Abstract
Besides being expressed on professional antigen-presenting cells, HLA class II antigens are expressed on various tumors of non-lymphoid origin, including a subset of colorectal cancers (CRC). Information about the regulation of HLA class II antigen expression is important for a better understanding of their role in the interactions between tumor and immune cells. Whether lack of HLA class II antigen expression in tumors reflects the selective immune destruction of HLA class II antigen-expressing tumor cells is unknown. To address this question, we tested whether lack of HLA class II antigen expression in CRC was associated with immune cell infiltration. We selected microsatellite-unstable (MSI-H) CRC, because they show pronounced tumor antigen-specific immune responses and, in a subset of tumors, lack of HLA class II antigen expression due to mutations inactivating HLA class II-regulatory genes. We examined HLA class II antigen expression, mutations in regulatory genes, and CD4-positive T cell infiltration in 69 MSI-H CRC lesions. Mutations in RFX5, CIITA, and RFXAP were found in 13 (28.9 %), 3 (6.7 %), and 1 (2.2 %) out of 45 HLA class II antigen-negative tumors. CD4-positive tumor-infiltrating lymphocyte counts were significantly higher in HLA class II antigen-negative tumors harboring mutations in HLA class II-regulatory genes (107.4 T cells per 0.25 mm2) compared to tumors without mutations (55.5 T cells per 0.25 mm2, p = 0.008). Our results suggest that the outgrowth of tumor cells lacking HLA class II antigen expression due to mutations of regulatory genes is favored in an environment of dense CD4-positive T cell infiltration.
Keywords: Colorectal cancer, HLA class II antigens, Immune evasion, Microsatellite instability, Tumor-infiltrating lymphocytes
Introduction
Human leukocyte antigen (HLA) class II antigens are expressed by professional antigen-presenting cells but can also be expressed at the surface of different types of solid tumor cells, although with marked differences in their frequency [1–3]. HLA class II antigens are in general heterogeneous in their expression within malignant lesions [3, 4]. In colorectal cancer (CRC), HLA class II antigen expression appears to have a clinical significance, since it is associated with improved prognosis [5–7].
HLA class II antigen expression by tumor cells requires two conditions: First, the presence of cytokines that induce HLA class II antigens in the tumor microenvironment [8], and second, functionality of genes which regulate HLA class II antigen expression upon cytokine stimulation. The latter can be impaired either by gene-inactivating mutations [9] or by silencing through promoter methylation [10].
Whether immune selection plays a role in the lack of HLA class II antigen expression by tumor cells with non-functional HLA class II antigen-regulatory genes is not known. To address this question, we have selected mismatch repair-deficient CRC with the high-level microsatellite instability (MSI-H) phenotype as a model system. The MSI-H phenotype occurs in about 15 % of sporadic CRC and in virtually all CRC lesions associated with the inherited Lynch syndrome [11–14]. MSI-H CRC lesions are a particularly well-suited model for studying immune selection because of three main reasons: (1) A relevant and measurable tumor antigen-specific immune response has been convincingly shown in these tumors [15, 16]. This immune response appears to be triggered by frameshift peptides, which are generated as a result of mismatch repair deficiency-induced insertion or deletion mutations at gene-encoding, short repetitive sequence stretches (coding microsatellites, cMS). MSI-H-associated frameshift peptides have been shown to induce HLA class I- and II-mediated T cell immune responses of the host, and frameshift peptide-derived HLA class I and II epitopes have been described [17, 18]. (2) HLA class II antigens are strongly expressed in about one-third of MSI-H CRC lesions [9], and signs of a pronounced local immune response are typical of these tumors [19, 20]. (3) Selection processes can be particularly well monitored in the model of MSI-H cancers, because mismatch repair deficiency-induced mutations occur at defined sets of cMS in the genome [21] (www.seltarbase.org [22]). Though these mutations occur randomly as a result of polymerase slippage events during DNA replication, tumor cell clones, which have acquired certain combinations of cMS mutations that favor their outgrowth [21], are selected for during MSI-H CRC progression. Consequently, tumor-promoting cMS mutations will be more frequent in manifesting MSI-H tumors than neutral or tumor-suppressive mutations [21].
The suitability of MSI-H CRC as a model for analyzing immune selection processes has become apparent in previous studies, which have demonstrated a high frequency of abnormalities in HLA class I antigen expression in MSI-H CRC lesions, mainly caused by mutations in the Beta2-microglobulin gene [23–25]. This observation strongly argues in favor of immune selection playing a role in MSI-H CRC development, as the cell clones which grow out are those, which have acquired the ability to escape from CD8-positive T cell recognition. However, whether this mechanism applies also to the differential HLA class II antigen expression in CRC lesions is not known.
Knowledge about the role of immunoselection in HLA class II antigen expression in solid tumors not only contributes to our understanding of the mechanisms involved in the expression of HLA class II antigens on tumor cells, but also of the role HLA class II antigens play in the interactions of tumor cells with the host’s immune system. We here investigated the expression of HLA class II antigens in MSI-H CRC lesions, analyzed HLA class II-regulatory genes for mutations, and measured the level of T cell infiltration in these tumors, with special emphasis on CD4-positive T cells as the direct interaction partners of HLA class II antigens.
Materials and methods
Patients and tumor specimens
Colorectal cancer samples were obtained from the Institute of Pathology, Westpfalz-Klinikum Kaiserslautern and the Institute of Pathology, University Hospital Heidelberg with informed consent from patients included in this study. The study was approved by the institutional ethics committee. The microsatellite instability status had been determined using marker panels described previously (BAT25, BAT26, D2S123, D5S346, D17S250, and CAT25) [26, 27]. Patients’ characteristics are summarized in Table 1.
Table 1.
