Summary
Diffuse large B-cell lymphoma (DLBCL) is the most common non Hodgkin lymphoma (NHL) in adults, and it accounts for about 30% of adult NHL cases. Newly diagnosed patients are treated with rituximab in combination with anthracycline-containing chemotherapy, but a significant number of patients relapse after initial treatment. New strategies for relapsed lymphomas are in development among which antibody–drug conjugates (ADCs) are currently in clinical trials. Polatuzumab vedotin is a novel ADC which binds to the commonly expressed B-cell antigen CD79b, and it delivers monomethyl auristatin E, a small molecule with anti-tubulin activity. Polatuzumab vedotin in combination with bendamustine and rituximab (BR) has been approved in the U.S. and the E.U. for use in patients with relapsed or refractory DLBCL ineligible for transplant. These approvals were based on a randomized study of patients treated with either polatuzumab vedotin plus BR or BR alone, where complete response was 40% in the polatuzumab vedotin + BR group versus 18% in the BR group. The most common adverse events of this treatment were cytopenias and peripheral neuropathy.
Keywords: Polatuzumab vedotin, Diffuse large B-cell lymphoma, Monoclonal antibodies, Antibody, drug conjugates, CD79b-targeted antibodies
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common non Hodgkin lymphoma (NHL) in adults. There are an estimated 5.6 new cases per 100,000 people in the U.S. each year and the median age of diagnosis is 66 (1). On the basis of gene expression profiling, DLBCL is further subdivided into germinal center B-cell (GCB) and activated B-cell (ABC) subtypes, ABC being associated with worse outcomes (2–4). This disease typically presents with lymphadenopathy, and about 50% of newly diagnosed cases are diagnosed with advanced stage (III or IV) (1).
DLBCL is diagnosed by tissue biopsy showing infiltration of large cells expressing B-cell antigens such as CD19, CD20, CD22, CD45 and CD79b, and in some cases, CD30 (5). Aside from cell of origin, other important prognostic information at the time of diagnosis includes overexpression of the oncogenes c-MYC and BCL2. Furthermore, the presence of gene rearrangements of these oncogenes defines specific subtypes of DLBCL called high-grade B-cell lymphoma, characterized by the presence of MYC and BCL2 rearrangements (“double-hit”) or the presence of MYC, BCL2 and BCL6 rearrangements (“triple-hit”). The presence of these gene rearrangements confers an unfavorable prognosis (6, 7).
The most commonly used chemotherapy regimen for newly diagnosed DLBCL patients is R-CHOP (rituximab-cyclophosphamide, doxorubicin, vincristine and prednisone). The 2-year overall survival (OS) for DLBCL patients treated with R-CHOP is 85.7% (8), and the 10-year OS for DLBCL patients has been estimated around 44% (9). Although long-term disease-free survival is now achieved in about half of the patients, there is still a significant number of patients with relapsed disease, particularly those with advanced stage at diagnosis. Standard therapy for these patients is salvage chemotherapy followed by autologous stem cell transplantation (10, 11). For those patients who are transplant-ineligible, treatment options are limited and OS is about 6 months (12). Prognosis is especially poor for patients who relapse within 6 months of induction chemotherapy with a 2-year OS of around 20% (12). The newly approved antibody–drug conjugate (ADC) polatuzumab vedotin has shown to have promising responses in relapsed, transplant-ineligible patients, thus increasing the repertoire of options in this subset of DLBCL patients.
Mechanism of Action and Design
ADCs have opened the opportunity to use small molecules with high potency that would otherwise be too toxic to use as systemic therapy (13). This strategy has been accomplished by linking a cytotoxic drug to a monoclonal antibody that is specific for a tumor marker or antigen. The antibody recognizes this antigen and this is followed by the release of the cytotoxic agent, thus leading to cell death (13, 14).
Polatuzumab vedotin (Genentech Inc.; Polivy, polatuzumab vedotin-piiq) belongs to a class of ADCs which recognizes cells expressing CD79b and delivers a drug called monomethyl auristatin E (MMAE). CD79b is a cell surface marker and a component of the B-cell receptor that is expressed in mature B-cell lymphomas (15–17). The B-cell receptor is a multimeric complex, which is composed of a surface immunoglobulin that provides antigen specificity, and two associated proteins, CD79a and CD79b (Igα and Igβ), which can form a heterodimer upon phosphorylation and induce signaling (18). Mutations in CD79a have been reported in about 20% of ABC DLBCL patients, and these mutations result in enhancement of B-cell receptor signaling (19).
