ABSTRACT
The extent of removal of the uremic toxins in hemodialysis (HD) therapies depends primarily on the dialysis membrane characteristics and the solute transport mechanisms involved. While designation of ‘flux’ of membranes as well toxicity of compounds that need to be targeted for removal remain unresolved issues, the relative role, efficiency and utilization of solute removal principles to optimize HD treatment are better delineated. Through the combination and intensity of diffusive and convective removal forces, levels of concentrations of a broad spectrum of uremic toxins can be lowered significantly and successfully. Extended clinical experience as well as data from several clinical trials attest to the benefits of convection-based HD treatment modalities. However, the mode of delivery of HD can further enhance the effectiveness of therapies. Other than treatment time, frequency and location that offer clinical benefits and increase patient well-being, treatment- and patient-specific criteria may be tailored for the therapy delivered: electrolytic composition, dialysate buffer and concentration and choice of anticoagulating agent are crucial for dialysis tolerance and efficacy. Evidence-based medicine (EBM) relies on three tenets, i.e. clinical expertise (i.e. doctor), patient-centered values (i.e. patient) and relevant scientific evidence (i.e. science), that have deviated from their initial aim and summarized to scientific evidence, leading to tyranny of randomized controlled trials. One must recognize that practice patterns as shown by Dialysis Outcomes and Practice Patterns Study and personalization of HD care are the main driving force for improving outcomes. Based on a combination of the three pillars of EBM, and particularly on bedside patient–clinician interaction, we summarize what we have learned over the last 6 decades in terms of best practices to improve outcomes in HD patients. Management of initiation of dialysis, vascular access, preservation of kidney function, selection of biocompatible dialysers and use of dialysis fluids of high microbiological purity to restrict inflammation are just some of the approaches where clinical experience is vital in the absence of definitive scientific evidence. Further, HD adequacy needs to be considered as a broad and multitarget approach covering not just the dose of dialysis provided, but meeting individual patient needs (e.g. fluid volume, acid–base, blood pressure, bone disease metabolism control) through regular assessment—and adjustment—of a series of indicators of treatment efficiency. Finally, in whichever way new technologies (i.e. artificial intelligence, connected health) are embraced in the future to improve the delivery of dialysis, the human dimension of the patient–doctor interaction is irreplaceable. Kidney medicine should remain ‘an art’ and will never be just ‘a science’.
Keywords: dialysis modalities, evidence-based medicine, patient outcome, personalized medicine
INTRODUCTION
Hemodialysis (HD) is a generic name that encompasses various kidney replacement treatment (KRT) modalities that share an extracorporeal circuit (blood circuit outside the body), a device for fluid and solute exchange (hemodialyzer or filter) and a solution (dialysis fluid) to enable exchange between the blood and fluid compartments. HD therapy is highly dependent on technology that evolved gradually from early experimental and perilous procedures to a sophisticated and safe technique today. Several authors have documented this remarkable journey of pioneers who overcame considerable adversity to develop and refine the various components of the extracorporeal circuit [1]. Today, improving long-term outcomes and adding quality to the lives of patients with end-stage kidney disease (ESKD) and on HD therapy depends essentially on the delivery of dialysis [2, 3]. Many variants of delivering HD are available, with the choice depending largely on the patient's clinical conditions and preferences as well as on practices implemented in different countries and individual centers. These variations in the choice of therapy modalities and mode of delivery have been shown to impact patient outcomes, with significant differences observed between countries and regions [4]. We address three interrelated aspects that need to be considered when treating patients with HD therapy: the variants and their options; prescription, personalization, and optimization; and application of principles of current evidence-based medicine (EBM) to HD therapies to improve outcomes and increase patient well-being.
HD therapy: modality variants
HD therapy options for ESKD patients can be categorized into five major features: mechanism and intensity of solute and fluid exchange, membrane specificity for the removal of solutes of different size ranges, treatment time and frequency, dialysis location and facility and selection of additional treatment options specific to patient needs. Some involve complex scientific concepts and considerations, but most pertain to the delivery of therapy based on clinical–patient interaction and experience acquired over several decades without a conclusive evidence base.
Mechanism of fluid and solute exchange
Based on the processes that control the removal of solutes from the bloodstream and membrane separation principles for solute and solvent exchange, there are three main therapy variants: HD, hemofiltration (HF) and hemodiafiltration (HDF) [5, 6]. Only the salient traits of each modality are outlined here, with references provided for a more detailed descriptions and comparisons of the three techniques [7].
HD relies on diffusive processes that transport solutes according to their gradient concentration between the blood and the dialysis fluid compartments. The size of the solutes (molecular weight) to be removed, membrane permeability features, blood and dialysate flow rates and treatment time determine the efficiency of diffusion [8]. Today, HD is still the most widely used option (i.e. the conventional treatment); worldwide, around 89% of patients on dialysis receive HD [9]. Diffusion-dependent HD removes mainly small molecular weight uremic retention solutes (URS, or uremic toxins) and it is less efficient for large molecular weight compounds [10]. It must be noted that solute mass transfer is a bidirectional process and some substances are added to dialysis fluids on purpose (i.e. electrolytes, glucose) or present inadvertently (i.e. microbial by products, contaminants) and may diffuse into the patient, particularly with the so-called high-flux membranes [11]. Hemodialyzer performance is defined according to the diffusive clearance (either instantaneously or time-integrated) of the solute of interest. The clinical performance (diffusive dialysis dose) of an HD session may be assessed by various indicators that include solute percent reduction per session [i.e. urea reduction rate (URR)], fractional solute removal (FSR, i.e. Kt/Vurea), mass solute removal index (SRI) or estimated glomerular filtration rate (eGFR) [12–14]. All these indicators reflect the patient–dialysis interaction and serve to characterize the relative efficiency of each dialysis session. Whatever the value of each of these indicators, it must be recognized that the Kt/Vurea indicator, despite its shortcomings, still represents the basis for quantifying HD efficiency in a quality assurance perspective [15–18]. Furthermore, standard weekly Kt/V is often used as a reference or comparator of treatment schedule effectiveness with different durations and/or treatment frequencies.
HF, like HDF, relies on the process of convection (‘solvent drag’), whereby removal of solutes occurs as they are pulled along with the volume of the fluid transported across the membrane, with ultrafiltration achieved by exerting a transmembrane pressure (TMP) on blood [6, 19, 20]. The magnitude of the transport depends on the hydraulic permeability [ultrafiltration coefficient (KUF)], as well as on the sieving properties of the membrane [sieving coefficient (SC)], the plasma solute concentration and treatment time or total ultrafiltered volume [21, 22]. Relying solely on the mechanism of convection, HF, like HDF, is highly effective for the removal of mainly large molecular weight compounds since they constraints imposed by the membrane hindrance are overcome by the applied TMP [23, 24]. To compensate for the ultrafiltrate produced, HF and HDF require replacement into the bloodstream of large volumes of substitution fluid produced ‘online’ by cold sterilizing processes [25]. HF is rarely used today for maintenance dialysis due to time or volume constraints and cost considerations. However, HF was developed in the 1960s to successfully enhance removal of ‘middle molecules’, which were not being removed by dialysis membranes available at the time, as well as to improve hemodynamic tolerance of dialysis [5].
HDF, a ‘hybrid’ therapy, relies on dual processes that combine diffusive removal by conventional HD and convective clearance (HF) in the same hemodialyzer. In other words, HDF brings the best of these two modalities by enhancing overall solute clearances and broadening the molecular weight spectrum of solutes removed, both small and large molecular weight [26]. Highly pure replacement fluid (to compensate for fluid removed from the patient to achieve convection) is prepared online from dialysis fluid [27]. Over the last 2 decades online external HDF has become the most popular convective treatment modality for economical and practical reasons, particularly outside the USA [28, 29]. Several clinical studies have confirmed the microbial safety of online production of substitution fluid and attest to the clinical performance superiority of HDF compared with conventional HD, provided an adequate convective dose is achieved [29]. Some other HD variants have been assessed clinically, but due to their low acceptance rate they will not be detailed here. This field includes hybrid modalities such as acetate-free biofiltration, paired filter HDF and push–pull HDF [30, 31, 32].
Membrane selectivity (‘sieving’ specificity of solute removal)
The selective elimination of uremic toxins achieved by manmade membranes is fundamental to the success of HD therapies and is theoretically analogous to the selective separation processes accomplished by the glomerular filtration apparatus, albeit considerably more complex than the sieving function of dialysis membranes [33, 34]. The semi permeability function of dialysis membranes depends on their manufacturing process which determines membrane morphology features that define which molecules traverse the membrane and which are retained in the blood [35]. This function depends on the size of the molecules relative to the average size of the pores at the innermost separating region of the membrane [36]. Those URS (often referred to as ‘uremic toxins’, although not all substances retained in uremia express toxicity) that are considered necessary for removal vary in size from small substances (e.g. water, Na+, phosphate, small peptides) to molecular weights of thousands of Daltons (Da) [37, 38].
Membranes having different pore size ranges are manufactured according to the desired solutes that are a clinician's target for removal during HD, usually rather loosely categorized as low, middle and high molecular weight [39]. Membrane classification schemes are highly arbitrary, as there is no consensus as to the precise definition of each of the three categories, and it is commonplace for authors in the literature and for manufacturers of membranes to establish their own classification boundaries [39–41]. Most commonly, and erroneously, the term ‘flux’ is used in conjunction with the three adjectival categories (low-, middle-, and high-flux) relating to the sieving potential of membranes according to the molecular weight size range of uremic toxins [22, 37]. More aptly, flux is a transport phenomenal term defining membrane separation processes and is a finite entity [expressed in HD as volume of fluid transported, i.e. ultrafiltration across the membrane per unit time per unit pressure (mL/h/mmHg = KUF)] [19, 36].
