Abstract
Our general understanding of plant responses to sub-zero temperatures focuses on mechanisms that mitigate stress to the plasma membrane. The plant cell wall receives comparatively less attention, and questions surrounding its role in mitigating freezing injury remain unresolved. Despite recent molecular discoveries that provide insight into acclimation responses, the goal of reducing freezing injury in herbaceous and woody crops remains elusive. This is likely due to the complexity associated with adaptations to low temperatures. Understanding how leaf cell walls of herbaceous annuals promote tissue tolerance to ice does not necessarily lead to understanding how meristematic tissues are protected from freezing by tissue-level barriers formed by cell walls in overwintering tree buds. In this mini-review, we provide an overview of biological ice nucleation and explain how plants control the spatiotemporal location of ice formation. We discuss how sugars and pectin side chains alleviate adhesive injury that develops at sub-zero temperatures between the matrix polysaccharides and ice. The importance of site-specific cell-wall elasticity to promote tissue expansion for ice accommodation and control of porosity to impede ice growth and promote supercooling will be presented. How specific cold-induced proteins modify plant cell walls to mitigate freezing injury will also be discussed. The opinions presented in this report emphasize the importance of a plant’s developmental physiology when characterizing mechanisms of freezing survival.
Keywords: Abiotic stress, Cell wall, Cold acclimation, Dehydration, Freezing tolerance, Supercooling
Introduction
At northern latitudes, a lack of adequate winter hardiness is the main environmental factor limiting plant distributions. Overwintering success is contingent on whether plant tissues can tolerate the formation of tissue ice or supercool to avoid freezing injury. Exposure to sub-lethal temperatures or endogenous abscisic acid (ABA) induces a cold acclimation response that alters numerous physiological traits. The up-regulation of several C-repeat binding factor/dehydrative response element binding transcription factors promotes the downstream modification of the plasma membrane and cell wall, the accumulation of cryoprotectant metabolites and proteins and the reduction of tissue water (see Uemura et al. 2006, Knight and Knight 2012, Wisniewski et al. 2014, 2020, Panter et al. 2020 for comprehensive reviews). Exposure to non-lethal freezing temperatures (−3°C) can induce an additional sub-zero acclimation response (Trunova 1965, Olien and Smith 1977, Livingston and Henson 1998, Takahashi et al. 2019, 2020). In acclimated plants, the disruption of the plasma membrane from freeze-thaw injury is generally accepted as the primary cause of lethal injury (Sakai and Larcher 1987, Olien and Livingston 2006, Uemura et al. 2006, Arora 2018). There is less agreement on the importance of the cell wall in mitigating plasma membrane damage.
Early experiments involving isolated plant protoplasts reported no significant difference (Siminovitch et al. 1978) or a higher freezing tolerance (Murai and Yoshida 1998) when compared to intact cells. This work drew two major conclusions that the firm attachment of the plasma membrane to the cell wall enhances freezing injury and the cell wall provides minimal tolerance to freezing. In contrast, observations with artificial matrices and intact plant tissues support the theory that the cell wall is an integral barrier to ice nucleation and propagation (Olien 1974, Ashworth and Abeles 1984, Wisniewski 1995, Yamada et al. 2002, McCully et al. 2004). Recent studies highlight the importance of tissue-specific variability in cell-wall elasticity as a mechanism to cope with tissue ice or promote supercooling (Neuner et al. 2019, Willick et al. 2019, Stegner et al. 2020, Steiner et al. 2020).
During cold and sub-zero acclimation, the cell wall and extracellular space, referred to as the apoplast, experience significant remodeling that leads to the acquisition of freezing tolerance and avoidance (Knight and Knight 2012, Panter et al. 2020). Treatment of Arabidopsis (Arabidopsis thaliana) at 4°C for 12 to 48 h downregulate cell-wall genes that promote cell expansion (Hannah et al. 2005). Prolonged exposure to 4°C (for days) upregulates transcripts signaling the accumulation of matrix polysaccharides, and wall remodeling enzymes that minimize matrix porosity, depending on the tissue, enhance or reduce cell-wall rigidity (Panter et al. 2020). Numerous studies report on the higher accumulation or specific activity of expansins, glycoside hydrolases (GHs), pectin methylesterases (PMEs) or xyloglucan endotransglucosylase/hydrolases (XTHs) in response to declining temperatures (Takahashi et al. 2016, 2019, Duruflé et al. 2017). These cell-wall modifications promote the development of tissue-level barriers responsible for the degree and depth of freezing survival.
The cell wall is not only a barrier against ice ingress to the plasma membrane, a primary site of freezing injury, but it also controls the propagation of ice. This mini-review will consider how low-temperature acclimation modifies the apoplast and discuss the significance of these changes in enhancing freezing tolerance and avoidance in herbaceous and woody plants.
Ice Nucleation and the Role of the Cell Wall in Tolerating Freezing Dehydration Injury
The attraction of water molecules to cell-wall polymers is a major determinant in the freezing behavior of individual cells. Under normal atmospheric pressures, a decline in temperature decreases the kinetic energy of water molecules. Water molecules cooled below 4°C have lower kinetic energy than what is required for the formation of hydrogen bonds. At 0°C, hydrogen bonds form more frequently resulting in metastable clusters of regularly ordered water molecules. A gradual reduction in temperature enhances the likelihood of a stable nucleus and the development of an extended ice lattice. Pure water can supercool to −38°C before undergoing homogeneous ice nucleation (Franks 1982). However, an ice nucleator can serve as a template for a stable nucleus and promote heterogeneous ice nucleation at temperatures close to 0°C. Common sources of ice nucleation include apoplastic bacterial epiphytes, intrinsic plant-derived substances, extrinsic ice nucleation active micro-organisms and soil particulates (reviewed by Wisniewski et al. 2014).
Under natural conditions, herbaceous freezing-tolerant plants cool at slow rates (<3°C h−1) and initiate ice nucleation in extracellular spaces between −0.5 and −4°C (Arora 2018). The ensuing equilibrium freezing is a result of small displacements in the energy required for water molecules to join (freeze) or escape (melt) the extracellular ice lattice. A gradual reduction in temperature promotes the diffusion of intracellular water to extracellular ice until the energy required for freezing and melting attains a new equilibrium. Injury from equilibrium freezing arises from interactions among the ice aggregates, the cell-wall matrix and the adjoining plasma membrane.
