ABSTRACT
Hemodialysis (HD) is a life-sustaining therapy as well as an intermittent and repetitive stress condition for the patient. In ridding the blood of unwanted substances and excess fluid from the blood, the extracorporeal procedure simultaneously induces persistent physiological changes that adversely affect several organs. Dialysis patients experience this systemic stress condition usually thrice weekly and sometimes more frequently depending on the treatment schedule. Dialysis-induced systemic stress results from multifactorial components that include treatment schedule (i.e. modality, treatment time), hemodynamic management (i.e. ultrafiltration, weight loss), intensity of solute fluxes, osmotic and electrolytic shifts and interaction of blood with components of the extracorporeal circuit. Intradialytic morbidity (i.e. hypovolemia, intradialytic hypotension, hypoxia) is the clinical expression of this systemic stress that may act as a disease modifier, resulting in multiorgan injury and long-term morbidity. Thus, while lifesaving, HD exposes the patient to several systemic stressors, both hemodynamic and non-hemodynamic in origin. In addition, a combination of cardiocirculatory stress, greatly conditioned by the switch from hypervolemia to hypovolemia, hypoxemia and electrolyte changes may create pro-arrhythmogenic conditions. Moreover, contact of blood with components of the extracorporeal circuit directly activate circulating cells (i.e. macrophages–monocytes or platelets) and protein systems (i.e. coagulation, complement, contact phase kallikrein–kinin system), leading to induction of pro-inflammatory cytokines and resulting in chronic low-grade inflammation, further contributing to poor outcomes. The multifactorial, repetitive HD-induced stress that globally reduces tissue perfusion and oxygenation could have deleterious long-term consequences on the functionality of vital organs such as heart, brain, liver and kidney. In this article, we summarize the multisystemic pathophysiological consequences of the main circulatory stress factors. Strategies to mitigate their effects to provide more cardioprotective and personalized dialytic therapies are proposed to reduce the systemic burden of HD.
Keywords: biocompatibility, cardiovascular disease, coagulation, complement system, hemodialysis, inflammation, intradialytic complications, systemic stress, volume status
INTRODUCTION
The success of hemodialysis (HD) as a life-sustaining therapy is undisputed: it allows most patients with end-stage kidney disease (ESKD) to lead reasonably normal and active lives for several years or even decades depending on their age and comorbid conditions. What is questioned, however, is the level of burden the disease represents to patients and to healthcare systems faced with the enormous costs of the therapy [1–3]. Patients on intermittent HD are at increased risk of cardiovascular disease (CVD), which is the leading cause of death among this population, with cardiovascular (CV) complications contributing significantly to the high incidences of hospitalization [4]. Much has been written about the poor long-term outcomes of dialysis patients compared with those with other chronic conditions and the need to develop strategies to overcome the discrepancy [5]. However, it is the intra- and interdialytic morbidity that patients encounter regularly that is debilitating [6–9]. The notion that HD per se contributes to this morbidity and mortality has been addressed only sporadically. HD is a systemic stress condition consisting of two main components, hemodynamic and non-hemodynamic experienced recurrently, usually thrice weekly, or more frequently depending on treatment schedules. There is growing recognition and evidence for the physiological burden and systemic cardiocirculatory stress that HD therapy represents [10] (Figure 1).
Figure 1:
Kidney replacement therapy (e.g. HD) represents only one component of a complex array of considerations in the management of end-stage CKD. The cyclical fluctuations of various physiological processes that accompany intermittent schedules represent a repetitive, unphysiological stress condition for the patient, impacting both outcomes and quality of life.
We examine the case for our assertion that intradialytic morbidity is the clinical expression of systemic stress associated with each HD session and, if unchecked, may act over the years of treatment as a disease modifier, aggravating cardio- and multi organ injury, in conjunction with pre-existent comorbidities, especially in elderly patients [11]. We propose strategies that could mitigate dialysis-induced systemic stress (DISS) as well as CV risks to improve patient well-being, particularly their quality of life.
The ‘unphysiology of hemodialysis’ (extreme physiology of hemodialysis treatment)
As early as the mid-1970s when dialysis had already established itself as a routine therapy (greatly facilitated by the development of the shunt some 15 years earlier), Kjellstrand et al. [12] first raised the specter of intermittent HD inducing debilitating side effects such as nephropathy. This ‘unphysiology’ of HD, which is the major determinant of the side effects of HD, was attributed, among various factors, to extremes in the cycling of water and electrolytes that occur in intermittent dialysis, particularly of uncooperative patients, and may be more dangerous than high levels of uremic ‘toxins’. At that time, resolution of technological restraints that had for so long impeded progress created enthusiasm to further increase the detoxification efficiency of the therapy—and profits—and any winds of caution were unwelcome [13]. Since then the cyclical fluctuations of various physiological processes that intermittent HD schedules (usually 4 h, 3/week) generate have been analyzed and debated. The unphysiology and limitations of intermittent HD schedules used today have also been expressed in mathematical terms [14–16]. The historical and scientific background of ‘dialysis unphysiology’ has been elegantly summarized by Kim [17]. Together with the ‘trade-off’ hypothesis of Bricker [18] that Depner [19] expounded upon to propose the ‘residual syndrome’ hypothesis, dialysis is now recognized as an imperfect therapy and a compromise, with benefits seemingly outweighing the perturbations HD creates.
Techniques to assess the impact of DISS on organ damage
The well-documented uremic and dialytic morbidity associated with the correction of uremia [removal of organic uremic retention solutes (URS) as well as inorganic compounds like water, sodium and phosphate] has repercussions beyond the observed intra- and interdialytic adverse symptoms [20, 21]. The cumulative effects of these aberrations that result from being on HD therapy for long periods of time are observed in several body organs, impairing their normal functioning and leading to a specific dialysis-related pathology (i.e. accelerated atherosclerosis, β2-microglobulin amyloidosis, vascular and valvular calcification, protein energy wasting, accelerated aging). In fact, the systemic circulatory stress exacerbated by the dialysis procedure starts very early into an HD session (usually the first 60 min) and increases over the course of the treatment, meaning that hypovolemia resulting from ultrafiltration may not be the only cause of this phenomenon [22–24].
The strides made in the refinement in recent years of non-invasive organ imaging techniques based on magnetic resonance imaging (MRI), computed tomography, echocardiography, angiography and ultrasound and their variants are today important tools to help better understand physiological as well as pathological processes [25, 26]. Together with sophisticated computer-aided software algorithms, astonishing functional details and mapping of organ or tissue damage can be studied with three-dimensional visualization and image acquisition and is being applied to nephrology and related fields [26, 27]. In conjunction with high-sensitivity laboratory cardiac biomarkers, the progressive effects of DISS can be monitored and studied in considerable detail [28]. A general measure of DISS that is relatively easy to assess is central venous oxygen saturation (ScvO2), which was shown to correlate with cardiac output in the chronic HD patient population studied [29, 30]. In most patients, ScvO2 declines during HD due to factors such as reduced preload, myocardial stunning and intermittent arrhythmias, and a high ScvO2 variability is associated with all-cause mortality in dialysis patients [31]. As well as the findings of organ-specific imaging techniques, the decline in ScvO2 during dialysis was found to be related to ultrafiltration volume and could evolve into a novel marker to monitor hemodynamic response to HD [31].
The multisystemic effects of hemodialysis-induced systemic stress: examples of organ damage
Cardiac stunning
Myocardial stunning is a transient post-ischemic cardiac dysfunction characterized by a temporary reduction in myocardial perfusion and contractility in various segmented areas; transient myocardial ischemia (MI) may lead to left ventricular (LV) dysfunction that can persist after the return of normal perfusion [32]. Three possible outcomes of MI are death of myocardial cells resulting in infarction and tissue scarring (i.e. myocardium fibrosis) with no recovery of cardiac functionality either contracting (systolic dysfunction) or relaxing (diastolic dysfunction) [33]; duration and severity of MI are not long or severe enough to kill cells and by reperfusion myocardium is viable but stunned, exhibiting contractile and biochemical dysfunction with potential sequelae; and chronic low blood flow that causes hibernating myocardium contractile abnormalities, viability and dedifferentiated cells [34].
The procedure of HD exerts acute stress upon the CV system that begins early (especially during the first 30–60 min) in a session and increases during the course of the treatment [10, 22, 35]. Conventional HD itself has been shown to be a sufficient CV functional stressor to precipitate such recurrent ischemic insults, leading to myocardial functional and structural changes [36]. Several factors contribute to cardiac stress in HD, including dialysis modality, treatment time, ultrafiltration volume and rate and electrolyte management (discussed below). In chronic HD, recurrent stunning contributes to heart failure and cardiac death, with ultrafiltration and intradialytic hypotension (IDH) being the principal determinants of this injury; even in critically ill acute kidney injury patients, initiation of continuous kidney replacement therapy was shown to be associated with cardiac stunning despite stable hemodynamics [37]. Importantly, the same process of dysregulated blood flow under the stress of HD that affects the myocardium also affects perfusion of a variety of vascular beds and may be an important element in the development of the poor outcomes in HD patients, manifested by dysfunction of key organ systems.
Brain
Kidney impairment and elements of the HD procedure itself may increase the risk of stroke; dialysis patients experience a 10-fold higher incidence, with case fatality rates reaching 90% [38]. High hemodynamic fluctuations, high variability of blood pressure, dialysate and anticoagulants, vascular access, dialysis amyloidosis, vascular calcification (VC) and years on dialysis may trigger both ischemic and hemorrhagic strokes [39]. Dialysis initiation constitutes the highest stroke risk period; in a 22-year, single-center study, among 151 HD patients with acute stroke, almost half of brain infarcts and more than one-third of brain hemorrhages occurred during or <30 min after the start of a dialysis session [40]. Patients undergoing long-term maintenance dialysis are at increased risk of stroke, with HD patients more likely to develop hemorrhagic stroke than those undergoing peritoneal dialysis [41]. A US analysis of ∼21 000 older dialysis patients found that stroke rates reached a peak during the first 30 days after dialysis initiation [42]. However, it is now well established that arrhythmias (i.e. atrial fibrillation) frequently unrecognized in HD patients may be the cause of ischemic stroke while the preventive use of anticoagulant (warfarin) in chronic atrial fibrillation may be associated with severe hemorrhagic stroke [43–48].