Membrane selectivity by sieving is determined predominantly by the mean pore size and is quantified and expressed by the sieving or rejection properties of membranes for solutes, i.e. by their SC or rejection coefficient (RC), respectively [19]. The two are essentially indicative of the same measure but are expressed in two different ways, as there is a reciprocal relationship between the two [42]:
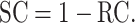 |
The most common indication of the sieving potential of membranes is obtained from the SC of β2-microglobulin (β2-m), an 11.8-kDa molecule widely recognized as a uremic toxin and a surrogate marker for what is termed as the group of ‘larger’ uremic toxins. However, the boundaries for what is considered ‘large’ have shifted recently, as membranes with much larger mean pore size allow almost total removal (i.e. SCβ2-m = 1) in attempts to target removal of molecules around in the size of albumin (molecular weight 66.5 kDa) [41, 42]. The strategy is highly contentious, as the more open the membrane pore structure, the greater the probability of uncontrolled loss of useful substances from patient's blood, with potential detrimental consequences for the patient [43, 44]. Consequently, such high permeability to middle and higher molecular weight solutes precludes their use in HDF or in any modality with high membrane pressure stress (TMP) [41].
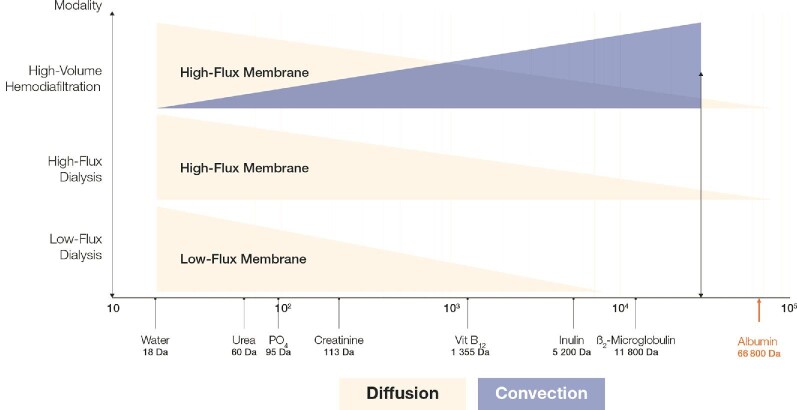
Based on the fluid/solute exchange mechanisms involved and membrane selectivity criteria, Figure 1 indicates the solute removal capabilities of the various HD therapy modalities in clinical use today.
Figure 1:
Schematic representation of HD therapy modalities and the approximate size range of uremic retention solutes they can remove according to the solute removal mechanism (diffusion and/or convection). The extent of removal of the solutes depends on the membrane characteristics as well as the treatment conditions used (e.g. treatment time, the volume of substitution fluid in HF and HDF). It should be noted that both designation of the ‘flux’ of membranes as well as the solutes that need to be targeted for removal (based on the toxicity they express) are highly controversial issues that divide opinion.
Treatment time and frequency
Based on treatment times per session (3–12 h) and frequency per week (2–6 sessions/week or daily dialysis), several permutations for treatment schedules are possible for the delivery of HD therapy. The duration–frequency debate is a highly subjective one, as cost implications often override the obvious clinical benefits (less morbidity and mortality) associated with longer and more frequent therapy [45–47]. The thrice-weekly schedule has, for decades, been considered ‘unphysiological’ and a contributor to the side effects of dialysis [48, 49]. The kinetics of removal of many molecules such as phosphate and larger compounds is time-dependent, and better volume control and smaller solute fluctuations are achieved with more intensive dialysis [50–52]. Other than the financial burden, increasing duration and frequency does have some downsides, such as increased risk of access malfunction [45]. As far as home care dialysis is concerned, widely different approaches and different modalities are favored by individual countries, making generalized recommendations difficult [53–55]. Schematically, treatment schedules can be placed in three main categories: conventional HD relying on thrice-weekly 4 h (12 h/week) sessions; shorter and/or less frequent HD relying on 1–3 sessions/week, each one lasting 2–4 h (6–9 h/week); longer and/or more frequent HD relying on 4–6 sessions/week, each one lasting 4–8 h (16–24 h/week) [56]. Conventional thrice-weekly HD is utilized in almost 80% of dialysis patients worldwide for practical and costs reasons. Shorter HD schedules are usually indicated in patients (e.g. elderly) with some residual kidney function (RKF) or in incremental programs [17, 57]. Longer dialysis schedules are usually indicated as rescue procedures, or for home or self-care programs [58, 59]. It is not our intent to describe the pros and cons of these treatment schedules and we refer interested readers to articles and expert forums that have addressed this topic.
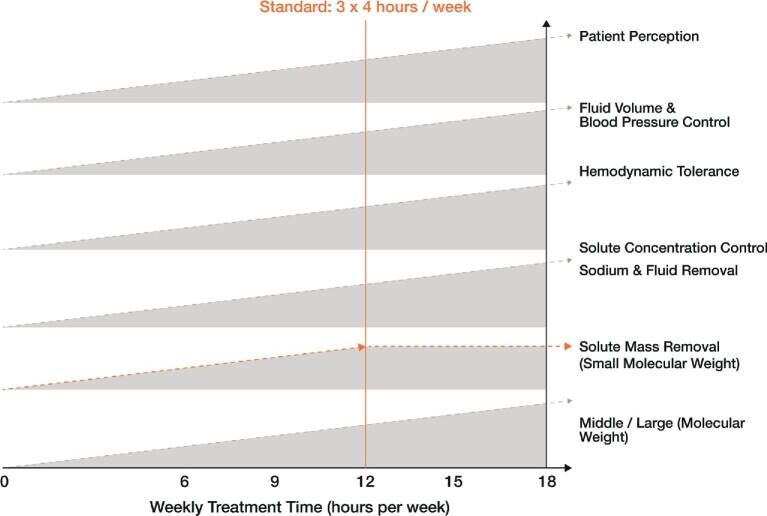
Various investigations show that longer weekly treatment time offers several clinical benefits that are shown in Figure 2. Our intention is to underline that the HD treatment schedule is always a compromise that should meet the patient's tolerance and metabolic needs, be the patient's choice (burden on the patient’s lifestyle) and finally be available in the healthcare system as a local care offering [60]. It is up to the referent nephrologist and/or caregiver team to adjust the prescription and dialysis treatment schedule to patient conditions and results [50].
Figure 2:
Impact of the duration of the therapy session (treatment time) on essential biological systems dialysis attempts to correct or which are affected or influenced by the procedure. Worldwide, a weekly treatment time of 12 h (3 sessions/week, each for 4 h) is most prescribed.
The combined effects of total weekly treatment time (sum of the number of sessions/week and their duration) are key indicators of therapy efficiency and benefits. Small solutes such as urea are removed quickly during HD; within 6–8 h almost 90% of circulating urea is removed, bringing circulating levels down to 3 mmol/L. Thus extending dialysis time over 8 h has no additional benefits. In contrast, middle or large solutes are slowly removed initially and are removed continuously after 8–12 h. This is the case of phosphate for example, for which time is more important than instantaneous flux for total solute removal. It is the same for β2-m for which the intracorporeal kinetics of the solute are the limiting factor and usually expressed by the intracorporeal mass transfer coefficient (ICMTC, i.e. intracorporeal clearance). The higher the ICMTC (e.g. urea), the lower the importance of time for solute removal. I contrast, the lower IMTC (β2-m or phosphate), the greater the importance of time to remove the solute [52].
Treatment location
Dialysis treatment is performed in the ambulatory mode most frequently in a center (dialysis facility being part of an outpatient clinic or hospital, dialysis material shared with other patients, medical and nursing staff), at home (personal HD equipment installed at home, self-care or assistance, telemonitoring) or in a self-care unit (small private-unit HD shared with other patients) dedicated to this activity. In recent years there has been a surge in interest for the provision of home care services for dialysis, with cost-effectiveness being a strong incentive for this therapy offering [54, 55, 61, 62]. It is not our intent to describe here the advantages and pitfalls of all these treatment modalities, but to highlight the fact that they should by law be part of the portfolio offering to dialysis patients, incorporating their clinical and personal situation and involvement in decision making through better education regarding treatment options [63].
Additional treatment options
After selecting from the various options discussed above, some treatment- and patient-specific criteria may be added or adjusted to the HD therapy being delivered. We briefly focus on three of them that are crucial for dialysis tolerance and efficacy [64].
First, the electrolytic composition refers to the concentration of each electrolyte constituent of the dialysis fluid. Unfortunately, electrolyte prescription is frequently neglected, but the patient's mass balance of each electrolyte and overall dialysis adequacy depends on this consideration. Dialysance and electrolyte mass balance relies on the dialysate–patient gradient and dialysis clinical performance. Dialysate sodium concentration may affect dialysis tolerance, fluid volume and hemodynamic management, blood pressure (BP) control and finally cardiovascular patient outcomes [65–67]. Dialysate potassium concentration is used to restore potassium homeostasis, but too fast changes may affect cardiac rhythm (arrhythmias) [68]. Dialysate calcium and magnesium are major divalent cations that are implicated in bone metabolism and vascular calcification in the long term, and hemodynamic response to dialysis on short term [68–70]. All these elements should be fine-tuned and adjusted to patient needs and tolerance on a regular basis with more frequent monitoring [68]. Individualized electrolyte management or prescription is a decisive strategy to ameliorate sudden cardiac death and improve overall patient well-being [71].
Second, dialysate buffer choice and concentration are of crucial importance to control acid–base derangements [64]. Dialysate bicarbonate has become a standard in contemporary dialysis. Dialysate bicarbonate concentration is usually around 35 mmol/L (range 30–35 mmol/L), but the concentration must be adjusted to the patient's acid–base status and tolerance [72]. The adjunction of an acidifier is required in dialysate bicarbonate to prevent precipitation of calcium and magnesium carbonate salts. A low concentration of acetic or citric acids is used for this purpose, each with its own different metabolic behavior that must be known by the user to prevent pitfalls. The composition and selection of dialysate buffers has been associated with cardiopulmonary events observed frequently during HD [73].