Below 0°C, there is a competitive interaction between ice and hydrophilic cell-wall polymers for a thin layer of intervening water molecules known as a quasi-liquid layer (QLL) (Olien 1974, Slater and Michaelides 2019). Equilibrium freezing reduces the kinetic freedom of water molecules (Franks 1982) while enhancing the adhesive tension of the ice lattice and the QLL. At sub-zero temperatures, a sufficient reduction in QLL thickness promotes adhesions of ice with cell-wall polymers (Olien 1974). This injurious stress can result in significant distortions of cell integrity that ruptures the plasma membrane as temperature-induced intracellular desiccation ensues (Olien and Smith 1977). Interestingly, between 0 and −100°C, the kinetic energy of adhesion stress is greater than the kinetic energy associated with freezing (Olien and Livingston 2006). One would think that this is the primary cause of freezing injury. Yet freezing-tolerant herbaceous plants experience lethal injury at −10 to −30°C from equilibrium freezing-induced dehydration (Olien 1974, Olien and Smith 1977, Olien and Livingston 2006, Wisniewski et al. 2020). Dehydration and adhesion likely occur simultaneously within adjacent tissues (Fig. 1).
Fig. 1.
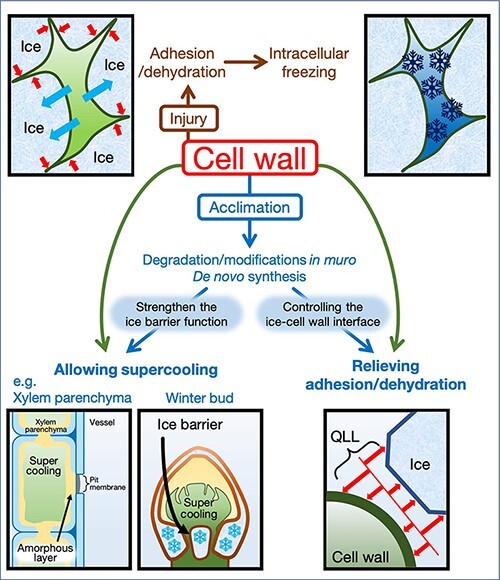
Schematic diagram of the effect of cell wall on freezing tolerance of plants. When plant cells or tissues are exposed to severe freezing. The QLL expands after the incorporation of soluble substances into the apoplastic space to relieve adhesion and dehydration stress. Reduction of the QLL can result in injurious adhesions forming between the cell wall and extracellular ice crystal. Simultaneously, the establishment of a vapor pressure gradient from the intracellular supercooled water to the extracellular ice crystal will promote dehydration injury. Specialized plants can avoid freezing altogether by reducing the porosity of the cell wall (e.g. xylem parenchyma cells) or by developing tissue-level barriers (e.g. winter bud of woody perennials).
One proposed mechanism for the alleviation of adhesive stress is that plants exude water-soluble solutes into the apoplast to enhance the width of QLL (Fig. 1). During exposure to mild frost (−2 to −5°C), winter cereals hydrolyze fructan oligosaccharides into their constituent hexose sugars (Trunova 1965). Based on this discovery, Olien hypothesized that plants exude soluble sugars into the extracellular space to relieve adhesive stress (Olien and Smith 1977). Subsequent experiments with winter oat and fall rye at −3°C confirmed the presence of extracellular fructan hydrolases and cell-wall invertases that hydrolyze fructan resulting in a concomitant increase in extracellular sucrose, glucose and fructose and a decline in intracellular fructan concentrations (Livingston and Henson 1998). It is, however, an open topic of debate whether sub-zero acclimation induces an increase in hexose sugars solely to minimize adhesions or to lower the availability of free water.
Factors other than simple sugars can mitigate damage caused by ice ingress. Isothermal calorimetric analysis indicated that, unlike the non-acclimated oat crown, the fraction of unfrozen water in cold-acclimated oat crowns did not follow the colligative freezing pattern observed in sucrose solutions (Livingston 2007). This suggests that cold acclimation promotes water sequestration in the intracellular space. Solutes exuded from the ice lattice in affiliation with the cell-wall matrix could serve as a barrier to ice. With magnetic resonance micro-imaging, Willick et al. (2019) identified a putative cell-level dehydrative barrier between the ice sensitive shoot apical meristem and freezing-tolerant vascularized tissues. In winter oat recovering from freezing, the same tissue region accumulates suberin and hypothetically operates as a physical barrier against secondary pathogenic injury (Livingston et al. 2013).
Intracellular water molecules will diffuse along the sugar osmotic gradient, through the hydrated cell-wall matrix and QLL toward the extracellular ice aggregate (Wisniewski et al. 2014, 2020). Prolonged dehydration at sub-zero temperatures can weaken cellular integrity through shifts in redox chemistry, protein and lipid denaturation (see review by Arora 2018). Severe dehydration can result in cytorrhysis or the deformation of the cell-wall matrix and separation from the plasma membrane (McCully et al. 2004, Stegner et al. 2020, Steiner et al. 2020). Lethal injury is avoidable if the cell wall can maintain its structure without separating from the plasma membrane or if the cell-wall matrix is elastic enough to allow contraction and expansion during dehydration and rehydration.
Recent evidence suggests that the cold-induced increase in cell-wall rigidity is heterogeneous and that herbaceous plants and algae reinforce the cell-wall matrix at specific junction zones to control the flexibility and direction of cell expansion during freezing (McCully et al. 2004, Stegner et al. 2020, Steiner et al. 2020). Jarvis et al. (2003) stresses the importance of these junctions for growth and cellular integrity and as reinforcements with various polymer compositions in the primary cell walls and the middle lamella. Thick cell-wall occlusions at cell corners of cold acclimated and frozen streptophyte green algae (Klebsormidium crenulatum) that maintain membrane and cell-wall adhesion were hypothesized to reinforce cells against cytorrhysis (Steiner et al. 2020). McCully et al. (2004) postulated that cell-wall juncture zones in the petioles of white clover (Trifolium repens L.) and California poppy (Eschscholzia californica Cham.) leaves maintain higher flexibility for separation to accommodate large ice lenses. Stegner and colleagues hypothesize a similar theory that glacier buttercup (Ranunculus glacialis L.) leaves accommodate ice lenses because of the inherent flexibility of the pectin-rich layer in mesophyll cell walls (Stegner et al. 2020). Herburger and Holzinger (2015) previously reported that callose deposition in cell walls reduced water loss. A similar mechanism could explain the cold-induced callose deposition in streptophyte green algae (Steiner et al. 2020). Additional immunostaining, Raman and Mid-infrared spectroscopy imaging studies will further elucidate the site-specific importance of cell-wall elasticity to better explain how plant cells tolerate the presence of ice within various tissues.
