Abstract
To assess the expected benefits of rapid reporting of respiratory viruses, we compared patients whose samples were processed using standard techniques such as enzyme immunoassays, shell vial assays, and culture tube assays (year 1) to patients whose samples were processed with the same standard techniques in addition to immunofluorescent testing (FA) directly on cytocentrifuged samples (year 2). The cytospin FA screened for influenza A and B viruses, respiratory syncytial virus (RSV), parainfluenza viruses 1 to 3, and adenovirus (DAKO Diagnostics Ltd.). The specificity of the cytospin FA for all viruses was 100%. The sensitivities for influenza A virus and RSV were 90 and 98%, respectively, but the sensitivities for influenza B virus and adenovirus were unacceptable (14.3 and 0%, respectively). However, since the former viruses account for >85% of our isolates from clinical specimens, the cytospin FA is an excellent screening test since the positive result was available within hours. The mean turnaround time for all positive viruses was 4.5 days in year 1 and 0.9 day in year 2 (P = 0.001). This rapid reporting resulted in physicians having access to information sooner, enabling more appropriate treatment. The mean length of stay in the hospital for inpatients with respiratory viral isolates was 10.6 days for year 1 versus 5.3 days for year 2. Mean variable costs for these patients was $7,893 in year 1 and $2,177 in year 2. After subtracting reagent costs and technological time, the savings in variable costs was $144,332/year. Summarizing, the cytospin FA markedly decreased turnaround time and was associated with decreased mortality, length of stay, and costs and with better antibiotic stewardship.
Because of managed-care issues, it is imperative that clinical virologists effectively demonstrate the impact of their contributions on patient care. Woo et al. have shown clinical and financial benefits of rapid detection of respiratory viral infections in a pediatric population in Hong Kong, but no other studies address the clinical and financial impact of virology data (5). Doing et al. described a technique of performing immunofluorescent testing (FA) on cytocentrifuged preparations which resulted in accurate, rapid detection of respiratory viral illnesses (4). In this study, we combined the methods used in these two studies to optimize rapid diagnosis and to evaluate its impact on patient care in a community teaching hospital in the United States. First, we evaluated the performance of FA on cytocentrifuged preparations. Second, we compared the turnaround time and patient outcomes, such as mortality, length of stay in the hospital, costs, and antibiotic prescribing patterns, for patients hospitalized during the winter of 1997 to 1998 (year 1, when rapid reporting was not attempted) and patients hospitalized during the winter of 1998 to 1999 (year 2, when rapid reporting was attempted using the cytospin FA technique).
MATERIALS AND METHODS
Study design.
Memorial Medical Center is a 500-bed community teaching hospital for Southern Illinois University School of Medicine. In a historical cohort analysis for two consecutive winters, we examined data from inpatients on whom viral studies on respiratory samples were performed. From 1 December 1997 to 19 April 1998 (year 1), samples were processed by standard viral techniques, including enzyme immunoassays (EIA), shell vial assays, and culture tube assays. From 1 December 1998 to 19 April 1999 (year 2), respiratory samples were processed more rapidly by using the standard techniques in addition to a cytospin FA technique (described below). For all the positive results during both years, an attempt was made to notify the physician immediately by telephone and/or page.
Methods used in year 1.
