Abstract
Pulmonary arterial hypertension is a kind of heart and lung vascular disease with low incidence and poor prognosis. Genetic variants are the important factors of pulmonary arterial hypertension. The mutations of activin receptor-like kinase-1 (ACVRL1) could cause pulmonary arteriole obstruction and occlusion in pulmonary arterial hypertension patients. The ACVRL1 gene mutation and clinical characteristics of Chinese idiopathic or hereditary pulmonary hypertension (IPAH/HPAH) patients are still unclear. This study aimed to retrospectively study the mutation characteristics of ACVRL1 gene in Chinese IPAH/HPAH patients and its effect on clinical prognosis. We analyzed the clinical, functional, hemodynamic and mutation characteristics of 12 IPAH/HPAH patients with ACVRL1 mutations and compared with 94 IPAH/HPAH patients (27 patients carried bone morphogenetic protein receptor type 2 (BMPR2) mutations and 67 without mutations). All ACVRL1 mutations of 12 patients were single nucleotide missense mutations. The ratio of male to female in 12 patients was 1:1. The diagnosis age of ACVRL1 mutation patients was younger than that of BMPR2 mutation patients (13.6 ± 11.3 years vs. 16.0 ± 12.9 years) but higher than that of patients without mutations (13.6 ± 11.3 years vs. 8.8 ± 8.5 years, p = 0.006). IPAH/HPAH patients with ACVRL1 mutation have rapid disease progresses, high overall mortality rate (approximately 50%) and no response to the acute pulmonary vasodilation test. In conclusion, this is the first study to analyze the ACVRL1 gene mutation and clinical characteristics of Chinese IPAH/HPAH patients. It is beneficial to screen ACVRL1 gene mutation for IPAH/HPAH patients to facilitate genetic counseling and early prevention and treatment.
Keywords: pulmonary hypertension, ACVRL1, gene mutation
Introduction
Pulmonary arterial hypertension (PAH) is a kind of heart and lung vascular disease with low incidence and poor prognosis. The unknown etiology PAH is classified as idiopathic pulmonary hypertension (IPAH). The annual incidence is one to two cases per million population in Europe and the United States.1,2 A study reported that the proportion of inpatient PAH was 6.63% and IPAH patients was 3.77%, 3 and the one-, three-, five-, seven-year survival rates of IPAH patients were 91% ± 2%, 74% ± 2%, 65% ± 3% and 59% ± 3%, respectively. 4 What’s more, PAH has a poor prognosis and high treatment cost, which causes a heavy burden on the family and society.
The pathogenesis of IPAH is unknown, but may be related to many factors, including hypoxic environment, toxicants and drug stimulation, immune disorders, cytokines, genetic polymorphism, gene defects, etc. Genetic variants are closely associated with IPAH. 5 The mutations of activin receptor-like kinase-1 (ACVRL1), a TGF-β receptor family member, have been found to be associated with PAH. 6 Dysfunction of the TGF-β pathway could promote pulmonary endothelial cell and smooth muscle cell dysfunction. 7 In addition, ACVRL1 mutation has been found in patients with hereditary hemorrhagic telangiectasia (HHT) combined with PAH. 8
ACVRL1 mutations were the important causes of the abnormal signal regulation of TGF-β/BMP-9 pathway, which causes pulmonary arteriole obstruction and occlusion in PAH patients and vascular dilation in HHT patients. 9 Therefore, the mutation characteristics of ACVRL1 gene in these two diseases are worth to study. However, the clinical and genetic characteristics of ACVRL1 mutation in HHT and PAH patients have been reported in other countries.6,10,11 But the ACVRL1 gene mutation and clinical characteristics of Chinese IPAH/HPAH patients are still unclear. Here, we used whole exome sequencing to detect the rare variants of ACVRL1 gene in 106 Chinese IPAH/HPAH patients, and we described the genetic and clinical characteristics of Chinese IPAH/HPAH patients for the first time.
Material and methods
Patients
There were 218 IPAH/HPAH patients (including adults and children) admitted to the Pediatric Cardiology Department of our Hospital between 1 January 2010 and 31 December 2020. We collected and analyzed the data of 106 IPAH/HPAH patients who underwent genetic screening. The patients were not related to each other. The diagnosis of IPAH was determined with hemodynamics measured by right heart catheterization. The patients who could not be examined by right heart catheterization (The peak velocity of tricuspid regurgitation was greater than 2.8 m/s) were diagnosed as PAH combined by echocardiography with clinical symptoms and other signs of right cardiac overload.12,13 Other etiologies of pulmonary hypertension were excluded based on the European Society of Cardiology guidelines. 12 The clinical diagnosis of HHT is based on the Curacao criteria, 14 which propose that three or more of the characteristic features described below define a definite diagnosis, whereas two of these features suggest a “possible or suspected” diagnosis and one or none of these features indicate unlikely HHT. Characteristic features of HHT include recurrent epistaxis, the presence of mucocutaneous telangiectasias at characteristic sites, arteriovenous malformations (AVMs) in internal organs and a first-degree relative with definite HHT. According to Curacao criteria, patients with no or only one of the above characteristic features in our study were excluded from HHT. Patients who screened for genetic mutations or their guardians signed informed consent and received genetic counseling. The research was approved by the Research Ethics Committee of our Hospital (No. 2020106X).
