Abstract
BACKGROUND:
European LeukemiaNet (ELN) 2017 risk stratification by genetics is prognostic of outcomes in patients with acute myeloid leukemia (AML). However, the prognostic impact of the 2017 ELN genetic risk stratification after allogeneic hematopoietic cell transplantation (alloHCT) is not well established.
OBJECTIVE:
We examined the effect of 2017 ELN genetic risk stratification on alloHCT outcomes of AML.
METHODS:
We included 500 adult (≥18 years) AML patients in first (n=370) or second (n=130) complete remission receiving alloHCT from 2005 to 2016. Patients were classified into favorable (12%), intermediate (57%), and adverse (32%) 2017 ELN risk groups. The Cox proportional hazard model was used to conduct the multivariable analyses of leukemia-free survival (LFS) and overall survival (OS). Relapse and non-relapse mortality were analyzed by the Fine-Gray regression model.
RESULTS:
OS at 2 years was 72% in the favorable vs. 60% in the intermediate vs. 45% in the adverse risk groups (p<0.001). In multivariable analyses, the 2017 ELN classifier was an independent predictor of OS after alloHCT with significantly higher overall mortality in the intermediate (HR=1.68, 95% CI 1.06–2.68; p=0.03) and adverse (HR=2.50, 95% CI 1.54–4.06; p<0.001) risk groups compared to the favorable risk group. Similarly, LFS was worse in the intermediate (HR=1.63, 95% CI 1.06–2.53; p=0.03) and adverse (HR 2.23, 95% CI 1.41–3.54; p<0.001) risk groups while relapse was higher in the adverse risk group (HR=2.36, 95% CI 1.28–4.35; p= 0.006) as compared to the favorable risk group.
CONCLUSION:
These data highlight the prognostic impact of the 2017 ELN genetic risk stratification on the survival of AML patients following alloHCT. Patients in the adverse risk group had the highest risk of relapse and worst survival. Thus, the 2017 ELN prognostic system can help identify AML patients who may benefit from clinical trials offering relapse mitigation strategies in order to improve transplant outcomes.
INTRODUCTION
Most adult patients with acute myeloid leukemia (AML), who enter remission following induction chemotherapy, still remain at considerable risk for relapse.1–4 While consolidation chemotherapy in patients achieving complete remission can be curative for those with favorable cytogenetic and molecular features, the majority of AML patients with intermediate or adverse genetic risk have no curative options outside of allogeneic hematopoietic stem cell transplantation (alloHCT).5, 6 Performing an alloHCT for AML in complete remission (CR) depends on assessment of disease related factors to include cytogenetic and molecular abnormalities, presence of measurable residual disease (MRD) and disease risk index (DRI).7–9,10–12 Given considerable progress achieved in the genomic landscape of AML, the European LeukemiaNet (ELN) provided updated recommendations in 2017 regarding standardization of response criteria and AML treatment.13 In the original 2010 ELN classification, the intermediate-I and intermediate-II risk groups, which constitute the majority of AML cases, were prognostically indistinguishable in older patients.9 Subsequently, the 2017 ELN genetic classification was simplified to include 3 genetic risk groups: favorable, intermediate, and adverse.13 In addition, the revised 2017 ELN classification included recently categorized AML subgroups with mutational abnormalities such as ASXL1, RUNX1, and TP53. In this study, we examined the prognostic significance of the 2017 ELN genetic risk stratification on clinical outcomes of alloHCT in adult patients with AML.
METHODS
Study Population
Adult patients (age ≥18 years) with AML in CR1 and CR2 receiving their first alloHCT at Moffitt Cancer Center between 2005 and 2016 were included in this study. All transplant recipients consented to an institutional long-term follow-up data collection protocol. The data abstraction was analyzed in this retrospective analysis which was approved by the institutional IRB (University of South Florida IRB). Data was collected from the Moffitt Cancer Center Blood and Marrow Transplant and Cellular Immunotherapy Research and Analysis Information Network (BRAIN) and supplemented by retrospective review of individual medical records. Patients with Acute Promyelocytic Leukemia (APL) and those without available cytogenetic information were excluded from our analyses.
All patients with AML in CR1 were assigned to favorable, intermediate and adverse risk groups by the 2017 ELN genetic risk classification applied at diagnosis of AML.13 In patients with AML in CR2, we considered both diagnostic and relapsed AML samples for 2017 ELN classification and did not identify any differences in categorization between the two samples. The favorable risk category included Core Binding Factor (CBF) abnormalities such as t(8;21), inv(16) or t(16;16) and molecular features such as mutated NPM1 without FLT3-ITD and mutated biallelic CEBPA.13 The 2017 ELN favorable risk group consisted of AML in CR2 (n=41) or in CR1 (n=17) with detectable MRD at alloHCT.
The adverse risk category included monosomal14 and complex karyotypes,9, 15 −5/del5q, −7, −17/abn(17p), inv3(q21.3q26.2 or t(3;3), t(6;9), t(v;11q23.3), t(9;22) and adverse molecular abnormalities such as FLT3-ITD with wild type NPM1, RUNX1, ASXL1 and TP53 mutations.13 The remaining cytogenetic and molecular risk abnormalities were considered as intermediate genetic risk.
A reverse transcription polymerase chain reaction (RT-PCR) or Sanger Sequencing molecular testing methods as previously reported have been used to test for FLT-3, NPM1 and CEBPA mutations since 2007.16–20 These tests were ordered separately even after introduction of Next-Generation Sequencing (NGS) due to the limitation of NGS to accurately detect FLT-3 and CEBPA mutations. Patients receiving alloHCT from 2005 and 2007 were not tested for the abovementioned risk defining mutations and were therefore classified based on the best available cytogenetic data into intermediate risk (N=36).
Mutational analysis by Next-Generation Sequencing (NGS) in a bone marrow aspirate was initially performed at a CLIA certified commercial lab (Genoptix) between May 2011 and October 2014 using a 5 gene panel then a 21-gene panel as we previously reported.20 Starting from October 2014, NGS was performed at Moffitt Cancer Center in a CAP/CLIA-certified environment using a custom TruSeq myeloid 32-gene panel which was transitioned to an Illumina TruSeq Myeloid 54-gene panel in 2016.21 Insertions/deletions were reported at validated variant allele frequency (VAF) >10%. Single nucleotide variants were reported with a variant allele frequency (VAF) ≥ 5% in all tests. Genetic risk-defining analysis by NGS for detection of RUNX1, ASXL1 and TP53 mutations was missing in 77 patients who had intermediate cytogenetics and wild type or unknown FLT3-ITD. MRD data from the bone marrow aspirates preceding alloHCT was available for the subset of patients (N=93) and assessed by multiparametric flow cytometry (MFC), cytogenetics, RT-PCR and/or NGS.