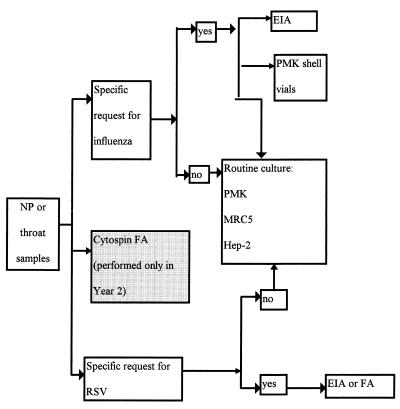
Respiratory samples from the throat, nose, and nasopharynx were submitted to the virology laboratory with three possible requests: culture, respiratory synctial virus (RSV) testing, and/or influenza virus testing. If a viral culture were requested, a culture with tubes only (no shell vials) was done. If testing for RSV were requested, either (on Monday through Saturday days) a direct FA (Imagen RSV; DAKO Corporation, Carpinteria, Calif.) or (on Sundays, evenings, or nights) an EIA for RSV (Directogen RSV kit; Becton Dickinson, Cockeysville, Md.) was done. If testing for influenza virus were requested, an EIA for influenza A virus (Directogen Influenza A kit, Becton Dickinson) as well as cultures of shell vials and tubes were done. Figure 1 shows the algorithm used for respiratory samples.
FIG. 1.
Workflow for respiratory samples submitted to the virology laboratory. NP, nasopharyngeal.
Standard culture methods were used. Samples were inoculated into one tube of Hep-2, two tubes of primary monkey kidney cells (PMK), and one or two tubes of MRC5 (one tube of MRC5 if influenza virus testing were requested) and incubated at 35°C. Cultures were held 2 weeks unless they became positive. If influenza virus testing were requested, additional culturing of two shell vials of PMK was done. The shell vials were examined generally after 24 and 48 h by immunofluorescent antibody staining for influenza A and B viruses (Imagen Influenza A and B; DAKO Corporation).
Turnaround time is defined as the time interval between arrival of the sample in the laboratory and the time the positive result is entered into the computer. Information on mortality, length of stay, and costs (not charges) was supplied by the clinical data management department. Total costs were the sum of fixed direct, variable direct, and fixed indirect costs. Fixed costs are those costs which do not change with an individual patient, such as overhead, costs of administration, etc. Variable costs are those costs which are associated directly with patient care, such as supplies used for a patient, laboratory or radiological tests performed on samples from a patient, etc.
Methods used in year 2.
At the onset of the study, some physicians were aware (via a newsletter) that an attempt would be made to process the samples more rapidly. Functionally, however, the vast majority of physicians were unaware of the attempt at rapid reporting. Physicians were notified of positive results by telephone and/or page the same way they were in year 1.
Samples were processed exactly as described above except that every throat, nasal, and nasopharyngeal sample also had a cytospin FA performed on it. The cytospin FA was performed 6 days per week. Samples were washed, cytocentrifuged, and then screened by indirect FA with a pool of immunofluorescent antisera to influenza A and B viruses, adenovirus, parainfluenza viruses 1 to 3, and RSV (Imagen respiratory screen; DAKO Diagnostics Ltd.). If the cytospin FA with the pooled antisera were positive, follow-up testing on duplicate cytospin slides with individual kits with monoclonal antisera (DAKO Diagnostics Ltd.) was done.
If the monoclonal FAs were negative, the cytospin FA was considered negative. Less than 10% of the cytospin FA tests had questionable positive or nonspecific results, necessitating further testing with the monoclonal antisera. Approximately one quarter of the samples with nonspecific or questionable staining were positive with a monoclonal antibody; half were true negatives compared to culture, and the remaining were false negatives.
Statistical analysis was performed by a doctoral-level biostatisitican with the Statistical Package for Social Sciences, Inc. (Chicago, Ill.), computer program. Fisher's exact test was used to analyze mortality; the Wilcoxon rank sum nonparametric test was used for all other analyses.
Comparability of year 1 and year 2.
To show the relative comparability between the two years, baseline parameters for all inpatients hospitalized in years 1 and 2 is provided in Table 1. The differences between years 1 and 2 are minimal, namely, (i) a negligible difference in mortality in year 2, (ii) a slight decrease in length of stay in year 2, and (iii) a slight increase in costs in year 2. The hospital is in a stable environment with only minor changes in contracts from year 1 to year 2 for managed-care populations, etc. The number of all respiratory samples processed in the virology laboratory was almost identical (293 in year 1 and 281 in year 2), as was the number of positive respiratory viral isolates (75 in year 1 and 74 in year 2).
