Abstract
Objective
To perform a meta-analysis of randomized controlled trials to evaluate the efficacy of vitamin D supplementation on thyroid autoimmunity markers in Hashimoto’s thyroiditis (HT).
Methods
This meta-analysis included randomized controlled clinical trials identified by a systematic search of electronic databases (PubMed®, MEDLINE®, EMBASE, The Cochrane Library, China National Knowledge Infrastructure) from inception to August 2020. All studies included patients with HT that received vitamin D supplementation irrespective of the doses administered or the duration of treatment. The primary and secondary outcome measures were thyroid peroxidase antibody (TPOAb) and/or thyroglobulin antibody (TGAb) titres.
Results
Eight studies (n = 652) were included. There was significant heterogeneity between the studies. Using a random-effect model, vitamin D supplementation reduced TPOAb titre (standardized mean difference [SMD]: –1.11; 95% confidence interval [CI]: 1–1.92, –0.29) and TGAb titre (SMD: –1.12; 95% CI: –1.96, –0.28). A subgroup analysis demonstrated that vitamin D supplementation for >3 months resulted in a decrease in TPOAb titre (SMD: –1.66, 95% CI: –2.91, –0.41) but treatment ≤3 months was ineffective. Treatment with vitamin D3 decreased TPOAb titre (SMD: –1.48; 95% CI: –2.53, –0.42) whereas vitamin D did not.
Conclusion
These data suggest that vitamin D reduces autoantibody titre in patients with HT.
Keywords: Hashimoto’s thyroiditis, vitamin D, meta-analysis
Introduction
Hashimoto’s thyroiditis (HT), also known as autoimmune thyroiditis and first described in 1912 by Hakaru Hashimoto, is the leading cause of primary hypothyroidism.1,2 Its incidence is estimated to be 0.3–1.5 per 1000 people per year and it is 5–10 times more common in women than men. 3 The disease is characterized by the presence of thyroid autoantibodies against thyroid peroxidase or thyroglobulin. 4 The antibody titre levels are positively correlated with hypothyroidism, 5 but there is no specific drug to reduce the antibody titre.
Vitamin D plays an important role in immune and endocrine processes. For example, a recent meta-analysis involving 26 observational studies found that the 25-hydroxyvitamin D (25(OH)D) level was significantly lower in patients with HT than in healthy controls. 6 The effect of vitamin D on immunity in patients with HT patients remains obscure. Some studies found that after supplementation with 25(OH)D3 in patients with HT with vitamin D deficiency, the titre of thyroid autoantibodies decreased significantly, suggesting that vitamin D treatment could delay the development of hypothyroidism.7–11 Other studies, however, reached the opposite conclusion that there was no significant effect on thyroid autoantibodies after supplementation with vitamin D in patients with HT.12–14 This current study aimed to systematically evaluate the effect of vitamin D on thyroid autoimmunity markers in patients with HT.
Materials and methods
Study methods
This meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. 15 It was registered into PROSPERO (CRD42020206000). This current study strictly followed a pre-set protocol.
Data source and search strategy
A systematic search of publications listed in electronic databases (PubMed®, MEDLINE®, EMBASE, The Cochrane Library, China National Knowledge Infrastructure [CNKI]) from inception to August 2020 was undertaken using a set of key words. The full search strategy for PubMed® was: (“hashimoto disease”[MeSH Terms] OR “hashimoto struma”[Title/Abstract] OR “hashimotos syndrome”[Title/Abstract] OR “chronic lymphocytic thyroiditis” [Title/Abstract] OR “hashimotos disease” [Title/Abstract]) AND (“vitamin D” [MeSH Terms] OR “Ergocalciferols” [MeSH Terms] OR “Cholecalciferol” [Title/Abstract] OR “Hydroxycholecalciferols” [Title/Abstract] OR “Ergocalciferols” [Title/Abstract] OR “25-hydroxyvitamin D3”[Title/Abstract] OR “Dihydrotachysterol” [Title/Abstract]) AND (“randomized controlled trials as topic”[MeSH Terms] OR “clinical study” [Publication Type] OR “clinical trial” [Publication Type] OR “controlled clinical trial”[Publication Type]). The search strategies for the other databases are presented in the appendix file (see supplementary materials). The work was conducted by two researchers (J.Z. & Y.C.) independently to ensure a comprehensive screening. No language restrictions or limitations were applied. A manual review of the reference lists from primary or review articles was undertaken to identify any additional relevant studies.
Study selection
Studies were considered eligible for inclusion if they met the following criteria: (i) study design: randomized controlled clinical trials; (ii) population: patients with HT; (iii) intervention: vitamin D to treat HT, regardless of its doses and courses; (iv) outcome variables: at least one of either thyroid peroxidase antibody (TPOAb) and/or thyroglobulin antibody (TGAb) titres. Studies were excluded based on the following criteria: (i) duplicate studies; (ii) case reports, conference abstracts or reviews; (iii) trials tested in animals; (iv) TPOAb or TGAb titres were not included in the results.