Polatuzumab vedotin has three components: an immunoglobulin G1 monoclonal antibody that recognizes human CD79b; the small molecule MMAE, which has anti-tubulin activity (20, 21); and a protease-cleavable linker that attaches MMAE to the antibody (22). Upon uptake of polatuzumab vedotin into the B cell, MMAE is released exerting its cytotoxic effect. A basic scheme of ADCs is shown in Figure 1.
Figure 1.
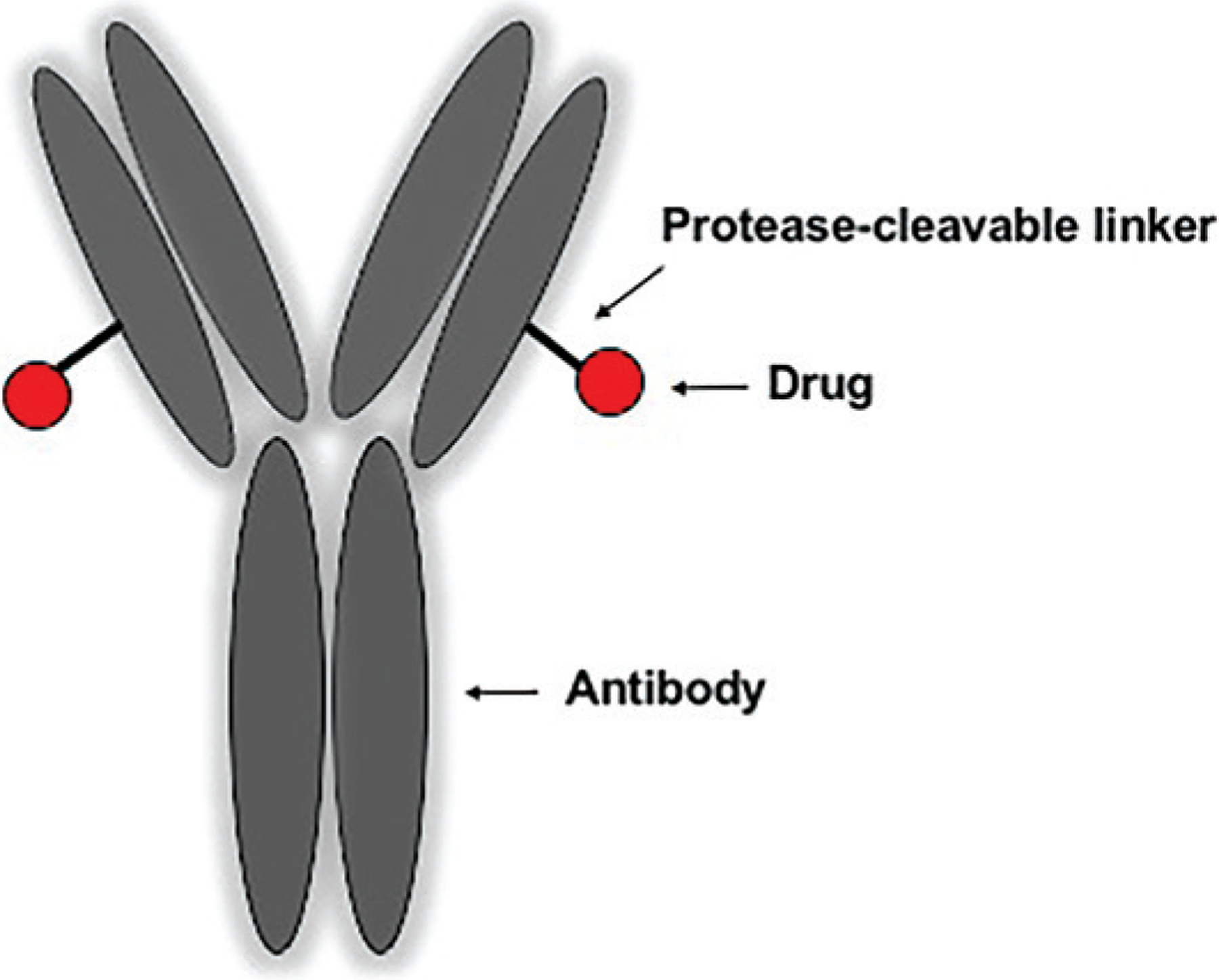
Antibody–drug conjugates are characterized by an antibody and a linker that attaches the drug of choice to the antibody.
