Abstract
Sequential diving by wild marine mammals results in a lifetime of rapid physiological transitions between lung collapse-reinflation, bradycardia-tachycardia, vasoconstriction-vasodilation, and oxygen store depletion-restoration. The result is a cycle of normoxia and hypoxia in which blood oxygen partial pressures can decline to <20–30 mmHg during a dive, a level considered injurious to oxygen-dependent human tissues (i.e., brain, heart). Safeguards in the form of enhanced on-board oxygen stores, selective oxygen transport, and unique tissue buffering capacities enable marine-adapted mammals to maintain physiological homeostasis and energy metabolism even when breathing and pulmonary gas exchange cease. This stands in stark contrast to the vulnerability of oxygen-sensitive tissues in humans that may undergo irreversible damage within minutes of ischemia and tissue hypoxia. Recently, these differences in protection against hypoxic injury have become evident in the systemic, multi-organ physiological failure during COVID-19 infection in humans. Prolonged recoveries in some patients have led to delays in the return to normal exercise levels and cognitive function even months later. Rather than a single solution to this problem, we find that marine mammals rely on a unique, integrative assemblage of protections to avoid the deleterious impacts of hypoxia on tissues. Built across evolutionary time, these solutions provide a natural template for identifying the potential for tissue damage when oxygen is lacking, and for guiding management decisions to support oxygen-deprived tissues in other mammalian species, including humans, challenged by hypoxia.
1. Introduction
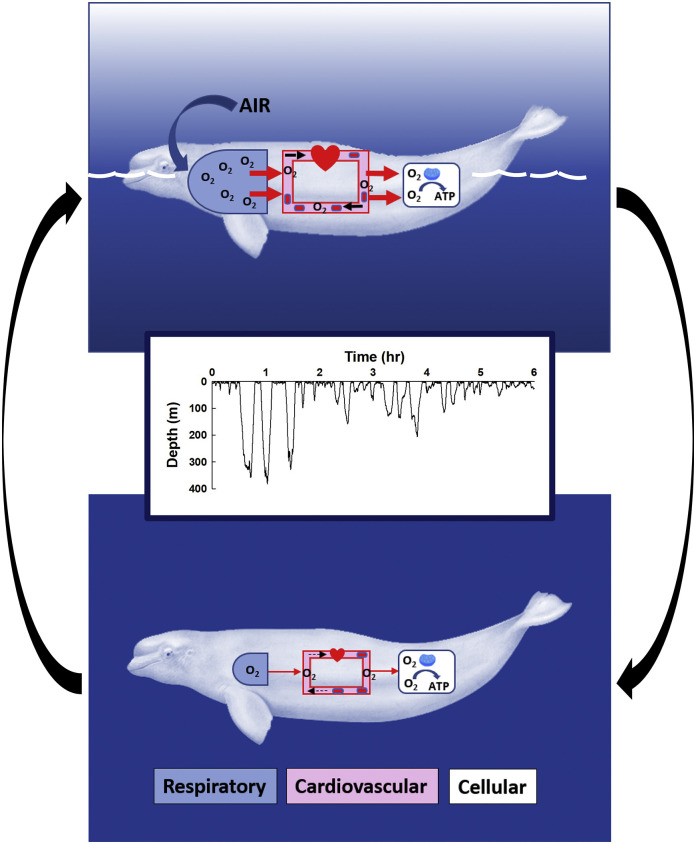
The diving response of air-breathing vertebrates results in abrupt, marked changes in physiological function across organs. Characterized by apnea, bradycardia, and vasoconstriction (Kooyman, 1989; Ponganis, 2015; Davis, 2019), the dive response is not a singular event for diving mammals. Rather, wild marine mammals may undergo dozens (i.e., narwhals and beluga whales) (Heide-Jørgensen et al., 2001) to over 100 (i.e., Antarctic fur seals) (Croxall et al., 1985) cycles of lung collapse-reinflation, bradycardia-tachycardia, vasoconstriction-vasodilation, and oxygen store depletion-restoration each day as they perform routine sequential dives during foraging or transiting. The result is a lifetime of rapid transitions between surface and submerged physiological states, that allows the maintenance of physiological homeostasis and energy metabolism even when breathing and pulmonary gas exchange cease (Fig. 1 ).
Fig. 1.
Transitions along the oxygen pathway for marine mammals breathing on the water surface (top) and diving (bottom). In this simplified model, the movement of oxygen travels sequentially through three major compartments: the respiratory system (blue box), the cardiovascular system (pink box) and into the cells of the tissues (white box) where O2 is taken up by the mitochondria during ATP production. The driving force for this cascade is the change in O2 partial pressures along the pathway. The size of each compartment and the red arrows denote the relative change in oxygen transport for each physiological state. Free-ranging marine mammals alternate between these states (black arrows) with each dive and interposing recovery period during sequential dive bouts as shown by a typical time-depth record for a wild narwhal (center inset). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Two important questions arise from this variation in physiological parameters. First, at the whole animal level, what do we consider the “normal” resting physiological state for marine mammals? And second, at the cellular level, how do oxygen-dependent tissues maintain function during such marked transitions in oxygen delivery? In this review, we use a comparative allometric approach to examine the range of metabolic and cardiovascular states that occur along the oxygen pathway in diving mammals. Recognizing that physiological variability characteristic of marine mammals may expose tissues to periods of low oxygen availability, we apply the Krogh Principle (Krogh, 1929) to examine how diving-adapted mammals avoid hypoxic tissue damage and how these adaptations may inform on the suite of hypoxia- related symptoms reported in patients during the current coronavirus 2019 (COVID-19) pandemic.