Hemodynamic monitoring and acute pulmonary vasodilation test
PAH was defined as mean pulmonary arterial pressure greater than 20 mmHg, accompanied by pulmonary arterial wedge pressure (PAWP) less than 15 mmHg and pulmonary vascular resistance (PVR) greater than 3 WU. 15 Acute pulmonary vasodilation test (AVT) was performed during right cardiac catheterization, and a positive reaction was defined as a decrease in mean pulmonary arterial pressure greater than 10 mmHg and a maximum mean pulmonary arterial pressure less than 40 mmHg in the case of increased or constant cardiac output.16,17
Genetic testing and WES data analysis
Whole exome sequencing was performed by BestNovo (Beijing) Medical Laboratory to detect the rare variants of ACVRL1 gene in 106 Chinese IPAH/HPAH patients. Genomic DNA was extracted from peripheral venous blood using a DNA Isolation Kit (Roche, Indianapolis, USA). GenCap enrichment kit (MyGenostics) was used to capture exons of candidate genes. 18 HiSeq 2500 (Illumina Inc., USA) was sequenced with an average effective sequencing depth of 100×. Results were compared with the reference sequence (GenBank M_000020), and bioinformatics analysis was performed. 18 The pathogenicity of the mutations was predicted using predictive software (Polyphen-2, SIFT, PANTHER, and Pmut). The variants were confirmed by Sanger sequencing.
Statistical analysis
We compared the demographic, hemodynamic and clinical characteristics of ACVRL1 mutation carriers, BMPR2 mutation carriers and non-mutation carriers. Continuous variables were expressed as mean ± standard deviation and classification variables as percentiles. Continuous variables that conform to normal distribution were compared by ANOVA, those that do not conform to normal distribution were compared by Kruskal-Wallis Test and classified variables were compared by chi-square test. Kaplan-Meier overall survival curve was constructed to show the difference of survival rate among the three groups, and the log-rank test was used for comparison. Cox proportional hazard regression model was used to study the relationship between mutations and all-cause mortality. A p value less than 0.05 was considered statistically significant. All analyses were performed with the SPSS version 23.0.
Results
Patient groups
A total of 106 IPAH/HPAH patients who underwent genetic screening at our Hospital during 31 December 2020 were enrolled in this study, including 59 female patients. There were 12 ACVRL1 mutation carriers, 27 BMPR2 mutation carriers and 67 non-mutation carriers. To limit the risk of misclassification, we excluded PAH patients with other mutations. There were 30 patients with PAH who did not undergo right heart catheterization, including three patients with ACVRL1 mutation. The reason was mainly due to the patient's severe condition and the patient's refusal.
Characteristics of PAH patients with ACVRL1 mutations
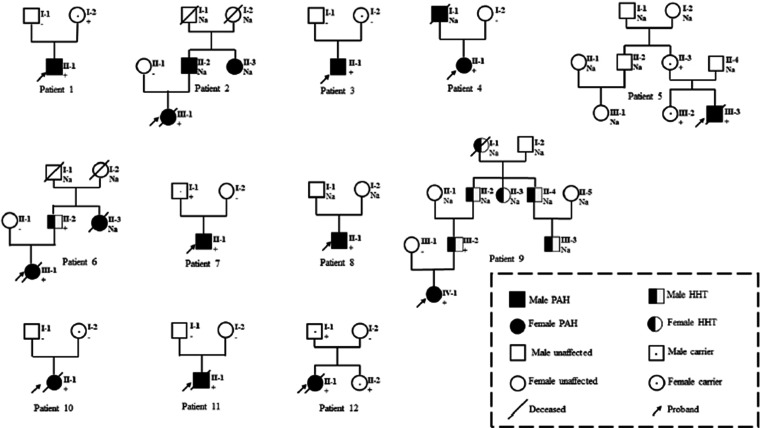
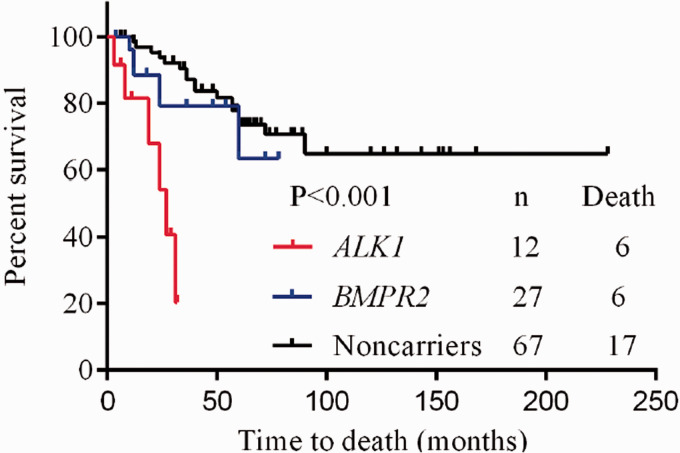
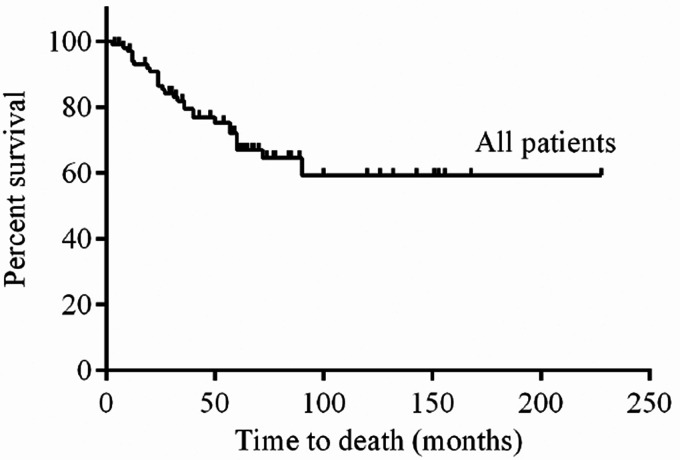