Preclinical Data
Pfeifer et al. initially showed that several DLBCL cell lines expressed CD79b, and this was also confirmed in 32 patient samples. Furthermore, 22 out of 27 DLBCL cell lines were sensitive to anti-CD79b-MMAE, with no preferential response based on ABC or GCB subtypes, and internalization of anti-CD79b-MMAE was not changed in cell lines with CD79b mutations (16). Later, Tiwari et al. tested the activity of combining polatuzumab vedotin with obinutuzumab in primary mediastinal B-cell lymphoma and Burkitt lymphoma cell lines. They showed that combination therapy led to increased cytotoxicity in these cell lines, and it improved survival in B-cell lymphoma xenografted mice (23).
Clinical Data
Polatuzumab vedotin was first studied in DLBCL in a phase I study in relapsed or refractory patients (24). This study explored polatuzumab vedotin with and without rituximab in NHL and chronic lymphocytic leukemia patients who were expected to express CD79b. For this trial, a 3 + 3 dose-escalation design was employed using doses of 0.1–2.4 mg/kg of polatuzumab vedotin every 21 days. The recommended phase II dose for NHL was 2.4 mg/kg. At this dose, objective responses were seen in 56% (14/25) of DLBCL patients treated with single-agent polatuzumab vedotin, including 4 complete responses (CR) and 10 partial responses (PR), with a progression-free survival (PFS) of 5 months and median duration of response (DoR) of 5.2 months. The combination arm of polatuzumab vedotin plus rituximab showed objective responses in 78% (7/9) of patients, although this arm did not include DLBCL patients (24). In a follow-up phase II study looking at relapsed or refractory DLBCL patients treated with polatuzumab vedotin plus rituximab, 54% of patients achieved a response with 21% achieving a CR (25).
Another study looked at polatuzumab vedotin in combination with other therapies in the relapsed and refractory setting (26). This phase Ib/II clinical trial evaluated polatuzumab vedotin plus bendamustine and rituximab (BR) or obinutuzumab in patients who were ineligible for a transplant. Most patients in this study had received two or more prior therapies. A total of 6 patients were included in the safety cohort, 27 patients in the expansion phase and 80 patients in the randomized phase.
In the dose-escalation and dose-expansion phases, the CRs were 50% (3/6) and 30% (8/27), respectively (26). In the randomized phase of the trial, patients were randomized in a 1:1 fashion to receive either polatuzumab vedotin plus BR or BR alone. Due to cumulative toxicity in prior studies using the 2.4 mg/kg dose, patients were treated with polatuzumab vedotin 1.8 mg/kg on day 2 of cycle 1 and day 1 of subsequent cycles, bendamustine 90 mg/m2 per day for 2 days and rituximab 375 mg/m2 on day 1 for each cycle for up to 6 cycles. After a follow-up of 22.3 months, the CR for polatuzumab vedotin + BR was 40%, with a median DoR of 10.3 months, median PFS of 7.6 months and OS of 12.4 months. These values were significantly better than those in the cohort receiving BR, which showed a CR of 18% with a median DoR of 4.1 months, PFS of 2 months and OS of 4.7 months. The outcomes favored polatuzumab vedotin irrespective of cell of origin (ABC or GCB) (26).
The toxicity profile of polatuzumab vedotin consisted mainly of neutropenia, anemia, peripheral neuropathy, and less commonly, diarrhea and respiratory tract infections as detailed below (26).
Pharmacokinetics
Antibody-conjugated MMAE is 71–77% bound to plasma proteins, and its plasma concentration increases proportionally with a polatuzumab vedotin dose range of 0.1–2.4 mg/kg. The AUC of antibody-conjugated MMAE at cycle 3 is expected to achieve more than 90% of the AUC at cycle 6, and its half-life is approximately 12 days after cycle 6. The metabolism of polatuzumab vedotin has not yet been studied but it is expected to be metabolized into peptides, unconjugated MMAE and other related catabolites. MMAE is a substrate of cytochrome P450 (CYP) enzyme CYP3A4, and although there have been no specific studies on drug–drug interactions, the activity of unconjugated MMAE is expected to increase with concomitant use of drugs such as ketoconazole, and decrease with concomitant use of drugs such as rifampin. Polatuzumab vedotin has been shown to have no relevant interactions when administered with regimens including rituximab or obinutuzumab and bendamustine (27).