Remodeling of the Woody Perennial Cell Wall Promotes Freezing Avoidance Mechanisms
Overwintering plants need to protect tissues critical for re-growth. Tissues of woody perennials are classically employed to study the primary adaptations to freezing. Cortical parenchyma cells survive exposure to sub-zero temperatures by tolerating extracellular ice formation, whereas some woody perennials develop xylem parenchyma cells that supercool to avoid freezing (Sakai and Larcher 1987).
The stability at ultra-low temperatures distinguishes deep supercooling from the transitory supercooling in annual plants. Supercooling from −1 to −12°C enhances the freezing avoidance of annuals that cannot tolerate freezing dehydration stress during the growing season. The depth of this transitory supercooling depends on leaf tissue wettability and the formation of tissue barriers against extrinsic ice nucleation (reviewed by Wisniewski et al. 2014). Some woody perennial leaves, rhizomes, overwintering tree buds and xylem parenchyma cells deep supercool to temperatures ranging from −15 to −60°C (Quamme et al. 1973, Sakai and Larcher 1987, Kuroda et al. 2003). These plants develop barriers against tissue water loss and ice ingress. The entrapped water remains in a liquid phase due to the lack of heterogeneous ice nucleators or the accumulation of anti-ice nucleating substances (Kasuga et al. 2008, Ishikawa et al. 2015).
Woody perennials that exhibit deep supercooling develop tissues lacking large extracellular spaces in favor of a continuous network of cell-wall microcapillaries (Sakai and Larcher 1987). Studies involving artificial matrices identified that microcapillaries with diameters of less than 100 nm impede the propagation of ice (Olien 1974, Ashworth and Abeles 1984). Within these microcapillaries, the strength of the adhesive forces between water and cell-wall polymers is greater than the forces of cohesion amongst water molecules (Wisniewski 1995, Slater and Michaelides 2019). In the absence of an intrinsic nucleator, the adhesive tension between the cell-wall matrix and water molecules in small microcapillaries (<4 nm) is sufficient to depress the ice nucleation temperature to −25°C and inhibit the evaporative loss of water to an adjacent ice sink (Olien 1974, Ashworth and Abeles 1984).
The importance of pore diameter was experimentally confirmed in peach (Prunus persica L. cv. Loring) xylem ray parenchyma cells treated with pectinase that resulted in the partial degradation of the cell wall, greater pore size and a reduction in the capacity to deep supercool (Wisniewski 1995). However, the xylem parenchyma cells of apple stems remain supercooled after the disruption of the plasma membrane by freeze-thawing, steam or a chloroform treatment (Quamme et al. 1973) suggesting that the cell wall, irrespective of the properties of the plasma membrane, can promote deep supercooling.
In xylem parenchyma, the largest microcapillaries are reportedly at the pit membranes (Wisniewski 1995). De-acclimation of peach stems at room temperature or treatment with pectinase, ethylene glycol tetraacetic acid (EGTA) and oxalic acid (Wisniewski 1995) reduces deep supercooling capacity, indicating that cold-induced cell-wall remodeling influences xylem pit diameter. Treatment of xylem parenchyma with EGTA reduces pit membrane JIM7 epitope labeling for pectin with 35–90% methyl esters (Wisniewski and Davis 1995), indicating that Ca2+ cross-linking reduces pore diameter. These examples underscore that homogalacturonan (HG) deposition within the amorphous layer might be related to the ability of xylem parenchyma to supercool (Fig. 1).
Visual observations with infrared video thermography illustrate that xylem parenchyma cells freeze intracellularly as single cells or in small cell groups (Neuner et al. 2010). One proposed mechanism for the maintenance of supercooling is that woody perennials accumulate solutes to depress the ice nucleation temperature. Infiltration of Japanese beech (Fagus crenata Blume.), katsura tree (Cercidiphyllum japonicum Siebold & Zucc.) and mulberry (Morus alba L.) stems with 0.5 M glucose enhances the supercooling capacity of xylem parenchyma cells (Kasuga et al. 2013). Regardless of species or degree of acclimation, infiltration of stems with water reduces the supercooling capacity. Interestingly, summer collected stems infiltrated with 0.5 M glucose have higher supercooling capacities as compared with non-infiltrated cold-acclimated winter stems. Although the cell wall influences the maintenance of supercooling, significant shifts in deep supercooling (>10°C) are likely induced by the accumulation of intracellular solutes. Further research will help clarify the role played by cell walls in the seasonal changes in freezing resistance in woody plant species.
Some overwintering buds undergo extraorgan freezing, where water from the floret or primordial meristem migrates along the vapor pressure gradient to ice aggregates in the subtending bud scales or shoot xylem (Sakai and Larcher 1987). Unlike xylem parenchyma cells, ice nucleation and rapid cooling (>5°C h−1) in dormant buds induce the intracellular freezing of the primordia (Sakai and Larcher 1987, Ishikawa et al. 2015). Interestingly, partially dehydrated grape hybrid (Vitis amurensis × Seibel 13,053 cv. Yamasachi) bud primordia remain supercooled at high (5 and 16°C h−1) rates of cooling (Kasuga et al. 2020).
To avoid freezing, dormant buds develop chemical and structural barriers to prevent ice ingress into primordial tissues (Fig. 1). Bud scales coated with hydrophobic compounds including cutin and suberin are likely barriers against extrinsic ice formation (Neuner et al. 2019). Dormant buds of Pinaceae, which exhibit extraorgan freezing, develop a crown plate between the bud and subtending shoot. Cells in the crown plate develop thick cell walls and remain tightly packed relative to cells in vascular and primordial tissues (Endoh et al. 2009, Kuprian et al. 2017). Cold acclimation of Norway spruce (Picea abies L.) buds promotes pectin accumulation in the crown plates (Kuprian et al. 2017). Independently, Lee et al. (2017) observed that shifting Norway spruce to short day (12 h) conditions induces the de-methyl esterification and Ca2+ cross-linking of HG in the crown plate. This mechanism was reversible by shifting Norway spruce into long day (24 h) conditions. A similar pattern of cold-induced de-methyl esterification occurs in cold-acclimated peach buds (Wisniewski and Davis 1995). These examples underscore the importance of the cell wall in preventing freezing injury.