Data extraction and quality evaluation
Important information from each study was extracted as follows: authors, publication year, country, sample size, mean age, sex ratio, intervention time and measures, levothyroxine (LT4) intake and main outcomes. Two independent researchers (J.Z. & Y.C.) extracted the relevant data from the articles using the same standard. Any disagreement was resolved by discussing with a third researcher (H.L.).
Two researchers (J.Z. & Y.C.) independently evaluated the methodological quality of the eligible trials using the Cochrane Collaboration Risk of Bias tool Statistical analysis. The study quality assessment covered: (i) random generation; (ii) allocation concealment; (iii) blinding of participants and personnel; (iv) blinding of outcome assessment; (v) incomplete outcome data; (vi) selective reporting; (vii) other factors that could impact the bias of studies such as the equality of baseline data between groups in the studies.
Statistical analyses
All analyses were undertaken using Stata® software (version 14.0; Stata Corporation, College Station, TX, USA). Continuous variables were evaluated using standardized mean difference (SMD) and 95% confidence interval (CI). In two-tailed tests, a P-value <0.05 was considered statistically significant. The χ2-test and I2 statistics were used to test for heterogeneity. If there was heterogeneity (P < 0.05, I2 >50%) in the results, a random-effect model was used. Otherwise, a fixed-effect model was used. If there was significant heterogeneity, a subgroup analysis was conducted using two factors (treatment duration, form of vitamin D administration) to find the source of the heterogeneity. Sensitivity analysis was made to test the stability of the primary outcomes. Begg’s test was also used to evaluate publication bias. P < 0.10 indicated the presence of publication bias.
Results
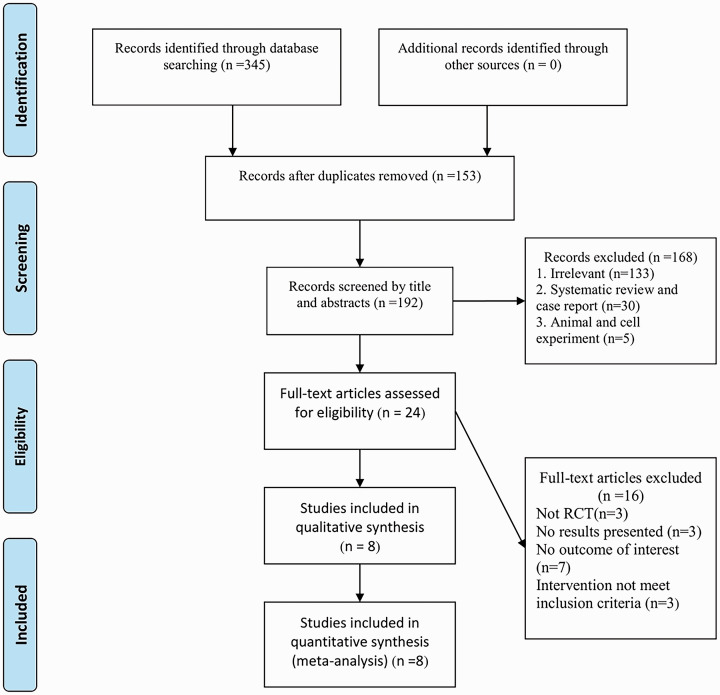
A flowchart showing the selection process for eligible studies is shown in Figure 1. Initially, 345 relevant articles were retrieved from five databases (PubMed®: 105; EMBASE: 11; The Cochrane Library: 21; MEDLINE®: 40; CNKI: 168). Of these, 153 duplicate studies were removed. Of the remaining 192 articles, 168 were excluded after reading the titles and abstracts. After reviewing the full text of 24 articles, eight eligible randomized controlled trials were included in the meta-analysis.7–14
Figure 1.
Literature search and study selection flowchart.
The study characteristics are shown in Table 1.7–14 These eight studies involved 652 patients with HT, including 332 in the intervention groups and 320 in the control groups. All were published between 2015 and 2020, with study periods ranging from 4 to 24 weeks. Among them, one study evaluated only females; 12 and fours studies evaluated the effect of LT4 tablets.8–11
Table 1.