The high burden of cognitive impairment in HD patients is also well established by several studies [49–51]. Up to 70% of elderly HD patients have moderate–severe chronic cognitive impairment depending on the tool used. A recent study exploring HD-associated brain injury by means of a sophisticated diffusion tensor MRI showed that conventional hyperthermic HD resulted in significant brain injury leading to brain white matter damage, which was prevented by hypothermic dialysis [52]. As this study suggests, recurrent and cumulative brain ischemic insults may contribute to acute decline in cognitive function during dialysis and may be prevented by hypothermic dialysis [53].
Gut
The acute circulatory stress effects of dialysis itself may contribute significantly to the development of gastrointestinal dysfunction to induce ‘gut stunning’ [10]. Ultrafiltration-induced gut ischemia may lead to bacterial endotoxin translocation from the gut to the bloodstream [54]. The link between endotoxins and systemic inflammatory reactions in dialysis is well established [55]. Increased gut permeability (also causing increased release of gut-derived uremic toxins such as indoxyl sulfate into the systemic circulation) and blood endotoxin has also been associated with cardiac stunning, with more frequent HD regimens being associated with lower endotoxin levels [56, 57]. HD-induced endotoxemia represents a plausible link between intradialytic hemodynamics and chronic inflammation.
Liver
The hepatic circulation is involved in adaptive systemic responses to circulatory stress and hepato-splanchnic circulatory stress has been proposed as an important effect of HD [58]. The liver receives a high proportion of cardiac output and holds a significant volume of blood (10–15% of total blood volume) and has a role in the maintenance of hemodynamic stability during circulatory stress and is also vulnerable to chronic hypervolemia and hypovolemia, which are common in HD patients [59, 60]. An observational study suggested that the complex hepatic response to ultrafiltration changes may play an important role in IDH and fluid shifts [61]. In contrast to the reduced total body water content, liver water content did not decrease post-HD, consistent with a diversion of blood to the hepatic circulation, in those patients with signs of greater circulatory stress.
Vasculature
Arterial stiffening is highly pronounced in patients with chronic kidney disease (CKD). Carotid artery stiffening is not only a powerful predictor of CV mortality and morbidity but correlates better with LV mass in dialysis patients than brachial artery blood pressure [62]. Arterial stiffening involves various mechanisms that include long-term ones such as atherosclerosis and VC in relation with the uremic milieu and the endothelial dysfunction, but also short-term ones such as those due to vascular sodium content increase or physical deconditioning [63–68]. VC is believed to be a major cause, but the phenomenon is complex, involving the interplay of promoters and inhibitors of calcification in a uremic milieu, dietary phosphate, use of vitamin D and calcimimetics, phosphate removal by binders and dialysis [69]. Dialysis vintage is a major risk factor for VC in CKD [70, 71]. Dialysis procedure and delivery impact vessel stiffening as changes in dialysate calcium levels result in changes in pulse wave velocity, an indicator of target organ damage [72]. Although calcium accumulation begins predialysis, dialysis accelerates VC through the induction of vascular smooth muscle cell (VSMC) apoptosis during HD, disabling VSMC defense mechanisms and leading to overt calcification [73].
The major stressors of systemic stress in hemodialysis
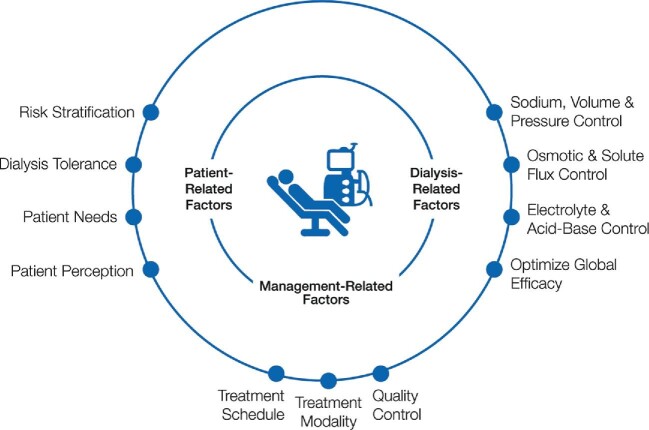
The multisystemic stress induced by HD has various origins (Figure 2). The mode of delivery of HD, components of the extracorporeal circuit (ECC) as well as patient-related factors collectively contribute to the stress load. Pathophysiological drivers of the phenomenon can be divided into two distinct categories, those of hemodynamic (i.e. cardiocirculatory) origin and non-hemodynamic drivers pertaining to the HD session (i.e. hypoxemia, osmotic and electrolytic changes, bioincompatibility). Significantly, these stress factors contribute to the symptom burden patients with kidney failure carry, negatively impacting their perception of the therapy and quality of life and impairing their cognitive functions, but they also contribute to the end damage of vital organs.
Figure 2:

The various origins of multisystemic stress induced by HD therapies can be divided into two main categories, hemodynamic and non hemodynamic. DISS acts as a negative disease modifier to worsen long-term outcomes and contributes significantly to end damage of vital organs and the symptom burden of HD patients.
Hemodynamic systemic stress factors
The current standard mode of delivery of HD (4 h sessions, thrice weekly) exposes patients to wide fluid volume fluctuations in the short intradialytic (∼4 h) and the extended interdialytic phases (∼44–68 h), a highly unphysiological profile. Mechanistically, these are two separate hemodynamic stress states, an acute intradialytic hemodynamic stress phase reflecting intravascular fluid depletion induced during the HD session (ultrafiltration and sodium removal) followed by the prolonged chronic interdialytic hemodynamic period of extracellular fluid accumulation and overload.
Intradialytic hypotension and hypovolemia
Acute hemodynamic stress is induced by ultrafiltration, sodium and fluid removal, which are fundamental to all HD sessions to meet the clinical need of removing excess water (equating to typically 1–4 kg weight gain, most of it water) that accumulates during the interdialytic period. Cyclical volemic changes (hypovolemia alternating with hypervolemia) result in chronic cardiac loading and acute unloading, causing repetitive stretching and shortening of the myocardium to promote cardiac fibrosis. Although less severe hypovolemia may go unnoticed by the physician, it may still have important consequences such as IDH or subclinical organ damage that can accumulate over the years of treatment [35]. Ultrafiltration and/or the HD procedure itself contributes to a decline in circulating blood volume that is partly compensated by blood redistribution and vascular refilling from the interstitial space. As during most HD sessions, the vascular refilling rate (driven, for example, by an increase in plasma protein concentration and oncotic pressure) does not fully compensate for the ultrafiltration rate and there is a decline of effective blood volume. The result is IDH and reduced tissue perfusion, potentially having a long-term structural and functional impact on all vital organs. The main causes of dialysis-induced hypotension are age hypovolemia (rapid ultrafiltration) and coexisting diseases (e.g. autonomic neuropathy, CVDs, diabetes) [74]. Cardiac stunning, a temporary reduction in myocardial perfusion and contractility, can occur in the absence of ultrafiltration (besides hypovolemia, dialysis-associated factors may be involved in the pathogenesis of HD-induced regional LV dysfunction) [23]. However, the dialysis treatment itself or rapid fluid removal by ultrafiltration—expressed in volume per time scaled to body weight—and hypovolemia contribute to cardiac stunning due to a decline in myocardial tissue perfusion [35, 75–78]. The systemic response to the patient–HD interaction and fluid removal is even more complex since it involves other factors such as thermal balance, reflecting dialysate-patient temperature gradient, electrolyte fluxes that depend on dialysate-patient gradients and also the individual patient's baseline cardiac and hemodynamic reserve and the neurohumoral stress response.
Furthermore, detailed intradialytic imaging studies have shown that it is causally related to the ultrafiltration rate and occurs even in the absence of coronary artery disease. In the face of ongoing ultrafiltration, dialysis-induced intravascular volemia and decreased stroke volume, arterial blood pressure and tissue perfusion are maintained by an increase in vascular tone, mainly in vasoconstriction in alpha-adrenoceptors, and venous return, although this response can be mitigated by a dialysis-induced increase in core temperature. A number of associations between mortality and ultrafiltration, change in blood pressure and end-organ ischemic injury have been reported [79]. Potential mechanistic pathways include ultrafiltration-related ischemia to the heart, brain and gut and volume overload–precipitated cardiac stress from reactive measures to ultrafiltration-induced hemodynamic instability [80].
Interdialytic fluid overload and hypertension
Sodium and fluid accumulation that occur in dialysis patients over time due to repetitive positive fluid imbalance is responsible for chronic extracellular fluid overload and its adverse effects, mainly hypertension and CV consequences leading to poor outcomes including frequent hospitalization for pulmonary edema [81]. Hypertension as part of this constellation of disorders is widely recognized as a leading cause for LV cardiomyopathy and accelerated atherosclerosis, including coronary artery disease, peripheral artery disease and cerebrovascular disease [82–84]. Interestingly, as shown in recent studies, the presence of fluid overload acts either as an independent risk factor or as an additive risk factor of hypertension to worsening outcomes [85]. In addition, apart from cardiac consequences, chronic fluid overload is associated with other derangements that include inflammation (fluid overload–inflammation axis) and related metabolic disorders (i.e. protein energy wasting, aging, insulin resistance) [86].