2. “Normal” physiological states of marine mammals
Cetaceans, pinnipeds, sea otters, sirenians, and polar bears are secondarily aquatic mammals that spend all or most of their lives in an environment where they only breathe periodically. Despite this, marine mammals maintain aerobic metabolism and physiological homeostasis similar to terrestrial mammals that have unlimited access to air (Davis, 2019). A major difference between these groups is that oxygenation by diving-adapted mammals is a transient event even during rest that can occur either while breathing at the water's surface or during apnea (breath-hold) on land or while submerged. In view of this, defining “normal” or “resting” basal states for marine mammals can be difficult. Similarly, the relationship between metabolic rates and heart rates during exercise differ when swimming on or near the water surface or at depth (Williams et al., 2015).
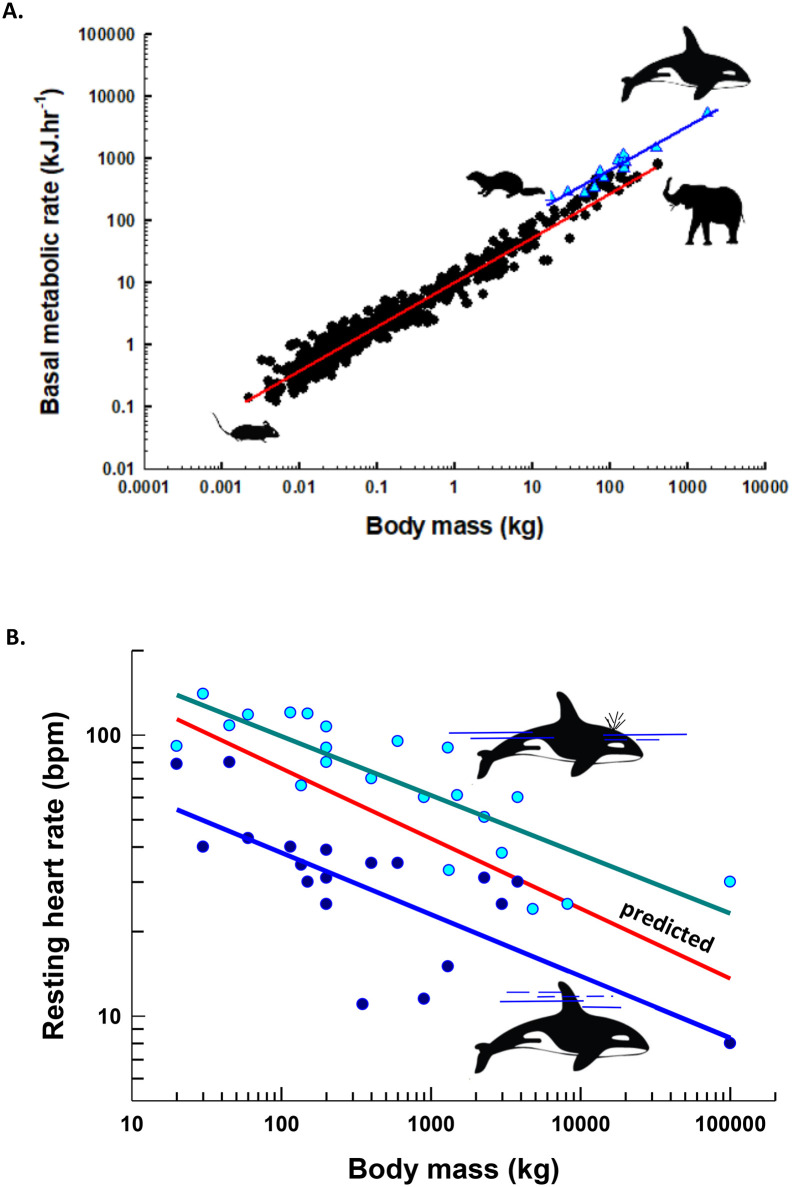
Comparative allometry with data from marine and terrestrial mammals demonstrates the variability in physiological states associated with an aquatic lifestyle (Fig. 2 ). In general, basal or resting metabolic rates measured in marine mammals during rhythmic breathing are higher than predicted for similarly-sized terrestrial mammals. While resting on the water, the metabolism of 14 species of marine mammals ranges from 0.3-times (West Indian manatee, Trichechus manatus) to 2.9-times (sea otter, Enhydra lutris) the allometric prediction for eutherian mammals of similar body mass. Considering only the carnivores (Cetacea, Pinnipedia and sea otters), marine mammals have a mean resting metabolic rate that is 2.3-fold higher than the allometric prediction for terrestrial eutherian mammals and 2.0-fold higher than predicted for terrestrial carnivores (Fig. 2A) (Davis, 2019). Experiments with trained pinnipeds (Hurley and Costa, 2001) show that these elevations in surface resting metabolism often diminish to predicted terrestrial levels or below when marine mammals are resting while submerged.