Definitions and Endpoints
The primary endpoint of the study was the overall survival (OS) after alloHCT. Secondary endpoints included cumulative relapse incidence (CRI), leukemia-free survival (LFS), and non-relapse mortality (NRM). Outcomes were measured from the time of stem cell infusion and surviving patients were censored at their last follow-up. OS was measured based on death from any cause, and LFS was defined as survival without disease progression or evidence of AML relapse. The intensity of conditioning chemotherapy was defined as previously reported.22, 23 The standard myeloablative regimen consisted of IV fludarabine 40 mg/m2 and IV busulfan (starting dose 130–145 mg/m2) each daily × 4 days. Busulfan pharmacokinetic samples were obtained, and the final two doses were adjusted to target an average daily busulfan area under the curve of 5300 μM/L*min per day. Reduced toxicity regimens included IV busulfan/fludarabine with targeted average daily area under the curve of 3500 μM/L*min per day, fludarabine (40 mg/m2/day × 4 days)/(IV busulfan 3.2 mg/kg × 2 days) and fludarabine (30 mg/m2/dayx4 days)/melphalan (140 mg/m2 single dose). Non-myeloablative regimens included fludarabine (25 mg/m2/day × 5 doses)/cyclophosphamide (50 mg/kg/day × 2 doses), fludarabine (30 mg/m2/day × 3 days)/2 Gy TBI, and cyclophosphamide 50 mg/kg/dayx4 days/equine ATG (30 mg/kgx3 doses).24 Hematopoietic stem cell transplant comorbidity index (HCT-CI) was defined as reported by Sorror et al,25 and DRI as reported by Armand et al8 and the Center for International Blood and Marrow Transplant Research (CIBMTR) Acute Leukemia Working Committee. CR was defined as absence of detectable leukemia morphologically with bone marrow blasts < 5%, no circulating blasts in peripheral blood or presence of extramedullary disease together with peripheral blood absolute neutrophil (≥ 1.0 × 109/L) and platelet count recovery (≥ 100 × 109/L).13 MRD positivity was defined as evidence of residual molecular (NGS or RT-PCR), MFC or cytogenetic abnormalities detected in pre-transplant bone marrow biopsy samples.10
Statistical Analysis
In this retrospective cohort study, we reported patient-, disease-, and transplantation-related characteristics descriptively. The competing risk modeling was used to estimate cumulative incidences for relapse and NRM in the 2017 ELN risk groups, with each treated as a competing risk for the other. The Kaplan-Meier method was used to estimate OS and LFS probabilities.26 The log-rank and Gray27 tests were used to assess the differences in the Kaplan-Meier and the cumulative incidence functions, respectively. The Cox proportional hazard model28 was employed to build the multivariable models for survival and leukemia-free survival, and the Fine-Gray regression model29 was employed for relapse and NRM. The backward elimination method with a threshold level of statistical significance of 5% was used to identify significant risk factors and to derive final multivariable models. The 2017 ELN genetic risk groups, regardless of level of significance, were included in all steps of model building. The ELN 2017 favorable risk group was used as a reference. All p-values were 2-sided. The statistical analyses were performed using R (version 3.5.2) and SAS 14.3 (Cary, NC) software.
RESULTS
Patient Characteristics
We identified 500 adult patients with AML in CR1 or CR2 who received their first alloHCT between 2005 and 2016 at Moffitt Cancer Center (Table 1). On the basis of 2017 ELN genetic risk stratification, 58 (12%) patients had favorable, 284 (57%) had intermediate, and 158 (32%) had adverse risk AML. Median age at transplantation was 55 years (range, 18–76 years). At the time of transplant, half of the study patients had HCT-CI ≥3, and 70% of all patients were CMV seropositive. Remission status at alloHCT was CR1 in 74% and CR2 in 26% of patients; 14% of all patients had high-risk DRI. Matched unrelated donor (MUD, 46%) transplants accounted for the majority of all alloHCT, followed by HLA-identical sibling (MSD, 28%), mismatched unrelated (MMUD, 16%), umbilical cord blood (UCB, 6%), and mismatched related (MMRD, 3%) transplants. The majority of the study patients (68%) received myeloablative conditioning, and tacrolimus in combination with sirolimus was the most common (44%) graft-versus-host disease (GVHD) prophylaxis regimen.
Table 1.
Patient characteristics
| Variable | Strata | N (%) Total N=500 |
|---|---|---|
| 2017 ELN genetic risk | ||
| Favorable | 58 (11.6) | |
| Intermediate | 284 (56.8) | |
| Adverse | 158 (31.6) | |
| Age, years | ||
| Median (range) | 55 (18–76) | |
| < 60 years | 332 (66.4) | |
| ≥ 60 years | 168 (33.6) | |
| Gender | ||
| Male | 261 (52.2) | |
| Female | 239 (47.8) | |
| Karnofsky performance score | ||
| ≥ 90 | 391 (78.2) | |
| < 90 | 109 (21.8) | |
| HCT-CI score | ||
| 0–2 | 248 (49.6) | |
| ≥ 3 | 252 (50.4) | |
| Disease type | ||
| De Novo | 390 (78.0) | |
| Secondary | 110 (22.0) | |
| Cytogenetic risk | ||
| Favorable | 37 (7.4) | |
| Intermediate | 342 (68.4) | |
| Adverse | 121 (24.2) | |
| Remission status at HCT | ||
| CR1 | 370 (74.0) | |
| CR2 | 130 (26.0) | |
| Pre-HCT MRD status* | ||
| Positive | 63 (12.6) | |
| Negative | 30 (6.0) | |
| DRI risk | ||
| Low | 38 (7.6) | |
| Intermediate | 393 (78.6) | |
| High | 69 (13.8) | |
| Conditioning intensity | ||
| Myeloablative | 338 (67.6) | |
| Reduced Intensity | 162 (32.4) | |
| Donor Type | ||
| MSD | 142 (28.4) | |
| MUD | 232 (46.4) | |
| MMUD | 82 (16.4) | |
| MMRD | 16 (3.2) | |
| UCB | 28 (5.6) | |
| GVHD Prophylaxis | ||
| T acrolimus/Sirolimus | 221 (44.0) | |
| Other | 279 (56.0) | |
| Recipient CMV serostatus | ||
| Positive | 350 (70.0) | |
| Negative | 150 (30.0) | |
| Year of HCT | ||
| 2005–2010 | 184 (36.8) | |
| 2011–2016 | 316 (63.2) | |
| Follow up of survivors, years | ||
| Median (range) | 4.7 (2.0 – 5.0) |
2017 ELN indicates European LeukemiaNet genetic risk classification; HCT-CI, hematopoietic stem cell transplantation comorbidity index; CR, complete remission; MRD*, measurable residual disease in subjects with available data at transplant; DRI, disease risk index; RIC, reduced-intensity conditioning; MSD, HLA-matched sibling donor; MUD, HLA-matched unrelated donor; MMUD, mismatched unrelated donor; MMRD, mismatched related donor; UCB, umbilical cord blood. GVHD, graft-versus-host disease; CMV, cytomegalovirus.
