ATP-III participant visits where participants were aged 40–79 years were included in analyses of ACC/AHA PCE since that is the age group for which the ACC/AHA PCE is valid.[25] FFP characteristics in MACS were ascertained at all 4 MACS sites during 2004–2019. In contrast, frailty in WIHS was assessed at 6 sites at a single time point between 2005–2006,[3, 33] in a sub-group of 3 of the 6 sites during 2012–2014, and then annually at 9 sites during 2015–2019. All components of CVD risk scores were available across all study visits.
Outcome ascertainment: Fried Frailty Phenotype
Participants were classified as frail if they exhibited three or more of five FFP characteristics: 1) slow walking speed, 2) weakness (reduced grip strength), and self-reported 3) physical exhaustion, 4) low physical activity and 5) unintentional weight loss. Walking speed was measured using a 3 or 4 meter timed gait test, and impaired walking speed was defined as ≥80th percentile of SN participants, as in prior studies.[3, 10, 34] Grip strength was measured using a dominant hand-held dynamometer with maximum force; reduced grip strength was mean grip strength (3 trials) ≤20th percentile of SN participants. Physical exhaustion was defined as a “Yes” response to: “During the past four weeks, as a result of your physical health, have you had difficulty performing your work or other activities (for example, it took extra effort)?” Low physical activity was a “Yes” to “Does your health now limit you in vigorous activities, such as running, lifting heavy objects, or participating in strenuous sports?” Unintentional weight loss was a “Yes” to: “Since your last visit, have you had unintentional weight loss of at least 10 pounds?”
Predictor ascertainment: Cardiovascular risk score calculations
For each participant, we calculated ATP-III FRS[24] and ACC/AHA PCE[25] cardiovascular risk scores using: age, race (Black or African American vs. other race), hypertension treatment and smoking status (yes/no); and clinically-measured SBP (mmHg), diabetes (fasting glucose ≥126 mg/dl or taking diabetes medication at the visit), and total and HDL cholesterol measured on a Roche Modular automated system (Roche Diagnostics Corporation, Indianapolis, IN). For ATP-III FRS, 10-year risk of CHD was defined as high (>20%), moderate (10–20%), or low (<10%).[24] Persons with ACC/AHA PCE ≥7.5% were considered at high 10-year risk of ASCVD while those with ACC/AHA PCE <7.5% were at low 10-year risk of ASCVD.[25] These definitions of risk are consistent with each respective guideline.
Covariates
A limited set of covariates was selected a priori for inclusion: education (<high school,high school,>high school), income (WIHS:≤$6,000,$6,001-$18,000,≥$18,001;MACS:≤$20,000,$20,000-$39,999,≥$40,000), cholesterol medication usage and hepatitis C virus (HCV) serostatus. HCV was selected because it affects cholesterol metabolism in SN[35] and PLWH[36] and predicts CVD events in PLWH.[37] A non-invasive measure of liver fibrosis (FIB-4) was also associated with frailty in WIHS[3] and HCV is associated with immune dysregulation in PLWH[38, 39] which could contribute to frailty. Among PLWH-only, models also included CD4 T cell count/mL, use of antiretroviral therapy (ART), and HIV viral load below the lower limit of quantification (LLQ or suppressed HIV viral load). LLQs for WIHS were 80(2004–2006) and 20(2011–2019) copies/mL and for MACS were 50(2004–2010) and 20(2011–2019) copies/mL.
Statistical Methods
Statistical analyses were stratified by cohort due to differences in participant sociodemographic characteristics beyond sex, as well as differences in MACS and WIHS protocols, including assessment of income using different thresholds and frequency of functional measures over time. We defined an index study visit for each participant as the first ascertainment of both ATP-III FRS and physical performance. Bivariate comparisons of continuous variables at the index visit (i.e., cross-sectional analyses) were analyzed using the Wilcoxon rank sum test and categorical variables using Fisher’s exact test. In both MACS and WIHS, associations of cardiovascular risk scores as the predictors with frailty as the outcome, including both index and follow-up visits (i.e., longitudinal analyses) were estimated using repeated measures logistic regression models, with generalized estimating equations (GEE) with exchangeable covariance structures.
Among MACS participants only, associations of cardiovascular risk scores at the index visit with incident frailty among men who were not frail at the index visit were estimated using Kaplan-Meier curves, global log-rank tests and Cox proportional hazards regression models. These analyses were restricted to men with ≥2 follow-up visits after the index visit and the Cox models included time-updated covariate information. Proportional hazards assumptions were verified. We did not assess associations of cardiovascular risk with incident frailty in women because of gaps in physical performance measures over time. SAS version 9.4 (SAS Institute Inc, Cary, NC) was used for analyses.
RESULTS
Cardiovascular risk scores in men by frailty status at the index visit
The ATP-III FRS and FFP were ascertained at ≥1 MACS visit among 1,265 SN men and 1,396 men living with HIV(MLWH). The median date of the index visit was 02/14/2006(IQR:10/29/2005–08/10/2006) for SN men and 03/20/2006(IQR:12/19/2005–05/24/2011) for MLWH. The ACC/AHA PCE risk score was available for 1010(80%) SN and 997(71%) MLWH at the index visit.
Prevalence of frailty was 6.1% in SN men and 9.0% in MLWH at the index visit (Table 1). In both SN men and MLWH, ATP-III FRS 10-year risk was higher in frail (median (IQR): 10%(5–16) and 6%(3–12),respectively) vs. non-frail (median (IQR):6%(2–10) and 4%(1–8),respectively) men (both P<0.001–Table 1). Similarly, in SN men and MLWH, ACC/AHA PCE 10-year risk was higher in frail (median(IQR)):10%(7–19%) and 8%(4–14%),respectively) vs. non-frail (median(IQR):6%(4–11%) and 6%(3–9%),respectively) men(both P<0.001). When considered by strata of 10-year risk, both higher ATP-III FRS and ACC/AHA PCE were associated with frailty in SN men and MLWH(all P<0.001).