Fig. 2.
Allometric comparisons for resting metabolic rate and heart rate in mammals. In A, basal and resting metabolic rates for terrestrial mammals from rodents to elephants (black symbols) and marine mammals from sea otters to killer whales (blue symbols) are compared. Measurements for marine mammals were taken as the animals rested on the water surface. Data, regressions and references are compiled in Davis (Davis, 2019). In B, resting heart rates of marine mammals lying sedentary on the water surface (cyan circles, heart rate = 260.6body mass −0.21, r2 = 0.69, p < 0.0001) or submerged/gliding (dark blue circles, heart rate = 105.1body mass −0.22, r2 = 0.54, p = 0.0016) are compared to the predicted values for terrestrial mammals (red line) from Stahl (Stahl, 1977). Lines in both panels are least squares regressions through the data points for each group plotted in relation to body mass. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The heart rates of resting marine mammals are also highly variable, which limits our ability to assign a single “normal” value. Unlike terrestrial mammals, which demonstrate a clear allometric relationship for resting heart rate (Stahl, 1977), marine mammals alternate between elevated rates relative to similarly-sized terrestrial mammals when breathing on the water surface to bradycardia when resting or gliding while submerged (Fig. 2B). On average, the resting heart rates of marine mammals are 42% higher than predicted for terrestrial mammals when on the water surface awake and breathing, and 43% lower than predicted when sedentary and submerged. Based on this, the movement of oxygen along the O2 pathway is clearly context specific for marine mammals.
3. Safeguarding function in oxygen-dependent tissues
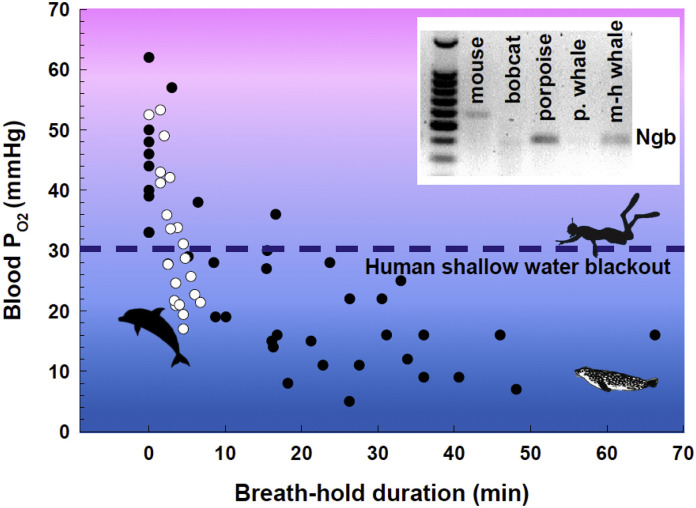
As would be expected, the variations in physiological function with diving and surfacing described above for marine mammals are associated with alterations in oxygen availability to tissues (Fig. 1). Depending on breath-hold duration, level of bradycardia, and exercise intensity, rapid declines in the partial pressure of blood oxygen (PO2; Fig. 3 ) and a concomitant increased reliance on tissue oxygen stores to support aerobic metabolic processes may occur. Compared to human divers, the minimum levels of arterial and mixed venous PO2 reached in marine mammals can be exceptionally low. For example, the minimum mixed venous PO2 of sedentary Pacific white-sided dolphins (Lagenorhynchus obliquidens), bottlenose dolphins (Tursiops truncatus), beluga whales (Delphinapterus leucas), and killer whales (Orcinus orca) declines to 17–31 mmHg during voluntary breath-holds, <40–50% of values measured when breathing on the water surface (Williams et al., 1999; Noren et al., 2012). Even lower levels (10–23 mmHg) are attained in actively diving, wild pinnipeds (Fig. 3) (Ponganis, 2015). Interestingly, blood PO2 during apnea in marine mammals routinely declines below the normoxia value of 35 mmHg in human brains (Carreau et al., 2011), reaching levels that would render a human diver unconscious. Yet, the marine mammals show no neural impairment, and continue to actively swim, hunt, and navigate under these conditions, revealing an exceptional tolerance to hypoxemia.
Fig. 3.