Characteristics of the eight randomized controlled trials included in a meta-analysis of the effect of vitamin D supplementation on thyroid autoimmunity markers in patients with Hashimoto’s thyroiditis (HT).7–14
| Author/year | Country | Interventions TG/CG | n | Age, years Mean ± SD | Female n (%) | Treatment duration | LT4 treatment | Main outcomes |
|---|---|---|---|---|---|---|---|---|
| Han et al. 11 /2015 | China | 1,25(OH)2D3 25 µg QD + LT4 | 32 | 34.8 ± 8.2 | 27 (84.4%) | 24 weeks | Yes | 25(OH)D2, 25(OH)D3, TPOAb, TGAb, FT3, FT4, TSH |
| LT4 | 30 | 35.2 ± 7.1 | 26 (86.7%) | |||||
| Simsek et al. 7 /2016 | Turkey | Vitamin D 1000 IU QD | 46 | 35.8 ± 12.0 | 37 (80.4%) | 1 month | No | Vitamin D, TSH, FT4, TPOAb, TGAb |
| Sunshine and diet | 36 | 39.7 ± 12.6 | 31 (86.1%) | |||||
| Zhao et al. 8 /2016 | China | 1,25(OH)2D3 25 µg QD + LT4 | 18 | 41.0 ± 5.0 | 14 (77.8%) | 24 weeks | Yes | 25(OH)D3, TPOAb, TGAb, FT3, FT4, TSH |
| LT4 | 18 | 42.0 ± 7.0 | 13 (72.2%) | |||||
| Vahabi Anaraki et al. 13 /2017 | Iran | Vitamin D 50000 IU QW | 33 | 43.6 ± 1.6 | 21 (63.6%) | 12 weeks | No | CRP, TPOAb, TSH, 25(OH)D, PTH, Ca |
| Placebo | 32 | 44.1 ± 1.6 | 15 (46.9%) | |||||
| Knutsen et al. 14 /2017 | Middle East, Africa, or South Asia | Vitamin D3 25 µg QD | 83 | Female: 35.0 ± 7.5Male: 40.0 ± 9.1 | 57 (68.7%) | 16 weeks | No | Vitamin D3, TSH, FT4, TPOAb, PTH |
| Placebo | 82 | Female: 38.0 ± 7.6 Male: 39.0 ± 7.8 | 63 (76.8%) | |||||
| Chahardoli et al. 12 /2019 | Iran | 1,25(OH)2D3 50000 IU QW | 19 | 36.4 ± 5.2 | 19 (100.0%) | 12 weeks | No | 25(OH)D, TPOAb, TGAb, T3, T4, TSH, Ca |
| Placebo | 21 | 35.9 ± 7.8 | 21 (100.0%) | |||||
| Yin et al. 10 /2019 | China | 1,25(OH)2D3 25 µg QD + LT4 | 50 | 45.6 ± 4.0 | 42 (84.0%) | 20 weeks | Yes | 25(OH)D3, TPOAb, TGAb, FT3, FT4, TSH |
| LT4 | 50 | 45.3 ± 3.8 | 45 (90.0%) | |||||
| Jia et al. 9 /2020 | China | 1,25(OH)2D3 25 µg QD | 51 | 42.0 ± 9.3 | 29 (56.9%) | 16 weeks | Yes | 25(OH)D2, 25(OH)D3, TPOAb, TGAb, FT3, FT4, TSH |
| LT4 | 51 | 42.20 ± 8.98 | 31 (60.8%) |