Third, the use of an anticoagulant agent is usually necessary to prevent clotting of the extracorporeal circuit. Standard or unfractionated heparin is most frequently used based on an intravenous bolus at dialysis initiation followed by a continuous infusion to keep activated clotting time 1.5–2 times the baseline value. Heparin-induced thrombocytopenia type II is a devastating complication associated with unfractionated heparins, but with low molecular weight heparins a lower incidence is observed [74]. Use of low molecular weight or fractionated heparins has steadily increased for this reason, as well as their use reduces antithrombotic handling burden and bleeding risks [75]. For patients with heparin allergies, nonheparin forms of anticoagulation need to be prescribed [69].
Prescription, personalization and optimization of HD therapy
HD initiation is indicated when kidney function has failed; this condition is usually taken as when eGFR is <15 mL/min, but because of variability in its measurement, the optimal timing for starting dialysis is unclear, with mean pre-dialysis eGFR varying among countries [50, 76]. Additional indicators for start of dialysis is when uremic symptomatology is present and/or intractable metabolic disorder has occurred (i.e. fluid overload, hypertension, congestive heart failure, metabolic acidosis) [77]. Some of the other indications are reduced energy levels, weight loss with no potential explanation, malnutrition, anorexia, acid–base or electrolyte abnormalities and an inability to control volume status or BP.
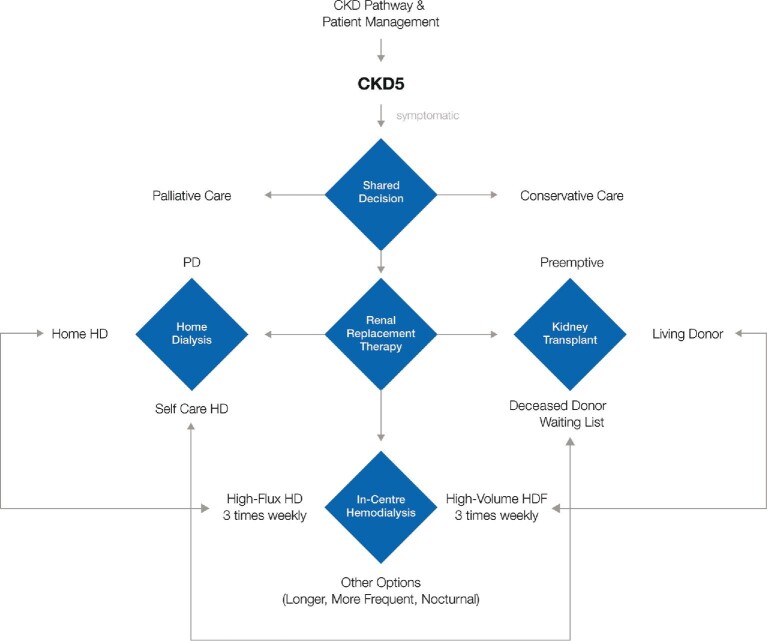
Peritoneal dialysis and kidney transplantation are the two other initial modality options in patients with kidney failure. Palliative and conservative care should also be considered as the two other important options for patients who do not prefer or are not suitable to start or continue dialysis or for transplantation. The circumstances of dialysis initiation and the choices of the initial modality and access can significantly affect patient experiences and outcomes [50, 78]. Ideally, HD start should be planned and prepared for in advance, meaning that chronic kidney patients might be regularly followed and managed by a nephrologist [79]. In this case, the treatment modality and option should have been discussed, shared, and planned with the patient (i.e. home HD, self-care HD, in-center HD), meaning that vascular access should have been prepared and constructed in due time; this complex pathway is summarized in the decisional algorithm presented in Figure 3. Initiation of HD treatment in an incident patient requires specific prescription over the first week aiming to deliver a low efficient frequent dialysis (daily) program to correct slowly uremic disorders and to prevent side effects of too fast correction [80].
Figure 3:
Summary of the different treatment options (modalities and delivery modes) available for end-stage CKD patients. Such a decisional algorithm to manage CKD stage 5 patients is centered around the patient's clinical condition(s), preferences and personal situation (family, work, living conditions, etc.) as well as on the recommendations of clinicians and practice patterns within countries or dialysis units.
Maintenance of the HD treatment schedule is established over the first few months by adjusting operating procedures (i.e. increase blood flow, high-flux hemodialyzer, modality switch to HDF) and increasing treatment time and/or frequency in order to achieve set clinical performance targets [81]. During this probing period the HD treatment schedule is fine-tuned to the patient's tolerance and intermediary clinical and biological results achieved.
Personalization and optimization of treatment in long-term HD consists of checking and adjusting if needed on a regular basis (i.e. monthly for in-center or quarterly for home treatment) so that clinical performance indicators (i.e. checklist) are in target ranges, that the patient's clinical condition (i.e. subjective global assessment) is maintained and that the patient's perception fits with the treatment program and schedule [50, 82]. This patient-oriented goal approach results from a long-standing well-established patient–physician interaction and trusted relationship with the caregiver team. Support of other patient's functions (i.e. psychologist, nutritionist, social worker) is needed in this context, since HD is only one component of a chronic and complex disease impacting all vital domains of such a patient. Additional support (i.e. nurses, technicians, pharmacist) and network expertise is needed in case of a home HD choice.
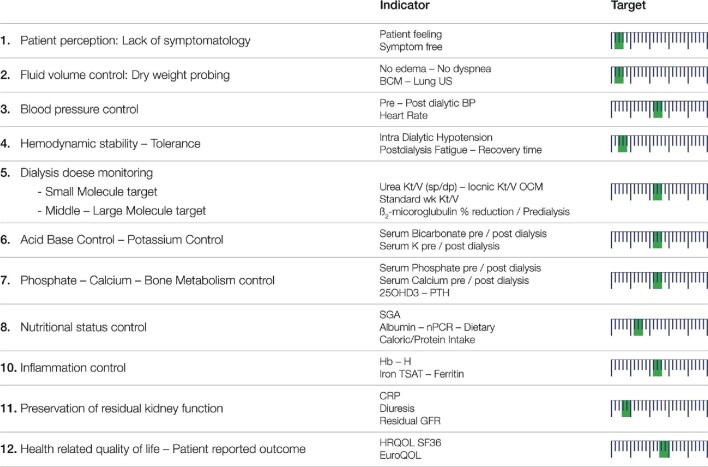
Garbelli et al. [83] recently described how implementation of continuous quality improvement (CQI) and medical patient review (MPR) processes through leverage of digital transformation can enhance clinical endpoints of dialysis patients. It is not our intent to review this major topic here and refer interested readers to the literature [84]. This approach should be integrated into dialysis adequacy assessment performed on a regular basis for patients and the dialysis unit [85]. Schematically, the quality assurance process consists of defining a list of key indicators (i.e. checklist and dashboard shown in Figure 4) reflecting domains of interest, choosing relevant clinical performance indicators, setting target ranges for each one, defining the methods and frequency of measurements and analyzing, aggregating and reporting results (i.e. dashboard, balanced score card) to stakeholders (i.e. patient, caregiver, provider, regulatory). Based on the results of this monthly (or quarterly for home HD) assessment, the efficacy of HD treatment can be ascertained and clinical decisions may be taken either to keep the treatment program as it is or to work out and correct the causes of deviations and/or to reset the treatment schedule.
Figure 4:
HD adequacy indicators as a broad and multitargeted approach covering individual patient needs. The targets that need to be realistically achieved for each indicator are based on the long-term (40–50 years) clinical experience of the author in the care of dialysis patients. The position of the green bar on the arbitrary scale (high on the left end, low on right end) suggests the value/importance of each target. Such a checklist of the indicators should be assessed on a regular basis to ensure treatment efficiency.
Preservation of RKF and clinical status should be considered as major aims in the management of advanced CKD patients [69]. This is the main task of clinicians when caring for CKD patients. A reduction in the GFR decline may be achieved by combining various actions that include BP control (medications such as renin—angiotensin—aldosterone system blocking agents, calcium channel blockers, diuretics, salt diet restrictions), diet and lifestyle adaptation (protein and salt diet restrictions, physical activity, stop smoking), reduction of proteinuria, phosphate and mineral and bone disease control and acidosis correction. Preservation of clinical status may be obtained by correcting uremia and metabolic disorders, including protein energy malnutrition, fluid volume control, anemia and iron deficiency, by means of adapted nutritional guidance and the use of erythropoietin and iron supplements [86–89].
EBM applied to HD
EBM was introduced in the early 1990s by UK physicians to rationalize medical practices facing rapid development of medicine knowledge to provide tangible evidence and promote better use of protocols to treat diseases, to support life with medical devices or to indicate surgical interventions. In 1995, Sackett and Rosenberg [90] defined clearly the scope of EBM whereby decisions are to be made on the best possible evidence. Subsequently EBM was delineated as being at the intersection of three domains: clinical expertise (i.e. doctor), patient-centered values (i.e. patient) and relevant scientific evidence (i.e. science) [91]. Unfortunately only one component of this triad appears to have been sustained, namely the scientific facet, and incorporated into the practice of supporting development of clinical guidelines, the volume of which has become unmanageable [69, 92, 93]. Clinical expertise and patient experience have been sidelined along the way, often leading to misconceptions and even misappropriation of the principles of EBM, causing further disillusionment and frustration within the clinical community [94, 95].
If one analyzes outcomes of HD therapies through the prism of the EBM principle, one will be surprised to discover that this essential and well-recognized life-sustaining therapy applied to millions of patients was developed initially without much scientific proof of efficacy or safety. In other words, in the case of HD, EBM relied in its early days on the clinical expertise of pioneering nephrologists and scientists taking risks and on the patients' willingness to survive without definitive proof [96–98]. This fact is not peculiar to HD but may be applied to several other major advances in the history of medicine (i.e. vaccination, antibiotic therapy, transplantation, hygiene).