Pre-transplant MRD status was available in 93 patients who were mostly treated after 2014: 63 (68%) were MRD positive and 30 (32%) were MRD negative at alloHCT. We found that MRD status at alloHCT was associated with 2017 ELN genetic risk (p=0.02). In particular, there was a higher proportion of patients with 2017 ELN adverse risk AML in the MRD-positive group (49%) than in the MRD-negative group (27%). Conversely, there was a lower proportion of patients with 2017 ELN intermediate risk AML in the MRD-positive group (38%) than in the MRD-negative group (60%). In addition, the MRD positive group had more patients (33%) with secondary AML than the MRD-negative (13%) group (p=0.04). We otherwise observed no significant differences among MRD groups across all patient- and transplant-related characteristics.
Relapse and NRM by 2017 ELN Genetic Risk Stratification
The cumulative incidence of relapse at 2 years after alloHCT was 30% (95% CI, 26–34) for the entire cohort: 26% in the favorable risk, 25% in the intermediate risk and 41% in the adverse risk 2017 ELN genetic risk groups (p=0.001, Figure 1 A). In multivariable analysis, increasing 2017 ELN genetic risk was associated with increased risk of relapse after alloHCT (Table 2). Compared with the 2017 ELN favorable risk group, the adverse risk group had a 2.4-fold (HR=2.36, 95% CI 1.28–4.35; p=0.006) greater risk of relapse after alloHCT. The CR2 remission status (HR=1.69; 95% CI 1.17–2.43; p=0.005) significantly increased the risk of relapse after alloHCT compared to CR1.
Figure 1.
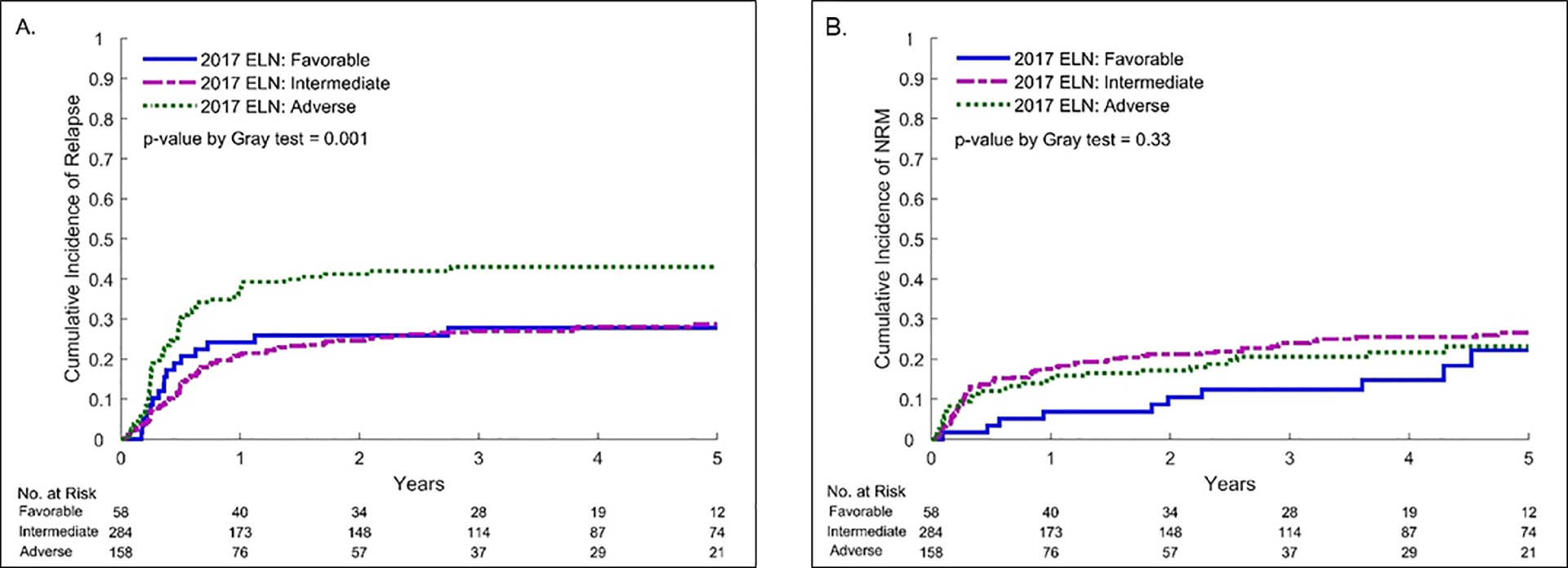
Relapse (A) and Non-Relapse Mortality (B) by 2017 ELN genetic risk for AML
Table 2:
Multivariable analysis of clinical outcomes by 2017 ELN genetic risk
| Variable | Stratification | N | HR | 95% CI | p-value |
|---|---|---|---|---|---|
| Relapse | |||||
| 2017 ELN | |||||
| Favorable | 58 | 1.0 | - | - | |
| Intermediate | 284 | 1.23 | 0.69–2.18 | 0.48 | |
| Adverse | 158 | 2.36 | 1.28–4.35 | 0.006 | |
| Remission status | |||||
| CR 1 | 370 | 1.0 | - | - | |
| CR 2 | 130 | 1.69 | 1.17–2.43 | 0.005 | |
| Non-Relapse Mortality | |||||
| 2017 ELN | |||||
| Favorable | 58 | 1.0 | |||
| Intermediate | 284 | 0.92 | 0.43–1.96 | 0.82 | |
| Adverse | 158 | 0.43 | 0.17–1.07 | 0.07 | |
| HCT-CI | |||||
| 0–2 | 248 | 1.0 | |||
| ≥ 3 | 252 | 1.47 | 1.00–2.18 | 0.05 | |
| DRI | |||||
| Low | 38 | 1.0 | |||
| Intermediate | 393 | 3.19 | 0.95–10.71 | 0.06 | |
| High | 69 | 7.75 | 1.92–31.30 | 0.004 | |
| Leukemia-Free Survival * | |||||
| 2017 ELN | |||||
| Favorable | 58 | 1.0 | |||
| Intermediate | 284 | 1.63 | 1.06–2.53 | 0.03 | |
| Adverse | 158 | 2.23 | 1.41–3.54 | < 0.001 | |
| Remission status | |||||
| CR 1 | 370 | 1.0 | |||
| CR 2 | 130 | 1.53 | 1.16–2.01 | 0.003 | |
| HCT-CI | |||||
| 0–2 | 248 | 1.0 | |||
| ≥ 3 | 252 | 1.56 | 1.23–1.99 | < 0.001 | |
| Overall Survival * | |||||
| 2017 ELN | |||||
| Favorable | 58 | 1.0 | |||
| Intermediate | 284 | 1.68 | 1.06–2.68 | 0.03 | |
| Adverse | 158 | 2.50 | 1.54–4.06 | <0.001 | |
| Remission status | |||||
| CR 1 | 370 | 1.0 | |||
| CR 2 | 130 | 1.48 | 1.12–1.97 | 0.007 | |
| HCT-CI | |||||
| 0–2 | 248 | 1.0 | |||
| ≥ 3 | 252 | 1.61 | 1.25–2.08 | < 0.001 | |
| Conditioning intensity | |||||
| Myeloablative | 338 | 1.0 | 0.01 | ||
| Reduced Intensity | 162 | 1.42 | 1.08–1.86 | 0.002 | |
| Year of HCT | |||||
| 2005–2010 | 184 | 1.0 | |||
| 2010–2016 | 316 | 0.71 | 0.53–0.93 | 0.02 | |
HR, hazard ratio.
HR denotes for increased risk of mortality. 2017 ELN indicates European LeukemiaNet genetic risk classification; HCT-CI, hematopoietic stem cell transplantation comorbidity index; CR, complete remission; MRD, measurable residual disease; DRI, disease risk index; RIC, reduced-intensity conditioning.
Adjusted for recipient age, HCT-CI, Karnofsky performance status, DRI, remission and MRD status prior to HCT, conditioning intensity, donor type, graft-versus-host disease prophylaxis, recipient CMV serostatus and year of HCT, as applicable based on the individual models.