TG, treatment group; CG, control group; LT4, levothyroxine; QD, once a day; TPOAb, thyroid peroxidase antibody; TGAb, thyroglobulin antibody; FT3, free triiodothyronine ; FT4, free thyroxine; TSH, thyroid-stimulating hormone; QW, once a week; CRP, C-reactive protein; PTH, parathormone; T3, total triiodothyronine; T4, thyroxine; Ca, calcium.
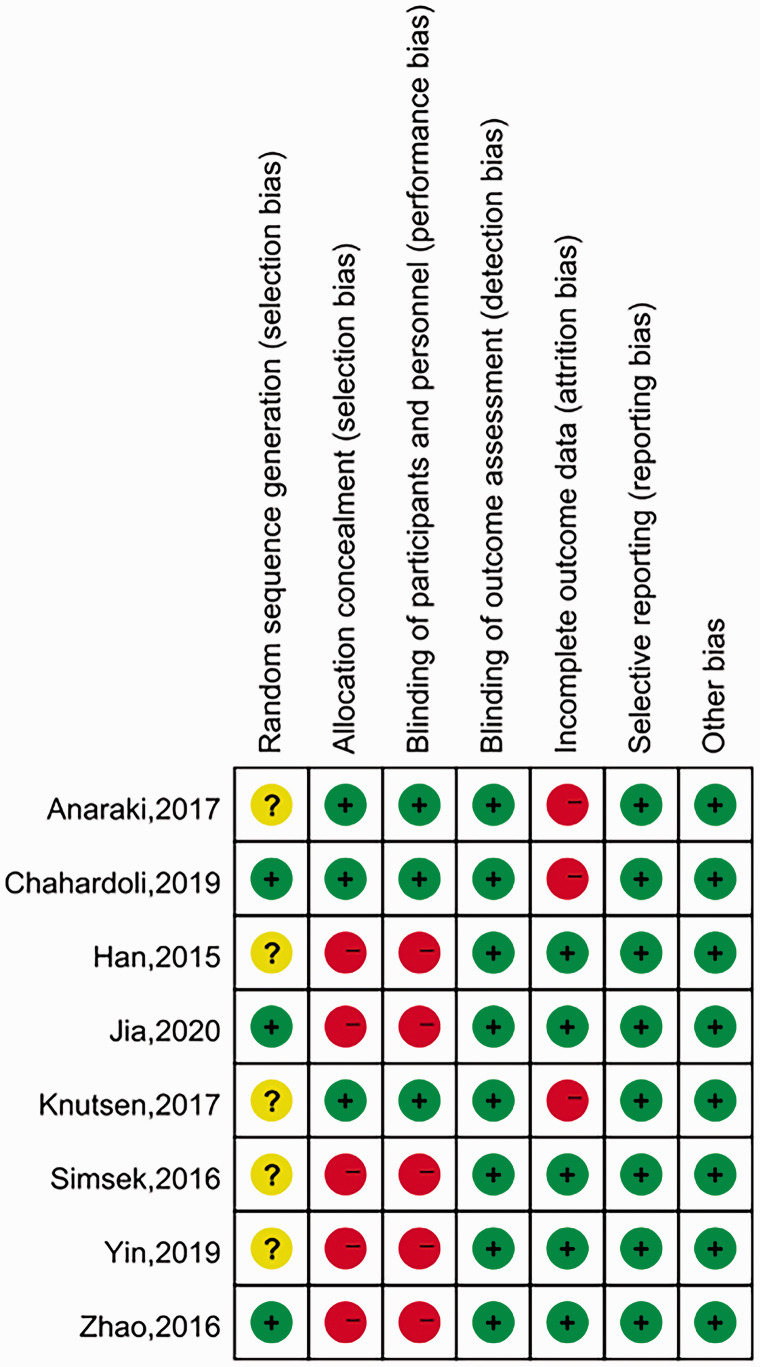
The bias assessment of all eight trials is detailed in Figure 2. The Cochrane risk of bias tool was used to assess the risk of bias of each study. Three studies explicitly described the random sequence generated by a random number table or block randomization. The other studies only mentioned the word ‘randomization’, thus their bias was considered to be ‘unclear’. Three articles described the ways of allocating concealment by sealed drug boxes and packages, thus their bias was assessed as ‘low risk’ for ‘allocation concealment’. The other studies did not mention if there was any allocation concealment. Only three studies used dummy placebos and neither patients nor researchers knew the grouping. Both TPOAb and TGAb were laboratory indicators, so all eight studies had a blinded outcome assessment. No patients were lost to follow-up or dropped out in five studies, thus their bias was assessed as ‘low risk’ for ‘incomplete outcome data’. Three studies reported that patients were lost to follow-up and discontinued intervention, therefore these studies were regarded as having a high risk in this domain. All included studies showed a low bias risk in ‘selective reporting’ because their statistical outcomes were prespecified in the protocol.
Figure 2.
Risk of bias graph and summary of bias assessment of the eight randomized controlled trials included in a meta-analysis of the effect of vitamin D supplementation on thyroid autoimmunity markers in patients with Hashimoto’s thyroiditis (HT).7–14 The colour version of this figure is available at: http://imr.sagepub.com.
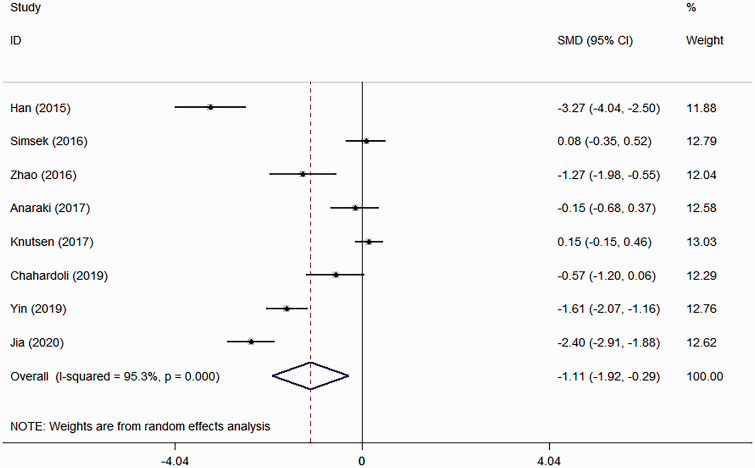
All eight studies reported data on TPOAb, but there was significant heterogeneity (χ2 = 150.02; I 2 = 95.3%, P < 0.001) (Figure 3) so a random-effect model was used.7–14 The pooled evidence showed that vitamin D supplementation was effective in reducing the TPOAb titre (SMD: –1.11; 95% CI: –1.92, –0.29; P = 0.008).
Figure 3.