Dosing and Administration
Dosing
The recommended dose of polatuzumab vedotin is 1.8 mg/kg by intravenous infusion over 90 minutes, every 21 days (22). This is given on day 2 of cycle 1 and on day 1 on subsequent cycles. The administration of BR is as follows: bendamustine is administered intravenously at 90 mg/m2 on days 2 and 3 of cycle 1 and on day 2 of subsequent cycles, and rituximab is administered intravenously at 375 mg/m2 on day 1 of each cycle.
Premedications and prophylaxis
It is recommended to administer an antihistamine and an antipyretic at least 30 minutes prior to administration of polatuzumab vedotin for potential infusion-related reactions. It is also recommended to administer prophylaxis for Pneumocystis jiroveci pneumonia and herpesvirus during treatment with polatuzumab vedotin. Other considerations include prophylactic treatment with granulocyte colony-stimulating factor (G-CSF), as well as tumor lysis syndrome prophylaxis for patients who are at increased risk (22).
Dose modifications
Dose modifications are recommended for peripheral neuropathy, cytopenias and infusion reactions (22).
For peripheral neuropathy that is Grade (Gr) 2–3, it is recommended to hold the medication until improved to Gr 1. If recovery to Gr 1 neuropathy occurs by day 14, polatuzumab vedotin can be resumed at a reduced dose of 1.4 mg/kg, and if Gr 2–3 neuropathy continues beyond day 14 polatuzumab vedotin should be permanently discontinued. For Gr 4 peripheral neuropathy, it is recommended that polatuzumab vedotin be completely discontinued (22).
For Gr 3–4 neutropenia, it is recommended to hold treatment until ANC > 1000/μL. If ANC recovers later than 7 days, the healthcare provider can consider using G-CSF prophylactically and dose-reduce polatuzumab vedotin to 1.4 mg/kg if bendamustine has already been dose-reduced. Similarly, for Gr 3–4 thrombocytopenia, treatment should be held until platelets recover to > 75,000/μL, and if thrombocytopenia takes longer than 7 days to recover, polatuzumab vedotin could be dose-reduced to 1.4 mg/kg if bendamustine has already been dose-reduced (22).
In the event of a Gr 4 infusion reaction, polatuzumab vedotin should be permanently discontinued. For Gr 1–3 infusion reactions, interruption of infusion and supportive treatment is recommended, and infusion may be restarted at 50% of the rate once symptoms have resolved. However, if initial symptoms are Gr 3 (or recurrent Gr 2) wheezing, bronchospasm or generalized urticaria, polatuzumab vedotin should be permanently discontinued (22).
Safety and Toxicity
In the phase I study led by Palanca-Wessels, single-agent polatuzumab vedotin at the recommended phase II dose of 2.4 mg/kg resulted in Gr 3–4 adverse events (AEs) in 58% of NHL patients. The most common Gr 3–4 AEs were neutropenia (40%), anemia (11%) and peripheral neuropathy (9%). Other common AEs included diarrhea (4%) and respiratory tract infections (4%). In the cohort treated with polatuzumab vedotin and rituximab, 77% of patients had Gr 3–4 AEs, and the most common AEs were neutropenia (56%), anemia (22%) and febrile neutropenia (22%) (24).
In the study combining polatuzumab vedotin with BR, Gr 3–4 hematological toxicities were more common in the investigational arm than in the control BR arm. The most common toxicities in the investigational arm were Gr 3–4 neutropenia (46.2% vs. 33.3%), anemia (28.2% vs. 17.9%) and thrombocytopenia (41% vs. 23.1%), while Gr 3–4 infections were similar in both arms (23.1% vs. 20.5%) (26). Although cytopenias were higher in the polatuzumab vedotin + BR arm than in the BR arm, transfusion rates were no different between both arms. For red blood cells, transfusion rates were 25.6% versus 20.5%, and for platelets they were 15.4% versus 15.4%. Dose reduction of polatuzumab vedotin only occurred in 2 patients in this trial and it was due to Gr 2 peripheral neuropathy. The overall incidence of peripheral neuropathy was 43.6% in the polatuzumab vedotin + BR arm compared with 7.7% in the BR arm (26).
