Abstract
Background:
Interventions with potential for broad reach in ambulatory settings are necessary to achieve a life course approach to advance care planning (ACP).
Objective:
To examine the effect of a computer-tailored, behavioral health model-based intervention on engagement of adults in ACP recruited from ambulatory settings.
Design:
Cluster randomized controlled trial with participant-level analysis.
Setting:
Ten pairs of primary and selected specialty care practices matched on patient sociodemographic information.
Participants:
English-speaking persons age 55 and older; 455 at practices randomized to usual care and 456 at practices randomized to intervention.
Intervention:
Brief telephone or web-based assessment generating a mailed individually tailored feedback report with a stage-matched brochure at baseline, two, and four months.
Measurements:
Primary outcome: completion of four ACP activities at six months: identifying and communicating with a trusted individual about views on quality versus quantity of life, assignment of a healthcare agent, completion of a living will, ensuring that documents are in the medical record assessed by a blinded interviewer. Secondary outcomes: completion of individual ACP activities.
Results:
Participants were 64% women, 76% white, with a mean age of 68.3 (8.3) years. Predicted probability of completing all ACP activities in usual care sites was 8.2% (95% confidence interval 4.9%, 11.4) compared to 14.1% (95% confidence interval 11.0%, 17.2%) in intervention sites (adjusted risk difference 5.2%, 95% confidence interval 1.6%, 8.8%). Prespecified subgroup analysis demonstrated no statistically significant interactions between the intervention and age, education, or race.
Limitations:
The study was conducted in a single region and excluded non-English speaking participants. No information was collected about non-participants.
Conclusions:
A brief and easily delivered tailored print intervention increased participation in ACP in ambulatory care settings.
Registration:
INTRODUCTION
Advance care planning (ACP), the process by which an individual can prepare for future treatment decisions at a time of decisional incapacity, is a key component of high-quality serious illness care (1). A recent consensus definition of ACP is “a process that supports adults at any age or stage of health in understanding and sharing their personal values, life goals, and preferences regarding future medical care.” It consists of: communication between an individual, their loved ones, and their clinicians about goals and preferences; the assignment of a trusted other to serve as a surrogate decision maker; and written documentation of medical choices in a manner that can be found when needed (2). Despite an increase in use over time (3), ACP remains underutilized. A systematic review examining studies conducted between 2011 and 2016 concluded that only approximately one-third of adults in the United States has completed advance directives (4). In one study using data from a nationally representative sample of community-living older adults age 65 and older, 38% had completed all three ACP activities and 27% had completed none (5).
A number of different intervention approaches have demonstrated efficacy in increasing engagement in at least one ACP activity. More intensive facilitator-led interventions appear to have the greatest effects (6) but also require the greatest resources (7). Many self-administered tools, including videos and websites, require internet access (8, 9). The National Academy of Medicine has endorsed a life course approach to ACP, in which it is introduced to all individuals before developing chronic or serious illness (1). This broad outreach may best be accomplished by approaches that bridge the gap between intensive clinician-led and self-administered tools for ACP engagement.
The Sharing and Talking about My Preferences (STAMP) program is designed to address gaps in existing programs for promoting ACP engagement. It is based on a health behavior change mode, the Transtheoretical Model (TTM), which has a long history of guiding the development of interventions effecting change across a wide range of behaviors (10). The intervention consists of a brief assessment followed by feedback reports tailored to individuals’ readiness to change, attitudes toward and beliefs about ACP with supplementary brochures. This cluster randomized controlled trial evaluated the efficacy of STAMP intervention in promoting completion of a full range of ACP activities in an ambulatory care population.
METHODS
Design and setting:
This was a single-blind cluster randomized controlled trial conducted within primary care and selected specialty practices (HIV, renal, cardiology, and pulmonary) for which patients often consider the specialty physician to be their primary provider (11). Randomization occurred at the level of the practice to avoid contamination. The study was approved by the Institutional Review Boards of Bridgeport Hospital and the Yale School of Medicine, and the protocol was registered at clinicaltrials.gov (NCT03137459). Full details regarding the trial have been published (12).
Randomization was performed within matched pairs of practice sites using the multi-attribute utility measurement approach (13). Sites were matched according to type of practice and proportion of patients aged 55 and older, non-white, and covered by Medicaid. The one site exclusion criterion was failure to recruit at least ten participants at a site. The randomization assignment was computer generated by an investigator not involved in data collection. With the exception of the matched set of HIV practices, which were hospital-based, all others were community-based and either privately owned or owned by an accountable care organization. Each practice was uniquely managed. No practice had any standardized approaches to ACP or modifications to the electronic health record facilitating ACP documentation, nor had their clinicians received any specialized ACP training.
Participants, recruitment, and enrollment:
The participant inclusion criteria were age 55 years or older and presentation for an in-person visit at a selected site. Exclusion criteria included urgent care clinical encounter, severe hearing or vision loss, moderate-to-severe cognitive impairment as identified by a clinician within the practice or inability to complete the process of informed consent, primary language other than English, or having completed all ACP activities. Consecutive potentially eligible participants were given an information sheet to review while waiting for their visit. Interested patients completed the process of written informed consent and baseline assessment administered by a research assistant following the visit. If the patient did not have time for the assessment, an appointment was made to complete it by telephone. These procedures were evaluated in a pilot study (14).