Characteristics of CRC patients
| Total | Sporadic CRCs | Hereditary CRCs | |
|---|---|---|---|
| Number of patients | 69 | 34 | 35 |
| Median age (range) | 49.5 (27–88) | 77 (52–88) | 49 (27–79) |
| NA | 3 | – | 3 |
| Gender | |||
| Male | 30 (43.5)a | 12 (35.3) | 18 (51.4) |
| Female | 39 (56.5) | 22 (64.7) | 17 (48.6) |
| Localization | |||
| Proximal | 45 (65.2) | 29 (85.3) | 16 (45.7) |
| Distal | 8 (11.6) | 3 (8.8) | 5 (14.3) |
| NA | 16 (23.2) | 2 (5.9) | 14 (40.0) |
| T stage | |||
| T1 | 1 (1.4) | 0 (0.0) | 1 (2.9) |
| T2 | 17 (24.6) | 7 (20.6) | 10 (28.6) |
| T3 | 35 (50.7) | 21 (31.8) | 14 (40.0) |
| T4 | 10 (14.5) | 5 (14.7) | 5 (14.3) |
| Tx | 6 (8.7) | 1 (2.9) | 5 (14.3) |
| N stage | |||
| N0 | 33 (47.8) | 22 (64.7) | 11 (34.4) |
| N1/N2 | 21 (30.4) | 11 (32.4) | 10 (28.6) |
| Nx | 15 (21.7) | 1 (2.9) | 14 (40.0) |
aPercentages are given in brackets
Antibodies
The HLA-DR-, HLA-DQ-, HLA-DP-specific monoclonal antibody (mAb) LGII-612.14 was developed and characterized as described previously [28]. mAb was purified from ascitic fluid by sequential precipitation with ammonium sulfate and caprylic acid [29]. The purity and activity of mAb preparation were monitored by SDS-PAGE and by a binding assay with cells expressing HLA class II antigens.
The CD4-specific mouse monoclonal antibody (clone IF6) and the CD8-specific mouse monoclonal antibody (clone 4B11) were obtained from Novocastra (Newcastle, UK). Biotinylated anti-mouse IgG xenoantibodies were obtained from Vector Laboratories (Burlingame, CA) and used as secondary antibodies.
Isolation of genomic DNA
Formalin-fixed, paraffin-embedded tissue sections (6–8 µm) were deparaffinized and stained with hematoxylin and eosin using standard protocols. For the isolation of genomic DNA, the Qiagen DNeasy Tissue Kit (Qiagen, Hilden, Germany) was used according to the manufacturer’s instructions.
Analysis of frameshift mutations
To identify cMS in HLA class II antigen-regulatory genes as potential mutational targets in mismatch repair-deficient cancers, a database search using publicly available information (www.seltarbase.org [22]) was performed for RFXAP (NM_000538) and RFXANK (NM_003721), with a minimum length of six mononucleotides. The cMS with the longest repeat within the respective gene was selected for mutation analysis. In addition, the previously reported C7 repeats in exon 3 of RFX5 (NM_000449) and in exon 11 of CIITA (NM_000246) [9] were also analyzed for cMS mutations in MSI-H CRCs. PCR was performed using the primers listed in Table 2, and PCR products were purified with the QIAquick PCR purification kit (Qiagen, Hilden, Germany). The sequencing reaction was performed using the Big Dye Terminator v1.1 Cycle Sequencing kit (Applied Biosystems, Darmstadt, Germany). Products for sequencing were precipitated, solved in 12 µl Hi-Di Formamide (Applied Biosystems, Darmstadt, Germany) and analyzed on an ABI3100 genetic analyzer.
Table 2.
Primer pairs used to amplify HLA class II-regulatory genes
| Gene | Repeat | Direction | Sequence (5′–3′) | Annealing (°C) | No. of cycles |
|---|---|---|---|---|---|
| RFX5 | C7 | s | CGGGATGGCAGAAGATGA | 58 | 40 |
| as | CAGGACTTGGAGATGTGATGA | ||||
| CIITA | C7 | s | GACTCTATGTCGGCCTGCT | 58 | 40 |
| as | GAACTGGTCCTCCTGTAGGG | ||||
| RFXAP | G61, G62 | s | AGGCGGACCTGTTAGACACT | 57 | 40 |
| as | CTTCGTAGGTGCAGGTCTTG | ||||
| RFXAP | A6 | s | TCGTCCTGCAAGACCTACTC | 55 | 40 |
| as | CAAAAATCTCAATTGTTCCATTT | ||||
| RFXANK | C6 | s | GGTGCAGCCTGGTGGTAT | 55 | 40 |
| as | GAACGGTCTCAATCTCTCCA |
s sense, as antisense
Immunohistochemistry (IHC)
HLA class II antigen and CD8 staining was performed following standard protocols [9]. Briefly, after deparaffinization and rehydration of paraffin tissue sections (2 µm), slides were boiled in 10 mM citrate buffer (pH 6.0) for 15 min for antigen retrieval and allowed to cool for 30 min. Endogenous peroxidase was blocked with 0.6 % H2O2 (v/v in methanol). For CD4 staining, antigen retrieval was performed after the peroxidase blocking step. Non-specific antibody binding was blocked by incubating tissue sections in 10 % horse serum (v/v in PBS, Vector Laboratories, Inc., Burlingame, CA, USA) for 10 min at room temperature. As a next step, tissue sections were incubated with the primary antibody (anti-CD4 mAb IF6, 1:40; anti-CD8 mAb 4B11, 1:40; mAb LGII-612.14, 1:50) overnight at +4 °C. After washing and incubation with the secondary antibody (biotinylated anti-mouse IgG/anti-rabbit IgG, 1:50, Vector Laboratories) for 30 min at room temperature, avidin–biotin-coupled horseradish peroxidase reagent (1:50, Vector Laboratories) was applied according to the manufacturer’s instructions. Antigen detection was performed by color reaction using 3,3-diaminobenzidine (Dako Liquid DAB + Chromogen Substrate, Dako, Glostrup, Denmark). Sections were counterstained with Mayer’s hematoxylin (AppliChem, Darmstadt, Germany).