Molecular Implications of in Muro Modifications for the Control of Freezing Behavior
Advancements in proteomic technologies have further expanded our understanding of the cold and sub-zero acclimation of plant cell walls. Several apoplastic proteins putatively associated with cell-wall degradation and/or modification in muro during cold acclimation treatment (Table 1). In Arabidopsis, XTH and PME/ PME inhibitor (PMEI) show diverse responses during cold and sub-zero acclimation. Loss of function of XTH21 or XTH19 has been shown to reduce freezing tolerance (Shi et al. 2014, Takahashi et al. 2020), suggesting that xyloglucan reorganization is linked to freezing tolerance. In cold-acclimated winter rape (Brassica napus subsp. oleifera), higher leaf tensile strength correlates with enhanced specific activity of PME and the concomitant de-methyl esterification of HG (Solecka et al. 2008). The tissue-specific calcium cross-linking of HG was experimentally observed to inhibit the propagation of ice in xylem parenchyma cells (Wisniewski and Davis 1995) and overwintering buds (Kuprian et al. 2017). A mur1 mutant, which lacks fucosylation of side chains necessary for efficient borate-diester linkages of pectin rhamnogalacturonan II (RG-II) dimerization, has a lower freezing tolerance as compared with wild-type plants (Panter et al. 2019). These findings suggest that xyloglucan reorganization and the cross-linking of HG and RG-II enhances freezing tolerance.
Table 1.
The effect of cold or sub-zero acclimation on the accumulation of select cell-wall-associated proteins. Listed proteins were retrieved from previous proteome studies
| Species | Tissue (fraction) | Treatment | Cell-wall-associated proteina | Responseb | Reference |
|---|---|---|---|---|---|
|
Arabidopsis thaliana
(Col-0) |
Leaves (apoplast) | 4°C for 7 d (cold) −3°C for 3 d (sub-zero) | Expansin | − (cold) | Takahashi et al. (2019) |
| Glycosyl hydrolase | − (sub-zero) | ||||
| Lipid transfer protein (GPI) | + (cold) | ||||
| Pectin methylesterase | ± | ||||
| Pectin methylesterase inhibitor | + (cold) | ||||
| Pectinacetylesterase | + (cold) | ||||
| Xyloglucan endotransglucosylase/hydrolase | ± | ||||
| α-l-arabinofuranosidase | − (cold) | ||||
| β-galactosidase | − (cold) | ||||
|
Arabidopsis thaliana
(Col-0 and Sha) |
Leaves (apoplast) | Grown at 15°C (cold) or 22°C (control) | Polygalacturonase | ± | Duruflé et al. (2017) |
| Endo-β-glucuronidase | + | ||||
| Expansin | + | ||||
| Lipid transfer protein (GPI) | − | ||||
| N-acetyl-β-glucosaminidase | − | ||||
| Pectin methylesterase | + | ||||
| β-1,3-glucosidase | − | ||||
|
Arabidopsis thaliana
(Col-0) |
Leaves (plasma membrane) | 4°C for 7 d | Fasciclin-like arabinogalactan protein (GPI) | + | Takahashi et al. (2016) |
| Lipid transfer protein (GPI) | + | ||||
| β-1,3-glucosidase (GPI) | + | ||||
| Pisum sativum | Leaves (total) | 10°C/4°C for 11 d | Caffeoyl-CoA O-methyltransferase | − (tolerant) | Dumont et al. (2011) |
| Brassica rapa var. rapa (susceptible/tolerant cultivars) | Roots (total) | 10°C/4°C for 48 h, −4°C for 8 h | Polygalacturonase inhibitor 1 | − (susceptible) | Zeng et al. (2018) |
| Allium cepa | Bulb scales (total) |
Frozen at −4.5°C and then thawed on ice | α-xylosidase-like | − (frozen) | Grene et al. (2012) |
| α-l-arabinofuranosidase | − (frozen) + (thawed) |
||||
| Secale cereale | Leaves (plasma membrane) | 4°C for up to 28 d | Fasciclin-like arabinogalactan protein (GPI) | + | Takahashi et al. (2013) |
| β-1,3-glucosidase (GPI) | + | ||||
| Triticum aestivum | Crown shoot apical meristem (SAM) and vascular tissues (apoplast) | 4°C for 21 or 42 d | α-glucosidase | + | Willick et al. (2018) |
| Apoplastic invertase | + | ||||
| Cell-wall-β-glucosidase | + | ||||
| Fasciclin-like arabinogalactan protein (GPI) | + | ||||
| Fructan exohydrolase | + | ||||
| Glucan endo-1,3-β-glucosidase | + (SAM only) | ||||
| Lipid transfer protein | + | ||||
| Pectin methylesterase | + | ||||
| Xylanase inhibitor | + | ||||
| α-xylosidase | + | ||||
| β-d-glucan exohydrolase | + | ||||
| β-expansin | + | ||||
|
Eucalyptus benthamii (tolerant) E. grandis (susceptible) |
Leaves (total) | 12.4°C for 20 d | β-galactosidase Caffeic acid 3-O-methyltransferase Caffeoyl-CoA O-methyltransferase Cinnamyl alcohol dehydrogenase Hydroxycinnamoyl transferase |
± (susceptible) − (tolerant) + (tolerant) − (both) + (susceptible) − (tolerant) |
Oberschelp et al. (2020) |
| Xyloglucan endotransglucosylase Xylosidase β-glucosidase |
− − + |
||||
| Hydrangea paniculata | Bark/xylem (total) | Early Jan to mid-Jun | Caffeoyl-CoA O-methyltransferase | + (winter) | Pagter et al. (2014) |
|
Rhododendron catawbiense
R. ponticum |
Leaves (total) | Aug to Dec | Cellulose synthase-like | + | Die et al. (2017) |
|
Vitis amurensis
V. vinifera |
Buds (total) | Late fall and early winter | Proteins associated with phenylpropanoid biosynthesis pathway | ± | Masocha et al. (2020) |
Putative glycosylphosphatidylinositol-anchored proteins denoted by (GPI) in parentheses.
Relative higher (>1.5-fold, +) or lower (<0.67-fold, −) accumulation of cold-induced cell-wall-associated proteins. If the response differs depending on the isoforms or fractions, ± is noted.
Cold-induced reductions in α-l-arabinofuranosidases and β-galactosidases abundance (Table 1) correspond with higher concentrations of cell-wall l-arabinose and galactose (Duruflé et al. 2017, Takahashi et al. 2020). These responses are presumably associated with the greater abundance of α-1,5-arabinan and β-1,4-galactan pectin RG-I sidechains (Montes et al. 2008, Moneo-Sánchez et al. 2018). It is currently unknown how pectin RG-I side chains mitigate freezing injury. In resurrection plants, arabinan side chains and arabinogalactan proteins (AGPs) act as ‘plasticizer’ to promote the convoluted folding of the cell wall to reduce cell size and maintain turgor (Moore et al. 2006) and after rehydration facilitate the restoration of cell shape by preventing the irreversible adhesion of pectin polysaccharides (Moore et al. 2013). Whereas the accumulation of truncated galactan side chains due to over expression of β-galactosidase in potato (Solanum tuberosum L.) causes reduction in the water-binding capacity and a decrease in the tolerance of tubers to dehydration (Klaassen and Trindade 2020).
