Disruptions in the delivery of oxygen and glucose impair the function of neural circuits, with lethal consequences commonly observed in stroke and cardiac arrest. Intense focus has been placed on understanding how to overcome neuronal failure during energy stress. Important insights into neuroprotective strategies have come from evolutionary adaptations for survival in hypoxic environments, such as those seen in turtles, naked mole-rats, and several other animals 1. Amphibians are not usually numbered among champion hypoxia-tolerant vertebrates, yet we demonstrate a massive conditional increase in the capacity of a neural circuit to produce activity following oxygen and glucose deprivation in adult bullfrogs. Rhythmic output from a brainstem circuit fails following minutes of severe hypoxia and simulated ischemia; however, after hibernation this network produces patterned activity for ~3.5 hrs during severe hypoxia and ~2 hrs in ischemia. This remarkable improvement was supported by a switch to brain glycogen to fuel anaerobic glycolysis, a pathway thought to support neuronal homeostasis for only a few minutes during ischemia 2. These results reveal that circuit activity can exhibit dramatic metabolic plasticity that minimizes the need for ATP synthesis and represent the greatest range in hypoxia tolerance within a vertebrate neural network. Uncovering the rules that allow the brain to flexibly run only on endogenous fuel reserves will reveal new insights into brain energetics, circuit evolution, and neuroprotection.
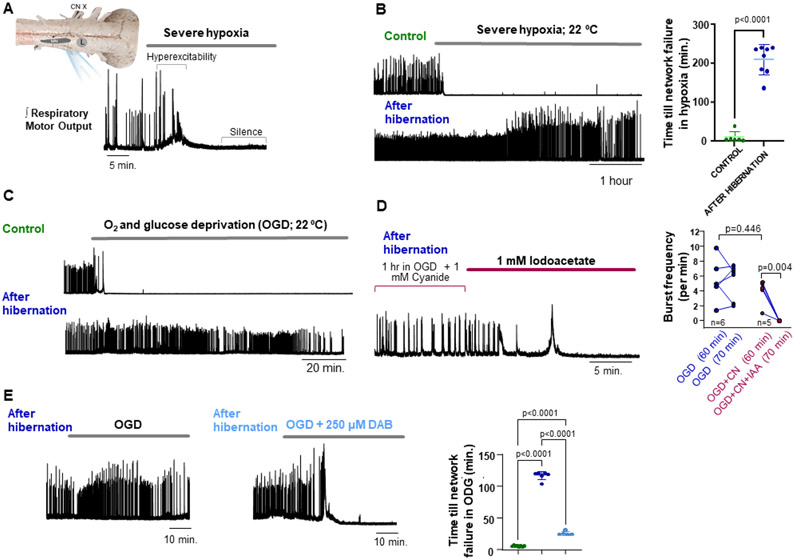
To address the response of a complete motor circuit to reduced ATP turnover, we bathed the brainstem respiratory network from American bullfrogs to severe hypoxia (~2% O2). Severe hypoxia typically induced hyperexcitability followed by silence within a few minutes (Figure 1A), a response outwardly similar to mammalian networks with high energetic demands 3. In contrast, under the same experimental setting, animals removed from a simulated overwintering environment produced network activity for an average of 210 minutes (range 137-≥240 min) (Figure 1B; Figure S1A). These results led us to test if networks transform to resist oxygen and glucose deprivation (OGD), an in vitro ischemia mimetic. During OGD, control preparations operated for~4 minutes, while hibernators continued for ~116 minutes (range 103-120), a ~30-fold increase in time until cessation of electrical activity (Figure 1C; Figure S1B). These data show the frog brainstem exhibits metabolic plasticity that dramatically prolongs function without oxygen or glucose delivery.
Figure 1. Dramatic improvement in circuit function during severe hypoxia (2% O2) and O2-glucose deprivation (OGD) after hibernation.
(A) Simplified schematic of the in vitro bullfrog brainstem preparation and activity produced by the respiratory network that generates breathing. Motor output is produced by a lung breathing rhythm generating network (L) that drives motor neuron discharge (MN; cranial nerve X recorded here). Integrated neurogram showing the typical response to severe hypoxia, involving hyperexcitability and shortly after, network silence. (B) Example integrated neurograms and mean time until silence of network output, illustrating the striking increase in duration of respiratory motor function during severe hypoxia following hibernation (control: n=6, hibernation: n=8). (C) Example oxygen and glucose deprivation (OGD) response before and following hibernation. (D) Example neurogram of a hibernation preparation exposed to cyanide (CN) + OGD to inhibit aerobic metabolism and then iodoacetate (IAA) to inhibit glycolysis (left). Plot on the right shows that at 60 minutes, OGD+CN have similar frequency to OGD alone indicating network function occurred without aerobic respiration. However, frequency falls following IAA exposure demonstrating glycolytic support of network activity. (E) Representative network responses to OGD in frogs after hibernation and after hibernation treated with DAB to inhibit glycogen phosphorylase. Mean data on the right showing the time until network failure during OGD in control (left, green, n=8), hibernation (middle, blue, n=6) and hibernation+ DAB (right, light blue, n=5). OGD. Dots represent data points from each preparation in individual experiments, horizontal bar is drawn at the mean, and error bars are S.E.M.
Network function without aerobic metabolism in hibernators was verified by exposing brainstems to OGD+ 1 mM sodium cyanide (CN), ensuring inhibition of mitochondrial respiration 4. After 1 hour of OGD+CN, burst frequency was comparable to OGD alone (Figure 1D). We then inhibited glycolysis using 1 mM iodoacetate to test whether glycolysis supported network function. Iodoacetate on the background of ODG+CN quickly led to hyperexcitability and then network silence (Figure 1D). Therefore, hibernation induces the ability for glycolysis to fuel network function with only local fuel stores.