Table 1 lists the clinical, functional and hemodynamic characteristics of all PAH patients in the study. Table 2 lists the ACVRL1 mutations in 12 patients. Twelve patients with ACVRL1 mutations came from different families (Fig. 1). Two patients had PAH family history. Two patients had a family history of HHT and presented with epistaxis, but HHT could not be diagnosed according to Curacao criteria (See Supplementary Table S3 for criteria). Characteristics of PAH patients with ACVRL1 mutations were shown in Supplementary Table S4. Nine patients had a history of syncope. No sex advantage was observed in patients with a 1:1 ratio of females to males who carrying the ACVRL1 mutation. As shown in Fig. 3, ACVRL1 mutation carriers had the highest overall mortality rate, and BMPR2 mutation carriers had lower three-year and five-year survival rates than non-mutation carriers (79.1% and 63.3%, respectively, compared to 87.1% and 73.7%, p < 0.001); PAH patients with ACVRL1 mutations were younger at diagnosis than those with BMPR2 mutations, but older than those without mutations (13.6 ± 11.3 years, 16.0 ± 12.9 years and 8.8 ± 8.5 years, respectively, p = 0.006). There was no significant difference in pulmonary systolic pressure measured by echocardiography among the three groups. Brain natriuretic peptide levels in ACVRL1 carriers were significantly higher than those in BMPR2 mutation carriers and non-mutation carriers (743.2 pg/ml, 214.0 pg/ml and 255.0 pg/ml, p = 0.017). Right heart catheterization and AVT were performed in nine ACVRL1 mutation carriers. Carriers with the ACVRL1 and BMPR2 mutations did not respond to the AVT. Thirty-two percent of patients without the mutation were positive for the AVT. There were no significant differences in mean pulmonary artery pressure, right atrial pressure and pulmonary artery wedge pressure among these three groups. Patients with ACVRL1 mutation had higher pulmonary vascular resistance than those without the mutation and lower pulmonary vascular resistance than those with BMPR2 mutation (19.1 ± 10.2 Wu, 14.3 ± 8.6 Wu and 22.5 ± 12.7 Wu, respectively, p = 0.016). The cardiac index of patients with ACVRL1 mutation was lower than those without the mutation and higher than those with BMPR2 mutation (2.9 ± 0.7 L/min/m2, 3.2 ± 1.0 L/min/m2 and 2.4 ± 0.5 L/min/m2, p = 0.012). The overall three-year, five-year and 10-year survival rates were 79.5%, 67%, and 59%, respectively, in all 106 PAH patients who received targeted PAH therapy (Fig. 2). Six of the 12 PAH patients who received targeted therapy with ACVRL1 mutations died, with a higher overall mortality rate than those with BMPR2 mutations or without mutations (p < 0.001) (Fig. 3), and the cause of death was directly related to PAH (Table 3). Univariate Cox proportional risk models of time of death showed that ACVRL1 mutation carriers had a significantly worse prognosis than those without mutations (HR = 13.89, p < 0.001). Patients with ACVRL1 mutations had a worse prognosis than those with BMPR2 mutations (HR = 4.51, p = 0.014) (Table 4). There was no significant difference in the effect of gender factors on the prognosis of the three groups.