The biomechanical hotspot model hypothesizes that the extensibility of the cell wall is manipulated at sites of close contact comprised of xyloglucan and possibly RG-I between cellulose microfibrils (Cosgrove 2016). The flexibility of cell walls in dehydrated resurrection plants (Moore et al. 2006, 2013) and the ice-accommodating tissues of herbaceous leaves (McCully et al. 2004) may result from the site-specific manipulation of cell-wall extensibility. Furthermore, desiccation-tolerant plants may utilize cell-wall folding as a general mechanism to tolerate freezing-induced dehydration injury. To accommodate cell wall folding, plants must adjust membrane elasticity and/or the site-specific connections between the cell wall and plasma membrane. In the absence of cold-induced wall folding, RG-I arabinan and galactan side chains could alleviate freezing-induced adhesive injury and play a role in maintaining hydration of the QLL. Additional research is needed to confirm these theories in cold-acclimated plants.
Post-translationally modified membrane-bound glycosylp hosphatidylinositol (GPI) anchored proteins maintain important roles in cold-induced cell-wall modification (Table 1). Among them, fasciclin-like AGPs and glycerophosphoryldiester phosphodiesterase-like proteins enhance primary cell-wall adhesion (Shi et al. 2003, Hayashi et al. 2008). Whereas some lipid transfer proteins promote the export and deposition of monomers required for the development of suberin and cuticular wax lipid barriers (Kim et al. 2012). The GPI anchored proteins associate with cell-wall polysaccharides via protein or O-glycan interactions (Yeats et al. 2018) and some of these can covalently bind both pectin and arabinoxylans (Tan et al. 2013). Artificial and seasonal cold acclimation of herbaceous annuals and woody perennials enhances the accumulation of GPI AGPs (Table 1). The mRNA levels of AGPs in the needles of Sitka spruce (Picea sitchensis Carr.) (Grene et al. 2012) and buds and roots of hybrid poplar (Populus simonii x P. nigra) (Zang et al. 2015) reach their highest levels in autumn and decline in the winter. Plasma membrane and the cell-wall interactions during freezing dehydration and thawing have a significant effect on recovery (Arora 2018). Therefore, the identification of proteins that regulate membrane-to-wall interactions as a mechanism of freezing tolerance in plants is an interesting area of future study.
Conclusion and Perspectives
Differences in the developmental biology of Arabidopsis as opposed to other herbaceous annuals, grasses or woody perennials will alter the cell-wall chemistry and its role in freezing tolerance or avoidance strategies. For example, fructan exohydrolases and cell-wall invertase-mediated freezing avoidance is proposed for monocotyledonous crops such as wheat (Livingston and Henson 1998), yet no such response is reported in trees. While it is proposed that the remodeling and accumulation of lignocellulosic polysaccharides enhance freezing tolerance in Rhododendron (Rhododendron catawbiense Michx.) (Wei et al. 2006), mutants of Arabidopsis that accumulate lower abundances of lignin exhibit greater freezing tolerance (Ji et al. 2015). These contrasting results highlight the importance of understanding the developmental history and physiology of your plant of interest. Mechanisms that enhance freezing tolerance or avoidance in a herbaceous annual may not translate into greater overwintering survival for a woody perennial. We must understand the developmental history and physiology of your plant of interest before engaging in cell-wall molecular studies. Without this basic knowledge, it is next to impossible to make any meaningful improvements in plant cold hardiness.
We must also recognize that cell walls are not homogenous structures. Understanding how tissue-specific cell-wall modifications in woody xylem parenchyma, dormant buds, tissues associated with plant meristems in grasses and herbs (Fig. 1) are key to advancing our understanding of plant freezing tolerance and avoidance. We can visualize tissue-level phenotypes using high-definition infrared thermography, nuclear magnetic resonance micro-imaging and cryo-scanning electron. Targeted Raman and Fourier transform infrared spectroscopy imaging techniques with omics approaches will provide deeper insight into mechanisms of freezing tolerance and avoidance.
Contributor Information
Daisuke Takahashi, Graduate School of Science and Engineering, Saitama University, Saitama, Japan.
Ian R Willick, Department of Plant Biology, Michigan State University, East Lansing, MI, USA; Plant Resilience Institute, Michigan State University, East Lansing, MI, USA; Great Lakes Bioenergy Research Center, Michigan State University, East Lansing, MI, USA.
Jun Kasuga, Research Center for Global Agro-Medicine, Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Hokkaido 080-8555, Japan.
David P Livingston III, USDA and Department of Crop Science, North Carolina State University, Raleigh, NC, USA.
Data Availability
Source data for table is provided in the paper.
Funding
The Great Lakes Bioenergy Research Center (U.S. Department of Energy, Office of Science, Office of Biological and Environmental Research, Award Number DE-SC0018409), Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (Grant/Award Numbers: 27328, 20K15494), Ichimura Foundation for New Technology and The Alexander-von-Humboldt Foundation Postdoctoral Fellowship.
Disclosures
The authors have no conflicts of interest to declare.