The effect of breath-hold duration on the partial pressure of blood oxygen in marine mammals. Sedentary bottlenose dolphins (n = 3, open circle) and Weddell seals freely diving beneath the Antarctic sea ice (n = 4, closed circle) are compared. Each point represents a single, mixed venous blood sample obtained within 2 min of surfacing. Data are from Williams et al. (Williams et al., 1999) for dolphins and (T.M. Williams, unpublished data) for Weddell seals. The dashed horizontal line denotes the circulating PO2 level associated with unconsciousness in humans. The inset shows a representative mRNA expression analysis for the cerebral cortex of five mammalian species, where total RNA was isolated and subjected to RT-PCR with primers specific for neuroglobin (Ngb). Note the presence of Ngb for the porpoise and melon-headed (m-h) whale and relative absence in mouse, bobcat and pilot (p) whale (Williams et al., 2008).
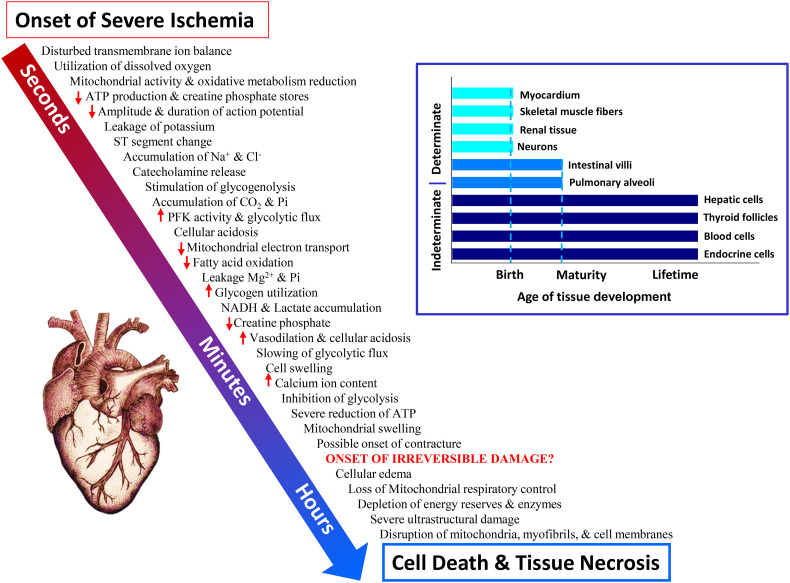
A concern, of course, is the potential for cellular injury in oxygen-dependent tissues, particularly those with determinate growth patterns such as the brain, heart, and kidneys (Fig. 4 ) (Ostadal and Kolar, 1999; Stankowski and Gupta, 2011). Sensitivity to oxygen deprivation coupled with limited ability to replace cells in these organs results in an increased risk of permanent damage with prolonged ischemia and tissue hypoxia. For example, the time course for molecular/cellular events associated with the onset of ischemia in mammalian myocardial cells reveals how quickly cellular deterioration ensues (Fig. 4). Irreversible damage can occur within minutes in human hearts; cell death leading to tissue necrosis happens within hours (Ostadal and Kolar, 1999). Likewise, a cascade of molecular events instigated by ischemic stroke in the human brain begins within minutes to ultimately end in neuronal death within hours to several days (Stankowski and Gupta, 2011).
Fig. 4.
Timeline for cellular events due to severe ischemia in myocardial tissues. Obstruction in blood flow to the heart and subsequent declines in oxygen availability and glycogen stores result in the initiation of a cascade (delineated by the arrow) of molecular, biochemical and functional events within seconds that ultimately can result in cellular death within hours. Redrawn from Ostadal and Kolar (Ostadal and Kolar, 1999). Determinate tissues including the myocardium and neurons with relatively finite growth set by birth (inset) and pulmonary alveoli set at maturity are especially vulnerable to irreversible damage with such events. Not surprisingly, these tissues have demonstrated long-term effects with COVID-19 infection.