Table 1.
| HIV− (n=1,265) | HIV+ (n=1,396) | |||||
|---|---|---|---|---|---|---|
| Not frail (n=1187) | Frail (n=78) | P a | Not frail (n=1270) | Frail (n=126) | P a | |
| Cardiovascular risk scores, median (IQR) | ||||||
| ATP-III FRS, %b | 6 (2–10) | 10 (5–16) | <0.001 | 4 (1–8) | 6 (3–12) | <0.001 |
| ACC/AHA PCE, %b,c | 6 (4–11) | 10 (7–19) | <0.001 | 6 (3–9) | 8 (4–14) | <0.001 |
| Cardiovascular risk scores, strata | ||||||
| ATP-III FRS, %b | <0.001 | 0.001 | ||||
| Low risk (<10%) | 813 (68%) | 32 (41%) | 983 (77%) | 79 (63%) | ||
| Moderate risk (10–20%) | 344 (29%) | 40 (51%) | 259 (20%) | 42 (33%) | ||
| High risk (>20%) | 30 (3%) | 6 (8%) | 28 (2%) | 5 (4%) | ||
| ACC/AHA PCE, %b,c | <0.001 | <0.001 | ||||
| Low risk (<7.5%) | 539 (57%) | 19 (29%) | 568 (64%) | 48 (46%) | ||
| High risk (≥7.5%) | 406(43%) | 46 (71%) | 325 (36%) | 56 (54%) | ||
| Cardiovascular risk score components, median (IQR) | ||||||
| Age | 50 (42–57) | 54 (45–58) | 0.03 | 45 (38–51) | 50 (44–55) | <0.001 |
| Total cholesterol, mg/dL | 188 (165–216) | 186 (160–220) | 0.86 | 180 (153–208) | 167 (147–187) | 0.006 |
| HDL cholesterol, mg/dL | 49 (41–58) | 46 (39–55) | 0.10 | 43 (36–53) | 40 (35–50) | 0.09 |
| SBP, mmHg | 128(118–137) | 130 (121–140) | 0.09 | 126 (117–134) | 128 (115–138) | 0.58 |
| Cardiovascular risk score components, strata | ||||||
| Race (Black or African American) | 0.02 | 0.55 | ||||
| No | 929 (78%) | 52 (67%) | 848 (67%) | 88 (70%) | ||
| Yes | 258 (22%) | 26 (33%) | 422 (33%) | 38 (30%) | ||
| Current smoker | 0.003 | 0.03 | ||||
| No | 888 (75%) | 46 (59%) | 830 (65%) | 70 (56%) | ||
| Yes | 299 (25%) | 32 (41%) | 440 (35%) | 56 (44%) | ||
| Antihypertensive medication use | <0.001 | <0.001 | ||||
| No | 923 (78%) | 44 (56%) | 1011 (80%) | 75 (60%) | ||
| Yes | 264 (22%) | 34 (44%) | 259 (20%) | 51 (40%) | ||
| Diabetes | 0.001 | 0.001 | ||||
| No | 1105 (93%) | 64 (82%) | 1176 (93%) | 105 (83%) | ||
| Yes | 82 (7%) | 14 (18%) | 94 (7%) | 21 (17%) | ||
ACC/AHA PCE: American College of Cardiology/American Heart Association pooled cohort equations; ATP-III FRS: Adult Treatment Program III Framingham risk score; HIV: human immunodeficiency virus; IQR: interquartile range; MACS: Multicenter AIDS Cohort Study; SBP: systolic blood pressure
Comparisons of continuous variables by Wilcoxon rank sum and categorical variables by Fisher’s exact test
ATP-III FRS and ACC/AHA PCE are in men 20–79 and 40–79 years of age, respectively
ACC/AHA PCE were available for 1010 (80%) HIV− and 997 (71%) HIV+ men at the index visit
CVD risk score components associated with frailty in both SN men and MLWH included age, smoking, antihypertensive medication use, and diabetes(all P<0.05). In addition, among SN men, those who were frail were more likely to be Black or African American, while frail MLWH had lower total cholesterol(P=0.006).
Cardiovascular risk scores in women by frailty status at the index visit
The ATP-III FRS and FFP were ascertained at ≥1 WIHS visit among 768 SN women and 1,924 WLWH. Of these, 60%(n=460) of SN women and 62%(n=1,202) of WLWH had an index visit from 04/04/2005–03/28/2006 with the remainder having an index visit from 01/09/2012–03/13/2019. ACC/AHA PCE were available for 487(63%) SN and 1,416(74%) WLWH at the index visit (because ACC/AHA PCE are only valid for persons ≥40 years of age).
Prevalence of frailty was 10.1% in SN women and 13.3% in WLWH at the index visit (Table 2). ATP-III FRS 10-year risk was higher in frail than non-frail WLWH (median (interquartile range (IQR)):1%(1–3)vs1%(0–2);P<0.001–Table 2). Results for ATP-III FRS were similar for SN women (2%(1–4)vs1% (0–2);P<0.001). ACC/AHA PCE 10-year risk was also higher in frail than non-frail WLWH (median (IQR)):4%(2–9)vs.3%(1–6);P<0.001). Results for ACC/AHA PCE were similar for SN women (median (IQR)):5%(2–11)vs.3%(1–8);P=0.01). However, when considered by strata of 10-year risk, ACC/AHA PCE and not the ATP-III FRS was associated with frailty status in WLWH and HIV SN women(Table 2).
Table 2.
