Abstract
Recent genetic studies suggest that ephrins may function in a kinase-independent Eph receptor pathway. Here we report that expression of EphA8 in either NIH 3T3 or HEK293 cells enhanced cell adhesion to fibronectin via α5β1- or β3 integrins. Interestingly, a kinase-inactive EphA8 mutant also markedly promoted cell attachment to fibronectin in these cell lines. Using a panel of EphA8 point mutants, we have demonstrated that EphA8 kinase activity does not correlate with its ability to promote cell attachment to fibronectin. Analysis using EphA8 extracellular and intracellular domain mutants has revealed that enhanced cell adhesion is dependent on ephrin A binding to the extracellular domain and the juxtamembrane segment of the cytoplasmic domain of the receptor. EphA8-promoted adhesion was efficiently inhibited by wortmannin, a phosphatidylinositol 3-kinase (PI 3-kinase) inhibitor. Additionally, we found that EphA8 had associated PI 3-kinase activity and that the p110γ isoform of PI 3-kinase is associated with EphA8. In vitro binding experiments revealed that the EphA8 juxtamembrane segment was sufficient for the formation of a stable complex with p110γ. Similar results were obtained in assay using cells stripped of endogenous ephrin A ligands by treatment with preclustered ephrin A5-Fc proteins. In addition, a membrane-targeted lipid kinase-inactive p110γ mutant was demonstrated to stably associate with EphA8 and suppress EphA8-promoted cell adhesion to fibronectin. Taken together, these results suggest the presence of a novel mechanism by which the EphA8 receptor localizes p110γ PI 3-kinase to the plasma membrane in a tyrosine kinase-independent fashion, thereby allowing access to lipid substrates to enable the signals required for integrin-mediated cell adhesion.
The Eph receptor tyrosine kinases (RTKs), together with their ephrin ligands, regulate diverse developmental patterning processes including axon guidance, cell migration, and cell segregation (13). However, in contrast to other families of receptor tyrosine kinases, the Eph RTKs do not appear to regulate cell proliferation and survival. It was recently reported that activation of the Eph RTKs by their cognate ligands leads to changes in cell adhesion to various extracellular matrix proteins. For example, EphB1 promoted cell attachment to fibronectin or fibrinogen, whereas neither a kinase-inactive EphB1 mutant nor EphB1 point mutants defective for binding to either Nck or low-molecular-weight protein tyrosine phosphatase (LMW-PTP) showed this effect (21, 35). EphB2 was also shown to indirectly control integrin activity by inducing tyrosine phosphorylation of R-Ras, possibly through a novel signaling intermediate, Src homology 2 (SH2) domain-containing Eph receptor binding protein 1 (SHEP1) (9, 43). More recently, EphA2 kinase was reported to regulate integrin function by causing focal adhesion kinase dephosphorylation (26). These results are consistent with the concept that the kinase activity of the Eph RTKs plays a pivotal role in regulation of cell adhesion through integrins. In contrast, some studies indicate that the Eph RTKs might function in a kinase activity-independent mechanism. Evidence supporting this possibility comes from genetic studies using the nematode Caenorhabditis elegans. For example, mutations in the Eph receptor VAB-1 kinase domain caused a less penetrant embryonic arrest phenotype than a null mutation in the same gene, suggesting that VAB-1 may function partly in a kinase-independent signaling pathway (15). Moreover, C. elegans ephrin vab-2 mutations enhanced vab-1 kinase mutations, resembling vab-1 null mutations in the resulting phenotype (6). Two different mechanisms of kinase activity-independent signaling by Eph RTKs are possible. First, the ephrin ligands could transmit signals via the Eph receptor cytoplasmic region in a way that does not involve the tyrosine kinase activity. Interestingly, the native EphB6/Mep protein lacks tyrosine kinase activity due to many amino acid substitutions in conserved kinase domain sequence motifs which are important for catalysis; this may reflect an intrinsic signaling function of the kinase-inactive receptor (17). Second, the Eph receptors could function in reverse signaling via ephrin ligands. Vertebrate ephrins either are membrane anchored by glycosylphosphatidylinositol (GPI) anchors (ephrin A subgroup) or are transmembrane proteins (ephrin B subgroup). (12). The observation that ephrin B protein become phosphorylated on cytoplasmic tyrosine residues in response to Eph receptor binding provides evidence for the reverse signaling hypothesis (4, 20). More recently, consistent with a guidance role for the EphB2 extracellular domain (19), it was shown that retinal ganglion cell axon pathfinding within the retina was partially mediated by EphB receptors acting in a kinase-independent manner (3). It was also demonstrated that upon receptor binding, the ephrin A5 ligand could induce a signaling event through the Fyn protein tyrosine kinase, concomitant with alterations in the adhesive properties of the ligand-expressing cells (8). However, there have been no indications to date that the known intracellular signals transmitted by mammalian Eph receptors elicit biological responses without the tyrosine kinase activity.
We have previously reported that the EphA8 receptor can regulate cellular cytoskeletal modification through a kinase-dependent signaling mechanism (7). For example, EphA8 defective in binding to Fyn kinase fails to attenuate cell attachment responses to uncoated glass surfaces. However, unlike the case for EphB receptors, we found no strong correlation between tyrosine phosphorylation of EphA8 and alterations in cell attachment to a specific extracellular matrix protein such as fibronectin. On the basis of this observation, we asked whether the EphA8-mediated cell attachment to fibronectin could be regulated in a kinase activity-independent manner. In this study, we used cell lines that express endogenous ephrin A ligand to evaluate whether expression of diverse EphA8 kinase mutants resulted in different responses to fibronectin. In these systems, we observed that expression of the EphA8 receptor significantly promoted cell attachment to fibronectin without altering cellular integrin levels. Surprisingly, no significant differences in the ability to promote cell attachment to fibronectin were observed between wild-type EphA8 and kinase-inactive mutants. Moreover, our results identify p110γ phosphotidylinositol (PI) 3-kinase as a key bridging molecule between EphA8 and integrins. Our results provide the first example of kinase-independent communication between the mammalian EphA receptors and integrins.
MATERIALS AND METHODS
Cell culture and transfection.
NIH 3T3 cells were maintained in Dulbecco's modified Eagle's medium (DMEM; Sigma) supplemented with 5% heat-inactivated fetal calf serum (Life Technologies. Inc) as described previously (30). HEK293 cells were routinely cultured in alpha-MEM (Sigma) containing 10% heat-inactivated fetal bovine serum (BioWhittaker). Tetracycline-free serum (Clontech) was used in experiments involving doxycycline-induced EphA8 expression to prevent leakage expression. The calcium phosphate precipitation method was used as described previously (16) to transfect cells with various expression plasmids. Stable G418-resistant clones were selected by supplementing the culture medium with G418 (400 μg/ml). The clones were periodically cultured in the same selection medium to maintain stable expression. Transient transfections were carried out using LipofectAMINE (Life Technologies) according to the manufacturer's instructions. Doxycycline-inducible EphA8 expression cell lines were constructed by cotransfection with the pTRE-EphA8 and pTet-On (Clontech) constructs, followed by selection with G418. Positive clones were directly selected by Western analysis on the basis of their ability to express EphA8 in the presence of doxycycline (2 μg/ml; Sigma). At least three independent clones of each of the wild-type and kinase-inactive EphA8 receptors were obtained. PI-specific phospholipase C (PI-PLC) was treated to eliminate GPI-linked ephrin A subgroup ligands from NIH 3T3 or HEK293 cells as described previously (30). For treatment of preclustered ephrin A5-Fc proteins, purified ephrin A5-Fc (30) was aggregated using anti-human Fc (Jackson Immunoresearch) for 1 h at 4°C, and stimulations were for 20 min at 37°C.
Construction of expression vectors.
The murine EphA8 cDNA (pSP38), EphA8-TrkB chimeric cDNA, and EphA8 cDNA tagged with the nine-amino-acid hemagglutinin (HA) epitope (YPYDVPDYA) at its COOH terminus have been described elsewhere (7, 30). The EphA8-ΔIII deletion mutant was generated as follows. First, a 689-bp EcoRI/NcoI fragment (nucleotides [nt] 2 to 690 of the insert in pSP38) was ligated to a 224-bp NcoI/XhoI-digested PCR product amplified using primers matching nt 899 to 918 and 1101 to 1120 of the insert in pSP38. The resulting EcoRI/XhoI fragment was subcloned into the corresponding region of full-length EphA8 cDNA. To construct the EphA8-ΔV-VII deletion mutant, a 1,095-bp PstI fragment (nt 814 to 1908 of the insert in pSP38) was subcloned into pGEM5z (Promega) and then subjected to two separate PCRs. A 248-bp PCR product was amplified using the 5′ T7 universal primer and a 3′ primer matching nt 1037 to 1054 of the pSP38 insert, and a 301-bp PCR product was amplified using a 5′ primer matching nt 1679 to 1696 of the pSP38 insert and the 3′ SP6 universal primer. The two partially complementary PCR fragments thus generated were annealed and used as the template in another PCR with the 5′ T7 and 3′ SP6 universal primers. The resulting 385-bp product was digested with PstI and subcloned into the corresponding region of full-length EphA8 cDNA. The EphA8 juxtamembrane region (JM) deletion mutant (EphA8-ΔJM) was also generated by PCR as follows: a 324-bp PCR product was amplified using primers matching nt 1524 to 1543 and 1822 to 1840 of the insert in pSP38, and a 255-bp PCR product was generated using primers nt 2008 to 2022 and 2230 to 2249 of the insert in pSP38. The partially complementary fragments generated by these PCRs were annealed and reamplified with the primers used for the 5′ end of the first PCR product and the 3′ end of the second PCR product. The resulting 481-bp product was digested with StuI and XbaI and subcloned into the corresponding region of full-length EphA8 cDNA. To construct the EphA8 sterile alpha motif (SAM) deletion mutant (EphA8-ΔSAM), a 1,010-bp XbaI/EcoRI fragment (nt 2235 to 3244 of the pSP38 insert) was subcloned into pGEM11z (Promega) and used as the template in two separate PCRs: a 634-bp PCR product was amplified using the 5′ SP6 universal primer and a 3′ primer matching nt 2839 to 2853 of the pSP38 insert, and a 239-bp PCR product was amplified using a 5′ primer matching nt 3049 to 3068 of the insert in pSP38 and the 3′ T7 universal primer. The resulting PCR fragments were annealed and used as the template in another PCR with the 5′ SP6 and 3′ T7 universal primers. The resulting 865-bp fragment was digested with NcoI and ApaI and subcloned into the corresponding region of the XbaI/EcoRI EphA8 fragment in pGEM11z. Regions amplified by PCR were sequenced to exclude the possibility of errors introduced by the polymerase.
Site-directed mutagenesis was performed using the Altered Sites II in vitro mutagenesis system (Promega) as described elsewhere (7). Briefly, to introduce a point mutation at the Tyr-792 codon, a 20-bp oligonucleotide (nt 2442 to 2461 of the insert in pSP38) incorporating TTC (Phe) instead of TAC (Tyr-792) was used together with amp repair and tet knockout oligonucleotides, according to the manufacturer's instructions. Point mutations at the Lys-666 codon were also generated using 20-bp oligonucleotides (nt 2068 to 2087 of the insert in pSP38) incorporating either ATG (Met) or AGG (Arg) instead of AAG (Lys-666). The resulting inserts containing Phe-792, Met-666, or Arg-666 codon were digested with NheI and EcoRI, and the 1,964-bp restriction fragments were isolated and subcloned into the corresponding region of full-length EphA8 cDNA.
The doxycycline-regulated EphA8 expression construct was generated by digesting the carboxy-terminal HA-tagged murine EphA8 cDNA with EcoRI and then subcloning the resulting fragment into the tetracycline-inducible vector pTRE (Clontech).
To generate a full-length murine p110γ cDNA, a partial fragment (nt 1668 to 4690 of the murine p110γ cDNA sequence (GenBank accession no. AJ24928)) was first isolated from an expressed sequence tag clone (GenBank accession no. AW761865; Research Genetics). The rest of the p110γ cDNA was generated using reverse transcription-PCR (RT-PCR) and mouse embryonic brain mRNA as a template. The first RT-PCR product was generated using Omniscript reverse transcriptase (Qiagen), a 5′ primer matching nt 1384 to 1408, and a 3′ primer matching nt 1805 to 1829. The resulting PCR fragment was digested with EcoRV, and this 353-bp fragment was then subcloned into pBluescript SK. The second RT-PCR product was generated using a 5′ primer (nt 300 to 319 of the p110γ cDNA plus an EcoRI site at its 5′ end) and a 3′ primer matching nt 1444 to 1468 of the p110γ cDNA. The resulting PCR fragment was digested with EcoRI and EcoRV, and the resulting 1,126-bp fragment was subcloned into pBluescript SK. These DNA fragments were then ligated together with the insert derived from the expressed sequence tag clone to generate the full-length p110γ cDNA in the vector pcDNA3. The construct was DNA sequenced in both directions to exclude the possibility of errors.
To introduce the eight-amino-acid FLAG epitope (DYKDDDDK) at the carboxy terminus of the murine p110γ protein, two oligonucleotides were synthesized. The first consisted of a 57-bp oligonucleotide with 3′ 20bp of the p110γ coding sequence, the FLAG tag sequence, a TGA stop codon, and an XhoI site at the 5′ end. The second consisted of a 20-bp oligonucleotide corresponding to nt 2607 to 2626 of the p110γ cDNA. PCR using these primers was performed with HotStar Taq polymerase (Qiagen), which amplified a 1,039-bp DNA fragment. The resulting PCR product was digested with HindIII and XhoI and subcloned into the corresponding region of the full-length p110γ in pcDNA3. DNA sequencing across the cloning site was performed to exclude the possibly of errors in sequence.
A point mutation at the Lys-833 codon of murine p110γ was generated using a 25-bp oligonucleotide (nt 2803 to 2827 of AJ24928) to incorporate AGA (Arg) instead of AAA (Lys).