TABLE 1.
Comparison of parameters for all inpatients hospitalized at Memorial Medical Center during years 1 and 2
| Parameter | Yr 1 | Yr 2 | Difference between yr 1 and 2a |
|---|---|---|---|
| Mortality (%) | 3.0 | 2.9 | −0.1 |
| Length of stay (day) | 5.8 | 5.5 | −0.3 |
| Total cost ($) | 7,219 | 7,386 | +167 |
| Variable cost ($) | 2,879 | 3,230 | +351 |
−, decrease was seen in year 2; +, increase was seen in year 2.
RESULTS
Of all the nose, throat, or nasopharynx samples for which viral testing was requested during year 1, 75 of 293 (25.6%) had positive results, namely, influenza A virus, 18.7%; untypeable influenza virus, 1.3%; and RSV, 80% (Table 2). During year 2, 74 of 281 samples (26.3%) had positive results, namely, influenza A virus, 27%; influenza B virus, 9.5%; untypeable influenza virus, 1.4%; RSV, 59.5%; and adenovirus, 2.7% (Table 2).
TABLE 2.
Distribution of all respiratory viruses isolated in years 1 and 2
| Virus | No. (%) of samples in year:
|
|
|---|---|---|
| 1 | 2 | |
| Influenza A virus | 14 (18.7) | 20 (27.0) |
| Influenza B virus | 0 | 7 (9.5) |
| Influenza virus (untypeable) | 1 (1.3) | 1 (1.4) |
| RSV | 60 (80) | 44 (59.5) |
| Adenovirus | 0 | 2 (2.7) |
| Parainfluenza virus | 0 | 0 |
| Total for all viruses | 75 (100) | 74 (100) |
The performance of the various tests used in the detection of viral respiratory viruses is shown in Tables 3 and 4. Compared to culture, the cytospin FA detected 18 out of 20 cultures positive for influenza A virus, giving a sensitivity of 90% and a specificity of 100% (Table 3). The cytospin FA did not detect most of the cases of influenza B virus or adenoviruses; there were no cases of parainfluenza virus in this study. Overall, the cytospin FA had a sensitivity of 65.6% for all viruses detected compared to culture, and it was 100% specific for all viruses tested. In our population, the predictive positive value of the cytospin FA is 100% for all viruses detected (influenza A and B viruses) and the predictive negative value is 97% for influenza A virus, 96.3% for influenza B virus, 98.7% for adenoviruses, and 92.8% for all three viruses. Compared to the EIA, the cytospin FA had a sensitivity of 97.6% and a specificity of 100% for the detection of RSV (Table 4).
TABLE 3.
Summary of tests for respiratory viruses, excluding samples with requests for RSV testing
| Virus | No. of specimens with results
|
Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | |||
|---|---|---|---|---|---|---|---|---|
| Cytospin FA positive and culture positive | Cytospin FA negative and culture positive | Cytospin FA positive and culture negative | Cytospin FA negative and culture negative | |||||
| Influenza A virus | 18 | 2 | 0 | 154 | 90 | 100 | 100 | 97 |
| Influenza B virus | 1 | 6 | 0 | 167 | 14.3 | 100 | 100 | 96.3 |
| Influenza virus (untypeable) | 0 | 1 | 0 | 173 | 0 | 100 | NAa | NA |
| Adenovirus | 0 | 2 | 0 | 172 | 0 | 100 | NA | 98.7 |
| RSV | 2 | 0 | 0 | 172 | 100 | 100 | NA | NA |
| All viruses | 21 | 11 | 0 | 142 | 65.6 | 100 | 100 | 92.8 |
NA, not applicable.
TABLE 4.