Forest plot showing the effects of vitamin D supplementation on thyroid peroxidase antibody titre in patients with Hashimoto’s thyroiditis using a random-effect model.7–14
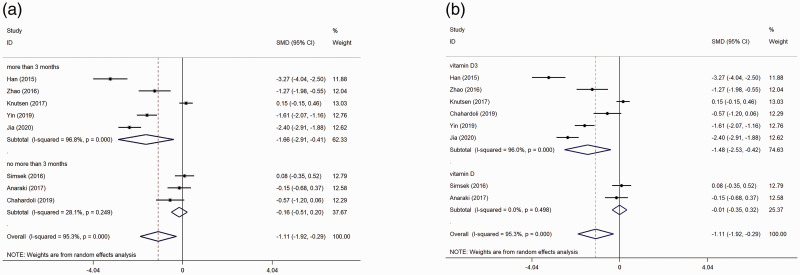
A subgroup analysis was performed based on treatment duration. The results showed that vitamin D supplementation could decrease TPOAb titre in the subgroups in whom the treatment duration >3 months (SMD: –1.66, 95% CI: –2.91, –0.41; P = 0.009), but not in the subgroups in whom the treatment duration was ≤3 months (SMD: –0.16; 95% CI: –0.51, 0.20; P = 0.392) (Figure 4A).
Figure 4.
Forest plots showing subgroup analyses of the effects of vitamin D supplementation on thyroid peroxidase antibody titre in patients with Hashimoto’s thyroiditis: (a) subgroup analysis based on treatment duration; (b) subgroup analysis based on the form of vitamin D administration.7–14
A subgroup analysis was performed based on the form of vitamin D administration. The results showed that vitamin D supplementation could decrease TPOAb titre in the subgroups that were treated with vitamin D3 (SMD: –1.48; 95% CI: –2.53, –0.42; P = 0.006), but not in the subgroups that were treated with vitamin D (SMD: –0.01; 95% CI: –0.35, 0.32; P = 0.945) (Figure 4B).
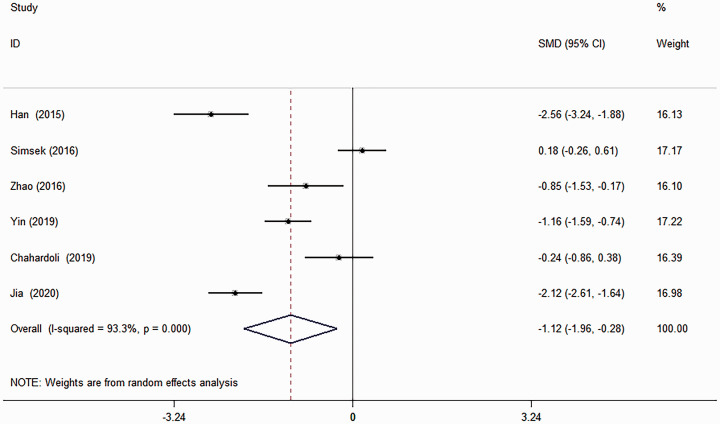
Six studies provided data on TGAb, but there was significant heterogeneity (χ2 = 75.12; I 2 = 93.3%, P < 0.001) (Figure 5) so a random-effect model was used.7–12 The pooled evidence showed that vitamin D supplementation was effective in reducing the TGAb titre (SMD: –1.12; 95% CI: –1.96, –0.28; P = 0.009). Due to limited data, no subgroup analysis and meta-regression analysis were performed.
Figure 5.
Forest plot showing the effects of vitamin D supplementation on thyroglobulin antibody titre in patients with Hashimoto’s thyroiditis using a random-effect model.7–12
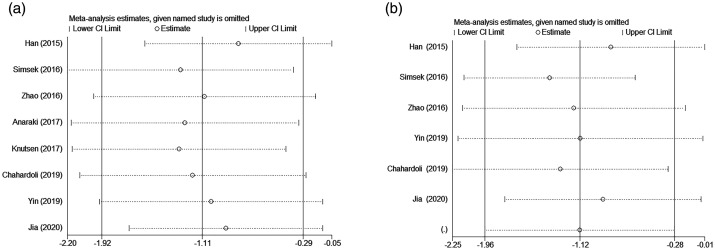
Sensitivity analyses were performed to examine the influence of each individual study on the overall effect size. The effect sizes after one-by-one elimination were all within the 95% CI of the total effect size, indicating that the results of this meta-analysis were stable and reliable (Figure 6).
Figure 6.
Sensitivity analyses of the included studies: (a) sensitivity analysis for the studies about the effect of vitamin D supplementation on thyroid peroxidase antibody titre; (b) sensitivity analysis for the studies about the effect of vitamin D supplementation on thyroglobulin antibody titre.7–14
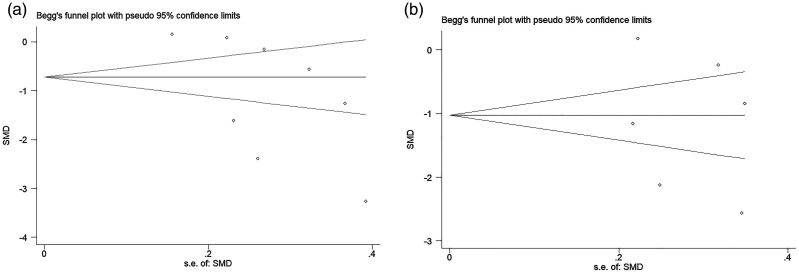
Begg’s test was used to measure publication bias. The TPOAb results showed Z = 1.61.pr > |Z| = 0.108, suggesting no obvious publication bias in the references included in this meta-analysis (Figure 7). The TGAb results showed Z = 0.00.pr > |Z|=1.000, suggesting no significance and no obvious bias.