| HIV− (n=768) | HIV+ (n=1924) | |||||
|---|---|---|---|---|---|---|
| Not frail (n=690) | Frail (n=78) | P a | Not frail (n=1668) | Frail (n=256) | P a | |
| Cardiovascular risk scores, median (IQR) | ||||||
| ATP-III FRS, %b | 1 (0–2) | 2 ((1–4) | <0.001 | 1 (0–2) | 1 (0–3) | <0.001 |
| ACC/AHA PCE, %b,c | 3 (1–8) | 5 (2–11) | 0.011 | 3 (1–6) | 4 (2–9) | <0.001 |
| Cardiovascular risk scores, strata | ||||||
| ATP-III FRS, %b | 0.83 | 0.06 | ||||
| Low risk (<10%) | 671 (97%) | 76 (95%) | 1634 (98%) | 245 (96%) | ||
| Moderate risk (10–20%) | 13 (2%) | 1 (1%) | 31 (2%) | 11 (4%) | ||
| High risk (>20%) | 6 (1%) | 1 (1%) | 3 (0%) | 0 | ||
| ACC/AHA PCE, %b,c | 0.006 | <0.001 | ||||
| Low risk (<7.5%) | 307 (73%) | 39 (57%) | 956 (80%) | 156 (70%) | ||
| High risk (≥7.5%) | 111 (27%) | 30 (43%) | 236 (20%) | 68 (30%) | ||
| Cardiovascular risk score components, median (IQR) | ||||||
| Age | 42 (33–50) | 48 (42–53) | <0.001 | 44 (38–51) | 48 (43–54) | <0.001 |
| Total cholesterol, mg/dL | 177 (154–203) | 187 (161–208) | 0.25 | 178 (152–203) | 171 (145–201) | 0.08 |
| HDL cholesterol, mg/dL | 53 (44–64) | 54 (42–69) | 0.69 | 48 (39–61) | 47 (37–62) | 0.27 |
| SBP, mmHg | 118 (109–132) | 123 (112–139) | 0.03 | 117 (107–131) | 119 (106–135) | 0.28 |
| Cardiovascular risk score components, strata | ||||||
| Race (Black or African American) | 0.15 | 0.67 | ||||
| No | 193 (28%) | 28 (36%) | 553 (33%) | 81 (32%) | ||
| Yes | 497 (72%) | 50 (64%) | 1115 (67%) | 175 (68%) | ||
| Current smoker | 0.02 | <0.001 | ||||
| No | 351 (51%) | 29 (37%) | 979 (59%) | 118 (46%) | ||
| Yes | 339 (49%) | 49 (63%) | 689 (41%) | 138 (54%) | ||
| Antihypertensive medication use | <0.001 | <0.001 | ||||
| No | 531 (77%) | 38 (49%) | 1187 (71%) | 131 (51%) | ||
| Yes | 159 (23%) | 40 (51%) | 481 (29%) | 125 (49%) | ||
| Diabetes | <0.001 | 0.002 | ||||
| No | 579 (84%) | 46 (59%) | 1413 (85%) | 196 (77%) | ||
| Yes | 111 (16%) | 32 (41%) | 255 (15%) | 60 (23%) | ||
ACC/AHA PCE: American College of Cardiology/American Heart Association pooled cohort equations; ATP-III FRS: Adult Treatment Program III Framingham risk score; HIV: human immunodeficiency virus; IQR: interquartile range; SBP: systolic blood pressure; WIHS: Women’s Interagency HIV Study
Comparisons of continuous variables by Wilcoxon rank sum and categorical variables by Fisher’s exact test
ATP-III FRS and ACC/AHA PCE are in women 20–79 and 40–79 years of age, respectively
ACC/AHA PCE were available for 487 (63%) HIV− and 1,416 (74%) HIV+ women at the index visit
Some components of ATP-III FRS and ACC/AHA PCE differed by frailty status at the index visit. Women who were frail were older, more likely to smoke, use antihypertensive medication and be diabetic than women who were not frail, regardless of HIV serostatus(all P<0.05); no differences were observed by frailty status in total and HDL cholesterol. SBP was higher among frail versus non-frail SN women(P=0.03) but not in WLWH(P=0.28).
Repeated measures associations of cardiovascular risk scores with frailty in men
The 1,265 SN men had a median of 15(IQR:5–22) visits during follow-up with ascertainment of ATP-III FRS and FFP at 17,349 visits. The 1,396 MLWH at the index visit along with n=46 MLWH who seroconverted during follow-up, had a median of 11(IQR:5–20) visits with ascertainment of ATP-III FRS and FFP at 17,771 visits.
As both continuous measures and by risk strata, ATP-III FRS and ACC/AHA PCE were associated with frailty in both SN men and MLWH, in unadjusted and adjusted analyses(all P<0.001–Table 3). Odds ratios (ORs) for frailty were uniformly higher in SN men vs. MLWH. The odds of frailty associated with high risk(≥7.5%) ACC/AHA PCE were OR=1.98(95%CI:1.68,2.34;P<0.001) in SN men and OR=1.44(95%CI:1.26,1.64;P<0.001) in MLWH. There was significant interaction by HIV serostatus for the association of ACC/AHA PCE high risk(PInteraction=0.003) with frailty in adjusted analyses. In contrast, there was no interaction by HIV serostatus for the associations of ATP-III FRS moderate (PInteraction=0.15) and high(PInteraction=0.46) risk strata with frailty in adjusted analyses.
Table 3.
| HIV− (n=17,349 study visits; 1,518 frail visits) | HIV+ (n=17,771 study visits, 2,138 frail visits) | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P a | OR | 95% CI | P a | |
| Cardiovascular risk scores, continuous | ||||||
| ATP-III FRS, % (unadjusted) | 1.05 | 1.04, 1.06 | <0.001 | 1.04 | 1.03, 1.05 | <0.001 |
| ATP-III FRS, % (adjusted) | 1.05 | 1.04, 1.06 | <0.001 | 1.04 | 1.03, 1.05 | <0.001 |
| ACC/AHA PCE, % (unadjusted) | 1.04 | 1.04, 1.05 | <0.001 | 1.03 | 1.03, 1.04 | <0.001 |
| ACC/AHA PCE, % (adjusted) | 1.04 | 1.03, 1.05 | <0.001 | 1.03 | 1.02, 1.04 | <0.001 |
| Cardiovascular risk scores, strata | ||||||
| ATP-III FRS, % | ||||||
| Low risk (<10%) | Ref | Ref | ||||
| Moderate risk (10–20%) (unadjusted) | 1.53 | 1.35, 1.75 | <0.001 | 1.30 | 1.15, 1.47 | <0.001 |
| Moderate risk (10–20%) (adjusted) | 1.54 | 1.34, 1.77 | <0.001 | 1.29 | 1.14, 1.47 | <0.001 |
| High risk (>20%) (unadjusted) | 2.33 | 1.75, 3.10 | <0.001 | 2.00 | 1.58, 2.53 | <0.001 |
| High risk (>20%) (adjusted) | 2.31 | 1.74, 3.07 | <0.001 | 2.03 | 1.60, 2.58 | <0.001 |
| ACC/AHA PCE, % | ||||||
| Low risk (<7.5%) | Ref | Ref | ||||
| High risk (≥7.5%) (unadjusted) | 2.02 | 1.74, 2.34 | <0.001 | 1.44 | 1.27, 1.63 | <0.001 |
| High risk (≥7.5%) (adjusted)d | 1.98 | 1.68, 2.34 | <0.001 | 1.44 | 1.26, 1.64 | <0.001 |
ACC/AHA PCE: American College of Cardiology/American Heart Association pooled cohort equations; ATP-III FRS: Adult Treatment Program III Framingham risk score; CI: confidence interval; HIV: human immunodeficiency virus; MACS: Multicenter AIDS Cohort Study; OR: odds ratio;
Adjusted analyses include as covariates: education, income, cholesterol medication use, HCV serostatus, and in HIV+ participants, CD4 count, ART therapy and suppressed HIV viral load
ATP-III FRS and ACC/AHA PCE are in men 20–79 and 40–79 years of age, respectively
ACC/AHA PCE were available for 15,681 (90%) HIV− and 15,394 (87%) HIV+ study visits
PInteraction = 0.003 by HIV serostatus
Repeated measures associations of cardiovascular risk scores with frailty in women
The 768 SN women had a median of 2(IQR:1–3) visits during follow-up with ascertainment of ATP-III FRS and FFP at 1,718 visits. The 1,924 WLWH had a median of 2(IQR:1–3) visits during follow-up with ascertainment of ATP-III FRS and FFP at 4,546 visits.