Intervention and control procedures:
Participants in practices randomized to the intervention arm of the study received the STAMP computer-tailored intervention, with assessments linked to personalized feedback. Assessments occurred at baseline, as described above, and at two and four months, either by research-assistant administration via telephone or self-administration via the web, based on participant preference. Development of STAMP has been previously described (15). Briefly, it is an expert system (a software system consisting of an assessment battery, normative data to make comparisons, decision rules for delivering feedback, and feedback components) based on the TTM (16). The system assessed key constructs of the TTM as they relate to the following four ACP behaviors: a) identifying a trusted individual and communicating with this person about views regarding quality versus quantity of life; b) formal assignment of a healthcare agent; c) completion of living will; d) ensuring that written documents are in the medical record.
The assessment, which took approximately ten minutes to complete, included the validated TTM constructs of stages of change (readiness to participate in each of the ACP behaviors or activities), decisional balance, and processes of change. The stages of change are precontemplation, or not thinking about engagement; contemplation, or thinking about engagement in the next six months; preparation, or preparing to engage; and action/maintenance, or engagement (17). We developed an additional construct for assessment of factors influencing ACP engagement, called ACP values/beliefs (18). The complete assessment is in the Appendix.
The original expert system provided a cross-sectional, or “normative,” report (14, 15). For this study, the system was further developed to give feedback based on simultaneous consideration of stage for all four behaviors (referred to as stage pattern) as well as to give longitudinal feedback by comparing stage at follow-up to stage at baseline (“ipsative” reporting). Feedback reports included: 1) introduction to ACP (common across reports); 2) figure illustrating stage of change for each behavior (normative) and changes in stage at follow-up (ipsative); 3) brief stage pattern-tailored feedback; 4) feedback for up to three endorsed values/beliefs items; 5) decisional balance by stage pattern feedback; 6) processes/strategies/efficacy or “next steps” stage pattern-tailored feedback; 7) summary. A sample feedback report is in the Appendix.
The feedback reports referenced one of two stage-matched brochures. The first brochure, provided to participants who had not completed any of the ACP behaviors, provided additional information promoting the reasons for engaging in ACP and addressing potential barriers. It contained two stories describing families who did and did not engage in ACP. The second brochure, provided to participants who had completed at least one ACP behavior, contained additional information about how to engage in each and an Advance Directives form. Participants received a pamphlet to give to potential surrogate decision makers to explain their role in ACP. Intervention participants received these materials directly following their baseline assessment. For subsequent assessments, they received materials by mail regardless of whether they completed the assessment by phone or web.
Participants in practices randomized to control completed assessments at baseline, two, and four months but did not receive reports or brochures. To minimize the effect of asking about ACP behaviors on participants’ engagement in these behaviors, control assessments concluded with questions about readiness to engage in physical activity
Outcomes:
The primary outcome was self-reported completion of all four ACP behaviors at six months either through a telephone assessment administered by a research assistant blinded to group assignment or through a web-based assessment. Secondary outcomes included self-reported completion of the individual ACP behaviors at six months among those who had not completed this behavior at baseline.
Additional measures:
Participant characteristics obtained during the baseline assessment included: age; gender; race/ethnicity; education; finances, assessed with a measure of economic strain (19); marital status; living alone or with others; self-rated health (20); attendance at religious services, assessed with a measure from the Duke University Religion Index (21); and variables included in a validated prognostic index for risk of four-year mortality: current tobacco use, hospitalizations, and functional status (22). Additional variables included in the prognostic index were obtained by chart review: body mass index, and presence of chronic conditions.
Power and sample size:
The sample size was calculated to detect an absolute increase of 10 percentage points for the primary outcome in the treatment group over the control group, estimating a 5% prevalence for the control group (17). The calculations assumed one-tailed significance alpha = .05 and were based on a one-way analysis of variance for proportions with arcsine transformation and nested random effects for sites to accommodate the cluster-randomized design with an estimate of the intraclass correlation of .05 (23). With a minimum of eight matched study sites, a sample size of 800 was needed to achieve 80% power. The baseline sample size was increased to 900 to account for an assumed 10% loss to follow up.
Statistical analysis:
Univariate statistics were used to describe the sample and observed outcome attainment at each time point. All analytic models utilized intention-to-treat analysis, with all participants who completed a baseline interview, regardless of adherence or missing data. Participants with missing six-month data were assumed to have not achieved the outcomes, an assumption supported by the finding that no participant with missing data at six months had achieved the primary outcome at a prior time point. SAS version 9.4 was used for all analyses. We conducted generalized estimating equations using PROC GENMOD with a binomial distribution and a logit link function, with an exchangeable correlation structure to account for clustering effects of participants within practices. We used the Margins macro to obtain predicted probabilities and risk differences. We then added adjustment for covariates differing between intervention and control participants at baseline: education, employment, and stage of change for each ACP behavior. We performed subgroup analyses with pre-specified variables to examine potential interactions between the intervention and covariates with demonstrated associations with engagement in ACP, including age, gender, education, and race (3, 5), adjusting for clustering. Further adjustment for covariates led to poorly fitting models with wide confidence intervals (Supplemental Table 2)
Role of the funding source:
The NIH had no role in the study design, conduct, or reporting.
