ABSTRACT
As the prevalence of chronic kidney disease is expected to rise worldwide over the next decades, provision of renal replacement therapy (RRT), will further challenge budgets of all healthcare systems. Most patients today requiring RRT are treated with haemodialysis (HD) therapy and are elderly. This article demonstrates the interdependence of clinical and sustainability criteria that need to be considered to prepare for the future challenges of delivering dialysis to all patients in need. Newer, more sustainable models of high-value care need to be devised, whereby delivery of dialysis is based on value-based healthcare (VBHC) principles, i.e. improving patient outcomes while restricting costs. Essentially, this entails maximizing patient outcomes per amount of money spent or available. To bring such a meaningful change, revised strategies having the involvement of multiple stakeholders (i.e. patients, providers, payers and policymakers) need to be adopted. Although each stakeholder has a vested interest in the value agenda often with conflicting expectations and motivations (or motives) between each other, progress is only achieved if the multiple blocs of the delivery system are advanced as mutually reinforcing entities. Clinical considerations of delivery of dialysis need to be based on the entire patient disease pathway and evidence-based medicine, while the non-clinical sustainability criteria entail, in addition to economics, the societal and ecological implications of HD therapy. We discuss how selection of appropriate modes and features of delivery of HD (e.g. treatment modalities and schedules, selection of consumables, product life cycle assessment) could positively impact decision-making towards value-based renal care. Although the delivery of HD therapy is multifactorial and complex, applying cost-effectiveness analyses for the different HD modalities (conventional in-centre and home HD) can support in guiding payability (balance between clinical value and costs) for health systems. For a resource intensive therapy like HD, concerted and fully integrated care strategies need to be urgently implemented to cope with the global demand and burden of HD therapy.
Keywords: haemodialysis, informed-decision making, sustainability, value-based healthcare
DELIVERY OF DIALYSIS BEYOND 2021
The burden of chronic kidney disease
Chronic kidney disease (CKD) is now one of the major non-communicable conditions worldwide [1]. Patients reaching the final stages of CKD (end-stage renal disease, ESRD) require renal replacement therapy (RRT), with a striking disparity of access worldwide in terms of the gap between needed and delivered RRT [2, 3]. If kidney transplant is not possible, the options are dialysis therapy, of which haemodialysis (HD) is preferred for most patients, or conservative care [4–6]. CKD is associated with adverse health outcomes, poor health-related quality of life (HRQoL) and disproportionately high costs. It is estimated that between 5.3 and 10.5 million people worldwide require dialysis or transplantation, although many do not receive these treatments due to lack of resources or financial barriers, especially in low income countries [4, 7–10]. Even high-income countries with established healthcare systems struggle to cope with the high (lifelong) costs of CKD and dialysis [11].
Prevalence and mortality from diseases or injuries is analysed in terms of summary measures of years of lost life (YLL), years lived with disability (YLD) or disability adjusted life years (DALYs), and healthy life expectancy [12]. The Global Burden of Diseases, Injuries and Risk Factors Study (GBD) provides a systematic scientific assessment of data on incidence, prevalence and mortality for a large list of diseases and injuries [13]. Although global health has steadily improved over the past 30 years as measured by the age-standardised DALY rates, CKD is now among the 10 most important drivers of increasing disease burden (i.e. the causes that had the largest absolute increases in number of DALYs between 1990 and 2019). CKD is one of the six causes that largely affect older adults (the others being ischaemic heart disease, diabetes, stroke, lung cancer and age-related hearing loss) [13]. Over a period of just under 30 years, CKD has come to be amongst the 10 leading causes of DALYs in the age groups 50–74 years and 75 years and older; CKD and diabetes are the only diseases in the top 10 causes in these two age groups showing an increase in age-standardized DALY rates (Table 1).
Table 1.
The increase in the prevalence (and burden) of CKD from 1999 to 2019 [13]. CKD is now among the 10 leading causes of death in both the 50– to 74-year and 75-years-and-older age groups according to the GBD 2019, injuries and risk factors study. DALYs, disability-adjusted life years due to 369 diseases and injuries for two sexes and for 204 countries and territories
| 1999 | 2019 | |||||
|---|---|---|---|---|---|---|
| Age group (years) | Position in leading 25 causes of death | % of DALYs 1999 | Position in leading 25 causes of death | % of DALYs 1999 | % Change in numbers of DALYs (1999–2019) | % Change in age-standardized DALY rate (1999–2019) |
| 10–24 | – | – | – | – | ||
| 25–49 | 21 | 1.3 (1.2 to 1.4) | 18 | 1.6 (1.4 to 1.8) | 67.3 (53.9 to 80.3) | 0.7 (–7.3 to 8.4) |
| 50–74 | 14 | 1.6 (1.4 to 1.7) | 8 | 2.3 (2.1 to 2.5) | 130.2 (113.0 to 145.6) | 12.1 (3.7 to 19.5) |
| ≥75 | 14 | 1.6 (1.5 to 1.8) | 9 | 2.5 (2.3 to 2.7) | 196.0 (173.9 to 211.1) | 21.6 (12.6 to 27.4) |
Values in columns 3, and 5-7 represent mean (range).
The prevalence of CKD is expected to rise across the world over the next decades, driven mainly by an ageing population and an increasing prevalence of diabetes and hypertension, the two main causes of renal failure [14]. Provision of RRT will further challenge most countries already facing healthcare budget restraints made worse by the current COVID-19 pandemic [15]. Moreover, dialysis therapies consume valuable and limited natural resources such as water and energy, and generate vast amounts of plastic disposable waste; ecological considerations are today an essential component of the chronic care setting [16]. Thus, the overall burden of CKD is higher compared with other chronic conditions and is projected to increase even further.
Himmelfarb et al. have recently reviewed the dilemma in alleviating the burden of disease both for the patient and healthcare systems struggling to cope with the exorbitant costs associated with the provision of dialysis [4]. Faced with the rapidly increasing prevalence of CKD, in this review we consider the interdependence of the clinical and sustainability criteria that need to be considered together to prepare for the future challenges of delivering dialysis [17–19]. Concerted treatment strategies and healthcare policies that incorporate at the outset the progression pathway of CKD and the comorbid conditions of the individual patient are urgently needed [1, 15, 20]. For those patients having reached end-stage CKD and dependent on regular therapy, newer, more sustainable models of high-value care need to be devised. We discuss how selection of appropriate modes of delivery of dialysis (treatment modalities, selection of consumables and machine systems), guided by health economics principles (value-based healthcare, VBHC) and ecological considerations, could positively impact both health outcomes in dialysis (based on evidence-based medicine, EBM) and long-term fiscal sustainability of providing dialysis [20–22].
The tenets of value-based healthcare and haemodialysis
The core tenet of VBHC is maximizing value for patients: achieving the best clinical outcomes at the lowest cost [23, 24]. The concept of linking patient outcomes to costs, particularly applicable to chronic conditions, was first described as a fundamentally new strategy by Porter [25, 26].
Another way to consider VBHC is that its goal is the ‘financially efficient delivery of quality’. Most models essentially consider one of three outcomes: improve quality while reducing cost, maintain quality while reducing cost or improve quality while maintaining cost. While the first is the most desirable it is hard to achieve, while in cost-conscious healthcare environments the second option is favoured over the third. All three are nevertheless centred around the value equation, i.e. value = quality (outcome)/cost [27].
Every healthcare system worldwide is struggling with the spiralling costs of medical care driven principally by high and rising prices of products and services [28, 29]. The burgeoning burden of diseases—and increased need for care—is further propelled by the dichotomous growth in incidence of diseases caused by abject poverty and poor nutrition on one hand and affluent lifestyle-induced conditions on the other. The increase in obesity for example in high-income populations globally is causally linked to the increase in diabetes, cardiovascular disease and hypertension—all causes of CKD [30]. Even as global health has improved for certain non-communicable diseases, the burden of healthcare expenditure and demands for health services continues to rise, with the ageing global population having disabling conditions adding to the burden of care [30]. Healthcare delivery has long been a supply-driven system centred around the work of physicians but is now transforming towards a patient-centred system around the needs of patients. The shift from volume and profitability of services provided to patient outcomes achieved, within the allocated budgets or financial restraints, is now the overarching health-delivery strategy adopted by healthcare systems, to cope with future needs [31, 32]. For CKD and HD, both components of the VBHC equation, i.e. outcomes and costs, are essentially unfavourable, reinforcing the need for transformative changes into higher value dialysis delivery and care with sustainable costs [33].
Outcomes in haemodialysis are generally acknowledged to be sub-optimal
Several investigations attest to the unsatisfactory outcomes of ESRD patients on HD therapies even after accounting for significant differences in dialysis practice patterns [4, 34–41]. For most ESRD patients worldwide treated with in-centre HD, overall survival is poor, but longer in some Asian countries than elsewhere in the world and longer in Europe than in the USA, although this gap may have reduced [42–44]. The poor outcomes have been attributed to diverse factors and documented in literature and will not be detailed here, but these do reflect the complexity of CKD and the difficulty in its effective treatment with different treatment strategies [38, 45–49].
Costs of haemodialysis therapies are regarded to be too high
Costs of CKD care and HD worldwide are high and will continue to rise, putting strains on patients as well as on healthcare system budgets [4, 25–29, 50–55]. Lifelong dialysis therapy is required for ESRD patients not able to receive a transplant; some patients may need dialysis for up to three decades or more. The increase in prevalence of CKD and ageing populations means more elderly dialysis patients and an increased burden on high-income countries [4, 56, 57]. Estimated annual costs for thrice weekly four-hour therapy vary among countries according to their overall economic strength (gross domestic product), with some being over US$100 000 per patient in some countries. In the European Union, 2% of the healthcare expenditure (representing about €15 billion) per year is devoted to the care of some 358 000 ESRD patients [58, 59].
Multiple stakeholders need to be considered and incorporated in the delivery of VBHC
Transforming healthcare delivery strategy that deals with both clinical and non-clinical considerations necessitates involvement of multiple categories of stakeholders (i.e. patients, providers, regulators and payers) [36]. Figure 1 presents the main categories of the stakeholders whose collective participation is required at different levels of the care delivery processes [22, 27]. Each stakeholder has a vested interest in the value agenda and with conflicting expectations and motivations (or motives) between each other, progress is only achieved if the multiple blocs of the delivery system are advanced as mutually reinforcing entities. An enabling information technology platform is vital as it ties together the other components of the healthcare delivery system into an independent and interconnected delivery system facilitating communication as well as transparency amongst the stakeholders [23]. In the USA, two additional issues emerge: firstly, all existing VBHC programs operate alongside transaction fee for service programs, thereby placing providers in a difficult position because the financial incentives are at odds. Secondly, evidence suggests that social determinants of health play a major role, and in countries like the USA that spend substantially more on healthcare than on social services there are concerns that ‘medicalization’ of social determinants of health may lead to wrong outcomes [60].