References
- Arora R. (2018) Mechanism of freeze-thaw injury and recovery: a cool retrospective and warming up to new ideas. Plant Sci. 270: 301–313.doi: 10.1016/j.plantsci.2018.03.002. [DOI] [PubMed] [Google Scholar]
- Ashworth E.N. and Abeles F.B. (1984) Freezing behavior of water in small pores and the possible role in the freezing of plant tissues. Plant Physiol. 76: 201–204.doi: 10.1104/pp.76.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove D.J. (2016) Plant cell wall extensibility: connecting plant cell growth with cell wall structure, mechanics, and the action of wall-modifying enzymes. J. Exp. Bot. 67: 463–476.doi: 10.1093/jxb/erv511. [DOI] [PubMed] [Google Scholar]
- Die J.V., Arora R. and Rowland L.J. (2017) Proteome dynamics of cold-acclimating Rhododendron species contrasting in their freezing tolerance and thermonasty behavior. PLoS One 12: e0177389.doi: 10.1371/journal.pone.0177389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont E., Bahrman N., Goulas E., Valot B., Sellier H., Hilbert J.-L., et al. (2011) A proteomic approach to decipher chilling response from cold acclimation in pea (Pisum sativum L.). Plant Sci. Plant and Microbe Adaptation to Cold 180: 86–98.doi: 10.1016/j.plantsci.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Duruflé H., Hervé V., Ranocha P., Balliau T., Zivy M., Chourré J., et al. (2017) Cell wall modifications of two Arabidopsis thaliana ecotypes, Col and Sha, in response to sub-optimal growth conditions: an integrative study. Plant Sci. 263: 183–193.doi: 10.1016/j.plantsci.2017.07.015. [DOI] [PubMed] [Google Scholar]
- Endoh K., Kasuga J., Arakawa K., Ito T. and Fujikawa S. (2009) Cryo-scanning electron microscopic study on freezing behaviors of tissue cells in dormant buds of larch (Larix kaempferi). Cryobiology 59: 214–222.doi: 10.1016/j.cryobiol.2009.07.010. [DOI] [PubMed] [Google Scholar]
- Franks F. (1982) The properties of aqueous solutions at subzero temperatures. InWater and Aqueous Solutions at Subzero Temperatures, Water. Edited by Franks, F. pp. 215–338. Springer US, Boston, MA. [Google Scholar]
- Grene R., Klumas C., Suren H., Yang K., Collakova E., Myers E., et al. (2012) Mining and visualization of microarray and metabolomic data reveal extensive cell wall remodeling during winter hardening in Sitka spruce (Picea sitchensis). Front. Plant Sci. 3: 241.doi: 10.3389/fpls.2012.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannah M.A., Heyer A.G. and Hincha D.K. (2005) A global survey of gene regulation during cold acclimation in Arabidopsis thaliana. PLoS Genet. 1: 18.doi: 10.1371/journal.pgen.0010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S., Ishii T., Matsunaga T., Tominaga R., Kuromori T., Wada T., et al. (2008) The glycerophosphoryl diester phosphodiesterase-like proteins SHV3 and its homologs play important roles in cell wall organization. Plant Cell Physiol. 49: 1522–1535.doi: 10.1093/pcp/pcn120. [DOI] [PubMed] [Google Scholar]
- Herburger K. and Holzinger A. (2015) Localization and quantification of callose in the streptophyte green algae Zygnema and Klebsormidium: correlation with desiccation tolerance. Plant Cell Physiol. 56: 2259–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa M., Ishikawa M., Toyomasu T., Aoki T. and Price W.S. (2015) Ice nucleation activity in various tissues of Rhododendron flower buds: their relevance to extraorgan freezing. Front. Plant Sci. 6: 149.doi: 10.3389/fpls.2015.00149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis M.C., Briggs S.P.H. and Knox J.P. (2003) Intercellular adhesion and cell separation in plants. Plant Cell Environ. 26: 977–989.doi: 10.1046/j.1365-3040.2003.01034.x. [DOI] [Google Scholar]
- Ji H., Wang Y., Cloix C., Li K., Jenkins G.I., Wang S., et al. (2015) The Arabidopsis RCC1 family protein TCF1 regulates freezing tolerance and cold acclimation through modulating lignin biosynthesis. PLoS Genet. 11: e1005471.doi: 10.1371/journal.pgen.1005471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasuga J., Endoh K., Yoshiba M., Taido I., Arakawa K., Uemura M., et al. (2013) Roles of cell walls and intracellular contents in supercooling capability of xylem parenchyma cells of boreal trees. Physiol. Plant. 148: 25–35.doi: 10.1111/j.1399-3054.2012.01678.x. [DOI] [PubMed] [Google Scholar]
- Kasuga J., Hashidoko Y., Nishioka A., Yoshiba M., Arakawa K. and Fujikawa S. (2008) Deep supercooling xylem parenchyma cells of katsura tree (Cercidiphyllum japonicum) contain flavonol glycosides exhibiting high anti-ice nucleation activity. Plant Cell Environ. 31: 1335–1348.doi: 10.1111/j.1365-3040.2008.01835.x. [DOI] [PubMed] [Google Scholar]
- Kasuga J., Tsumura Y., Kondoh D., Jitsuyama Y., Horiuchi R. and Arakawa K. (2020) Cryo-scanning electron microscopy reveals that supercooling of overwintering buds of freezing-resistant interspecific hybrid grape ‘Yamasachi’ is accompanied by partial dehydration. J. Plant Physiol. 253: 153248.doi: 10.1016/j.jplph.2020.153248. [DOI] [PubMed] [Google Scholar]
- Kim H., Lee S.B., Kim H.J., Min M.K., Hwang I. and Suh M.C. (2012) Characterization of glycosylphosphatidylinositol-anchored lipid transfer protein 2 (LTPG2) and overlapping function between LTPG/LTPG1 and LTPG2 in cuticular wax export or accumulation in Arabidopsis thaliana. Plant Cell Physiol. 53: 1391–1403.doi: 10.1093/pcp/pcs083. [DOI] [PubMed] [Google Scholar]
- Klaassen M.T. and Trindade L.M. (2020) RG-I galactan side-chains are involved in the regulation of the water-binding capacity of potato cell walls. Carbohydr. Polym. 227: 115353.doi: 10.1016/j.carbpol.2019.115353. [DOI] [PubMed] [Google Scholar]
- Knight M.R. and Knight H. (2012) Low-temperature perception leading to gene expression and cold tolerance in higher plants. New Phytol. 195: 737–751.doi: 10.1111/j.1469-8137.2012.04239.x. [DOI] [PubMed] [Google Scholar]