In this section, based on a combination of the three pillars of EBM, but particularly on the bedside patient–clinician interaction, we summarize what we have learned over the last 6 decades in terms of best practices to improve the outcome of HD patients. They are aggregated in 10 main sections:
Initiation of HD
It is currently recognized that HD therapy should be prepared along pathways of CKD development and patient management [i.e. arteriovenous fistula (AVF) creation, shared modality decision], while dialysis launch should be based on a combination of low GFR (<10 mL/min), uremic clinical manifestations and/or intractable symptoms or life-threatening complications. Dialysis start could not be decided only on ‘numbers’ or low GFR [50, 99]. Other treatment options may be discussed with CKD patients (share decision process) at this stage according to their informed choice, risk profile and local care offerings [8]. They include conservative management (delaying as much as possible the start of dialysis), palliative care (for patients with a poor or short-term prognosis), peritoneal dialysis or home therapy, incremental dialysis (stepwise increase of dialysis frequency and time compensating for GFR decline) and preemptive kidney transplantation. All these options have their pro and con arguments, with their defenders and opponents, and will not be discussed here since we are focusing on HD, the therapy [63].
Vascular access
Autologous AVF is the vascular access of choice and should be preferred as a first option since it provides the best results in terms of flow performances, long-term survival and lowest morbidity [3, 100]. Arteriovenous graft (AVG) is the second choice when AVF creation is not possible or when it failed repeatedly. Central venous catheter or port catheter devices should be reserved to specific indications and experienced teams as a bridging or rescue solution. Vascular access monitoring and maintenance are crucial to prevent dysfunction or to reduce vascular access-related morbidity.
Hemodialyzer, membrane flux and biocompatibility
Synthetic polymer membranes (i.e. polysulfone and the polyarylsulfone family of polymers) are the most used worldwide, with a sustained increase representing up to 70% of market share. High-flux synthetic membranes should be the first choice, as indicated by guidelines, since they offer higher clinical performances in terms of solute removal and better long-term patient survival [101, 102].
Dialysate buffer
Bicarbonate-buffered dialysate has become over time the new standard in in-center hemodialysis, as dialysate buffer comes in a powder bicarbonate form in disposable closed containers. Sodium bicarbonate as a physiologic buffer does not need any intermediary metabolism, meaning that its use is associated with better cardiovascular and overall dialysis tolerance.
HD modality
High-flux HD is the standard of care, representing 60–70% of HD patients worldwide. Online HDF represents a natural evolution of high-flux HD to increase clinical performance and to enhance removal of a broad large molecular weight spectrum of solutes retained during kidney failure. Online HDF acceptance in Europe and Asia is growing fast, with a patient average growth rate of 12–24%, with high usage in Japan [103]. HDF is recommended as a first-choice therapy by the National Institute for Health and Care Excellence guidelines [104].
HD adequacy
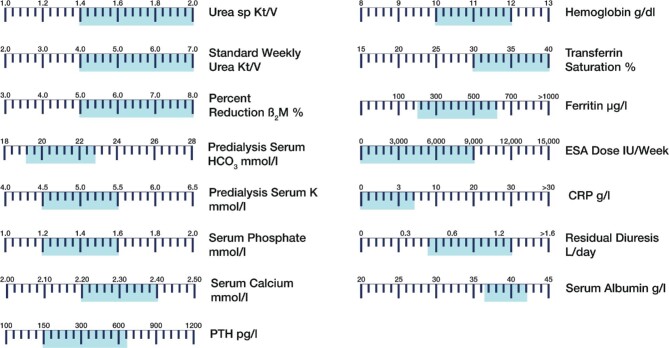
HD adequacy should be considered as a broad and multitargeted approach covering patient's needs [105, 106]. It is not our intent to review here the scientific background of this crucial and often controversially discussed pillar, but to highlight the key indicators—and the biochemical targets that need to be achieved (Figure 5)—reflecting the array of clinical performances of RRT [107–109]. The following is a checklist of the indicators that should be assessed on a regular basis to ensure treatment effectiveness:
Figure 5:
Dashboard of key treatment and biochemical indicators used for assessing the adequacy and efficacy of dialysis in individual patients. For each indicator, the currently recommended target values are displayed by the zone shaded in blue (simulation).
Patient perception and lack of symptoms and/or complaints
This subjective global assessment is the first step and a highly clinically relevant approach to ensure that the dialysis patient is adequately treated [110]. In other words, the patient should be symptom free, feeling well and maintain usual functionalities and lifestyle-related activities [111].
Fluid volume control
Fluid overload issues remain prevalent in the majority (40–60%) of dialysis patients [88, 112]. Cumulative scientific evidence indicates that chronic fluid overload is a strong enhancer of cardiovascular disease. Precise monitoring and management of fluid status are therefore strongly recommended. This is integrated in the clinical dry weight probing approach [87]. Figure 6 represents the central pillars and targets of sodium, fluid and BP management in HD as essential cardioprotective measures and for patient well-being.
Figure 6:
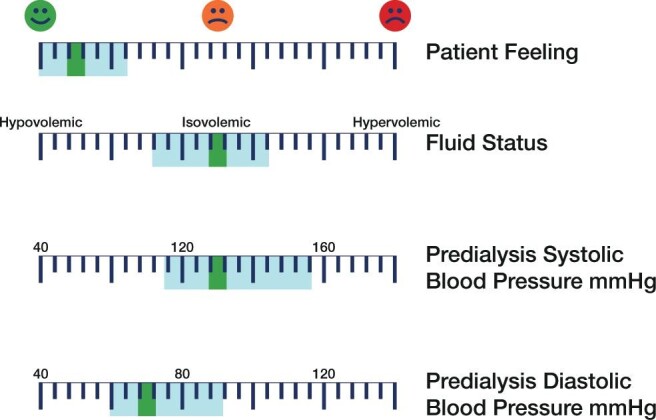
An example of targeted fluid and pressure management indicative of a simulated patient. Key indicators are shown with currently recommended target values (blue zone represents the accepted target range). The patient's value appears as a green bar.
BP control (pre- and post dialysis BP, home BP)
High BP is well established as a main factor of cardiovascular disease progression, particularly for cardiac remodeling (left ventricular hypertrophy or dilation), heart failure and accelerated atherosclerosis (vascular stiffness) [113]. Precise monitoring and management of BP, including new tools such as ambulatory or home BP devices, is appealing [114–116]. In this context, personalized targeted BP based on age and comorbid conditions is the next step to optimize cardiac risk.
Hemodynamic stability (intradialytic hypotension, tolerance)
Ultrafiltration (rate and volume) due to the intermittency of dialysis treatment creates volume fluctuations with hemodynamic response that may be impaired by advanced age, comorbid conditions or medications. In this context, intradialytic hypotension episodes are involved in hemodynamic-induced stress and multiorgan end-organ damage [117–119].
Dialysis dose control
Assessment of dialysis dose to be delivered on a regular basis is crucial for monitoring HD patients. Dialysis dose delivered based either on small (i.e. urea Kt or Kt/V; percent reduction of urea) and middle or large molecule uremic solutes (i.e. percent reduction of β2-m) removal capacity or on circulating levels of biomarkers of interest are currently recommended to assess and objectively quantify dialysis effectiveness [102]. Unfortunately, dialysis dose is synonymously associated with urea Kt/V. That should not be the case anymore since urea (i.e. Kt/V, stdKt/V; PRU) solely reflects the exposure risk associated with the accumulation of low molecular weight uremic toxins and not with larger molecular weight compounds [106, 120–122]. Therefore additional indicators reflecting the effectiveness of removal of middle or large molecular weight uremic solutes should be incorporated in the panel of biomarkers to address this neglected aspect [43]. As an example, total ultrafiltered volume is currently proposed as a clinical surrogate of the convective dose delivered in HDF, considering that it reflects the clearance of middle molecular weight substances such as β2-m [26]. Also, by incorporating dialysis frequency, the hemodialysis product (HDP) is considered by Scribner et al. [123] to be a better index of dialysis adequacy than Kt/V, as it has advantages in that HDP does not depend on any blood test and has a built-in margin of safety. The squared-frequency Kt/V may be even more appropriate, as this index reflects peak concentrations of urea and β2-m, taking into account dialysis frequency, session duration, dialyser clearance and the body weight of the patient [124].
Acid–base control (serum bicarbonate and potassium)
Metabolic acidosis requires tight control of serum bicarbonate to prevent further metabolic derangements such as bone disease or protein energy malnutrition [125, 126]. Serum bicarbonate correction is ensured by buffering and adjusting the dialysate sodium bicarbonate concentration to the patient's needs. The dialysate potassium concentration is an important adjustable factor for ensuring potassium homeostasis control [68, 127]. Dialysate bicarbonate and potassium concentrations should be adjusted and probed regularly to serum patient concentrations to prevent too high gradients.
Phosphate, calcium and bone disease metabolism control (PO4, ALP, PTH, 25(0H)D3)
Phosphate control relies on three factors: HD effectiveness, dietary protein intake and phosphate binders [128–130]. It is of utmost importance to highlight that phosphate mass removal is poorly reflected by its instantaneous clearance, but rather by treatment time and HD modality. Longer treatment and more frequent sessions as well as HDF enhance phosphate mass removal, facilitating serum phosphate control. Positive calcium mass balance is required to control the bone metabolism of HD patients. This is achieved mainly by adjusting dialysate calcium concentrations to a targeted calcium mass balance. It must be noted that the intensity of calcium mass transfer relies on the ionized dialysate–plasma calcium gradient and HD operating conditions.
Nutritional status control (nPCR, SGA, albumin, diet survey)
Protein energy wasting is a highly prevalent condition in HD patients that is associated with poor outcomes [131–133]. Regular nutritional monitoring is crucial in this fragile population that relies on several tools, including clinical subjective global assessment, dietary survey, somatic protein concentrations (albumin, transthyretin) and instrumental measures (bioimpedance, dual-energy X-ray absorptiometry).