Microscopic evaluation and statistical analysis
Tissue sections stained for HLA class II antigens were scored as high expression of HLA class II antigens, when strong and homogeneous staining was observed (Fig. 1a), or as lack of or barely detectable expression of HLA class II antigens, when staining was not detectable or faint and patchy (Fig. 1b), according to previously published evaluation protocols [9]. From the tissue samples stained for CD4-positive T cells, three to five representative tumor regions were analyzed for T cell infiltration utilizing a 10 × 10 ocular grid at a magnification of ×200 (analyzed area: 0.25 mm2) and a Leica DMRBE microscope (Leica, Solms, Germany). To differentiate T cells from dendritic cells or monocytes/macrophages, which can express low levels of cytoplasmic CD4 [30, 31], only cells showing strong membraneous staining were recorded as CD4-positive T cells. HLA class II antigen-positive lesions with circumscribed cMS mutation-induced lack of HLA class II antigen expression (n = 5) were not evaluable for CD4-positive T cell infiltration, because the affected areas, which did not express HLA class II antigens, were too small for a proper assessment of CD4-positive T cell counts. Stromal and epithelial compartments were evaluated separately. Relative infiltration was determined as previously described [32]. Box–Whisker plots were generated, and statistical evaluation was performed utilizing Statistica software (release 7, StatSoft Europe, Hamburg, Germany). Mann–Whitney rank sum test and Kruskal–Wallis test were used to calculate the statistical significance of the differences. P values smaller than 0.05 were considered as statistically significant.
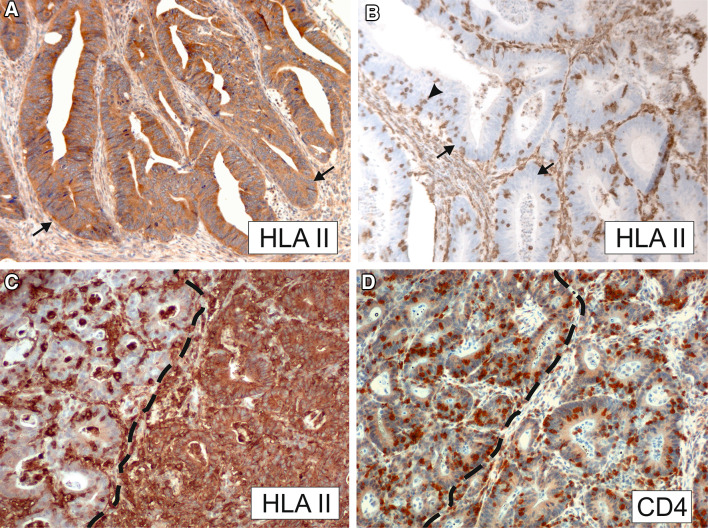
Fig. 1.
Representative immunohistochemical stainings with the HLA class II antigen-specific mAb LGII-612.14 (panels A–C) and CD4-specific mAb IF6 (panel D). a High HLA class II antigen expression in tumor cells (arrows). b HLA class II antigens are not detectable in tumor cells (arrows) but are expressed in stromal cells and in intraepithelial, tumor-infiltrating lymphocytes (arrowhead). c Tumor section displaying adjacent areas with strong HLA class II antigen expression (right) and lack of HLA class II antigen expression (left, areas are separated by a dashed line). d Tumor section stained for CD4-positive T cells (region corresponding to C). CD4-positive T cell counts were elevated in the HLA class II antigen-negative area (left) compared to the HLA class II antigen-positive area (right)
Results
HLA class II antigen expression in MSI-H CRC lesions
HLA class II antigen expression was analyzed in 69 MSI-H CRC lesions; 35 of them were Lynch syndrome-associated MSI-H CRCs, and the remaining 34 were sporadic MSI-H CRCs. Clinical characteristics of the lesions are summarized in Table 1.
High HLA class II antigen expression was detected in 24 (34.8 %) out of 69 MSI-H CRCs lesions. The frequency of HLA class II antigen expression in sporadic and hereditary CRC lesions was similar (sporadic 13/34, 38.2 %; hereditary 11/35, 31.4 %; p = 0.62) as shown in Table 3. Representative HLA class II antigen staining patterns are shown in Fig. 1.
Table 3.