Current FDA Label and Boxed Warnings
On the basis of the results from the study by Sehn et al. (26), the U.S. Food and Drug Administration (FDA) granted accelerated approval to polatuzumab vedotin (Genentech Inc.; Polivy, polatuzumab vedotin-piiq) on June 6, 2019, and its indication is in combination with BR for adult patients with relapsed or refractory DLBCL after at least two prior therapies (22).
The FDA label includes warnings for peripheral neuropathy, infusion-related reactions, myelo-suppression and serious and opportunistic infections (22). There is also a warning for progressive multifocal leukoencephalopathy (PML), as 1/173 patients was reported to have PML following polatuzumab vedotin administration (26), and monitoring of worsening neurological, cognitive and behavioral changes should occur during treatment. The warning also includes tumor lysis syndrome for patients that have a high tumor burden. Liver enzymes and bilirubin should be monitored given possible hepatotoxicity, and this medication has an embryo-fetal toxicity warning, therefore females of reproductive potential should use effective contraception during treatment and for 3 months after the last dose is administered (22).
On January 16, 2020, the European Commission also granted conditional marketing authorization for the combination of polatuzumab vedotin plus BR for the treatment of adult patients with relapsed or refractory DLBCL who are not candidates for a hematopoietic stem cell transplant (28).
Future Directions
Although polatuzumab vedotin is currently approved only in the relapsed and refractory setting, it is likely its indication will be expanded on the basis of ongoing clinical trials testing its use in the upfront setting. A recent phase Ib/II clinical trial looked at the use of this agent in combination with cyclophosphamide, doxorubicin, prednisone and either rituximab or obinutuzumab in newly diagnosed DLBCL patients. The overall response rate was 89%, with CR of 77% and PR of 12%. The most common Gr 3–4 AEs were neutropenia (30%), febrile neutropenia (18%) and thrombocytopenia (9%) (29). Furthermore, polatuzumab vedotin is currently being tested in the front-line setting in a double-blind, placebo-controlled, randomized phase III clinical trial (POLARIX study), where R-CHOP is being compared to R-CHP (rituximab-cyclophosphamide, doxorubicin and prednisone) + polatuzumab vedotin. This study is currently enrolling (ClinicalTrials.gov Identifier NCT03274492).
A number of studies testing polatuzumab vedotin in combination with other agents in DLBCL are in progress (registered at www.clinicaltrials.gov), but do not have results yet. For example, polatuzumab vedotin is being studied in the relapsed and refractory setting in combination with rituximab and venetoclax. Similarly, polatuzumab vedotin is currently being tested in combination with mosunetuzumab in relapsed and refractory NHL. Polatuzumab vedotin has also shown activity in follicular lymphoma (25), and its indication may be expanded to other types of lymphoma in the future on the basis of ongoing studies. A list of ongoing clinical trials of polatuzumab vedotin is outlined in Table I.
Table I.
Ongoing clinical trials with polatuzumab vedotin.
| Study title | Conditions | NCT number |
|---|---|---|
| A phase III, randomized, double-blind, placebo-controlled study comparing the efficacy, safety, and pharmacokinetics of polatuzumab vedotin plus R-CHP vs R-CHOP in patients with previously untreated DLBCL |
|
NCT03274492 |
| An open-label, randomized, multicenter, phase Ib/II trial evaluating the safety, tolerability, pharmacokinetics, and efficacy of mosunetuzumab (BTCT4465A) in combination with polatuzumab vedotin in patients with B-cell non-Hodgkin lymphoma |
|
NCT03671018 |
| A phase Ib/II study evaluating the safety and efficacy of obinutuzumab in combination with polatuzumab vedotin and venetoclax in patients with relapsed or refractory follicular lymphoma and rituximab in combination with polatuzumab vedotin and venetoclax in patients with relapsed or refractory diffuse large B-cell lymphoma |
|
NCT02611323 |
| A phase Ib/II study investigating the safety, tolerability, pharmacokinetics, and efficacy of mosunetuzumab (BTCT4465A) in combination with CHOP or CHP-polatuzumab vedotin in participants with B-cell non-Hodgkin lymphoma |
|
NCT03677141 |
| A study of obinutuzumab, polatuzumab vedotin, and lenalidomide in relapsed or refractory follicular lymphoma (FL) and rituximab in combination with polatuzumab vedotin and lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (DLBCL) |
|
NCT02600897 |
| A phase III study to evaluate the safety and efficacy of polatuzumab vedotin in combination with rituximab, gemcitabine and oxaliplatin compared to rituximab, gemcitabine and oxaliplatin alone in participants with relapsed or refractory diffuse large B-cell lymphoma |
|
NCT04182204 |
| An open-label phase Ib study of RO7082859 and atezolizumab or polatuzumab vedotin in adult patients with relapsed/refractory B-cell non-Hodgkin’s lymphoma |
|
NCT03533283 |
| A study evaluating safety and efficacy of obinutuzumab, polatuzumab vedotin (Pola), and atezolizumab (atezo) in participants with relapsed or refractory follicular lymphoma and rituximab, atezo, and Pola in participants with relapsed or refractory diffuse large B-cell lymphoma |
|
NCT02729896 |
DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; NHL, non Hodgkin lymphoma; R-CHOP, rituximab-cyclophosphamide, doxorubicin, vincristine and prednisone; R-CHP, rituximab-cyclophosphamide, doxorubicin and prednisone; R/R, relapsed or refractory.