Anemia and iron status control
Anemia correction relies mainly on the use of erythropoietic-stimulating agents (ESAs) in HD patients [134]. However, it should be noted that HD efficiency, blood saving during each dialysis session, nutritional support, prevention of inflammation and iron repletion are also very important factors to consider in achieving this important target [135, 136].
Restricting inflammation (CRP)
Detection and prevention of additional treatment-related inflammation is of critical importance in the dialysis patient since this is a recognized marker and enhancer of poor outcome [137–139]. Regular monitoring of a sensitive biomarker such as CRP is recommended in clinical practice.
Preservation of residual kidney function
This clinical fact or is associated with better outcomes in HD patients [140, 141] and linked to better control of circulating uremic toxins (i.e. β2-m; protein-bound uremic toxins) and fluid volume management. Approaches to preserve kidney function are discussed elsewhere, but preventing hemodynamic insult associated with intermittent dialysis is likely one of the best methods [142].
Health-related quality of life
Cumulative clinical evidence tends to show that patient perceptions and patient-reported outcomes are important factors to judge dialysis treatment quality and the anticipated outcome [143, 144].
Treatment time and frequency
Conventional HD therapy relying on a thrice-weekly 3–4 h treatment schedule is accepted as the standard of care in most patients. However, it must acknowledged that this short treatment schedule was developed for logistical, practical and economic reasons as a viable compromise to prevent treatment shortage and ensure acceptable long-term outcomes. However, it is also largely recognized that a more personalized approach with more flexible treatment times and frequencies is needed. Also, it has been shown that longer treatment time (5–8 h) and more frequent HD (4–6 per week) is associated with better outcomes and improved results in terms of patient experiences [145–147]. In this context, longer and more frequent HDF treatment schedules have been shown to significantly enhance intermediary and patient outcomes in a relatively long-term study [148].
Ultrapurity of dialysis fluid
Ultrapurity of HD fluids has become a new standard of care in HD to improve biocompatibility and to prevent the development of low-grade chronic inflammation. Dialysis fluid ultrapurity relies on an integrated approach consisting of production and distribution of ultrapure water to dialysis machines, ensuring final cold sterilization of dialysate through sterilizing filters and ensuring strict hygienic rules with regular disinfection of the entire treatment chain [149, 150]. Regular use of ultrapure dialysis fluid in HD and HDF is associated with a significant reduction in inflammation markers and provides clinical benefits [151]. Dialysis fluid ultrapurity is strongly recommended in most guidelines [152].
Practice patterns and quality assurance
The international Dialysis Outcomes and Practice Patterns Study has clearly shown that practice patterns at all levels (local, national, international) have a tremendous impact on dialysis patient outcomes [153]. Exploring various domains of clinical practice patterns has clearly identified areas of potential improvement, including management of vascular access, anemia, BP and fluid volume control, mineral and bone disorders, nutritional aspects and various others [4]. An integrated approach consisting of monitoring, analyzing and reporting key clinical performance indicators (i.e. dashboard or balance scorecard) represents the basis of a quality assurance process for improving patient care and global outcomes [85, 154, 155].
Preservation of residual kidney function
RKF has emerged in the last few years as a major factor contributing to improving dialysis patient outcomes [86, 156]. Residual diuresis has the capacity to facilitate fluid volume control, reduce circulating levels of middle molecules and protein-bound uremic toxins and significantly improve patient outcomes. As suggested by some recent imaging studies, prevention of repetitive kidney ischemic insults due to intradialytic hypotension episodes would be the most appealing approach [157]. All clinical efforts should be deployed to preserve kidney function, including the use of HDF, volemia-controlled algorithms, thermal balance control and further innovative tools when available.
CONCLUSIONS
As highlighted in this article, HD and RRT practices have changed considerably over the last 5 decades. HD therapy has incrementally adapted to the growing body of scientific findings (e.g. uremic toxins, ESA, mineral and bone disorders, cardiovascular complications), to changes in the patients' medical profiles (i.e. aging, comorbidities) and to their lifestyle-related needs by implementing highly effective and safe technologies (i.e. high-flux dialyzers, online-HDF) and by improving medical practices (i.e. education, quality control tools) [4, 158–160]. Currently, practitioners are at the stage of delivering more personalized dialysis treatment in a more patient-centered care approach by including various patient dimensions (i.e. metabolic, perception, social, family). It has become apparent that ‘one size fits all’, as was essentially the case in the earlier years of the evolution of dialysis, does not deliver individualized state-of-the-art dialysis treatment to patients [69]. Significantly, new value-based dialysis approaches need to be considered from the holistic healthcare perspective, i.e. incorporating care offerings, outcomes, budget constraints and cost-effectiveness as well societal and environmental responsibility considerations for the provision of sustainable kidney care [161, 162]. Finally, it must be remembered that in whatever way new technologies (i.e. artificial intelligence, connected health) are embraced for the improved delivery of dialysis, the human dimension of the patient–doctor interaction is irreplaceable [159, 163]. As we have outlined in this article, the highly complex and multifactorial nature of CKD and its treatment require the judgment of knowledgeable physicians to adjust and personalize prescriptions based on individual patient needs. Despite technological advancements in the future, we strongly believe kidney medicine should continue to remain more of ‘an art’ rather than just ‘a science’ [164].
ACKNOWLEDGEMENT
This article is published as part of a supplement supported by Fresenius Medical Care.
Contributor Information
Bernard Canaud, Montpellier University, Montpellier, France; Global Medical Office, FMC Deutschland, Bad Homburg, Germany.
Stefano Stuard, Global Medical Office, Fresenius Medical Care, Bad Homburg, Germany.
Frank Laukhuf, Global Medical Office, Fresenius Medical Care, Bad Homburg, Germany.
Grace Yan, Fresenius Kidney Care, China.
Maria Ines Gomez Canabal, Clínica NephroCare sede Servicio Renal, Colombia.
Paik Seong Lim, Tungs Taichung Metroharbour Hospital, Taiwan.
Michael A Kraus, Indiana University Medical School, Indianapolis, Indiana, USA; Global Medical Office, Fresenius Medical Care, Waltham, Massachusetts, USA.
CONFLICT OF INTEREST STATEMENT
B.C., S.S., F.L., G.Y., M.I.G.C., P.S.L. and M.A.K. are employees of Fresenius Medical Care.
REFERENCES
- 1. Ing TS, Rahman MA, Kjellstrand CM. Dialysis: History, Development and Promise. Hackensack, NJ: World Scientific; 2012 [Google Scholar]
- 2. Sargent JA. Shortfalls in the delivery of dialysis. Am J Kidney Dis 1990; 15: 500–510 [DOI] [PubMed] [Google Scholar]
- 3. Kerr PG. International differences in hemodialysis delivery and their influence on outcomes. Am J Kidney Dis 2011; 58: 461–470 [DOI] [PubMed] [Google Scholar]
- 4. Robinson BM, Akizawa T, Jager KJ et al. Factors affecting outcomes in patients reaching end-stage kidney disease worldwide: differences in access to renal replacement therapy, modality use, and haemodialysis practices. Lancet 2016; 388: 294–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Henderson W, Henderson L. Kinetics new of hemodiafiltration. II. Characterization of a new blood cleansing modality. J Lab Clin Med 1975; 85: 372–391 [PubMed] [Google Scholar]
- 6. Henderson LW. The beginning of clinical hemofiltration: a personal account. ASAIO J 2003; 49: 513–517 [DOI] [PubMed] [Google Scholar]
- 7. Locatelli F, Altieri P, Andrulli S et al. Hemofiltration and hemodiafiltration reduce intradialytic hypotension in ESRD. J Am Soc Nephrol 2010; 21: 1798–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Williams A. Hemodialysis and peritoneal dialysis. In: Godbole PP, Koyle MA, Wilcox DT (eds). Pediatric Urology: Surgical Complications and Management. Hoboken. NJ: Wiley-Blackwell, 2015: 307–314 [Google Scholar]
- 9. Himmelfarb J, Vanholder R, Mehrotra R et al. The current and future landscape of dialysis. Nat Rev Nephrol 2020; 16: 573–585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bowry SK. Dialysis membranes today. Int J Artif Organs 2002; 25: 447–460 [DOI] [PubMed] [Google Scholar]
- 11. Soltys PJ, Zydney A, Leypoldt JK et al. Potential of dual-skinned, high-flux membranes to reduce backtransport in hemodialysis. Kidney Int 2000; 58: 818–828 [DOI] [PubMed] [Google Scholar]