To generate the Myr-p110γ construct, we first designed a pcDNA-Myr vector, a modified pcDNA3 expression vector encoding the amino-terminal 15 amino acids of chicken c-Src, which includes the c-Src amino-terminal myristoylation signal. The pcDNA-Myr vector was generated with two complementary oligomers. The 57-bp forward primer consisted of an EcoRI site at the 5′ end, 45 nt encoding the amino-terminal 15 amino acids of chicken c-Src, and an XhoI site at the 3′ end. The two oligonucleotides were phosphorylated at their 5′ ends using T4 polynucleotide kinase (Epicentre Technologies) and were then self-annealed prior to being inserted into pcDNA3.
RT-PCR analysis.
For RT-PCR analysis, total RNA was isolated from transfected HEK293 cells using an RNeasy mini kit (Qiagen) and then used to synthesize first-strand cDNA using oligo (dT) primers and Omniscript reverse transcriptase (Qiagen). The resulting cDNA was then used in PCR to amplify murine p110γ, EphA8, and human p110γ DNA fragments. A 546-bp DNA fragment of EphA8 was amplified using a 5′ primer matching nt 2569 to 2588 of the insert in pSP38 and a 3′ primer matching the HA epitope sequence appended at the carboxy terminus of EphA8. A 330-bp DNA fragment of murine p110γ was amplified using a 5′ primer matching nt 3322 to 3341 of the murine p110γ cDNA and a 3′ primer matching the FLAG epitope sequence appended at the carboxy terminus of p110γ. A 229-bp DNA fragment of the human p110γ was amplified using a 5′ primer matching nt 3401 to 3420 and a 3′ primer matching nt 3609 to 3628 of the human p110γ cDNA sequence (GenBank accession no. X83368). PCR was performed using HotStar Taq DNA polymerase and 18 cycles of amplification with a 1-min denaturation step at 94°C, a 1-min annealing step at 56°C, and a 1.5-min extension step at 72°C.
Immunoprecipitation, Western blotting, cell surface biotinylation, and pulse-chase experiments.
Immunoprecipitation and Western blotting were performed as previously described (7). For cell surface biotinylation, confluent cells were detached by trypsin treatment, washed once with complete medium (DMEM or alpha-MEM containing 10% heat-inactivated fetal bovine serum) and counted prior to cell surface biotinylation. The cells were placed on ice, washed five times with phosphate-buffered saline (PBS) and biotinylated by incubation with sulfo–N-hydroxysuccinimide–biotin (0.5 mg/ml; Pierce) for 30 min. The reaction was stopped by washing three times with PBS. Labeled cells were lysed with PLC lysis buffer as described above. Extracted cell proteins were immunoprecipitated with the relevant antibodies, and the immunocomplexes were bound to protein A-Sepharose (integrin α5 polyclonal antibody) or rabbit anti-mouse immunoglobulin G (IgG)-Sepharose (integrin α5β1 monoclonal antibody) beads. Bound material was eluted by boiling the beads in 5% sodium dodecyl sulfate (SDS) and resolved by electrophoresis on SDS–7.5 polyacrylamide gels (7.5% SDS-PAGE) under nonreducing conditions. Biotinylated integrins were detected by incubating blots with streptavidin-horseradish peroxidase (HRP) (Pierce). For pulse-chase experiments, cells were transiently transfected with FLAG-tagged p110γ and then treated with doxycycline to induce the wild-type EphA8 protein. Cells were washed once with PBS and starved for 2 h in methionine- and cysteine-free medium containing 10% dialyzed fetal calf serum. The cells were then pulse-labeled for 15 min with 200 μCi of [35S]methionine and [35S]cysteine (NEN), per ml, washed once to remove unincorporated radioactivity, and chased for 30 min in unlabeled normal medium with or without preclustered EphA5-Fc. At the indicated times, cells were detergent extracted and subjected to immunoprecipitation with anti-FLAG antibody.
Binding assay, GST-mixing experiment, far-Western analysis, and PI 3-kinase assay.
Binding assays using EphA5-Fc proteins and glutathione S-transferase (GST)-mixing experiments were performed as described previously (7, 30). Far-Western blot assays were performed as described previously (11). GST-JM fusion protein was radiolabeled using [γ-32P]ATP and anti-HA immunoprecipitates containing wild-type EphA8 as described previously (7). The labeled GST-JM proteins were purified using a PD-10 column (Amersham-Pharmacia) and then used to probe membranes containing proteins transferred from 7.5% SDS-polyacrylamide gels. After incubation at room temperature for 3 h, the membranes were washed five times with Tris-buffered saline (10 mM Tris-HCl [pH 7.5], 0.9% NaCl) before exposure to film. PI 3-kinase activity was measured as previously described (14), with some modifications. Briefly, proteins were immunoprecipitated by incubating cell extracts with an anti-HA antibody or a p85 subunit-specific PI 3-kinase antibody and protein A-Sepharose as described above. The Sepharose beads were washed three times with HNTG buffer (7), once with 1% Nonidet P-40 in PBS, once with 100 mM Tris-HCl (pH 7.5) containing 500 mM LiCl, and once with 50 mM Tris-HCl buffer (pH 7.2) containing 150 mM NaCl. After removal of the last wash, the beads were resuspended in kinase buffer (20 mM HEPES [pH 7.2], 50 mM NaCl, 1 mM EGTA) containing 4 μg of PI (Sigma), 10 μM ATP, 5 mM MnCl2, and 10 μCi of [γ-32P]ATP and incubated for 20 min at 30°C. The reaction was stopped by the addition of 100 μl of 1 N HCl and 200 μl of a 1:1 mixture of chloroform and methanol. The lipids were extracted and resolved on potassium oxalate-pretreated thin-layer chromatography (TLC) plates (EM Sciences) with 35 ml of 2 N acetic acid and 65 ml of 1-propanol as the mobile phase. Dried plates were exposed to either Fuji-Imaging plates for quantitation or Kodak X-ray film for autoradiography. Analysis of ephrin A5-stimulated accumulation of 3′-phosphorylated inositol phospholipids was performed as described elsewhere (18, 32). Briefly, HEK293 cells transiently transfected with p110γ were treated with doxycyline for 12 h to induce the expression of the EphA8 receptors. These cells were serum starved in phosphate-deficient DMEM for 2 h prior to labeling with 32Pi (2 mCi/ml for 2 h in a 60-mm-diameter dish) and then treated with preclustered ephrin A5-Fc for the indicated times. Lipid extraction, deacylation, and analysis of the deacylated lipids by anion-exchange high-pressure liquid chromatography (HPLC) were performed as previously described (32).
Cell attachment assays.
Attachment assays were performed on 22-mm2 coverslips coated with different matrix proteins. Coverslips were placed in 35-mm-diameter dishes, coated by overnight incubation at 4°C, and postcoated for 1 h with 1% bovine serum albumin in PBS. Fibronectin (Promega) and laminin (Upstate Biotechnology, Inc.) were used at 100 and 125 μg/ml, respectively, in PBS. in cell attachment assays using PI 3-kinase inhibitors, wortmannin (stored in dimethyl sulfoxide [DMSO] in the dark at −20°C) was added directly to the culture medium for 30 min. Cells were collected by brief trypsinization, washed twice with complete medium, and then replated in triplicate onto coverslips (5 × 105 cells/coverslip). After 15 (NIH 3T3) or 30 (HEK293) min at 37°C, unattached cells were removed by washing twice with complete medium, and adherent cells were incubated until 2 h after replating. Adherent cells on coverslips were counted directly by a hemocytometer. Alternatively, cells were fixed in 4% paraformaldehyde, stained with 0.5% crystal violet in 20% methanol, and quantified by measurement of the optical density at 570 nm. Control coverslips were incubated for 2 h without washing and used to measure total cell numbers. The ratio of attached cells to total cell numbers was calculated for each of three coverslips. Data are expressed as mean ± standard error (SE) and are representative of three independent experiments. In experiments using integrin blocking antibodies, cells were preincubated with the relevant antibodies (5 μg/ml) for 5 min at room temperature before replating.
Antibodies.
Polyclonal rabbit antibody specific for the EphA8 JM was described previously (7). Monoclonal antiphosphotyrosine antibody (4G10) and polyclonal anti-p85 subunit PI 3-kinase antibody were purchased from Upstate Biotechnology. Polyclonal rabbit anti-p110α, -p110β, and -p110γ antibodies were from Santa Cruz Biotechnology. Polyclonal rabbit anti-HA antibody was obtained from Zymed. Monoclonal mouse anti-FLAG antibody was from Sigma. Monoclonal anti-human integrin α5β1 (JBS5) and murine α5β1 (BMA5) and polyclonal anti-murine integrin α5 antibodies were from Chemicon. Monoclonal anti-murine integrin β3 antibody was from Transduction Laboratories. The HRP-conjugated secondary antibodies were from Amersham Pharmacia Biotech, and streptavidin-HRP was from Pierce.
RESULTS
Both wild-type and kinase-inactive EphA8 proteins promote cell attachment to fibronectin.
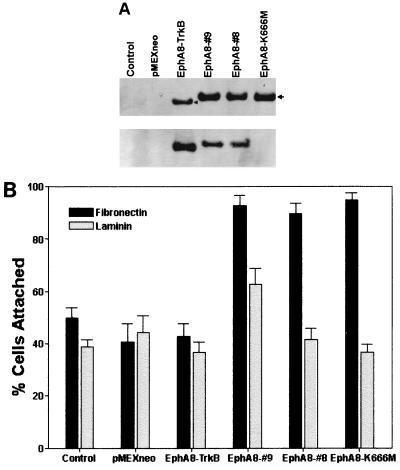
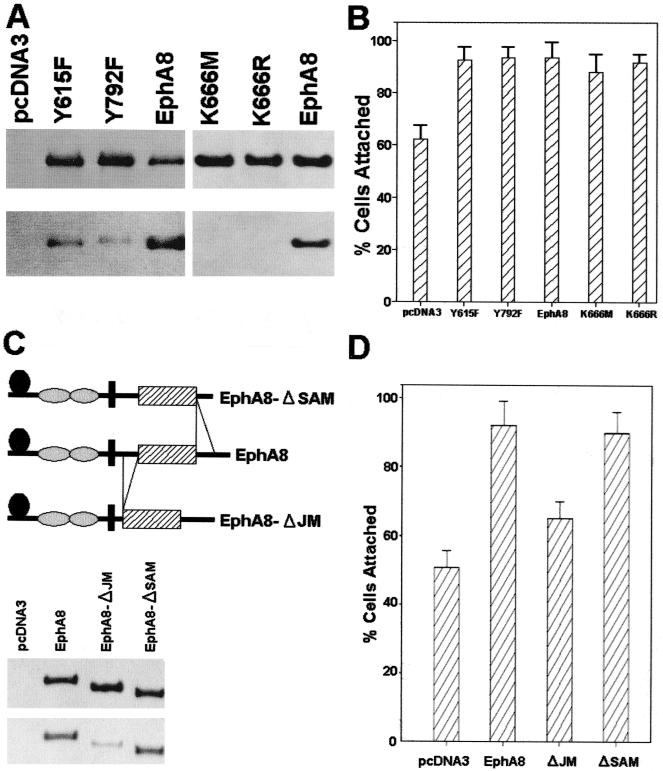
To determine whether EphA8 signaling can promote cell adhesion through integrins, wild-type and kinase-inactive EphA8 receptors and a chimeric EphA8-TrkB receptor consisting of the extracellular domain of the EphA8 receptor plus the transmembrane and cytoplasmic domains of TrkB were stably expressed in NIH 3T3 fibroblasts. Lysates prepared from representative cell lines were analyzed by immunoprecipitation with either anti-TrkB or anti-EphA8 antibodies and then assayed by immunoblotting using a mixture of anti-EphA8 and anti-TrkB antibodies as a probe (Fig. 1A, top). High levels of chimeric EphA8-TrkB receptor, wild-type EphA8, and kinase-inactive EphA8 were detected in the correspondingly transfected fibroblasts. When the same blot was stripped and reprobed with antiphosphotyrosine antibody (Fig. 1A, bottom), it was evident that both EphA8-TrkB and EphA8 proteins were highly tyrosine phosphorylated due to the endogenous expression of EphA subgroup ligands as described previously (Fig. 1A, bottom, lanes 3 to 5) (30). In contrast, the EphA8 mutant containing Met in place of Lys-666, the putative ATP binding residue, was not tyrosine phosphorylated (Fig. 1A, bottom, lane 6). The kinase-inactive EphA8 mutant appeared to be weakly tyrosine phosphorylated because it was very weakly detected after long exposure (data not shown). This phosphorylation is possibly due to cross-phosphorylation induced by heterodimerization of EphA8 with other EphA family members present in low levels in these cells, as assessed by a binding assay using ephrin A5-Fc (see Fig. 4B). Cell attachment assays were performed by detaching the cells from culture dishes and replating them onto coverslips coated with fibronectin or laminin. As shown in Fig. 1B, adhesion of two independent NIH 3T3 clones onto a fibronectin matrix was significantly enhanced by expression of the wild-type EphA8 receptor, whereas the EphA8-TrkB chimeric receptor did not promote cell attachment to fibronectin. Unexpectedly, expression of a kinase-inactive EphA8 receptor also markedly promoted cell adhesion to fibronectin, producing an effect similar to that exerted by the wild-type EphA8 receptor. No consistent differences in adhesion to laminin-coated surfaces were observed in response to EphA8. In our cell adhesion assays where cells were seeded onto coverslips, most attached to the surface as well-separated cells that did not aggregate. These results strongly suggest that the intracytoplasmic region of the EphA8 receptor plays a critical role in promoting cell attachment to fibronectin and that the kinase activity of the receptor is not necessary for this action.
FIG. 1.