Management of sodium and fluid excess to restore fluid status homeostasis is frequently summarized by the ‘dry weight’ probing approach [87]. Although this clinical approach has been associated with benefits in CV outcomes, it is now challenged by studies showing that the intensity or aggressiveness to remove sodium and fluid excess (i.e. ultrafiltration volume, dialysate sodium concentration) in conventional thrice-weekly HD might induce excessive hemodynamic stress and potential organ damage, with potentially deleterious consequences on long-term outcomes. Interestingly, it is widely recognized that a long interdialytic gap in a thrice-weekly treatment schedule is associated with a significant increase in hospitalization and mortality from CV origins [88–90]. From a clinical perspective, it is well perceived that assessment and management of the fluid status of HD patients is not an easy task. Whatever the complexity, that should remain the basic fundamental and permanent aim in HD patient management, to control blood pressure and to restore hemodynamic equilibrium. In that context, it is clear that precise sodium, fluid and pressure management will rely in the future on a stepwise approach including clinical assessment and management (i.e. patient probing), instrumental tools support (i.e. inferior vena cava diameter, relative blood volume change, bioimpedance, lung ultrasound), cardiac and volemic biomarkers (i.e. B-type natriuretic peptide, copeptin, troponins) and advanced analytic tools (i.e. artificial intelligence, deep learning) [91, 92]. Furthermore, management of HD patients will also require new treatment perspectives, including a more personalized approach (i.e. treatment option, time, frequency) and new tools (i.e. dialysate sodium management, blood volume monitoring) [93].
Cardiac arrhythmias
Sudden cardiac death (SCD) and cardiac arrhythmias (CA) are frequent in HD patients [94]. According to US Renal Data System data [95], SCD and CA are the leading causes of death in HD patients, accounting for 26.9% of all-cause mortality in prevalent HD patients. The prevalence of symptomatic atrial and ventricular arrhythmias has been reported to range from 20 to 70%, with an average of 26%, in HD patients depending on the markers or tools used [96]. Most of studies exploring CA in HD patients have involved short-term Holter monitoring and reported that atrial arrhythmias are most prevalent, not necessarily requiring treatment [97, 98]. However, the prevalence of arrhythmias in intermittent HD patients is likely to be underestimated when it is based on symptomatic arrhythmias. When continuous cardiac rhythm monitoring over a prolonged period of time is performed using implantable loop recorders, the prevalence of significant asymptomatic arrhythmias is much more frequent than expected [94, 99]. In a recent prospective study involving 66 HD patients using such recorders to detect asymptomatic arrhythmias, 1678 CA events were recorded in 44 patients. The majority were bradycardias (n = 1461), with 14 episodes of asystole and only 1 of sustained ventricular tachycardia. The CA rate was highest during the first dialysis of thrice-weekly HD sessions and increased during the last 12 h of each interdialytic interval, particularly the long interval. Atrial fibrillation, although not defined as clinically significant arrhythmias, was detected in 41% of patients and was highest during the HD session. HD conditions and efficiency contributed to favor arrhythmia occurrence, including warm dialysate ≥37°C (hyperthermic dialysis), low dialysate calcium (<1.25 mmol/L), low dialysate potassium (<2 mmol/L) and sodium modeling [100, 101]. Although the CKD5 dialysis patients are known to be at increased risk for arrhythmias, the underlying mechanisms of CA and its association with SCD have not been completely delineated. Various pathogenetic factors, including hypertension, pre-existing cardiomyopathy or coronary artery disease, and HD conditions have been implicated in triggering CAs. Indeed, among them, cyclical cardiac structural changes (stretching and shortening) due to volemic fluctuations induced by intermittent ultrafiltration, pulmonary arterial hypertension, electrolyte and acid-base disorders as well as acute changes are believed to be important contributing factors [102]. Furthermore, dialysate calcium and magnesium concentration changes, interacting with other ion changes (i.e. potassium, acid–base), as well as hemodynamic insults linked to HD sessions are recognized as pro-arrhythmogenic conditions [103, 104].
Non-hemodynamic systemic stress factors
Hypoxemia (impaired respiratory function)
Other than reduced tissue perfusion, hypoxemia observed in patients is believed to contribute to increased mortality, CA and CV events [105]. The phenomenon of hypoxemia has been known for some time in relation to sequestration of neutrophils in the pulmonary vasculature due to bioincompatibility and is usually apparent within the first hour of initiation of dialysis, suggesting the occurrence of respiratory stress resulting from impaired pulmonary gas exchange or an impaired ventilator drive. With the advent of more biocompatible dialysis, reduced ventilatory drive due to a rapid increase in plasma bicarbonate likely plays a larger role. The causes of hypoxemia are thus multifactorial, with the interplay of both direct HD-related factors as well as pathologies related to kidney failure and underlying lung disease [106]. Pulmonary lung hypertension due to chronic fluid overload may also be considered as a potential cause of impaired gas exchange [102]. Hypoxia has also been implicated in an increase in sympathetic tone, oxidative stress and inflammation [107]. Recently hypoxemia has been associated with intradialytic and peridialytic paradoxical hypertension with increased mortality [108], possibly mediated by sympathetic activation and endothelin-1 secretion [109, 110]. Extended intradialytic hypoxemia is likely to aggravate end-organ damage by reducing further tissue oxygen delivery, although it is difficult to distinguish the effects of intradialytic hypoxemia from those of pre-existing respiratory pathologies, sleep apnea and fluid overload in relation to outcome. In addition to these factors, capillary rarefaction and reduced mitochondrial efficacy can further affect the balance between cellular oxygen supply and demand contributing to aggravate cell damage [111].
Thermal (hyper- or hypothermic dialysis)
The reduction in frequency of symptomatic hypotension and slowing of the rate of decrease in blood pressure by cool dialysate has been known for some time [112]. Regional systolic LV function was significantly less impaired at low dialysate temperatures, with fewer episodes of hypotension as a result of higher peripheral resistance and no difference in stroke volume [113]. Adjusting HD thermal balance by reducing dialysate temperature is thus a simple, low-cost strategy to improve hemodynamic tolerance with a low degree of patient discomfort [113, 114]. The potential mechanisms and determinants of extracorporeal energy balance and the hemodynamic consequence of HD-induced thermal stress have been described by Kooman et al. [35].
Biochemical stressors: solute, electrolyte and osmotic imbalances
The success of HD as a therapy depends on the bidirectional transport processes occurring across the semipermeable membrane. Facilitating the movement of substances in both directions, i.e. from blood to the dialysate compartment and vice versa, is fundamental to HD: in removing uremic toxins and water from the blood in one direction, blood has to be simultaneously replenished with electrolytes and buffers in the opposite direction. These transport processes, either passive (diffusion) or forced (convection), are highly unphysiological and the biological fluctuations carried out intensely and intermittently during each session alter body composition, inducing large osmotic and biochemical composition changes, but also removing useful substances such as glucose, amino acids and nutrients (i.e. vitamin E as a natural antioxidant), to aggravate other stressors of circulatory stress through their deleterious action on endothelial cells. The intradialytic biochemical changes, large osmotic pressure fluctuations (e.g. due to urea), acid–base imbalance and electrolyte and water shifts are described as ‘dialysis disequilibrium syndrome’ [19, 115]. Clinical manifestations of these shifts range from minor (fatigue, headache) to severe (impaired cognition, arrhythmias), including neurologic manifestations that progress sequentially as cerebral edema worsens and intracranial pressure rises, and if not promptly recognized and managed, can lead to coma and even death [116].
Blood-incompatibility reactions including inflammation and complement
In leaving the protective environment of the endothelium that lines all vessels of the circulation, blood contacts air and interacts with different artificial surfaces of the ECC, triggering a series of reactions. While the dialysis membrane is the focus of the separation processes of HD and of blood–material interactions, the ECC comprises several polymeric materials, each of which represents a different stimulus for the activation of blood cells and diverse biochemical pathways. Activation of the coagulation, complement, immune and inflammation pathways has been the most widely studied of reactions during HD. The resultant blood incompatibility that occurs has been documented to have severe effects on patients and the functioning (solute removal) of the therapy.
Dialysis necessitates the use of anticoagulants, which themselves trigger unwanted reactions. Heparins, the most widely used anticoagulants worldwide, trigger heparin-induced thrombocytopenia in certain patients; although the incidence of these reactions is very low, the consequences can be severe. Long-term use of heparins has been associated with some metabolic side effects (i.e. hypoaldosteronism, osteoporosis and bone fracture) and other potential safety issues [117–120]. Heparin is also problematic for patients on other medications such as antithrombotic agents (e.g. warfarin and aspirin) or at high-bleeding risk and needs to be administered and monitored with care due to the potential hemorrhagic risk [121, 122]. Contact of blood with the ECC induces complement activation and generation of pro-inflammatory cytokines that are also produced by endotoxins entering the bloodstream by the mechanism of backtransport from dialysis fluids with bacterial contamination. Components of the polymers of the ECC may leach into the blood and cause cytotoxic reactions. Direct physical trauma to red cells (hemolysis) by pumps releases substances that cause platelet aggregation, and denaturation of plasma proteins occurs at the blood–air interface, especially in the bubble trap chamber.
Approaches to mitigate hemodialysis-induced circulatory stress: cardioprotective strategies
The multifactorial and multisystemic effects of circulatory stress that creates an unphysiological environment are believed to be largely responsible for the array of symptoms in HD patients [123]. The result is both direct, short-term physical discomfort (headaches, nausea, itching, fatigue) that patients regularly must endure, as well as sustained multiorgan damage that impairs normal functioning of various organs to impact patient survival and general quality of life. Both can be alleviated by a combination of judicious selection of patient- and treatment-specific conditions to prevent dialysis-induced cardio- and multiorgan damage [93] (Figure 3).
Figure 3:
Potential approaches to mitigate the effects of DISS. HD treatment factors [reduced blood flow, treatment schedule (i.e. increased time or frequency of treatment)] can reduce DISS. In addition, a more personalized approach, coupled with ‘smart machine’ options to adjust therapy conditions according to patient characteristics, would help alleviate multisystemic stress for the patient.