- 12. Waniewski J, Debowska M, Lindholm B. Can the diverse family of dialysis adequacy indices be understood as one integrated system? Blood Purif 2010; 30: 257–265 [DOI] [PubMed] [Google Scholar]
- 13. Waniewski J, Debowska M, Lindholm B. Are dialysis adequacy indices independent of solute generation rate? ASAIO J 2014; 60: 90–94 [DOI] [PubMed] [Google Scholar]
- 14. Waniewski J, Lindholm B. Fractional solute removal and Kt/V in different modalities of renal replacement therapy. Blood Purif 2004; 22: 367–376 [DOI] [PubMed] [Google Scholar]
- 15. Locatelli F, Buoncristiani U, Canaud B et al. Dialysis dose and frequency. Nephrol Dial Transplant 2005; 20: 285–296 [DOI] [PubMed] [Google Scholar]
- 16. Tattersall J, Martin-Malo A, Pedrini L et al. EBPG guideline on dialysis strategies. Nephrol Dial Transplant 2007; 22(Suppl 2): ii5–ii21 [DOI] [PubMed] [Google Scholar]
- 17. Tattersall J. Hemodialysis time and Kt/V: less may be better. Semin Dial 2017; 30: 10–14 [DOI] [PubMed] [Google Scholar]
- 18. Vanholder R, Glorieux G, Eloot S. Once upon a time in dialysis: the last days of Kt/V? Kidney Int 2015; 88: 460–465 [DOI] [PubMed] [Google Scholar]
- 19. Jaffrin MY. Convective mass transfer in hemodialysis. Artif Organs 1995; 19: 1162–1171 [DOI] [PubMed] [Google Scholar]
- 20. Neri M, Villa G, Garzotto F et al. Nomenclature for renal replacement therapy in acute kidney injury: basic principles. Crit Care 2016; 20: 318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leypoldt JK. Solute fluxes in different treatment modalities. Nephrol Dial Transplant 2000; 15(Suppl 1): 3–9 [DOI] [PubMed] [Google Scholar]
- 22. Haroon S, Davenport A. Choosing a dialyzer: what clinicians need to know. Hemodial Int 2018; 22(Suppl 2): S65–S74 [DOI] [PubMed] [Google Scholar]
- 23. Pedrini LA, Cozzi G, Faranna P et al. Transmembrane pressure modulation in high-volume mixed hemodiafiltration to optimize efficiency and minimize protein loss. Kidney Int 2006; 69: 573–579 [DOI] [PubMed] [Google Scholar]
- 24. Feliciani A, Riva MA, Zerbi S et al. New strategies in haemodiafiltration (HDF): prospective comparative analysis between on-line mixed HDF and mid-dilution HDF. Nephrol Dial Transplant 2007; 22: 1672–1679 [DOI] [PubMed] [Google Scholar]
- 25. Canaud B. Online hemodiafiltration. Contrib Nephrol 2007; 158: 110–122 [DOI] [PubMed] [Google Scholar]
- 26. Tattersall JE, Ward RA. Online haemodiafiltration: definition, dose quantification and safety revisited. Nephrol Dial Transplant 2013; 28: 542–550 [DOI] [PubMed] [Google Scholar]
- 27. Canaud B. The early years of on-line HDF: how did it all start? How did we get here? Contrib Nephrol 2011; 175: 93–109 [DOI] [PubMed] [Google Scholar]
- 28. Locatelli F, Canaud B. Dialysis adequacy today: a European perspective. Nephrol Dial Transplant 2012; 27: 3043–3048 [DOI] [PubMed] [Google Scholar]
- 29. Ward RA, Vienken J, Silverstein DM et al. Regulatory considerations for hemodiafiltration in the United States. Clin J Am Soc Nephrol 2018; 13: 1444–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santoro A, Guarnieri F, Ferramosca E et al. Acetate-free biofiltration. Contrib Nephrol 2007; 158: 138–152 [DOI] [PubMed] [Google Scholar]
- 31. Conti P. [Paired hemodiafiltration]. G Ital di Nefrol 2012; 29(Suppl 5): S83–S88. [PubMed] [Google Scholar]
- 32. Shinzato T, Maeda K. Push/pull hemodiafiltration. Contrib Nephrol 2007; 158: 169–176 [DOI] [PubMed] [Google Scholar]
- 33. Haraldsson B, Nyström J, Deen WM. Properties of the glomerular barrier and mechanisms of proteinuria. Physiol Rev 2008; 88: 451–487 [DOI] [PubMed] [Google Scholar]
- 34. Rippe B, Davies S. Permeability of peritoneal and glomerular capillaries: what are the differences according to pore theory? Perit Dial Int 2011; 31: 249–258 [DOI] [PubMed] [Google Scholar]
- 35. Vienken J, Bowry S. Quo vadis dialysis membrane? Artif Organs 2002; 26: 152–159 [DOI] [PubMed] [Google Scholar]
- 36. Mulder M. Basic Principles of Membrane Technology. Dordrecht: Kluwer Academic, 1996: 155–162, 358–361 [Google Scholar]
- 37. Glorieux G, Vanholder R. New uremic toxins – which solutes should be removed? Contrib Nephrol 2011; 168: 117–128 [DOI] [PubMed] [Google Scholar]
- 38. Dhondt A, Vanholder R, Van Biesen W et al. The removal of uremic toxins. Kidney Int Suppl 2000; 76: S47–S59 [DOI] [PubMed] [Google Scholar]
- 39. Vanholder RC, Glorieux GL, De Smet RV. Back to the future: middle molecules, high flux membranes, and optimal dialysis. Hemodial Int 2003; 7: 52–57 [DOI] [PubMed] [Google Scholar]
- 40. Golper TA, Chaudary R, Ogu I Schulman G. High-efficiency and high-flux hemodialysis. In: Lerma EV, Weir MR (eds). Henrich's Principles and Practice of Dialysis. Philadelphia: Wolters Kluwer, 2017: 114–121 [Google Scholar]
- 41. Storr M, Ward RA. Membrane innovation: closer to native kidneys. Nephrol Dial Transplant 2018; 33(Suppl 3): iii22–iiii27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ronco C, Clark WR. Haemodialysis membranes. Nat Rev Nephrol 2018; 14: 394–410 [DOI] [PubMed] [Google Scholar]
- 43. Masakane I, Sakurai K. Current approaches to middle molecule removal: room for innovation. Nephrol Dial Transplant 2018; 33(Suppl 3): iii12–iii21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kratochwill K. The extracorporeal proteome—the significance of selective protein removal during dialysis therapy. Proteomics Clin Appl 2018; 12: e1800078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hakim RM, Saha S. Dialysis frequency versus dialysis time, that is the question. Kidney Int 2014; 85: 1024–1029 [DOI] [PubMed] [Google Scholar]
- 46. Chazot C, Jean G. The advantages and challenges of increasing the duration and frequency of maintenance dialysis sessions. Nat Clin Pract Nephrol 2009; 5: 34–44 [DOI] [PubMed] [Google Scholar]
- 47. Jefferies HJ, Virk B, Schiller B et al. Frequent hemodialysis schedules are associated with reduced levels of dialysis-induced cardiac injury (myocardial stunning). Clin J Am Soc Nephrol 2011; 6: 1326–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gul A, Miskulin DC, Harford A et al. In-center hemodialysis: time for a paradigm shift. J Am Soc Nephrol 2018; 29: 2452–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. McFarlane PA. More of the same: improving outcomes through intensive hemodialysis. Semin Dial 2009; 22: 598–602 [DOI] [PubMed] [Google Scholar]
- 50. Chan CT, Blankestijn PJ, Dember LM et al. Dialysis initiation, modality choice, access, and prescription: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2019; 96: 37–47 [DOI] [PubMed] [Google Scholar]
- 51. Kuhlmann MK. Phosphate elimination in modalities of hemodialysis and peritoneal dialysis. Blood Purif 2010; 29: 137–144 [DOI] [PubMed] [Google Scholar]
- 52. Ward RA, Greene T, Hartmann B et al. Resistance to intercompartmental mass transfer limits β2-microglobulin removal by post-dilution hemodiafiltration. Kidney Int 2006; 69: 1431–1437 [DOI] [PubMed] [Google Scholar]
- 53. Aydede SK, Komenda P, Djurdjev O et al. Chronic kidney disease and support provided by home care services: a systematic review. BMC Nephrol 2014; 15: 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Morfín JA, Yang A, Wang E et al. Transitional dialysis care units: a new approach to increase home dialysis modality uptake and patient outcomes. Semin Dial 2018; 31: 82–87 [DOI] [PubMed] [Google Scholar]
- 55. Liu FX, Treharne C, Culleton B et al. The financial impact of increasing home-based high dose haemodialysis and peritoneal dialysis. BMC Nephrol 2014; 15: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Twardowski ZJ. Treatment time and ultrafiltration rate are more important in dialysis prescription than small molecule clearance. Blood Purif 2006; 25: 90–98 [DOI] [PubMed] [Google Scholar]
- 57. Han SS, Park JY, Kang S et al. Dialysis modality and mortality in the elderly: a meta-analysis. Clin J Am Soc Nephrol 2015; 10: 983–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sulowicz W, Radziszewski A. Pathogenesis and treatment of dialysis hypotension. Kidney Int 2006; 70(Suppl 104): S36–S39 [Google Scholar]
- 59. Ng TG, Tan SH. Novel trends in haemodialysis: where are we heading? Ann Acad Med Singap 2010; 39: 482–488 [PubMed] [Google Scholar]
- 60. Vanholder R, Annemans L, Brown E et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol 2017; 13: 393–409 [DOI] [PubMed] [Google Scholar]
- 61. Walker RC, Hanson CS, Palmer SC et al. Patient and caregiver perspectives on home hemodialysis: a systematic review. Am J Kidney Dis 2015; 65: 451–463 [DOI] [PubMed] [Google Scholar]
- 62. Petrovic J, Canaud B, Kendzia D et al. Are current reimbursement schemes preventing more patients going home? Kidney Int Rep 2020; 5(3 Suppl): S95–S96 [Google Scholar]
- 63. St. Clair Russell J, Boulware LE. End-stage renal disease treatment options education: what matters most to patients and families. Semin Dial 2018; 31: 122–128 [DOI] [PubMed] [Google Scholar]
- 64. Van Buren PN PB. Dialysate composition in hemodialysis and peritoneal dialysis. In: Lerma, EV, Weir, MR (eds). Henrich's Principles and Practice of Dialysis. Philadelphia: Wolters Kluwer, 2017: 15–31 [Google Scholar]
- 65. Basile C, Lomonte C. It is time to individualize the dialysate sodium prescription. Semin Dial 2016; 29: 24–27 [DOI] [PubMed] [Google Scholar]
- 66. Colì L, La Manna G, Comai G et al. Automatic adaptive system dialysis for hemodialysis-associated hypotension and intolerance: a noncontrolled multicenter trial. Am J Kidney Dis 2011; 58: 93–100 [DOI] [PubMed] [Google Scholar]