Summary of tests for which RSV testing was requested
| Result | No. of samples with result by:
|
|
|---|---|---|
| Cytospin FA | EIA | |
| Positive | 41 | 42 |
| Negative | 56 | 55 |
| Total | 97 | 97 |
We studied parameters on all inpatients who had positive respiratory viral results. There were 11 such inpatients in year 1 and 27 such inpatients in year 2. Over half the patients in year 1 shared common diagnoses with patients in year 2. These common diagnoses included chronic obstructive pulmonary disease, pneumonia, otitis media, and upper respiratory tract infection. The mean age of patients in year 1 was 58.5 years, and for patients in year 2 it was 46.3 years, not statistically different (P = 0.872) (Table 5). The mean turnaround time for positive viral results on inpatients during year 1 was 4.5 days; during year 2, it was 0.9 day, a statistically significant difference (P = 0.001) (Table 5). For two-thirds of the patients with positive findings, the result was available within 6 h after the sample arrived in the laboratory because of the cytospin FA.
TABLE 5.
Summary of parameters examined for all inpatients with respiratory virus isolated in years 1 and 2
| Parameter | Mean in yr 1 | SD in yr 1 | Mean in yr 2 | SD in yr 2 | Difference between yrs 1 and 2b | P |
|---|---|---|---|---|---|---|
| Mean age (yr) | 58.5 | 22.6 | 46.3 | 33.8 | −12.2 | 0.872 |
| Mean turnaround time (day) | 4.5 | 4.2 | 0.9 | 1.6 | −3.6 | 0.001 |
| Mortality rate (%) | 18.2 | NAa | 7.4 | NA | −10.8 | 0.326 |
| Mean length of stay (day) | 10.6 | 9.3 | 5.3 | 5.1 | −5.3 | 0.065 |
| Mean total cost ($) | 17,358 | 20,033 | 5,541 | 5,522 | −11,817 | 0.104 |
| Mean variable cost ($) | 7,893 | 9,603 | 2,177 | 2,223 | −5,716 | 0.079 |
NA, not applicable.
−, decrease was seen in year 2.
We then evaluated parameters supplied by clinical data management which were potentially impacted by this faster turnaround time. For inpatients testing positive for respiratory viruses, the mortality in year 1 was 18.2%; in year 2, it was 7.4%, a decrease of 10.8% in year 2 (not statistically significant [P = 0.326]) (Table 5). The mean length of stay in the hospital for patients with viral isolates was 10.6 days in year 1 and 5.3 days in year 2, a decrease of 5.3 days in year 2 (P = 0.065). The mean total cost of these patients was $17,358 in year 1 and $5,541 in year 2. This was a difference of $11,817 less per patient in year 2 (P = 0.104). The mean variable cost of these patients was $7,893 in year 1 and $2,177 in year 2. This was a difference in variable costs of $5,716 less per patient in year 2 (P = 0.079) (Table 5).
In order to eliminate the potential influence of severity of disease caused by the different viruses, we examined a subset of the inpatients described above. Table 6 shows a comparison of only those inpatients from whom influenza A virus or RSV was isolated. Approximately three-fourths (71%) of the influenza A patients in year 1 shared identical discharge diagnoses with corresponding patients in year 2. The same trends in the subsets are seen—decreased turnaround time, decreased length of stay, and decreased total and variable costs—for patients in year 2.
TABLE 6.