Better patient management: targeting fluid volume control and restoring sodium, volume and pressure homeostasis
Reducing and preventing dialysis-induced hemodynamic stress is crucial toward more multiorgan-protective therapy approaches and improving patient experiences. The quest for optimal fluid volume of HD patients related to hemodynamic management—intradialytic as well as interdialytic—is a cornerstone of dialysis adequacy and prescription. The highly contentious topic remains a matter of concern as opinions vary regarding how best to restore homeostasis of extracellular volume, achieving adequate blood pressure control and preserving hemodynamic equilibrium [105, 124, 125]. Achieving patient-specific fluid balance is challenging in dialysis patients, as chronic fluid overload is commonly associated with increased CVD mortality and morbidity, whereas excessive or too fast fluid removal can lead to multiorgan damage (e.g. myocardial, gut, brain ‘stunning’) [126–128]. Extracellular fluid status should be a component of sufficient dialysis, such that approaching normalization of extracellular fluid volume is a primary goal of dialysis care.
The conventional approach to optimal management of fluid and sodium imbalance in dialysis patients is achieved by adjusting salt and fluid removal through dialysis and salt intake restriction, and fluid gain between HD sessions. This ‘dry weight adjustment approach’ may provide benefits in CV outcomes but is not conducive because of the discontinuous nature of the HD treatment and/or patient intolerance to fluid and sodium removal. Recent studies have shown that the intensity or aggressiveness of removing fluid during conventional thrice-weekly dialysis might induce excessive hemodynamic stress and potential organ damage, with potentially deleterious consequences in the long term [10, 126]. Longer session treatment times and/or increased frequency of therapy sessions are the obvious answer and evidence suggests reduced levels of dialysis-induced cardiac injury [129]. As circulatory stress is mainly time-dependent, prolonged or more dialysis treatment may reduce the homeostatic burden on the patient. In this regard, compressing the 3-day interdialytic interval by moving to every other day dialysis treatment would have the potential to mitigate this additional CV risk [130]. The benefits of increased HD time and frequency, particularly for better removal of uremic toxins, are undisputed [131–133]; however, the increased costs related to nursing time, consumables and logistics is an impediment to the approach [92, 134]. Thus fluid removal should be gradual and routine HD treatment duration should not be <4 h without justification based on individual patient factors [124].
Ultrafiltration is the denominator that is common to most of the circulatory stressors that are believed to contribute to multiorgan damage [135]. Considering the complexity of the hemodynamic response to ultrafiltration, it has been suggested that the time of dialysis should be adjusted in such a way that patients would not suffer from symptoms related to rapid ultrafiltration, which is associated with higher mortality risk [76, 80, 133, 136, 137]. Barbieri et al. [138] have reviewed different approaches based on the absolute or relative blood volume (automated or closed-loop feedback control) of ultrafiltration to prevent critical reductions in circulating blood volume and improve hemodynamic stability and reduce the incidence of hypotension. Adapting the ultrafiltration rate, dialysate sodium and treatment time (i.e. dialysis prescription) in a more precise and personalized way with the application of modern technology and analytical tools to ensure optimal fluid status control would help minimize the risks associated with hemodynamic stress [139].
An increase in core temperature could induce an undesired hemodynamic response (vasodilation, tachycardia or drop in ejection fraction). An effective strategy to improve hemodynamic tolerance is adjusting thermal balance by delivering iso- or hypothermic HD (both effectively reduce hypotension rates) to prevent the patient from warming during the HD session and is most easily achieved by setting the dialysate temperature 0.5–1°C below the patient's core temperature. Isothermic dialysis requires the use of a blood temperature monitor device embedded on the HD machine that can precisely control the patient's thermal balance by adjusting the dialysate temperature in response to the blood temperature in the ECC [140].
Strategies to reduce blood incompatibility reactions of the extracorporeal circuit
Interaction of plasma proteins and blood cells with artificial surfaces of the ECC is an inevitable ‘unphysiological’ reality of all extracorporeal blood purification treatment modalities [141]. Biomaterials science has advanced considerably to provide materials that are more compatible with biological substances, but constraints related to the special demands of each therapy application mean that blood components are altered and undesirable biochemical reactions are triggered. The consequences are both short term (relevant for the duration of the therapy) and longer term (persistent insult over several years). Dialysis-induced blood incompatibility can add to the high hemodynamic stress burden dialysis patients already carry, i.e. traditional (hypertension, diabetes, age, obesity, hyperlipidemia, smoking, etc.) and uremia-related risk factors (inflammation, oxidative stress, salt water overload, anemia, malnutrition dyslipidemia) [142, 143]. Mitigation of the intensity of dialysis-related CV burden is clearly crucial to patient well-being.
An effective conceptual approach to diminish the effects of the blood–ECC circuit interaction is the engineering and design philosophy. First, improvement of the ECC involves using the minimal possible amount of polymer material(s) for the tubing system taking blood to and from the dialyser. By using compact, specially designed blood cassettes incorporating a minimal blood trauma-causing pumping system, blood is exposed to markedly less tubing material. Circuitry geometry is an important consideration in the overall blood incompatibility equation, as blood rheology in the ECC impacts the intensity of the blood–material interaction. Short tubing systems are also designed to minimize the blood–air interface (especially in the venous bubble trap chamber). which is known to denature proteins and create air microemboli and result in serious sequelae.
Anticoagulation
Prevention of activation of coagulation is mandatory to prevent the risk of thrombosis complications within the body and clot formation within the ECC that could, together with increased protein adsorption, impair the functioning of the dialyser [120]. Constriction of the lumen of the hollow fibers, particularly in the header region of the filters (where blood flow stagnation regions promote clotting), and plugging of the pores by fibrin clots reduces the effectiveness of the device in clearing uremic toxins from the blood. It should be noted that well before clotting is visible in the ECC, soluble fibrin formation occurs through a combination of inadequate anticoagulation and factor XII activation–initiated coagulation [144]. Heparin, either unfractionated or low molecular weight fractions, is used universally except in rare cases where patients need alternative anticoagulants. The heparin dosage should be titrated to each patient's individual needs; however, there is a general tendency to give suboptimal doses to patients to prevent post-dialysis bleeding events. Heparin-induced thrombocytopaenia, although relatively infrequent, can have severe consequences [120].
Two possible approaches to mitigate the effects of ECC-induced coagulation that have been attempted since the early days of HD are surface modification of polymers used for the manufacture of the membranes and tubing materials. Attachment of either chemical groups or heparin onto surfaces to reduce their thrombogenicity have met with limited success so far [145]. In many instances, significant amounts of heparin have been shown in the laboratory to be attached to surfaces either passively or via covalent attachment [146, 147]. However, heparin (or its derivatives or heparin-like compounds) attached to surfaces is biologically inactive and unable to freely bind antithrombin III to achieve effective and consistent anticoagulation. Recently a novel non thrombogenic material system has been developed that shows promising results toward the goal of having ‘heparin-poor dialysis’ [148].
Complement and immune response
Complement proteins are perceived as sensors and transmitters of ‘danger signals’ that trigger and modulate immune responses to pathological conditions [149]. The proteolytic cascade of events and cell activation with the release of various pro-inflammatory mediators represents a ‘biologic or cytokine storm’ or circulatory stress situation. The complement activation pathway has an historical association with HD and together with thrombogenicity is the stimulus for the blood incompatibility and hypersensitivity phenomena associated with HD membranes [150–152]. Although the clinical significance of HD membrane-initiated complement activation and associated leukopenia remains to be fully elucidated, it is widely seen as an undesirable stress factor in HD and needs to be kept to a minimum [153].
Inflammation
CKD, like most chronic diseases, is essentially an inflammatory condition; any additional pro-inflammatory reactions during the delivery of HD or by the ECC increases the CV load for the patient [154]. Other than membrane or material-related stimuli, a well-recognized and potent source of inflammation in HD is the presence in dialysis fluids of endotoxins that arise from bacterial contamination of the water treatment systems of dialysis units [55, 155, 156]. During growth and lysis, the outer leaflet of Gram-negative bacteria releases lipopolysaccharide (LPS, chemical designation of endotoxins) fragments and other compounds of various sizes into the water. As transport processes in HD membranes are bidirectional, LPS may diffuse into or be forced into the bloodstream by the process of backtransport to trigger systemic inflammation [54, 157, 158]. Other than ensuring that microbiological water quality standards are met from ‘tap to machine’, the CV risk presented endotoxins entering the blood can be reduced by using special endotoxin-retention filters that are today an essential part of modern HD machines [159]. However, the final line of defense is offered by certain types of dialysers that contain membranes that are capable of retaining endotoxins should the ultrafilters be used in ways not recommended by the manufacturers [160].
Mitigating the effects of systemic stress effects of solute fluxes
The ‘biochemical stress’ that reflects rapid biochemical changes occurs because of solute, water and osmotic fluxes (e.g. disequilibrium syndrome). The intensity of such alterations is directly related to the plasma–dialysate gradient and operating conditions (e.g. blood and dialysate flow) during the HD treatment. The systemic stress induced by fluctuations in solute gradients and fluxes (e.g. of dialysate electrolyte levels of sodium, calcium, magnesium, potassium, bicarbonate) depends on their intensity and magnitude and are potentially modifiable factors of HD prescription [17, 161–163] (Figure 3). Approaches to mitigate their effects include reducing blood flow or increasing treatment time or frequency of treatment, which are generally better tolerated and result in less circulatory stress compared with short HD schedules. More personalized approaches based on the use of smart HD machines to adjust solute fluxes according to patient characteristics are currently under clinical evaluation. Today, the most advanced option relies on automated sodium and water management (i.e. isonatremic or zero diffusive sodium dialysis) using dialysate conductivity sensors coupled to specific algorithms embedded in the HD machine [164–166].