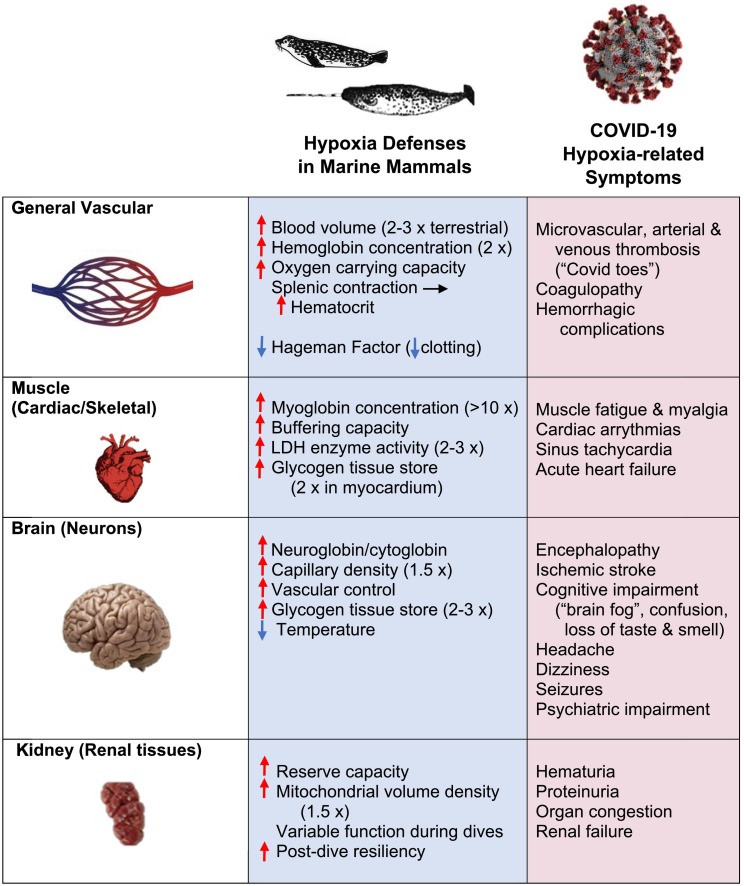
In contrast to humans and terrestrial mammals, the tissues of diving-adapted mammals appear comparatively resistant to hypoxemic injury when convective oxygen transport declines during apnea (Ponganis, 2015; Davis, 2019; Ramirez et al., 2007). For some deep-diving species (i.e., Curvier's beaked whale, Ziphius cavirostris), apnea can exceed three hours, theoretically depleting onboard tissue oxygen stores (Quick et al., 2020) and potentially increasing the risk to injury. How marine mammals protect oxygen sensitive tissues under these conditions is the product of over 50 million years of evolution. It began with terrestrial ancestors re-entering the oceans (Davis, 2019) and has led to an assemblage of physiological adaptations that enhance aerobic metabolism as well as permit unique resilience to hypoxia and anaerobic byproducts (Fig. 5 ). Three major, interrelated processes are involved, 1) enhanced tissue oxygen stores, 2) altered oxygen transport to select organs, and 3) increased buffering capacities conferring greater tolerance to hypoxia in marine mammals compared to terrestrial mammals.
Fig. 5.
Hypoxia defense and vulnerability in oxygen sensitive tissues of mammals. Blue boxes indicate the breadth of protective mechanisms for preventing tissue injury during hypoxia in the general vascular system, cardiac and skeletal muscle, brain and neural tissues, and renal tissues of diving-adapted mammals. Red arrows indicate increased levels relative to terrestrial mammals (proportional increases shown in parentheses); blue arrows denote decreases. Pink boxes show the broad range of hypoxia-related symptoms reported for patients with COVID-19 infections. (Note mild to marked cases are combined for this general review.) See Machhi et al. (Machhi et al., 2020) for a review of the full list of symptoms relative to severity of infection. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
At the vascular level, elevated blood volumes and hemoglobin concentrations promote an increase in oxygen carrying capacity in the blood of marine mammals. During deep dives by some species, most notably Weddell seals (Leptonychotes weddellii), this may be further enhanced by splenic contraction which releases a store of oxygenated erythrocytes into the circulation (Hurford et al., 1996). A similar diving adaptation has recently been proposed for an Indonesian tribe of sea nomads known as the Bajau, that have evolved larger spleens to deliver more oxygen-carrying blood cells when diving (Ilardo et al., 2018). Higher capillary densities in the brain and myocardium of marine mammals compared to terrestrial mammals also promote shorter diffusion distances for oxygen transfer in these oxygen-dependent tissues.
With prolonged breath-holds, declines in circulating oxygen can lead to progressive hypoxic conditions within tissues. However, rather than an obligate detriment, lower blood PO2 in marine mammals is necessary in tissues such as skeletal muscles that rely on enhanced stores of oxygen-carrying globins to support aerobic metabolism. In these cases, a reduction in arterial PO2 results in muscle hypoxia that promotes the dissociation of oxygen from stored myoglobin for use in aerobic pathways (Ponganis, 2015; Davis, 2019). This is a significant adaptation in both locomotory skeletal muscles and the myocardium of diving mammals (Berenbrink, 2020). The concentration of oxygen-linked globins in the form of myoglobin in these muscles is approximately 10-fold higher in marine mammals compared to terrestrial mammals (Fig. 5), thus providing a critical “SCUBA” tank of oxygen for supporting aerobic metabolism during a dive. Likewise, higher concentrations of oxygen-linked globins in the brain (e.g., neuroglobin and cytoglobin) of marine mammals have been reported (Fig. 3A) (Williams et al., 2008), although their importance to oxygen transport or neuroprotection during hypoxia are less known.
As oxygen stores decline during the course of a dive, the distribution of blood as well as tissue perfusion may be altered (Kooyman, 1989; Ponganis, 2015; Davis, 2019). In response, organ function is adjusted in some tissues (kidneys, liver) (Davis, 2019), while others can enter into a state of anaerobiosis (heart) as evident from lactate accumulation (Ponganis, 2015; Ramirez et al., 2007). Note that the latter is unsustainable for long periods and likely only occurs on dives exceeding aerobic diving limits (Kooyman et al., 2020).