Table 1.
Baseline characteristics and treatment in patients with pulmonary arterial hypertension.
| Variable | ACVRL1 mutation carriers (n = 12) | BMPR2 mutation carriers (n = 27) | Mutation non-carriers (n = 67) | All patients (n = 106) | p |
|---|---|---|---|---|---|
| NYHA FC III or IV | 9/12 (75%) | 14/27 (52%) | 28/67 (42%) | 51/106 (48%) | 0.105 |
| Death | 6/12 (50%) | 6/27 (22.2%) | 17/67 (25.4%) | 29/106 (27%) | 0.169 |
| Female gender | 6 (50%) | 17 (63%) | 36 (54%) | 59 (56%) | 0.368 |
| Age at onset (years) | 13.6 ± 11.3 | 16.0 ± 12.9 | 8.8 ± 8.5 | 11.1 ± 10.6 | 0.006 |
| Brain natriuretic peptide (pg/ml) | 743.2 (471.3–1179.9) | 214.0 (117.0–556.0) | 255.0 (56.8–844.5) | 306.0 (92.0–795.0) | 0.017 |
| Systolic PAP (cardiac echocardiography) (mmHg) | 91.3 ± 23.2 | 83.8 ± 16.7 | 81.0 ± 22.0 | 82.9 ± 21.1 | 0.301 |
| Mean pulmonary artery pressure (mm Hg) | 70.2 ± 14.7 (n = 9) | 72.3 ± 18.4 (n = 23) | 63.9 ± 23.7 (n = 44) | 67.2 ± 21.4 (n = 76) | 0.285 |
| Right atrial pressure (mm Hg) | 9.2 ± 4.4 (n = 9) | 9.5 ± 3.4 (n = 23) | 8.0 ± 2.8 (n = 44) | 8.3 ± 3.2 (n = 76) | 0.149 |
| Pulmonary vascular resistance (Wood units) | 19.1 ± 10.2 (n = 9) | 22.5 ± 12.7 (n = 23) | 14.3 ± 8.6 (n = 44) | 17.7 ± 10.9 (n = 76) | 0.016 |
| Pulmonary artery wedge pressure (mm Hg) | 9.9 ± 4.0 (n = 9) | 11.4 ± 2.9 (n = 23) | 11.0 ± 2.9 (n = 44) | 11.1 ± 3.1 (n = 76) | 0.462 |
| Cardiac index (L/min/m2) | 2.9 ± 0.7 (n = 9) | 2.4 ± 0.5 (n = 23) | 3.2 ± 1.0 (n = 44) | 2.8 ± 0.8 (n = 76) | 0.012 |
| Acute vasodilator responder | 0/9 | 0/23 | 14/44 (32%) | 14/76 (18%) | |
| PAH therapy(targeted drug) | |||||
| None, n (%) | 0 | 0 | 4 (6%) | 4 (3.8%) | |
| Monotherapy, n (%) | 3 (25%) | 1 (3.7%) | 33 (49.3%) | 37 (34.9%) | |
| ERAs | 1 | 1 | 15 | 17 | |
| PDE-5is | 2 | 0 | 15 | 17 | |
| Prostanoids | 0 | 0 | 0 | 0 | |
| CCBs | 0 | 0 | 3 | 3 | |
| Combination therapy, n (%) | 9 (75%) | 26 (96.3%) | 30 (44.7%) | 65 (61.3%) | |
| ERAs + PDE-5is | 5 | 18 | 21 | 44 | |
| ERAs + Prostanoids | 0 | 2 | 1 | 3 | |
| PDE-5is + Prostanoids | 1 | 1 | 2 | 4 | |
| CCBs + Prostanoids | 0 | 0 | 2 | 2 | |
| ERAs + PDE-5is + Prostanoids | 3 | 5 | 4 | 12 | |
Note: Data are expressed as number (percentage), as mean ± SD, or as median (interquartile range).
NYHA FC: New York Heart Association Function Class; CCBs: calcium channel blockers; ERAs: endothelin receptor antagonists; PDE-5is: phosphodiesterase-5 inhibitors; PAH: pulmonary arterial hypertension.
Table 2.