As continuous measures, ATP-III FRS and ACC/AHA PCE risk scores were associated with frailty among SN women and WLWH in unadjusted analyses. Continuous ATP-III FRS and ACC/AHA PCE risk scores remained associated with frailty in adjusted analyses in WLWH and SN, respectively(both P<0.05–Table 4). However, moderate(10–20%) and high(>20%) risk strata of ATP-III FRS were not statistically associated with frailty in SN women or WLWH in either unadjusted or adjusted analyses (Table 4). In contrast, high (≥7.5%) risk ACC/AHA PCE was associated with frailty in both SN women and WLWH, in unadjusted and adjusted analyses – adjusted ORs were 1.43(95%CI:1.02,2.00;P=0.04) for SN women and 1.36(95%CI:1.08,1.71;P=0.01) for WLWH.
Table 4.
Repeated measures logistic regression of cardiovascular risk scores with frailty in WIHS women a,b,c
| HIV− (n=1,718 study visits, 185 frail visits) | HIV+ (n=4,546 study visits, 494 frail visits) | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P a | OR | 95% CI | P a | |
| Cardiovascular risk scores, continuous | ||||||
| ATP-III FRS, % (unadjusted) | 1.06 | 1.02, 1.10 | 0.003 | 1.05 | 1.02, 1.08 | <0.001 |
| ATP-III FRS, % (adjusted) | 1.02 | 0.98, 1.07 | 0.32 | 1.04 | 1.01, 1.07 | 0.009 |
| ACC/AHA PCE, % (unadjusted) | 1.03 | 1.02, 1.04 | <0.001 | 1.02 | 1.01, 1.03 | <0.001 |
| ACC/AHA PCE, % (adjusted) | 1.02 | 1.00, 1.03 | 0.01 | 1.01 | 1.00, 1.02 | 0.07 |
| Cardiovascular risk scores, strata | ||||||
| ATP-III FRS, % | ||||||
| Low risk (<10%) | Ref | Ref | ||||
| Moderate risk (10–20%) (unadjusted) | 1.19 | 0.46, 3.05 | 0.72 | 1.23 | 0.76, 2.00 | 0.40 |
| Moderate risk (10–20%) (adjusted) | 0.83 | 0.33, 2.09 | 0.69 | 1.11 | 0.68, 1.81 | 0.67 |
| High risk (>20%) (unadjusted) | 1.65 | 0.48, 5.62 | 0.42 | 0.78 | 0.19, 3.24 | 0.73 |
| High risk (>20%)(adjusted) | 1.03 | 0.30, 3.49 | 0.96 | 0.86 | 0.23, 3.20 | 0.82 |
| ACC/AHA PCE, % | ||||||
| Low risk (<7.5%) | Ref | Ref | ||||
| High risk (≥7.5%) (unadjusted) | 1.77 | 1.29, 2.43 | <0.001 | 1.52 | 1.22, 1.90 | <0.001 |
| High risk (≥7.5%) (adjusted) | 1.43 | 1.02, 2.00 | 0. 04 | 1.36 | 1.08, 1.71 | 0.01 |
ACC/AHA PCE: American College of Cardiology/American Heart Association pooled cohort equations; ATP-III FRS: Adult Treatment Program III Framingham risk score; CI: confidence interval; HIV: human immunodeficiency virus; OR: odds ratio; WIHS: Women’s Interagency HIV Study
Adjusted analyses include as covariates: education, income, cholesterol medication use, HCV serostatus, and in HIV+ participants, CD4 count, ART therapy and suppressed HIV viral load
ATP-III FRS and ACC/AHA PCE are in women 20–79 and 40–79 years of age, respectively
ACC/AHA PCE were available for 1,432 (83%) HIV− and 4,029 (89%) HIV+ study visits
Cardiovascular risk score associations with incident frailty in men
The 1,072 SN men without frailty at the index visit and with ≥2 follow-up visits after the index visit had a median follow-up time of 9.9 years(IQR:4.5–12.8), with incident frailty in 318. The 1,119 MLWH without frailty at the index visit and with ≥2 follow-up visits after the index visit had a median follow-up time of 6.5 years(IQR:3.5–12.2), with incident frailty in 391. Kaplan-Meier curves of frailty-free survival in SN men and MWLH by ATP-III FRS and ACC/AHA PCE risk strata are shown in Figure 1, panels A-D. Incident frailty differed by ATP-III FRS and ACC/AHA PCE risk strata in SN men and MWLH using global log-rank tests(Figure 1).
Figure 1.