Numerous safety factors and biochemical buffers enable even the most oxygen-dependent tissues in marine mammals to not only withstand these physiological transitions, but maintain function and avoid injury during ischemia and reperfusion. For example, normal mammalian renal and hepatic function exceeds what is needed for physiological homeostasis (i.e., a reserve capacity) such that a decrease in blood flow to these organs during dives within aerobic dive limits does not disrupt homeostasis. As reported for freely-diving Weddell seals, a moderate bradycardia and reduction in renal and hepatic blood flow during routine dives does not disrupt glomerular filtration by the kidneys or intermediary metabolism in the liver (Davis, 2019). However, because the energy metabolism of these organs is dependent on blood flow, there is a modest savings in oxygen consumption, which decreases overall metabolic rate relative to levels while breathing at the surface. In these diving specialists, the splanchnic organs can tolerate a 75–80% reduction in blood flow relative to resting rates and still maintain aerobic function for digestion and assimilation during most dives.
For critical organs such as the brain, neuro-protection may also be enhanced by selective cooling to preserve the functioning of essential neural networks (Ramirez et al., 2007). In addition, the redistribution of blood via fine control of the microvasculature ensures adequate flow to the brain during most dives (Dormer et al., 1972; McKnight et al., 2019). Other safety factors in marine mammals include increased glycogen levels in the heart and brain to support short periods of anaerobic function, hypoxia tolerant enzyme systems (Ramirez et al., 2007), and increased non-bicarbonate buffering capacity in the skeletal muscles of deep divers and fast swimmers (Noren, 2006).
One of the most important adaptative safety factors in marine mammals occurs after the dive in the form of tissue resiliency that allows sequential transitions between ischemia and reperfusion as occur with continuous diving and recovery (Fig. 1). Unlike human tissues in which oxidative stress associated with such transitions initiates the formation of oxygen radicals and antioxidant cascades that challenge cell function during reperfusion (Ramirez et al., 2007), marine mammals appear uniquely protected through specialized antioxidants that prevent tissue injury following prolonged breath-holds (Tift and Ponganis, 2019). This is especially important for prolonged dives exceeding aerobic dive limits when lactate accumulation and the potential for intracellular ion imbalances and pH shifts would be most likely to occur.
4. Conclusion: insights for COVID-19 injury
When pulmonary ventilation is reduced (Fig. 1) and dives exceed aerobic capacity, marine mammals can enter into the beginnings of true asphyxia. In this physiological state, the oxygen pathway of the animals resembles the disrupted respiratory gas exchange and reduced convective oxygen transport characteristic of COVID-19 patients. However, the ability of marine mammals to avoid or cope with reduced oxygen delivery stands in stark contrast to the vulnerability of oxygen deprived tissues in humans. This has been especially apparent in the myriad of symptoms displayed by patients during the COVID-19 pandemic (Fig. 5). Using the Krogh Principle (Krogh, 1929) in which “for a large number of problems there will be some animal of choice, or a few such animals, on which it can be most conveniently studied” we find that marine mammals provide a novel perspective on how nature has solved the problem of avoiding hypoxic tissue injury in some mammals, while leaving humans comparatively defenseless.
Although the original focus of concern for COVID-19 patients was on reduced oxygen delivery by the pulmonary system due to extensive alveolar damage (Bussani et al., 2020), it is now clear that co-occurring immune responses to infection can instigate systemic physiological failure with homeostatic disruption across multiple organs (Fig. 5) (Machhi et al., 2020). Mediated in part by angiotensin-converting enzyme 2 (ACE2) receptors, many disorders involve vascular complications (i.e., coagulopathies, inflammation, thrombosis formation, altered capillary permeability, edema, ischemic stroke) resulting in localized hypoxemia (Bussani et al., 2020; Szelenberger et al., 2020). In contrast to diving marine mammals, the resulting disruption in oxygen availability is manifested in humans as a rapid loss of organ function and in some cases permanent cell damage particularly for determinate, oxygen-dependent tissues (Fig. 4). In the absence of hypoxia protection, the response to COVID-19 infection in humans can be marked, and irreparable in severe cases for vulnerable organs like the alveoli of the lungs, heart and brain (Bussani et al., 2020; Machhi et al., 2020; Szelenberger et al., 2020). Because the ability of the human body to repair these tissues is uncertain, it is not surprising that recovery to normal exercise levels (Bussani et al., 2020; Wilson et al., 2020) and cognitive function (Barker-Davies, 2020) can be prolonged for some patients even with mild infection. In view of this comparatively low tissue resiliency in humans, protections that reduce infection rates become increasingly important, and a level of caution is added to planned herd immunity programs.