FIGURE 1:
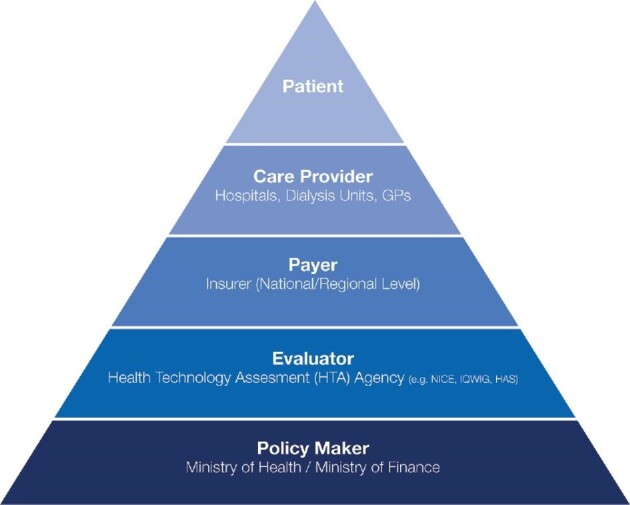
Transformation to a high-value care delivery system has physicians and provider organizations taking the lead, but each of the other stakeholders—including the patient—has a role to play in improving the value of care and hastening transformation by aligning incentives across stakeholders for mutual benefit. GP, general practitioner; IQWIG, Institute for Quality and Efficiency in Health Care; HAS, Haute Autorité de Santé (or French National Authority for Health).
DECISION-MAKING FOR DELIVERY OF HAEMODIALYSIS: TWO SIDES TO THE COIN
Delivery of HD therapy has traditionally been the domain of the nephrologists (or advanced practitioners under the supervision of nephrologists), relying primarily on clinical criteria [37, 61–63]. Based on the experience accumulated over years in treating dialysis patients and recommendations from guidelines (e.g. KDIGO, European Best Practice Group and Kidney Disease Outcomes Quality Initiative) prepared by groups of experts and physicians strove towards standardized therapy for all patients with ESRD [36, 41, 64, 65]. This approach of optimizing dialysis delivery for all patients irrespective of their constitution, comorbid conditions or age and was necessary in the developmental stages of dialysis before several variables, issues and controversies arose [37]. Additionally, the procedure of HD itself is technologically complex and the quality of the detoxification (removal of uraemic toxins and fluid) depends on several procedure-related factors and permutations [66, 67]. Membrane type (flux and blood compatibility), dialyser surface areas, blood and dialysis fluid flow rates, electrolyte and buffer prescription, fluid removal rates and targets, type and dose of anticoagulant required for the extracorporeal circuit and effects of numerous medications required to correct uraemia or associated imbalances created by the procedure are just some of the considerations for each treatment session. If diverse comorbid conditions (and their severity) are considered together with modality options (low- and high-flux, online haemodiafiltration, etc.) and treatment regimens (of variable duration and frequency), the goals of devizing standardization of dialysis practices was understandable. We now know that such an approach has had limited success in improving patient outcomes, which remain unsatisfactory [68].
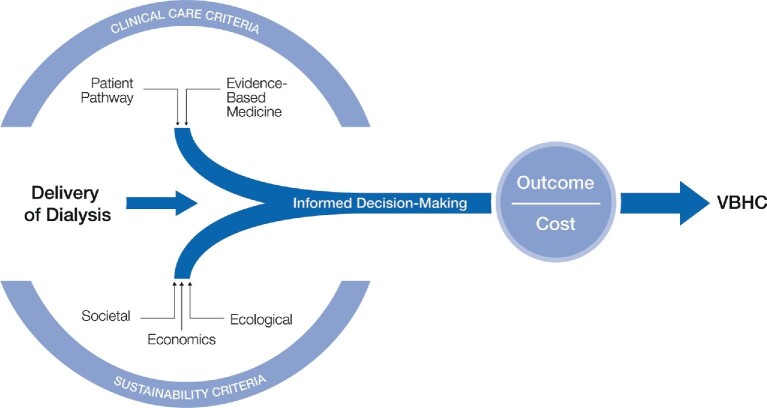
The alternative to the current one-size-fits-all optimized or standardized therapy delivery is to move towards a value-for-money and personalized approach [36, 68]. For this more patient-centric and flexible form of delivery accommodating for individualized targets, the responsibility of achieving better outcomes at reduced costs lies with all stakeholders [36]. Informed decision-making is crucial to the successful implementation of all facets of value-based delivery of dialysis, not just for physicians who rely on the principles of EBM (on which guidelines are based) but for all stakeholders and policymakers [69]. Provision of value-based care, considering both clinical and sustainability criteria, needs to be based on robust evidence or data to support informed decision-making (Figure 2). Sustainable HD within the VBHC framework in the long-term is not just about keeping the costs of therapy in check, but is also about managing societal burden and ecological impact of HD therapy [4, 68].
FIGURE 2:
Delivery of personalized medicine and VBHC necessitates evidence-informed decision-making, not just by physicians, but also by policymakers and stakeholders. Successful implementation of VBHC (outcomes divided by costs) is achieved by assessing evidence and data for clinical outcomes and sustainability decision-making.
Clinical care criteria for evidence-informed health policymaking for value-based care
Clinical decisions according to the patient pathway of CKD
The KDIGO classification stages based on glomerular filtration rate (GFR) and albuminuria are commonly used to define, identify or predict the progression of CKD [64]. However, the true prognosis of CKD for an individual patient is variable as underlying cause of disease and other risk factors, e.g. elevated blood pressure (BP), hyperglycaemia, dyslipidaemia, smoking, obesity, history of cardiovascular disease, ongoing exposure to nephrotoxic agents as well as age, gender and ethnicity, determine the patient-specific progression pathway of CKD [70]. Management of progression and complications of CKD are highly complex and not always easily fulfilled by the prescription of standardized protocols or formal recommendations from guidelines [21, 70].
With the burden of CKD increasing, future long-term sustainability cannot be achieved by creating value only for end-stage CKD when patients are on RRT. An inherently systemic and complex condition like CKD can only be managed effectively if the entire patient disease pathway is considered and managed cohesively, from the initial occurrence of symptoms backed by diagnosis of disease (GFR/albuminuria), to its progression until renal function has deteriorated such that RRT is indicated [68, 71]. While recognizing the fact that only a small proportion of CKD patients will progress to ESRD and require RRT, disease management according to the stage of CKD and overall condition of the patient is a cost-effective strategy to reduce the healthcare burden of renal disease [72]. For example, early management of CKD (together with comorbid conditions) when GFR stages G3a/G3b are reached will delay progression of disease to CKD stage G5 and alleviate the burden of disease for patient, provider and healthcare systems [18, 73]. Effectively managed, such a strategy increases the probability of better long-term outcomes and a significant reduction in overall costs throughout the course of disease [33]. By providing the appropriate treatment according to the individual, changing clinical needs at each stage of the disease, those patients eventually requiring dialysis are prepared in advance about the therapy (options) through informed and shared decision-making [74–76].
Evidence-based medicine and real-world evidence for decision-making
Decision-making guided by the best possible scientific and medical evidence is the cornerstone of clinical practice. Of the three domains that constitute the ideals of EBM, i.e. clinical expertise, patient-centered values and relevant scientific evidence, only the last of these facets is usually acknowledged [77]. Clinical guidelines and recommendations rely solely on published scientific data, with emphasis on evidence from prospective randomized controlled trials (RCTs) on which systematic reviews or metanalyses acquire their ultimate validity. In nephrology and HD, the majority of RCTs display negative outcomes, yet such studies still carry the weight of opinion within the scientific community despite some trials being based on flawed or biased methodologies [78–82]. Even metanalysis and systematic scientific reviews from critically appraised sources such as the Cochrane organization are contentious and have revealed methodologically inconsistencies [83, 84]. The overemphasis on EBM and some of its negative unintended consequences need to be acknowledged; the dogma that RCTs are of absolute necessity has been challenged [85, 86].
The scarcity of data from sound clinical trials, much needed as they are, is unlikely to be adequately filled to guide the improvement and future direction of dialysis therapy or its provision, especially in low income countries [73, 87]. As long ago as 2004, the Cochrane Renal Group reported fewer published RCTs in nephrology than any other subspecialty of internal medicine [81, 86]. The logistical effort and financial restraints in a climate of stringent ethical and regulatory control has all but halted the desire to conduct large RCTs to resolve clinical issues requiring validation or further proof. Other than some studies initiated by industry, few HD-related RCTs are currently in the pipeline. Real world evidence (RWE) is an alternative to data from RCTs that is either not available at all to address specific issues, or, confounded by virtue of being inadequately powered (e.g. small sample size) or selection (inclusion/exclusion criteria) and information bias. RWE has been widely accepted for decades as supporting evidence particularly for safety monitoring, with regulators today being increasingly open to real world data where RCTs are not feasible. For example, health technology assessment (HTA) agencies (e.g. National Institute for Health and Care Excellence, NICE) have processes in place to include data on a case-by-case basis where a clinical–economic benefit is foreseeable [88].
With the imperfect nature of most RCTs and estimated costs (in 2009) of conducting an RCT reaching US$15 000 per participant, the validity of alternative sources of clinical evidence is being reassessed by regulators [86]. The major dialysis providers have implemented patient-centered holistic care models coordinated by a physician-led network and routinely acquire data from every patient for each treatment session. In striving to enhance patient experience and long-term outcomes by applying clinical best practices, CKD management tools, data scorecards, a wealth of real-situation information on diverse areas of HD care is available [89–91]. Derived from tens of thousands of patients and millions of treatment sessions over a period of several years that RCTs are unable to match, RWE is considered to provide more meaningful insights towards the effective management and personalization of HD therapy as well as the complex comorbid conditions of CKD patients [92]. RWE can be analysed in low-income countries where RCT data are less readily available. The merits of real evidence-based medicine (rEBM) where the ethical care of the patient is the top priority as emphasized by Greenhalgh et al. need to be considered by policy makers [85]. Usable as well as robust rEBM is now the most feasible option to mitigate the oft-cited concerns regarding the suboptimal clinical outcomes of HD patients and ensure the future sustainability of the HD therapies.
Sustainability criteria for decision-making in value-based healthcare
Economic criteria for delivery of haemodialysis
Even in the early pioneering and developmental stages of HD it was apparent that provision of routine dialysis would incur high costs particularly as it would be needed at regular intervals throughout the patient's life [93]. In the 1960s in the USA, a committee decided who should receive HD, and payments were required—from those patients who could afford it—in advance [52]. At the outset it was recognized that clinical decision-making and delivery of dialysis are intricately linked with costs and profitability for it to be sustainable. In fact, the Kt/V concept, based solely on urea kinetics) and devised primarily as a measure of the dialysis dose and adequacy of treatment, became an instrument to reduce treatment times preferred by patients, or to allow treatment of more patients [94]. By increasing blood flow rates or using larger surface areas dialysers to increase urea clearance, weekly Kt/V target values (for three sessions per week) could mathematically be met, reducing treatment duration from 4 to 3 h [94]. It has since been established that rapid removal of fluid from blood within a short time is a highly unphysiological stress situation for the patient (as refilling from extracellular fluid is not equally fast) and responsible for intradialytic hypotensive episodes often requiring hospitalization—an important cost factor. The dilemma of having to balance clinical practices and economic considerations remains and is expected to be even more challenging in the future.