(A) Stable expression and in vivo tyrosine phosphorylation of EphA8, EphA8-TrkB, and the EphA8 mutant containing Met in place of Lys-666. NIH 3T3 cells were stably transfected with pMEXneo-derived expression plasmids containing, the indicated cDNAs, and individual G418-resistant clones were isolated. Cells were lysed in PLC lysis buffer, and proteins from each cell lysate were immmuprecipitated with either anti-TrkB or anti-EphA8 antibody. Immunoprecipitates were separated by 7.5% SDS-PAGE and immunoblotted with a mixture of anti-TrkB and anti-EphA8 antibodies (top); the same blot was stripped and reprobed with antiphosphotyrosine antibody (bottom). (B) Cell attachment responses to two different extracellular matrix proteins. Control NIH 3T3 fibroblasts (either parental or vector transfected) or stably transfected cells expressing the indicated proteins were plated on coverslips coated with fibronectin or laminin. Cells were allowed to adhere for 15 min. then nonadherent cells were removed by washing, and incubation was continued for 105 min. The percentage of cells attached after 15 min is shown. Data from three separate experiments are presented as means ± SE.
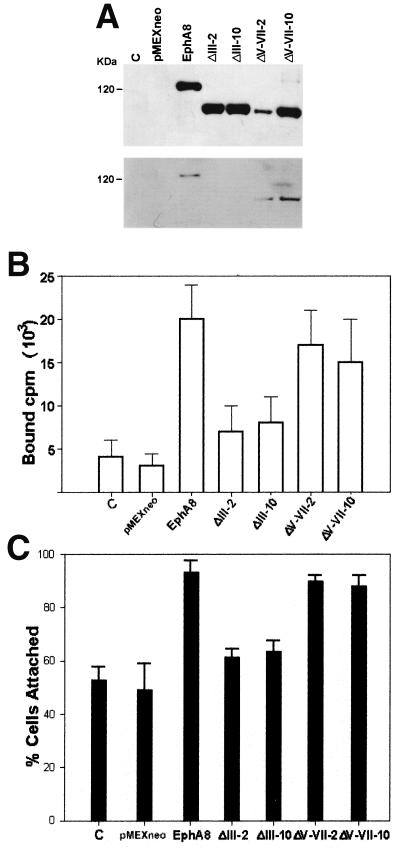
FIG. 4.
(A) Expression and tyrosine phosphorylation of the exogenous EphA8ΔIII and EphA8ΔV-VII deletion mutants in NIH 3T3 fibroblasts. NIH 3T3 cells were transfected with pMEXneo-derived expression plasmids containing recombinant cDNAs encoding the indicated deletion proteins and selected in G418-containing medium. Two independent cell lines per constructs were analyzed by immunoprecipitation with anti-EphA8 antibody. Western blot analysis was performed with anti-EphA8 antibody (top); the same blot was stripped and then reprobed with antiphosphotyrosine antibody (bottom). C, control. (B) The domain encoded by exon III of EphA8 is critical for efficient binding of the ephrin A5 ligand. NIH 3T3 cells stably expressing wild-type EphA8 or the indicated deletion mutants were treated with 40 nM chimeric ephrin A5-Fc proteins followed by radioiodinated goat anti-human lgG, washed, and solubilized for determination of specific binding. Nonspecific binding was assessed as binding in the absence of chimeric Fc proteins. Specific binding, shown on the y axis, was calculated by subtracting nonspecific binding from total counts bound. (C) Deletion of exon III abrogates the EphA8-stimulated cell attachment to fibronectin. Cell attachment assays were performed as described in previous figure legends.
EphA8-stimulated cell attachment is mediated through α5β1 and β3 integrins in NIH 3T3 fibroblasts.
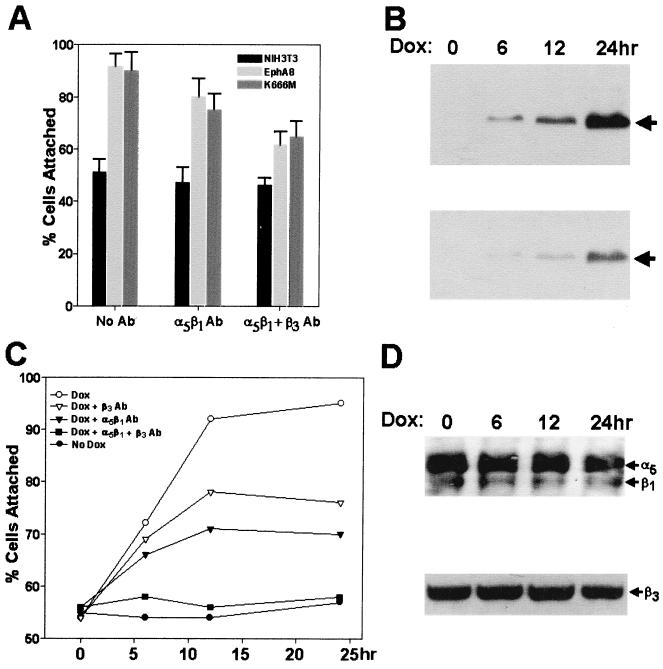
We next investigated whether the observed promotion of cell attachment by EphA8 was mediated by integrins by testing the effects of monoclonal anti-α5β1 and anti-β3 integrin blocking antibodies on EphA8-stimulated cell attachment to fibronectin. Neither anti-α5β1 nor anti-β3 integrin antibodies had any significant effect on the attachment of a parental NIH 3T3 fibroblasts to fibronectin, which stayed at a consistent level of 45 to 55% (Fig. 2A). This level of adhesion seems to be the basal level, and it is possible that it is not integrin mediated, since it could not be reduced by the use of the integrin blocking antibodies, including those specific for α4 and αv integrins (data not shown). In contrast, EphA8-stimulated cell adhesion in NIH 3T3 fibroblasts expressing the wild-type or kinase-inactive EphA8 receptor was partially inhibited by anti-α5β1 integrin blocking antibodies. The simultaneous addition of anti-α5β1 and anti-β3 integrin blocking antibodies markedly inhibited adhesion of these transfected fibroblasts onto a fibronectin matrix. The β3 integrin subunit is known to heterodimerize with the αv or αIIb integrin subunit (1). Since αIIbβ3 integrin is expressed only in platelets, it is likely that αvβ3 integrin is involved in EphA8 modulation of cell adhesion in NIH 3T3 fibroblasts. This possibility could not be confirmed in the present study, as no specific blocking antibody against murine αvβ3 integrin was available. Nonetheless, our data strongly suggest that EphA8 action modulates cell adhesion through α5β1 and possibly αvβ3 integrin proteins in NIH 3T3 fibroblasts.
FIG. 2.
(A) Effects of anti-α5β1 and anti-β3 integrin blocking antibodies (Ab) on NIH 3T3 cell adhesion to fibronectin. NIH 3T3 fibroblasts stably expressing wild-type or kinase-inactive EphA8 were detached and incubated for 5 min with either anti-α5β1 or anti-β3 integrin blocking antibody (5 μg/ml). The cells were then replated on fibronectin-coated coverslips and allowed to adhere for 15 min. Data from three independent experiments are presented as means ± SE. (B) Induction of wild-type EphA8 protein expression in NIH 3T3 fibroblasts. Cells were incubated with 2 μg of doxycycline (Dox) per ml for the indicated periods of time and then lysed, and proteins from the lysates were immunoprecipitated with anti-EphA8 antibody. Immunoprecipitates were separated by 7.5% SDS-PAGE and immunoblotted with anti-EphA8 antibody (top); the same blot was stripped of antibodies and reprobed with antiphosphotyrosine antibody (bottom). (C) Effects of anti-α5β1 and anti-β3 integrin blocking antibodies on NIH 3T3 cell attachment stimulated by the induced expression of EphA8. Cells were treated with doxycycline for the indicated periods of time, and cell attachment assays were performed after incubation with either anti-α5β1 or anti-β3 blocking antibodies as described above. (D) Analysis of α5β1 and β3 integrins expressed in NIH 3T3 cells, concomitant with the induced expression of EphA8. EphA8 expression was induced for the indicated times, then the cells were harvested and cell surface proteins were biotinylated; integrins were immunoprecipitated with anti-α5 polyclonal antibody, and biotinylated integrins were detected using streptavidin-HRP (top). Cells were lysed in PLC lysis buffer, and protein concentrations were equalized (bottom). Cell lysates were fractionated by SDS-PAGE and then analyzed by immunobloting using anti-β3 antibody as a probe.
Long-term forced overexpression of EphA8 in NIH 3T3 fibroblasts may result in complex modifications of cell adhesion molecules. We therefore adopted a doxycycline-inducible expression system for the EphA8 receptor in NIH 3T3 fibroblasts. In this system, no EphA8 was detected in the absence of doxycycline, the level of EphA8 expression correlated with the time of incubation with doxycycline (Fig. 2B, top), and a marked increase in tyrosine phosphorylation of EphA8 was observed in response to doxycycline treatment (Fig. 2B, bottom), demonstrating that expression of EphA8 is tightly regulated by doxycycline. To investigate whether integrin activity was regulated by doxycycline-induced expression of EphA8, we carried out cell attachment assays after treatment with doxycycline for various periods of time. In the absence of doxycycline, transfected and parental cells adhered to fibronectin to similar extents, whereas the transfected cells treated with doxycycline for 6 h showed a significant increase in adhesion to fibronectin (Fig. 2C). The increase in cell attachment was maximal after 12 h of induction. Similar to the effects observed in NIH 3T3 fibroblasts stably expressing the EphA8 receptor, anti-α5β1 and anti-β3 integrin blocking antibodies partially inhibited doxycycline-induced cell adhesion to fibronectin. In addition, the simultaneous addition of anti-α5β1 and anti-β3 integrin blocking antibodies markedly inhibited adhesion of these NIH 3T3 fibroblasts onto a fibronectin matrix.
To test whether EphA8 expression affected integrin expression, we analyzed cell surface α5β1 integrin levels by immunoprecipitation of biotin-labeled cells with an anti-α5 polyclonal antibody (Fig. 2D, top). This result revealed that overall cell surface α5β1 integrin was not significantly altered by the induced expression of wild-type EphA8 receptor. Likewise, as demonstrated by Western blot analysis of total cell lysates with the anti-β3 integrin antibody as a probe, total levels of β3 integrin expression were not changed by the doxycycline-induced expression of EphA8 (Fig. 2D, bottom). Taken together, our results indicate that EphA8 expression modulates cell adhesion through α5β1 and β3 integrin proteins in NIH 3T3 fibroblasts without altering the integrin expression level.
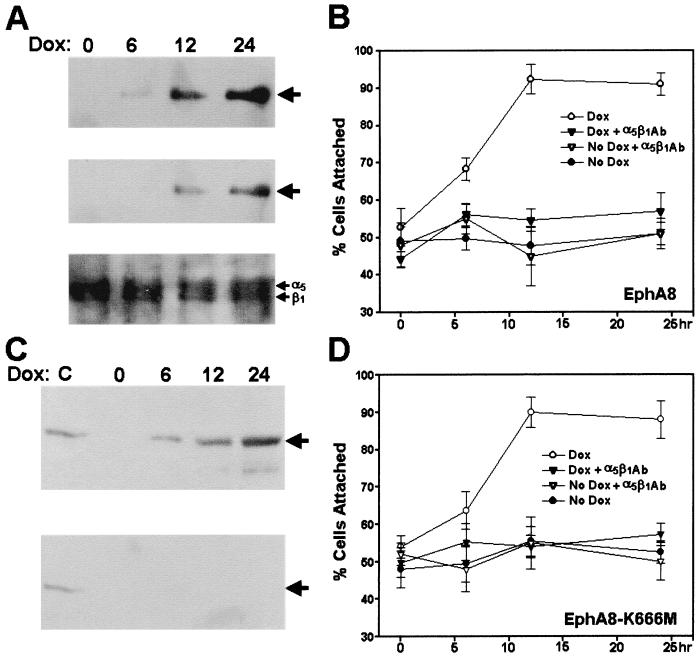
EphA8-stimulated cell attachment is mediated mainly through α5β1 integrin in HEK293 epithelial cells.
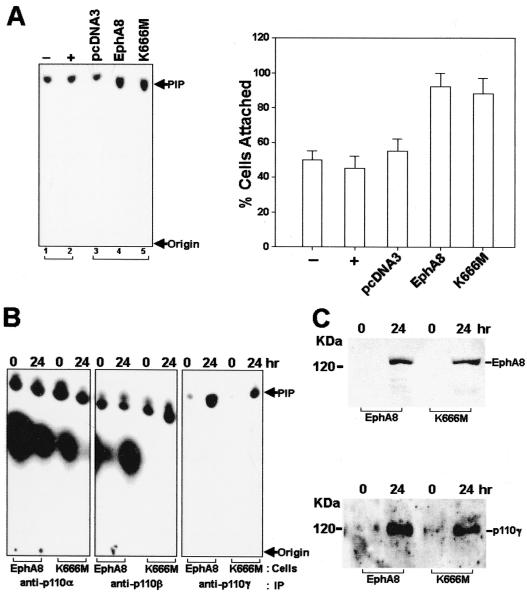
To investigate whether regulation of integrin activity by the EphA8 receptor is dependent on cell type, we also expressed wild-type or kinase-inactive EphA8 receptors in HEK293 epithelial cells, once again using a powerful doxycycline-inducible expression system. In response to doxycycline, the time-dependent EphA8 induction (Fig. 3A, top) and tyrosine phosphorylation (Fig. 3A, middle) profiles in HEK293 cells were very similar to the patterns observed for the EphA8 receptor inducibly expressed in NIH 3T3 fibroblasts. Likewise, the kinase-inactive EphA8 mutant was also induced by doxycycline (Fig. 3C, top), and as we expected, no tyrosine phosphorylation of the mutant EphA8 protein was detectable (Fig. 3C, bottom). The adhesive properties of these cells in response to doxycycline were tested in cell attachment assays. Induction of the EphA8 proteins in these cells dramatically promoted cell attachment to a fibronectin matrix within 12 h, regardless of the EphA8 kinase activity (Fig. 3B and D). To test whether the cells adhered to fibronectin through α5β1 integrin receptors, we examined the effects of anti-α5β1 blocking antibodies on cell adhesion to fibronectin at each induction time point (Fig. 3B and D). It was evident that anti-α5β1 blocking antibodies alone greatly inhibited cell adhesion to fibronectin. In contrast, no inhibition of cell adhesion onto fibronectin could be detected by adding anti-αvβ3 blocking antibodies (data not shown). These results indicate that in HEK293 cells, α5β1 integrin is a major fibronectin receptor which interacts with EphA8 in a kinase activity-independent fashion. In addition, as observed in NIH 3T3 fibroblasts, the expression of EphA8 did not alter the surface expression of α5β1 integrin in HEK293 epithelial cells (Fig. 3A, bottom). Taken together, our data indicate that EphA8 modulation of cell adhesion through alteration of integrin activity is a global mechanism and not restricted to a small subset of cell types.