Figure 7.
Begg’s method for investigating publication bias: (a) Begg’s funnel plot of the publication bias for studies about the effect of vitamin D supplementation on thyroid peroxidase antibody titre; (b) Begg’s funnel plot of the publication bias for studies about the effect of vitamin D supplementation on thyroglobulin antibody titre. SMD, standardized mean difference; s.e., standard error.7–14
Discussion
Hashimoto’s thyroiditis is characterized by specific immune responses against the thyroid gland, which usually manifests as increased levels of serum TPOAb and TGAb. 4 In general, the antibody titre is positively correlated with the extent of hypothyroidism. 5 The present meta-analysis demonstrated that vitamin D supplementation could reduce TPOAb and TGAb titres in patients with HT. A meta-analysis of six randomized controlled trials published in 2018 showed that vitamin D supplementation could decrease serum antibody titres in patients with HT in the short-term (approximately 6 months). 16 This current meta-analysis evaluated additional recent randomized controlled trials and reached a similar conclusion. 16 This current meta-analysis showed that taking vitamin D3 was more effective than taking vitamin D, but this finding needs to be demonstrated by more randomized controlled trials.
Hashimoto’s thyroiditis is characterized by the homing of leukocytes, such as cluster of differentiation (CD) 4+ T cells, cytotoxic T lymphocytes, B cells and natural killer cells, to the thyroid gland. 17 These leukocytes proceed to destroy thyroid follicular cells via antibody-dependent cytotoxicity and/or apoptosis. 18 Once the type 1 helper T (Th1) cell/type 2 helper T (Th2) cell immune balance is disrupted, Th1 cell-dominated cellular immunity is initiated, which is the main mechanism of the pathogenesis of HT. 19 Moreover, complement is activated and thyroid autoantibodies are produced, leading to thyroid follicular cell damage and ultimately hypothyroidism. 20 TGAb and TPOAb are two types of thyroid tissue autoantibodies, the levels of which are positively associated with the severity of thyroid inflammation and hypothyroidism. 21 In particular, TPO, a key enzyme located in thyroid cells, is involved in iodinated tyrosine synthesis, iodinated tyrosine coupling and other processes. 22 TPO is the most important autoantigen involved in the induction of autoimmune thyroid disease.23–25 TGAb and TPOAb positivity, especially almost 100% positivity for TPOAb, is one of the necessary criteria for the diagnosis of Hashimoto's encephalopathy.26–29 However, it has not been determined whether antithyroid antibodies play a direct role in the pathogenesis of Hashimoto's encephalopathy or simply serve as a marker of autoimmune reactivity.26–29
As an active form of vitamin D, 1,25-dihydroxyvitamin D3 can cooperate with vitamin D receptors to regulate calcium and phosphorus metabolism, control the proliferation and differentiation of cells and generate immunomodulatory effects. 30 1,25 (OH)2D3 down-regulates the expression of the major histocompatibility complex class II and stimulating molecules (CD40, CD80, CD86), increases the production of interleukin (IL)-10 and reduces the secretion of IL-12, thus repressing the Th1 cell immune response. 31 In contrast, 1,25(OH)2D3 inhibits plasma cell generation, memory cell formation and subsequent secretion of immunoglobulin (Ig)G and IgM by activated B cells. 32 Vitamin D also regulates regulatory B (Breg) cells involved in immunological tolerance by producing IL-10, IL-35 and transforming growth factor-β. 33 Therefore, these current findings suggest that the effect of vitamin D supplementation in reducing thyroid tissue autoantibodies may be related to its regulation of immune function.
This current meta-analysis had several limitations as follows: (i) the power of the analysis may be restricted because of the limited study numbers and population sizes; (ii) there was significant heterogeneity among the included studies; (iii) most of the included studies were conducted in China, which affects the extrapolation of the conclusions to other populations to some extent; (iv) only published data were included, which may lead to a reporting bias by overestimating the effect of vitamin D.
The findings of this current meta-analysis suggest that vitamin D supplementation reduces TGAb and TPOAb titre in patients with HT. The effect was increased if patients received vitamin D3 and the duration of treatment was >3 months. More randomized, double-blind, placebo-controlled trials with longer follow-up durations are required to confirm these preliminary findings.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605211060675 for Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: systematic review and meta-analysis by Jingwen Zhang, Yuting Chen, Hongyan Li and Hong Li in Journal of International Medical Research
Footnotes
Author contributions: Jingwen Zhang and Hong Li designed the study. Jingwen Zhang and Yuting Chen independently carried out the literature search and screening of articles. Hongyan Li analysed the data. Jingwen Zhang and Yuting Chen wrote the manuscript. Hongyan Li helped edit the manuscript; Hong Li revised the manuscript. All authors read and approved the final version of the manuscript.