Patient-related factors (risk stratification, personalized treatment)
It is important to consider that the response to systemic stress factors detailed in this article may be modulated according to various patient-related considerations, e.g. age, gender, comorbidity, medication. These may explain individual or temporal variations in hemodynamic adaptation. Whatever the reason, the hemodynamic stress of dialysis must be considered as a potent disease modifier, especially in an already highly vulnerable population, e.g. decreased body cell mass associated with aging and muscle attenuation. Patient involvement is crucial to the alleviation of adverse effects of stress factors. The benefits of dietary counseling through patient education programs to advise patients with consistently high interdialytic weight gain to practice salt restriction can help toward achievement of optimal fluid volume status [124, 167]. Personalized dialysis treatment will take advantage in the future of new technologies relying on pervasive smart sensor monitoring devices (i.e. connected watches) and/or point-of-care testing and/or online monitoring devices integrated in dialysis machines [168–170].
CONCLUSIONS
The cost-conscious rather than physiology- and/or personalized-based delivery of modern HD therapies is characterized by poor treatment tolerability and suboptimal long-term outcome [10, 12, 13]. This observation reflects the dual intrinsic limitation of current short HD treatment schedules due to their intermittent character and their limited global efficiency when compared with native kidney functions. In this report we addressed specifically the role of conventional thrice-weekly HD treatment as a potential negative disease modifier, the so called dialysis adequacy criteria, and voluntarily excluded the impact of global treatment efficiency that has been addressed in other reports.
DISS leads to both a significant reduction in overall quality of life and direct physical impacts with potential end-organ damaging effects abstracted in intradialytic morbidity complex syndrome. Conventional HD treatment is a sufficient CV functional stressor to provoke significant systemic circulatory stress [36]. This appears to affect most HD patients and cuts across age and comorbidity-defined groupings. Although significant progress has been achieved over the last few years in improving hemodynamic stability and reducing CV mortality, a more balanced and precise approach is required to reduce DISS in this high-risk population [171–173].
To satisfy this unmet need, it is time to move to a broader approach embracing the entire management of HD patients in a more physiologically based and holistically oriented way rather than focusing only on one component, such as hemodynamic management or HD efficiency. DISS results from multifactorial components that may act as a disease modifier, resulting in multiorgan injury superimposed on pre-existent comorbidities [91]. Intradialytic morbidity is the clinical expression of a composite phenomenon that includes hemodynamic stress (i.e. hypovolemia, IDH, arrhythmias) and non-hemodynamic factors (i.e. osmotic changes, electrolytes changes, hypoxia, inflammation).
Recognition that intradialytic morbidity has variable causes may allow tailoring of personalized treatments and multitargeted interventions in the future to improve outcomes. In this context, three main streams are envisaged: first, establishing initial RRT schedules and various options, based on the patient's risk stratification profile and choice would probably improve short- and mid-term patient perceptions and outcomes; second, positioning kidney replacement treatment in a patient's management integrated trajectory (i.e. incremental HD, home therapy, kidney transplantation) would definitively improve long-term outcomes; third, using innovative technologies based either on smart HD machines and on support of artificial intelligence and i-health-connected tools.
ACKNOWLEDGEMENT
This article is published as part of a supplement supported by Fresenius Medical Care.
Contributor Information
Bernard Canaud, Montpellier University, Montpellier, France; Global Medical Office, FMC Deutschland, Bad Homburg, Germany.
Melanie P Stephens, MSL & Medical Strategies for Innovative Therapies, Fresenius Medical Care, Waltham, MA, USA.
Milind Nikam, Global Medical Office, Fresenius Medical Care, Hong Kong.
Michael Etter, Global Medical Office, Fresenius Medical Care, Hong Kong.
Allan Collins, Global Medical Office, Fresenius Medical Care, Waltham, MA, USA.
CONFLICT OF INTEREST STATEMENT
B.C., M.P.S., M.K., M.E. and A.C. are employees of Fresenius Medical Care.
REFERENCES
- 1. Mitema D, Jaar BG. How can we improve the quality of life of dialysis patients? Semin Dial 2016; 29: 93–102 [DOI] [PubMed] [Google Scholar]
- 2. Versino E, Piccoli GB. Chronic kidney disease: the complex history of the organization of long-term care and bioethics. Why now, more than ever, action is needed. Int J Environ Res Public Health 2019; 16: 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Meyer TW, Hostetter TH. Uremia. N Engl J Med 2007; 357: 1316–1325 [DOI] [PubMed] [Google Scholar]
- 4. Moradi H, Sica DA, Kalantar-Zadeh K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am J Nephrol 2013; 38: 136–148 [DOI] [PubMed] [Google Scholar]
- 5. Jager KJ, Fraser SDS. The ascending rank of chronic kidney disease in the global burden of disease study. Nephrol Dial Transplant 2017; 32: ii121–ii128 [DOI] [PubMed] [Google Scholar]
- 6. Hecking M, Moissl U, Genser B et al. Greater fluid overload and lower interdialytic weight gain are independently associated with mortality in a large international hemodialysis population. Nephrol Dial Transplant 2018; 33: 1832–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Salem M, Ivanovich PT, Ing TS et al. Adverse effects of dialyzers manifesting during the dialysis session. Nephrol Dial Transplant 1994; 9 (Suppl 2): 127–137 [PubMed] [Google Scholar]
- 8. Williams A. Hemodialysis and peritoneal dialysis. In: Godbole PP, Koyle MA, Wilcox DT (eds). Pediatric Urology: Surgical Complications and Management. Hoboken, NJ: John Wiley & Sons, 2015: 307–314 [Google Scholar]
- 9. Correa S, Pena-Esparragoza JK, Scovner KM et al. Predictors of intradialytic symptoms: an analysis of data from the hemodialysis study. Am J Kidney Dis 2020; 76: 331–339 [DOI] [PubMed] [Google Scholar]
- 10. McIntyre CW. Recurrent circulatory stress: the dark side of dialysis. Semin Dial 2010; 23: 449–451 [DOI] [PubMed] [Google Scholar]
- 11. Canaud B, Kooman JP, Selby NM et al. Dialysis-Induced cardiovascular and multiorgan morbidity. Kidney Int Rep 2020; 5: 1856–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kjellstrand CM, Evans RL, Petersen RJ et al. The “unphysiology” of dialysis: a major cause of dialysis side effects? Kidney Int Suppl 1975; 2: 30–34 [PubMed] [Google Scholar]
- 13. Kjellstrand CM. My addiction: the artificial kidney, the rise and fall of dialysis. Artif Organs 2012; 36: 575–580 [DOI] [PubMed] [Google Scholar]
- 14. Lopot F, Válek A. Quantification of dialysis unphysiology. Nephrol Dial Transplant 1998; 13 Suppl 6: 74–78 [DOI] [PubMed] [Google Scholar]
- 15. Lopot F, Válek A. Mathematical concept of dialysis unphysiology. Home Hemodial Int (1997) 1998; 2: 18–21 [DOI] [PubMed] [Google Scholar]
- 16. Lopot F, Nejedlý B, Sulková S. Physiology in daily hemodialysis in terms of the time average concentration/time average deviation concept. Hemodial Int 2004; 8: 39–44 [DOI] [PubMed] [Google Scholar]
- 17. Kim GH. Dialysis unphysiology and sodium balance. Electrolyte Blood Press 2009; 7: 31–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bricker NS. On the pathogenesis of the uremic state. An exposition of the “trade-off hypothesis”. N Engl J Med 1972; 286: 1093–1099 [DOI] [PubMed] [Google Scholar]
- 19. Depner TA. Uremic toxicity: urea and beyond. Semin Dial 2001; 14: 246–251 [DOI] [PubMed] [Google Scholar]
- 20. Almeras C, Argilés A. The general picture of uremia. Semin Dial 2009; 22: 329–333 [DOI] [PubMed] [Google Scholar]
- 21. Teschan PE. On the pathogenesis of uremia. Am J Med 1970; 48: 671–677 [DOI] [PubMed] [Google Scholar]
- 22. Assa S, Hummel YM, Voors AA et al. Hemodialysis-induced regional left ventricular systolic dysfunction and inflammation: a cross-sectional study. Am J Kidney Dis 2014; 64: 265–273 [DOI] [PubMed] [Google Scholar]
- 23. Assa S, Kuipers J, Ettema E et al. Effect of isolated ultrafiltration and isovolemic dialysis on myocardial perfusion and left ventricular function assessed with 13N-NH3 positron emission tomography and echocardiography. Am J Physiol Renal Physiol 2018; 314: F445–F452 [DOI] [PubMed] [Google Scholar]
- 24. Butani L, Calogiuri G. Hypersensitivity reactions in patients receiving hemodialysis. Ann Allergy Asthma Immunol 2017; 118: 680–684 [DOI] [PubMed] [Google Scholar]
- 25. Gómez-Gaviro MV, Sanderson D, Ripoll J et al. Biomedical applications of tissue clearing and three-dimensional imaging in health and disease. iScience 2020; 23: 101432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang JL, Morrell G, Rusinek H et al. New magnetic resonance imaging methods in nephrology. Kidney Int 2014; 85: 768–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tian T, Yang Z, Li X. Tissue clearing technique: recent progress and biomedical applications. J Anat 2021; 238: 489–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Passino C, Aimo A, Masotti S et al. Cardiac troponins as biomarkers for cardiac disease. Biomark Med 2019; 13: 325–330 [DOI] [PubMed] [Google Scholar]