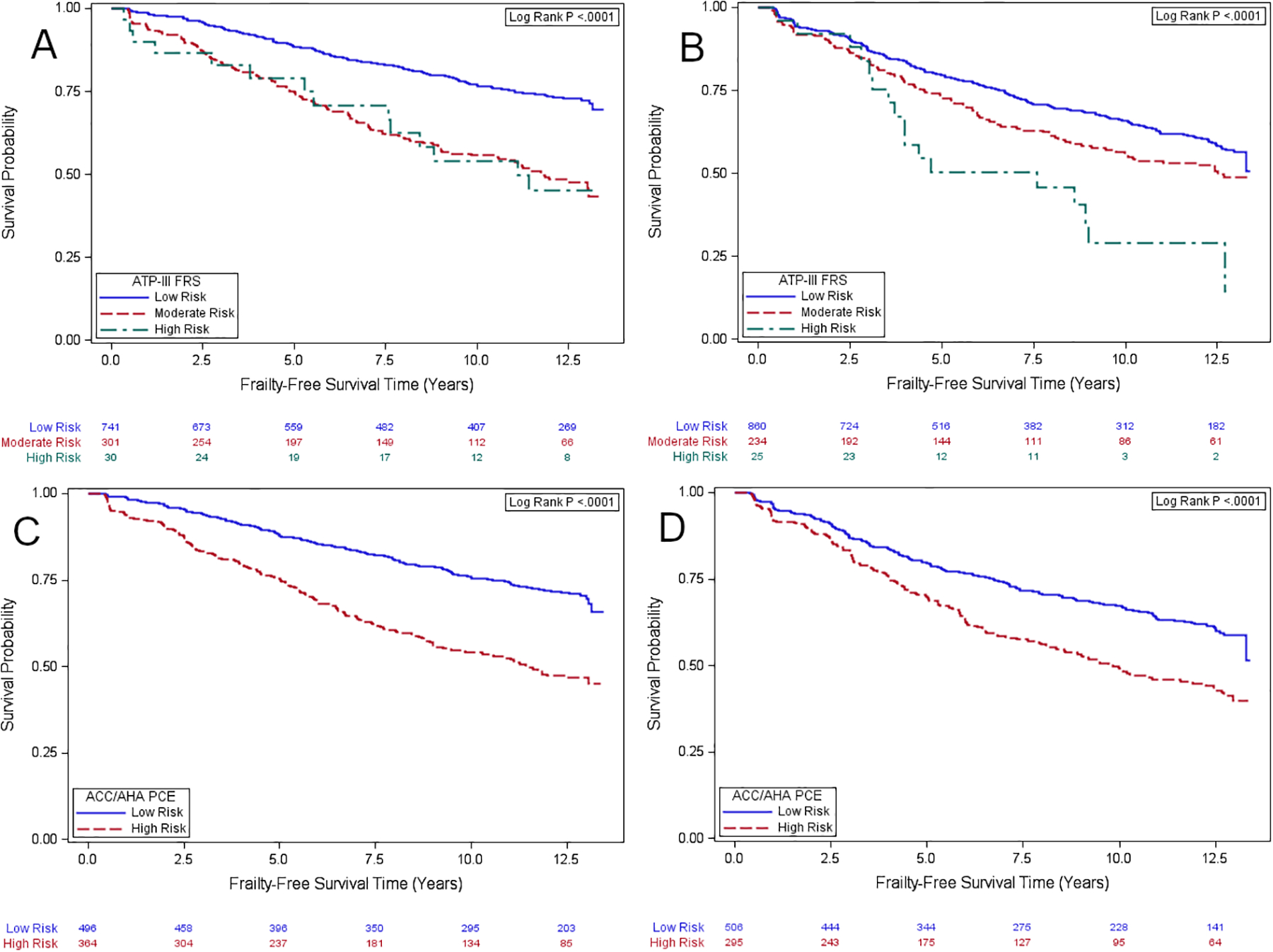
Kaplan-Meier curves for frailty-free survival: (A) HIV(−) men by ATP-III FRS risk; (B) HIV(+) men by ATP-III FRS risk; (C) HIV(−) men by ACC/AHA PCE risk; (D) HIV(+) men by ACC/AHA PCE risk. For ATP-III FRS, individuals with >20% estimated risk of CHD within 10 years are high risk; persons with 10–20% risk are moderate risk, and persons with <10% risk are low risk. Individuals with ACC/AHA PCE ≥7.5% have high 10-year risk of ASCVD while those with ACC/AHA PCE <7.5% have low 10-year risk of ASCVD
As continuous measures, ATP-III FRS and ACC/AHA PCE at the index visit were associated with incident frailty among both HIV serostatus groups, in unadjusted and adjusted analyses(all P<0.001–Supplementary Table 1). ATP-III FRS and ACC/AHA PCE risk strata were also associated with incident frailty among these groups in unadjusted analyses (Supplementary Table 1). In adjusted analyses, moderate risk ATP-III FRS(10–20%) status at index visit was associated with incident frailty in SN men(hazard ratio(HR)=2.09,95%CI:1.65, 2.64;P<0.001) but not among MLWH(HR=1.28,95%CI:1.00,1.63;P=0.05)). High risk(>20%) ATP-III FRS status was associated with incident frailty in both SN men(HR=2.12,95%CI:1.22,3.69;P=0.008) and MLWH(HR=2.19,95%CI:1.33,3.61;P=0.002) in adjusted analysis. Similarly, high risk(≥7.5%) ACC/AHA PCE status at index visit was associated with incident frailty in SN men(HR=1.88,95%CI:1.48,2.39;P<0.001) and MLWH(HR=1.59,95%CI:1.26,2.00;P<0.001) in adjusted analyses(Supplementary Table 1). While unadjusted and adjusted HRs for incident frailty were sometimes higher in SN men compared to MLWH, there was no interaction by HIV serostatus for associations of moderate and high risk ATP-III FRS(PInteraction=0.17 and 0.37,respectively) or high risk ACC/AHA PCE(PInteraction=0.68) in adjusted analyses.
DISCUSSION
Associations of individual CVD risk factors including hypertension, diabetes, smoking and abdominal obesity have been observed with prevalent and/or incident frailty in middle-aged and older PLWH and SN adults.[3, 16, 34, 40–42] Few studies, however, have considered CVD risk scores as used in clinical practice in relation to frailty.[43] We observed that high 10-year CVD risk as defined by 2013 ACC/AHA guidelines was positively associated with frailty among both men and women regardless of HIV serostatus. In contrast, high CHD risk as defined by 2001 ATP-III guidelines was positively associated with frailty among men only. These findings are important because frailty is common in middle-aged populations of PLWH like MACS and WIHS in the US, and among some middle-aged PLWH living in low- and middle-income countries.[7]
ATP-III FRS risk scores were low (medians:1%−2%) at the index visit among women regardless of HIV serotatus. Age is included not only as a component of the ATP-III FRS risk score equations but also serves as a modifier of the contribution of smoking and total cholesterol.[24] It is possible that the relatively young age of WIHS women with and without frailty (median ages:42–48 years) contributed to these low FRS risk scores. Age is included differently in ACC/AHA PCE risk score calculations,[25] as PCE predicts both CHD and stroke. This could explain why ACC/AHA PCE risk scores were higher (medians:3%−5%) than those of ATP-III FRS at the WIHS index visit. However, in a sample comprised of predominantly male Dutch PLWH, ATP-III FRS risk scores were higher than ACC/AHA PCE risk scores,[18] while among MACS SN men the two algorithms yielded similar CVD risk. Another difference between ATP-III FRS and ACC/AHA PCE is inclusion of African American race in ACC/AHA PCE equations – WIHS women are mostly African American whereas MACS includes majority White men.