Mutation frequencies of HLA class II-regulatory genes
| All CRC | Mutation (%) | RFX5 | CIITA | RFXANK | RFXAP | |||
|---|---|---|---|---|---|---|---|---|
| C7 in exon 3 (%) | C7 in exon 11 (%) | C6 in exon 6 (%) | G61* in exon 1 (%) | G62** in exon 1 (%) | A6 in exon 2 (%) | |||
| 69 | 18/69 (26.1) | 14/69 (20.3) | 3/69 (4.3) | 0/69 (0.0) | 1/69 (1.4) | 0/69 (0.0) | 0/69 (0.0) | |
| HLA class II antigen expression | ||||||||
| All MSI-H CRC | ||||||||
| High | 24 | 1/24 (4.2)# | 1/24 (4.2)# | 0/24 (0.0) | 0/24 (0.0) | 0/24 (0.0) | 0/24 (0.0) | 0/24 (0.0) |
| Lack or barely detectable | 45 | 17/45 (37.8) | 13/45 (28.9) | 3/45 (6.7) | 0/45 (0.0) | 1/45 (2.2) | 0/45 (0.0) | 0/45 (0.0) |
| Sporadic MSI-H CRC | ||||||||
| High | 13 | 0/13 (0.0) | 0/13 (0.0) | 0/13 (0.0) | 0/13 (0.0) | 0/13 (0.0) | 0/13 (0.0) | 0/13 (0.0) |
| Lack or barely detectable | 21 | 9/21 (42.9) | 9/21 (42.9) | 0/21 (0.0) | 0/21 (0.0) | 0/21 (0.0) | 0/21 (0.0) | 0/21 (0.0) |
| Hereditary MSI-H CRC | ||||||||
| High | 11 | 1/11 (9.1)# | 1/11 (9.1)# | 0/11 (0.0) | 0/11 (0.0) | 0/11 (0.0) | 0/11 (0.0) | 0/11 (0.0) |
| Lack or barely detectable | 24 | 8/24 (33.3) | 4/24 (16.7) | 3/24 (12.5) | 0/24 (0.0) | 1/24 (4.2) | 0/24 (0.0) | 0/24 (0.0) |
* Position c.297 to c.302 ** Position c.354 to c.359
#Includes a tumor with regional lack of HLA class II antigen expression
Frameshift mutations in cMS of HLA class II-regulatory genes
The database search for cMS located in HLA class II-regulatory genes revealed three cMS in RFXAP; two were G6 repeats located in exon 1, and one was an A6 repeat in exon 2. One C6 repeat was identified in exon 6 of RFXANK. Based on the results of the database search, the 69 MSI-H CRC lesions were analyzed for cMS mutations in the HLA class II-regulatory genes CIITA, RFX5, RFXAP, and RFXANK. The results, which include RFX5 and CIITA mutation data in a subset of the specimens published previously [9], revealed that 17 (37.8 %) out of 45 CRC lesions with lack of or barely detectable HLA class II antigen expression harbored mutations in one of the regulatory genes analyzed. The frequency of mutations affecting HLA class II-regulatory genes was significantly higher in tumors with lack of or barely detectable HLA class II antigen expression compared to tumors showing high HLA class II antigen expression (17 out of 45, 37.8 %; 1 out of 24, 4.2 %; p = 0.003, Table 3).
Coding microsatellite mutations in RFX5 and CIITA were detected in 13 (28.9 %) and 3 (6.7 %) of the 45 tumors with lack of or barely detectable HLA class II antigen expression, respectively. A novel mutation affecting the G6 repeat starting at nucleotide position 297 of the RFXAP-encoding gene region was identified in one MSI-H CRC (1 out of 45, 2.2 %). In contrast, no mutation was found in RFXANK. The overall frequency of mutations affecting HLA class II-regulatory genes was not significantly different between sporadic and hereditary MSI-H CRCs. The mutation frequency of CIITA was higher in hereditary (3 out of 24, 12.5 %) than in sporadic tumors (0 out of 21, 0 %, p = 0.24), while that of RFX5 was lower in hereditary (4 out of 24, 16.7 %) than in sporadic MSI-H CRC lesions (9 out of 21, 42.9 %, p = 0.10, Table 3). However, neither difference reached the level of statistical significance.
Tumor infiltration with CD4-positive T cells
To analyze whether HLA class II antigen expression was associated with CD4-positive T cell infiltration, we measured the density of infiltrating CD4-positive T cells in the epithelial and stromal compartment of 58 MSI-H CRC lesions.
High-level microsatellite instability CRC lesions showed infiltration with CD4-positive T cells at a median density of 86.3 T cells per 0.25 mm2. Separate analysis of the epithelial and stromal compartment revealed significantly higher numbers of CD4-positive T cells in the tumor stroma in comparison with the tumor epithelium (median: 124.8 T cells vs. 54.5 T cells per 0.25 mm2, p < 0.001). Comparison of CD4-positive T cell infiltration between sporadic and hereditary MSI-H CRC detected no significant difference in epithelial (median: 68.9 T cells vs. 53.4 T cells per 0.25 mm2, p = 0.90) and stromal (median: 141.2 T cells vs. 119.4 T cells per 0.25 mm2, p = 0.90) T cell infiltration (data not shown).
Correlation of tumor-infiltrating CD4-positive T cells with HLA class II antigen expression and mutations in HLA class II- regulatory genes
The CD4-positive T cell infiltration in tumors with high HLA class II antigen expression was not significantly different from tumors with lack of or barely detectable HLA class II antigen expression, when mutation data were not taken into account (median: 98.9 T cells vs. 78.9 T cells per 0.25 mm2, p = 0.335; Fig. 2a). However, CD4-positive T cell infiltration in lesions with strong HLA class II antigen expression was significantly higher than that in lesions with lack of or barely detectable HLA class II antigen expression if only lesions without mutations in HLA class II-regulatory genes were considered (median: 98.9 T cells vs. 55.5 T cells per 0.25 mm2, p = 0.027).
Fig. 2.