- Kuprian E., Munkler C., Resnyak A., Zimmermann S., Tuong T.D., Gierlinger N., et al. (2017) Complex bud architecture and cell-specific chemical patterns enable supercooling of Picea abies bud primordia. Plant Cell Environ. 40: 3101–3112.doi: 10.1111/pce.13078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda K., Kasuga J., Arakawa K. and Fujikawa S. (2003) Xylem ray parenchyma cells in boreal hardwood species respond to subfreezing temperatures by deep supercooling that is accompanied by incomplete desiccation. Plant Physiol. 131: 736–744.doi: 10.1104/pp.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y., Karunakaran C., Lahlali R., Liu X., Tanino K.K. and Olsen J.E. (2017) Photoperiodic regulation of growth-dormancy cycling through induction of multiple bud–shoot barriers preventing water transport into the winter buds of Norway spruce. Front. Plant Sci. 8: 2109.doi: 10.3389/fpls.2017.02109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston D.P. (2007) Quantifying liquid water in frozen plant tissues by isothermal calorimetry. Thermochim. Acta 459: 116–120.doi: 10.1016/j.tca.2007.04.019. [DOI] [Google Scholar]
- Livingston D.P. and Henson C.A. (1998) Apoplastic sugars, fructans, fructan exohydrolase, and invertase in winter oat: responses to second-phase cold hardening. Plant Physiol. 116: 403–408.doi: 10.1104/pp.116.1.403. [DOI] [Google Scholar]
- Livingston D.P., Henson C.A., Tuong T.D., Wise M.L., Tallury S.P. and Duke S.H. (2013) Histological analysis and 3D reconstruction of winter cereal crowns recovering from freezing: a unique response in oat (Avena sativa L.). PLoS One 8: e53468.doi: 10.1371/journal.pone.0053468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masocha V.F., Li Q., Zhu Z., Chai F., Sun X., Wang Z., et al. (2020) Proteomic variation in Vitis amurensis and V. vinifera buds during cold acclimation. Sci. Hortic. 263: 109143.doi: 10.1016/j.scienta.2019.109143. [DOI] [Google Scholar]
- McCully M.E., Canny M.J. and Huang C.X. (2004) The management of extracellular ice by petioles of frost-resistant herbaceous plants. Ann. Bot. 94: 665–674.doi: 10.1093/aob/mch191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moneo-Sánchez M., Izquierdo L., Martín I., Hernández-Nistal J., Albornos L., Dopico B., et al. (2018) Knockout mutants of Arabidopsis thaliana β-galactosidase. Modifications in the cell wall saccharides and enzymatic activities. Biol. Plant. 62: 80–88.doi: 10.1007/s10535-017-0739-2. [DOI] [Google Scholar]
- Montes R.A.C., Ranocha P., Martinez Y., Minic Z., Jouanin L., Marquis M., et al. (2008) Cell wall modifications in Arabidopsis plants with altered α-l-arabinofuranosidase activity. Plant Physiol. 147: 63–77.doi: 10.1104/pp.107.110023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.P., Nguema-Ona E., Chevalier L., Lindsey G.G., Brandt W.F., Lerouge P., et al. (2006) Response of the leaf cell wall to desiccation in the resurrection plant Myrothamnus flabellifolius. Plant Physiol. 141: 651–662.doi: 10.1104/pp.106.077701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J.P., Nguema-Ona E.E., Vicré-Gibouin M., Sørensen I., Willats W.G.T., Driouich A., et al. (2013) Arabinose-rich polymers as an evolutionary strategy to plasticize resurrection plant cell walls against desiccation. Planta 237: 739–754.doi: 10.1007/s00425-012-1785-9. [DOI] [PubMed] [Google Scholar]
- Murai M. and Yoshida S. (1998) Evidence for the cell wall involvement in temporal changes in freezing tolerance of Jerusalem artichoke (Helianthus tuberosus L.) tubers during cold acclimation. Plant Cell Physiol. 39: 97–105.doi: 10.1093/oxfordjournals.pcp.a029295. [DOI] [PubMed] [Google Scholar]
- Neuner G., Monitzer K., Kaplenig D. and Ingruber J. (2019) Frost survival mechanism of vegetative buds in temperate trees: deep supercooling and extraorgan freezing vs. ice tolerance. Front. Plant Sci. 10: 537.doi: 10.3389/fpls.2019.00537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuner G., Xu B. and Hacker J. (2010) Velocity and pattern of ice propagation and deep supercooling in woody stems of Castanea sativa, Morus nigra and Quercus robur measured by IDTA. Tree Physiol. 30: 1037–1045.doi: 10.1093/treephys/tpaq059. [DOI] [PubMed] [Google Scholar]
- Oberschelp G.P.J., Guarnaschelli A.B., Teson N., Harrand L., Podestá F.E. and Margarit E. (2020) Cold acclimation and freezing tolerance in three Eucalyptus species: a metabolomic and proteomic approach. Plant Physiol. Biochem. 154: 316–327.doi: 10.1016/j.plaphy.2020.05.026. [DOI] [PubMed] [Google Scholar]
- Olien C.R. (1974) Energies of freezing and frost desiccation. Plant Physiol. 53: 764–767.doi: 10.1104/pp.53.5.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olien C.R. and Livingston D.P. (2006) Understanding freeze stress in biological tissues: thermodynamics of interfacial water. Thermochim. Acta 451: 52–56.doi: 10.1016/j.tca.2006.08.014. [DOI] [Google Scholar]
- Olien C.R. and Smith M.N. (1977) Ice adhesions in relation to freeze stress. Plant Physiol. 60: 499–503.doi: 10.1104/pp.60.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagter M., Sergeant K., Møller S.M., Bertram H.C. and Renaut J. (2014) Changes in the proteome and water state in bark and xylem of Hydrangea paniculata during loss of freezing tolerance. Environ. Exp. Bot. The Biology of Plant Cold Hardiness: Adaptive Strategies 106: 99–111.doi: 10.1016/j.envexpbot.2013.11.009. [DOI] [Google Scholar]
- Panter P.E., Kent O., Dale M., Smith S.J., Skipsey M., Thorlby G., et al. (2019) MUR1-mediated cell-wall fucosylation is required for freezing tolerance in Arabidopsis thaliana. New Phytol. 224: 1518–1531.doi: 10.1111/nph.16209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panter P.E., Panter J.R. and Knight H. (2020) Impact of cell wall structure and composition on plant freezing tolerance. Annu. Plant Rev. Online 3: 607–642. [Google Scholar]
- Quamme H., Weiser C.J. and Stushnoff C. (1973) The mechanism of freezing injury in xylem of winter apple twigs. Plant Physiol. 51: 273–277.doi: 10.1104/pp.51.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai A., Larcher W. (1987) Low temperature and frost as environmental factors. InFrost Survival of Plants: Responses and Adaptation to Freezing Stress. Edited by Sakai, A. and Larcher, W. pp. 1–20. Springer, Berlin, Heidelberg. [Google Scholar]
- Shi H., Kim Y., Guo Y., Stevenson B. and Zhu J.-K. (2003) The Arabidopsis SOS5 locus encodes a putative cell surface adhesion protein and is required for normal cell expansion. Plant Cell. 15: 19–32.doi: 10.1105/tpc.007872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Ye T., Zhong B., Liu X., Jin R. and Chan Z. (2014) AtHAP5A modulates freezing stress resistance in Arabidopsis through binding to CCAAT motif of AtXTH21. New Phytol. 203: 554–567.doi: 10.1111/nph.12812. [DOI] [PubMed] [Google Scholar]