Cost drivers of CKD care and cost components per treatment session
Healthcare providers in each country have their own system of analysing key variables involved in the overall care of patients with CKD and for those who eventually require regular dialysis therapy. A trade-off between cost containment and quality is an objective for all diseases but particularly necessary for dialysis that consumes disproportionately higher resources per patient over their treatment lifetime [95–97]. It is not the objective of this communication to examine the influence of multiple covariates on costs (which vary according to regions, countries, demographics, comorbid conditions, and types of treatment and services provided) related to HD, but to examine the key cost drivers. Table 2 reflects a combination of healthcare provider service delivery costs and costs incurred by purchasers in healthcare, although in some countries like USA, unit costs are the principal driver.
Table 2.
The principal cost-incurring components involved in the care of CKD patients. The components go beyond clinical considerations (therapy, medications, hospitalization). The overall cost equation includes several other components that healthcare providers need to consider when making decisions for the most appropriate delivery of CKD care
| Cost driver category | Components |
|---|---|
| Infrastructure | Physician/nurse fees; unit (outpatient) setting; catering |
| HD treatment | Disposables (dialyser/tubing set); solutions; machines/equipment |
| Medications | Heparin; various classes of medications (including for comorbidities) |
| Hospitalization | Adverse episodes; cardiovascular complications; vascular access issuesa |
| Transport | Six trips a week |
| Diagnostics, lab testing | Regular blood monitoring; dialysis efficiency measures; dialysis fluid safety |
| Waste disposal | Packaging material; ‘contaminated’ dialysers; tubing sets; dialysis fluids |
Vascular access is one-off cost.
Improving the management of CKD patients both before and after the onset of dialysis treatment as well as their comorbid conditions can potentially reduce redundancies and leads to cost savings [55]. In a budget constraint environment, it is apparent from Table 2 that each component listed could contribute to cost savings by improving efficiencies or reducing waste. Cost optimization processes, if conducted injudiciously without due consideration to the physiological and medical consequences, are often to the detriment of the patient who then receive suboptimal therapy leading to poor outcomes. The use of Kt/V as an instrument to reduce treatment times compromised patient well-being has already been described [94, 98]. Such measures often have quite the opposite effect in that they incur higher total cost of care for every patient in the long run.
The dialyser costs example: impact of specifications on tenders and reimbursement
The dialyser, and the membrane within, is not only the centrepiece of HD therapies to lower concentrations of uraemic toxins but also the focal point from a cost perspective. Dialyser performance specifications equate to the efficiency of the detoxification processes and together with other criteria are considered to impact clinical outcomes [99, 100]. An enormous volume of literature deals with the effect of dialysers (particularly the membrane) on patient morbidity, adverse events and mortality [101, 102]. Dialyser specifications and choice are thus important from the clinical as well as a business perspective as they have a bearing on the price manufacturers can ask for in a highly competitive field. Although dialyser costs may in some countries be a fraction of the overall treatment costs, they are nevertheless a cornerstone of reimbursement, decision-making for tenders and overall sustainability of HD and thus receive more attention.
Public procurement procedures list several dialyser criteria that are conventionally part of the product specification sheets to facilitate clinical decision-making to meet the needs of patients to achieve the best possible outcomes. However modern calls for tenders still include outdated requirements that are either irrelevant for current clinical practices, or, have questionable medical value without sound scientific evidence. For example, the ultrafiltration coefficient (KUF; mL/min mmHg) still features prominently in data specification sheets and tender requirements [103]. The KUF value is a measure of the efficiency of dialysis: higher values indicate lower transmembrane pressure (TMP) to achieve the same rate of ultrafiltration (fluid removed) [104]. The parameter is redundant in modern dialysis delivery terms: all dialysis machines automatically adjust the TMP (unlike manual settings of the past) to achieve the desired ultrafiltration rate to remove the volume of fluid a patient has accumulated during the interdialytic period [105]. As Ronco and Clark describe, few clinicians today have an appreciation of KUF as a measure: it is simply not needed for clinicians [106]. Another example of questionable clinical value is the inclusion in specification sheets of data on substances suggested for removal during dialysis but have no relevance in terms of uraemic toxicity. For example, free kappa- or lambda-light chains are elevated in certain forms of cancer effectively and negated by chemotherapy; in the small number of patients with myeloma, dialysis is used as a supportive therapy in intensive care settings [107–109]. Even though free kappa- or lambda-chains are not considered uraemic toxins in chronic HD, they are being introduced into tenders as marketing ploys for manufacturers seeking unique selling proposition.
Such tactics that go unchallenged add extra financial burden on healthcare systems and have a negative impact on the sustainability of dialysis therapy that is perceived as low value-for-money form of chronic care by payers. To facilitate evidence-informed clinical decision making, dialyser performance specifications and selection must (i) relate to clear medical needs of dialysis patients (achievable blood flow according to vascular access) and (ii) be defined objectively, without bias or ambiguity [99, 110, 111]. Incorrect selection of dialysers based on specifications having little value to CKD or that are scientifically redundant leads eventually to poor outcomes and incurs unnecessary costs to healthcare systems. Many procurement procedures are outdated with specifications having questionable clinical value, but are included either from a historical perspective or without due scrutiny. Procurement and reimbursement decisions need to be made based on evidence and relevant data.
Societal criteria for delivery of haemodialysis
The substantial societal costs associated with treatment of CKD and ESRD are predominantly discussed in monetary terms [50]. While it is patently clear that sustaining life artificially for several years or even decades is associated with costs that have eventually to be borne by the ‘society,’ i.e. the country and healthcare system the patient is part of, there is a much broader impact of the cost of a disease that has no cure (in terms of reversing failed kidney function). Medical ethics and costs are intertwined: the entire endeavour of striving to improve the suboptimal outcomes of patients by prolonging survival rates is proactively adding a financial burden to society or healthcare at large. The same is achieved in attempting to correct inequity of access to dialysis therapies in low-income countries where unaffordability of RRT leads to the deaths of millions of people annually [4]. Making dialysis available to sustain life of ESRD patients increases per se the cost burden to individuals, insurers or governments.
There is, however, a humanistic ‘cost’ of being on dialysis therapy. The unphysiological nature of intermittent dialysis that induces circulatory stress, both haemodynamic and non-haemodynamic, results in symptoms that impact the QoL of the patients [112–116]. Their collective day-to-day stress is extremely debilitating both physically and psychologically, contributing to the well-documented poor HRQoL measures [117]. Implementation of patient-reported outcomes measures (PROM) are important to understand the patient perspective of dialysis care and are increasingly being regarded as highly valuable instruments for quality improvement and optimization of CKD care. Six PROM domains for determining the HRQoL have been proposed for patients with CKD: general HRQoL reflecting overall burden and well-being, pain, fatigue, physical function, depression and daily activity [27]. These can be tracked by a variety of tools each of which has its own merits and limitations and recently summarized by Verberne et al. [27]. Because of the burden associated with practicalities of data collection and their processing, the main challenge is to convince various stakeholders of the significance of such patient-centric value-based outcome measures in an increasingly cost-conscious environment. The medications (pill) burden of patients to combat the effects of not only the uraemic syndrome but also other conditions associated with renal failure and dialysis therapy is an impediment to patients in terms of poor QoL. Family members of patients often must make cost-impacting adjustments to their lives, especially for elderly dialysis patients. Inability to work is a loss to society especially when patients have trained as professionals in areas with shortage of workforce (e.g. healthcare). Walker et al. describe a number of rarely reported socio-economic considerations such as employment retention, housing and other out-of-pocket expenses patients incur when making treatment choices [118, 119].
Ecological criteria for delivery of haemodialysis cannot be ignored anymore
Living in times of global warming, with the impact of pollution and awareness that resources like water and energy from mineral oil are limited, provision of healthcare today has to be achieved with minimal damage to the environment [52]. As the annual increase in the number of patients on HD corresponds to the growth (∼6%) in the prevalence of CKD, a parallel increase in the ecological burden the therapy creates is to be expected. It is evident that both peritoneal dialysis and HD, whether provided in-centre or at home, will create a higher need for natural resources [120]. Moreover, a greater generation of plastic waste from disposable products such as solution bags, tubing sets and dialysers is to be expected [2]. After treatment, dialysers, tubing sets, used dialysis fluids, syringe needles, gloves, etc., all of which are classified as biological waste (and potentially hazardous), need to be disposed of separately and safely involving protocols that are both stringent and costly [16]. Together with manufacturers, dialysis care providers have taken initiatives that focus on reducing water- and energy-consumption and the overall carbon footprint along the entire life-cycle of each product (development, production, transport, storage, application and waste management) as well as during dialysis care (facility heating, air-conditioning, lighting, waste disposal and investment in new technologies that are water and energy saving) [6, 121, 122].
The scale of the problem facing healthcare systems is best exemplified by examining the environmental impact of the dialyser, the central element of each HD session. If around 3.2 million patients worldwide receive thrice weekly HD therapy, some 500 million dialyser units are produced—and disposed of annually. In an assessment of the overall environmental impact of dialysers during each stage of their product life cycle, inputs and outputs from the initial stages of acquisition and transport of raw materials to manufacturing, distribution, usage and product end-of-life management were compiled and evaluated (Table 3). According to the impact categories defined by ISO (reference CH) 15 product life cycle assessment values were applied to compare a modern environment-friendly dialyser with its predecessor model manufactured by less environment-conscious manufacturing processes. The recent model (FX-Class of dialysers, Fresenius Medical Care, Germany) performs 42% better than its predecessor, thereby greatly reducing the (negative) environmental impact. Moreover, as the recent model has a housing made from lighter material (polypropylene) compared with its predecessor that had a heavier polycarbonate housing, the reduced weight of the dialyser positively impacts the carbon footprint during its transport. Another example of efforts to reduce the impact on the environment of dialysers relates to creating devices with novel design features. By creating micro-undulations along the length of each fibre, uniform dialysis fluid flow around each fibre in the bundle provides high performances (clearance) at reduced dialysis fluid flow rates, leading to significant savings in terms of reduced water usage [123].
Table 3.