The cumulative incidence of NRM at 2 years was 19% (95% CI, 15–22) for the entire cohort: 10% in the favorable risk, 21% in the intermediate risk and 17% in the adverse risk 2017 ELN genetic risk groups (p=0.33, Figure 1 B). In multivariable analysis, the 2017 ELN genetic risk had no significant impact on NRM. High risk DRI (HR=7.75, 95% CI 1.92–31.30; p=0.004) and HCT-CI ≥3 (HR=1.47, 95% CI 1.00–2.18; p=0.05) were additional factors associated with increased risk of NRM after alloHCT.
LFS and OS by 2017 ELN Genetic Risk Stratification
LFS probability at 2 years after alloHCT for the entire cohort was 51% (95% CI 47–55): 64% in the favorable, 54% in the intermediate and 42% in the adverse risk 2017 ELN groups (p=0.002; Figure 2 A). In multivariable analysis, 2017 ELN genetic risk remained strongly prognostic for LFS. Compared to the 2017 ELN favorable risk group, treatment failure (inverse of LFS) after alloHCT was 1.6-fold (95% CI 1.06–2.53; p=0.03) greater with intermediate risk and 2.2-fold (95% CI 1.41–3.54; p<0.001) greater with adverse genetic risk. Other factors that were associated with worse LFS included HCT-CI ≥3 (HR=1.56, 95% CI 1.23–1.99; p<0.001) and CR2 remission status (HR=1.53, 95% CI 1.16–2.01; p=0.003).
Figure 2.
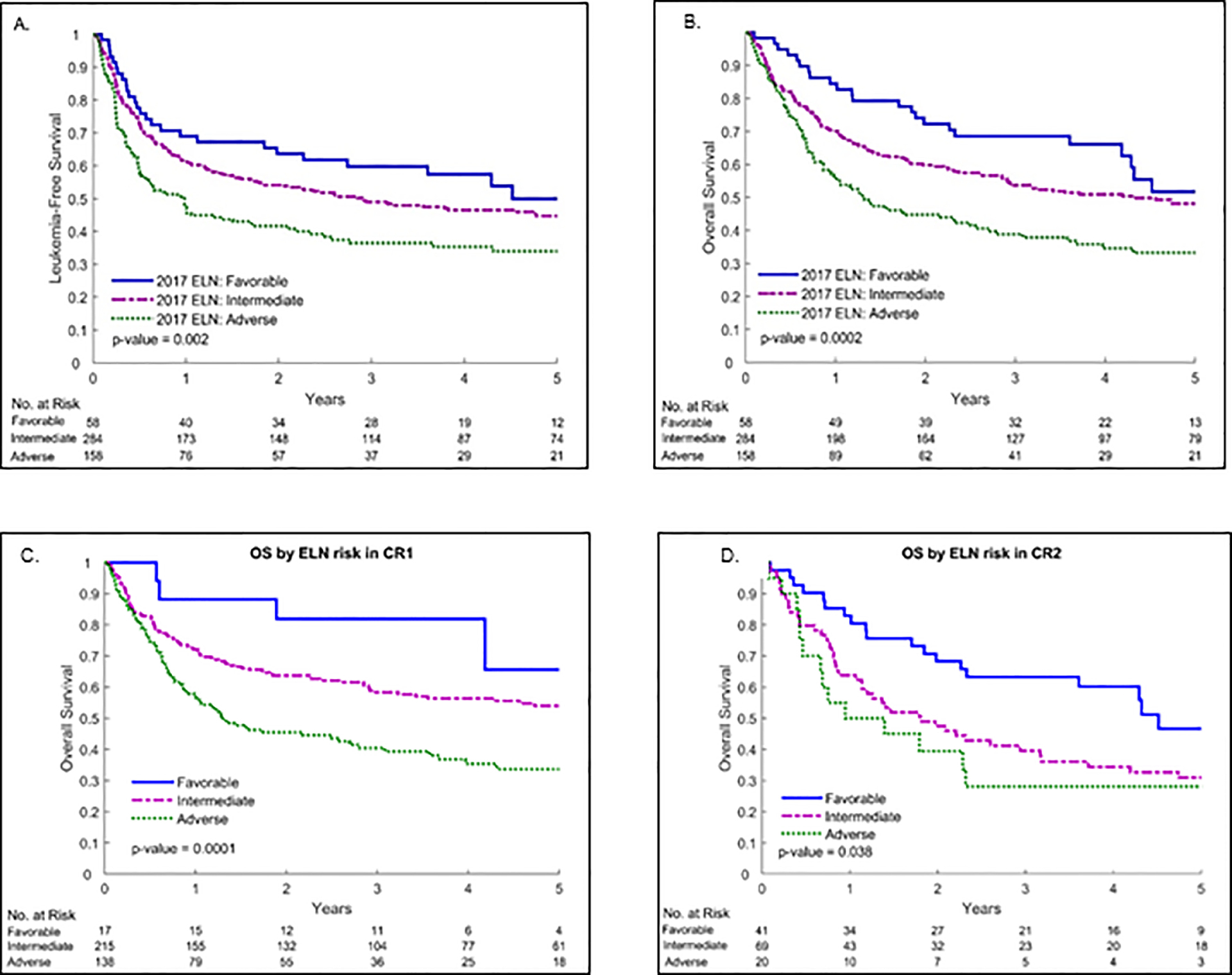
Leukemia-Free Survival of all patients (A), Overall Survival of all patients (B), Overall Survival of CR1 patients (C), and Overall Survival of CR2 patients (D) by 2017 ELN genetic risk for AML
The probability of OS at 2 years after alloHCT for the entire cohort was 56% (95% CI, 52–61): 72% in the favorable, 60% in the intermediate and 45% in the adverse risk 2017 ELN genetic risk groups (p<0.001; Figure 2 B). In multivariable analysis, 2017 ELN genetic risk remained a significant independent predictor of OS after alloHCT. Overall mortality was 1.7-fold greater with the intermediate (95% CI 1.06–2.68; p=0.03) and 2.5-fold greater with the adverse (95% CI 1.54–4.06; p<0.001) 2017 ELN genetic risk groups as compared to the favorable risk group. Factors associated with lower OS after alloHCT included HCT-CI ≥3 (HR=1.61, 95% CI 1.25–2.08; p<0.001) and the use of RIC (HR=1.42, 95% CI 1.08–1.86; p=0.01), while alloHCT performed after 2010 was associated with improved OS (HR=0.71, 95% CI 0.53–0.93; p=0.02). In addition, CR2 remission status (HR=1.48, 95% CI 1.12–1.97; p=0.007) compared to CR1 was associated with worse OS. We also studied the effect of the 2017 ELN classification on CR1 and CR2 subsets separately. We found that the 2017 ELN classification is prognostic for overall survival of patients in both subsets. In univariable analysis of patients with AML in CR1, the overall mortality risk was 2.2-fold higher in the intermediate risk and 3.8-fold higher in the adverse risk group compared to the favorable risk group (p<0.001; Figure 2 C). In patients with AML in CR2, the overall mortality risk was 1.8-fold higher in the intermediate risk and 2.2-fold higher in the adverse risk group compared to the favorable risk group (p=0.04; Figure 2 D).
DISCUSSION
In this study, we identified the significant independent prognostic impact of the 2017 ELN genetic risk stratification on OS of AML patients receiving alloHCT in CR1 and CR2. Survival was the highest in the favorable risk group, followed by the intermediate risk group, while the adverse risk group had the worst survival after alloHCT. In addition, our analysis showed that increasing 2017 ELN genetic risk is associated with greater risk of relapse and worse LFS after transplant. NRM, however, was not affected by the 2017 ELN genetic risk stratification.