- 29. Harrison LE, Selby NM, McIntyre CW. Central venous oxygen saturation: a potential new marker for circulatory stress in haemodialysis patients? Nephron Clin Pract 2014; 128: 57–60 [DOI] [PubMed] [Google Scholar]
- 30. Walley KR. Use of central venous oxygen saturation to guide therapy. Am J Respir Crit Care Med 2011; 184: 514–520 [DOI] [PubMed] [Google Scholar]
- 31. Zhang H, Campos I, Chan L et al. Association of central venous oxygen saturation variability and mortality in hemodialysis patients. Blood Purif 2019; 47: 246–253 [DOI] [PubMed] [Google Scholar]
- 32. Guaricci AI, Bulzis G, Pontone G et al. Current interpretation of myocardial stunning. Trends Cardiovasc Med 2018; 28: 263–271 [DOI] [PubMed] [Google Scholar]
- 33. Escoli R, Carvalho MJ, Cabrita A. et al. Diastolic dysfunction, an underestimated new challenge in dialysis. Ther Apher Dial 2019; 23: 108–117 [DOI] [PubMed] [Google Scholar]
- 34. Kloner RA. Stunned and hibernating myocardium: where are we nearly 4 decades later? J Am Heart Assoc 2020; 9: e015502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kooman JP, Katzarski K, van der Sande FM et al. Hemodialysis: a model for extreme physiology in a vulnerable patient population. Semin Dial 2018; 31: 500–506 [DOI] [PubMed] [Google Scholar]
- 36. McIntyre CW. Haemodialysis-induced myocardial stunning in chronic kidney disease — a new aspect of cardiovascular disease. Blood Purif 2010; 29: 105–110 [DOI] [PubMed] [Google Scholar]
- 37. Slessarev M, Salerno F, Ball IM et al. Continuous renal replacement therapy is associated with acute cardiac stunning in critically ill patients. Hemodial Int 2019; 23: 325–332 [DOI] [PubMed] [Google Scholar]
- 38. Power A. Stroke in dialysis and chronic kidney disease. Blood Purif 2013; 36: 179–183 [DOI] [PubMed] [Google Scholar]
- 39. Miglinas M, Cesniene U, Janusaite MM et al. Cerebrovascular disease and cognition in chronic kidney disease patients. Front Cardiovasc Med 2020; 7: 96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Toyoda K, Fujii K, Fujimi S et al. Stroke in patients on maintenance hemodialysis: a 22-year single-center study. Am J Kidney Dis 2005; 45: 1058–1066 [DOI] [PubMed] [Google Scholar]
- 41. Wang HH, Hung SY, Sung JM et al. Risk of stroke in long-term dialysis patients compared with the general population. Am J Kidney Dis 2014; 63: 604–611 [DOI] [PubMed] [Google Scholar]
- 42. Murray AM, Seliger S, Lakshminarayan K et al. Incidence of stroke before and after dialysis initiation in older patients. J Am Soc Nephrol 2013; 24: 1166–1173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roy-Chaudhury P, Tumlin JA, Koplan BA et al. Primary outcomes of the monitoring in dialysis study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int 2018; 93: 941–951 [DOI] [PubMed] [Google Scholar]
- 44. Wetmore JB, Ellerbeck EF, Mahnken JD et al. Atrial fibrillation and risk of stroke in dialysis patients. Ann Epidemiol 2013; 23: 112–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yoon CY, Noh J, Jhee JH et al. Warfarin use in patients with atrial fibrillation undergoing hemodialysis: a nationwide population-based study. Stroke 2017; 48: 2472–2479 [DOI] [PubMed] [Google Scholar]
- 46. Brancaccio D, Neri L, Bellocchio F et al. Warfarin in CKD patients with atrial fibrillation. Kidney Int 2017; 92: 766–767 [DOI] [PubMed] [Google Scholar]
- 47. Brancaccio D, Neri L, Bellocchio F et al. Patients' characteristics affect the survival benefit of warfarin treatment for hemodialysis patients with atrial fibrillation. A historical cohort study. Am J Nephrol 2016; 44: 258–267 [DOI] [PubMed] [Google Scholar]
- 48. Belley-Cote EP, Eikelboom JW. Anticoagulation for stroke prevention in patients with atrial fibrillation and end-stage renal disease-first, do no harm. JAMA Netw Open 2020; 3: e202237. [DOI] [PubMed] [Google Scholar]
- 49. Murray AM. Cognitive impairment in the aging dialysis and chronic kidney disease populations: an occult burden. Adv Chronic Kidney Dis 2008; 15: 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Seliger SL, Weiner DE. Cognitive impairment in dialysis patients: focus on the blood vessels? Am J Kidney Dis 2013; 61: 187–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kurella Tamura M, Unruh ML, Nissenson AR et al. Effect of more frequent hemodialysis on cognitive function in the frequent hemodialysis network trials. Am J Kidney Dis 2013; 61: 228–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Eldehni MT, Odudu A, McIntyre CW. Randomized clinical trial of dialysate cooling and effects on brain white matter. J Am Soc Nephrol 2015; 26: 957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Drew DA, Weiner DE, Sarnak MJ. Cognitive impairment in CKD: pathophysiology, management, and prevention. Am J Kidney Dis 2019; 74: 782–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. McIntyre CW, Harrison LE, Eldehni MT et al. Circulating endotoxemia: a novel factor in systemic inflammation and cardiovascular disease in chronic kidney disease. Clin J Am Soc Nephrol 2011; 6: 133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Lonnemann G. Chronic inflammation in hemodialysis: the role of contaminated dialysate. Blood Purif 2000; 18: 214–223 [DOI] [PubMed] [Google Scholar]
- 56. Jefferies HJ, Crowley LE, Harrison LE et al. Circulating endotoxaemia and frequent haemodialysis schedules. Nephron Clin Pract 2014; 128: 141–146 [DOI] [PubMed] [Google Scholar]
- 57. McIntyre CW, Odudu A. Hemodialysis-associated cardiomyopathy: a newly defined disease entity. Semin Dial 2014; 27: 87–97 [DOI] [PubMed] [Google Scholar]
- 58. Grant CJ, Huang SS, McIntyre CW. Hepato-splanchnic circulatory stress: an important effect of hemodialysis. Semin Dial 2019; 32: 237–242 [DOI] [PubMed] [Google Scholar]
- 59. Lautt WW. Hepatic vasculature: a conceptual review. Gastroenterology 1977; 73: 1163–1169 [PubMed] [Google Scholar]
- 60. Lautt WW. Hepatic Circulation: Physiology and Pathophysiology. San Rafael, CA: Morgan & Claypool Life Sciences; 2009 [PubMed] [Google Scholar]
- 61. Grant CJ, Wade TP, McKenzie CA et al. Effect of ultrafiltration during hemodialysis on hepatic and total-body water: an observational study. BMC Nephrol 2018; 19: 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Covic A, Goldsmith DJ, Panaghiu L et al. Analysis of the effect of hemodialysis on peripheral and central arterial pressure waveforms. Kidney Int 2000; 57: 2634–2643 [DOI] [PubMed] [Google Scholar]
- 63. Lioufas N, Hawley CM, Cameron JD et al. Chronic kidney disease and pulse wave velocity: a narrative review. Int J Hypertens 2019; 2019: 9189362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med 2011; 17: 1410–1422 [DOI] [PubMed] [Google Scholar]
- 65. Safar ME, Temmar M, Kakou A et al. Sodium intake and vascular stiffness in hypertension. Hypertension 2009; 54: 203–209 [DOI] [PubMed] [Google Scholar]
- 66. Grigorova YN, Wei W, Petrashevskaya N et al. Dietary sodium restriction reduces arterial stiffness, vascular TGF-β-dependent fibrosis and marinobufagenin in young normotensive rats. Int J Mol Sci 2018; 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Nowak KL, Chonchol M, Jovanovich A et al. Serum sodium and pulse pressure in SPRINT. Am J Hypertens 2019; 32: 649–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Gijsbers L, Dower JI, Mensink M et al. Effects of sodium and potassium supplementation on blood pressure and arterial stiffness: a fully controlled dietary intervention study. J Hum Hypertens 2015; 29: 592–598 [DOI] [PubMed] [Google Scholar]
- 69. Ruderman I, Holt SG, Hewitson TD et al. Current and potential therapeutic strategies for the management of vascular calcification in patients with chronic kidney disease including those on dialysis. Semin Dial 2018; 31: 487–499 [DOI] [PubMed] [Google Scholar]
- 70. Cozzolino M, Ciceri P, Galassi A et al. The key role of phosphate on vascular calcification. Toxins (Basel) 2019; 11: 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Chen NC, Hsu CY, Chen CL. The strategy to prevent and regress the vascular calcification in dialysis patients. Biomed Res Int 2017; 2017: 9035193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Charitaki E, Davenport A. Do higher dialysate calcium concentrations increase vascular stiffness in haemodialysis patients as measured by aortic pulse wave velocity? BMC Nephrol 2013; 14: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shroff RC, McNair R, Figg N et al. Dialysis accelerates medial vascular calcification in part by triggering smooth muscle cell apoptosis. Circulation 2008; 118: 1748–1757 [DOI] [PubMed] [Google Scholar]
- 74. Sulowicz W, Radziszewski A. Pathogenesis and treatment of dialysis hypotension. Kidney Int 2006; 70 (Suppl 104): S36–S39 [Google Scholar]
- 75. Flythe JE, Kimmel SE, Brunelli SM. Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 2011; 79: 250–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Assimon MM, Wenger JB, Wang L et al. Ultrafiltration rate and mortality in maintenance hemodialysis patients. Am J Kidney Dis 2016; 68: 911–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Flythe JE, Assimon MM, Wang L. Ultrafiltration rate scaling in hemodialysis patients. Semin Dial 2017; 30: 282–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. McIntyre CW, Burton JO, Selby NM et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 2008; 3: 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Inrig JK. Beware intradialytic hypotension: how low is too low? Clin J Am Soc Nephrol 2018; 13: 1453–1454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Assimon MM, Flythe JE. Rapid ultrafiltration rates and outcomes among hemodialysis patients: re-examining the evidence base. Curr Opin Nephrol Hypertens 2015; 24: 525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dekker MJ, Marcelli D, Canaud BJ et al. Impact of fluid status and inflammation and their interaction on survival: a study in an international hemodialysis patient cohort. Kidney Int 2017; 91: 1214–1223 [DOI] [PubMed] [Google Scholar]