RESULTS
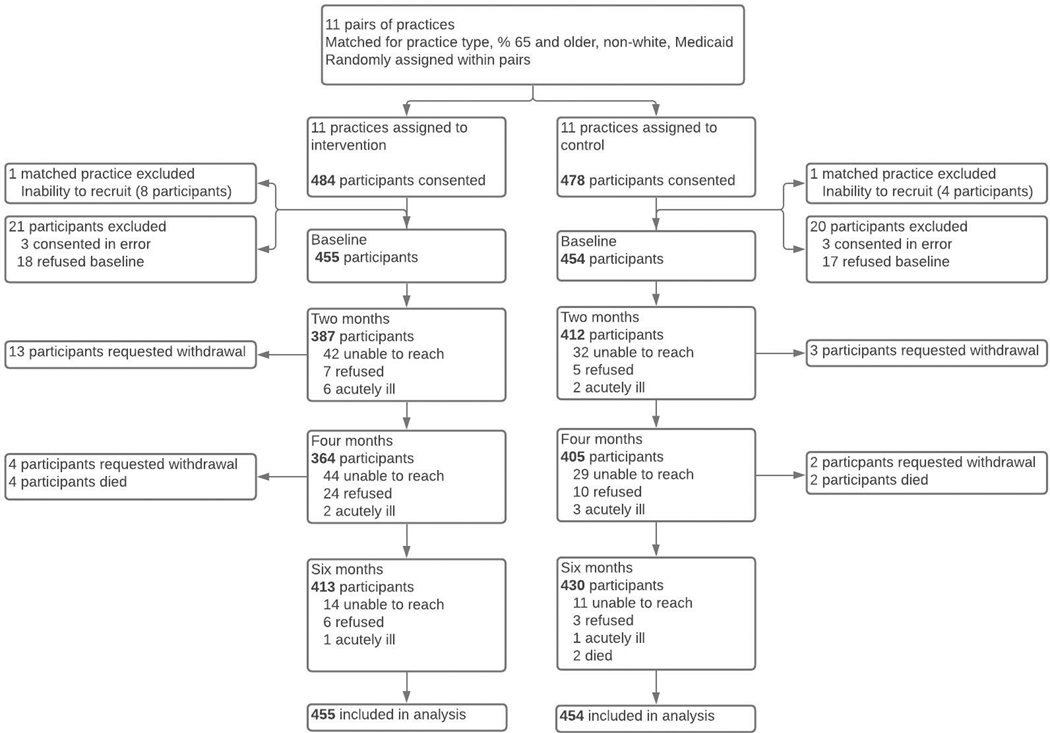
A total of twenty-one sites were matched into pairs and then randomized within pair, with one matched pair of primary care practices located within assisted living facilities subsequently excluded for failure to recruit because of a high prevalence of cognitive impairment (Figure 1). Among intervention sites, 455 participants completed a baseline assessment and among usual care sites, 454 participants completed a baseline assessment. At six months, 413 participants at intervention sites (91%) and 430 participants at usual care sites (95%) completed final interviews. No sites dropped out, and participants with missing outcome data were distributed across sites (Supplemental Table 1). Among participants completing both the two- and four-month assessments, 43% did so by telephone, 46% by web, and 11% used both modalities.
Figure 1:
Flow of participants through study
Table 1 presents participant baseline characteristics. A total of 36% were age 55–64, 42% were 65–74, and 22% were 75 or older. Participants were 64% women and 24% non-white. Approximately one-quarter had completed a high-school education or less, 35% reported just enough or not enough income at the end of each month, and 20% rated their health as fair or poor. A total of 28% had completed no ACP activities, 21% had assigned a health care agent, 25% had completed a living will, and 65% had discussed quality versus quantity of life with a loved one. Intervention and usual care participants were well matched on most characteristics. Larger proportion of participants at intervention sites had a college education (79% versus 70%) and were employed (43% versus 35%) compared to participants at usual care sites. Although the number of ACP behaviors in action/maintenance was well matched across groups, proportions of individuals in different stages of change for each ACP behavior differed across groups. Table 2 presents the characteristics of the participating practices.