FIG. 3.
(A) Induction of wild-type EphA8 protein expression in HEK293 epithelial cells. Cells were incubated with 2 μg of doxycycline (Dox) per ml for the indicated times. Proteins from cell lysates were immmunoprecipitated with anti-EphA8 antibody, then separated by 7.5% SDS-PAGE, and immunoblotted with the same antibody (top); the same blot was stripped and then reprobed with antiphosphotyrosine antibody (middle); Bottom, analysis of α5β1 integrin expressed in HEK293 cells, concomitant with the induced expression of EphA8. EphA8 expression was induced for the indicated times, then the cells were harvested, and cell surface proteins were biotinylated. Labeled integrins were immunoprecipitated with anti-α5β1 monoclonal antibody and then detected with streptavidin-HRP. (B) Effect of anti-α5β1 integrin blocking antibodies (Ab) on the cell attachment stimulated by the induced expression of EphA8 in HEK293 cells. The cells were treated with doxycycline for the indicated times, and then cell attachment assays were performed after incubation with anti-α5β1 blocking antibodies as described in the legend to Fig. 2A. Note that HEK293 cells were allowed to adhere for 30 min before being washed to remove nonadherent cells, and then incubation was continued for 90 min. The percentage of cells attached after 30 min is shown. Data from three independent experiments are presented as means ± SE. (C) Induction of kinase-inactive EphA8 protein expression in HEK293 epithelial cells. As a positive control, HEK293 cells inducibly expressing the wild-type EphA8 protein were treated with doxycycline for 12 h. Proteins from cell lysates were immmunoprecipitated with anti-EphA8 antibody, then separated by 7.5% SDS-PAGE, and immunoblotted with anti-EphA8 antibody (top); the same blot was stripped and then reprobed with antiphosphotyrosine antibody (bottom). (D) Effects of anti-α5β1 integrin blocking antibodies on cell attachment stimulated by the induced expression of kinase-inactive EphA8 receptor in HEK293 cells. Cell attachment assays were performed as described for panel B.
Ligand binding of EphA8 is critical for the increased cell attachment to fibronectin.
To investigate whether regulation of integrin activity by EphA8 is a ligand-dependent process, we constructed two different deletion mutants lacking structural motifs of the EphA8 ectodomain. According to the recently published genomic organization of the murine EphA8 gene, the globular domain and a stretch of cysteine-rich sequences are encoded, possibly as a functional unit, by exon III, whereas two fibronectin type III (FNIII) domains are encoded by exons V to VII (22). It is well known that the N-terminal globular domain of Eph receptors is critical for ligand binding (23). It has also been suggested that FNIII domains play a role in receptor dimerization (24). Thus, exon boundaries were used to generate EphA8 deletion mutants lacking these two important structural domains. The deletion mutants were named EphA8ΔIII and EphA8ΔV-VII, according to their deleted exons. These deletion mutants were stably expressed in NIH 3T3 fibroblasts, and two independent clones of each deletion mutant were studied in order to eliminate the possibility of clonal selection for the results.
Expression of the corresponding proteins with the expected molecular masses was demonstrated by Western blot analysis (Fig. 4A, top), but the EphA8ΔIII protein was not detectably tyrosine phosphorylated, possibly due to inefficient ligand binding (Fig. 4A, bottom, lanes 4 and 5). The EphA8ΔV-VII protein became highly tyrosine phosphorylated without further stimulation with exogenous ligands (Fig. 4A, bottom, lanes 6 and 7). To test whether ephrin A subgroup ligands could interact with these deletion mutants, ephrin A5-Fc chimeric protein was purified and used as a probe in binding assays. EphA5-Fc was added in excess to cells expressing wild-type or deletion mutant EphA8 proteins, and the cells were incubated with radioiodinated goat anti-human IgG. As shown in Fig. 4B, the ephrin A5-Fc chimeric proteins did not bind efficiently to EphA8ΔIII-expressing cells but did bind in a specific manner to cells expressing EphA8ΔV-VII. However, we reproducibly observed that binding of ephrin A5 to the EphA8 protein lacking two FNIII domains was reduced relative to wild-type EphA8 receptor binding, suggesting that FNIII domains are partially required for ligand binding. Alternatively, it is also possible that the ligand binding-inhibitory effect of deleting the FNIII domains was due to the decreased distance between the N-terminal ligand binding domain and the membrane, resulting in a reduction in its accessibility by ephrin A. We next performed a cell attachment assay to test whether expression of EphA8 deletion mutants could promote cell adhesion to fibronectin. As shown in Fig. 4C, cells expressing either wild-type EphA8 or EphA8ΔV-VII bound efficiently to fibronectin. However, in multiple experiments, we consistently observed that cells expressing the EphA8ΔIII protein adhered to fibronectin slightly more than the parental NIH 3T3 fibroblasts did. Thus, our data strongly indicate that the specific interaction between the ephrin A ligand and the EphA8 receptor is crucial for modulation of integrin activity.
Tyrosine phosphorylations in EphA8 do not correlate with EphA8-stimulated cell attachment to fibronectin
To test whether the EphA8-stimulated integrin activity was independent of tyrosine phosphorylation of EphA8, the codons for Tyr-615 and Tyr-792 were replaced with phenylalanine codons in the EphA8 cDNA to construct EphA8Y615F and EphA8Y792F point mutants. We have previously demonstrated that Tyr-615 constitutes a major autophosphorylation site and mediates preferential binding to the Fyn SH2 domain (7). Tyr-792 is located in the activation loop of the EphA8 tyrosine kinase domain, and corresponding tyrosine residues are found in all known members of the Eph receptor family. HEK293 cells were transiently transfected with pcDNA3 constructs containing wild-type or mutated EphA8 cDNAs. In contrast to the wild-type protein, the EphA8 mutant proteins immunoprecipitated from lysates of EphA8Y615F- or EphA8Y792F-transfected cells contained markedly reduced levels of phosphotyrosine (Fig. 5A, bottom left) compared with the overall expression levels of these proteins (Fig. 5A, top left). Nonetheless, the attachment responses of HEK293 cells expressing EphA8Y615F or EphA8Y792F were stimulated to a degree similar to that observed in cells expressing the wild-type EphA8 protein (Fig. 5B).
FIG. 5.
(A) Transient expression and tyrosine phosphorylation of EphA8 point mutants and kinase-inactive proteins. HEK293 cells were transiently transfected with pcDNA3-derived expression plasmids containing the recombinant cDNAs encoding the indicated EphA8 mutant proteins and were lysed for immunoprecipitation with anti-EphA8 antibody at 24 h posttransfection. Western blot analysis was performed with anti-EphA8 antibody to verify the level of EphA8 expression (top); the same blot was stripped and then reprobed with antiphosphotyrosine antibody to evaluate the level of autophosphorylation of the transfected EphA8 mutant proteins (bottom). (B) Mutations of two major tyrosine phosphorylation sites and the ATP binding site lysine of the EphA8 receptor do not affect EphA8-stimulated cell attachment to fibronectin. Cell attachment assays were performed as described in previous figure legends. (C) Top, schematic representation of the EphA8 cytoplasmic domain deletion mutants. Middle, HEK293 cells were transiently transfected with the indicated constructs, and cells were lysed for immunoprecipitation with anti-HA polyclonal antibody at 24 h posttransfection. Western blot analysis was performed with same antibody to verify the expression level of the deletion mutant. Note that the anti-EphA8 antibody does not recognize the EphA8 mutant lacking the JM encoded by exon X. Bottom, the same blot was stripped and then reprobed with antiphosphotyrosine antibody. (D) Cell attachment assays performed as described in previous figure legends.
In the case of the kinase-inactive mutant EphA8K666M, replacement of a lysine residue with methionine may structurally alter the EphA8 protein, leading to constitutive stimulation of integrin activity. In other studies of EphB1 and EphB2, the corresponding lysine residue was replaced with arginine, and these kinase-inactive EphB mutants failed to confer EphB1-induced cell attachment responses (9, 21, 35, 43). This difference led us to investigate whether another kinase-inactive mutant, EphA8K666R, could stimulate integrin activity. Despite the fact that similarly high levels of expression of each kinase-inactive mutant protein were observed (Fig. 5A, right), the EphA8K666R mutant promoted cell attachment to fibronectin similarly to wild-type EphA8 or the EphA8K666M mutant (Fig. 5B). Taken together, these results demonstrate that phosphorylation on tyrosine residues of EphA8 does not play a critical role in the EphA8-stimulated cell attachment to fibronectin.
The JM encoded by exon X is crucial for interaction with integrin
To investigate the structural determinant of EphA8 that controls integrin activity, we used exon-intron boundaries of the murine EphA8 gene to design deletion mutants. The exon X- and XVI-encoded portions of the murine EphA8 receptor correspond to a highly conserved JM and a sterile alpha motif (SAM), respectively (22). The EphA8 cytoplasmic domain deletion mutants were transiently expressed as HA epitope-tagged proteins in HEK293 cells. The HA epitope-tagged deletion mutants were expressed at the expected molecular masses and at similar levels, indicating the stability of these constructs (Fig. 5C, middle). Although the JM deletion mutant contained reduced levels of phosphotyrosine (Fig. 5C, bottom, lane 3) due to the lack of a major autophosphorylation site, Tyr-615, it had retained efficient tyrosine kinase activity, as demonstrated by its action on an exogenous substrate (enolase) in an in vitro kinase assay (data not shown). We then monitored the effects of each deletion mutant on the adhesive properties of the cells. HEK293 cells expressing wild-type EphA8 or the SAM deletion mutant showed increased adhesion to fibronectin relative to cells transiently transfected with empty vector (Fig. 5D). In contrast, cells expressing the JM deletion mutant showed only a slight increase in adhesion to fibronectin, much less than the increase observed in cells expressing the SAM deletion mutant or wild-type EphA8. This result indicates that the EphA8 JM encoded by exon X is required for interaction with integrin.
The p85 subunit-associated PI 3-kinase does not contribute to EphA8-stimulated integrin activation.
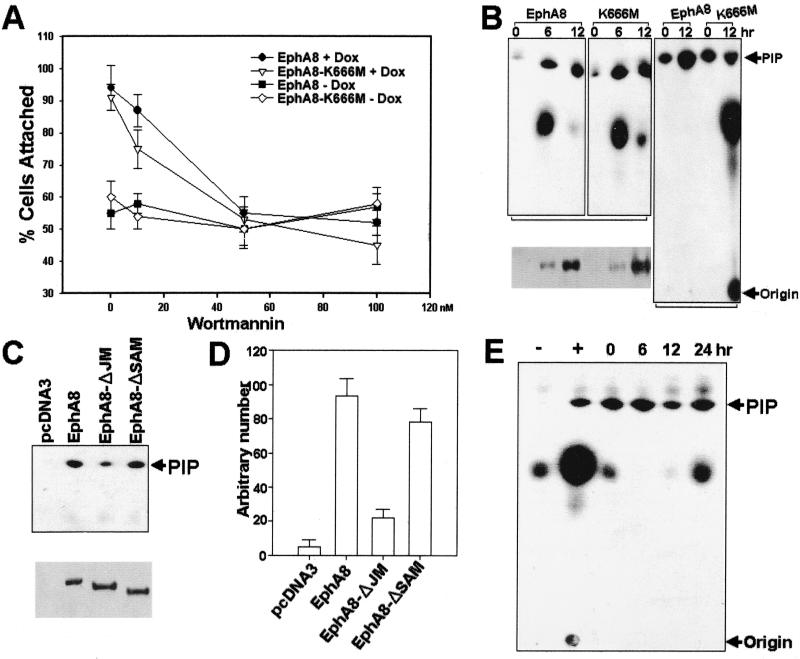
In a preliminary approach to identifying the signaling mechanism by which EphA8 regulates integrin function, we assessed the effects of the PI 3-kinase inhibitor wortmannin on HEK293 cells that had been induced to express wild-type or kinase-inactive EphA8 receptors. Treatment of HEK293 cells with up to 100 nM wortmannin had no effect on the basal adhesion to fibronectin (Fig. 6A). Regardless of the kinase activity, doxycycline-induced expression of EphA8 proteins in HEK293 cells stimulated cell attachment to fibronectin in the absence of wortmannin. However, EphA8-stimulated cell attachment was partially inhibited by 10 nM wortmannin and almost completely abolished by wortmannin concentrations of 50 to 100 nM (Fig. 6A). We also observed that the PI 3-kinase inhibitor LY294002 had a similar inhibitory effect on EphA8-stimulated cell attachment (data not shown). These results imply that PI 3-kinase may couple the EphA8 receptor to integrin activation. To further explore the possibility of an association between the EphA8 receptor and PI 3-kinase activity, we performed in vitro kinase assays using HEK293 cells induced to express EphA8 proteins. After induction of EphA8 proteins with doxycycline, extracts were immunoprecipitated with anti-HA antibody to capture the activated EphA8 proteins, and the immunoprecipitates were then assayed for the ability to phosphorylate PI in vitro. An increase in PI 3-kinase activity, as indicated by the appearance of PI(3)P, was observed upon expression of the wild-type or kinase-inactive EphA8 protein (Fig. 6B, first and second panels). Interestingly, an in vitro PI 3-kinase assay using antiPhosphotyrosine immunoprecipitates has revealed that PI 3-kinase activity was significantly increased by the induction of expression of the wild-type EphA8 but not when kinase-inactive EphA8 was induced (Fig. 6B, third panel). More importantly, wild-type or SAM deletion EphA8 proteins stimulated PI 3-kinase activity to a greater extent than did the JM deletion EphA8 mutant in HEK293 transfectants (Fig. 6C and D). However, we observed that transient expression of the PI 3-kinase p85 subunit lacking the p110 binding site (Δp85) did not inhibit EphA8-promoted cell adhesion to fibronectin (data not shown). Consistent with this observation, the EphA8-expressing cells did not show any significant alteration in PI 3-kinase activity in anti-p85 subunit immunoprecipitates, whereas p85-associated PI 3-kinase activity in the same cells was elevated in response to serum stimulation (Fig. 6E). We also generated stable HEK293 cell lines expressing 10-fold-excess amounts of Δp85. The anti-p85 subunit immunoprecipitates from these cells showed no changes in PI 3-kinase activity in response to serum stimulation (Fig. 7A, left, lanes 1 and 2). In contrast, transient expression of the wild-type or kinase-inactive EphA8 proteins in the same cells reproducibly induced a marked increase in PI 3-kinase activity (Fig. 7A, left, lanes 4 and 5) and also promoted cell attachment to a fibronectin matrix (Fig. 7A, right). In addition, immunoprecipitates obtained with antiserum against the p110α or p110β PI 3-kinase catalytic subunit showed similar levels of PI 3-kinase activity, regardless of the auto-kinase activity of the expressed EphA8 protein (Fig. 7B, first and second panels). These results strongly indicate that the EphA8 receptor can regulate integrin activity independently of the p85 regulatory subunit by interacting with PI 3-kinase.