Summary of parameters examined for patients with the same virus isolated in years 1 and 2
| Virus isolated | Parameter | Mn of yr 1 | SD of yr 1 | Mean of yr 2 | SD of yr 2 | Difference between yrs 1 and 2b | P |
|---|---|---|---|---|---|---|---|
| Influenza A | No. of patients | 7 | 15 | NAa | |||
| Age (yr) | 67.9 | 10.5 | 70.2 | 13.5 | +2.3 | 0.69 | |
| Turnaround time (day) | 2.5 | 1.7 | 0.4 | 0.5 | −2.1 | 0.02 | |
| Mortality rate (%) | 14 | NA | 13 | NA | −1 | 0.95 | |
| Length of stay (day) | 11.1 | 10.4 | 8.1 | 5.3 | −3.0 | 0.36 | |
| Total cost ($) | 15,489 | 19,089 | 8,040 | 6,353 | −7,449 | 0.18 | |
| Variable cost ($) | 6,916 | 9,049 | 3,045 | 2,630 | −3,871 | 0.13 | |
| RSV | No. of patients | 1 | 7 | NA | |||
| Age (yr) | 0.2 | 0 | 0.9 | 0.1 | +0.7 | 0.0004 | |
| Turnaround time (day) | 0.8 | 0 | 0.3 | 0.6 | −0.5 | 0.39 | |
| Mortality rate (%) | 0 | NA | 0 | NA | 0 | NA | |
| Length of stay (day) | 2.0 | 0 | 1.1 | 0.4 | −0.9 | 0.08 | |
| Total cost ($) | 3,085 | 0 | 1,719 | 513 | −1,367 | 0.05 | |
| Variable cost ($) | 1,202 | 0 | 724 | 251 | −478 | 0.13 |
NA, not applicable.
−, decrease was seen in year 2; +, increase was seen in year 2.
The most logical reason for these changes is that physicians could treat the viral infections more appropriately, e.g., by discontinuing unnecessary antibiotics (therefore decreasing the possibility of an adverse drug reaction) as well even discharging their patients sooner once they are known to be infected with a virus. A fellow in pulmonology (N.L.) did a chart review on all inpatients to determine if antibacterial therapy were discontinued within 24 h of the report of viral detection. For patients in year 1, 1 of 11 (9.1%) of the patients had unnecessary antibacterial therapy discontinued within 24 h after the positive virus result was reported; in year 2, this figure was 8 of 28 (28.6%) of the patients. The average age of the patients whose antibiotics were discontinued was 29.3 years (which is younger than the average age of all the patients studied).
DISCUSSION
Because managed care often results in the downsizing of clinical microbiology laboratories, it is essential that the contribution of microbiology data on patient outcomes be recognized by administrators (2). In the United States and in Hong Kong, rare studies have shown the financial and clinical benefits of rapid reporting of bacterial and viral results (1, 3, 5). In the setting of a community teaching hospital in the United States, this study supports the concept that there are clinical and financial benefits of rapid reporting of viral respiratory tests. While it is difficult to prove a causal relationship (because of multiple uncontrolled variables in two different time periods), this study shows a consistent positive clinical and financial impact for all variables studied. By performing a cytospin FA on respiratory samples submitted for viral testing, we were able to decrease turnaround time of positive viral results. Decreased mortality, length of stay, and hospital costs and better antibiotic stewardship resulted. The decrease in costs was found despite the overall trend of increasing costs between years 1 and 2 (Tables 1, 5, and 6). The 5.3-day decrease in length of stay in year 2 cannot be explained by the current local and national trend of an overall decrease in length of stay. At Memorial Medical Center, the length of stay between years 1 and 2 decreased by 5% (1 − [5.5 days/5.8 days]) for all patients admitted (Table 1). By that reasoning, the decrease in length of stay during year 2 would be expected to be 0.5 day (5% of 10.6 days); in fact the decrease was 5.3 days. Further, this difference in length of stay cannot be explained by the difference in age of our study group versus control group (mean ages 46 and 58 years, respectively). For 1998, the average length of stay for all 46-year-old patients in our hospital was 5.5 days; for all 58-year-old patients, it was 5.6 days. In addition, the difference in mortality between the two groups (10.8%) cannot be explained by the differences in their ages. The mortality for 46-year-old patients for year 1 was 1.2%, and for 58-year-old patients, it was 2.8%. For year 2, the mortality for 46 year old patients was 0%, and for 58 year old patients, it was 2.1%.