- 67. Kuhlmann U, Maierhofer A, Canaud B et al. Zero diffusive sodium balance in hemodialysis provided by an algorithm-based electrolyte balancing controller: a proof of principle clinical study. Artif Organs 2019; 43: 150–158 [DOI] [PubMed] [Google Scholar]
- 68. Pun PH, Middleton JP. Dialysate potassium, dialysate magnesium, and hemodialysis risk. J Am Soc Nephrol 2017; 28: 3441–3451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ashby D, Borman N, Burton J et al. Renal association clinical practice guideline on haemodialysis. BMC Nephrol 2019; 20: 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Toussaint N, Boddington J, Simmonds R et al. Calcium phosphate metabolism and bone mineral density with nocturnal hemodialysis. Hemodial Int 2006; 10: 280–286 [DOI] [PubMed] [Google Scholar]
- 71. Rhee CM, Chou JA, Kalantar-Zadeh K. Dialysis prescription and sudden death. Semin Nephrol 2018; 38: 570–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Basile C, Rossi L, Lomonte C. The choice of dialysate bicarbonate: do different concentrations make a difference? Kidney Int 2016; 89: 1008–1015 [DOI] [PubMed] [Google Scholar]
- 73. Munger MA, Ateshkadi A, Cheung AK et al. Cardiopulmonary events during hemodialysis: effects of dialysis membranes and dialysate buffers. Am J Kidney Dis 2000; 36: 130–139 [DOI] [PubMed] [Google Scholar]
- 74. Suranyi M, Chow JSF. Review: anticoagulation for haemodialysis. Nephrology 2010; 15: 386–392 [DOI] [PubMed] [Google Scholar]
- 75. Lazrak HH, René É, Elftouh N et al. Safety of low-molecular-weight heparin compared to unfractionated heparin in hemodialysis: a systematic review and meta-analysis. BMC Nephrol 2017; 18: 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Leurs P, Machowska A, Lindholm B. Timing of dialysis initiation: when to start? which treatment? J Ren Nutr 2015; 25: 238–241 [DOI] [PubMed] [Google Scholar]
- 77. Mehrotra R, Rivara M, Himmelfarb J. Initiation of dialysis should be timely: neither early nor late. Semin Dial 2013; 26: 644–649 [DOI] [PubMed] [Google Scholar]
- 78. Rosansky SJ, Cancarini G, Clark WF et al. Dialysis initiation: what's the rush? Semin Dial 2013; 26: 650–657 [DOI] [PubMed] [Google Scholar]
- 79. Liberek T, Warzocha A, Galgowska J et al. When to initiate dialysis—is early start always better? Nephrol Dial Transplant 2011; 26: 2087–2091 [DOI] [PubMed] [Google Scholar]
- 80. Twardowski ZJ. Fallacies of high-speed hemodialysis. Hemodial Int 2003; 7: 109–117 [DOI] [PubMed] [Google Scholar]
- 81. Stopper A, Amato C, Gioberge S et al. Managing complexity at dialysis service centers across Europe. Blood Purif 2007; 25: 77–89 [DOI] [PubMed] [Google Scholar]
- 82. Canaud B. Adequacy target in hemodialysis. J Nephrol 2004; 17(Suppl 8): S77–S86 [PubMed] [Google Scholar]
- 83. Garbelli M, Ion Titapiccolo J, Bellocchio F et al. Leveraging digital transformation to empower clinical governance: enhancement in intermediate clinical endpoints and patients' survival after implementation of a continuous quality improvement program in a large dialysis network. Nephrol Dial Transplant 2021; doi: 10.1093/ndt/gfab160 [DOI] [PubMed] [Google Scholar]
- 84. Alquist M, Bosch JP, Barth C et al. Knowing what we do and doing what we should: quality assurance in hemodialysis. Nephron Clin Pract 2014; 126: 135–143 [DOI] [PubMed] [Google Scholar]
- 85. Kliger AS. Quality measures for dialysis: time for a balanced scorecard. Clin J Am Soc Nephrol 2016; 11: 363–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mathew AT, Fishbane S, Obi Y, Kalantar-Zadeh K. Preservation of residual kidney function in hemodialysis patients: reviving an old concept. Kidney Int 2016; 90: 262–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Canaud B, Chazot C, Koomans J et al. Fluid and hemodynamic management in hemodialysis patients: challenges and opportunities. J Bras Nefrol 2019; 41: 550–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hecking M, Moissl U, Genser B et al. Greater fluid overload and lower interdialytic weight gain are independently associated with mortality in a large international hemodialysis population. Nephrol Dial Transplant 2018; 33: 1832–1842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Arndt U, Kaltwasser JP, Gottschalk R et al. Correction of iron-deficient erythropoiesis in the treatment of anemia of chronic disease with recombinant human erythropoietin. Ann Hematol 2005; 84: 159–166 [DOI] [PubMed] [Google Scholar]
- 90. Sackett DL, Rosenberg WM. The need for evidence-based medicine. J R Soc Med 1995; 88: 620–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sackett DL, Rosenberg WM, Gray JA et al. Evidence based medicine: what it is and what it isn't. BMJ 1996; 312: 71–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Vanholder R. Approach for guideline development. Nephrol Dial Transplant 2007; 22(Suppl 2): ii1–ii4 [DOI] [PubMed] [Google Scholar]
- 93. Watanabe Y, Kawanishi H, Suzuki K et al. Japanese Society for Dialysis Therapy Clinical Guideline for “maintenance hemodialysis: hemodialysis prescriptions”. Ther Apher Dial 2015; 19(Suppl 1): 67–92 [DOI] [PubMed] [Google Scholar]
- 94. Greenhalgh T, Howick J, Maskrey N et al. Evidence based medicine: a movement in crisis? BMJ 2014; 348: g3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Ioannidis JP. Evidence-based medicine has been hijacked: a report to David Sackett. J Clin Epidemiol 2016; 73: 82–86 [DOI] [PubMed] [Google Scholar]
- 96. Scribner BH, Buri R, Caner JE et al. The treatment of chronic uremia by means of intermittent hemodialysis: a preliminary report. Trans Am Soc Artif Intern Organs 1960; 6: 114–122 [PubMed] [Google Scholar]
- 97. Nosé Y. Home hemodialysis: a crazy idea in 1963: a memoir. ASAIO J 2000;46: 13–17 [DOI] [PubMed] [Google Scholar]
- 98. Blagg CR. The early years of chronic dialysis: the Seattle contribution. Am J Nephrol 1999; 19: 350–354 [DOI] [PubMed] [Google Scholar]
- 99. Levey AS, Coresh J. Chronic kidney disease. Lancet 2012; 379: 165–180 [DOI] [PubMed] [Google Scholar]
- 100. Tordoir J, Canaud B, Haage P et al. EBPG on vascular access. Nephrol Dial Transplant 2007; 22(Suppl 2): ii88–ii117 [DOI] [PubMed] [Google Scholar]
- 101. Tattersall J, Canaud B, Heimburger O et al. High-flux or low-flux dialysis: a position statement following publication of the membrane permeability outcome study. Nephrol Dial Transplant 2010; 25: 1230–1232 [DOI] [PubMed] [Google Scholar]
- 102. Tattersall J, Martin-Malo A, Pedrini L et al. EBPG guideline on dialysis strategies. Nephrol Dial Transplant 2007; 22(Suppl 2): ii5–ii21 [DOI] [PubMed] [Google Scholar]
- 103. Canaud B, Köhler K, Sichart JM et al. Global prevalent use, trends and practices in haemodiafiltration. Nephrol Dial Transplant 2020; 35: 398–407 [DOI] [PubMed] [Google Scholar]
- 104. National Institute for Health and Care Excellence. Renal replacement therapy and conservative management. Guideline 107. London: National Institute for Health and Care Excellence, 2018. https://www.nice.org.uk/guidance [PubMed]
- 105. Canaud B, Wabel P, Tetta C. Dialysis prescription: a modifiable risk factor for chronic kidney disease patients. Blood Purif 2010; 29: 366–374 [DOI] [PubMed] [Google Scholar]
- 106. Perl J, Dember LM, Bargman JM et al. The use of a multidimensional measure of dialysis adequacy-moving beyond small solute kinetics. Clin J Am Soc Nephrol 2017; 12: 839–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. National Kidney Foundation . KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis 2015; 66: 884–930 [DOI] [PubMed] [Google Scholar]
- 108. KDOQI hemodialysis adequacy clinical practice guideline update 2015: erratum. Am J Kidney Dis 2016; 67: 534 [Google Scholar]
- 109. European Best Practice Guidelines for Haemodialysis (Part 1) . 2002; 17(Suppl 7): 1–111 [Google Scholar]
- 110. Pifer TB, McCullough KP, Port FK et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int 2002; 62: 2238–2245 [DOI] [PubMed] [Google Scholar]
- 111. Verberne WR, Das-Gupta Z, Allegretti AS et al. Development of an international standard set of value-based outcome measures for patients with chronic kidney disease: a report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD working group. Am J Kidney Dis 2019; 73: 372–384 [DOI] [PubMed] [Google Scholar]
- 112. Zoccali C, Moissl U, Chazot C et al. Chronic fluid overload and mortality in ESRD. J Am Soc Nephrol 2017; 28: 2491–2497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Charra B, Bergström J, Scribner BH. Blood pressure control in dialysis patients: importance of the lag phenomenon. Am J Kidney Dis 1998; 32: 720–724 [DOI] [PubMed] [Google Scholar]
- 114. Agarwal R, Flynn J, Pogue V et al. Assessment and management of hypertension in patients on dialysis. J Am Soc Nephrol 2014; 25: 1630–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Levin NW, Kotanko P, Eckardt KU et al. Blood pressure in chronic kidney disease stage 5D—report from a kidney disease: improving global outcomes controversies conference. Kidney Int 2010; 77: 273–284 [DOI] [PubMed] [Google Scholar]