FIG. 6.
(A) Inhibition of EphA8-stimulated cell attachment by wortmannin. Expression of wild-type or kinase-inactive EphA8 proteins in HEK293 cells was induced with doxycycline (Dox) for 24 h, and cells were incubated with wortmannin at concentrations ranging from 10 to 100 nM for 30 min prior to cell attachment assays. Cells that had not been treated with doxycycline were included as controls. Wortmannin was dissolved in DMSO. Note that cells were treated with DMSO for 30 min prior to the cell attachment assay in the absence of wortmannin (0 nM on the x axis). Treatment of cells with DMSO (5 to 10 μl) had no effect on the basal level of adhesion or EphA8-stimulated cell adhesion. (B) The EphA8 proteins were induced with doxycycline for the indicated times and then immunoprecipitated with either anti-HA antibody (top, left and middle) or antiphosphotyrosine antibody (top, right). Immunoprecipitates were incubated with 4 μg of PI and 10 μCi of [γ-32P]ATP for 10 min, and the reaction was analyzed by TLC followed by autoradiography. Western blot analysis of anti-HA immunoprecipitates was performed with the same antibody to verify the expression levels of wild-type and kinase-inactive EphA8 proteins at the indicated induction times (bottom). (C and D) The EphA8 JM is necessary for association with PI 3-kinase activity. HEK293 cells were transiently transfected with the indicated constructs, and cells were lysed for immunoprecipitation of the EphA8 deletion mutants with anti-HA polyclonal antibody at 24 h posttransfection. Western blot analysis was performed with same antibody to verify the expression level of the deletion mutant (C, bottom) PI 3-kinase activity was determined by TLC and autoradiography (C, top) and also by measuring formation of radiolabeled PIP from PI, using a phosphorimaging system (D). Shown are mean values (±SE) of three independent experiments. (E) p85-dependent heterodimeric PI 3-kinase is not stimulated to response to EphA8 expression. The kinase-inactive EphA8 protein was induced with doxycycline for the indicated times, and then p85-p110 heterodimeric PI 3-kinase was immunoprecipitated with anti-p85 polyclonal antibody. As a control, the same cells were starved of serum for 24 h and then stimulated with (+) or without (−) serum for 15 min in the absence of doxycycline. Immunoprecipitates were analyzed by in vitro PI 3-kinase assay, and lipids were extracted and analyzed by TLC and autoradiography as described above.
FIG. 7.
Evidence that p110γ PI 3-kinase is tightly associated with the EphA8 receptor. (A) Left, HEK293 cells were transfected with the Δp85 cDNA, and individual G418-resistant clones were isolated. A representative cell line was treated with (+) or without (−) serum for 15 min, cells were lysed in PLC lysis buffer, and p110-p85 heterodimers from the cell lysates were immmuprecipitated with anti-p85 polyclonal antibody (lanes 1 and 2). The stable Δp85 transfectants were transiently transfected with the indicated constructs and lysed for immunoprecipitation of the EphA8 proteins with anti-HA polyclonal antibody at 24 h posttransfection (lanes 3 to 5). PI-3 kinase activity was measured as described in previous figure legends. Right, cell attachment assays performed as in previous figure legends. (B) EphA8 protein expression in HEK293 cells was induced with doxycycline treatment for the indicated times, and then PI 3-kinases were immunoprecipitated (IP) with the indicated anti-p110 isotype antibodies. PI 3-kinase activity was measured as described in previous figure legends. (C) Expression of wild-type or kinase-inactive EphA8 proteins in HEK293 cells was induced with doxycycline for the indicated times. Top, proteins from cell lysates were immunoprecipitated with anti-p110γ antibody and then analyzed by immunoblot using anti-EphA8 antibody as a probe (top); the same blot was stripped and reprobed with anti-p110γ antibody (bottom).
The tight association between p110γ PI 3-kinase and the EphA8 receptor.
To identify the EphA8-specific PI 3-kinase isotype, we performed immunoprecipitations using a p110γ-specific antibody (Fig. 7B, third panel). Anti-p110γ immunoprecipitates contained low levels of PI-3 kinase activity in the absence of EphA8 protein (lanes 1 and 3). Expression of the wild-type or kinase-inactive EphA8 proteins resulted in a substantial increase in p110γ PI 3-kinase activity (lanes 2 and 4). Moreover, stringently washed anti-p110γ immunoprecipitates contained high levels of wild-type or kinase-inactive EphA8 protein (Fig. 7C, top, lanes 2 and 4). Interestingly, we reproducibly observed that the p110γ protein level was substantially increased in EphA8-expressing cells (Fig. 7C, bottom), suggesting that the p110γ PI 3-kinase may be stabilized by the EphA8 receptor. In addition, these results suggest that the increase in PI 3-kinase activity from either EphA8 or p110γ immunoprecipitates was due to the presence of more p110γ protein that had been stabilized by the activated EphA8 receptor. Taken together, our results demonstrate that p110γ PI 3-kinase mediates EphA8-dependent regulation of integrins.
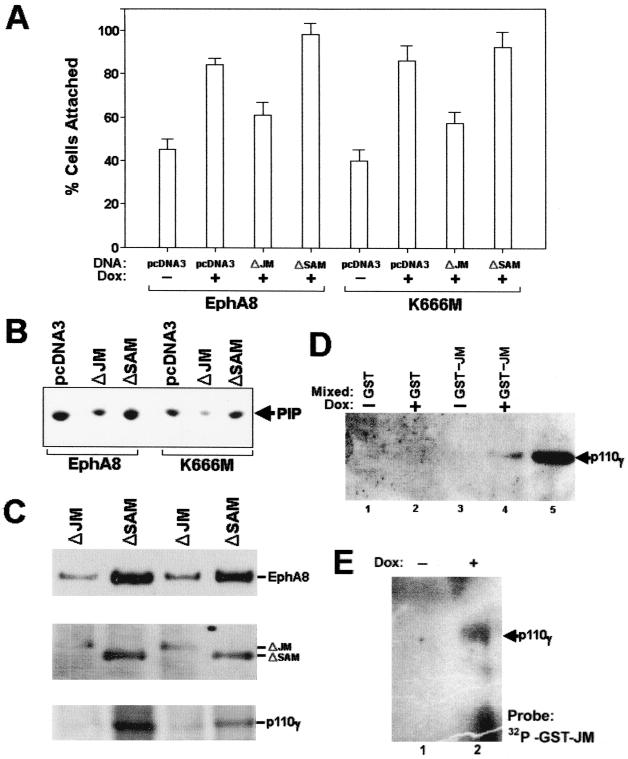
To further investigate whether deletion of the JM region of EphA8 weakens EphA8 regulation of integrin activity, we transiently expressed JM or SAM deletion EphA8 proteins in HEK293 cells and treated the cells with doxycycline for 12 h to induce wild-type or kinase-inactive EphA8 protein. The effects of each deletion mutant on EphA8-stimulated cell adhesion, p110γ PI 3-kinase activity, and EphA8 association with p110γ were then examined (Fig. 8). Regardless of the level of EphA8 kinase activity, fibronectin adhesion activity was much lower for cells expressing the JM deletion EphA8 mutant than for cells transfected with the SAM deletion mutant (Fig. 8A). In addition, the JM deletion mutant resulted in a substantial inhibition of EphA8-stimulated p110γ PI 3-kinase activity, whereas the SAM deletion mutant did not (Fig. 8B). We have consistently observed that the JM deletion EphA8 mutant partially inhibits the association of wild-type or kinase-inactive EphA8 protein with p110γ (Fig. 8C, top, lanes 1 and 3). However, it appears that association of the JM deletion EphA8 mutant with p110γ was not completely defective (Fig. 8C, middle). More importantly, in cells expressing both full-length EphA8 and the JM deletion mutant protein, the p110γ protein level was significantly reduced, suggesting that the JM deletion mutant heterodimerizes with the full-length EphA8 to weaken the ability of EphA8 to associate with cytoplasmic p110γ. To further determine whether the EphA8 JM is sufficient for interaction with p110γ, we performed in vitro binding assays using a bacterially expressed GST-JM fusion protein comprising the entire JM of EphA8. In these experiments, we transiently expressed FLAG-tagged murine p110γ protein and treated the cells with doxycycline for 24 h to induce wild-type EphA8 protein (Fig. 8D, lanes 2 and 4). Approximately equal amounts of purified GST-JM or GST alone were bound to glutathione-Sepharose beads and then mixed with equivalent amounts of lysate prepared from HEK293 cells expressing p110γ with or without EphA8 induction. Proteins from the cell lysates that bound to each GST fusion protein were then analyzed by immunoblot using anti-FLAG antibody as a probe (Fig. 8D). Data obtained from these experiments clearly show that the of EphA8 JM forms a stable complex with the p110γ protein that is highly enriched in doxycycline-treated cells (Fig. 8D, lane 4). To further assess whether GST-JM protein directly associate with p110γ, FLAG-tagged p110γ protein was transiently expressed in HEK293 cells expressing wild-type EphA8 in response to treatment with doxycycline. Lysates prepared from the cells treated with or without doxycycline were immunoprecipitated with anti-FLAG antibody and then subjected to far-Western blot analysis using 32P-labeled GST-JM protein as a probe. In these experiments, the purified GST-JM fusion protein containing Tyr-615 (Fig. 5C) was phosphorylated in vitro by incubation with immunoprecipitates containing wild-type EphA8 and then purified using a gel filtration column. As shown in Fig. 8E, 32P-labeled GST-JM protein bound to p110γ protein that was highly enriched in doxycycline-treated cells, indicating that the EphA8 receptor JM is able to directly associate with p110γ (Fig. 8E, lane 2). Taken together, these findings provide evidence that the EphA8 JM plays a critical role in forming a stable complex with p110γ PI-3 kinase.
FIG. 8.
The JM deletion EphA8 mutant suppresses EphA8-promoted p110γ PI 3-kinase activity and integrin activation by inhibiting the association of EphA8 with p110γ. HEK293 cells expressing EphA8 in response to doxycycline (Dox) were transiently transfected with the indicated constructs; 12 h after transfection, EphA8 protein expression was induced for 12 h. (A) Cell attachment assays performed as described in previous figure legends. (B) Proteins from cell lysates were immunoprecipitated with anti-p110γ antibody, and then PI-3 kinase activity was measured as described in previous figure legends. (C) Proteins from cell lysates were immunoprecipitated with anti-p110γ antibody and then analyzed by immunoblotting with anti-EphA8 antibody as a probe (top); the same blot was stripped and reprobed with anti-HA and anti-p110γ antibodies (middle and bottom, respectively). (D) The EphA8 JM is sufficient for association with p110γ. A FLAG-tagged murine p110γ construct was transiently transfected into parental cells (lane 5) or HEK293 cells expressing EphA8 in response to doxycycline treatment (lanes 1 to 4). As a control, the FLAG-tagged p110γ protein transiently expressed in parental HEK293 cells was directly immunoprecipitated with anti-FLAG antibody (lane 5). The induction of EphA8 began 12 h after transfection and continued for 24 h (lanes 2 and 4). Proteins from each cell lysate were mixed with approximately equal amounts of purified GST or with GST-JM fusion protein bound to glutathione-Sepharose beads (lanes 1 to 4). The washed beads were separated by 7.5% SDS-PAGE and Western blotted using anti-FLAG antibody as a probe. (E) The EphA8 JM is sufficient for direct association with p110γ. HEK293 cells expressing both exogenous p110γ and wild-type EphA8 were prepared as described for panel D. FLAG-tagged p110γ was directly immunoprecipitated with anti-FLAG antibody, and then the washed beads were directly separated by 7.5% SDS-PAGE and transferred to membranes. The blots were probed with 32P-labeled GST-JM protein to detect p110γ. The labeling of GST-JM protein was performed in an in vitro kinase assay with [γ-32P]ATP and anti-HA immunoprecipitates containing wild-type EphA8 as previously described. Note that the GST-JM fusion protein contained Tyr-615, which is a major phosphorylation site.