Details of ACVRL1 mutations.
| Patient | Mutation location | Nucleotide changea | Amino acid changeb | Mutation category | Reference |
|---|---|---|---|---|---|
| 1 | Exon3 | c.77C>T | p.P26L | Missense | Reported |
| 2 | Exon5 | c.601C>A | p.Q201K | Missense | 17 |
| 3 | Exon6 | c.643G>A | p.E215K | Missense | Reported |
| 4 | Exon6 | c.665A>C | p.H222P | Missense | Novel |
| 5 | Exon6 | c.676G>A | p.V226M | Missense | Reported |
| 6 | Exon7 | c.955G>C | p.G319R | Missense | 18 |
| 7 | Exon8 | c.1120C>T | p.R374W | Missense | 9,19 |
| 8 | Exon8 | c.1135G>A | p.E379K | Missense | 20 |
| 9 | Exon8 | c.1217G>T | p.W406L | Missense | Novel |
| 10 | Exon8 | c.1231C>T | p.R411W | Missense | 21 |
| 11 | Exon10 | c.1450C>T | p.R484W | Missense | 6 |
| 12 | Exon10 | c.1451G>A | p.R484Q | Missense | 22–25 |
aThe mutation nomenclature follows current guidelines as recommended by the Human Genome Variation Society. Transcript is NM-000020. The letter c. indicates the numbering of the base change.
bThe letter p. is used to indicate the change at the protein level.
Fig. 1.
Family trees of patients with pulmonary arterial hypertension (PAH) and carrying an activin A receptor-type II-like kinase-1 (ACVRL1).
Fig. 3.
Survival of BMPR2 and ALK1 mutation carriers and mutation non-carriers with PAH (log-rank test, p < 0.001).
Fig. 2.
Kaplan-Meier curves for survival of all patients. Three-, five- and 10-year survival probabilities were 79.5%, 67% and 59%, respectively.
Table 3.
Follow-up of patients with pulmonary arterial hypertension carrying an ACVRL1 mutation.
| Patient | PAH therapy | NYHA class | Follow-up (months after diagnosis) | Cause of death |
|---|---|---|---|---|
| 1 | Bosentan plus sildenafil | III | Alive at month 29 | – |
| 2 | Ambrisentan plus tadalafil | III | Death at month 19 | Right heart failure |
| 3 | Bosentan plus tadalafil and treprostinil | III | Alive at month 8 | – |
| 4 | Bosentan plus sildenafil | IV | Alive at month 6 | – |
| 5 | Bosentan | II | Death at month 24 | Right heart failure |
| 6 | Sildenafil | II | Death at month 27 | Right heart failure |
| 7 | Bosentan plus sildenafil and treprostinil | IV | Death at month 8 | Right heart failure |
| 8 | Ambrisentan plus tadalafil | III | Alive at month 11 | – |
| 9 | Macitentan plus tadalafil and treprostinil | III | Alive at month 6 | – |
| 10 | Sildenafil | IV | Death at month 3 | Right heart failure |
| 11 | Sildenafil plus beraprost sodium | III | Death at month 31 | Right heart failure |
| 12 | Bosentan plus tadalafil | II | Alive at month 32 | – |
PAH: pulmonary arterial hypertension; NYHA: New York Heart Association.
Table 4.
Univariate Cox proportional-hazards model for time to death in patients with pulmonary arterial hypertension.
| Characteristic | Hazard ratio (95% Confidence Interval) | p |
|---|---|---|
| ACVRL1 mutation vs. mutation non-carriers | 13.89 (4.15–46.48) | <0.001 |
| BMPR2 mutation vs. mutation non-carriers | 1.58 (0.61–4.10) | 0.348 |
| ACVRL1 mutation vs. BMPR2 mutation | 4.51 (1.36–14.93) | 0.014 |
| Gender (male vs. female) | 0.92 (0.59–1.45) | 0.729 |
Targeted drug therapy
Until now, drug therapy targeting the endothelin, nitric oxide and prostacyclin pathways is the mainly used treatment method. 12 The application of targeted drugs in patients with PAH is mainly based on the patient's condition and economic conditions (shown in Table 1).
ACVRL1 gene mutations in IPAH/HPAH patients
We found 12 different ACVRL1 exon mutations, all of which were single nucleotide missense mutations. And there were no patients who have copy number variants or exonic deletion in ACVRL1. Sanger sequencing were performed for these structural variants (See Supplementary Figs. 2 and 3). Ten mutations occurred in the intracellular kinase domain of ACVRL1, of which four (33%) were located on exon 8 and one was located on the GS-rich region of ACVRL1 (exon 5). Two previously unreported ACVRL1 mutations were found, c.665A>C (p.His222Pro) and c.1217G>T (p.Trp406Leu). Table 2 describes all mutations. Amino acids at all mutant sites were highly conserved in multiple species (see Supplementary Fig. 1). Two patients also carried other PAH-related gene mutations. One patient carried a heterozygous mutation of both ACVRL1 (c.665A>C, p.H222P) and EIF2AK4 (c.2320-4T>G, splicing). One patient carried a heterozygous mutation of both ACVRL1 (c.601C>A, p.Q201K) and NOTCH3 (c.224G>A, p.Arg75Gln).