Life-cycle assessment (LCA) of dialysers to evaluate the potential environmental impact during their life cycle. Beginning with acquisition of resources, transport of raw materials, manufacturing, distribution, usage and end-of-life management (safe waste disposal), all stages are considered (EN ISO 14040/44: 2006). The 15 environmental impact categories are grouped into five areas. The LCA of FX class dialysers (environment awareness manufacturing) is shown in relation to its predecessor model (housing made from polycarbonate). On average, the FX class dialysers showed a 42% improvement in terms of reduced impact on the environment
| Performance improvement versus reference for individual categories (%) | Overall performance improvement | ||
|---|---|---|---|
| Human health preservation | Human toxicity, non-cancerous effects | 50 | 42% |
| Human toxicity, cancerous effects | 44 | ||
| Ionising radiation | –39 | ||
| Terrestrial conservation | Acidification | 54 | |
| Terrestrial eutrophication | 54 | ||
| Water protection | Freshwater eutrophication | 51 | |
| Marine eutrophication | 52 | ||
| Freshwater ecotoxicity | 44 | ||
| Atmosphere protection | Photochemical ozone formation | 53 | |
| Particulate matter | 66 | ||
| Ozone depletion | 21 | ||
| Climate change | 52 | ||
| Resource conservation | Land use | 35 | |
| Mineral, fossil and renewable resource depletion | 34 | ||
| Water resource depletion | 51 |
Such product life-cycle assessments, carried out for each component of dialysis, enable identification of environment-influencing stages where even minor optimization steps could contribute towards the long-term ecological sustainability of every aspect of delivery of dialysis [124]. Certain areas of dialysis that adversely affect the environment and add unnecessary fiscal burden for the therapy, however, have yet to addressed adequately [20]. For example, the environmental impact of the pills (∼20–30 pills per day, required lifelong) prescribed to patients is seldom considered. The contribution of large number of drugs used or their metabolites to the municipal sewage systems and ground water may be significant. And as compliance with medications is only ∼50%, it can be presumed that a significant proportion of the unused drugs and supplements enter normal household waste and enter into the environment. The unused medications prescribed to patients also have two implications: firstly, about half of the substantial costs incurred for the prescription of the medications are unnecessary, and secondly, patient non-compliance contributes to poorer outcomes as suboptimal dosage does not have its intended effect of correcting the condition the physician intended. While payers cover all aspects of HD therapy, nephrologists, who prescribe dialysis modalities and medication, are reimbursed for performing the dialysis treatment itself, but the costs of medication in some countries, unlike in the USA, are not covered within this reimbursement. The payment for medication comes from a separate budget and goes to pharmacies.
VALUE-BASED RENAL CARE
Cost effectiveness and willingness to pay for therapies
Nephrologists today need to select from dialysis therapy options based not only on clinical criteria but on economic considerations as well [125, 126]. The choice of the most appropriate therapy for the patient is primarily made on the strength of published evidence (EBM) particularly based on RCTs, systematic reviews and meta-analyses, all of which provide the basis for guidelines recommended by groups of experts. The economic evaluation of therapies carried out by three types of methods (cost-effectiveness, cost-minimization and cost–benefit analyses) is often challenging from the standpoint of the clinician, who remains an important stakeholder in decision-making [127]. While each of these approaches has its own merits and limitations and involves different health benefit metrics, the relative usefulness of each approach is not immediately obvious to clinicians, whose primary focus is on clinical matters than on economic emphasis [50, 118].
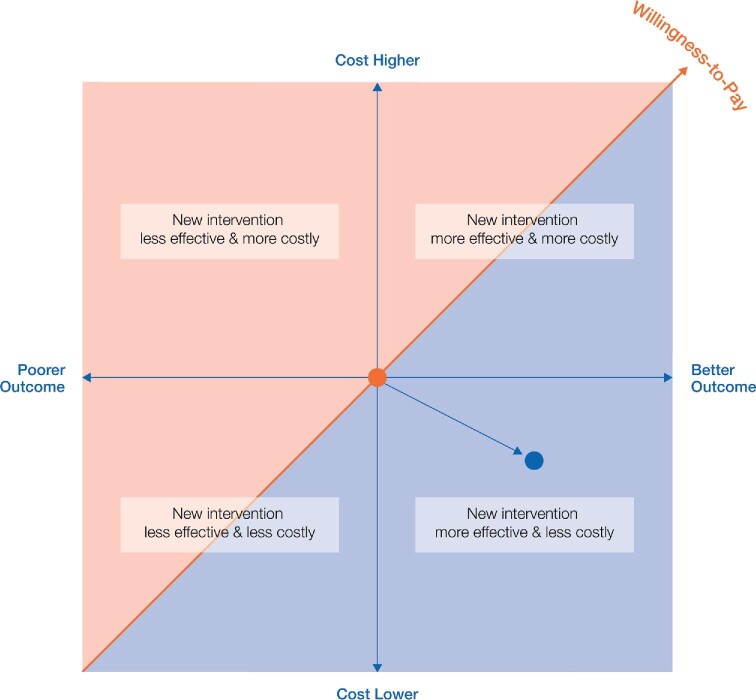
Typically, comparative cost-effectiveness is captured as cost per quality-adjusted life year (cost/QALY). The willingness-to-pay concept is a pragmatic way to assess the health benefit (i.e. outcome-based EBM) in monetary terms for cost–benefit analysis, the most common form of economic evaluation [73]. It allows assessment of the value of a new intervention on a plot of costs (y axis) against clinical (health) effectiveness or outcomes (x axis); frequently, the comparator (‘current standard of care’) is plotted at the origin [128, 129]. In Figure 3, the graph illustrating that the ‘willingness to pay’ of a health system for additional value a new intervention is delivering, runs diagonally across the four quadrants. If the intervention is above the line (red region) it is scrutinized by payers as being unfavourable. Payers prefer (new) interventions that either deliver same outcomes at lower costs, or even higher outcomes at same costs. The methodology allows assessment of clinical effectiveness of the therapy relative to costs; significantly, it is centred around the foundations of EBM decision-making that clinicians are familiar with [130]. For CKD and HD, the International Consortium for Health Outcomes Measurement (ICHOM) CKD Working Group proposes hospitalization and cardiovascular complications as key outcome measures as they are significant cost-increasing factors [27].
FIGURE 3:
The cost-effectiveness plane, showing that ‘willingness to pay’ is one of the most important payer tools to assess the value (cost effectiveness) of an intervention. The fundamental premise of VBHC is that if value improves, patients, payers, providers and suppliers can all benefit while the economic sustainability of the healthcare system increases.
Cost-effectiveness of more intensive and home haemodialysis treatment schedules
Improving outcomes by changing HD practice patterns was recognized in the formative years of dialysis and has been advocated ever since [131]. The conventional thrice weekly 4 h treatment regimen used for most HD patients is a compromise between adequate dialysis and affordability; regardless of the causes, the uraemic state persists in many patients even when reaching their dialysis adequacy targets and a typical dialysis patient faces a life of poor quality and a significantly shortened survival. More intensive HD could improve patient outcomes [132–135]. HD can be intensified by increasing duration (time), frequency (number of sessions/week) or both. Alternatively, dialysis efficiency can be increased without changing duration or frequency by selecting more efficient modalities (e.g. haemodiafiltration, HDF). All intensification methods increase removal of small solute, but not of certain larger molecular weight uraemic toxins whose removal is time dependent. Due to their compartmentalization within the body, the kinetics of removal of β2-microglobulin and phosphate for example is slower than urea clearance (used to calculate Kt/V) [136, 137].
Several dialysis delivery strategies have been considered and implemented in recent years focussed mainly towards improving patient outcomes [34, 41, 68, 130]. Modalities such as short daily or long intermittent and quotidian nocturnal HD have been reported to be associated with a variety of clinical improvements, as well as improvements in QoL and lower standardized mortality ratio [116, 138–140]. We examine the health economic implications of such strategies with some examples.
Cost effectiveness of intensive or home versus conventional haemodialysis
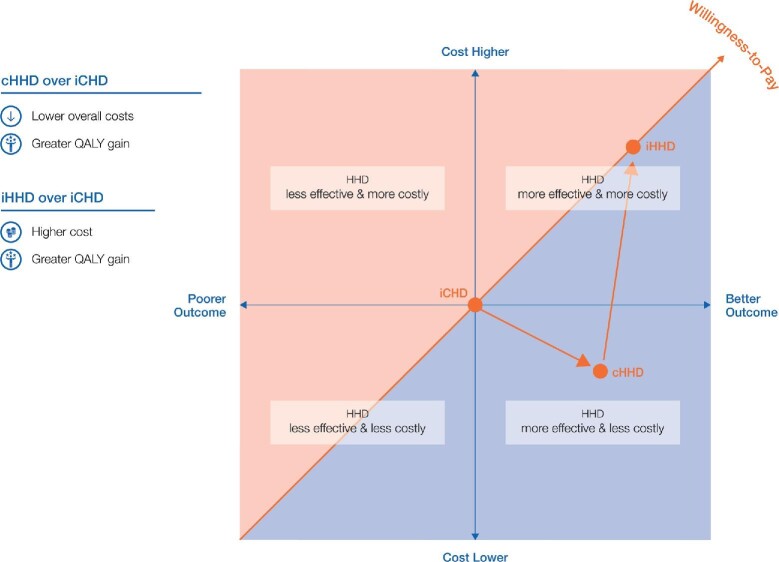
A cost-effectiveness analysis can be used to compare conventional in-centre dialysis (iCHD) with conventional home haemodialysis (cHHD), having the same duration (4 h sessions) and frequency (thrice weekly). In Figure 4, iCHD as the current the ‘current standard of care’ is at the origin of the plane. Moving patients from in-centre to home HD (but maintaining the time and duration of treatment) would result in cost savings (no transport required, or less cost related to nurse and physician time) and would thus be positioned below the point of origin (iCHD) on the y axis. As a better QoL (patient in comfort of own home and surroundings, flexible timing, presence of partner/family) is perceived by the patient, the point moves to the right on the x axis. Taken together (costs and outcome), the therapy from the payer's perspective is in the lower right quadrant of the CE-plane.
FIGURE 4:
The cost-effectiveness plane, showing the payers ‘willingness to pay’ for cHHD (3×/week, 4 h) and iHHD (e.g. 8 h thrice weekly/day or nocturnal and 5 × 3 h) compared with conventional (3×/week, 4 h) iCHD.
However, maintaining the conventional 3 × 4 h regimen does not fully exploit the full potential of having HD therapy at home, where higher frequency or longer duration can be achieved. If more intensive (e.g. longer duration of 8 h but 3×/week, or 3 h sessions 5×/week) home HD therapy (iHHD) is delivered, the effectiveness-cost relationship changes. Both the QoL and costs (more disposables if treatment frequency is increased; more heparin, water, concentrates and energy requirements for both) would increase, positioning the therapy in the upper right quadrant in Figure 4. As in iHHD the additional medical benefits outweigh the higher costs; iHHD is still considered cost-effective over iCHD. In the USA, the dialysis provider charges the payer for additional treatments, even though payers may be reluctant to pay for additional treatments beyond the thrice weekly prescription.