Conclusions
Polatuzumab vedotin is a new FDA- and E.U.-approved ADC that recognizes the commonly expressed B-cell antigen CD79b and subsequently delivers MMAE, a small molecule with anti-tubulin activity. Its current indication is for use in combination with BR in patients with relapsed or refractory DLBCL ineligible for transplant. This approval was based on a phase Ib/II study comparing polatuzumab vedotin plus BR versus BR alone, which resulted in a CR of 40%, a median PFS of 7.6 months and OS of 12.4 months for polatuzumab vedotin + BR, compared with a CR of 18%, a median PFS of 2 months and OS of 4.7 months in the BR arm. We anticipate the indications for polatuzumab vedotin will expand, pending the results of ongoing clinical trials.
Acknowledgments
M.L. Amaya is supported by the Cancer Biology T32CA190216.
Footnotes
Disclosures
M. Kamdar has served on the speakers’ bureau of Seattle Genetics; has acted as a consultant for Pharmacyclics, Celgene and AstraZeneca; and has served as one of the coinvestigators on the study “Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma” (26). M.L. Amaya and A. Jimeno state no conflicts of interest.
References
- 1.Cancer stat facts: NHL - Diffuse large B-cell lymphoma (DLBCL). National Institutes of Health (NIH) – National Cancer Institute, https://seer.cancer.gov/statfacts/html/dlbcl.html. [Google Scholar]
- 2.Rosenwald A, Wright G, Chan WC et al. The use of molecular profiling to predict survival after chemotherapy for diffuse large-B-cell lymphoma. N Engl J Med 2002, 346(25): 1937–47. [DOI] [PubMed] [Google Scholar]
- 3.Gutiérrez-García G, Cardesa-Salzmann T, Climent F et al. Gene-expression profiling and not immune-phenotypic algorithms predicts prognosis in patients with diffuse large B-cell lymphoma treated with immunochemotherapy. Blood 2011, 117(18): 4836–43. [DOI] [PubMed] [Google Scholar]
- 4.Scott DW, Mottok A, Ennishi D et al. Prognostic significance of diffuse large B-cell lymphoma cell of origin determined by digital gene expression in formalin-fixed paraffin-embedded tissue biopsies. J Clin Oncol 2015, 33(26): 2848–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu Y, Barta SK Diffuse large B-cell lymphoma: 2019 update on diagnosis, risk stratification, and treatment. Am J Hematol 2019, 94(5): 604–16. [DOI] [PubMed] [Google Scholar]
- 6.Green TM, Young KH, Visco C et al. Immuno-histochemical double-hit score is a strong predictor of outcome in patients with diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012, 30(28): 3460–7. [DOI] [PubMed] [Google Scholar]
- 7.Johnson NA, Slack GW, Savage KJ et al. Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol 2012, 30(28): 3452–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartlett NL, Wilson WH, Jung SH et al. Dose-adjusted EPOCH-R compared with R-CHOP as frontline therapy for diffuse large B-cell lymphoma: clinical outcomes of the phase III Intergroup Trial Alliance/CALGB50303. J Clin Oncol 2019, 37(21): 1790–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coiffier B, Thieblemont C, Van Den Neste E et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d’Etudes des Lymphomes de l’Adulte. Blood 2010, 116(12): 2040–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gisselbrecht C, Glass B, Mounier N et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol 2010, 28(27): 4184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Philip T, Guglielmi C, Hagenbeek A et al. Autologous bone marrow transplantation as compared with salvage chemotherapy in relapses of chemotherapy-sensitive non-Hodgkin’s lymphoma. N Engl J Med 1995, 333(23): 1540–5. [DOI] [PubMed] [Google Scholar]