Discussion
Mutations in ACVRL1 are one of the causes of HHT and PAH. HHT is a non-fatal disease, and its penetrance increases with age. 19 PAH is a rare and fatal disease. The relationship between the penetrance of PAH caused by ACVRL1 mutation and age has not been reported. Girerd et al. 6 reported that the diagnosis age of ACVRL1 mutation carriers in PAH patients was 21.8 ± 16.7 years old. Our study found that the mean age of diagnosis of ACVRL1 mutation carriers was 13.6 ± 11.3 years old (n = 12), and nine of the 12 patients were children, which suggests that PAH associated with ACVRL1 mutations tend to develop clinical phenotype at a younger age. Western scholars' studies have reported that the ratio of female to male in HHT patients was 1:1, 19 but PAH patients was 1.9:1 to 4.1:1. 20 The ratio of female to male in ACVRL1 mutation patients was 1:1 in this study, showing no gender advantage. Females accounted for 63% of BMPR2 mutation carriers, 54% of those without mutations and 56% of the 106 PAH patients. The main reason for the lack of female gender preponderance is that the cohort of this study is dominated by children.
In this study, BMPR2 mutation carriers had the worst pulmonary vascular resistance and cardiac index among the three groups of patients, while ACVRL1 mutation carriers were worse than those without mutation. Although baseline hemodynamic characteristics of ACVRL1 mutation carriers were less severe than those of BMPR2 mutation carriers, they had the highest overall mortality, indicated rapid progression and poor prognosis. The specific reasons need further observation and research. Nine ACVRL1 mutation carriers were tested for acute pulmonary vasodilation and all were negative, indicated that PAH patients with the ACVRL1 mutation had a poor response to pulmonary vasodilation drugs. More aggressive treatments were recommended for PAH patients with ACVRL1 mutations.
Among the 12 PAH patients with ACVRL1 mutation, 10 patients were the only PAH patients in the family, and two patients had epistaxis after PAH symptoms, indicated that PAH could be the first or only manifestation of ACVRL1 mutation carriers, which was consistent with the study conclusions of Fujiwara et al. 21 The ACVRL1 mutation of eight patients was derived from the previous generation (see Fig. 1), and the parents of the patients carried ACVRL1 mutation without clinical manifestations of PAH, which emphasized that ACVRL1 gene mutation was not all the factors contributing to PAH, and there may be other stimulating factors, such as additional abnormal genes, environmental factors and abnormal autoimmune function, etc. This is consistent with the theory of "second strike". 22 Long-term follow-up observation and genetic counseling are necessary for PAH patients with ACVRL1 mutation and their families.
In our study, one case of mutation c.601C>A (p.Q201K) was located on exon 5, and the patient died two years after treatment. Tørring et al. 23 reported an 84-year-old HHT patient with this mutation. However, there were no report about this mutation and PAH. Exon 5 is located on the glycine–serine-rich domain of ACVRL1, which is a key regulatory region that regulates the catalytic activity of ALK1 protein or its interaction with substrate. 24 Pathogenic mutations occurring in exon 5 affect the function of ALK1 protein, leading to the pathogenesis of HHT and PAH and may be associated with poor prognosis of PAH.
ACVRL1 mutations of seven cases found in this study have been reported,6,7,10,11,21,24– 29 including c.601C>A, c.955G>C, c.1120C>T, c.1135G>A, c.1231C>T, c.1450C>T, c.1451G>A. Two novel mutations were found, c.665A>C (p.His222Pro) and c.1217G>T (p.Trp406Leu). Mutations in c.665A>C (p.His222Pro) is located on the ACVRL1 kinase domain. The missense mutations near this site, such as c.647T>G (p.Val216Gly), c.677T>A (p.Val226Glu), c.682G>A (p.Val228Ile), c.686A>T (p.Lys229Met) were assessed as pathogenic or possibly pathogenic (NM_000020, Clinvar database) and were predicted to be deleterious by predictive software (Polyphen-2, SIFT, PANTHER, and Pmut). The amino acid at this site is highly conserved in vertebrates (see Supplementary Fig. 1). Therefore, according to American College of Medical Genetics and Genomics (ACMG) guideline, ACVRL1 c.665A>C was a likely pathogenic mutation. The children who carrying ACVRL1 mutation c.1217G>T (p.Trp406Leu) had a family history of HHT, their great-grandmothers and grandfathers had recurrent epistaxis, and their fathers carried the same mutation and had recurrent epistaxis and pulmonary AVM, but none of them showed clinical features of PAH. c.1217G>T located on the ACVRL1 kinase domain, was predicted as deleterious by predictive software. The ACMG grade of ACVRL1 c.665A>C was likely pathogenic. In addition, a protein function verification test is conducted to prove the pathogenicity of the two novel mutations at the molecular and cellular level.