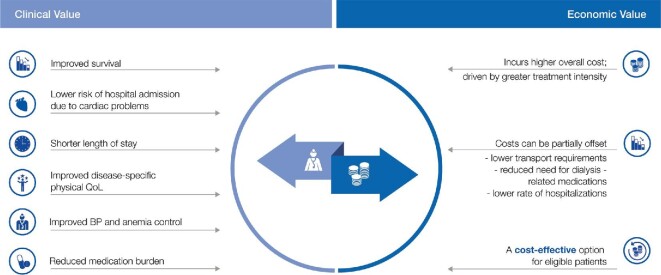
From such cost effectiveness analysis carried out by HTA agencies on behalf of payers or governments, the balance between the clinical value (effectiveness) of an intervention and the costs associated with it are analysed side-by-side (Figure 5). Significantly, this analysis is based on informed decision-making for the clinical therapy-related considerations (EBM, publications, guidelines, systematic reviews), as well as economic deliberations involving costs (direct product, personnel, dialysis centre, transport, medications, etc.).
FIGURE 5:
Clinical and economic value of iHHD (relative to conventional iCHD). Such analyses are the basis of VBHC and are carried out by health technology assessment agencies to guide the payability of therapies based on the clinical benefits obtained based on evidence.
Cost effectiveness of high-volume haemodiafiltration versus high-flux haemodialysis
Together with the therapy delivery conditions (e.g. duration and frequency of therapy), the other important determinant of efficiency removal of uraemic toxins and excess fluid is the choice of dialytic treatment modality. Collectively, based on the patient's individual overall condition, these factors are considered decisive factors impacting both patient outcomes and costs incurred. Online HDF (OL-HDF), used worldwide for thousands of patients, is a treatment modality that has been shown to present multiple clinical advantages for patients [141–145]. OL-HDF involves the mechanism of convection to achieve more efficient removal of larger uraemic toxins and the degree of convection is related to the convective volume achieved for each patient [146–152]. The cost-effectiveness of high-volume OL-HDF (HV-HDF) has been analysed and Figure 6 summarizes its economic and clinical value [144, 153]. While there is variability between countries in terms of the relative economic value of components shown in Table 2, it must be noted that any increase in the total costs affects the healthcare system in its entirety.
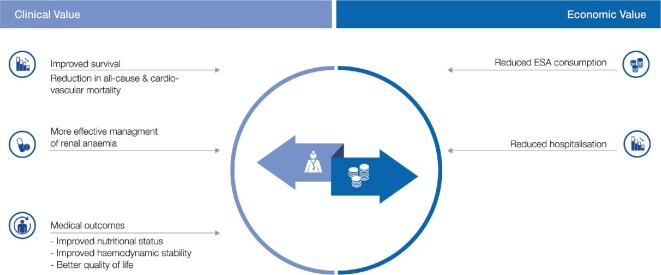
FIGURE 6:
Clinical and economic value of HV-HDF relative to conventional high-flux HD. ESA, erythropoiesis-stimulating agent.
CONCLUSION
Mitigating the overall burden of chronic kidney disease to ensure future sustainability of haemodialysis
About 89% of patients on dialysis worldwide receive HD [4]. It keeps patients alive for several years, some for decades, yet their burden of disease is reflected by substantially lower HRQoL than for the general population, or compared with patients with other chronic conditions [154]. Healthy longevity for end-stage CKD on dialysis patients needs to be achieved. Dialysis is a resource-intensive therapy [52]. Even before CKD patients reach the stage when dialysis is required, the condition incurs high healthcare costs with a graded association between severity of CKD and costs [53]. As dialysis consumes disproportionately higher proportions of healthcare budgets, healthcare systems of all countries irrespective of the size of their economies have concerns regarding future sustainability. The rising prevalence of underlying causes of ESRD, the number of dialysis patients living longer, aging multimorbid patients and shortages of medical personnel are expected to greatly increase the burden for healthcare systems and payers [155, 156].
Unless the focus shifts from costs to investing in value—maximizing the best outcomes out of the amount spent—sustaining future provision of dialysis therapy will be more challenging. To bring about a meaningful change that balances improved patient well-being while curtailing the high costs, revised strategies having the buy-in of all stakeholders (patients, providers, payers and policymakers) need to be adapted. The process of successful implementation of value-based systems begins with measuring value (outcomes meaningful for patients and associated costs), setting and communicating value (benchmarking) and coordinating care around patient profile, and culminates in development of payment models that reward value. Above all, a divergence from current practices all stakeholders are accustomed to needs to be considered across the entire value chain landscape.
Management of the condition of CKD requires early recognition and intervention—from the outset when reduced GFR and/or albuminuria are diagnosed, to progression to advanced stages of disease; interventions at the earlier stages of CKD delay progression of disease to the more severe phases [73, 157]. For example, glycaemic control may delay progression of CKD and increases the probability of more favourable long-term prognosis [21]. Such an approach also has the benefit of more patient involvement and awareness in their overall condition that could lead them to make life style adjustments to help control the multimorbidity. Interventions targeting specific symptoms, or aimed at supporting educational or lifestyle considerations, make a positive difference to people living with CKD. To better manage the challenges of a life with a debilitating condition, the holistic renal care of along the entire patient disease pathway thus needs to shift beyond providing RRT. In committing to allow CKD patients to live purposeful lives with meaning, all delivery of care (diagnostics, therapy intervention, addressing associated comorbid conditions) needs to be addressed collectively—with social needs and environmental and affordability considerations.
With a deeper appreciation of the physiological stresses imposed by intermittent dialysis on patient wellbeing and outcomes, a ‘one size fits all’ approach—currently the rule rather than the exception—is favourable neither to patients nor to healthcare systems [158]. Dialysis modality and dose, treatment time and frequency, electrolyte and prescription of medications are prescribed in a generalized manner, relying on the guidance provided by professional organizations without due consideration of the patient's specific clinical profile. While such a direction was necessary in the formative years of dialysis when better scientific understanding and technological improvements of the CKD and dialysis had to be delineated, the situation is quite different now. Dialysis is a routine procedure now where the key determinants of therapy quality are well known even if not totally resolved. Improvement of outcomes—and hence value—requires adapting these therapy determinants to patient needs (rather than vice versa) by tailoring prescription according to the patient's symptoms, metabolic changes over the course of the disease and treatment tolerance. Identification of a patient's risks helps define more appropriate and individualized therapy by stratifying patients according to their risk of adverse short- or mid-term outcomes. The studies of Couchoud et al. and Peeters et al. have shown the predictive value of such validated risk stratification algorithms to improve patient-centred outcomes [159, 160]. For example, by harnessing data pools and knowledge acquired from scoring systems key treatment features specific (e.g. incremental dialysis, nocturnal dialysis or adapted dialysis schedule) can be altered to reduce premature mortality of CKD stage V patients transitioning to dialysis. More personalized or precision medicine targeting optimization of fluid and sodium control, blood pressure management and customizing electrolyte prescription could lead to better treatment tolerance and acceptability by HD patients.
Today's technology, involving biosensors and sophisticated analytics, provides tools that help individualize patient treatments and facilitate adjustment of treatment parameters according to the patient's specific clinical profile and needs. Automated, self-adapting systems adapted within ‘smart machines’ and clinical workflows governed by adaptive algorithms incorporating feedback control loops are no longer a vision. Recently, Kuhlmann et al. described a conductivity-based algorithmic electrolyte balancing module (EBC) embedded within the HD machine that helps achieve zero diffusive sodium balance [161]. Restoring and controlling fluid volume homeostasis has always been a major challenge in the delivery of haemodialysis as uniform dialysate sodium prescription still gives rise to intradialytic symptoms or postdialytic thirst, depending on the patient's natremia gradients. With the EBC, the machine automatically individualizes dialysate sodium to patient plasma sodium with an absence of clinical manifestations, increasing patient comfort. The potential cost savings of such automated monitoring systems are additional benefits while resolving clinical dilemmas and improving patient QoL [162]. Artificial intelligence has been proposed recently for clinical guidance and decision-making support in adapting dialysis prescription (e.g. ultrafiltration rate, dialysate sodium, treatment time) to ensure an optimal fluid status control and to minimize a haemodynamic stress. Improving efficiency of treatment during each session and impacting improved outcomes are the benefits of the approach of individualized comorbidity management.
The challenge of applying this framework to the renal setting (value-based renal care) is that CKD itself is caused by other major chronic conditions such as diabetes and hypertension, but also directly contributes to cardiovascular disease, the major cause of death in CKD and dialysis patients [22]. Additionally, as CKD is an extraordinarily complex set of physiological conditions intricately linked to numerous metabolic disorders and perturbations, measurement of ‘meaningful outcomes’ is extremely challenging. Hence, there is currently a high variability in measures of patient outcomes that also differ in terms of their number and composition, e.g. those proposed by the ERA-EDTA registry and those proposed by ICHOM [27]. Additionally, international standards are needed to guide aspects like tendering, to re-focus on delivering value (maximizing outcomes per amount of money spent) that can be achieved in different areas for patients, payers and providers by reducing inefficiencies in today's healthcare systems A stepwise approach from simple to complex is thus required for a systematic implementation of a strategy to transition from the current fragmented care to a fully integrated care model that harmonizes outcome measures along the entire patient pathway [68].
ACKNOWLEDGEMENT
This article is published as part of a supplement supported by Fresenius Medical Care.
Contributor Information
Christian Apel, Health Economics and Market Access EMEA, Fresenius Medical Care, Bad Homburg, Germany.
Carsten Hornig, Health Economics and Market Access EMEA, Fresenius Medical Care, Bad Homburg, Germany.
Frank W Maddux, Global Medical Office, Fresenius Medical Care, Waltham, MA, USA.
Terry Ketchersid, Integrated Care Group, Fresenius Medical Care, Waltham, MA, USA.
Julianna Yeung, Health Economics & Market Access Asia-Pacific, Fresenius Medical Care, Hong Kong.
Adrian Guinsburg, Global Medical Office, Fresenius Medical Care, Buenos Aires, Argentina.
CONFLICT OF INTEREST STATEMENT
C.A., C.H., F.W.M., T.K., J.Y. and A.G. are employees of Fresenius Medical Care.