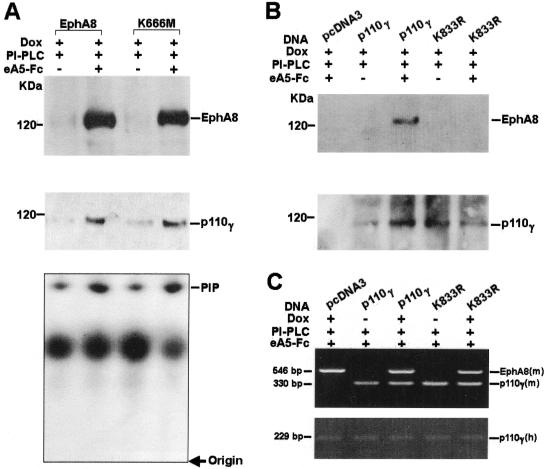
Association of p110γ with EphA8 is rapidly induced by oligomeric ephrin A5-Fc added to cells stripped of ephrin A ligands.
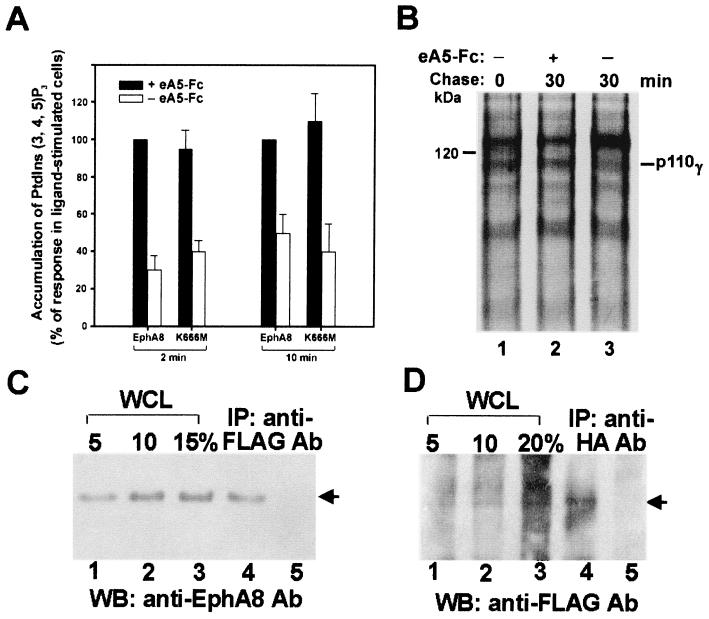
To further exclude the possibility that EphA8 promotes integrin activity by activating ligand signaling pathways, NIH 3T3 cells were treated with PI-PLC, which eliminates GPI-linked ephrin A subgroup ligands from the cell surface as previously reported (30). We then compared the ability of the ephrin A-stripped cells to adhere to surfaces coated with fibronectin with or without preclustered ephrin A5-Fc soluble ligand stimulation (see Fig. 11A). As we expected, a prominent increase in cell attachment was induced in cells stimulated by preclustered ephrin A5-Fc. Furthermore, the ability of wild-type or kinase-inactive EphA8 receptor to associate with the p110γ protein was markedly enhanced by stimulation with preclustered ephrin A5-Fc (Fig. 9A, top, lanes 2 and 4). The p110γ PI 3-kinase activity was also significantly increased in the cells stimulated by preclustered ephrin A5-Fc (Fig. 9A. bottom, lanes 2 and 4). We also transiently expressed wild-type or lipid kinase-inactive p110γ in HEK293 cells expressing induced wild-type EphA8. These cells were also treated with PI-PLC and preclustered ephrin A5-Fc. Interestingly, we reproducibly observed that transient expression of a murine p110γ mutant lacking lipid kinase activity, K833R, did not significantly alter cell attachment promoted by EphA8 activation (See Fig. 11B). Consistent with these results, association of the lipid kinase-inactive p110γ protein with EphA8 was not detected in cells stimulated with preclustered ephrin A5-Fc (Fig. 9B, lane 5). These results suggest that the lipid kinase-active form of p110γ may be important for stable association with EphA8 at the plasma membrane. In contrast, exogenous wild-type p110γ expression markedly strengthened cell attachment promoted by EphA8 activation until 4 h after EphA8 induction (see Fig. 11B). In addition, the rapid and stable association of the FLAG-tagged wild-type p110γ PI-3 kinase with EphA8 occurred upon stimulation with preclustered ephrin A5-Fc (Fig. 9B, lane 3). We also investigated whether EphA8 increases the level of 3′ phosphoinositides in vivo upon stimulation with preclustered ephrin A5-Fc (Fig. 10A). For these experiments, we transiently expressed wild-type p110γ in HEK293 cells expressing induced wild-type or kinase-inactive EphA8. These cells were treated with PI-PLC during the period of serum starvation for 2 h, then labeled with 32Pi, and exposed to preclustered ephrin A5-Fc for the indicated times. The 32P-labeled phospholipids were then analyzed as described previously (18, 32). As expected, the ephrin A5-Fc treatment markedly increased the amount of PI(3, 4, 5)P3 in HEK293 cells expressing either wild-type or kinase-inactive EphA8. The EphA5-stimulated accumulation of PI(3, 4)P2 in these cell lines was similar to that of PI(3, 4, 5)P3, but the levels of PI(3)P PI(4)P and PI(4, 5)P2 did not significantly increase with ephrin A5 treatment (data not shown). From results obtained from multiple experiments, it became evident that endogenously expressed p110γ protein levels were significantly elevated upon stimulation with preclustered ephrin A5-Fc (Fig. 9A, middle). In addition, the exogenously expressed wild-type p110γ protein level was consistently increased by EphA8 activation (Fig. 9B, bottom, lane 3). These results suggest that ephrin A ligands enhance the stability of p110γ bound to EphA8 rather than increasing p110γ mRNA because the p110γ cDNA used for exogenous expression did not contain its own basal promoter and transcriptionally regulated sequences. To further investigate whether p110γ mRNA levels can be upregulated by activated EphA8 expression in HEK293 cells, we used a semiquantitative RT-PCR approach to measure p110γ mRNA levels. Primer sets that discriminate between exogenous murine p110γ and endogenous human p110γ were used (Fig. 9C). In all PCR experiments, the quality and amount of cDNA were initially assessed by PCR with primers specific for β-actin (data not shown). Similar levels of human p110γ mRNA were detected regardless of activated EphA8 induction (Fig. 9C, bottom). In addition, a similar level of murine p110γ mRNA was observed in cells transfected with wild-type murine p110γ or the lipid kinase-inactive p110γ mutant (Fig. 9C, top). As expected, in the absence of doxycycline treatment, the murine EphA8 mRNA level was barely detectable. In contrast, it was abundant in the same cells treated with doxycycline. To further analyze the rate of degradation of the exogenously expressed p110γ protein, we transiently expressed wild-type EphA8 in HEK293 cells expressing induced wild-type EphA8. These cells were serum starved and treated with PI-PLC simultaneously and then pulsed labeled with [35S]cysteine and [35S]methionine for 15 min without preclustered ephrin A5-Fc treatment. The amount of p110γ was then measured in the 30 min following the chase with or without preclustered ephrin A5-Fc treatment. As shown in Fig. 10B, the rate of degradation of p110γ was much higher in untreated cells (lane 3). The half-life of p110γ in untreated cells was estimated to be less than 10 min (data not shown). In contrast, degradation of p110γ in the ligand-treated cells was almost undetectable for at least 30 min (lane 2). These results strongly suggest that the level of p110γ in cells is increased by the activated EphA8 receptor through protein stabilization. It should be noted that in these experiments, the Eph receptor was not efficiently labeled, probably because of the absence of doxycycline treatment during the period of serum starvation and pulse-chase. Taken together, these results strongly indicate that induced EphA8 expression does not affect p110γ mRNA levels; rather, it probably stabilizes the p110γ protein that is associated with EphA8 at the plasma membrane. We also estimated the fraction of EphA8 associated with the exogenously expressed p110γ protein in HEK293 cells that had been transiently transfected with p110γ and then treated with doxycycline for 12 h. These cells were also treated with PI-PLC and preclustered ephrin A5-Fc. The relative amount of EphA8 was calculated by comparing the amount of EphA8 recovered using anti-FLAG antibody to the total cellular EphA8 in the same lysate. These amounts were estimated using immunoblot analysis and anti-EphA8 antibody as a probe (Fig. 10C). To achieve maximum recovery of p110γ, three consecutive immunoprecipitations of the same lysate were combined and analyzed. The results indicate that about 10% of total cellular EphA8 was recovered by anti-FLAG antibody through association with p110γ (Fig. 10C, lane 4). The lysate supernatant after the third immunoprecipitation was subjected to further round of immunoprecipitation using anti-FLAG antibody to confirm that most of the EphA8 associated with p110γ was recovered in the previous immunoprecipitations (Fig. 10C, lane 5). In reverse experiments using a similar experimental procedure, we observed that about 20% of the exogenously expressed p110γ protein was recovered by anti-HA antibody through association with EphA8 (Fig. 10D).
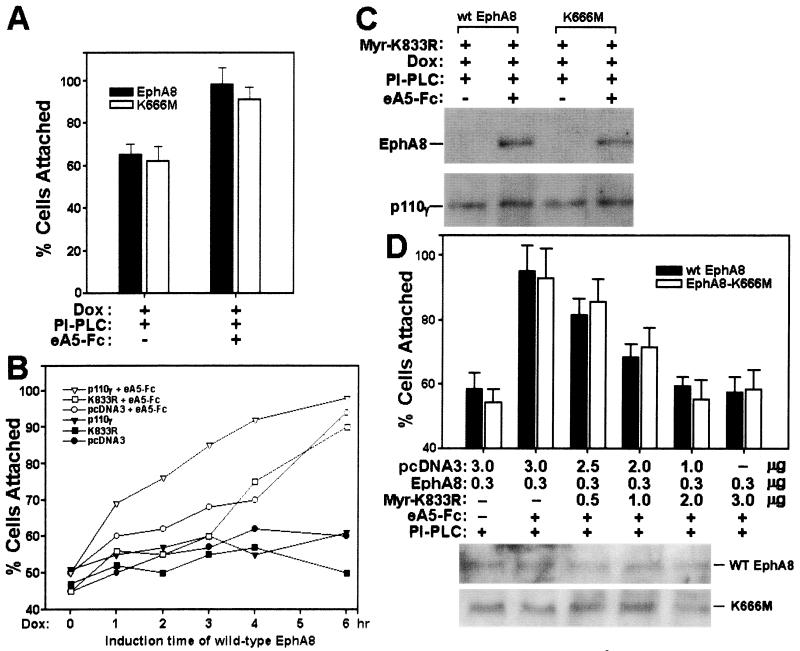
FIG. 11.
(A and B) Rapid increase of fibronectin adhesion activity by clustered ephrin A5-Fc (e5A-Fc) in cells stripped of endogenous ephrin A ligands. (A) Cell attachment assays were performed with NIH 3T3 cells prepared as described in the legend to Fig. 9A. Dox, doxycyline. (B) FLAG-tagged wild-type or K833R p110γ constructs were transiently tranfected into HEK293 cells expressing wild-type EphA8 in response to doxycycline treatment. At 24 h following transfection, induction of EphA8 began. Treatment with PI-PLC was then started, and the cells were further stimulated by clustered ephrin A5-Fc for 20 min. In the presence of clustered ephrin A5-Fc and 6 h after induction of EphA8 expression, fibronectin adhesion activities were not significantly different between the three cell lines containing different transfected DNAs (pcDNA3, wild-type p110γ, and K833R mutant). Note that expression of wild-type p110γ, together with ligand stimulation, strengthened EphA8-stimulated cell attachment to a significant extent at times ranging from 1 to 4 h after the EphA8 induction time point. Data from three independent experiments are presented as means; SEs are not shown to avoid complexity. (C and D) A membrane-targeted K833R p110γ mutant efficiently blocked the fibronectin adhesion activity stimulated by clustered ephrin A5-Fc. Myr-K833R was generated as described in Materials and Methods. (C) Procedures for transfections with p110γ constructs followed by incubation with PI-PLC and clustered ephrin A5-Fc were essentially as described in the legend to Fig. 9B. Proteins from cell lysates were immunoprecipitated with anti-FLAG antibody and then analyzed by immunoblotting with anti-EphA8 antibody as a probe (top); the same blot was stripped and reprobed with anti-FLAG antibody (bottom). wt, wild type. (D) Parental NIH 3T3 cells were cotransfected with mixed DNAs containing the indicated constructs. At 12 h following transfection, cells were incubated with PI-PLC and grown for a further 12 h. At this time, cells were treated with clustered ephrin A5-Fc for 20 min, and then cell attachment assays were performed as described in previous figure legends (top). Proteins from cell lysates were immunoprecipitated with anti-EphA8 antibody and then analyzed by immunoblotting with the same antibody as a probe (bottom).
FIG. 9.
Rapid induction of an association between EphA8 and p110γ by clustered ephrin A5-Fc in cells stripped of endogenous ephrin A ligands. (A) Expression of wild-type or kinase-inactive EphA8 protein in NIH 3T3 cells was induced with doxycycline (Dox) for 12 h, incubation with PI-PLC for 2 h, and then treatment with preclustered ephrin A5-Fc (eA5-Fc) chimeric ligands for 20 min. Proteins from cell lysates were immunoprecipitated with anti-p110γ antibody and then analyzed by immunoblotting with anti-EphA8 antibody as a probe (top); the same blot was stripped and reprobed with anti-p110γ antibody (middle). Proteins from cell lysates were immunoprecipitated with anti-p110γ antibody, and then PI 3-kinase activity was measured as described in previous figure legends (bottom). (B) FLAG-tagged wild-type or K833R (lipid kinase-inactive mutant) p110γ constructs were transiently transfected into HEK293 cells expressing wild-type EphA8 in response to doxycycline (lanes 2 to 5). At 12 h following transfection, EphA8 induction began and continued for another 12 h (lanes 1 to 5). Cells were then incubated with PI-PLC for 2 h (lanes 1 to 5), followed by clustered ephrin A5-Fc for 20 min (lanes 1, 3, and 5). Proteins from cell lysates were immunoprecipitated with anti-FLAG antibody and then analyzed by immunoblotting with anti-EphA8 antibody as a probe (top); the same blot was stripped and reprobed with anti-FLAG antibody (bottom). (C) Semiquantitative RT-PCR analysis of EphA8 and p110γ mRNA in response to doxycycline treatment. Procedures for transfections with p110γ constructs followed by incubation with PI-PLC and clustered ephrin A5-Fc were essentially the same as described for panel B except that EphA8 was not induced in some of the transfected cells (lanes 2 and 4). mRNA from HEK293 cells was extracted, and all PCR products were obtained from oligo(dT)-primed reverse-transcribed cDNAs. The top panel shows both murine EphA8- and p110γ-specific PCR products (18 cycles) coamplified under the same condition. Note that the 546-bp EphA8-specific band began to appear after 20 cycles of PCR when we used mRNA extracted from cells that had not been treated with doxycycline (lanes 2 and 4). The bottom panel shows the 229-bp endogenous human p110γ-specific PCR products (18 cycles) amplified under the same conditions used for amplification of the murine EphA8 and p110γ.