Several genetic risk stratification systems have been examined in the past to prognosticate the outcomes of AML patients receiving alloHCT including models proposed by the Medical Research Council (MRC),30 Cancer and Leukemia Group B (CALGB),31 Southwest Oncology Group/Eastern Cooperative Oncology Group (SWOG/ECOG)32 monosomal karyotype based on cytogenetic information14, 33 and the modified 2010 ELN prognostic model34. However, most of these prior models were largely based on the AML cytogenetic risk and did not consider molecular aberrations. While the 2010 ELN prognostic model considered both cytogenetic and molecular abnormalities and stratified AML patients into 4 distinct risk groups (favorable, intermediate-I, intermediate-II, and adverse),9 it required modification in 2017 to include 3 genetic AML risk groups (favorable, intermediate, and adverse) for better prognostic separation.13, 35 In addition, the 2010 ELN did not include ASXL1, RUNX1, and TP53 molecular risk features, which were later assigned to the 2017 ELN adverse risk group as recent reports showed their independent influence on worse outcomes in AML.13, 36, 37
In two prior studies, the 2010 ELN genetic risk classification had no significant prognostic impact on relapse or survival outcomes among patients with AML.34, 35 A study that included 1550 adults with newly diagnosed AML treated on CALGB first-line clinical trials demonstrated similar LFS and OS outcomes between intermediate-I and intermediate-II groups across younger (< 60 years) and older (≥ 60 years) age cohorts, except for better OS in the intermediate-II group compared to intermediate-I in younger patients.35 Similarly, survival outcome discrepancies were observed in another report of 464 patients receiving alloHCT for AML when the 2010 ELN genetic risk stratification was applied.34 In that study, the best post-alloHCT survival outcomes were observed in the favorable and intermediate-II groups in younger (< 60 years) patients with no significant difference between these two groups. In addition, older (≥ 60 years) patients in the favorable and intermediate-I groups had similar survival.34 In our analysis, age was found not be an independent predictor for clinical outcomes. In addition, in contrast to above studies, the updated 2017 ELN risk stratification in our analysis showed clear prognostic separation of the 3 genetic risk groups and it was highly predictive of relapse and survival outcomes of patients receiving alloHCT for AML in CR1 and CR2. A recent study by Grimm et al. in 234 patients with AML in CR1 also showed the prognostic effect of the 2017 ELN risk stratification on clinical outcomes after alloHCT.38
We also considered MRD status prior to transplant in our analysis, which in prior several reports influenced alloHCT outcomes in AML.7, 12, 39–42 Although MRD information in our study was only available in a subset of patients who were mostly treated in recent years, we observed an association between increasing 2017 ELN genetic risk and MRD positivity prior to alloHCT. A larger independent patient cohort is required to further explore the interaction between 2017 ELN genetic risk and MRD in predicting outcomes of alloHCT in AML. Nevertheless, our analysis demonstrated that the 2017 ELN genetic risk prognosticates relapse after alloHCT independent of MRD status and thereby identifies patients who would most likely benefit from relapse risk reduction strategies. In addition, as noted in ELN MRD consensus document, no standardized methods for the high-sensitivity quantification of MRD prior to HCT are yet available for AML.43 Uniform approach for detection of MRD is not yet available, however, flow cytometry and NGS have both been used. Challenges associated with MRD assessment by NGS include clonal evolution of tumor cells and concerns about each assay’s sensitivity. Standardization and validation of MRD assessment, which is currently being addressed by the ELN MRD Working Party, can help to improve patient selection for alloHCT and for clinical trial participation to reduce AML relapse.