REFERENCES
- 1. Coresh J. Update on the burden of CKD. J Am Soc Nephrol 2017; 28: 1020–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coresh J, Jafar TH. Disparities in worldwide treatment of kidney failure. Lancet 2015; 385: 1926–1928 [DOI] [PubMed] [Google Scholar]
- 3. Liyanage T, Ninomiya T, Jha V et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet 2015; 385: 1975–1982 [DOI] [PubMed] [Google Scholar]
- 4. Himmelfarb J, Vanholder R, Mehrotra R et al. The current and future landscape of dialysis. Nat Rev Nephrol 2020; 16: 573–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wongrakpanich S, Susantitaphong P, Isaranuwatchai S et al. Dialysis therapy and conservative management of advanced chronic kidney disease in the elderly: a systematic review. Nephron 2017; 137: 178–189 [DOI] [PubMed] [Google Scholar]
- 6. Wong SPY, Boyapati S, Engelberg RA et al. Experiences of US nephrologists in the delivery of conservative care to patients with advanced kidney disease: a national qualitative study. Am J Kidney Dis 2020; 75: 167–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gonzalez-Bedat MC, Rosa-Diez G, Ferreiro-Fuentes A et al. Burden of disease: closing the gaps in the burden of end-stage kidney disease in Latin America. Clin Nephrol 2020; 93: 55–59 [DOI] [PubMed] [Google Scholar]
- 8. Rosa-Diez G, Gonzalez-Bedat M, Pecoits-Filho R et al. Renal replacement therapy in Latin American end-stage renal disease. Clin Kidney J 2014; 7: 431–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vanholder R, Annemans L, Brown E et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017; 13: 393–409 [DOI] [PubMed] [Google Scholar]
- 10. International Society of Nephrology 2020 . More than 850 million worldwide have some form of kidney disease: help raise awareness. ISN News, 27 November.https://www.theisn.org/blog/2020/11/27/more-than-850-million-worldwide-have-some-form-of-kidney-disease-help-raise-awareness/ (28 July 2021, date last accessed)
- 11. Lee VS, Kawamoto K, Hess R et al. Implementation of a value-driven outcomes program to identify high variability in clinical costs and outcomes and association with reduced cost and improved quality. JAMA 2016; 316: 1061–1072 [DOI] [PubMed] [Google Scholar]
- 12. Murray CJL, Lopez AD. Measuring the global burden of disease. N Engl J Med 2013; 369: 448–457 [DOI] [PubMed] [Google Scholar]
- 13. Vos T, Lim SS, Abbafati C et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet 2020; 396: 1204–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hill NR, Fatoba ST, Oke JL et al. Global prevalence of chronic kidney disease—a systematic review and meta-analysis. PLoS One 2016; 11: e0158765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lv JC, Zhang LX. Prevalence and disease burden of chronic kidney disease. Adv Exp Med Biol 2019; 1165: 3–15 [DOI] [PubMed] [Google Scholar]
- 16. Piccoli GB, Mery D. Sister earth, our common home: toward a sustainable, planet friendly approach to dialysis, a paradigm of high technology medicine. J Ren Nutr 2017; 27: 478–484 [DOI] [PubMed] [Google Scholar]
- 17. Jager KJ, Fraser SDS. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant 2017; 32 (Suppl 2): ii121–ii128 [DOI] [PubMed] [Google Scholar]
- 18. Kiberd B. The chronic kidney disease epidemic: stepping back and looking forward. J Am Soc Nephrol 2006; 17: 2967–2973 [DOI] [PubMed] [Google Scholar]
- 19. St Peter WL. Introduction: chronic kidney disease: a burgeoning health epidemic. J Manag Care Pharm 2007; 13 (Suppl 9D): S2–S5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Piccoli GB, Cupisti A, Aucella F et al. Green nephrology and eco-dialysis: a position statement by the Italian Society of Nephrology. J Nephrol 2020; 33: 681–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA 2019; 322: 1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Busink E, Canaud B, Schröder-Bäck P et al. Chronic kidney disease: exploring value-based healthcare as a potential viable solution. Blood Purif 2019; 47: 156–165 [DOI] [PubMed] [Google Scholar]
- 23. Porter ME, Lee TH. The strategy that will fix health care. Harv Bus Rev 2013; 91: 1–39 [Google Scholar]
- 24. NEJM Catalyst . 2017. What is value-based healthcare? NEJM Catalyst, 1 January. https://catalyst.nejm.org/doi/full/10.1056/CAT.17.0558 (28 July 2021, date last accessed) [Google Scholar]
- 25. Porter ME. A strategy for health care reform–toward a value-based system. N Engl J Med 2009; 361: 109–112 [DOI] [PubMed] [Google Scholar]
- 26. Porter ME. Value-based health care delivery. Ann Surg 2008; 248: 503–509 [DOI] [PubMed] [Google Scholar]
- 27. Verberne WR, Das-Gupta Z, Allegretti AS et al. Development of an international standard set of value-based outcome measures for patients with chronic kidney disease: a report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD working group. Am J Kidney Dis 2019; 73: 372–384 [DOI] [PubMed] [Google Scholar]
- 28. Yong PL, Saunders RS, Olsen L. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: National Academic Press, 2010, 1–852 [PubMed] [Google Scholar]
- 29. Hunt A. The price isn't right: startegies to address high and rising healthcare prices. Altar Health Value Hub 2021; 1–8. https://www.healthcarevaluehub.org/advocate-resources/publications/price-isnt-right-strategies-address-high-and-rising-healthcare-prices. (11 Nov 2021, date last accessed) [Google Scholar]
- 30. Vos T, Allen C, Arora M et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the global burden of disease study 2015. Lancet 2016; 388: 1545–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Porter ME, Lee TH. Why strategy matters now. N Engl J Med 2015; 372: 1681–1684 [DOI] [PubMed] [Google Scholar]
- 32. Wilson NE. For-profit status and industry evolution in health care markets: evidence from the dialysis industry. Int J Heal Econ Manag 2016; 16: 297–319 [DOI] [PubMed] [Google Scholar]
- 33. Stopper A, Raddatz A, Grassmann A et al. Delivering quality of care while managing the interests of all stakeholders. Blood Purif 2011; 32: 323–330 [DOI] [PubMed] [Google Scholar]
- 34. Lameire N, Van Biesen W, Vanholder R. Did 20 years of technological innovations in hemodialysis contribute to better patient outcomes? Clin J Am Soc Nephrol 2009; 4 (Suppl 1): S30–S40 [DOI] [PubMed] [Google Scholar]
- 35. Go AS, Chertow GM, Fan D et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 2004; 351: 1296–1305 [DOI] [PubMed] [Google Scholar]
- 36. Chan CT, Blankestijn PJ, Dember LM et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) controversies conference. Kidney Int 2019; 96: 37–47 [DOI] [PubMed] [Google Scholar]
- 37. Kerr PG. International differences in hemodialysis delivery and their influence on outcomes. Am J Kidney Dis 2011; 58: 461–470 [DOI] [PubMed] [Google Scholar]
- 38. Rastogi A, Linden A, Nissenson AR. Disease management in chronic kidney disease. Adv Chronic Kidney Dis 2008; 15: 19–28 [DOI] [PubMed] [Google Scholar]
- 39. Herzog CA, Asinger RW, Berger AK et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 2011; 80: 572–586 [DOI] [PubMed] [Google Scholar]
- 40. Canaud B. Adequacy target in hemodialysis. J Nephrol 2004; 17 (Suppl 8): S77–S86 [PubMed] [Google Scholar]
- 41. Tattersall J, Martin-Malo A, Pedrini L et al. EBPG guideline on dialysis strategies. Nephrol Dial Transplant 2007; 22 (Suppl 2): ii5–ii21 [DOI] [PubMed] [Google Scholar]
- 42. Robinson BM, Akizawa T, Jager KJ et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016; 388: 294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Foley RN, Hakim RM. Why is the mortality of dialysis patients in the United States much higher than the rest of the world? J Am Soc Nephrol 2009; 20: 1432–1435 [DOI] [PubMed] [Google Scholar]
- 44. Luxardo R, Kramer A, González-Bedat MC et al. The epidemiology of renal replacement therapy in two different parts of the world: the Latin American dialysis and transplant registry versus the European Renal Association-European Dialysis and Transplant Association registry. Rev Panam Salud Publica 2018; 42: e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malta D, Petersen KS, Johnson C et al. High sodium intake increases blood pressure and risk of kidney disease. From the science of salt: a regularly updated systematic review of salt and health outcomes (August 2016 to March 2017). J Clin Hypertens 2018; 20: 1654–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Vilar E, Fry AC, Wellsted D et al. Long-term outcomes in online hemodiafiltration and high-flux hemodialysis: a comparative analysis. Clin J Am Soc Nephrol 2009; 4: 1944–1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Grooteman M, Nubé M. Dialysis: membrane flux, dialysate purity and cardiovascular outcomes. Nat Rev Nephrol 2013; 9: 439–441 [DOI] [PubMed] [Google Scholar]
- 48. Asci G, Töz H, Ozkahya M et al. The impact of membrane permeability and dialysate purity on cardiovascular outcomes. J Am Soc Nephrol 2013; 24: 1014–1023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Eknoyan G, Beck GJ, Cheung AK et al. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2002; 347: 2010–2019 [DOI] [PubMed] [Google Scholar]
- 50. Vanholder R, Annemans L, Brown E et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017; 13: 393–409 [DOI] [PubMed] [Google Scholar]
- 51. Vanholder R, Van Biesen W, Lameire N. Renal replacement therapy: how can we contain the costs? Lancet 2014; 383: 1783–1785 [DOI] [PubMed] [Google Scholar]
- 52. Versino E, Piccoli GB. Chronic kidney disease: the complex history of the organization of long-term care and bioethics. Why now, more than ever, action is needed. Int J Environ Res Public Health 2019; 16: 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Manns B, Hemmelgarn B, Tonelli M et al. The cost of care for people with chronic kidney disease. Can J Kidney Health Dis 2019; 6: 2054358119835521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Wong CKH, Chen J, Fung SKS et al. Direct and indirect costs of end-stage renal disease patients in the first and second years after initiation of nocturnal home haemodialysis, hospital haemodialysis and peritoneal dialysis. Nephrol Dial Transplant 2019; 34: 1565–1576 [DOI] [PubMed] [Google Scholar]