In summary, physiological homeostasis is the driving principle for survival in mammals, whether as an adaptive mechanism in marine mammals or as the foundation of efforts to restore it in the Intensive Care Units of COVID-19 patients who are poorly equipped for periods of reduced tissue oxygenation. Nature has clearly provided marine mammals with an arsenal of protections to safeguard tissue oxygen levels in the face of hypoxia that we continue to discover (Fig. 5). Although they provide insights into potential protective mechanisms for other mammals, the solution to assisting COVID-19 patients will not be as simple as turning humans into diving dolphins or Bajau fishermen. Behind nature's solutions are associated safety factors that must be taken into account. For example, in dolphins and killer whales, the ability to release oxygenated red blood cells into the circulatory system to prevent hypoxia during a dive is accompanied by the absence of the Hageman Factor (Factor XII) that typically initiates coagulation in mammalian blood (Robinson et al., 1969). In this way, cetaceans avoid the threat of clots or stroke instigated by elevated hematocrits, that can occur in COVID-19 patients (Fig. 5). Importantly, the resiliency of marine mammal tissues under conditions of limited oxygen availability depends on a suite of coordinated adaptations honed over millions of years. Such an evolutionary foundation provides a natural template for understanding the potential for damage in oxygen deprived tissues, and for guiding the management of oxygen-sensitive tissues of other mammalian species challenged by hypoxia.
Declaration of Competing Interest
None
Acknowledgements
Support for this manuscript was provided by the Office of Naval Research (grants N00014-13-1-0808 and N00014-17-1-2737 to T.M.W.)
References
- Barker-Davies R.M., O’Sullivan O., Senaratne K.P.P., Baker P., Cranley M., Dharm-Datta S., Ellis H., Goodall D., Gough M., Lewis S., Norman J., Papadopoulou T., Roscoe D., Sherwood D., Turner P., Walker T., Mistlin A., Nicol A.M., Bennett A.N., Bahadur S. The Stanford Hall consensus statement for post-COVID-rehabilitation. Br. J. Sports Med. 2020;54:949–959. doi: 10.1136/bjsports-2020-102596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenbrink M. The role of myoglobin in the evolution of mammalian diving capacity – The August Krogh principle applied in molecular and evolutionary physiology. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020 doi: 10.1016/j.cbpa.2020.110843. [DOI] [PubMed] [Google Scholar]
- Bussani R., Schneider E., Zentilin L., Collesi C., Ali H., Braga L., Concetta Volpe M., Colliva A., Zanconati F., Berlot G., Silvestri F., Zacchigna S., Giacca M. Persistence of viral RNA, pneumocyte syncytia and thrombosis are hallmarks of advanced COVID-19 pathology. EBioMedicine. 2020:103104. doi: 10.1016/j.ebiom.2020.103104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carreau A., El Hafny-Rahbi B., Matejuk A., Grillon C., Kieda C. Why is the partial oxygen pressure of human tissues a crucial parameter? Small molecules and hypoxia. J. Cell. Mol. Med. 2011;15:1239–1253. doi: 10.1111/j.1582-4934.2011.01258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croxall J.P., Everson I., Kooyman G.L., Ricketts C., Davis R.W. Fur seal diving behaviour in relation to vertical distribution of krill. J. Anim. Ecol. 1985;54:1–8. doi: 10.2307/4616. [DOI] [Google Scholar]
- Davis R.W. Springer Nature; Switzerland AG: 2019. Marine Mammals: Adaptations for an Aquatic Life. (302 pp) [Google Scholar]
- Dormer K.J., Denn M.J., Stone H.L. Cerebral blood flow in the sea lion (Zalophus californianus) during voluntary dives. Comp. Biochem. Physiol. 1972;58A:11–18. [Google Scholar]
- Heide-Jørgensen M.P., Hammeken N., Dietz R., Orr J., Richard P.R. Surfacing times and dive rates for narwhals (Monodon monoceros) and belugas (Delphinapterus leucas) Arctic. 2001;54:284–298. Belugas and Narwhals: Application of New Technology to Whale Science in the Arctic. [Google Scholar]
- Hurford W.E., Hochachka P.W., Schneider R.C., Guyton G.P., Stanek K.S., Zapol D.G., Liggins G.C., Zapol W.M. Splenic contraction, catecholamine release, and blood volume redistribution during diving in the Weddell seal. J. Appl. Physiol. 1996;80:298–306. doi: 10.1152/jappl.1996.80.1.298. [DOI] [PubMed] [Google Scholar]
- Hurley J.A., Costa D.P. Standard metabolic rate at the surface and during trained submersions in adult California sea lions (Zalophus californianus) J. Exp. Biol. 2001;204:3273–3281. doi: 10.1242/jeb.204.19.3273. [DOI] [PubMed] [Google Scholar]