Table 1:
Characteristics of study participants at baseline
| N (%) | |||
|---|---|---|---|
|
| |||
| Characteristic | Control (n=454) | Intervention (n=455) | Total (n=909) |
|
| |||
| Age, mean (SD) | 67.8 (8.0) | 68.9 (8.5) | 68.3 (8.3) |
| Women | 262 (57.7) | 292 (64.4) | 554 (64.2) |
| Race | |||
| White | 339 (74.7) | 352 (77.4) | 691 (76.0) |
| African-American | 91 (20.0) | 76 (16.7) | 167 (18.4) |
| Other | 24 (5.3) | 27 (5.9) | 51 (5.6) |
| Education ≤ 12th grade | 96 (21.1) | 137 (30.1) | 233 (25.3) |
| Not enough or just enough money to make ends meet | 159 (35.0) | 159 (34.9) | 318 (35.0) |
| Married/partnered | 242 (53.3) | 243 (53.2) | 484 (53.2) |
| Employed | 194 (43.1) | 157 (34.5) | 351 (38.9) |
| Lives alone | 141 (31.1) | 136 (29.9) | 277 (30.5) |
| Attends religious service ≥ 1x/week* | 140 (30.9) | 170 (37.5) | 351 (38.7) |
| Self-rated health fair/poor | 97 (21.4) | 81 (17.8) | 178 (19.6) |
| Probability of 4-year mortality | |||
| 4% risk | 278 (61.2) | 274 (60.2) | 552 (60.7) |
| 15% risk | 133 (29.3) | 127 (27.9) | 260 (28.6) |
| 42% risk | 36 (7.9) | 47 (10.3) | 83 (9.1) |
| 64% risk | 7 (1.5) | 7 (1.5) | 14 (1.5) |
| Number of ACP activities completed | |||
| 0 | 134 (29.5) | 121 (26.6) | 255 (28.1) |
| 1 | 294 (42.7) | 227 (49.9) | 421 (46.3) |
| 2 | 58 (12.8) | 53 (11.6) | 111 (12.2) |
| 3 | 68 (15.0) | 54 (11.9) | 122 (13.4) |
| Stage of change for communication with surrogate about quality versus quantity of life | |||
| Precontemplation | 112 (24.7) | 80 (17.6) | 192 (21.2) |
| Contemplation | 46 (10.1) | 60 (13.2) | 106 (11.7) |
| Preparation | 5 (1.1) | 11 (2.4) | 16 (1.7) |
| Action/maintenance | 289 (63.7) | 304 (66.8) | 593 (65.2) |
| Stage of change for assigning a healthcare agent | |||
| Precontemplation | 99 (22.0) | 69 (15.2) | 168 (18.5) |
| Contemplation | 217 (48.0) | 227 (49.9) | 444 (48.8) |
| Preparation | 31 (6.9) | 76 (16.7) | 107 (11.8) |
| Action/maintenance | 105 (23.2) | 83 (18.2) | 188 (20.9) |
| Stage of change for completing a living will | |||
| Precontemplation | 86 (18.9) | 64 (14.1) | 150 (16.5) |
| Contemplation | 224 (49.3) | 224 (49.2) | 448 (49.3) |
| Preparation | 21 (4.6) | 59 (13.0) | 80 (8.8) |
| Action/maintenance | 120 (26.) | 108 (23.7) | 228 (25.0) |
1 participant in a control site and 2 participants in intervention sites did not respond
Table 2:
Characteristics of participating practices
| Intervention (N=10) | Control (N=10) | Total (N=20) | |
|---|---|---|---|
|
| |||
| Mean (SD) | |||
|
| |||
| Number of clinicians | 3.3 (3.8) | 4.5 (4.9) | 4.1 (4.4) |
| Proportion of patients 55+ years | 51.8 (5.0) | 59.5 (12.7) | 55.5 (11.7) |
| Proportion of non-white patients | 23.4 (12.2) | 23.8 (9.9) | 22.8 (9.0) |
| Proportion of patients receiving Medicaid | 4.2 (.96) | 4.9 (2.1) | 4.5 (1.9) |
Table 3 presents the observed proportions of participants completing ACP activities at each follow up. Table 4 presents intention-to-treat analysis of the six-month study outcomes. The predicted probability of completing all ACP activities (completing a living will, naming a health care agent, communicating with a loved one about quality versus quantity of life, and ensuring documents were in the health record) in usual care sites was 8.2% (95% confidence interval (CI) 4.9%, 11.4%) compared to 14.1% (95% CI 11.0%, 17.2%) in intervention sites (risk difference accounting for clustering 5.9%, 95% confidence interval (95% CI) 1.4%, 10.5%). After additional adjustment for covariates, the risk difference was 5.2% (95% CI 1.6%, 8.8%).
Table 3:
Proportions of participants who completed advance care planning activities at 2, 4, and 6 months from all observed data
| Two months | Four months % (n/N) | Six months | |
|---|---|---|---|
|
| |||
| Completion of all ACP activities | |||
| Intervention | 3.6 (15/412) | 9.9 (36/364) | 15.5 (64/413) |
| Usual care | 3.9 (15/372) | 5.7 (23/405) | 8.6 (37/430) |
| Completion of living will* | |||
| Intervention | 15.6 (45/288) | 26.8 (72/269) | 31.9 (99/310) |
| Usual care | 12.9 (38/295) | 15.6 (46/294) | 21.5 (67/311) |
| Assignment of healthcare agent* | |||
| Intervention | 22.4 (70/313) | 32.5 (95/292) | 36.3 (122/336) |
| Usual care | 16.5 (52/315) | 18.4 (57/310) | 20.9 (68/325) |
| Communication about quality versus quantity of life* | |||
| Intervention | 56.9 (70/123) | 69.7 (76/109) | 69.9 (93/133) |
| Usual care | 50.3 (75/149) | 55.5 (81/146) | 57.1 (89/156) |
Denominators are individuals who had not completed this activity at baseline.