Declaration of conflicting interest: The authors declare that there are no conflicts of interest.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and publication of this article: This research was supported by grants from the Shanghai Committee of Science and Technology Research Projects (no.19140904600, no. 18401900800) and the 2018–2020 Three-year Action Plan for Traditional Chinese Medicine Further Development in Shanghai (no. ZY2018-2020-CCCX-2002-08).
ORCID iD: Hong Li https://orcid.org/0000-0002-9614-2964
Supplemental material: Supplemental material for this article is available online.
References
- 1.Lorini R, Gastaldi R, Traggiai C, et al. Hashimoto's Thyroiditis. Pediatr Endocrinol Rev 2003; 1: 205–211. [PubMed] [Google Scholar]
- 2.Caturegli P, Kimura H, Rocchi R, et al. Autoimmune thyroid diseases. Curr Opin Rheumatol 2007; 19: 44–48. DOI: 10.1097/BOR.0b013e3280113d1a. [DOI] [PubMed] [Google Scholar]
- 3.Hiromatsu Y Satoh H andAmino N.. Hashimoto's thyroiditis: history and future outlook. Hormones (Athens) 2013; 12: 12–18. DOI: 10.1007/bf03401282. [DOI] [PubMed] [Google Scholar]
- 4.Ralli M, Angeletti D, Fiore M, et al. Hashimoto's thyroiditis: An update on pathogenic mechanisms, diagnostic protocols, therapeutic strategies, and potential malignant transformation. Autoimmun Rev 2020; 19: 102649. DOI: 10.1016/j.autrev.2020.102649. [DOI] [PubMed] [Google Scholar]
- 5.Bossowski A andUrban M.. Serum levels of cytokines in children and adolescents with Graves' disease and non-toxic nodular goiter. J Pediatr Endocrinol Metab 2001; 14: 741–747. DOI: 10.1515/jpem.2001.14.6.741. [DOI] [PubMed] [Google Scholar]
- 6.Štefanić M andTokić S.. Serum 25-hydoxyvitamin D concentrations in relation to Hashimoto's thyroiditis: a systematic review, meta-analysis and meta-regression of observational studies. Eur J Nutr 2020; 59: 859–872. DOI: 10.1007/s00394-019-01991-w. [DOI] [PubMed] [Google Scholar]
- 7.Simsek Y, Cakir I, Yetmis M, et al. Effects of Vitamin D treatment on thyroid autoimmunity. J Res Med Sci 2016; 21: 85. DOI: 10.4103/1735-1995.192501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao L Wang L andFu B.. Clinical application of calcitriol in Hashimoto's thyroiditis with hypothyroidism. Medical Review 2016; 22: 2410–2412. [Google Scholar]
- 9.Jia R. Observation on the efficacy of calcitriol combined with levothyroxine sodium tablets in Hashimoto's thyroiditis with hypothyroidism. Journal of Medical Forum 2020; 41: 133–135. [Google Scholar]
- 10.Yin H, Gu J, Guo X, et al. Application of calcitriol in hypothyroidism patients with Hashimoto's thyroiditis. Journal of Medical Forum 2019; 40: 140–142. [Google Scholar]
- 11.Han Y, Chen YJ, Li YM, et al. Effect of calcitriol treatment on Hashimoto's thyroiditis with hypothyroidism. Chinese Clinical Research 2015; 28: 17–19. DOI:10.13429/j.cnki.cjcr.2015.01.006. [Google Scholar]
- 12.Chahardoli R, Saboor-Yaraghi AA, Amouzegar A, et al. Can Supplementation with Vitamin D Modify Thyroid Autoantibodies (Anti-TPO Ab, Anti-Tg Ab) and Thyroid Profile (T3, T4, TSH) in Hashimoto's Thyroiditis? A Double Blind, Randomized Clinical Trial. Horm Metab Res 2019; 51: 296–301. DOI: 10.1055/a-0856-1044. [DOI] [PubMed] [Google Scholar]
- 13.Vahabi Anaraki P, Aminorroaya A, Amini M, et al. Effect of Vitamin D deficiency treatment on thyroid function and autoimmunity markers in Hashimoto's thyroiditis: A double-blind randomized placebo-controlled clinical trial. J Res Med Sci 2017; 22: 103. DOI: 10.4103/jrms.JRMS_1048_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knutsen KV, Madar AA, Brekke M, et al. Effect of Vitamin D on Thyroid Autoimmunity: A Randomized, Double-Blind, Controlled Trial Among Ethnic Minorities. J Endocr Soc 2017; 1: 470–479. DOI: 10.1210/js.2017-00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. DOI: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Wu Y, Zuo Z, et al. The effect of vitamin D supplementation on thyroid autoantibody levels in the treatment of autoimmune thyroiditis: a systematic review and a meta-analysis. Endocrine 2018; 59: 499–505. [DOI] [PubMed] [Google Scholar]