- 55. Damien P, Lanham HJ, Parthasarathy M et al. Assessing key cost drivers associated with caring for chronic kidney disease patients. BMC Health Serv Res 2016; 16: 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Nakayama T, Imanaka Y, Okuno Y et al. Analysis of the evidence-practice gap to facilitate proper medical care for the elderly: investigation, using databases, of utilization measures for National Database of Health Insurance Claims and Specific Health Checkups of Japan (NDB). Environ Health Prev Med 2017; 22: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Han SS, Park JY, Kang S et al. Dialysis modality and mortality in the elderly: a meta-analysis. Clin J Am Soc Nephrol 2015; 10: 983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vanholder R, Annemans L, Brown E et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017; 13: 393–409 [DOI] [PubMed] [Google Scholar]
- 59. European Kidney Health Alliance . Recommendations for sustainable kidney care. http://ekha.eu/wp-content/uploads/2016/01/EKHA-Recs-for-Sustainable-Kidney-Care-25.08.2015.pdf (11 November 2021, date last accessed)
- 60. Weinstein AM, Kimmel PL. Social determinants of health in people with kidney disease. Clin J Am Soc Nephrol 2021; 16: 803–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sargent JA. Shortfalls in the delivery of dialysis. Am J Kidney Dis 1990; 15: 500–510 [DOI] [PubMed] [Google Scholar]
- 62. Saran R, Bragg-Gresham JL, Levin NW et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int 2006; 69: 1222–1228 [DOI] [PubMed] [Google Scholar]
- 63. Locatelli F, Buoncristiani U, Canaud B et al. Dialysis dose and frequency. Nephrol Dial Transplant 2005; 20: 285–296 [DOI] [PubMed] [Google Scholar]
- 64. Eknoyan G, Lameire N, Eckhardt K et al. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 5–14 [DOI] [PubMed] [Google Scholar]
- 65. National Kidney Foundation . K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39 (2 suppl_1): S1–S266 [PubMed] [Google Scholar]
- 66. Eloot S, Vanholder R, van Biesen W et al. The patient as a limit to dialysis technology. Clin J Am Soc Nephrol 2011; 6: 2105–2107 [DOI] [PubMed] [Google Scholar]
- 67. Ronco C. Evolution of technology for continuous renal replacement therapy: forty years of improvement. Contrib Nephrol 2018; 194: 1–14 [DOI] [PubMed] [Google Scholar]
- 68. Canaud B, Collins A, Maddux F. The renal replacement therapy landscape in 2030: reducing the global cardiovascular burden in dialysis patients. Nephrol Dial Transplant 2020; 35 (suppl_2): ii51–ii57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lavis JN, Tugwell P. David Sackett's unintended impacts on health policy. Milbank Q 2015; 93: 867–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Levin A, Stevens PE. Summary of KDIGO 2012 CKD guideline: behind the scenes, need for guidance, and a framework for moving forward. Kidney Int 2014; 85: 49–61 [DOI] [PubMed] [Google Scholar]
- 71. Zoccali C, Vanholder R, Massy ZA et al. The systemic nature of CKD. Nat Rev Nephrol 2017; 13: 344–358 [DOI] [PubMed] [Google Scholar]
- 72. El Nahas AM, Bello AK. Chronic kidney disease: the global challenge. Lancet 2005; 365: 331–340 [DOI] [PubMed] [Google Scholar]
- 73. Webster AC, Nagler EV, Morton RL et al. Chronic kidney disease. Lancet 2017; 389: 1238–1252 [DOI] [PubMed] [Google Scholar]
- 74. Murray MA, Brunier G, Chung JO et al. A systematic review of factors influencing decision-making in adults living with chronic kidney disease. Patient Educ Couns 2009; 76: 149–158 [DOI] [PubMed] [Google Scholar]
- 75. Cassidy BP, Getchell LE, Harwood L et al. Barriers to education and shared decision making in the chronic kidney disease population: a narrative review. Can J Kidney Health Dis 2018; 5: 2054358118803322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Cabrera VJ, Hansson J, Kliger AS et al. Symptom management of the patient with CKD: the role of dialysis. Clin J Am Soc Nephrol 2017; 12: 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Sackett DL, Rosenberg WMC. The need for evidence-based medicine. J R Soc Med 1995; 88: 620–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Locatelli F, Di Filippo S, Pozzoni P. A critical assessment of uremia research. Blood Purif 2006; 24: 71–76 [DOI] [PubMed] [Google Scholar]
- 79. Locatelli F, Gauly A, Czekalski S et al. The MPO study: just a European HEMO study or something very different? Blood Purif 2008; 26: 100–104 [DOI] [PubMed] [Google Scholar]
- 80. Varela Lema L, Ruano Raviña A. Effectiveness and safety of different hemodialysis modalities: a review. J Nephrol 2007; 20: 525–542 [PubMed] [Google Scholar]
- 81. Yaseen M, Hassan W, Awad R et al. Impact of recent clinical trials on nephrology practice: are we in a stagnant era? Kidney Dis 2019; 5: 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shen JI, Lum EL, Chang TI. Balancing the evidence: how to reconcile the results of observational studies vs. Randomized clinical trials in dialysis. Semin Dial 2016; 29: 342–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nistor I, Palmer SC, Craig JC et al. Convective versus diffusive dialysis therapies for chronic kidney failure: an updated systematic review of randomized controlled trials. Am J Kidney Dis 2014; 63: 954–967 [DOI] [PubMed] [Google Scholar]
- 84. Bowry SK, Apel C, Canaud B. Assessment of clinical evidence for convective dialysis therapies. Am J Kidney Dis 2014; 64: 820. [DOI] [PubMed] [Google Scholar]
- 85. Greenhalgh T, Howick J, Maskrey N et al. Evidence based medicine: a movement in crisis? BMJ 2014; 348: g3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Harman C. Putting clinical trials on trial. Nat Rev Nephrol 2009; 5: 301. [DOI] [PubMed] [Google Scholar]
- 87. Qarni B, Osman MA, Levin A et al. Kidney care in low- and middle-income countries. Clin Nephrol 2020; 93: 21–30 [DOI] [PubMed] [Google Scholar]
- 88. National Institute for Health and Care Excellence . Renal replacement therapy and conservative management. NICE Guideline 107. https://www.nice.org.uk/guidance/ng107/resources/renal-replacement-therapy-and-conservative-management-pdf-66141542991301 (11 November 2021, date last accessed) [PubMed] [Google Scholar]
- 89. Marcelli D, Kirchgessner J, Amato C et al. EuCliD (European clinical database): a database comparing different realities. J Nephrol 2001; 14 (Suppl 4): S94–S100 [PubMed] [Google Scholar]
- 90. Canaud B, Tetta C, Marcelli D et al. Implementation and management of strategies to set and to achieve clinical targets. In: Hiromichi S (ed.) Hemodialysis. Rijeka, Croatia: IntechOpen, 2013, 439–453 [Google Scholar]
- 91. Maddux DW, Usvyat LA, DeFalco D et al. Effects of renal care coordinator case management on outcomes in incident dialysis patients. Clin Nephrol 2016; 85: 152–158 [DOI] [PubMed] [Google Scholar]
- 92. Kooman JP, Usvyat LA, Dekker MJE et al. Cycles, arrows and turbulence: time patterns in renal disease, a path from epidemiology to personalized medicine? Blood Purif 2019; 47: 171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Henderson LW. Dialysis in the 21st century. Am J Kidney Dis 1996; 28: 951–957 [DOI] [PubMed] [Google Scholar]
- 94. Kjellstrand CM. My addiction: the artificial kidney, the rise and fall of dialysis. Artif Organs 2012; 36: 575–580 [DOI] [PubMed] [Google Scholar]
- 95. Schreyögg J, Stargardt T. The trade-off between costs and outcomes: the case of acute myocardial infarction. Health Serv Res 2010; 45 (6 Pt 1): 1585–1601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Carey K, Burgess JFJ. On measuring the hospital cost/quality trade-off. Health Econ 1999; 8: 509–520 [DOI] [PubMed] [Google Scholar]
- 97. Braun LA, Sood V, Hogue S et al. High burden and unmet patient needs in chronic kidney disease. Int J Nephrol Renovasc Dis 2012; 5: 151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lacson EJ, Brunelli SM. Hemodialysis treatment time: a fresh perspective. Clin J Am Soc Nephrol 2011; 6: 2522–2530 [DOI] [PubMed] [Google Scholar]
- 99. Saitō A. Definition of high-performance membranes—from a clinical point of view. Contrib Nephrol 2011; 173: 1–10 [DOI] [PubMed] [Google Scholar]
- 100. Kerr P, Toussaint N. KHA-CARI guidline: dialysis adequacy (haemodialysis): dialysis membranes. Nephrology 2013; 18: 485–488 [DOI] [PubMed] [Google Scholar]
- 101. Subramanian S, Venkataraman R, Kellum JA. Influence of dialysis membranes on outcomes in acute renal failure: a meta-analysis. Kidney Int 2002; 62: 1819–1823 [DOI] [PubMed] [Google Scholar]
- 102. Vanholder R, Glorieux G, Van Biesen W. Advantages of new hemodialysis membranes and equipment. Nephron Clin Pract 2010; 114: 165–172 [DOI] [PubMed] [Google Scholar]
- 103. Keshaviah PR, Constantini EG, Luehmann DA et al. Dialyzer ultrafiltration coefficients: comparison between in vitro and in vivo values. Artif Organs 1982; 6: 23–26 [DOI] [PubMed] [Google Scholar]
- 104. Ficheux A, Kerr PG, Brunet P et al. The ultrafiltration coefficient of a dialyser (KUF) is not a fixed value, and it follows a parabolic function: the new concept of KUF max. Nephrol Dial Transplant 2011; 26: 636–640 [DOI] [PubMed] [Google Scholar]
- 105. Ficheux A, Ronco C, Brunet P et al. The ultrafiltration coefficient: this old “grand inconnu” in dialysis. Nephrol Dial Transplant 2015; 20: 204–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ronco C, Clark WR. Haemodialysis membranes. Nat Rev Nephrol 2018; 14: 394–410 [DOI] [PubMed] [Google Scholar]
- 107. Singh G. Concentrations of serum free light chains in kappa and lambda lesions in light-chain myelomas. Lab Med 2019; 50: 189–193 [DOI] [PubMed] [Google Scholar]
- 108. Gondouin B, Hutchison CA. High Cut-off dialysis membranes: current uses and future potential. Adv Chronic Kidney Dis 2011; 18: 180–187 [DOI] [PubMed] [Google Scholar]
- 109. Jolly M, Francis S, Aggarwal R et al. Serum free light chains, interferon-alpha, and interleukins in systemic lupus erythematosus. Lupus 2014; 23: 881–888 [DOI] [PubMed] [Google Scholar]
- 110. Clark WR, Ronco C. Determinants of haemodialyser performance and the potential effect on clinical outcome. Nephrol Dial Transplant 2001; 16 (Suppl 5): 56–60 [DOI] [PubMed] [Google Scholar]
- 111. Vienken J. Polymers in nephrology. Characteristics and needs. Int J Artif Organs 2002; 25: 470–479 [DOI] [PubMed] [Google Scholar]