Table 4:
Likelihood of participation in advance care planning at the six-month follow-up
| Outcome | Participants included in analysis (n)* | Predicted probability of completion (95% CI)** | Risk difference (95% CI)** | Adjusted risk difference (95% CI)*** |
|---|---|---|---|---|
|
| ||||
| Completion of all ACP activities | 5.9% (1.4%, 10.5%) | 5.2% (1.6%, 8.8%) | ||
| Intervention | 455 | 14.1% (11.0%, 17.2%) | ||
| Usual care | 454 | 8.2% (4.9%, 11.4%) | ||
| Completion of living will | 8.2% (1.4%, 15.0%) | 6.5% (0.1%, 12.8%) | ||
| Intervention | 347 | 28.5% (24.5%, 32.6%) | ||
| Usual care | 334 | 20.4% (15.0%, 25.7%) | ||
| Assignment of health care agent | 13.3% (6.6%, 20.1%) | 12.2% (2.8%, 21.5%) | ||
| Intervention | 372 | 32.8% (30.0%, 35.5%) | ||
| Usual care | 349 | 19.5% (13.3%, 25.7%) | ||
| Communication about quality versus quantity of life | 7.0% (−4.7%, 18.8%) | 2.8% (−7.9%, 13.5%) | ||
| Intervention | 151 | 61.6% (52.6%, 70.6%) | ||
| Usual care | 165 | 54.5% (46.9%, 62.1%) | ||
CI = confidence interval
All analyses are intention-to-treat, in which participants with missing values assigned to failure to achieve the outcome
Adjusted for clustering within practice site; risk difference = difference in proportions of completing ACP activities between the intervention and usual care groups.
Adjusted for clustering within practice site and for the following covariates: education, employment status, and baseline stage of change (for outcome of completion of all ACP activities, this is baseline stage for living will completion, assignment of healthcare agent, and communication about quality versus quantity of life; for other outcomes this is baseline stage for the single behavior); risk difference = difference in proportions of completing ACP activities between the intervention and usual care groups.
Among participants who had not completed the particular ACP activity at baseline, 28.5% (95% CI 24.5%, 32.6) of participants at intervention sites versus 20.4% (95% CI 15.0%, 25.7%) at control sites completed a living will (adjusted risk difference 6.5%, 95% CI .01%, 12.8%) at six months; 32.8% (95% CI 30.0%, 35.5%) versus 19.5% (95% CI 13.3%, 25.7%) assigned a health care agent (adjusted risk difference 12.2%, 95% CI 2.8%, 21.5%); and 61.6% (95% CI 52.6%, 70.6% versus 54.7% (95% CI 46.9%, 62.1%) communicated with loved ones about quality versus quantity of life (adjusted risk difference 2.8%, 95% CI −4.7%, 13.5%).
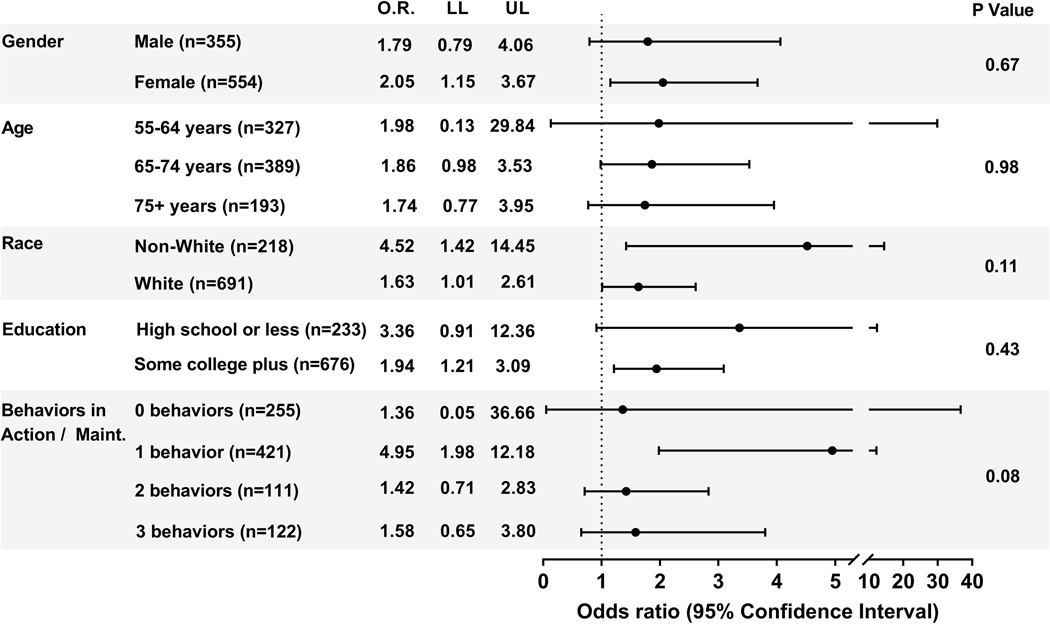
In subgroup analyses, there were no statistically significant interactions between demographic characteristics and intervention assignment (Figure 2). While the effect size was larger for non-whites (adjusted odds ratio (AOR) 4.52, 95% CI 1.42, 14.45) than for whites (AOR 1.63, 95% CI 1.01, 2.61), the difference did not reach statistical significance (interaction P = .11). The effect size was larger among those who had completed one ACP activity at baseline (AOR 4.95, 95% CI 1.98, 12.18) than among those completing none, two, or three, but this interaction was also not statistically significant.