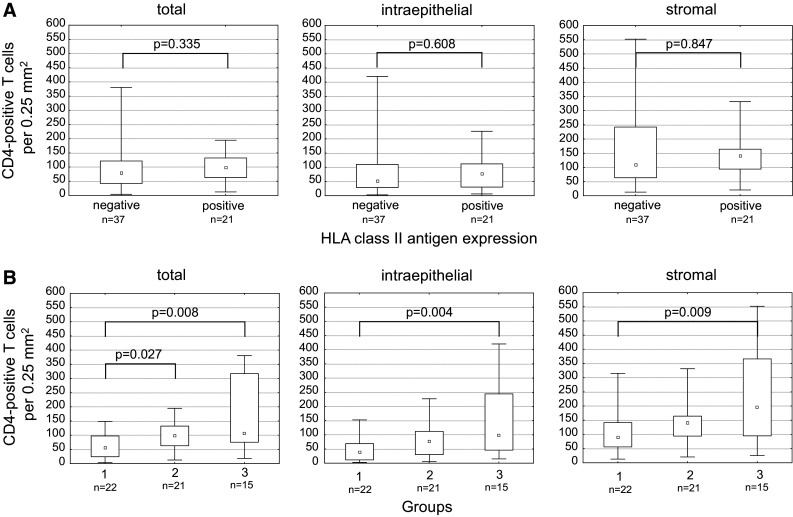
Correlation of CD4-positive T cell infiltration with HLA class II antigen expression not accounting (a) and accounting for (b) mutations in HLA class II-regulatory genes. a There was no significant correlation of CD4-positive T cells with HLA class II antigen expression. b When tumors were grouped accounting for cMS mutations in HLA class II-regulatory genes, significant differences of CD4-positive T cell infiltration emerged between the three groups: group 1—tumors with lack of or barely detectable HLA class II antigen expression (“negative”) without cMS mutations in one of the HLA class II-regulatory genes, group 2—HLA class II antigen-positive tumors, group 3—HLA class II antigen-negative tumors with cMS mutations in one of the HLA class II-regulatory genes. Infiltration (total, intraepithelial, and stromal) was highest in cMS-mutant group 3
In tumors with lack of or barely detectable HLA class II antigen expression, the number of CD4-positive T cells infiltrating tumors harboring a mutation in one of the regulatory genes was significantly higher than that in tumors that were wild type for the same genes (median: 107.4 T cells vs. 55.5 T cells per 0.25 mm2, p = 0.008). The same differences were observed when analyzing intraepithelial and stromal lymphocyte counts separately (Fig. 2). Densities of CD4-positive T cell infiltration in MSI-H CRC lesions and their relation to HLA class II antigen expression are shown in Fig. 2.
Generally, mutations in HLA class II-regulatory genes were more frequent in MSI-H CRC lesions showing infiltration with CD4-positive T cells above median than in those showing infiltration below median (14 out of 29, 48.3 %, vs. 7 out of 29, 24.1 %, p = 0.10).
Correlation of tumor-infiltrating CD8-positive T cells with HLA class II antigen expression
To evaluate whether the association between HLA class II antigen phenotype and T cell infiltration was specifically restricted to CD4-positive T cells, CD8-positive T cells were counted in a subset of the tumors (n = 29), from which sufficient material was available.
Overall, MSI-H CRC lesions showed infiltration with CD8-positive T cells at a median density of 57.0 T cells per 0.25 mm2. Akin to CD4-positive T cells, separate analysis of the epithelial and stromal compartment revealed significantly higher numbers of CD8-positive T cells in the tumor stroma in comparison with the tumor epithelium (median: 98.9 T cells vs. 37.6 T cells per 0.25 mm2, p < 0.001).
A trend toward an increased CD8-positive T cell infiltration was observed in HLA class II antigen-positive tumors compared to HLA class II antigen-negative tumors (p = 0.094) (Fig. 3). CD8-positive T cell infiltration in lesions with high HLA class II antigen expression, but without detectable mutations was significantly higher than that in lesions with lack of or barely detectable HLA class II antigen expression (median: 65.7 T cells vs. 19.6 T cells per 0.25 mm2, p = 0.013). However, in contrast to the results obtained for CD4-positive T cell infiltration, the comparison between mutation-positive and mutation-negative tumors with lack of or barely detectable HLA class II antigen expression did not reach statistical significance (median: 61.6 T cells vs. 19.6 T cells per 0.25 mm2, p = 0.051). The results are summarized in Fig. 3. Similarly, T cell infiltration was not significantly associated with HLA class I antigen expression. The latter was determined using B2M mutation status as a surrogate parameter in a subset of lesions (data not shown).
Fig. 3.
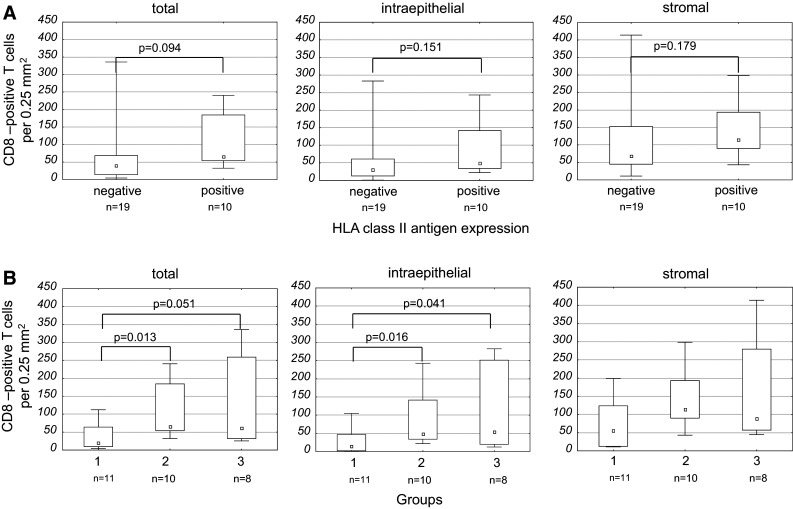
Correlation of CD8-positive T cell infiltration with HLA class II antigen expression not accounting (a) and accounting for (b) mutations in HLA class II-regulatory genes. a There was no significant correlation of CD8-positive T cells with HLA class II antigen expression. b When tumors were grouped accounting for cMS mutations in HLA class II-regulatory genes, significant differences of CD8-positive T cell infiltration emerged between the groups. Tumors with lack of or barely detectable HLA class II antigen expression (“negative”) without cMS mutations in one of the HLA class II-regulatory genes (group 1) and HLA class II antigen-positive tumors (group 2)
Discussion
The present study has shown for the first time an association between the density of tumor-infiltrating CD4-positive T cells and lack of HLA class II antigen expression in MSI-H CRC lesions harboring mutations inactivating HLA class II-regulatory genes. We demonstrate that MSI-H CRC lesions, which lack HLA class II antigen expression, consist of two groups: One includes tumors with a low density of local immune cell infiltration and lack of HLA class II antigen expression not caused by mutations in HLA class II-regulatory genes. The other group includes tumors with pronounced CD4-positive T cell infiltration and lack of HLA class II antigen expression due to mutations in HLA class II-regulatory genes.