Associations of ATP-III FRS and ACC/AHA PCE risk scores with frailty were stronger among the SN men as compared to MLWH, similar to data from a MACS study in which FFP was associated with subclinical coronary atherosclerosis among SN men but not MLWH.[10] One hypothesis to explain these observations is that a greater proportion of the variance in frailty is explained by CVD in SN men. HIV infection, including in MLWH who consume ART with 100% adherence, may be associated with immune activation and systemic inflammation,[44, 45] which may also contribute to frailty, but to a lesser extent than CVD. Regardless, our findings are consistent with the concept that CVD contributes to frailty.[11] For example, among participants in the AIDS Clinical Trials Group A5322 HIV Infection, Aging, Immune Function Long-Term Observational Study, frailty at baseline was associated with incident CVD with incidence rate ratio of 3.83(95%CI:1.59–9.23) over a median of 4.0 years of follow-up.[46]
This study has a number of strengths including clinically standardized frailty measurements and CVD risk factors, every six month follow-up, multi-site representation across the US, and inclusion of control groups of both women and men living without HIV. These strengths notwithstanding, limitations should be considered in interpretating our findings. First, there is no consensus on a single definition of frailty. The FFP reflects a physical phenotype whereas other measures are based on accumulation of deficits or other frameworks.[47, 48] Moreover, optimal cut-points and algorithms to define ‘weakness’ (e.g., grip/body mass index, grip/body weight) and ‘slowness’ (e.g., walking speed/body height) in relation to the frailty outcome are an area of active investigation.[49, 50] Different methods to define the FFP components might lead to different findings as compared to FFP based on average grip and walk speed and comparisons by HIV serostatus, as in this investigation and prior studies.[3, 10, 34] Second, while ATP-III FRS and 2013 ACC/AHA PCE have been widely used in clinical practice, they represent two of several validated CVD risk scores (e.g.,FRS ASCVD,[51] 2019 ACC/AHA PCE,[52] Systematic Coronary Risk Evaluation(SCORE),[53] and Data collection on Adverse Effects of Anti-HIV Drugs Study(D:A:D)[54]). Different CVD risk equations may have different associations with frailty as for CVD events[22, 23] and subclinical CVD[20] which can vary by race/ethnicity.[19] Notably, the 2019 ACC/AHA guideline uses the same PCE as the 2013 ACC/AHA guideline. However, ≥7.5% 10-year ASCVD risk is considered high risk in the 2013 guideline but ≥7.5% to <20% is considered intermediate risk in the 2019 guideline, and HIV infection is considered a risk enhancing factor that would favor initiation of statin therapy particularly in borderline (5% to <7.5%) and intermediate risk groups.[52] Finally, our study of frailty did not exclude participants who might have been excluded in a study of cardiovascular endpoints – those with self-reported CVD (myocardial infarction or heart attack, revascularization or angioplasty, transient ischemic attack/stroke, angina or hospitalization for heart condition).
In conclusion, these data suggest that CVD risk prediction tools may be valuable in clinical practice to identify PLWH and SN adults who may benefit from frailty phenotyping to reduce consequences of frailty onset including falls, hospitalization, disability, and mortality. Unfortunately, one of the reasons that frailty is not assessed more often clinically is lack of consensus around a single reliable metric. Frailty screening under accumulation of deficits models may be well-suited to screening patients in intensive care units.[55] For example, 234,568 critically-ill patients were screened under a single protocol.[56] Protocols for physical frailty assessment have included hospitalized patients as well as community-dwelling participants, those living in long term care/assisted living facilities and out-patients.[57] It may be that optimal frailty screening strategies will differ according to the population to be screened. Ultimately implementation science strategies are needed to assess if, how and when CVD risk assessment should be part of best practices for frailty identification and intervention in diverse settings among men and women with or at risk for HIV.
Supplementary Material
ACKNOWLEDGEMENTS:
The authors gratefully acknowledge the contributions of the study participants and dedication of the staff at the MWCCS sites.
SOURCES OF SUPPORT
The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH). MWCCS (Principal Investigators): Atlanta CRS (Ighovwerha Ofotokun, Anandi Sheth, and Gina Wingood), U01-HL146241; Baltimore CRS (Todd Brown and Joseph Margolick), U01-HL146201; Bronx CRS (Kathryn Anastos and Anjali Sharma), U01-HL146204; Brooklyn CRS (Deborah Gustafson and Tracey Wilson), U01-HL146202; Data Analysis and Coordination Center (Gypsyamber D’Souza, Stephen Gange and Elizabeth Golub), U01-HL146193; Chicago-Cook County CRS (Mardge Cohen and Audrey French), U01-HL146245; Chicago-Northwestern CRS (Steven Wolinsky), U01-HL146240; Northern California CRS (Bradley Aouizerat, Jennifer Price, and Phyllis Tien), U01-HL146242; Los Angeles CRS (Roger Detels and Matthew Mimiaga), U01-HL146333; Metropolitan Washington CRS (Seble Kassaye and Daniel Merenstein), U01-HL146205; Miami CRS (Maria Alcaide, Margaret Fischl, and Deborah Jones), U01-HL146203; Pittsburgh CRS (Jeremy Martinson and Charles Rinaldo), U01-HL146208; UAB-MS CRS (Mirjam-Colette Kempf, Jodie Dionne-Odom, and Deborah Konkle-Parker), U01-HL146192; UNC CRS (Adaora Adimora), U01-HL146194. The MWCCS is funded primarily by the National Heart, Lung, and Blood Institute (NHLBI), with additional co-funding from the Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Allergy And Infectious Diseases (NIAID), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Mental Health (NIMH), National Institute On Drug Abuse (NIDA), National Institute Of Nursing Research (NINR), National Cancer Institute (NCI), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institute on Minority Health and Health Disparities (NIMHD), and in coordination and alignment with the research priorities of the National Institutes of Health, Office of AIDS Research (OAR). MWCCS data collection is also supported by UL1-TR000004 (UCSF CTSA), UL1-TR003098 (JHU ICTR), UL1-TR001881 (UCLA CTSI), P30-AI-050409 (Atlanta CFAR), P30-AI-073961 (Miami CFAR), P30-AI-050410 (UNC CFAR), P30-AI-027767 (UAB CFAR), and P30-MH-116867 (Miami CHARM).
CONFLICTS OF INTEREST:
MHK reports consulting for Sanofi. PCT received grant support from Merck. AS received grant support from Gilead Sciences. FJP has received support from Gilead Sciences, Janssen, ViiV and Merck. AAAdimora has received personal funds from Merck and Gilead; Merck and Gilead have also provided her institution with funding for her research. All other authors declare no conflicts.