Figure 2:
Effect of the intervention in pre-specified subgroups, adjusting for clustering within practice site. OR = Odds ratio LL = Lower Limit UL = Upper Limit. See Supplement Table 2 for results additionally adjusted for education, employment, and baseline stage of change for completion of a living will, assignment of a health care agent, and communication about quality versus quantity of life
DISCUSSION
In this cluster randomized controlled trial of English-speaking persons age 55 years and older recruited from ambulatory practices, practices were randomized either to usual care or to intervention. Participants in intervention practices received an individually tailored report providing feedback regarding their readiness to engage in different specific ACP activities, their progression over time, their attitudes and beliefs about ACP, and personalized next steps along with one of two brochures based on their overall readiness for ACP engagement. The predicted probability of completing a full set of ACP activities was 14.1% (95% CI 11.0%, 17.2%) in intervention sites compared to 8.2% (95% CI 4.9%, 11.4%) in usual care sites, with an adjusted risk difference of 5.2% (95% CI 1.6%, 8.8%)..
These results demonstrate that a theory-informed behavioral health approach to ACP engagement was successful in promoting engagement in a full range of ACP activities. Most studies of interventions to increase engagement in ACP examine only one component or consider a successful outcome participation in any single component (24). The more comprehensive outcome used in the current study closely mirrors a consensus definition from a multidisciplinary Delphi panel on the definition of (2) and outcomes identifying (25) successful ACP. In addition, while there has been a movement away from communication regarding specific treatment preferences and toward what is commonly referred to as “goals of care,” there is uncertainty and ambiguity about what is meant by this term (26). This study asked participants about whether they had discussed their views on quality versus quantity of life as a specific component of goals of care communication. The consideration of quality versus quantity of life reflects the existence of trade-offs between life extension and a decreased quality of life in many end-of-life decisions (27), and asking individuals about their priorities in the face of a trade-off is an effective way of helping them to clarify what is most important (28). Even individuals who have not given much thought to their goals and preferences understand this trade-off (29), and consideration of quality versus quantity of life is a component of several other ACP tools (30, 31).
While there are many studies examining approaches to increasing engagement in ACP, an overview of systematic reviews concluded that much of the data are of low quality (32). A scoping review of recent randomized controlled trials highlights the heterogeneity in populations studied, approaches used, and outcomes measured (33). Arguably, the most meaningful and important effects of engagement in ACP are measured with patient and caregiver outcomes such as the receipt of goal-concordant care and improved caregiver bereavement (34). Demonstrating the effects of early ACP on these outcomes would require longitudinal studies conducted over long time periods to follow individuals and their surrogates until the time of serious illness and death. Interventions that have been shown to improve these outcomes, such as The Respecting Choices program (35). have been targeted at patients at high risk of death. This program consists of discussions between patients and their surrogates led by a trained facilitator, requiring substantial implementation resources (36).
With the goal of reaching a broader population, the National Academy of Medicine put out a call to incorporate ACP throughout the lifespan (1). The inclusion of ACP in routine primary wellness care may help to normalize its conduct, counteract suspicion that its purpose is to ration health care, and help better prepare patients and their surrogates for more intensive programs with illness progression (37). Only a minority of studies have been conducted in primary care settings (33). The STAMP program provides an important alternative to other approaches conducted in these settings, including videos (9) and interactive websites (30, 38). In one trial, over 50% of participants had no access to the internet (8), and there is additional evidence that digital supports are less likely to be utilized by older and minority individual (39). The STAMP intervention accommodated both participants who preferred web-based and telephone contact. US physician practices are increasing their use of components of the medical home model, including team-based approaches and care management (40). The resources required by STAMP, consisting of a modest licensing fee for the software platform, a 10-minute assessment that can be performed by a case manager or other appropriate healthcare team member or completed by a patient while waiting for a visit, followed by provision of written materials can allow STAMP to reach broad and diverse patient populations. Combined with preliminary evidence of at least equal efficacy among non-white and white participants, the STAMP intervention demonstrates potential to overcome persistent disparities in ACP engagement (41).
The effect size for the STAMP intervention was largest among participants who had completed one ACP activity when they entered the study, although it was not statistically different from effect sizes in other participants. It might have been expected that the intervention would have had the largest effect among those who had completed three ACP activities, since all that remained for these participants was to get their documents were in the electronic health record. This finding highlights the importance of initiatives to eliminate the health system barriers to this task (44). For some patients, the six-month follow up time may not have been long enough to include follow-up visits to check on this. Additional work is needed to determine whether repeated contact over longer time periods would result in even greater engagement and the optimal timing and use of different clinical and population-based approaches to ACP over the lifespan.