Several lines of evidence support the possibility that lack of HLA class II antigen expression in the latter group reflects the outgrowth of cells, which do not express HLA class II antigens as a result of the selective pressure imposed by tumor-infiltrating CD4-positive T cells. The observation that mutations inactivating HLA class II-regulatory genes that are detectable in tumors with a particularly high density of CD4-positive T cells suggest that a pronounced local immune response may eliminate HLA class II antigen-positive tumor cells and favor the outgrowth of tumor cells lacking HLA class II antigen expression due to mutations in regulatory genes. In this regard, CD4-positive T cells as direct interaction partners of HLA class II antigens have previously been shown to be capable of exerting cytotoxic activity and contribute directly to tumor cell lysis [33–36]. Accordingly, mutations inactivating HLA class II-regulatory genes may mirror an immunoselective pressure imposed by CD4-positive T cells, which shape the MSI-H CRC phenotype during tumor progression.
In our study, the comparative analysis of CD8-positive T cell infiltration revealed results similar to CD4-positive T cells, though statistical significance was not reached (Fig. 3), potentially due to the limited number of tumor samples. Unfortunately, no material for additional analyses was available from the remaining cases. The present study is underpowered to examine a potential association between HLA class I antigen expression and T cell infiltration. In a subset of samples, tested for HLA class I antigen expression by B2M mutation analysis, no significant association was detected between HLA class I antigen expression and degree of T cell infiltration (data not shown). This finding is in agreement with previous observations that in colorectal cancer, unlike in melanoma [37], lack of HLA class I antigen expression is not associated with a reduction in tumor-infiltrating lymphocytes. Some studies even suggested an increased T cell infiltration in HLA class I antigen-negative compared to HLA class I antigen-positive CRCs [38]. This difference, which may be caused by immune selection, parallels the results of this study: CD4-positive T cell infiltration was increased in CRCs, which do not express HLA class II antigen expression because of mutations in HLA class II-regulatory genes. Furthermore, in the subgroup of HLA class II antigen-negative CRC lesions, no significant association was found between HLA class I antigen expression and T cell infiltration, suggesting that HLA class II antigen expression is more closely linked to T cell infiltration than HLA class I antigen expression. Additional studies are required to identify the mechanisms contributing to T cell retention or expansion in CRCs that lack HLA class II antigen expression due to mutations in regulatory genes.
The hypothesis of immune selection and functional significance of lack of HLA class II antigen expression is further supported by the observation that three different genes encoding proteins involved in the regulation of HLA class II antigen expression have been found to be mutated in MSI-H CRC. Based on bioinformatics predictions (www.seltarbase.org [22]), the occurrence of mutations affecting three genes, which harbor short cMS (microsatellite length of seven nucleotides) and which are involved in one pathway, is highly unlikely in a neutral environment and thus strongly compatible with the presence of a selective pressure.
To comprehensively analyze mutations in HLA class II-regulatory genes in MSI-H CRCs and to correlate with HLA class II antigen expression and CD4-positive T cell infiltration, we have performed a systematic database search and mutation analysis of cMS in HLA class II-regulatory genes. So far, RFX5 and CIITA mutations are known to cause lack of HLA class II antigen expression in a subset of MSI-H CRC lesions [9]. In the present study, three additional cMS were identified in RFXAP and RFXANK, and a so far unknown mutation in RFXAP was detected in one MSI-H CRC lesion that lacked HLA class II antigen expression. Thus, the present study identified somatic mutations of the RFXAP gene as a novel mechanism underlying lack of HLA class II antigen expression in solid tumors.
Similar to the observations by Cabrera et al. [4], no correlation was observed between HLA class II antigen expression and tumor-infiltrating CD4-positive T cell counts, when mutation status of HLA class II-regulatory genes was not taken into account. This, however, is in contrast to a previous study [7], most likely reflecting the higher proportion of tumors harboring mutations in HLA class II-regulatory genes in our study, which results from the inclusion of only MSI-H CRC lesions.
In summary, our results demonstrate that mutations in distinct HLA class II-regulatory genes including RFX5, CIITA, and RFXAP occur frequently in MSI-H CRC. Mutations in HLA class II antigen-regulatory genes cause lack of HLA class II antigen expression and are associated with an increased infiltration with CD4-positive T cells. The association of mutations in HLA class II antigen-regulatory genes with high CD4-positive T cell infiltration suggests that MSI-H CRC cells lacking HLA class II antigen expression due to mutations in regulatory genes may be selected for in tumors with dense immune infiltration. Our study thus provides indirect evidence that lack of HLA class II antigen expression may be a relevant mechanism favoring the outgrowth of certain solid cancers.
Acknowledgments
We are grateful to Nina Nelius, Beate Kuchenbuch and Petra Höfler for the excellent technical support they provided. This work was supported by the Public Health Service (PHS) Grants R01 CA110249 and R01 CA138188 (Soldano Ferrone), awarded by the National Cancer Institute and by a Grant of the Deutsche Forschungsgemeinschaft (German Research Foundation).