REFERENCES
- 1.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001; 56:M146–156. [DOI] [PubMed] [Google Scholar]
- 2.Desquilbet L, Jacobson LP, Fried LP, Phair JP, Jamieson BD, Holloway M, et al. HIV-1 infection is associated with an earlier occurrence of a phenotype related to frailty. J Gerontol A Biol Sci Med Sci 2007; 62:1279–1286. [DOI] [PubMed] [Google Scholar]
- 3.Gustafson DR, Shi Q, Thurn M, Holman S, Minkoff H, Cohen M, et al. Frailty and Constellations of Factors in Aging HIV-infected and Uninfected Women--The Women’s Interagency HIV Study. J Frailty Aging 2016; 5:43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piggott DA, Muzaale AD, Mehta SH, Brown TT, Patel KV, Leng SX, et al. Frailty, HIV infection, and mortality in an aging cohort of injection drug users. PLoS One 2013; 8:e54910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kooij KW, Wit FW, Schouten J, van der Valk M, Godfried MH, Stolte IG, et al. HIV infection is independently associated with frailty in middle-aged HIV type 1-infected individuals compared with similar but uninfected controls. AIDS 2016; 30:241–250. [DOI] [PubMed] [Google Scholar]
- 6.Ding Y, Lin H, Liu X, Wong FY, Sun YV, Marconi VC, et al. Higher Prevalence of Frailty Among a Sample of HIV-Infected Middle-aged and Older Chinese Adults Is Associated With Neurocognitive Impairment and Depressive Symptoms. J Infect Dis 2017; 215:687–692. [DOI] [PubMed] [Google Scholar]
- 7.Pathai S, Gilbert C, Weiss HA, Cook C, Wood R, Bekker LG, et al. Frailty in HIV-infected adults in South Africa. J Acquir Immune Defic Syndr 2013; 62:43–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stewart R. Cardiovascular Disease and Frailty: What Are the Mechanistic Links? Clin Chem 2019; 65:80–86. [DOI] [PubMed] [Google Scholar]
- 9.Ferrucci L, Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 2018; 15:505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Korada SKC, Zhao D, Tibuakuu M, Brown TT, Jacobson LP, Guallar E, et al. Frailty and subclinical coronary atherosclerosis: The Multicenter AIDS Cohort Study (MACS). Atherosclerosis 2017; 266:240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guaraldi G, Raggi P. Atherosclerosis in frailty: Not frailty in atherosclerosis. Atherosclerosis 2017; 266:226–227. [DOI] [PubMed] [Google Scholar]
- 12.Searle SD, Mitnitski A, Gahbauer EA, Gill TM, Rockwood K. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Afilalo J, Karunananthan S, Eisenberg MJ, Alexander KP, Bergman H. Role of frailty in patients with cardiovascular disease. Am J Cardiol 2009; 103:1616–1621. [DOI] [PubMed] [Google Scholar]
- 14.Marengoni A, Zucchelli A, Vetrano DL, Aloisi G, Brandi V, Ciutan M, et al. Heart failure, frailty, and pre-frailty: A systematic review and meta-analysis of observational studies. Int J Cardiol 2020; 316:10. [DOI] [PubMed] [Google Scholar]
- 15.Newman AB, Gottdiener JS, McBurnie MA, Hirsch CH, Kop WJ, Tracy R, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci 2001; 56:M158–166. [DOI] [PubMed] [Google Scholar]
- 16.Wong TY, Massa MS, O’Halloran AM, Kenny RA, Clarke R. Cardiovascular risk factors and frailty in a cross-sectional study of older people: implications for prevention. Age Ageing 2018; 47:714–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Agostino RB Sr. Cardiovascular risk estimation in 2012: lessons learned and applicability to the HIV population. J Infect Dis 2012; 205 Suppl 3:S362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krikke M, Hoogeveen RC, Hoepelman AI, Visseren FL, Arends JE. Cardiovascular risk prediction in HIV-infected patients: comparing the Framingham, atherosclerotic cardiovascular disease risk score (ASCVD), Systematic Coronary Risk Evaluation for the Netherlands (SCORE-NL) and Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) risk prediction models. HIV Med 2016; 17:289–297. [DOI] [PubMed] [Google Scholar]
- 19.Liang Y, Ketchum NS, Turner BJ, Flores J, Bullock D, Villarreal R, et al. Cardiovascular Risk Assessment Varies Widely by Calculator and Race/Ethnicity in a Majority Latinx Cohort Living with HIV. J Immigr Minor Health 2020; 22:323–335. [DOI] [PubMed] [Google Scholar]
- 20.Monroe AK, Haberlen SA, Post WS, Palella FJ Jr., Kinsgley LA, Witt MD, et al. Cardiovascular disease risk scores’ relationship to subclinical cardiovascular disease among HIV-infected and HIV-uninfected men. AIDS 2016; 30:2075–2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosepele M, Regan S, Massaro J, Meigs JB, Zanni MV, D’Agostino RB Sr., et al. Impact of the American College of Cardiology/American Heart Association Cholesterol Guidelines on Statin Eligibility Among Human Immunodeficiency Virus-Infected Individuals. Open Forum Infect Dis 2018; 5:ofy326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson-Paul AM, Lichtenstein KA, Armon C, Palella FJ Jr., Skarbinski J, Chmiel JS, et al. Cardiovascular Disease Risk Prediction in the HIV Outpatient Study. Clin Infect Dis 2016; 63:1508–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Triant VA, Perez J, Regan S, Massaro JM, Meigs JB, Grinspoon SK, et al. Cardiovascular Risk Prediction Functions Underestimate Risk in HIV Infection. Circulation 2018; 137:2203–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Expert Panel on Detection E, Treatment of High Blood Cholesterol in A. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA 2001; 285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 25.Goff DC Jr., Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014; 129:S49–73. [DOI] [PubMed] [Google Scholar]
- 26.Pencina MJ, Navar-Boggan AM, D’Agostino RB Sr., Williams K, Neely B, Sniderman AD, et al. Application of new cholesterol guidelines to a population-based sample. N Engl J Med 2014; 370:1422–1431. [DOI] [PubMed] [Google Scholar]
- 27.D’Souza G, Bhondoekhan F, Benning L, Margolick JB, Adedimeji AA, Adimora AA, et al. Characteristics Of The Macs-Wihs Combined Cohort Study: Opportunities For Research On Aging With Hiv In The Longest Us Observational Study Of HIV. Am J Epidemiol 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adimora AA, Ramirez C, Benning L, Greenblatt RM, Kempf MC, Tien PC, et al. Cohort Profile: The Women’s Interagency HIV Study (WIHS). Int J Epidemiol 2018; 47:393–394i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bacon MC, von Wyl V, Alden C, Sharp G, Robison E, Hessol N, et al. The Women’s Interagency HIV Study: an observational cohort brings clinical sciences to the bench. Clin Diagn Lab Immunol 2005; 12:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barkan SE, Melnick SL, Preston-Martin S, Weber K, Kalish LA, Miotti P, et al. The Women’s Interagency HIV Study. WIHS Collaborative Study Group. Epidemiology 1998; 9:117–125. [PubMed] [Google Scholar]