- Siminovitch D., Singh J. and De La Roche I.A. (1978) Freezing behavior of free protoplasts of winter rye. Cryobiology 15: 205–213.doi: 10.1016/0011-2240(78)90025-1. [DOI] [PubMed] [Google Scholar]
- Slater B. and Michaelides A. (2019) Surface premelting of water ice. Nat. Rev. Chem. 3: 172–188.doi: 10.1038/s41570-019-0080-8. [DOI] [Google Scholar]
- Solecka D., Zebrowski J. and Kacperska A. (2008) Are pectins involved in cold acclimation and de-acclimation of winter oil-seed rape plants? Ann. Bot. 101: 521–530.doi: 10.1093/aob/mcm329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegner M., Lackner B., Schäfernolte T., Buchner O., Xiao N., Gierlinger N., et al. (2020) Winter nights during summer time: stress physiological response to ice and the facilitation of freezing cytorrhysis by elastic cell wall components in the leaves of a nival species. Int. J. Mol. Sci. 21: 7042.doi: 10.3390/ijms21197042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner P., Obwegeser S., Wanner G., Buchner O., Lütz-Meindl U. and Holzinger A. (2020) Cell wall reinforcements accompany chilling and freezing stress in the streptophyte green alga Klebsormidium crenulatum. Front. Plant Sci. 11: 873.doi: 10.3389/fpls.2020.00873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D., Gorka M., Erban A., Graf A., Kopka J., Zuther E., et al. (2019) Both cold and sub-zero acclimation induce cell wall modification and changes in the extracellular proteome in Arabidopsis thaliana. Sci. Rep. 9: 2289.doi: 10.1038/s41598-019-38688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi D., Johnson K.L., Hao P., Tuong T., Erban A., Sampathkumar A., et al. (2020) Cell wall modification by the xyloglucan endotransglucosylase/hydrolase xth19 influences freezing tolerance after cold and sub‐zero acclimation. Plant Cell Environ. 44: 915–930.doi: 10.1111/pce.13953. [DOI] [PubMed] [Google Scholar]
- Takahashi D., Kawamura Y. and Uemura M. (2013) Changes of detergent-resistant plasma membrane proteins in oat and rye during cold acclimation: association with differential freezing tolerance. J. Proteome Res. 12: 4998–5011.doi: 10.1021/pr400750g. [DOI] [PubMed] [Google Scholar]
- Takahashi D., Kawamura Y. and Uemura M. (2016) Cold acclimation is accompanied by complex responses of glycosylphosphatidylinositol (GPI)-anchored proteins in Arabidopsis. J. Exp. Bot. 67: 5203–5215.doi: 10.1093/jxb/erw279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan L., Eberhard S., Pattathil S., Warder C., Glushka J., Yuan C., et al. (2013) An Arabidopsis cell wall proteoglycan consists of pectin and arabinoxylan covalently linked to an arabinogalactan protein. Plant Cell. 25: 270–287.doi: 10.1105/tpc.112.107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trunova T. (1965) Light and temperature systems in the hardening of winter wheat and the significance of oligosaccharides for frost resistance. FizjolRast 12: 70–77. [Google Scholar]
- Uemura M., Tominaga Y., Nakagawara C., Shigematsu S., Minami A. and Kawamura Y. (2006) Responses of the plasma membrane to low temperatures. Physiol. Plant. 126: 81–89.doi: 10.1111/j.1399-3054.2005.00594.x. [DOI] [Google Scholar]
- Wei H., Dhanaraj A.L., Arora R., Rowland L.J., Fu Y. and Sun L. (2006) Identification of cold acclimation-responsive Rhododendron genes for lipid metabolism, membrane transport and lignin biosynthesis: importance of moderately abundant ESTs in genomic studies. Plant Cell Environ. 29: 558–570.doi: 10.1111/j.1365-3040.2005.01432.x. [DOI] [PubMed] [Google Scholar]
- Willick I.R., Gusta L.V., Fowler D.B. and Tanino K.K. (2019) Ice segregation in the crown of winter cereals: evidence for extraorgan and extratissue freezing. Plant Cell Environ. 42: 701–716.doi: 10.1111/pce.13454. [DOI] [PubMed] [Google Scholar]
- Willick I.R., Takahashi D., Fowler D.B., Uemura M. and Tanino K.K. (2018) Tissue-specific changes in apoplastic proteins and cell wall structure during cold acclimation of winter wheat crowns. J. Exp. Bot. 69: 1221–1234.doi: 10.1093/jxb/erx450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisniewski M. (1995) Deep supercooling in woody plants and the role of cell wall structure. InBiological Ice Nucleation and Its Applications. Edited by Lee, R.E., Warren, G.J. and Gusta, L.V. pp. 163–181. APS Press, Minneapolis, MN. [Google Scholar]
- Wisniewski M. and Davis G. (1995) Immunogold localization of pectins and glycoproteins in tissues of peach with reference to deep supercooling. Trees 9: 253–260.doi: 10.1007/BF00202015. [DOI] [Google Scholar]
- Wisniewski M., Gusta L. and Neuner G. (2014) Adaptive mechanisms of freeze avoidance in plants: a brief update. Environ. Exp. Bot. 99: 133–140.doi: 10.1016/j.envexpbot.2013.11.011. [DOI] [Google Scholar]
- Wisniewski M., Willick I.R., Duman J.G., Livingston D., Newton S.S. (2020) Plant antifreeze proteins. InAntifreeze Proteins Volume 1: Environment, Systematics and Evolution. Edited by Ramløv, H. and Friis, D.S. pp. 189–226. Springer International Publishing, Cham. [Google Scholar]
- Yamada T., Kuroda K., Jitsuyama Y., Takezawa D., Arakawa K. and Fujikawa S. (2002) Roles of the plasma membrane and the cell wall in the responses of plant cells to freezing. Planta 215: 770–778.doi: 10.1007/s00425-002-0814-5. [DOI] [PubMed] [Google Scholar]
- Yeats T.H., Bacic A. and Johnson K.L. (2018) Plant glycosylphosphatidylinositol anchored proteins at the plasma membrane-cell wall nexus. J. Integr. Plant Biol. 60: 649–669.doi: 10.1111/jipb.12659. [DOI] [PubMed] [Google Scholar]
- Zang L., Zheng T., Chu Y., Ding C., Zhang W., Huang Q., et al. (2015) Genome-wide analysis of the Fasciclin-Like Arabinogalactan Protein gene family reveals differential expression patterns, localization, and salt stress response in Populus. Front. Plant Sci. 6: 1140.doi: 10.3389/fpls.2015.01140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X., Xu Y., Jiang J., Zhang F., Ma L., Wu D., et al. (2018) iTRAQ-based comparative proteomic analysis of the roots of TWO winter turnip rapes (Brassica rapa L.) with different freezing-tolerance. Int. J. Mol. Sci. 19: 4077.doi: 10.3390/ijms19124077. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Source data for table is provided in the paper.