FIG. 10.
(A) Ephrin A5-stimulated accumulation of PI(3, 4, 5)P3 in HEK293 cells expressing p110γ and EphA8. A FLAG-tagged p110γ construct was transiently transfected into HEK293 cells expressing wild-type EphA8 in response to doxycycline. The induction of EphA8 began 12 h after transfection and continued for 24 h. Cells were serum starved and treated with PI-PLC simultaneously for 2 h, labeled with 32Pi for 2 h, washed, and treated with preclustered ephrin A5-Fc (eA5-Fc) for the indicated times at 37°C. Incubations were then terminated, and the lipids were extracted and analyzed. The response of [32P]PI(3, 4, 5)P3 in HEK293 cell expressing wild-type EphA8 in the presence of the preclustered EphA5-Fc is represented as 100%; the response of [32P]PI(3, 4, 5)P3 in cells without ephrin A5-Fc treatment or in cells expressing ephrin A8-K666M is presented as a percentage thereof (n = 3). (B) Stability of exogenously expressed p110γ protein in the presence of preclustered ephrin A5-Fc. HEK293 cells prepared as described above were pulse-labeled with 200 μCi of [35S]cysteine and [35S]methionine per ml for 15 min in the absence of ephrin A5-Fc treatment and then chased for the indicated times either with or without ephrin A5-Fc treatment for 30 min. The cell extracts were immunoprecipitated with the FLAG antibody. (C and D) Amounts of EphA8 associated with p110γ or vice versa. Cells prepared as described above were treated with PI-PLC for 2 h prior to incubation with preclustered ephrin A5-Fc for 20 min. Whole-cell lysates (WCL) were prepared in PLC lysis buffer. For comparison, 5, 10, 15, and 20% aliquots of the whole cell lysate were directly analyzed on the same blot using the indicated antibodies. For immunoprecipitation (IP), proteins were immunoprecipitated three times with the indicated antibodies (Ab) (lane 4 of each panel). The supernatant after the third immunoprecipitation was then immunoprecipitated once more with the indicated antibodies (lane 5 of each panel). WB, Western blot.
The lipid kinase activity of p110γ is required for EphA8-stimulated cell adhesion.
To further demonstrate that p110γ is required for EphA8-induced adhesion, we generated a dominant negative p110γ mutant (Myr-K833R). This was accomplished by fusing the coding region of the lipid kinase-inactive p110γ (K833R) mutant to that of the NH2-terminal myristoylation, membrane localization signal from c-Src (38). We first tested whether this potential dominant negative p110γ mutant is able to associate with EphA8 in HEK293 cells upon stimulation with clustered ephrin A5-Fc. HEK293 cells were transiently transfected with the Myr-K833R p110γ construct, then wild-type or kinase-inactive EphA8 was induced, and the cells were then stimulated with or without clustered ephrin A5-Fc. As shown in Fig. 11C, stable association of wild-type or kinase-inactive EphA8 with the Myr-K833R p110γ mutant was rapidly induced by stimulation with ephrin A5-Fc. These results contradict our observation that the K833R p110γ protein does not complex with the activated EphA8 receptor. However, this difference may suggest that the membrane localization of K833R p110γ mutant is able to overcome its weak association with the activated EphA8 receptor. We also cotransfected wild-type or kinase-inactive EphA8 DNA together with the Myr-K833R p110γ construct into NIH 3T3 cells for use in cell attachment assays. It was evident that the level of wild-type or kinase-inactive EphA8 was equivalent irrespective of the cotransfected p110γ construct (Fig. 11D, bottom). As shown in the top panel of Fig. 11D, transient expression of Myr-K833R p110γ mutant was highly effective in inhibiting cell adhesion stimulated by preclustered ephrin A5-Fc, in a dose-dependent manner and regardless of the auto-kinase activity of the expressed EphA8 proteins. In addition, transient expression of myristoylated wild-type p110γ in NIH 3T3 cells markedly stimulated cell adhesion to fibronectin irrespective of preclustered ephrin A5-Fc treatment and EphA8 expression (data not shown). Similar results were observed for HEK293 cells (data not shown). These results strongly suggest that Myr-K833R p110γ mutant acts as a dominant negative mutant by blocking endogenous p110γ PI 3-kinase access to the EphA8 receptor and its lipid substrates at plasma membrane. In addition, our results clearly show that the lipid kinase function of p110γ is critical for EphA8-induced cell adhesion.
DISCUSSION
In this present study, we used NIH 3T3 fibroblasts and HEK293 epithelial cell to study the regulation of integrins by the EphA8 receptor. These cell lines express endogenous ephrin A subgroup ligands and thus exhibit increased tyrosine phosphorylation of ectopically expressed EphA8 proteins. Our results clearly demonstrate that EphA8 transduces a signal that increases the activity of α5β1 and, possibly, αvβ3 integrins. The binding of ephrin A-type ligands is critical for this event, and the EphA8 JM encoded by exon X plays a major role in modifying integrin activity. The observation that the regulation of specific integrin activity does not require the EphA8 auto-kinase function is novel and, perhaps, physiologically relevant. It is interesting that unlike other Eph members, the native EphB6/Mep protein lacks tyrosine kinase activity due to the large number of amino acid substitutions within the conserved kinase sequence motifs which are important for catalysis (17). It will be important to examine whether EphB6/Mep can stimulate selective integrin activity in cultured cells. We also observed that the kinase-inactive EphA4 protein was able to stimulate cell attachment to fibronectin, suggesting that the integrin regulatory properties of EphA8, which we have shown to be independent of its phosphotyrosine activity, are shared by other members of the Eph family (C. Gu and S. Park, unpublished results). The finding that a kinase-inactive EphA8 mutant protein could regulate integrin function was somewhat surprising and contradictory to previous reports, which indicated that kinase-dependent signaling of EphB1 through Nck and Ste20 kinase, or LMW-PTP, is absolutely required for stimulation of cell attachment to fibrinogen (2, 21, 35). More recently, it was reported that EphB2 attenuates integrin activity by inducing tyrosine phosphorylation of R-Ras, which may be indirectly recruited to the activated EphB2 protein through SHEP1 (9, 43). In contrast, EphA2 was shown to mediate some of its functions by negatively regulating integrins and focal adhesion kinase (26). In view of these seemingly contradictory results, which may be due to the different cell types, assay conditions, or Eph genes used, it is not possible at present to draw definitive conclusions as to the relative importance of each Eph family member in the positive or negative control of integrin activity. We also previously reported that the kinase-active EphA8 receptor stably expressed in HEK293 epithelial cells could attenuate cell attachment to uncoated surfaces (7). In contrast, results of the present study show that cell attachment to fibronectin can be stimulated by the transient or induced expression of EphA8 receptors in HEK293 cells or by the stable expression of EphA8 receptors in NIH 3T3 cells. The disparity in the reported levels of cell adhesion mediated by EphA8 could be due to differences in the levels of EphA8 overexpression in these cells. In stably transfected HEK293 cells, the EphA8 receptor was expressed at least 5- to 10-fold more than in NIH 3T3 cells (S. Choi and S. Park, unpublished results). Therefore, in stably transfected HEK293 cells, it is possible that the constitutive EphA8 receptor above a certain threshold may downregulate integrin activity and possibly other cell adhesion functions as well. Transient activation of the EphA8 receptor for a short period of time using either transient transfection or tetracycline-inducible expression systems might be more physiologically relevant since ephA8 gene expression is temporally and spatially regulated during mouse embryonic development. In contrast, understanding how the abnormal expression of EphA8 deactivates integrin function might yield important insights into the connection between decreased matrix adhesion and malignant transformation.
Two possible mechanisms can be envisaged for EphA8 receptor in kinase-independent pathways. In the first, which is analogous to the signaling functions of transmembrane ephrins, ephrin A8 could mediate a reverse signal via GPI-linked ephrins to promote association of these ligands with transmembrane coreceptors. Recent reports support this hypothesis by showing that EphA5 transduces reverse signals to regulate cellular adhesion (8). However, our observations may not be consistent with this hypothesis because EphA8 receptor mutants lacking the entire cytoplasmic region or a specific portion of the JM did not efficiently promote cellular adhesion, although these deletions did not affect the binding affinity of GPI-linked ligands. One may argue that deleting cytoplasmic portions of the receptor reduces the ability of EphA8 to signal in reverse through the ephrin A proteins. This could be due to a change in protein conformation or a change in subcellular localization, for example. However, EphA8 promoted integrin-mediated cell adhesion in a tyrosine kinase-independent and p110γ-dependent fashion reproducibly in cells stripped of endogenous ephrin A ligands and then treated with exogenously added oligomerized ephrin A5-Fc. This finding strongly supports the second mechanism in which GPI-linked ligands would transmit signals via the EphA8 cytoplasmic domain without any requirement for EphA8 tyrosine kinase activity.
Many effectors interacting with activated Eph receptors have been isolated by using the yeast two-hybrid system and identifying tyrosine-phosphorylated proteins and their interaction partners in Eph-stimulated cells. Most of these proteins are SH2 domain-containing effectors. They include cytoplasmic tyrosine kinases of the Src family (42); adapter proteins such as the Src-like adapter (29), Grb2 (34), Grb10 (34), Nck (35), and the p85 subunit of PI 3-kinase (28); the Ras GTPase-activating protein (20); and, more recently, SHEP1 (9). It has been clearly demonstrated that Tyr-594 and Tyr-929 in EphB1 are required for binding to Nck and LMW-PTP, respectively, and the site of Nck binding is also used by SHEP1 to bind to EphB2 through the SH2 domain. In EphA8, the tyrosine residue corresponding to Tyr-594 of EphB1 is replaced by phenylalanine, and the site equivalent to Tyr-929 of EphB1 is poorly phosphorylated (Gu and Park, unpublished results). Therefore, these signaling proteins may not be involved in EphA8-mediated integrin regulation. The EphA8 receptor does not appear to regulate integrins directly, because we observed no coprecipitation of α5β1 or αvβ3 integrins with EphA8 (data not shown). Instead, a novel signaling intermediate may connect the EphA8 receptor to integrin receptors in a phosphotyrosine-independent manner. In T lymphocytes, a variety of cell surface receptors have been shown to regulate PI 3-kinase activity by recruiting the p85/p110 isoform via SH2 domain-phosphotyrosine interactions (40). All of these receptors are capable of inducing integrin activation, suggesting that the PI 3-kinase play a major role in the upregulation of cell adhesiveness (5, 10, 27, 33, 41). Similar to these reports, our results of assays using wortmannin provide evidence that the coupling of the EphA8 receptor to the PI 3-kinase signaling pathway promotes cell adhesion through activation of integrins. An important finding of the present study is the tight association between PI 3-kinase activity and the EphA8 receptor, which is unaffected by the latter's kinase activity. Quite surprisingly, the p85 subunit-associated PI 3-kinase activity did not contribute to EphA8-stimulated integrin activation. Instead, our findings indicate the existence of a novel mechanism for the membrane recruitment of the p110γ PI 3-kinase isotype through its interaction with the EphA8 receptor. It is likely that a stable association between EphA8 and p110γ involves at least the EphA8 JM and the p110γ lipid kinase in its active conformation. It remains to be determined how ephrin binding to EphA8 induces association of p110γ in a kinase-independent fashion. Possibly the JM segment is masked in an EphA8 receptor that has no ligand bound to its extracellular domain and becomes exposed to p110γ when ligand binds to its extracellular domain. We have also demonstrated that the level of p110γ is elevated in cells expressing the activated EphA8 receptor, as a result of increased stability of the protein. In fact, membrane-targeted p110γ forms a stable complex with EphA8 even without lipid kinase activity. These results suggest that the increase of PI 3-kinase activity detected in EphA8 or p110γ immunocomplexes may be due to increased levels of p110γ protein rather than stimulation of the lipid kinase activity. Nevertheless, our results of assays using wortmannin and the membrane-targeted K833R p110γ mutant have conclusively demonstrated that the lipid kinase activity of p110γ is required for EphA8-stimulated cell adhesion. To explain these results, our current model suggests that the activated EphA8 receptor localizes p110γ to the plasma membrane in a phosphotyrosine-independent manner, thereby allowing the stabilized p110γ protein access to lipid substrates. A p110γ PI 3-kinase isotype has been implicated in the heterotrimeric G protein-linked receptor signaling mechanism in which the α or βγ subunit or both directly modulate the activity of p110γ (25, 36, 37, 39). In this respect, our findings have revealed a novel signaling mechanism whereby Eph receptor regulation of the p110γ isotype leads to modification of integrin function. Further experiments are required to determine whether any p101 regulatory subunit or G protein βγ subunit has a biological effect on EphA8-mediated modulation of p110γ PI 3-kinase activity. In addition, our study suggests a role for phosphoinositides in the inside-out signaling by EphA8 for integrin activation. The phosphorylated lipids are likely to constitute an important upstream intracellular signal, provoking changes in the integrin extracellular domain and resulting in activation, as manifested by an increase in affinity for extracellular ligands.