Although both the number of all respiratory samples processed in the virology laboratory and the number of viruses isolated were almost identical in both years, there were over twice the number of inpatients with respiratory viral isolates in year 2. This suggests the possibility that the patients in year 2 were sicker than their counterparts in year 1 and required hospitalization. In spite of this possibility, patients in year 2 showed clinical and financial benefits compared to patients in year 1.
Of particular note is the decrease in variable costs of $5,716 per patient in year 2. Administrators consider these variable costs responsible for the actual cost savings realized by the hospital. There were 27 inpatients who benefited from the cytospin FA. Extrapolating this data, Memorial Medical Center could expect to save $154,332 (27 × $5,716) annually as a result of rapid reporting of viral respiratory infections. Estimated technologist time was 150 h to perform the cytospin FA on 281 respiratory samples (this testing includes inpatients as well as outpatients). Assuming a technologist costs $20/h, the cost of technological time was $3,000 (150 h × $20). The estimated cost of reagents was $7,000. In total, the extra effort of doing a cytospin FA cost the hospital approximately $10,000 ($3,000 + $7,000). If this cost is subtracted from the expected annual savings from variable costs, the net cost reduction in 1999 is $144,332 ($154,332 − $10,000). In fact, this cost savings could be increased even more by limiting the cytospin FA only to inpatients. However, advantages of the cytospin FA are still applicable to outpatients, but the benefits are extremely difficult to evaluate in a hospital setting.
Because of the small numbers of patients, the differences in length of stay and both cost variables did not reach the typical significance level of 0.05 but were near it (Table 5). The biostatician said that had there been 27 patients in year 1, the differences would have been significant at 0.05. Some statistically significant differences did emerge when data from patients infected with the same virus were analyzed (Table 6).
Our results are consistent with the findings of Doing et al., who found 80.4% sensitivity for direct FA for influenza A virus and 81.9% for RSV compared to culture (5). The cytospin FA is a procedure which can markedly decrease turnaround time for diagnosing respiratory viral disease. Although it has a sensitivity of 65.6% for all viruses detected compared to culture, it is extremely specific. There were no false positives. False-negative results may have been caused by the presence of fragile cells disrupted during the cytospin procedure. The cytospin FA gave negative results for the two cases of adenovirus, but adenovirus is notorious for testing negative by direct specimen testing and positive on follow-up culturing. There are no sufficiently sensitive assays for the detection of adenovirus for use directly on clinical samples. Although the sensitivity of the cytospin FA for all viruses detected is only 65.6%, the vast majority of the viruses detected in clinical specimens are RSV and influenza A. The sensitivity for these two viruses is high (98 and 90%, respectively) so the overall sensitivity underestimates the real value in detecting viruses found in most of the clinical samples. However, since the sensitivity of the cytospin FA is only 65.6%, the question arises whether this is a clinically useful test. Other rapid tests with this low sensitivity and high specificity are extremely useful in clinically managing patients. For instance, the widely used “rapid strep” tests have a sensitivity and specificity similar to those of the cytospin FA. The problem with false negatives for group A streptococci is handled by following up all negative rapid tests with culture. This is what must be done with the rapid test described here—one must follow up all cytospin FA-negative samples with culture.