Another limitation of our study is the missing data on several prognostic mutations in a subset of patients within the intermediate risk cytogenetic group. However, since the expression of adverse-risk defining mutations (ASXL1, RUNX1, TP53 or FLT3-ITD) within the intermediate risk cytogenetic group is expected to affect the genetic risk group assignment in <10% of our study population, we believe that the current categorization would not likely significantly influence the results of our analysis.
RIC compared to MAC in our study resulted in worse survival as consistent with previous reports.44 CR2 status compared to CR1 in our study was another factor that independent of the 2017 ELN genetic risk significantly increased the relapse risk and mortality after alloHCT. These findings are consistent with those from a previous, large registry report by the European Group for Bone Marrow Transplantation (EBMT) showing higher relapse risk after transplant with CR2 status compared to CR1 in AML.45 Since AML relapse remains the leading cause of treatment failure and mortality after alloHCT, accurate characterization of patients who are at increased risk of relapse is important in order to design clinical trials with novel relapse reduction approaches such as immunotherapy or targeting agent maintenance.46–51
In conclusion, our study findings indicate that the 2017 ELN genetic risk stratification can serve as a readily available prognostic tool for alloHCT outcomes in patients with AML in CR1 and CR2. In addition, the 2017 ELN genetic risk can help to stratify patients with AML for participation in alloHCT clinical trials. Relapse reduction strategies can be of benefit to all, but particularly to patients with adverse risk AML, and CR2 status at transplant.
HIGHLIGHTS.
2017 ELN risk stratification by genetics is an independent predictor of survival after allogeneic transplantation in patients with AML.
2017 ELN risk stratification significantly increased mortality and treatment failure in the adverse and intermediate risk groups compared to the favorable risk group.
Relapse after allogeneic transplantation was the highest in the adverse risk genetic group.
Acknowledgements:
Editorial assistance was provided by the Moffitt Cancer Center’s Scientific Editing Department by Dr. Paul Fletcher and Daley Drucker. No compensation was given beyond their regular salaries.
This work has been supported in part by the Biostatistics and Bioinformatics Core at the H. Lee Moffitt Cancer Center and Research Institute, a comprehensive cancer center designated by the National Cancer Institute and funded in part by Moffitt’s Cancer Center Support Grant (P30-CA076292).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict-of-interest disclosure: The authors report no conflicts of interest in the analysis or report of the data.
REFERENCES
- 1.Rashidi A, Weisdorf DJ, Bejanyan N. Treatment of relapsed/refractory acute myeloid leukaemia in adults. Br J Haematol 2018; 181(1): 27–37. e-pub ahead of print 2018/01/11; doi: 10.1111/bjh.15077 [DOI] [PubMed] [Google Scholar]
- 2.Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M et al. Survival of patients with acute myeloid leukemia relapsing after allogeneic hematopoietic cell transplantation: a center for international blood and marrow transplant research study. Biol Blood Marrow Transplant 2015; 21(3): 454–459. e-pub ahead of print 2014/12/03; doi: 10.1016/j.bbmt.2014.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood 2012; 119(6): 1599–1606. doi: 10.1182/blood-2011-08-375840 [DOI] [PubMed] [Google Scholar]
- 4.Thanarajasingam G, Kim HT, Cutler C, Ho VT, Koreth J, Alyea EP et al. Outcome and prognostic factors for patients who relapse after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant 2013; 19(12): 1713–1718. e-pub ahead of print 2013/10/01; doi: 10.1016/j.bbmt.2013.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. Jama 2009; 301(22): 2349–2361. e-pub ahead of print 2009/06/11; doi: 10.1001/jama.2009.813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasu S, Kohlschmidt J, Mrozek K, Eisfeld AK, Nicolet D, Sterling LJ et al. Ten-year outcome of patients with acute myeloid leukemia not treated with allogeneic transplantation in first complete remission. Blood advances 2018; 2(13): 1645–1650. e-pub ahead of print 2018/07/12; doi: 10.1182/bloodadvances.2017015222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thol F, Gabdoulline R, Liebich A, Klement P, Schiller J, Kandziora C et al. Measurable residual disease monitoring by NGS before allogeneic hematopoietic cell transplantation in AML. Blood 2018; 132(16): 1703–1713. e-pub ahead of print 2018/09/08; doi: 10.1182/blood-2018-02-829911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood 2014; 123(23): 3664–3671. e-pub ahead of print 2014/04/20; doi: 10.1182/blood-2014-01-552984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dohner H, Estey EH, Amadori S, Appelbaum FR, Buchner T, Burnett AK et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood 2010; 115(3): 453–474. e-pub ahead of print 2009/11/03; doi: 10.1182/blood-2009-07-235358 [DOI] [PubMed] [Google Scholar]
- 10.Walter RB, Gooley TA, Wood BL, Milano F, Fang M, Sorror ML et al. Impact of pretransplantation minimal residual disease, as detected by multiparametric flow cytometry, on outcome of myeloablative hematopoietic cell transplantation for acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2011; 29(9): 1190–1197. e-pub ahead of print 2011/02/02; doi: 10.1200/jco.2010.31.8121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grimwade D, Freeman SD. Defining minimal residual disease in acute myeloid leukemia: which platforms are ready for “prime time”? Hematology Am Soc Hematol Educ Program 2014; 2014(1): 222–233. e-pub ahead of print 2015/02/20; doi: 10.1182/asheducation-2014.1.222 [DOI] [PubMed] [Google Scholar]