- 112. Kjellstrand CM, Evans RL, Petersen RJ et al. The “unphysiology” of dialysis: a major cause of dialysis side effects? Hemodial Int 2004; 8: 24–29 [DOI] [PubMed] [Google Scholar]
- 113. Kjellstrand CM, Rosa AA, Shideman JR et al. Optimal dialysis frequency and duration: the “unphysiology hypothesis”. Kidney Int Suppl 1978; 13 (Suppl 8): S120–S124 [PubMed] [Google Scholar]
- 114. Lopot F, Válek A. Mathematical concept of dialysis unphysiology. Home Hemodial Int 1998; 2: 18–21 [DOI] [PubMed] [Google Scholar]
- 115. Lopot F, Nejedlý B, Sulková S. Physiology in daily hemodialysis in terms of the time average concentration/time average deviation concept. Hemodial Int 2004; 8: 39–44 [DOI] [PubMed] [Google Scholar]
- 116. Chan CT. Nocturnal hemodialysis: an attempt to correct the “unphysiology” of conventional intermittent renal replacement therapy. Clin Invest Med 2002; 25: 233–235 [PubMed] [Google Scholar]
- 117. Yong DSP, Kwok AOL, Wong DML et al. Symptom burden and quality of life in end-stage renal disease: a study of 179 patients on dialysis and palliative care. Palliat Med 2009; 23: 111–119 [DOI] [PubMed] [Google Scholar]
- 118. Walker RC, Howard K, Tong A et al. The economic considerations of patients and caregivers in choice of dialysis modality. Hemodial Int 2016; 20: 634–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Walker R, Marshall MR, Morton RL et al. The cost-effectiveness of contemporary home haemodialysis modalities compared with facility haemodialysis: a systematic review of full economic evaluations. Nephrology (Carlton) 2014; 19: 459–470 [DOI] [PubMed] [Google Scholar]
- 120. Agar JWM. Personal viewpoint: hemodialysis–water, power, and waste disposal: rethinking our environmental responsibilities. Hemodial Int 2012; 16: 6–10 [DOI] [PubMed] [Google Scholar]
- 121. Piccoli GB, Nazha M, Ferraresi M et al. Eco-dialysis: the financial and ecological costs of dialysis waste products: is a “cradle-to-cradle” model feasible for planet-friendly haemodialysis waste management? Nephrol Dial Transplant 2015; 30: 1018–1027 [DOI] [PubMed] [Google Scholar]
- 122. Bendine G, Autin F, Fabre B et al. Haemodialysis therapy and sustainable growth: a corporate experience in france. Nephrol Dial Transplant 2020; 35: 2154–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Ronco C, Bowry SK, Brendolan A et al. Hemodialyzer: from macro-design to membrane nanostructure; the case of the FX-class of hemodialyzers. Kidney Int Suppl 2002; 61: 126–142 [DOI] [PubMed] [Google Scholar]
- 124. Ferraresi M, Nazha M, Vigotti FN et al. Ecodialysis: first strategies to limit damages and reduce costs. G Ital Nefrol 2014; 31: gin/31.5.16. [PubMed] [Google Scholar]
- 125. Alquist M, Bosch JP, Barth C et al. Knowing what we do and doing what we should: quality assurance in hemodialysis. Nephron Clin Pract 2014; 126: 135–143 [DOI] [PubMed] [Google Scholar]
- 126. Haller MC, Vanholder R, Oberbauer R et al. Health economics and European renal best practice—is it time to bring health economics into evidence-based guideline production in Europe? Nephrol Dial Transplant 2014; 29: 1994–1997 [DOI] [PubMed] [Google Scholar]
- 127. Klarenbach SW, Tonelli M, Chui B et al. Economic evaluation of dialysis therapies. Nat Rev Nephrol 2014; 10: 644–652 [DOI] [PubMed] [Google Scholar]
- 128. Black WC. The CE plane: a graphic representation of cost-effectiveness. Med Decis Making 1990; 10: 212–214 [DOI] [PubMed] [Google Scholar]
- 129. Sendi PP, Briggs AH. Affordability and cost-effectiveness: decision-making on the cost-effectiveness plane. Health Econ 2001; 10: 675–680 [DOI] [PubMed] [Google Scholar]
- 130. Canadian Agency for Drugs and Technologies in Health . Dialysis modalities for the treatment of end-stage kidney disease: recommendations. CADTH Optimal Use Report. CADTH, Ottawa, 2017 [PubMed] [Google Scholar]
- 131. Fissell R, Hakim RM. Improving outcomes by changing hemodialysis practice patterns. Curr Opin Nephrol Hypertens 2013; 22: 675–680 [DOI] [PubMed] [Google Scholar]
- 132. McFarlane PA. More of the same: improving outcomes through intensive hemodialysis. Semin Dial 2009; 22: 598–602 [DOI] [PubMed] [Google Scholar]
- 133. Copland M, Komenda P, Weinhandl ED et al. Intensive hemodialysis, mineral and bone disorder, and phosphate binder use. Am J Kidney Dis 2016; 68: S24–S32 [DOI] [PubMed] [Google Scholar]
- 134. Morfin JA, Fluck RJ, Weinhandl ED et al. Intensive hemodialysis and treatment complications and tolerability. Am J Kidney Dis 2016; 68: S43–S50 [DOI] [PubMed] [Google Scholar]
- 135. McCullough PA, Chan CT, Weinhandl ED et al. Intensive hemodialysis, left ventricular hypertrophy, and cardiovascular disease. Am J Kidney Dis 2016; 68: S5–S14 [DOI] [PubMed] [Google Scholar]
- 136. Ward RA, Greene T, Hartmann B et al. Resistance to intercompartmental mass transfer limits β2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int 2006; 69: 1431–1437 [DOI] [PubMed] [Google Scholar]
- 137. Minutolo R, Bellizzi V, Cioffi M et al. Postdialytic rebound of serum phosphorus: pathogenetic and clinical insights. J Am Soc Nephrol 2002; 13: 1046–1054 [DOI] [PubMed] [Google Scholar]
- 138. Diaz-Buxo JA, White SA, Himmele R. Frequent hemodialysis: a critical review. Semin Dial 2013; 26: 578–589 [DOI] [PubMed] [Google Scholar]
- 139. Williams AW, Chebrolu SB, Ing TS et al. Early clinical, quality-of-life, and biochemical changes of “daily hemodialysis” (6 dialyses per week). Am J Kidney Dis 2004; 43: 90–102 [DOI] [PubMed] [Google Scholar]
- 140. Moeller S. ESRD patients in 2001: global overview of patients, treatment modalities and development trends. Nephrol Dial Transplant 2002; 17: 2071–2076 [DOI] [PubMed] [Google Scholar]
- 141. Canaud B, Bowry S, Stuard S. Hemodiafiltration. In: Daugirdas JT, Blake PG, Ing TS (eds) Handbook of Dialysis. Philadelphia, PA: Wolters Kluwer Health, 2014, 321–332 [Google Scholar]
- 142. Canaud B. Online hemodiafiltration. Contrib Nephrol 2007; 158: 110–122 [DOI] [PubMed] [Google Scholar]
- 143. Nubé MJ, Peters SAE, Blankestijn PJ et al. Mortality reduction by post-dilution online-haemodiafiltration: a cause-specific analysis. Nephrol Dial Transplant 2017; 32: 548–555 [DOI] [PubMed] [Google Scholar]
- 144. Canaud PB, Bowry SK et al. Emerging clinical evidence on online hemodiafiltration: does volume of ultrafiltration matter? Blood Purif 2013; 35: 55–62 [DOI] [PubMed] [Google Scholar]
- 145. Lornoy W, Becaus I, Billiouw JM et al. On-line haemodiafiltration. Remarkable removal of beta2-microglobulin. Long-term clinical observations. Nephrol Dial Transplant 2000; 15 (Suppl 1): 49–54 [DOI] [PubMed] [Google Scholar]
- 146. Bowry SK, Canaud B. Achieving high convective volumes in on-line hemodiafiltration. Blood Purif 2013; 35 (Suppl 1): 23–28 [DOI] [PubMed] [Google Scholar]
- 147. Basile C, Davenport A, Blankestijn PJ. Why choose high volume online post-dilution hemodiafiltration? J Nephrol 2017; 30: 181–186 [DOI] [PubMed] [Google Scholar]
- 148. De Roij van Zuijdewijn CL, Nubé MJ, ter Wee PM et al. Treatment time or convection volume in HDF: what drives the reduced mortality risk? Blood Purif 2015; 40: 53–58 [DOI] [PubMed] [Google Scholar]
- 149. Maduell F. Is there an “Optimal dose” of hemodiafiltration? Blood Purif 2015; 40 (Suppl 1): 17–23 [DOI] [PubMed] [Google Scholar]
- 150. Penne EL, Van Der Weerd NC, Bots ML et al. Patient-and treatment-related determinants of convective volume in post-dilution haemodiafiltration in clinical practice. Nephrol Dial Transplant 2009; 24: 3493–3499 [DOI] [PubMed] [Google Scholar]
- 151. Canaud B, Barbieri C, Marcelli D et al. Optimal convection volume for improving patient outcomes in an international incident dialysis cohort treated with online hemodiafiltration. Kidney Int 2015; 88: 1108–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Chapdelaine I, de Roij van Zuijdewijn CL, Mostovaya IM et al. Optimization of the convection volume in online post-dilution haemodiafiltration: practical and technical issues. Clin Kidney J 2015; 8: 191–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Oates T, Cross J, Davenport A. Cost comparison of online haemodiafiltration with high-flux haemodialysis. J Nephrol 2012; 25: 192–197 [DOI] [PubMed] [Google Scholar]
- 154. Mitema D, Jaar BG. How can we improve the quality of life of dialysis patients? Semin Dial 2016; 29: 93–102 [DOI] [PubMed] [Google Scholar]
- 155. Ross J, Jones J, Callaghan P et al. A survey of stress, job satisfaction and burnout among haemodialysis staff. J Ren Care 2009; 35: 127–133 [DOI] [PubMed] [Google Scholar]
- 156. Osman MA, Alrukhaimi M, Ashuntantang GE et al. Global nephrology workforce: gaps and opportunities toward a sustainable kidney care system. Kidney Int Suppl 2018; 8: 52–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Basile JN. Recognizing the link between CKD and CVD in the primary care setting: accurate and early diagnosis for timely and appropriate intervention. South Med J 2007; 100: 499–505 [DOI] [PubMed] [Google Scholar]
- 158. Canaud B, Chazot C, Koomans J et al. Fluid and hemodynamic management in hemodialysis patients: challenges and opportunities. J Bras Nefrol 2019; 41: 550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Couchoud CG, Beuscart JB, Aldigier JC et al. Development of a risk stratification algorithm to improve patient-centered care and decision making for incident elderly patients with end-stage renal disease. Kidney Int 2015; 88: 1178–1186 [DOI] [PubMed] [Google Scholar]
- 160. Peeters P, Van Biesen W, Veys N et al. External validation of a risk stratification model to assist shared decision making for patients starting renal replacement therapy. BMC Nephrol 2016; 17: 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Kuhlmann U, Maierhofer A, Canaud B et al. Zero diffusive sodium balance in hemodialysis provided by an algorithm-based electrolyte balancing controller: a proof of principle clinical study. Artif Organs 2019; 43: 150–158 [DOI] [PubMed] [Google Scholar]
- 162. Daugirdas JT, Tattersall JE. Automated monitoring of hemodialysis adequacy by dialysis machines: potential benefits to patients and cost savings. Kidney Int 2010; 78: 833–835 [DOI] [PubMed] [Google Scholar]