The study has several limitations. Because non-English speaking participants were excluded, the generalizability of the results is limited to English-speaking adults. Self-reported measures are subject to social desirability bias, with participants feeling pressured to report completion of ACP. However, chart review for ACP is also subject to error (45). The study was conducted in a single geographical location. Although the assessment conducted with participants at control sites included questions about readiness to engage in exercise as well as ACP, the assessment may have prompted participants with sufficient readiness to engage in ACP. In order to minimize the study’s impact on practice workflow, we did not collect information on potentially eligible patients who chose not to participate and patients who were not eligible because they had already completed full ACP engagement, which limits conclusions about the generalizability of the study results. While overall ascertainment of the primary outcome was high, there was greater loss to follow-up in the intervention group. Our analyses utilized the assumption that these participants had not completed ACP, providing a conservative estimate of efficacy
In conclusion, a brief assessment resulting in a health behavior model-based tailored feedback report supplemented by additional information provided in a brochure demonstrated efficacy in increasing participation in ACP among middle-age and older adults recruited from primary care and specialty clinics. The intervention can be delivered both using the web and by telephone and mail, supporting the use of the STAMP program as a feasible population-based approach to increasing engagement in ACP.
Supplementary Material
Acknowledgments
Funding Source: National Institute of Nursing Research (R01 NR016007), National Institute of Aging (P30 AG21342)
Footnotes
Reproducible Research Statement:
Study protocol: Available from Dr. Fried (e-mail, terri.fried@yale.edu)
Statistical code: Available from Ms. Drohan (e-mail, mrisi@uri.edu)
Data set: Available from Dr. Paiva (e-mail, apaiva@uri.edu)
REFERENCES
- 1.Institute of Medicine. Dying in America: Improving and Honoring Individual Preferences Near the End of Life. Washington, DC: The National Academies Press; 2014. [PubMed] [Google Scholar]
- 2.Sudore RL, Lum HD, You JJ, Hanson LC, Meier DE, Pantilat SZ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage. 2017;53:821–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Block BL, Jeon SY, Sudore RL, Matthay MA, Boscardin WJ, Smith AK. Patterns and trends in advance care planning among older adults who received intensive care at the end of life. JAMA Intern Med. 2020;180:786–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yadav KN, Gabler NB, Cooney E, Kent S, Kim J, Herbst N, et al. Approximately one in three US adults completes any type of advance directive for end-of-life care. Health Aff (Millwood). 2017;36:1244–51. [DOI] [PubMed] [Google Scholar]
- 5.Harrison KL, Adrion ER, Ritchie CS, Sudore RL, Smith AK. Low completion and disparities in advance care planning activities among older Medicare beneficiaries. JAMA Intern Med. 2016;176:1872–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houben CHM, Spruit MA, Groenen MTJ, Wouters EFM, Janssen DJA. Efficacy of advance care planning: A systematic review and meta-analysis. J Am Med Dir Assoc. 2014;15:477–89. [DOI] [PubMed] [Google Scholar]
- 7.MacKenzie MA, Smith-Howell E, Bomba PA, Meghani SH. Respecting Choices and related models of advance care planning: A systematic review of published evidence. Am J Hosp Palliat Med. 2017;35:897–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sudore RL, Boscardin J, Feuz MA, McMahan RD, Katen MT, Barnes DE. Effect of the PREPARE website vs an easy-to-read advance directive on advance care planning documentation and engagement among veterans: A randomized clinical trial. JAMA Intern Med. 2017;177:1102–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volandes AE, Paasche-Orlow MK, Davis AD, Eubanks R, El-Jawahri A, Seitz R. Use of video decision aids to promote advance care planning in Hilo, Hawai’i. J Gen Intern Med. 2016;31:1035–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prochaska JO, Redding CA, Evers KE. The Transtheoretical Model and Stages of Change. In: Glanz K, Rimer BK, Viswanath KV, eds. Health Behavior and Health Education: Theory, Research, and Practice. 4th edition. San Francisco: Jossey-Bass; 2008:170–222. [Google Scholar]
- 11.Fried TR, Bradley EH, O’Leary J. Prognosis communication in serious illness: perceptions of older patients, caregivers, and clinicians. J Am Geriatr Soc. 2003;51:1398–403. [DOI] [PubMed] [Google Scholar]
- 12.Fried TR, Redding CA, Martino S, Paiva A, Iannone L, Zenoni M, et al. Increasing engagement in advance care planning using a behaviour change model: study protocol for the STAMP randomised controlled trials. BMJ Open. 2018;8:e025340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham JW, Flay BR, Anderson Johnson C, Hansen WB, Collins LM. Group comparability: A multiattribute utility measurement approach to the use of random assignment with small numbers of aggregated units. Eval Rev. 1984;8:247–60. [Google Scholar]
- 14.Paiva A, Redding CA, Iannone L, Zenoni M, O’Leary JR, Fried TR. Feasibility of delivering a tailored intervention for advance care planning in primary care practice. J Am Geriatr Soc. 2019;67:1917–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried TR, Redding CA, Robbins ML, Paiva AL, O’Leary JR, Iannone L. Development of personalized health messages to promote engagement in advance care planning. J Am Geriatr Soc. 2016;64:359–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Velicer WF, Prochaska JO, Bellis JM, DiClemente CC, Rossi JS, Fava JL, et al. An expert system intervention for smoking cessation. Addict Behav. 1993;18:269–90. [DOI] [PubMed] [Google Scholar]