Glial cells store glycogen in mammals and amphibians 5, 6 but are generally thought to fuel network activity for only a few minutes [2]. However, brain glycogen increases in winter frogs 7 suggesting the network can switch to glycogen as an alternative fuel source. Thus, we inhibited glycogen phosphorylase, the rate-limiting step of glycogenolysis using 1,4-dideoxy-1,4-imino-D-arabinitol (DAB; 250 μM). In the presence of DAB, hibernation preparations became hyperexcitable and stopped after ~25 min of ODG, significantly shorter than ~116 min with OGD alone but longer than controls (Figure 1E). In addition, no controls recovered following restoration of oxygen and glucose, while 5 of 6 hibernators resumed near-normal rhythmic activity. No preparations recovered with glycogenolysis inhibited by DAB (Figures S1C-D).
Circuit function requires ongoing ATP production through aerobic metabolism due to the high cost of electrical signaling 8. Indeed, most animals succumb to severe hypoxia and ischemia within minutes, or in the case of inherently hypoxia-tolerant species, use adaptations to oppose ATP depletion and tissue damage 1. It was, therefore, surprising that a complete glutamatergic network with high aerobic demands 4 (Figure 1A) can transform to produce motor activity for hours without aerobic metabolism or glucose delivery. Thus, we reveal that adult vertebrate circuits can exhibit metabolic plasticity, switching between aerobic and highly glycolytic states to support network function even without glucose delivery.
While we identified glycogenolysis as a requirement of metabolic plasticity (Figure 1D), we speculate that additional network adjustments must contribute. Circuit activity represents a balance between ATP supply and the energetic cost largely associated with excitatory synaptic transmission 9. Given that anaerobic glycolysis produces far less ATP compared to aerobic metabolism, we reason that synaptic function must become substantially more efficient or else glycolysis/glycogenolysis could not support network activity as long as we observed. Therefore, along with enhancing the use of alternative fuels most likely supplied by glia 5, the energy used for synaptic vesicle cycling and ion regulation may be reduced as low as network function permits.
Bullfrogs overwinter under water which dramatically lowers blood O2 due to a sole reliance on skin gas exchange 10. Therefore, metabolic plasticity in the respiratory network may serve to restart breathing upon emergence when O2 is otherwise below the aerobic requirement of circuit function, a problem compounded by metabolic rate increases associated with rising body temperature. More broadly, these results imply circuits may not always exist in their most energetically efficient state, which adds a layer of complexity to the understanding of circuit evolution. Circuit function is thought to reflect natural selection for optimized information transfer relative to ATP consumption 8. However, the large capacity for metabolic plasticity we observed brings into question the advantage of network designs with such high metabolic requirements: Why settle on an expensive network if less costly possibilities achieve the same function? Do networks with ultra-high efficiency come at a cost? Can other networks transition to work during bouts of low ATP synthesis? At its core, metabolic plasticity here seems to represent the overarching goal for human neuroprotection: preemptively transform circuits to avoid a collapse in integrity during an energy crisis. Therefore, learning how to modify circuits to function during oxygen and glucose deprivation may uncover new principles for both circuit energetics and neuroprotection alike.
Supplementary Material
Acknowledgements
Research funded by the Department of Defense (W911NF2010275) and NIH (1R15NS112920-01A1) to J.S.
Footnotes
Declaration of Interests: The authors have no competing interest to declare.
Inclusion and Diversity Statement: One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in science. One or more of the authors of this paper self-identifies as living with a disability. One or more of the authors of this paper received support from a program designed to increase minority representation in science.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Larson J, Drew KL, Folkow LP, Milton SL, and Park TJ (2014). No oxygen? No problem! Intrinsic brain tolerance to hypoxia in vertebrates. J. Exp. Biol 217, 1024–1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen NJ, Káradóttir R, and Attwell D (2005). A preferential role for glycolysis in preventing the anoxic depolarization of rat hippocampal area CA1 pyramidal cells. J. Neurosci 25, 848–859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somjen GG Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol. Rev 2001; 81, 1065–1096. [DOI] [PubMed] [Google Scholar]
- 4.Adams S, Zubov T, Bueschke N, and Santin JM (2021). Neuromodulation or energy failure? Metabolic limitations silence network output in the hypoxic amphibian brainstem. Am. J. Physiol.-Reg., Int., Comp. Physiol 320.2, R105–R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rossi DJ, Brady JD, and Mohr C (2007). Astrocyte metabolism and signaling during brain ischemia. Nat. Neurosci 10, 1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samosudova N, Reutov V, and Larionova N (2010). Role of glycogen in processes of cerebellar glial cells under conditions of its damage with sodium nitrite. Bul Exp. Biol. Med 150, 247–250. [DOI] [PubMed] [Google Scholar]
- 7.McDougal D Jr, Holowach J, Howe M, Jones E, and Thomas C (1968). The effects of anoxia upon energy sources and selected metabolic intermediates in the brains of fish, frog and turlte. J. Neurochem 15, 577–588. [DOI] [PubMed] [Google Scholar]
- 8.Harris JJ, Jolivet R, and Attwell D (2012). Synaptic energy use and supply. Neuron 75, 762–777. [DOI] [PubMed] [Google Scholar]
- 9.Rangaraju V, Calloway N, and Ryan TA (2014). Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tattersall GJ, and Ultsch GR (2008). Physiological ecology of aquatic overwintering in ranid frogs. Biol. Rev 83, 119–140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.