- 12.Hourigan CS, Dillon LW, Gui G, Logan BR, Fei M, Ghannam J et al. Impact of Conditioning Intensity of Allogeneic Transplantation for Acute Myeloid Leukemia With Genomic Evidence of Residual Disease. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019: JCO1903011. e-pub ahead of print 2019/12/21; doi: 10.1200/JCO.19.03011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129(4): 424–447. e-pub ahead of print 2016/11/30; doi: 10.1182/blood-2016-08-733196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breems DA, Van Putten WL, De Greef GE, Van Zelderen-Bhola SL, Gerssen-Schoorl KB, Mellink CH et al. Monosomal karyotype in acute myeloid leukemia: a better indicator of poor prognosis than a complex karyotype. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2008; 26(29): 4791–4797. e-pub ahead of print 2008/08/13; doi: 10.1200/JCO.2008.16.0259 [DOI] [PubMed] [Google Scholar]
- 15.Sabattini E, Bacci F, Sagramoso C, Pileri SA. WHO classification of tumours of haematopoietic and lymphoid tissues in 2008: an overview. Pathologica 2010; 102(3): 83–87. e-pub ahead of print 2010/12/22; [PubMed] [Google Scholar]
- 16.Mack EKM, Marquardt A, Langer D, Ross P, Ultsch A, Kiehl MG et al. Comprehensive genetic diagnosis of acute myeloid leukemia by next-generation sequencing. Haematologica 2019; 104(2): 277–287. e-pub ahead of print 2018/09/08; doi: 10.3324/haematol.2018.194258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spencer DH, Abel HJ, Lockwood CM, Payton JE, Szankasi P, Kelley TW et al. Detection of FLT3 internal tandem duplication in targeted, short-read-length, next-generation sequencing data. J Mol Diagn 2013; 15(1): 81–93. e-pub ahead of print 2012/11/20; doi: 10.1016/j.jmoldx.2012.08.001 [DOI] [PubMed] [Google Scholar]
- 18.Ivey A, Hills RK, Simpson MA, Jovanovic JV, Gilkes A, Grech A et al. Assessment of Minimal Residual Disease in Standard-Risk AML. The New England journal of medicine 2016; 374(5): 422–433. e-pub ahead of print 2016/01/21; doi: 10.1056/NEJMoa1507471 [DOI] [PubMed] [Google Scholar]
- 19.Kim B, Cho YU, Bae MH, Jang S, Seo EJ, Chi HS et al. The added values of multiplex reverse transcriptase-PCR followed by mutation screening in the initial evaluation of acute leukemia. Int J Lab Hematol 2016; 38(4): 444–453. e-pub ahead of print 2016/06/21; doi: 10.1111/ijlh.12521 [DOI] [PubMed] [Google Scholar]
- 20.Hussaini MO, Mirza AS, Komrokji R, Lancet J, Padron E, Song J. Genetic Landscape of Acute Myeloid Leukemia Interrogated by Next-generation Sequencing: A Large Cancer Center Experience. Cancer Genomics Proteomics 2018; 15(2): 121–126. e-pub ahead of print 2018/03/03; doi: 10.21873/cgp.20070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Au CH, Wa A, Ho DN, Chan TL, Ma ES. Clinical evaluation of panel testing by next-generation sequencing (NGS) for gene mutations in myeloid neoplasms. Diagnostic pathology 2016; 11: 11. e-pub ahead of print 2016/01/23; doi: 10.1186/s13000-016-0456-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant 2009; 15(3): 367–369. e-pub ahead of print 2009/02/11; doi: 10.1016/j.bbmt.2008.12.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bejanyan N, Brunstein CG, Cao Q, Lazaryan A, Ustun C, Warlick ED et al. Predictive value of disease risk comorbidity index for overall survival after allogeneic hematopoietic transplantation. Blood advances 2019; 3(3): 230–236. e-pub ahead of print 2019/01/25; doi: 10.1182/bloodadvances.2018018549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khimani F, Kim J, Chen L, Dean E, Rizk V, Betts B et al. Predictors of overall survival among patients treated with sirolimus/tacrolimus vs methotrexate/tacrolimus for GvHD prevention. Bone marrow transplantation 2017; 52(7): 1003–1009. e-pub ahead of print 2017/04/04; doi: 10.1038/bmt.2017.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood 2005; 106(8): 2912–2919. e-pub ahead of print 2005/07/05; doi: 10.1182/blood-2005-05-2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaplan EL, Meier P. Nonparametric-Estimation from Incomplete Observations. J Am Stat Assoc 1958; 53(282): 457–481. doi: Doi 10.2307/2281868 [DOI] [Google Scholar]
- 27.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. The Annals of statistics 1988: 1141–1154. [Google Scholar]
- 28.Cox DR. Regression models and life‐ tables. Journal of the Royal Statistical Society: Series B (Methodological) 1972; 34(2): 187–202. [Google Scholar]
- 29.Fine JP, Gray RJ. A Proportional Hazards Model for the Subdistribution of a Competing Risk. Journal of the American Statistical Association 1999; 94(446): 496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 30.Grimwade D, Walker H, Oliver F, Wheatley K, Harrison C, Harrison G et al. The importance of diagnostic cytogenetics on outcome in AML: analysis of 1,612 patients entered into the MRC AML 10 trial. The Medical Research Council Adult and Children’s Leukaemia Working Parties. Blood 1998; 92(7): 2322–2333. e-pub ahead of print 1998/09/25; [PubMed] [Google Scholar]
- 31.Byrd JC, Mrozek K, Dodge RK, Carroll AJ, Edwards CG, Arthur DC et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood 2002; 100(13): 4325–4336. e-pub ahead of print 2002/10/24; doi: 10.1182/blood-2002-03-0772 [DOI] [PubMed] [Google Scholar]
- 32.Slovak ML, Kopecky KJ, Cassileth PA, Harrington DH, Theil KS, Mohamed A et al. Karyotypic analysis predicts outcome of preremission and postremission therapy in adult acute myeloid leukemia: a Southwest Oncology Group/Eastern Cooperative Oncology Group Study. Blood 2000; 96(13): 4075–4083. e-pub ahead of print 2000/12/09; [PubMed] [Google Scholar]
- 33.Oran B, Dolan M, Cao Q, Brunstein C, Warlick E, Weisdorf D. Monosomal karyotype provides better prognostic prediction after allogeneic stem cell transplantation in patients with acute myelogenous leukemia. Biol Blood Marrow Transplant 2011; 17(3): 356–364. e-pub ahead of print 2010/06/18; doi: 10.1016/j.bbmt.2010.05.012 [DOI] [PubMed] [Google Scholar]