- 17.Fried TR, Redding CA, Robbins ML, Paiva A, O’Leary JR, Iannone L. Stages of change for the component behaviors of advance care planning. J Am Geriatr Soc. 2010;58:2329–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fried TR, Redding CA, Robbins ML, Paiva A, O’Leary JR, Iannone L. Promoting advance care planning as health behavior change: Development of scales to assess Decisional Balance, Medical and Religious Beliefs, and Processes of Change. Patient Educ Couns. 2012;86:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. 1981;22:337–56. [PubMed] [Google Scholar]
- 20.Idler EL, Benyamini Y. Self-rated health and mortality: a review of twenty-seven community studies. J Health Soc Behav. 1997;38:21–37. [PubMed] [Google Scholar]
- 21.Koenig HG, Büssing A. The Duke University Religion Index (DUREL): A five-item measure for use in epidemological studies. Religions. 2010;1:78–85. [Google Scholar]
- 22.Lee SJ, Lindquist K, Segal MR, Covinsky KE. Development and validation of a prognostic index for 4-year mortality in older adults. JAMA. 2006;295:801–8. [DOI] [PubMed] [Google Scholar]
- 23.Rossi JS. Statistical power analysis. In: Schinka JA, Velicer WF, eds. Handbook of Psychology. Volume 2: Research Methods in Psychology, 2nd ed. New York: John Wiley & Sons; 2013:71–108. [Google Scholar]
- 24.Bravo G, Dubois M-F, Wagneur B. Assessing the effectiveness of interventions to promote advance directives among older adults: A systematic review and multi-level analysis. Soc Sci Med. 2008;67:1122. [DOI] [PubMed] [Google Scholar]
- 25.Sudore RL, Heyland DK, Lum HD, Rietjens JAC, Korfage IJ, Ritchie CS, et al. Outcomes that define successful advance care planning: A delphi panel consensus. J Pain Sympt Manage. 2018;55:245–55.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edmonds KP, Ajayi TA. Do we know what we mean? An examination of the use of the phrase “goals of care” in the literature. J Palliat Med. 2019. [DOI] [PubMed] [Google Scholar]
- 27.Kaldjian LC. Clarifying core content of goals of care discussions. J Gen Int Med. 2020;35:913–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wright DR. Distinguishing wants vs preferences for end-of-life care: can you tell me what you want, what you really, really want? JAMA Netw Open. 2020;3:e2010907-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fried TR, Bullock K, Iannone L, O’Leary JR. Understanding advance care planning as a process of health behavior change. J Am Geriatr Soc. 2009;57:1547–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sudore RL, Knight SJ, McMahan RD, Feuz M, Farrell D, Miao Y, et al. A novel website to prepare diverse older adults for decision making and advance care planning: A pilot study. J Pain Sympt Manage. 2014;47:674–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Green MJ, Levi BH. Development of an interactive computer program for advance care planning. Health Expect. 2009;12:60–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jimenez G, Tan WS, Virk AK, Low CK, Car J, Ho AHY. Overview of systematic reviews of advance care planning: Summary of evidence and global lessons. J Pain Sympt Manage. 2018;56:436–59.e25. [DOI] [PubMed] [Google Scholar]
- 33.McMahan RD, Tellez I, Sudore RL. Deconstructing the complexities of advance care planning outcomes: What do we know and where do we go? A scoping review. J Am Geriatr Soc. 2021;69:234–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Detering K. Measuring success in advance care planning. What outcomes are measurable, and what is important? BMJ Support Palliat Care. 2012;2:182. [Google Scholar]
- 35.Detering KM, Hancock AD, Reade MC, Silvester W. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ. 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson KS, Kottke TE, Schettle S. Honoring Choices Minnesota: Preliminary data from a community-wide advance care planning model. J Am Geriatr Soc. 2014;62:2420–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fried TR, Drickamer M. Garnering support for advance care planning. JAMA. 2010;303:269–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sudore RL, Schillinger D, Katen MT, Shi Y, Boscardin WJ, Osua S, et al. Engaging diverse English-and Spanish-speaking older adults in advance care planning: the PREPARE randomized clinical trial. JAMA Int Med. 2018;178:1616–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker DM, Hefner JL, Fareed N, Huerta TR, McAlearney AS. Exploring the digital divide: Age and race disparities in use of an inpatient portal. Telemed J e-Health. 2019;26:603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiley JA, Rittenhouse DR, Shortell SM, Casalino LP, Ramsay PP, Bibi S, et al. Managing chronic illness: Physician practices increased the use of care management and medical home processes. Health Aff (Millwood). 2015;34:78–86. [DOI] [PubMed] [Google Scholar]
- 41.Bazargan M, Bazargan-Hejazi S. Disparities in palliative and hospice care and completion of advance care planning and directives among non-hispanic blacks: a scoping reviews of recent literature. Am J Hosp Palliat Med 2021;38:688–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamas D, Panariello N, Henrich N, Hammes B, Hanson LC, Meier DE, et al. Advance care planning documentation in electronic health records: Current challenges and recommendations for change. J Palliat Med. 2018;21:522–8. [DOI] [PubMed] [Google Scholar]
- 43.Garner KK, Dubbert P, Lensing S, Sullivan DH, Aslakson RA, Ast K, et al. Concordance between veterans’ self-report and documentation of surrogate decision makers: Implications for quality measurement. J Pain Sympt Manage. 2017;53:1–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.