- 12.Crump M, Neelapu SS, Farooq U et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood 2017, 130(16): 1800–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bakhtiar R Antibody drug conjugates. Biotechnol Lett 2016, 38(10): 1655–64. [DOI] [PubMed] [Google Scholar]
- 14.Beck A, Goetsch L, Dumontet C, Corvaia N Strategies and challenges for the next generation of antibody–drug conjugates. Nat Rev Drug Discov 2017, 16(5): 315–37. [DOI] [PubMed] [Google Scholar]
- 15.Dornan D, Bennett F, Chen Y et al. Therapeutic potential of an anti-CD79b antibody–drug conjugate, anti-CD79b-vc-MMAE, for the treatment of non-Hodgkin lymphoma. Blood 2009, 114(13): 2721–9. [DOI] [PubMed] [Google Scholar]
- 16.Pfeifer M, Zheng B, Erdmann T et al. Anti-CD22 and anti-CD79B antibody drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia 2015, 29(7): 1578–86. [DOI] [PubMed] [Google Scholar]
- 17.Polson AG, Calemine-Fenaux J, Chan P et al. Antibody-drug conjugates for the treatment of non-Hodgkin’s lymphoma: target and linker-drug selection. Cancer Res 2009, 69(6): 2358–64. [DOI] [PubMed] [Google Scholar]
- 18.Dal Porto JM, Gauld SB, Merrell KT, Mills D, Pugh-Bernard AE, Cambier J B cell antigen receptor signaling 101. Mol Immunol 2004, 41(6–7): 599–613. [DOI] [PubMed] [Google Scholar]
- 19.Rossi D, Ciardullo C, Gaidano G Genetic aberrations of signaling pathways in lymphomagenesis: revelations from next generation sequencing studies. Semin Cancer Biol 2013, 23(6): 422–30. [DOI] [PubMed] [Google Scholar]
- 20.Bai RL, Pettit GR, Hamel E Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and vinca alkaloid sites. J Biol Chem 1990, 265(28): 17141–9. [PubMed] [Google Scholar]
- 21.Doronina SO, Toki BE, Torgov MY et al. Development of potent monoclonal antibody auristatin conjugates for cancer therapy. Nat Biotechnol 2003, 21(7): 778–84. [DOI] [PubMed] [Google Scholar]
- 22.Polivy. Highlights of prescribing information. Food and Drug Administration (FDA), 2019. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2019/761121Orig1s000Lbl.pdf. [Google Scholar]
- 23.Tiwari A, Edani D, Ayello J, Klein C, Lee DA, Cairo MS Polatuzumab vedotin alone or in combination with obinutuzumab synergistically enhances in-vitro cytotoxicity and cytokine release against CD20+/CD79b+ Burkitt lymphoma (BL)/primary mediastinal large B-cell lymphoma (PMBL). Blood [59th Annu Meet Am Soc Hematol (Dec 9–12, 2017] 2017, 130: Abst 1540. [Google Scholar]
- 24.Palanca-Wessels MC, Czuczman M, Salles G et al. Safety and activity of the anti-CD79B antibody–drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol 2015, 16(6): 704–15. [DOI] [PubMed] [Google Scholar]
- 25.Morschhauser F, Flinn IW, Advani R et al. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS). Lancet Haematol 2019, 6(5): e254–e65. [DOI] [PubMed] [Google Scholar]
- 26.Sehn LH, Herrera AF, Flowers CR et al. Polatuzumab vedotin in relapsed or refractory diffuse large B-cell lymphoma. J Clin Oncol 2020, 38(2): 155–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deeks ED Polatuzumab vedotin: first global approval. Drugs 2019, 79(13): 1467–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polivy: polatuzumab vedotin. European Medicines Agency (EMA), 2020. https://www.ema.europa.eu/en/medicines/human/EPAR/polivy. [Google Scholar]
- 29.Tilly H, Morschhauser F, Bartlett NL et al. Polatuzumab vedotin in combination with immunochemotherapy in patients with previously untreated diffuse large B-cell lymphoma: an open-label, non-randomised, phase 1b-2 study. Lancet Oncol 2019, 20(7): 998–1010. [DOI] [PubMed] [Google Scholar]