- 82. Wanner C, Amann K, Shoji T. The heart and vascular system in dialysis. Lancet 2016; 388: 276–284 [DOI] [PubMed] [Google Scholar]
- 83. McCullough PA, Chan CT, Weinhandl ED et al. Intensive hemodialysis, left ventricular hypertrophy, and cardiovascular disease. Am J Kidney Dis 2016; 68: S5-s14 [DOI] [PubMed] [Google Scholar]
- 84. Hung SC, Kuo KL, Peng CH et al. Volume overload correlates with cardiovascular risk factors in patients with chronic kidney disease. Kidney Int 2014; 85: 703–709 [DOI] [PubMed] [Google Scholar]
- 85. Tsai YC, Chiu YW, Tsai JC et al. Association of fluid overload with cardiovascular morbidity and all-cause mortality in stages 4 and 5 CKD. Clin J Am Soc Nephrol 2015; 10: 39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dekker MJE, van der Sande FM, van den Berghe F et al. Fluid overload and inflammation axis. Blood Purif 2018; 45: 159–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Ohashi Y, Sakai K, Hase H et al. Dry weight targeting: the art and science of conventional hemodialysis. Semin Dial 2018; 31: 551–556 [DOI] [PubMed] [Google Scholar]
- 88. Foley RN, Gilbertson DT, Murray T et al. Long interdialytic interval and mortality among patients receiving hemodialysis. N Engl J Med 2011; 365: 1099–1107 [DOI] [PubMed] [Google Scholar]
- 89. Zhang H, Schaubel DE, Kalbfleisch JD et al. Dialysis outcomes and analysis of practice patterns suggests the dialysis schedule affects day-of-week mortality. Kidney Int 2012; 81: 1108–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fotheringham J, Fogarty DG, El Nahas M et al. The mortality and hospitalization rates associated with the long interdialytic gap in thrice-weekly hemodialysis patients. Kidney Int 2015; 88: 569–575 [DOI] [PubMed] [Google Scholar]
- 91. Pinter J, Chazot C, Stuard S et al. Sodium, volume and pressure control in haemodialysis patients for improved cardiovascular outcomes. Nephrol Dial Transplant 2020; 35: ii23–ii30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Canaud B, Chazot C, Koomans J et al. Fluid and hemodynamic management in hemodialysis patients: challenges and opportunities. J Bras Nefrol 2019; 41: 550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Canaud B, Collins A, Maddux F. The renal replacement therapy landscape in 2030: reducing the global cardiovascular burden in dialysis patients. Nephrol Dial Transplant 2020; 35: ii51–ii57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kalra PA, Green D, Poulikakos D. Arrhythmia in hemodialysis patients and its relation to sudden death. Kidney Int 2018; 93: 781–783 [DOI] [PubMed] [Google Scholar]
- 95. US Renal Data System 2018 Annual Data Report . Volume 2–ESRD in the United States. Chapter 5: mortality. Am J Kidney Dis 2019; 73 (3 Suppl 1): S411–S426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ansari N, Manis T, Feinfeld DA. Symptomatic atrial arrhythmias in hemodialysis patients. Ren Fail 2001; 23: 71–76 [DOI] [PubMed] [Google Scholar]
- 97. deMello VR, Malone D, Thanavaro S et al. Cardiac arrhythmias in end-stage renal disease. South Med J 1981; 74: 178–180 [DOI] [PubMed] [Google Scholar]
- 98. Winkelmayer WC, Patrick AR, Liu J et al. The increasing prevalence of atrial fibrillation among hemodialysis patients. J Am Soc Nephrol 2011; 22: 349–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. El Hage N, Jaar BG, Cheng A et al. Frequency of arrhythmia symptoms and acceptability of implantable cardiac monitors in hemodialysis patients. BMC Nephrol 2017; 18: 309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Charytan DM, Foley R, McCullough PA et al. Arrhythmia and sudden death in hemodialysis patients: protocol and baseline characteristics of the monitoring in dialysis study. Clin J Am Soc Nephrol 2016; 11: 721–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Schneditz D. Temperature and thermal balance in hemodialysis. Semin Dial 2001; 14: 357–364 [DOI] [PubMed] [Google Scholar]
- 102. Zile MR, Bennett TD, St John Sutton M et al. Transition from chronic compensated to acute decompensated heart failure: pathophysiological insights obtained from continuous monitoring of intracardiac pressures. Circulation 2008; 118: 1433–1441 [DOI] [PubMed] [Google Scholar]
- 103. Kim ED, Watt J, Tereshchenko LG et al. Associations of serum and dialysate electrolytes with QT interval and prolongation in incident hemodialysis: the Predictors of Arrhythmic and Cardiovascular Risk in End-Stage Renal Disease (PACE) study. BMC Nephrol 2019; 20: 133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Tumlin JA, Roy-Chaudhury P, Koplan BA et al. Relationship between dialytic parameters and reviewer confirmed arrhythmias in hemodialysis patients in the monitoring in dialysis study. BMC Nephrol 2019; 20: 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Chou JA, Streja E, Nguyen DV et al. Intradialytic hypotension, blood pressure changes and mortality risk in incident hemodialysis patients. Nephrol Dial Transplant 2018; 33: 149–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Campos I, Chan L, Zhang H et al. Intradialytic hypoxemia in chronic hemodialysis patients. Blood Purif 2016; 41: 177–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kooman JP, Dekker MJ, Usvyat LA et al. Inflammation and premature aging in advanced chronic kidney disease. Am J Physiol Renal Physiol 2017; 313: F938–F950 [DOI] [PubMed] [Google Scholar]
- 108. Meyring-Wösten A, Zhang H, Ye X et al. Intradialytic hypoxemia and clinical outcomes in patients on hemodialysis. Clin J Am Soc Nephrol 2016; 11: 616–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Meyring-Wösten A, Luo Y, Zhang H et al. Intradialytic hypertension is associated with low intradialytic arterial oxygen saturation. Nephrol Dial Transplant 2018; 33: 1040–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Inrig JK, Patel UD, Toto RD et al. Association of blood pressure increases during hemodialysis with 2-year mortality in incident hemodialysis patients: a secondary analysis of the Dialysis Morbidity and Mortality Wave 2 study. Am J Kidney Dis 2009; 54: 881–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Kooman JP, Stenvinkel P, Shiels PG et al. The oxygen cascade in patients treated with hemodialysis and native high-altitude dwellers: lessons from extreme physiology to benefit patients with end-stage renal disease. Am J Physiol Renal Physiol 2021; 320: F249–F261 [DOI] [PubMed] [Google Scholar]
- 112. Sherman RA, Rubin MP, Cody RP et al. Amelioration of hemodialysis-associated hypotension by the use of cool dialysate. Am J Kidney Dis 1985; 5: 124–127 [DOI] [PubMed] [Google Scholar]
- 113. Selby NM, Burton JO, Chesterton LJ et al. Dialysis-induced regional left ventricular dysfunction is ameliorated by cooling the dialysate. Clin J Am Soc Nephrol 2006; 1: 1216–1225 [DOI] [PubMed] [Google Scholar]
- 114. Chesterton LJ, Selby NM, Burton JO et al. Cool dialysate reduces asymptomatic intradialytic hypotension and increases baroreflex variability. Hemodial Int 2009; 13: 189–196 [DOI] [PubMed] [Google Scholar]
- 115. Cerra FB, Anthone R, Anthone S. Colloid osmotic pressure fluctuations and the disequilibrium syndrome during hemodialysis. Nephron 1974; 13: 245–252 [DOI] [PubMed] [Google Scholar]
- 116. Mistry K. Dialysis disequilibrium syndrome prevention and management. Int J Nephrol Renovasc Dis 2019; 12: 69–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Oster JR, Singer I, Fishman LM. Heparin-induced aldosterone suppression and hyperkalemia. Am J Med 1995; 98: 575–586 [DOI] [PubMed] [Google Scholar]
- 118. Yang S, Niu Q, Gan L et al. Effect of long-term use of unfractionated or low-molecular-weight heparin on bone mineral density in maintenance hemodialysis patients. Hemodial Int 2020; 24: 374–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lazrak HH, René É, Elftouh N et al. Safety of low-molecular-weight heparin compared to unfractionated heparin in hemodialysis: a systematic review and meta-analysis. BMC Nephrol 2017; 18: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Suranyi M, Chow JS. Review: anticoagulation for haemodialysis. Nephrology (Carlton) 2010; 15: 386–392 [DOI] [PubMed] [Google Scholar]
- 121. Swartz RD, Port FK. Preventing hemorrhage in high-risk hemodialysis: regional versus low-dose heparin. Kidney Int 1979; 16: 513–518 [DOI] [PubMed] [Google Scholar]
- 122. Shen JI, Mitani AA, Winkelmayer WC. Heparin use in hemodialysis patients following gastrointestinal bleeding. Am J Nephrol 2014; 40: 300–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kjellstrand CM, Evans RL, Petersen RJ et al. The “unphysiology” of dialysis: a major cause of dialysis side effects? Hemodial Int 2004; 8: 24–29 [DOI] [PubMed] [Google Scholar]
- 124. Weiner DE, Brunelli SM, Hunt A et al. Improving clinical outcomes among hemodialysis patients: a proposal for a “volume first” approach from the chief medical officers of US dialysis providers. Am J Kidney Dis 2014; 64: 685–695 [DOI] [PubMed] [Google Scholar]
- 125. Chou JA, Kalantar-Zadeh K. Volume balance and intradialytic ultrafiltration rate in the hemodialysis patient. Curr Heart Fail Rep 2017; 14: 421–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. London GM. Ultrafiltration intensification for achievement of dry weight and hypertension control is not always the therapeutic gold standard. J Nephrol 2011; 24: 395–397 [DOI] [PubMed] [Google Scholar]