Conflict of interest
The authors declare that they have no conflict of interest.
Abbreviations
- cMS
Coding microsatellite
- CRC
Colorectal cancer
- HE
Hematoxylin and eosin
- HLA
Human leukocyte antigen
- HNPCC
Hereditary nonpolyposis colorectal cancer
- IHC
Immunohistochemistry
- mAb
Monoclonal antibody
- MSI-H
High-level microsatellite instability
- PCR
Polymerase chain reaction
Footnotes
Eva-Maria Surmann and Anita Y. Voigt have contributed equally to the manuscript.
References
- 1.Campoli M, Ferrone S. HLA antigen changes in malignant cells: epigenetic mechanisms and biologic significance. Oncogene. 2008;27:5869–5885. doi: 10.1038/onc.2008.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferrone S, Campoli M. A fresh look at an old story: revisiting HLA class II antigen expression by melanoma cells. Expert Rev Dermatol. 2006;1:805–823. doi: 10.1586/17469872.1.6.805. [DOI] [Google Scholar]
- 3.Gutierrez J, Lopez-Nevot MA, Cabrera T, Oliva R, Esquivias J, Ruiz-Cabello F, Garrido F. Class I and II HLA antigen distribution in normal mucosa, adenoma and colon carcinoma: relation with malignancy and invasiveness. Exp Clin Immunogenet. 1987;4:144–152. [PubMed] [Google Scholar]
- 4.Cabrera T, Ruiz-Cabello F, Garrido F. Biological implications of HLA-DR expression in tumours. Scand J Immunol. 1995;41:398–406. doi: 10.1111/j.1365-3083.1995.tb03584.x. [DOI] [PubMed] [Google Scholar]
- 5.Lovig T, Andersen SN, Thorstensen L, Diep CB, Meling GI, Lothe RA, Rognum TO. Strong HLA-DR expression in microsatellite stable carcinomas of the large bowel is associated with good prognosis. Br J Cancer. 2002;87:756–762. doi: 10.1038/sj.bjc.6600507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morita M, Tanaka K, Kawanishi H, Tsuji M, Ookusa T, Takada H, Okamura A, Hioki K. Immunohistochemically demonstrated expression of HLA-DR antigen in colorectal adenocarcinomas and its relation to clinicopathological features. J Surg Oncol. 1995;59:233–238. doi: 10.1002/jso.2930590407. [DOI] [PubMed] [Google Scholar]
- 7.Walsh MD, Dent OF, Young JP, et al. HLA-DR expression is associated with better prognosis in sporadic Australian clinicopathological Stage C colorectal cancers. Int J Cancer. 2009;125:1231–1237. doi: 10.1002/ijc.24484. [DOI] [PubMed] [Google Scholar]
- 8.LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, Acha-Orbea H, Reith W. Mini-review: specificity and expression of CIITA, the master regulator of MHC class II genes. Eur J Immunol. 2004;34:1513–1525. doi: 10.1002/eji.200424964. [DOI] [PubMed] [Google Scholar]
- 9.Michel S, Linnebacher M, Alcaniz J, et al. Lack of HLA class II antigen expression in microsatellite unstable colorectal carcinomas is caused by mutations in HLA class II regulatory genes. Int J Cancer. 2010;127:889–898. doi: 10.1002/ijc.25106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Satoh A, Toyota M, Ikeda H, et al. Epigenetic inactivation of class II transactivator (CIITA) is associated with the absence of interferon-gamma-induced HLA-DR expression in colorectal and gastric cancer cells. Oncogene. 2004;23:8876–8886. doi: 10.1038/sj.onc.1208144. [DOI] [PubMed] [Google Scholar]
- 11.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138(2073–87):e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ionov Y, Peinado MA, Malkhosyan S, Shibata D, Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993;363:558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- 13.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 14.Herman JG, Umar A, Polyak K, et al. Incidence and functional consequences of hMLH1 promoter hypermethylation in colorectal carcinoma. Proc Natl Acad Sci USA. 1998;95:6870–6875. doi: 10.1073/pnas.95.12.6870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwitalle Y, Kloor M, Eiermann S, Linnebacher M, Kienle P, Knaebel HP, Tariverdian M, Benner A, von Knebel Doeberitz M. Immune response against frameshift-induced neopeptides in HNPCC patients and healthy HNPCC mutation carriers. Gastroenterology. 2008;134:988–997. doi: 10.1053/j.gastro.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 16.Kloor M, Michel S, von Knebel Doeberitz M. Immune evasion of microsatellite unstable colorectal cancers. Int J Cancer. 2010;127:1001–1010. doi: 10.1002/ijc.25283. [DOI] [PubMed] [Google Scholar]
- 17.Linnebacher M, Gebert J, Rudy W, Woerner S, Yuan YP, Bork P, von Knebel Doeberitz M. Frameshift peptide-derived T-cell epitopes: a source of novel tumor-specific antigens. Int J Cancer. 2001;93:6–11. doi: 10.1002/ijc.1298. [DOI] [PubMed] [Google Scholar]