- Ilardo M.A., Moltke I., Korneliussen T.S., Cheng J., et al. Physiological and genetic adaptations to diving in sea nomads. Cell. 2018;173:569–580. doi: 10.1016/j.cell.2018.03.054. [DOI] [PubMed] [Google Scholar]
- Kooyman G.L. Springer-Verlag; Berlin: 1989. Diverse Divers: Physiology and Behavior. 200 pp. [Google Scholar]
- Kooyman G.L., McDonald B.I., Williams C.L., Meir J.U., Ponganis P.J. The aerobic dive limit: after 40 years, still rarely measured but commonly used. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2020 doi: 10.1016/j.cbpa.2020.110841. [DOI] [PubMed] [Google Scholar]
- Krogh A. The progress of physiology. Am. J. Phys. 1929;90:243–251. [Google Scholar]
- Machhi J., Herskovitz J., Senan A.M., et al. The natural history, pathobiology, and clinical manifestations of SARS-CoV-2 infections. J. NeuroImmune Pharmacol. 2020;15:359–386. doi: 10.1007/s11481-020-09944-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight J.C., et al. Shining new light on mammalian diving physiology using wearable near-infrared spectroscopy. PLoS Biol. 2019;17:e3000306. doi: 10.1371/journal.pbio.3000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noren S.R. Buffering capacity of the locomotor muscle in cetaceans: correlates with postpartum development, dive duration, and swim performance. Mar. Mamm. Sci. 2006;20:808–822. doi: 10.1111/j.1748-7692.2004.tb01194.x. [DOI] [Google Scholar]
- Noren S.R., Williams T.M., Ramirez K., Boehm J., Glenn M., Cornell L. Changes in partial pressures of respiratory gases during submerged voluntary breath hold across odontocetes: is body mass important? J. Comp. Physiol. B. 2012;182:299–309. doi: 10.1007/s00360-011-0612-0. [DOI] [PubMed] [Google Scholar]
- Ostadal B., Kolar F. Springer Science + Business Media; New York: 1999. Cardiac Ischemia: From Injury to Protection. [Google Scholar]
- Ponganis P.J. Cambridge University Press; 2015. Diving Physiology of Marine Mammals and Seabirds. UK. 333 pp. [Google Scholar]
- Quick N.J., Cioffi W.R., Shearer J.M., Fahlman A., Read A.J. Extreme diving in mammals: first estimates of behavioural aerobic dive limits in Cuvier's beaked whales. J. Exp. Biol. 2020;223:jeb222109. doi: 10.1242/jeb.222109. [DOI] [PubMed] [Google Scholar]
- Ramirez J.-M., Folkow L.P., Blix A.S. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu. Rev. Physiol. 2007;69:113–143. doi: 10.1146/annurev.physiol.69.031905.163111. [DOI] [PubMed] [Google Scholar]
- Robinson A.J., Kropatkin M., Aggeler P.M. Hageman Factor (Factor XII) deficiency in marine mammals. Science. 1969;166:1420–1422. doi: 10.1126/science.166.3911.1420. [DOI] [PubMed] [Google Scholar]
- Stahl W.R. Scaling of respiratory variable in mammals. J. Appl. Physiol. 1977;22:453–460. doi: 10.1152/jappl.1967.22.3.453. [DOI] [PubMed] [Google Scholar]
- Stankowski J.N., Gupta R. Therapeutic targets and neuroprotection in acute ischemic stroke: lost in translation? Antioxid. Redox Signal. 2011;14:1841–1851. doi: 10.1089/ars.2010.3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szelenberger R., Saluk-Bijak J., Bijak M. Ischemic stroke among the symptoms caused by the COVID-19 infection. J. Clin. Med. 2020;9:2688. doi: 10.3390/jcm9092688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tift M.S., Ponganis P.J. Time domains of hypoxia adaptation—elephant seals stand out among divers. Front. Physiol. 2019;10:677. doi: 10.3389/fphys.2019.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.M., Haun J.E., Friedl W.A. The diving physiology of bottlenose dolphins, (Tursiops truncatus) I. Balancing the demands of exercise for energy conservation at depth. J. Exp. Biol. 1999;202:2739–2748. doi: 10.1242/jeb.202.20.2739. [DOI] [PubMed] [Google Scholar]
- Williams T.M., Zavanelli M., Miller M.M., Goldbeck R.A., Morledge M., Caper D., Pabst D.A., McLellan W., Cantin L.P., Kliger D.S. Running, swimming and diving modifies neuroprotecting globins in the mammalian brain. Proc. R. Soc. B. 2008;275:751–758. doi: 10.1098/rspb.2007.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams T.M., Fuiman L.A., Kendall T., Berry P., Thometz N., Richter B., Noren S.R., Shattock M.J., Farrell E., Stamper A.M., Davis R.W. Exercise at depth alters bradycardia and incidence of cardiac anomies in deep-diving marine mammals. Nat. Commun. 2015 doi: 10.1038/ncomms7055. [DOI] [PubMed] [Google Scholar]
- Wilson M.G., et al. Cardiorespiratory considerations for return-to-play in elite athletes after COVID-19 infection: a practical guide for sport and exercise medicine physicians. Br. J. Sports Med. 2020;54:1157–1161. doi: 10.1136/bjsports-2020-102710. [DOI] [PMC free article] [PubMed] [Google Scholar]