Our findings have yet to be demonstrated in a cell type that expresses the EphA8 receptor naturally, and therefore their true biological significance is not known. Unfortunately, cell lines that show endogenous expression of EphA8 have yet to be found. This is probably because the gene for EphA8 is expressed only in a subpopulation of midbrain neurons during mouse embryonic development. It has been suggested that the EphA8 receptor plays an important role in axonal pathfinding for a subset of tectal commissural axons (31). We have also observed that the EphA8 receptor is temporally expressed in a subset of mesencephalic neural crest cells (S. Shim and S. Park, unpublished results). Based on our results and this observation, the effects of EphA8 on cell attachment may be crucial for axon guidance and/or neural crest cell migration. However, the mechanisms through which EphA8 receptors mediate axonal pathfinding or cell migration remain to be determined. It is possible that the modulation of integrin between high- and low-affinity states through signaling from EphA8 receptors might influence pathfinding of migrating axons or neural crest cells. The results presented in this paper suggest that integrin modulation through inside-out signaling involving the EphA8 receptor and p110γ PI 3-kinase might be the one of the molecular mechanisms of axon pathfinding and cell migration. Further studies to identify the promoter region containing spatial and temporal information for EphA8 gene expression will be an important part of elucidating an exact role for EphA8. We are planning further studies whereby various EphA8 mutants and the p110γ isotype are expressed in transgenic mice under the control of the EphA8 promoter. This may further elucidate the biological relevance of our current findings.
ACKNOWLEDGMENTS
We thank Seung Namkoong, Sungbo Shim, Jaemin Jeong, and Sunga Choi for excellent technical assistance, Jongkyung Chung for providing the Δp85 subunit cDNA, and Seokjoo Park for HPLC analysis.
This work was supported by grants 981-0505-026-2 and 98-0501-006-1 from the Korea Science and Engineering Research Foundation (KOSEF).
REFERENCES
- 1.Aplin A E, Howe A K, Juliano R. Cell adhesion molecules, signal transduction and cell growth. Curr Opin Cell Biol. 1999;11:737–744. doi: 10.1016/s0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- 2.Becker E, Huynh-Do U, Holland S, Pawson T, Daniel T O, Skolnik E Y. Nck-interacting Ste20 kinase couples Eph receptors to c-Jun N-terminal kinase and integrin activation. Mol Cell Biol. 2000;20:1537–1545. doi: 10.1128/mcb.20.5.1537-1545.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birgbauer E, Cowan C A, Sretavan D W, Henkemeyer M. Kinase independent function of EphB receptors in retinal axon pathfinding to the optic disc from dorsal but not ventral retina. Development. 2000;127:1231–1241. doi: 10.1242/dev.127.6.1231. [DOI] [PubMed] [Google Scholar]
- 4.Bruckner K, Pasquale E B, Klein R. Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science. 1997;275:1640–1643. doi: 10.1126/science.275.5306.1640. [DOI] [PubMed] [Google Scholar]
- 5.Chan A S, Mobley J L, Fields G B, Shimizu Y. CD7-mediated regulation of integrin adhesiveness on human T cells involves tyrosine phosphorylation-dependent activation of phosphatidylinositol 3-kinase. J Immunol. 1997;159:934–942. [PubMed] [Google Scholar]
- 6.Chin-Sang I D, George S E, Ding M, Moseley S L, Lynch A S, Chisholm A D. The ephrin VAB-2/EFN-1 functions in neuronal signaling to regulate epidermal morphogenesis in C. elegans. Cell. 1999;99:781–790. doi: 10.1016/s0092-8674(00)81675-x. [DOI] [PubMed] [Google Scholar]
- 7.Choi S, Park S. Phosphorylation at Tyr-838 in the kinase domain of EphA8 modulates Fyn binding to the Tyr-615 site by enhancing tyrosine kinase activity. Oncogene. 1999;18:5413–5422. doi: 10.1038/sj.onc.1202917. [DOI] [PubMed] [Google Scholar]
- 8.Davy A, Gale N W, Murray E W, Klinghoffer R A, Soriano P, Feuerstein C, Robbins S M. Compartmentalized signaling by GPI-anchored ephrin-A5 requires the Fyn tyrosine kinase to regulate cellular adhesion. Genes Dev. 1999;13:3125–3135. doi: 10.1101/gad.13.23.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dodelet V C, Pazzagli C, Zisch A H, Hauser C A, Pasquala E B. A novel signaling intermediate, SHEP1, directly couples Eph receptors to R-Ras and Rap1A. J Biol Chem. 1999;274:31941–31946. doi: 10.1074/jbc.274.45.31941. [DOI] [PubMed] [Google Scholar]
- 10.Driessens M H, van Hulten P E, van Rijthoven E A, Soede R D, Roos E. Activation of G-proteins with AIF-4 induces LFA-1-mediated adhesion of T-cell hybridoma cells to ICAM-1 by signal pathways that differ from phorbol ester- and manganese-induced adhesion. Exp Cell Res. 1997;231:242–250. doi: 10.1006/excr.1996.3463. [DOI] [PubMed] [Google Scholar]
- 11.Einarson M B. Detection of protein-protein interactions using Far Western with GST fusion proteins. In: Sambrook J, Russel D W, editors. Molecular cloning. Vol. 3. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2001. pp. 1848–1854. [Google Scholar]
- 12.Eph Nomenclature Committee. Unified nomenclature for Eph family receptors and their ligands, the ephrins. Cell. 1997;90:403–404. doi: 10.1016/s0092-8674(00)80500-0. [DOI] [PubMed] [Google Scholar]
- 13.Flanagan J G, Vanderhaeghen P. The ephrins and Eph receptors in neural development. Annu Rev Neurosci. 1998;21:309–345. doi: 10.1146/annurev.neuro.21.1.309. [DOI] [PubMed] [Google Scholar]
- 14.Fukui Y, Hanafusa H. Phosphatidylinositol kinase activity associates with viral p60src protein. Mol Cell Biol. 1989;9:1651–1658. doi: 10.1128/mcb.9.4.1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George S E, Simokat K, Hardin J, Chisholm A D. The VAB-1 Eph receptor tyrosine kinase functions in neural and epithelial morphogenesis in C. elegans. Cell. 1998;6:633–643. doi: 10.1016/s0092-8674(00)81131-9. [DOI] [PubMed] [Google Scholar]
- 16.Graham F L, van der Eb A J. Transformation of rat cells by DNA of human adenovirus 5. Virology. 1973;54:536–539. doi: 10.1016/0042-6822(73)90163-3. [DOI] [PubMed] [Google Scholar]
- 17.Gurniak C B, Berg L J. A new member of the Eph family of receptors that lacks protein tyrosine kinase activity. Oncogene. 1996;13:777–786. [PubMed] [Google Scholar]
- 18.Hawkins T R, Jackson T R, Stephens L R. Platelet-derived growth factor stimulates synthesis of Ptdlns(3, 4, 5)P3 by activating a Ptdlns(4, 5)P2 3-OH kinase. Nature. 1992;358:157–159. doi: 10.1038/358157a0. [DOI] [PubMed] [Google Scholar]
- 19.Henkemeyer M, Orioli D, Henderson J T, Saxton T M, Roder J, Pawson T, Klein R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 20.Holland S J, Gale N W, Mbamalu G, Yancopoulos G D, Henkemeyer M, Pawson T. Bidirectional signaling through the EPH-family receptor Nuk and its transmembrane ligands. Nature. 1996;383:722–725. doi: 10.1038/383722a0. [DOI] [PubMed] [Google Scholar]
- 21.Huynh-Do U, Stein E, Lane A A, Liu H, Cerretti D P, Daniel T O. Surface densities of ephrin-B1 determine EphB1-coupled activation of cell attachment through αvβ3 and α5β1 integrins. EMBO J. 1999;18:2165–2173. doi: 10.1093/emboj/18.8.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeong J, Choi S, Gu C, Lee H, Park S. Genomic structure and promoter analysis of the mouse EphA8 receptor tyrosine kinase gene. DNA Cell Biol. 2000;19:291–300. doi: 10.1089/10445490050021203. [DOI] [PubMed] [Google Scholar]
- 23.Labrador J P, Brambilla R, Klein R. The N-terminal globular domain of Eph receptors is sufficient for ligand binding and receptor signaling. EMBO J. 1997;16:3889–3897. doi: 10.1093/emboj/16.13.3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lackmann M, Oates A C, Dottori M, Smith F M, Do C, Power M, Kravets L, Boyd A W. Distinct subdomains of the EphA3 receptor mediate ligand binding and receptor dimerization. J Biol Chem. 1998;273:20228–20237. doi: 10.1074/jbc.273.32.20228. [DOI] [PubMed] [Google Scholar]
- 25.Lopez-Ilasaca M, Crespo P, Pellici P G, Gutkind J S, Wetzker R. Linkage of G protein-coupled receptors to the MAPK signaling pathway through P13-kinase gamma. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 26.Miao H, Burnett E, Kinch M, Simon E, Wang B. Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat Cell Biol. 2000;2:62–69. doi: 10.1038/35000008. [DOI] [PubMed] [Google Scholar]
- 27.Nielsen M, Svejgaard A, Skov S, Dobson P, Bendtzen K, Geisler C, Odum N. IL-2 induces beta2-integrin adhesion via a wortmannin/LY294002-sensitive, rapamycin-resistant pathway. Phosphorylation of a 125-kilodalton protein correlates with induction of adhesion, but not mitogenesis. J Immunol. 1996;157:5350–5358. [PubMed] [Google Scholar]
- 28.Pandey A, Lazar D F, Saltiel A R, Dixit V M. Activation of the Eck receptor protein tyrosine kinase stimulates phosphatidylinositol 3-kinase activity. J Biol Chem. 1994;269:30154–30157. [PubMed] [Google Scholar]
- 29.Pandey A, Duan H, Dixit V M. Characterization of a novel Src-like adapter protein that associates with the Eck receptor tyrosine kinase. J Biol Chem. 1995;270:19201–19204. doi: 10.1074/jbc.270.33.19201. . 19201–19204. [DOI] [PubMed] [Google Scholar]
- 30.Park S, Sanchez M P. The Eek receptor, a member of the Eph family of tyrosine protein kinases, can be activated by three different Eph family ligands. Oncogene. 1997;14:533–542. doi: 10.1038/sj.onc.1200857. [DOI] [PubMed] [Google Scholar]
- 31.Park S, Frisen J, Barbacid M. Aberrant axonal projections in mice lacking EphA8 (Eek) tyrosine protein kinase receptors. EMBO J. 1997;16:3106–3114. doi: 10.1093/emboj/16.11.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Serunian L A, Auger K R, Cantley L C. Identification and quantification of polyphosphoinositides produced in response to platelet-derived growth factor stimulation. Methods Enzymol. 1991;198:78–87. doi: 10.1016/0076-6879(91)98010-4. [DOI] [PubMed] [Google Scholar]
- 33.Shimizu Y, Mobley J L, Finkelstein L D, Chan A S. A role for phosphatidylinositol 3-kinase in the regulation of beta 1 integrin activity by the CD2 antigen. J Cell Biol. 1995;131:1867–1880. doi: 10.1083/jcb.131.6.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stein E, Cerretti D P, Daniel T O. Ligand activation of ELK receptor tyrosine kinase promotes its association with Grb10 and Grb2 in vascular endothelial cells. J Biol Chem. 1996;271:23588–23593. doi: 10.1074/jbc.271.38.23588. [DOI] [PubMed] [Google Scholar]
- 35.Stein E, Lane A A, Cerretti D P, Schoecklmann H O, Schroff A D, Van Etten R L, Daniel T O. Eph receptors discriminate specific ligand oligomers to determine alternative signaling complexes, attachment, and assembly responses. Genes Dev. 1998;12:667–678. doi: 10.1101/gad.12.5.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stephens L, Smrcka A, Cooke F T, Jackson T R, Sternweis P C, Hawkins P T. A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by protein beta gamma subunits. Cell. 1994;77:83–93. doi: 10.1016/0092-8674(94)90237-2. [DOI] [PubMed] [Google Scholar]
- 37.Stoyanov B, Volinla S, Hanck T, Rubio I, Loubtchenkov M, Malek D, Stoyanova S, Vanhaesebroeck B, Dhand R, Nurnberg B, Gierschik P, Seedorf K, Hsuan J J, Waterfield M D, Wetzker R. Cloning and characterization of a G protein-activated human phosphoinositide-3 kinase. Science. 1995;269:690–693. doi: 10.1126/science.7624799. [DOI] [PubMed] [Google Scholar]
- 38.Takeya T, Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983;32:881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- 39.Tang X, Downes C P. Purification and characterization of Gβγ-responsive phosphoinositide 3-kinases from pig platelet cytosol. J Biol Chem. 1997;272:14193–14199. doi: 10.1074/jbc.272.22.14193. [DOI] [PubMed] [Google Scholar]
- 40.Ward S G, June C H, Olive D. PI 3-kinase: a pivotal pathway in T-cell activation? Immunol Today. 1996;17:187–197. doi: 10.1016/0167-5699(96)80618-9. [DOI] [PubMed] [Google Scholar]
- 41.Zell T, Hunt S W, Mobley J L, Finkelstein L D, Shimizu Y. CD28-mediated up-regulation of beta 1-integrin adhesion involves phosphatidylinositol 3-kinase. J Immunol. 1996;156:883–886. [PubMed] [Google Scholar]
- 42.Zisch A H, Kalo M S, Chong L D, Pasquale E B. Complex formation between EphB2 and Src requires phosphorylation of tyrosine 611 in the EphB2 juxtamembrane region. Oncogene. 1998;16:2657–2670. doi: 10.1038/sj.onc.1201823. [DOI] [PubMed] [Google Scholar]
- 43.Zou J X, Wang B, Kalo M S, Zisch A H, Pasquale E B, Ruoslahti E. An Eph receptor regulates integrin activity through R-Ras. Proc Natl Acad Sci USA. 1999;96:13813–13818. doi: 10.1073/pnas.96.24.13813. [DOI] [PMC free article] [PubMed] [Google Scholar]