- 17.Pearce EN Farwell AP andBraverman LE.. Thyroiditis. N Engl J Med 2003; 348: 2646–2655. DOI: 10.1056/NEJMra021194. [DOI] [PubMed] [Google Scholar]
- 18.Ajjan RA andWeetman AP.. The Pathogenesis of Hashimoto's Thyroiditis: Further Developments in our Understanding. Horm Metab Res 2015; 47: 702–710. DOI: 10.1055/s-0035-1548832. [DOI] [PubMed] [Google Scholar]
- 19.Safdari V, Alijani E, Nemati M, et al. Imbalances in T Cell-Related Transcription Factors Among Patients with Hashimoto's Thyroiditis. Sultan Qaboos Univ Med J 2017; 17: e174–e180. DOI: 10.18295/squmj.2016.17.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ząbczyńska M, Polak K, Kozłowska K, et al. The Contribution of IgG Glycosylation to Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) and Complement-Dependent Cytotoxicity (CDC) in Hashimoto's Thyroiditis: An in Vitro Model of Thyroid Autoimmunity. Biomolecules 2020; 10: 171. DOI: 10.3390/biom10020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pyzik A, Grywalska E, Matyjaszek-Matuszek B, et al. Immune disorders in Hashimoto's thyroiditis: what do we know so far? J Immunol Res 2015; 2015: 979167. DOI: 10.1155/2015/979167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramhøj L, Svingen T, Frädrich C, et al. Perinatal exposure to the thyroperoxidase inhibitors methimazole and amitrole perturbs thyroid hormone system signaling and alters motor activity in rat offspring. Toxicol Lett 2021: S0378-4274(21)00863-8. DOI: 10.1016/j.toxlet.2021.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Gerenova JB Manolova IM andTzoneva VI.. Clinical significance of autoantibodies to parietal cells in patients with autoimmune thyroid diseases. Folia Med (Plovdiv) 2013; 55: 26–32. DOI: 10.2478/folmed-2013-0014. [DOI] [PubMed] [Google Scholar]
- 24.Aliesky H, Courtney CL, Rapoport B, et al. Thyroid autoantibodies are rare in nonhuman great apes and hypothyroidism cannot be attributed to thyroid autoimmunity. Endocrinology 2013; 154: 4896–4907. DOI: 10.1210/en.2013-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Kim J, Diana T, et al. A novel bioassay for anti-thyrotrophin receptor autoantibodies detects both thyroid-blocking and stimulating activity. Clin Exp Immunol 2013; 173: 390–397. DOI: 10.1111/cei.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattozzi S, Sabater L, Escudero D, et al. Hashimoto encephalopathy in the 21st century. Neurology 2020; 94: e217–e224. [DOI] [PubMed] [Google Scholar]
- 27.Ercoli T Defazio G andMuroni A.. Cerebellar Syndrome Associated with Thyroid Disorders. Cerebellum 2019; 18: 932–940. [DOI] [PubMed] [Google Scholar]
- 28.Valencia-Sanchez C, Pittock SJ, Mead-Harvey C, et al. Brain dysfunction and thyroid antibodies: autoimmune diagnosis and misdiagnosis. Brain Commun 2021; 3: fcaa233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ercoli T Defazio G andMuroni A.. Status epilepticus in Hashimoto's encephalopathy. Seizure 2019; 70: 1–5. [DOI] [PubMed] [Google Scholar]
- 30.Alagarasu K. Immunomodulatory effect of vitamin D on immune response to dengue virus infection. Vitam Horm 2021; 117: 239–252. DOI: 10.1016/bs.vh.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 31.Lopez D, Al-Jaberi F, Woetmann A, et al. Macrophages Control the Bioavailability of Vitamin D and Vitamin D-Regulated T Cell Responses. Front Immunol 2021; 12: 722806. DOI: 10.3389/fimmu.2021.722806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen S, Sims GP, Chen XX, et al. Modulatory effects of 1,25-dihydroxyvitamin D3 on human B cell differentiation. J Immunol 2007; 179: 1634–1647. DOI: 10.4049/jimmunol.179.3.1634. [DOI] [PubMed] [Google Scholar]
- 33.Heine G, Niesner U, Chang HD, et al. 1,25-dihydroxyvitamin D(3) promotes IL-10 production in human B cells. Eur J Immunol 2008; 38: 2210–2218. DOI: 10.1002/eji.200838216. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605211060675 for Effects of vitamin D on thyroid autoimmunity markers in Hashimoto’s thyroiditis: systematic review and meta-analysis by Jingwen Zhang, Yuting Chen, Hongyan Li and Hong Li in Journal of International Medical Research