The distribution of ACVRL1 mutations is the focus of research in recent years. Girerd et al. 6 found that mutations on exon 10 of ACVRL1 were common in PAH patients (accounting for 33.3% of ACVRL1 mutations). Zhao et al. 10 found that 47.1% of ACVRL1 mutations in HHT patients were distributed on exon 8, and they believed that exon 8 was the hot mutation region of HHT. Chen et al. 11 found that in eight HHT-associated PAH patients with ACVRL1 mutations, three cases of mutations occurred on exon 8. Of the 12 patients with ACVRL1 mutations in this study, four patients (33%) had mutations on exon 8, c.1120C>T (p.R374W), c.1135G>A (p.E379K), c.1217G>T (p.W406L), c.1231C>T (p.R411W), indicated that exon 8 of ACVRL1 gene was a highly correlated mutation region with PAH.
This study has some limitations and belongs to a single-center retrospective study. Some patients did not have a right heart catheterization, which need improve follow-up. The higher proportion of children results in a lower mean age at diagnosis for patients without mutations. The sample size of PAH patients with ACVRL1 mutation was small, and the follow-up time of some patients was short, leading to no statistical difference in some observed indicators. Multicenter, larger sample sizes and long-term follow-up studies are needed.
Conclusion
In conclusion, this study found that ACVRL1 mutations were mainly missense mutations in Chinese IPAH/HPAH patients, and exon 8 of ACVRL1 gene was a mutation region with high PAH correlation, and two cases of novel mutation sites were found that may be related to the formation of IPAH. PAH patients with ACVRL1 mutation have a young onset age, no significant gender difference, but with rapid disease progression and poor prognosis and more active treatment is recommended. It is suggested that IPAH patients and their families should be screened for ACVRL1 mutation as soon as possible to facilitate genetic counseling and early prevention and treatment.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-2-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-3-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-4-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-5-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-6-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-7-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Acknowledgements
We thank patients for providing clinical examination data. We appreciate Dr Kaisheng Lai of BestNovo (Beijing) Medical Laboratory Co., Ltd for the technical support and bioinformatics analysis of WES data.
Footnotes
Authors’ contribution: Hong Gu is responsible for enrolling patients with clinically characterizes, study design and perform surgery. Xinyu Zhang improved the study design and wrote the manuscript. Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang collected the samples and clinical information. All authors contributed to and discussed the results and critically reviewed the article. All authors read and approved the final article.
Conflict of interest: The author(s) declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the fund of National Natural Science Foundation of China (82070243).
ORCID iD: Hong Gu https://orcid.org/0000-0002-3557-7147
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Vorselaars VMM, Hosman AE, Westermann CJJ, et al . Pulmonary arterial hypertension and hereditary haemorrhagic telangiectasia. Int J Mol Sci 2018; 19: 3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Germain M, Eyries M, Montani D, et al. Genome-wide association analysis identifies a susceptibility locus for pulmonary arterial hypertension. Nat Genet 2013; 45: 518–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng XS, Guo YH, He JG, et al . Analysis on inpatient pulmonary hypertension surveillance in Fuwai Cardiovascular Hospital from 1996 to 2005. Zhonghua Xin Xue Guan Bing Za Zhi 2007; 35: 251–254. [PubMed] [Google Scholar]
- 4.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456. [DOI] [PubMed] [Google Scholar]
- 5.Gore B, Izikki M, Mercier O, et al. Key role of the endothelial TGF-β/ALK1/endoglin signaling pathway in humans and rodents pulmonary hypertension. PLoS One 2014; 9: e100310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Girerd B, Montani D, Coulet F, et al. Clinical outcomes of pulmonary arterial hypertension in patients carrying an ACVRL1 (ALK1) mutation. Am J Respir Crit Care Med 2010; 181: 851–861. [DOI] [PubMed] [Google Scholar]
- 7.Brenner L andChung WK.. Clinical and molecular genetic features of hereditary pulmonary arterial hypertension. Compr Physiol 2011; 1: 1721–1728. [DOI] [PubMed] [Google Scholar]
- 8.Simonneau G, Gatzoulis MA, Adatia I, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013; 62: D34–D41. [DOI] [PubMed] [Google Scholar]