- 18.Saeterdal I, Bjorheim J, Lislerud K, et al. Frameshift-mutation-derived peptides as tumor-specific antigens in inherited and spontaneous colorectal cancer. Proc Natl Acad Sci USA. 2001;98:13255–13260. doi: 10.1073/pnas.231326898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolcetti R, Viel A, Doglioni C, et al. High prevalence of activated intraepithelial cytotoxic T lymphocytes and increased neoplastic cell apoptosis in colorectal carcinomas with microsatellite instability. Am J Pathol. 1999;154:1805–1813. doi: 10.1016/S0002-9440(10)65436-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buckowitz A, Knaebel HP, Benner A, Blaker H, Gebert J, Kienle P, von Knebel Doeberitz M, Kloor M. Microsatellite instability in colorectal cancer is associated with local lymphocyte infiltration and low frequency of distant metastases. Br J Cancer. 2005;92:1746–1753. doi: 10.1038/sj.bjc.6602534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woerner SM, Kloor M, von Knebel Doeberitz M, Gebert JF. Microsatellite instability in the development of DNA mismatch repair deficient tumors. Cancer Biomark. 2006;2:69–86. doi: 10.3233/cbm-2006-21-208. [DOI] [PubMed] [Google Scholar]
- 22.Woerner SM, Yuan YP, Benner A, Korff S, von Knebel Doeberitz M, Bork P. SelTarbase, a database of human mononucleotide-microsatellite mutations and their potential impact to tumorigenesis and immunology. Nucleic Acids Res. 2010;38:D682–D689. doi: 10.1093/nar/gkp839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bicknell DC, Kaklamanis L, Hampson R, Bodmer WF, Karran P. Selection for beta 2-microglobulin mutation in mismatch repair-defective colorectal carcinomas. Curr Biol. 1996;6:1695–1697. doi: 10.1016/S0960-9822(02)70795-1. [DOI] [PubMed] [Google Scholar]
- 24.Kloor M, Michel S, Buckowitz B, et al. Beta2-microglobulin mutations in microsatellite unstable colorectal tumors. Int J Cancer. 2007;121:454–458. doi: 10.1002/ijc.22691. [DOI] [PubMed] [Google Scholar]
- 25.Kloor M, von Knebel Doeberitz M, Gebert JF. Molecular testing for microsatellite instability and its value in tumor characterization. Expert Rev Mol Diagn. 2005;5:599–611. doi: 10.1586/14737159.5.4.599. [DOI] [PubMed] [Google Scholar]
- 26.Boland CR, Thibodeau SN, Hamilton SR, et al. A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer. Cancer Res. 1998;58:5248–5257. [PubMed] [Google Scholar]
- 27.Findeisen P, Kloor M, Merx S, et al. T25 repeat in the 3′ untranslated region of the CASP2 gene: a sensitive and specific marker for microsatellite instability in colorectal cancer. Cancer Res. 2005;65:8072–8078. doi: 10.1158/0008-5472.CAN-04-4146. [DOI] [PubMed] [Google Scholar]
- 28.Temponi M, Kekish U, Hamby CV, Nielsen H, Marboe CC, Ferrone S. Characterization of anti-HLA class II monoclonal antibody LGII-612.14 reacting with formalin fixed tissues. J Immunol Methods. 1993;161:239–256. doi: 10.1016/0022-1759(93)90300-V. [DOI] [PubMed] [Google Scholar]
- 29.Temponi M, Kageshita T, Perosa F, Ono R, Okada H, Ferrone S. Purification of murine IgG monoclonal antibodies by precipitation with caprylic acid: comparison with other methods of purification. Hybridoma. 1989;8:85–95. doi: 10.1089/hyb.1989.8.85. [DOI] [PubMed] [Google Scholar]
- 30.Lee B, Sharron M, Montaner LJ, Weissman D, Doms RW. Quantification of CD4, CCR5, and CXCR4 levels on lymphocyte subsets, dendritic cells, and differentially conditioned monocyte-derived macrophages. Proc Natl Acad Sci USA. 1999;96:5215–5220. doi: 10.1073/pnas.96.9.5215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wood GS, Warner NL, Warnke RA. Anti-Leu-3/T4 antibodies react with cells of monocyte/macrophage and Langerhans lineage. J Immunol. 1983;131:212–216. [PubMed] [Google Scholar]
- 32.Michel S, Benner A, Tariverdian M, Wentzensen N, Hoefler P, Pommerencke T, Grabe N, von Knebel Doeberitz M, Kloor M. High density of FOXP3-positive T cells infiltrating colorectal cancers with microsatellite instability. Br J Cancer. 2008;99:1867–1873. doi: 10.1038/sj.bjc.6604756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finke JH, Rayman P, Alexander J, Edinger M, Tubbs RR, Connelly R, Pontes E, Bukowski R. Characterization of the cytolytic activity of CD4+ and CD8+ tumor-infiltrating lymphocytes in human renal cell carcinoma. Cancer Res. 1990;50:2363–2370. [PubMed] [Google Scholar]
- 34.Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207:637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Williams NS, Engelhard VH. Identification of a population of CD4 + CTL that utilizes a perforin- rather than a Fas ligand-dependent cytotoxic mechanism. J Immunol. 1996;156:153–159. [PubMed] [Google Scholar]
- 36.Xie Y, Akpinarli A, Maris C, Hipkiss EL, Lane M, Kwon EK, Muranski P, Restifo NP, Antony PA. Naive tumor-specific CD4(+) T cells differentiated in vivo eradicate established melanoma. J Exp Med. 2010;207:651–667. doi: 10.1084/jem.20091921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Al-Batran SE, Rafiyan MR, Atmaca A, et al. Intratumoral T-cell infiltrates and MHC class I expression in patients with stage IV melanoma. Cancer Res. 2005;65:3937–3941. doi: 10.1158/0008-5472.CAN-04-4621. [DOI] [PubMed] [Google Scholar]
- 38.de Miranda NF, Goudkade D, Jordanova ES, Tops CM, Hes FJ, Vasen HF, van Wezel T, Morreau H. Infiltration of Lynch colorectal cancers by activated immune cells associates with early staging of the primary tumor and absence of lymph node metastases. Clin Cancer Res. 2012;18:1237–1245. doi: 10.1158/1078-0432.CCR-11-1997. [DOI] [PubMed] [Google Scholar]