- 31.Becker JT, Kingsley LA, Molsberry S, Reynolds S, Aronow A, Levine AJ, et al. Cohort Profile: Recruitment cohorts in the neuropsychological substudy of the Multicenter AIDS Cohort Study. Int J Epidemiol 2015; 44:1506–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaslow RA, Ostrow DG, Detels R, Phair JP, Polk BF, Rinaldo CR Jr. The Multicenter AIDS Cohort Study: rationale, organization, and selected characteristics of the participants. Am J Epidemiol 1987; 126:310–318. [DOI] [PubMed] [Google Scholar]
- 33.Terzian AS, Holman S, Nathwani N, Robison E, Weber K, Young M, et al. Factors associated with preclinical disability and frailty among HIV-infected and HIV-uninfected women in the era of cART. J Womens Health (Larchmt) 2009; 18:1965–1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Althoff KN, Jacobson LP, Cranston RD, Detels R, Phair JP, Li X, et al. Age, comorbidities, and AIDS predict a frailty phenotype in men who have sex with men. J Gerontol A Biol Sci Med Sci 2014; 69:189–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butt AA, Yan P, Simon TG, Chung RT, Abou-Samra AB, team Es. Changes in circulating lipids level over time after acquiring HCV infection: results from ERCHIVES. BMC Infect Dis 2015; 15:510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuniholm MH, Liang H, Anastos K, Gustafson D, Kassaye S, Nowicki M, et al. Association of a 3’ untranslated region polymorphism in proprotein convertase subtilisin/kexin type 9 with HIV viral load and CD4+ levels in HIV/hepatitis C virus coinfected women. AIDS 2017; 31:2483–2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Osibogun O, Ogunmoroti O, Michos ED, Spatz ES, Olubajo B, Nasir K, et al. HIV/HCV coinfection and the risk of cardiovascular disease: A meta-analysis. J Viral Hepat 2017; 24:998–1004. [DOI] [PubMed] [Google Scholar]
- 38.Kuniholm MH, O’Brien TR, Prokunina-Olsson L, Augenbraun M, Plankey M, Karim R, et al. Association of Hepatitis C Virus Infection With CD4/CD8 Ratio in HIV-Positive Women. J Acquir Immune Defic Syndr 2016; 72:162–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuniholm MH, Xie X, Anastos K, Kaplan RC, Xue X, Kovacs A, et al. Association of chronic hepatitis C infection with T-cell phenotypes in HIV-negative and HIV-positive women. J Acquir Immune Defic Syndr 2014; 67:295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brothers TD, Kirkland S, Theou O, Zona S, Malagoli A, Wallace LMK, et al. Predictors of transitions in frailty severity and mortality among people aging with HIV. PLoS One 2017; 12:e0185352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hawkins KL, Zhang L, Ng DK, Althoff KN, Palella FJ Jr., Kingsley LA, et al. Abdominal obesity, sarcopenia, and osteoporosis are associated with frailty in men living with and without HIV. AIDS 2018; 32:1257–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Umbleja T, Brown TT, Overton ET, Ribaudo HJ, Schrack JA, Fitch KV, et al. Physical Function Impairment and Frailty in Middle-Aged People Living With Human Immunodeficiency Virus in the REPRIEVE Trial Ancillary Study PREPARE. J Infect Dis 2020; 222:S52–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gale CR, Cooper C, Sayer AA. Framingham cardiovascular disease risk scores and incident frailty: the English longitudinal study of ageing. Age (Dordr) 2014; 36:9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castillo-Mancilla JR, Brown TT, Palella FJ Jr., Macatangay BJC, Breen EC, Jacobson LP, et al. Partial Normalization of Biomarkers of Inflammation and Immune Activation Among Virally Suppressed Men With HIV Infection and High ART Adherence. Open Forum Infect Dis 2020; 7:ofaa099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wada NI, Jacobson LP, Margolick JB, Breen EC, Macatangay B, Penugonda S, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kelly SG, Wu K, Tassiopoulos K, Erlandson KM, Koletar SL, Palella FJ. Frailty Is an Independent Risk Factor for Mortality, Cardiovascular Disease, Bone Disease, and Diabetes Among Aging Adults With Human Immunodeficiency Virus. Clin Infect Dis 2019; 69:1370–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dent E, Kowal P, Hoogendijk EO. Frailty measurement in research and clinical practice: A review. Eur J Intern Med 2016; 31:3–10. [DOI] [PubMed] [Google Scholar]
- 48.Martin FC, O’Halloran AM. Tools for Assessing Frailty in Older People: General Concepts. Adv Exp Med Biol 2020; 1216:9–19. [DOI] [PubMed] [Google Scholar]
- 49.Cawthon PM, Manini T, Patel SM, Newman A, Travison T, Kiel DP, et al. Putative Cut-Points in Sarcopenia Components and Incident Adverse Health Outcomes: An SDOC Analysis. J Am Geriatr Soc 2020; 68:1429–1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Erlandson KM, Travison TG, Zhu H, Magaziner J, Correa-de-Araujo R, Cawthon PM, et al. Application of Selected Muscle Strength and Body Mass Cut Points for the Diagnosis of Sarcopenia in Men and Women With or at Risk for HIV Infection. J Gerontol A Biol Sci Med Sci 2020; 75:1338–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Agostino RB Sr., Vasan RS, Pencina MJ, Wolf PA, Cobain M, Massaro JM, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation 2008; 117:743–753. [DOI] [PubMed] [Google Scholar]
- 52.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019; 140:e596–e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Conroy RM, Pyorala K, Fitzgerald AP, Sans S, Menotti A, De Backer G, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J 2003; 24:987–1003. [DOI] [PubMed] [Google Scholar]
- 54.Friis-Moller N, Thiebaut R, Reiss P, Weber R, Monforte AD, De Wit S, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil 2010; 17:491–501. [DOI] [PubMed] [Google Scholar]
- 55.De Biasio JC, Mittel AM, Mueller AL, Ferrante LE, Kim DH, Shaefi S. Frailty in Critical Care Medicine: A Review. Anesth Analg 2020; 130:1462–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Darvall JN, Bellomo R, Paul E, Bailey M, Young PJ, Reid A, et al. Routine Frailty Screening in Critical Illness: A Population-Based Cohort Study in Australia and New Zealand. Chest 2021. [DOI] [PubMed] [Google Scholar]
- 57.Theou O, Cann L, Blodgett J, Wallace LM, Brothers TD, Rockwood K. Modifications to the frailty phenotype criteria: Systematic review of the current literature and investigation of 262 frailty phenotypes in the Survey of Health, Ageing, and Retirement in Europe. Ageing Res Rev 2015; 21:78–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