- 116. McCallum W, Sarnak MJ. Blood pressure target for the dialysis patient. Semin Dial 2019; 32: 35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. McIntyre CW, Burton JO, Selby NM et al. Hemodialysis-induced cardiac dysfunction is associated with an acute reduction in global and segmental myocardial blood flow. Clin J Am Soc Nephrol 2008; 3: 19–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Burton JO, Jefferies HJ, Selby NM et al. Hemodialysis-induced cardiac injury: determinants and associated outcomes. Clin J Am Soc Nephrol 2009; 4: 914–920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Buchanan C, Mohammed A, Cox E et al. Intradialytic cardiac magnetic resonance imaging to assess cardiovascular responses in a short-term trial of hemodiafiltration and hemodialysis. J Am Soc Nephrol 2017; 28: 1269–1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Daugirdas JT, Greene T. Dialysis dose as a determinant of adequacy. Semin Nephrol 2005; 25: 76–80 [DOI] [PubMed] [Google Scholar]
- 121. Daugirdas JT. Dialysis dosing for chronic hemodialysis: beyond Kt/V. Semin Dial 2014; 27: 98–107 [DOI] [PubMed] [Google Scholar]
- 122. Scribner BH, Blagg CR. Effect of dialysis dose and membrane flux in maintenance hemodialysis. N Engl J Med 2003; 348: 1491–1494 [DOI] [PubMed] [Google Scholar]
- 123. Scribner BH, Oreopoulos DG. The hemodialysis product (HDP): a better index of dialysis adequacy than Kt/V. Dial Transplant 2011; 40: 431–433 [Google Scholar]
- 124. Murakami K, Kokubo K, Hirose M et al. Squared frequency-Kt/V: a new index of hemodialysis adequacy-correlation with solute concentrations by computer simulation. Ren Replace Ther 2019; 5: 8 [Google Scholar]
- 125. Tovbin D, Sherman RA. Correcting acidosis during hemodialysis: current limitations and a potential solution. Semin Dial 2016; 29: 35–38 [DOI] [PubMed] [Google Scholar]
- 126. Tentori F, Karaboyas A, Robinson BM et al. Association of dialysate bicarbonate concentration with mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2013; 62: 738–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Karaboyas A, Zee J, Brunelli SM et al. Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2017; 69: 266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hamano T. Mineral and bone disorders in conventional hemodialysis: challenges and solutions. Semin Dial 2018; 31: 592–598 [DOI] [PubMed] [Google Scholar]
- 129. Locatelli F, Cannata-Andía JB, Drüeke TB et al. Management of disturbances of calcium and phosphate metabolism in chronic renal insufficiency, with emphasis on the control of hyperphosphataemia. Nephrol Dial Transplant 2002; 17: 723–731 [DOI] [PubMed] [Google Scholar]
- 130. Moe SM, Drüeke TB. Management of secondary hyperparathyroidism: the importance and the challenge of controlling parathyroid hormone levels without elevating calcium, phosphorus, and calcium-phosphorus product. Am J Nephrol 2003; 23: 369–379 [DOI] [PubMed] [Google Scholar]
- 131. Fouque D, Kalantar-Zadeh K, Kopple J et al. A proposed nomenclature and diagnostic criteria for protein-energy wasting in acute and chronic kidney disease. Kidney Int 2008; 73: 391–398 [DOI] [PubMed] [Google Scholar]
- 132. Ikizler TA, Cano NJ, Franch H et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: a consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int 2013; 84: 1096–1107 [DOI] [PubMed] [Google Scholar]
- 133. Sabatino A, Piotti G, Cosola C et al. Dietary protein and nutritional supplements in conventional hemodialysis. Semin Dial 2018; 31: 583–591 [DOI] [PubMed] [Google Scholar]
- 134. Collister D, Rigatto C, Tangri N. Anemia management in chronic kidney disease and dialysis: a narrative review. Curr Opin Nephrol Hypertens 2017; 26: 214–218 [DOI] [PubMed] [Google Scholar]
- 135. Drüeke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s). Kidney Int 2012; 82: 952–960 [DOI] [PubMed] [Google Scholar]
- 136. Locatelli F, Bárány P, Covic A et al. Kidney Disease: Improving Global Outcomes guidelines on anaemia management in chronic kidney disease: a European Renal Best Practice position statement. Nephrol Dial Transplant 2013; 28: 1346–1359 [DOI] [PubMed] [Google Scholar]
- 137. Qureshi AR, Alvestrand A, Divino-Filho JC et al. Inflammation, malnutrition, and cardiac disease as predictors of mortality in hemodialysis patients. J Am Soc Nephrol 2002; 13(Suppl 1): S28–S36 [PubMed] [Google Scholar]
- 138. Cobo G, Lindholm B, Stenvinkel P. Chronic inflammation in end-stage renal disease and dialysis. Nephrol Dial Transplant 2018; 33(Suppl 3): iii35–iii40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Borges MC, Vogt BP, Martin LC et al. Malnutrition Inflammation Score cut-off predicting mortality in maintenance hemodialysis patients. Clin Nutr ESPEN 2017; 17: 63–67 [DOI] [PubMed] [Google Scholar]
- 140. Canaud B. Residual renal function: the delicate balance between benefits and risks. Nephrol Dial Transplant 2008; 23: 1801–1805 [DOI] [PubMed] [Google Scholar]
- 141. Obi Y, Rhee CM, Mathew AT et al. Residual kidney function decline and mortality in incident hemodialysis patients. J Am Soc Nephrol 2016; 27: 3758–3768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Vilar E, Farrington K. Emerging importance of residual renal function in end-stage renal failure. Semin Dial 2011; 24: 487–494 [DOI] [PubMed] [Google Scholar]
- 143. Mapes DL, Bragg-Gresham JL, Bommer J et al. Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 2004; 44(Suppl 2): 54–60 [DOI] [PubMed] [Google Scholar]
- 144. Morena M, Jaussent A, Chalabi L et al. Treatment tolerance and patient-reported outcomes favor online hemodiafiltration compared to high-flux hemodialysis in the elderly. Kidney Int 2017; 91: 1495–1509 [DOI] [PubMed] [Google Scholar]
- 145. Held PJ, Levin NW, Bovbjerg RR et al. Mortality and duration of hemodialysis treatment. JAMA 1991; 265: 871–875 [PubMed] [Google Scholar]
- 146. Saran R, Bragg-Gresham JL, Levin NW et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int 2006; 69: 1222–1228 [DOI] [PubMed] [Google Scholar]
- 147. Lacson EJ, Brunelli SM. Hemodialysis treatment time: a fresh perspective. Clin J Am Soc Nephrol 2011; 6: 2522–2530 [DOI] [PubMed] [Google Scholar]
- 148. Maduell F, Ojeda R, Arias-Guillen M et al. Eight-year experience with nocturnal, every-other-day, online haemodiafiltration. Nephron 2016; 133: 98–110 [DOI] [PubMed] [Google Scholar]
- 149. European Best Practice guidelines for haemodialysis (part 1). Section IV. Dialysis fluid purity. Nephrol Dial Transplant 2002; 17(Suppl 7): 45–62 [PubMed] [Google Scholar]
- 150. Hasegawa T, Nakai S, Masakane I et al. Dialysis fluid endotoxin level and mortality in maintenance hemodialysis: a nationwide cohort study. Am J Kidney Dis 2015; 65: 899–904 [DOI] [PubMed] [Google Scholar]
- 151. Schiffl H. High-flux dialyzers, backfiltration, and dialysis fluid quality. Semin Dial 2011; 24: 1–4 [DOI] [PubMed] [Google Scholar]
- 152. Masakane I, Kawanishi H, Mineshima M et al. 2011 JSDT standard on the management of endotoxin retentive filter for dialysis and related therapies. Ther Apher Dial 2013; 17: 229–240 [DOI] [PubMed] [Google Scholar]
- 153. Robinson BM, Bieber B, Pisoni RL et al. Dialysis Outcomes and Practice Patterns Study (DOPPS): its strengths, limitations, and role in informing practices and policies. Clin J Am Soc Nephrol 2012;7: 1897–1905 [DOI] [PubMed] [Google Scholar]
- 154. Cattinelli I, Bolzoni E, Barbieri C et al. Use of self-organizing maps for balanced scorecard analysis to monitor the performance of dialysis clinic chains. Health Care Manag Sci 2012; 15: 79–90 [DOI] [PubMed] [Google Scholar]
- 155. Stopper A, Raddatz A, Grassmann A et al. Delivering quality of care while managing the interests of all stakeholders. Blood Purif 2011; 32: 323–330 [DOI] [PubMed] [Google Scholar]
- 156. Kong J, Davies M, Mount P. The importance of residual kidney function in haemodialysis patients. Nephrology (Carlton) 2018; 23: 1073–1080 [DOI] [PubMed] [Google Scholar]
- 157. Marants R, Qirjazi E, Grant CJ et al. Renal perfusion during hemodialysis: intradialytic blood flow decline and effects of dialysate cooling. J Am Soc Nephrol 2019; 30: 1086–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Chen TK, Knicely DH, Grams ME. Chronic kidney disease diagnosis and management: a review. JAMA 2019; 322: 1294–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Barbieri C, Cattinelli I, Neri L et al. Development of an artificial intelligence model to guide the management of blood pressure, fluid volume, and dialysis dose in end-stage kidney disease patients: proof of concept and first clinical assessment. Kidney Dis (Basel) 2019; 5: 28–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Davenport A. New dialysis technology and biocompatible materials. Contrib Nephrol 2017; 189: 130–136 [DOI] [PubMed] [Google Scholar]
- 161. Busink E, Canaud B, Schröder-Bäck P et al. Chronic kidney disease: exploring value-based healthcare as a potential viable solution. Blood Purif 2019; 47: 156–165 [DOI] [PubMed] [Google Scholar]
- 162. Canaud B, Collins A, Maddux F. The renal replacement therapy landscape in 2030: reducing the global cardiovascular burden in dialysis patients. Nephrol Dial Transplant 2020; 35(Suppl 2): ii51–ii57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Germain MJ, Davison SN, Moss AH. When enough is enough: the nephrologist's responsibility in ordering dialysis treatments. Am J Kidney Dis 2011; 58: 135–143 [DOI] [PubMed] [Google Scholar]
- 164. Schnabel TG. Is medicine still an art? N Engl J Med 1983; 309: 1258–1261 [DOI] [PubMed] [Google Scholar]