Other commercially available methods to detect RSV and influenza virus rapidly are available now, but generally these methods are used only with specific requests of testing for a particular virus. This constitutes a serious drawback since many viral syndromes are indistinguishable clinically. Cavalieri et al. (S. J. Cavalieri, A. R. Sambol, S. M. Flor, K. M. Shuck, and C. Harrison, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. C318, 1999) reported that 16% of their positive respiratory viral specimens would have been missed by directing detection only to the specific viruses (RSV and influenza A virus) requested. In addition, they found numerous dual infections. Since only RSV testing was requested, the first case of influenza heralding the beginning of the influenza epidemic in our community would have gone undetected without the screening for the common respiratory viruses. Detection of several other cases of influenza would have been delayed because only RSV testing was requested. Similarly, we were able to diagnose RSV infection rapidly in an elderly patient for whom only influenza virus testing had been requested. The mortality rate of RSV infection in the elderly is substantial (17%), but a specific request for RSV is rarely made in this population (J. Flamaing, I. Engelmann, M. Van Ranst, and W. E. Peetermans, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 287, 1999). The disadvantage of substituting the EIA for the cytospin FA is that each virus must be tested for separately; e.g., one EIA must be performed to detect influenza A virus, one EIA must be performed to detect RSV, etc. In addition, no commercially available EIA even exist for parainfluenza virus (or adenovirus). Early detection of multiple viruses (without the need for specifying which virus) has major implications on patient management, especially on starting, stopping, and altering anti-infective therapy. Given the fact that there are several antiviral agents available now and the urgent need for good antibiotic stewardship to prevent multidrug resistance in bacteria, early diagnosis is crucial to the ideal care of the patient as well as the community.
An announcement to physicians was posted when the first cases of influenza A virus infection were detected. Before this announcement, 16 of 66 samples (24%) had specific orders for influenza virus testing; for the month following the announcement, 62 of 128 samples (48%) had specific orders for influenza virus testing. This reflects the importance that physicians place on detecting this virus and the need for communication of this when influenza is present in the community.
Solely from a laboratory point of view, the added work of the cytospin FA had a disadvantage because it was during our busiest time in the virology laboratory, the winter respiratory virus season. However, the “downstream” effects (the clinical and financial benefits) clearly offset this drawback.
In summary, this study confirms the previously described sensitivity and specificity of the cytospin FA and supports the concept that there are clinical and financial benefits associated with rapid reporting of respiratory viral diseases. By using the cytospin FA technique, 67% of the inpatients were diagnosed with a viral disease within 6 h of submission of their sample to the virology laboratory. The clinical impact of this rapid reporting is significant because it resulted in physicians having access to crucial information sooner, enabling them to treat viral diseases more appropriately. The average length of stay in the hospital was 5.3 days less than that for similar patients hospitalized the previous winter. Variable costs (which administrators look at as potential savings) were decreased by $5,716/patient. Even after subtracting the cost of reagents and technological time, the potential savings in variable costs to the hospital was $144,332 annually. While the cost savings generally do not achieve statistical significance because of the small number of patients in the control group, our administrators were sufficiently convinced that rapid reporting of respiratory viruses justified the extra technological time and costs.
ACKNOWLEDGMENTS
This work was supported by a grant from the Memorial Medical Center Foundation.
Reagents were supplied by DAKO Corporation.
REFERENCES
- 1.Barenfanger J, Drake C, Kacich G. Clinical and financial benefits of rapid bacterial identification and antimicrobial susceptibility testing. J Clin Microbiol. 1999;37:1415–1418. doi: 10.1128/jcm.37.5.1415-1418.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Check W. Managed care deeply affecting clinical microbiology. ASM News. 1998;64:495–500. [Google Scholar]
- 3.Doern G, Vautour R, Gaudet M, Levy B. Clinical impact of rapid in vitro susceptibility testing and bacterial identification. J Clin Microbiol. 1994;32:1757–1762. doi: 10.1128/jcm.32.7.1757-1762.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doing K M, Jerkofsky M A, Dow E G, Jellison J A. Use of fluorescent antibody staining of cytocentrifuge-prepared smears in combinations with cell culture for direct detection of respiratory viruses. J Clin Microbiol. 1998;36:2112–2114. doi: 10.1128/jcm.36.7.2112-2114.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woo P C, Chiu S S, Seto W, Peiris M. Cost-effectiveness of rapid diagnosis of viral respiratory tract infection in pediatric patients. J Clin Microbiol. 1997;35:1579–1581. doi: 10.1128/jcm.35.6.1579-1581.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]