- 34.Oran B, Jimenez AM, De Lima M, Popat UR, Bassett R, Andersson B et al. Age and Modified European LeukemiaNet Classification to Predict Transplant Outcomes: An Integrated Approach for Acute Myelogenous Leukemia Patients Undergoing Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant 2015; 21(8): 1405–1412. e-pub ahead of print 2015/04/04; doi: 10.1016/j.bbmt.2015.03.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mrozek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2012; 30(36): 4515–4523. e-pub ahead of print 2012/09/19; doi: 10.1200/jco.2012.43.4738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. The New England journal of medicine 2016; 374(23): 2209–2221. e-pub ahead of print 2016/06/09; doi: 10.1056/NEJMoa1516192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paschka P, Schlenk RF, Gaidzik VI, Herzig JK, Aulitzky T, Bullinger L et al. ASXL1 mutations in younger adult patients with acute myeloid leukemia: a study by the German-Austrian Acute Myeloid Leukemia Study Group. Haematologica 2015; 100(3): 324–330. e-pub ahead of print 2015/01/18; doi: 10.3324/haematol.2014.114157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimm J, Jentzsch M, Bill M, Goldmann K, Schulz J, Niederwieser D et al. Prognostic impact of the ELN2017 risk classification in patients with AML receiving allogeneic transplantation. Blood advances 2020; 4(16): 3864–3874. e-pub ahead of print 2020/08/19; doi: 10.1182/bloodadvances.2020001904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Araki D, Wood BL, Othus M, Radich JP, Halpern AB, Zhou Y et al. Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia: Time to Move Toward a Minimal Residual Disease-Based Definition of Complete Remission? Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016; 34(4): 329–336. doi: 10.1200/JCO.2015.63.3826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Buckley SA, Wood BL, Othus M, Hourigan CS, Ustun C, Linden MA et al. Minimal residual disease prior to allogeneic hematopoietic cell transplantation in acute myeloid leukemia: a meta-analysis. Haematologica 2017; 102(5): 865–873. doi: 10.3324/haematol.2016.159343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kongtim P, Hasan O, Perez JMR, Varma A, Wang SA, Patel KP et al. Novel Disease Risk Model for Patients with Acute Myeloid Leukemia Receiving Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2020; 26(1): 197–203. e-pub ahead of print 2019/09/14; doi: 10.1016/j.bbmt.2019.09.006 [DOI] [PubMed] [Google Scholar]
- 42.Walter RB, Gyurkocza B, Storer BE, Godwin CD, Pagel JM, Buckley SA et al. Comparison of minimal residual disease as outcome predictor for AML patients in first complete remission undergoing myeloablative or nonmyeloablative allogeneic hematopoietic cell transplantation. Leukemia 2015; 29(1): 137–144. e-pub ahead of print 2014/06/03; doi: 10.1038/leu.2014.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuurhuis GJ, Heuser M, Freeman S, Bene MC, Buccisano F, Cloos J et al. Minimal/measurable residual disease in AML: a consensus document from the European LeukemiaNet MRD Working Party. Blood 2018; 131(12): 1275–1291. e-pub ahead of print 2018/01/14; doi: 10.1182/blood-2017-09-801498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017; 35(11): 1154–1161. e-pub ahead of print 2017/04/06; doi: 10.1200/jco.2016.70.7091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frassoni F, Labopin M, Gluckman E, Prentice HG, Gahrton G, Mandelli F et al. Are patients with acute leukaemia, alive and well 2 years post bone marrow transplantation cured? A European survey. Acute Leukaemia Working Party of the European Group for Bone Marrow Transplantation (EBMT). Leukemia 1994; 8(6): 924–928. e-pub ahead of print 1994/06/01; [PubMed] [Google Scholar]
- 46.Schmid C, Labopin M, Veelken H, Schaap N, Schleuning M, Stadler M et al. Efficacy, Safety and Long Term Results of Prophylactic and Preemptive Donor Lymphocyte Infusion after Allogeneic Stem Cell Transplantation for Acute Leukemia: A Registry-Based Evaluation on 343 Patients By the Acute Leukemia Working Party of EBMT. Blood 2015; 2015(126:863). [Google Scholar]
- 47.Solomon SR, Sizemore CA, Zhang X, Brown S, Holland HK, Morris LE et al. Preemptive DLI without withdrawal of immunosuppression to promote complete donor T-cell chimerism results in favorable outcomes for high-risk older recipients of alemtuzumab-containing reduced-intensity unrelated donor allogeneic transplant: a prospective phase II trial. Bone marrow transplantation 2014; 49(5): 616–621. doi: 10.1038/bmt.2014.2 [DOI] [PubMed] [Google Scholar]
- 48.Ciurea SO, Schafer JR, Bassett R, Denman CJ, Cao K, Willis D et al. Phase 1 clinical trial using mbIL21 ex vivo-expanded donor-derived NK cells after haploidentical transplantation. Blood 2017; 130(16): 1857–1868. e-pub ahead of print 2017/08/25; doi: 10.1182/blood-2017-05-785659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen YB, Li S, Lane AA, Connolly C, Del Rio C, Valles B et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2014; 20(12): 2042–2048. e-pub ahead of print 2014/09/23; doi: 10.1016/j.bbmt.2014.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pusic I, Choi J, Fiala MA, Gao F, Holt M, Cashen AF et al. Maintenance Therapy with Decitabine after Allogeneic Stem Cell Transplantation for Acute Myelogenous Leukemia and Myelodysplastic Syndrome. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2015; 21(10): 1761–1769. e-pub ahead of print 2015/06/10; doi: 10.1016/j.bbmt.2015.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Lima M, Oran B, Champlin RE, Papadopoulos EB, Giralt SA, Scott BL et al. CC-486 Maintenance after Stem Cell Transplantation in Patients with Acute Myeloid Leukemia or Myelodysplastic Syndromes. Biology of blood and marrow transplantation : journal of the American Society for Blood and Marrow Transplantation 2018; 24(10): 2017–2024. e-pub ahead of print 2018/06/23; doi: 10.1016/j.bbmt.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]