- 127. McIntyre C, Crowley L. Dying to feel better: the central role of dialysis-induced tissue hypoxia. Clin J Am Soc Nephrol 2016; 11: 549–551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Garg N, Fissell WH. Intradialytic hypotension: a case for going slow and looking carefully. Nephrol Dial Transplant 2013; 28: 247–249 [DOI] [PubMed] [Google Scholar]
- 129. Jefferies HJ, Virk B, Schiller B et al. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning). Clin J Am Soc Nephrol 2011; 6: 1326–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Georgianos PI, Sarafidis PA, Sinha AD et al. Adverse effects of conventional thrice-weekly hemodialysis: is it time to avoid 3-day interdialytic intervals? Am J Nephrol 2015; 41: 400–408 [DOI] [PubMed] [Google Scholar]
- 131. Chazot C, Jean G. The advantages and challenges of increasing the duration and frequency of maintenance dialysis sessions. Nat Clin Pract Nephrol 2009; 5: 34–44 [DOI] [PubMed] [Google Scholar]
- 132. Greene T, Daugirdas JT, Depner TA et al. Solute clearances and fluid removal in the frequent hemodialysis network trials. Am J Kidney Dis 2009; 53: 835–844 [DOI] [PubMed] [Google Scholar]
- 133. Saran R, Bragg-Gresham JL, Levin NW et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int 2006; 69: 1222–1228 [DOI] [PubMed] [Google Scholar]
- 134. Hakim RM, Saha S. Dialysis frequency versus dialysis time, that is the question. Kidney Int 2014; 85: 1024–1029 [DOI] [PubMed] [Google Scholar]
- 135. Perl J, Dember LM, Bargman JM et al. The use of a multidimensional measure of dialysis adequacy-moving beyond small solute kinetics. Clin J Am Soc Nephrol 2017; 12: 839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Flythe JE, Brunelli SM. The risks of high ultrafiltration rate in chronic hemodialysis: implications for patient care. Semin Dial 2011; 24: 259–265 [DOI] [PubMed] [Google Scholar]
- 137. Twardowski ZJ. Treatment time and ultrafiltration rate are more important in dialysis prescription than small molecule clearance. Blood Purif 2007; 25: 90–98 [DOI] [PubMed] [Google Scholar]
- 138. Barbieri C, Cattinelli I, Neri L et al. Development of an artificial intelligence model to guide the management of blood pressure, fluid volume, and dialysis dose in end-stage kidney disease patients: proof of concept and first clinical assessment. Kidney Dis (Basel) 2019; 5: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Daugirdas JT, Tattersall JE. Automated monitoring of hemodialysis adequacy by dialysis machines: potential benefits to patients and cost savings. Kidney Int 2010; 78: 833–835 [DOI] [PubMed] [Google Scholar]
- 140. Maggiore Q, Pizzarelli F, Santoro A et al. The effects of control of thermal balance on vascular stability in hemodialysis patients: results of the European randomized clinical trial. Am J Kidney Dis 2002; 40: 280–290 [DOI] [PubMed] [Google Scholar]
- 141. Lane DA, Bowry SK. The scientific basis for selection of measures of thrombogenicity. Nephrol Dial Transplant 1994; 9 (Suppl 2): 18–28 [PubMed] [Google Scholar]
- 142. Bowry SK, Kuchinke-Kiehn U, Ronco C. The cardiovascular burden of the dialysis patient: the impact of dialysis technology. Contrib Nephrol 2005; 149: 230–239 [DOI] [PubMed] [Google Scholar]
- 143. Ronco C, Bowry S, Tetta C. Dialysis patients and cardiovascular problems: can technology help solve the complex equation? Blood Purif 2006; 24: 39–45 [DOI] [PubMed] [Google Scholar]
- 144. Matata BM, Courtney JM, Sundaram S et al. Determination of contact phase activation by the measurement of the activity of supernatant and membrane surface-adsorbed factor XII (FXII): its relevance as a useful parameter for the in vitro assessment of haemodialysis membranes. J Biomed Mater Res 1996; 31: 63–70 [DOI] [PubMed] [Google Scholar]
- 145. Albanese A, Barbucci R, Belleville J et al. In vitro biocompatibility evaluation of a heparinizable material (PUPA), based on polyurethane and poly(amido-amine) components. Biomaterials 1994; 15: 129–136 [DOI] [PubMed] [Google Scholar]
- 146. Baumann H, Kokott A. Surface modification of the polymers present in a polysulfone hollow fiber hemodialyser by covalent binding of heparin or endothelial cell surface heparan sulfate: flow characteristics and platelet adhesion. J Biomater Sci Polym Ed 2000; 11: 245–272 [DOI] [PubMed] [Google Scholar]
- 147. Ren X, Xu L, Xu J et al. Immobilized heparin and its anti-coagulation effect on polysulfone membrane surface. J Biomater Sci Polym Ed 2013; 24: 1707–1720 [DOI] [PubMed] [Google Scholar]
- 148. Meyer JM SD, Weber LA et al. Clinical study to assess the performance of a novel dialyzer with Endexo™ in ESRD subjects [FR-P0474]. J Am Soc Nephrol 2019; 30: 561–562 [Google Scholar]
- 149. Reis ES, Mastellos DC, Hajishengallis G et al. New insights into the immune functions of complement. Nat Rev Immunol 2019; 19: 503–516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Bowry SK, Gatti E, Vienken J. Contribution of polysulfone membranes to the success of convective dialysis therapies. Contrib Nephrol 2011; 173: 110–118 [DOI] [PubMed] [Google Scholar]
- 151. Krieter DH, Canaud B. Dialysis membranes today. Int J Artif Organs 2002; 25: 816. [DOI] [PubMed] [Google Scholar]
- 152. Lemke HD, Heidland A, Schaefer RM. Hypersensitivity reactions during haemodialysis: role of complement fragments and ethylene oxide antibodies. Nephrol Dial Transplant 1990; 5: 264–269 [DOI] [PubMed] [Google Scholar]
- 153. Poppelaars F, Faria B, Gaya da Costa M et al. The complement system in dialysis: a forgotten story? Front Immunol 2018; 9: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Jofré R, Rodriguez-Benitez P, López-Gómez JM et al. Inflammatory syndrome in patients on hemodialysis. J Am Soc Nephrol 2006; 17 (12 Suppl 3): S274–S280 [DOI] [PubMed] [Google Scholar]
- 155. Hasegawa T, Nakai S, Masakane I et al. Dialysis fluid endotoxin level and mortality in maintenance hemodialysis: a nationwide cohort study. Am J Kidney Dis 2015; 65: 899–904 [DOI] [PubMed] [Google Scholar]
- 156. Lonnemann G. When good water goes bad: how it happens, clinical consequences and possible solutions. Blood Purif 2004; 22: 124–129 [DOI] [PubMed] [Google Scholar]
- 157. Schiffl H. High-flux dialyzers, backfiltration, and dialysis fluid quality. Semin Dial 2011; 24: 1–4 [DOI] [PubMed] [Google Scholar]
- 158. Ronco C, Orlandini G, Brendolan A et al. Enhancement of convective transport by internal filtration in a modified experimental hemodialyzer: technical note. Kidney Int 1998; 54: 979–985 [DOI] [PubMed] [Google Scholar]
- 159. Glorieux G, Neirynck N, Veys N et al. Dialysis water and fluid purity: more than endotoxin. Nephrol Dial Transplant 2012; 27: 4010–4021 [DOI] [PubMed] [Google Scholar]
- 160. Weber V, Linsberger I, Rossmanith E et al. Pyrogen transfer across high- and low-flux hemodialysis membranes. Artif Organs 2004; 28: 210–217 [DOI] [PubMed] [Google Scholar]
- 161. Basile C, Rossi L, Lomonte C. Dialysate bicarbonate concentration: too much of a good thing? Semin Dial 2018; 31: 576–582 [DOI] [PubMed] [Google Scholar]
- 162. Trinh E, Weber C. The dialysis sodium gradient: a modifiable risk factor for fluid overload. Nephron Extra 2017; 7: 10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Brunelli SM, Spiegel DM, Du Mond C et al. Serum-to-dialysate potassium gradient and its association with short-term outcomes in hemodialysis patients. Nephrol Dial Transplant 2018; 33: 1207–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Kuhlmann U, Maierhofer A, Canaud B et al. Zero diffusive sodium balance in hemodialysis provided by an algorithm-based electrolyte balancing controller: a proof of principle clinical study. Artif Organs 2019; 43: 150–158 [DOI] [PubMed] [Google Scholar]
- 165. Ságová M, Wojke R, Maierhofer A et al. Automated individualization of dialysate sodium concentration reduces intradialytic plasma sodium changes in hemodialysis. Artif Organs 2019; 43: 1002–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Ponce P, Pinto B, Wojke R et al. Evaluation of intradialytic sodium shifts during sodium controlled hemodialysis. Int J Artif Organs 2020; 43: 620–624 [DOI] [PubMed] [Google Scholar]
- 167. Başer E, Mollaoğlu M. The effect of a hemodialysis patient education program on fluid control and dietary compliance. Hemodial Int 2019; 23: 392–401 [DOI] [PubMed] [Google Scholar]
- 168. Wieringa FP, Kooman JP. Smart sensors for real-time monitoring of patients on dialysis. Nat Rev Nephrol 2020; 16: 554–555 [DOI] [PubMed] [Google Scholar]
- 169. Tricoli A, Neri G. Miniaturized Bio- and chemical-sensors for point-of-care monitoring of chronic kidney diseases. Sensors (Basel) 2018; 18: 942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Sharma MK, Wieringa FP, Frijns AJ et al. On-line monitoring of electrolytes in hemodialysis: on the road towards individualizing treatment. Expert Rev Med Devices 2016; 13: 933–943 [DOI] [PubMed] [Google Scholar]
- 171. Wakasugi M, Kazama JJ, Narita I. Mortality trends among Japanese dialysis patients, 1988–2013: a joinpoint regression analysis. Nephrol Dial Transplant 2016; 31: 1501–1507 [DOI] [PubMed] [Google Scholar]
- 172. Storey BC, Staplin N, Harper CH et al. Declining comorbidity-adjusted mortality rates in English patients receiving maintenance renal replacement therapy. Kidney Int 2018; 93: 1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Pippias M, Stel VS, Abad Diez JM et al. Renal replacement therapy in Europe: a summary of the 2012 ERA-EDTA Registry Annual Report. Clin Kidney J 2015; 8: 248–261 [DOI] [PMC free article] [PubMed] [Google Scholar]