- 9.Humbert M andGhofrani HA.. The molecular targets of approved treatments for pulmonary arterial hypertension. Thorax 2016; 71: 73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao Y, Zhang Y, Wang X, et al. Variant analysis in Chinese families with hereditary hemorrhagic telangiectasia. Mol Genet Genomic Med 2019; 7: e893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen YJ, Yang QH, Liu D, et al. Clinical and genetic characteristics of Chinese patients with hereditary haemorrhagic telangiectasia-associated pulmonary hypertension. Eur J Clin Invest 2013; 43: 1016–1024. [DOI] [PubMed] [Google Scholar]
- 12.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 13.Bossone E, D'Andrea A, D'Alto M, et al. Echocardiography in pulmonary arterial hypertension: from diagnosis to prognosis. J Am Soc Echocardiogr 2013; 26: 1–14. [DOI] [PubMed] [Google Scholar]
- 14.Shovlin CL, Guttmacher AE, Buscarini E, et al. Diagnostic criteria for hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber syndrome). Am J Med Genet 2000; 91: 66–67. [DOI] [PubMed] [Google Scholar]
- 15.Baumgartner H, De Backer J, Babu-Narayan SV, et al. 2020 ESC Guidelines for the management of adult congenital heart disease. Eur Heart J 2021; 42: 563–645. [DOI] [PubMed] [Google Scholar]
- 16.Sitbon O, Humbert M, Jaïs X, et al. Long-term response to calcium channel blockers in idiopathic pulmonary arterial hypertension. Circulation 2005; 111: 3105–3111. [DOI] [PubMed] [Google Scholar]
- 17.Douwes JM, Humpl T, Bonnet D, et al. Acute vasodilator response in pediatric pulmonary arterial hypertension: current clinical practice from the TOPP registry. J Am Coll Cardiol 2016; 67: 1312–1323. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Jiang T, Piao C, et al. Screening mutations of MYBPC3 in 114 unrelated patients with hypertrophic cardiomyopathy by targeted capture and next-generation sequencing. Sci Rep 2015; 5: 11411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdalla SA andLetarte M.. Hereditary haemorrhagic telangiectasia: current views on genetics and mechanisms of disease. J Med Genet 2006; 43: 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badesch DB, Raskob GE, Elliott CG, et al. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest 2010; 137: 376–387. [DOI] [PubMed] [Google Scholar]
- 21.Fujiwara M, Yagi H, Matsuoka R, et al. Implications of mutations of activin receptor-like kinase 1 gene (ALK1) in addition to bone morphogenetic protein receptor II gene (BMPR2) in children with pulmonary arterial hypertension. Circ J 2008; 72: 127–133. [DOI] [PubMed] [Google Scholar]
- 22.Machado RD, James V, Southwood M, et al. Investigation of second genetic hits at the BMPR2 locus as a modulator of disease progression in familial pulmonary arterial hypertension. Circulation 2005; 111: 607–613. [DOI] [PubMed] [Google Scholar]
- 23.Tørring PM, Brusgaard K, Ousager LB, et al. National mutation study among Danish patients with hereditary haemorrhagic telangiectasia. Clin Genet. 2014; 86: 123–133. [DOI] [PubMed] [Google Scholar]
- 24.Attisano L, Cárcamo J, Ventura F, et al. Identification of human activin and TGF beta type I receptors that form heteromeric kinase complexes with type II receptors. Cell 1993; 75: 671–680. [DOI] [PubMed] [Google Scholar]
- 25.Machado RD, Southgate L, Eichstaedt CA, et al. Pulmonary arterial hypertension: a current perspective on established and emerging molecular genetic defects. Hum Mutat 2015; 36: 1113–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Canzonieri C, Centenara L, Ornati F, et al. Endoscopic evaluation of gastrointestinal tract in patients with hereditary hemorrhagic telangiectasia and correlation with their genotypes. Genet Med 2014; 16: 3–10. [DOI] [PubMed] [Google Scholar]
- 27.Brakensiek K, Frye-Boukhriss H, Mälzer M, et al. Detection of a significant association between mutations in the ACVRL1 gene and hepatic involvement in German patients with hereditary haemorrhagic telangiectasia. Clin Genet. 2008; 74: 171–177. [DOI] [PubMed] [Google Scholar]
- 28.Pfarr N, Fischer C, Ehlken N, et al. Hemodynamic and genetic analysis in children with idiopathic, heritable, and congenital heart disease associated pulmonary arterial hypertension. Respir Res 2013; 14: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chida A, Shintani M, Yagi H, et al. Outcomes of childhood pulmonary arterial hypertension in BMPR2 and ALK1 mutation carriers. Am J Cardiol 2012; 110: 586–593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-2-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-3-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-4-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-5-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-6-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation
Supplemental material, sj-pdf-7-pul-10.1177_20458940211044577 for Clinical characteristics and prognosis analysis of idiopathic and hereditary pulmonary hypertension patients with ACVRL1 gene mutations by Xinyu Zhang, Chen Zhang, Qiangqiang Li, Chunmei Piao, Hongsheng Zhang and Hong Gu in Pulmonary Circulation