Abstract
Pregnancy is an inflammatory process that is carefully regulated by the placenta via immunomodulation and cell-to-cell communication of maternal and fetal tissues. Exosomes, types of extracellular vesicles, facilitate the intercellular communication and traffic biologically modifying cargo within the maternal-placental-fetal axis in normal and pathologic pregnancies. Chorioamnionitis is characterized by inflammation of chorioamniotic membranes that produces systemic maternal and fetal inflammatory responses of cytokine dysregulation and has been associated with brain injury and neurodevelopmental disorders. This review focuses on how pathologic placental exosomes propagate acute and chronic inflammation leading to brain injury. The evidence reviewed here highlights the need to investigate exosomes from pathologic pregnancies and those with known brain injury to identify new diagnostics, biomarkers, and potential therapeutic targets.
Keywords: Perinatal brain injury, Chorioamnionitis, Inflammatory signal transduction, Exosomes
1. Introduction
In utero inflammation leading to perinatal brain injury (PBI) contributes directly to neurodevelopmental disorders and long term central nervous system (CNS) injuries, such as cerebral palsy (CP) (Anblagan et al., 2016; Dammann & Leviton, 1997; Leviton et al., 2016; O’Shea et al., 2012; Venkatesh et al., 2020). Chorioamnionitis is a common etiology of in utero inflammation and is known to elicit a fetal inflammatory response syndrome (FIRS) via the maternal-placental-fetal axis (Cappelletti et al., 2020; Kallapur et al., 2014; Romero et al., 2016). The development of FIRS contributes to PBI by leading to a systemic and neurotoxic inflammatory response during a critical period of neurodevelopment (Salas et al., 2013; Tang et al., 2019). Though immune and cytokine dysregulation certainly contribute to the inflammatory response, the underlying mechanisms facilitating inflammatory signal transduction and modes of cellular communication are poorly understood (Jung et al., 2020; Madsen-Bouterse et al., 2010). There is increasing evidence that extracellular vesicles, specifically exosomes, play a large role in immune modulation, cell-to-cell signaling, and transportation of both immunostimulatory and immunoinhibitory cargo (i.e. micro-RNA) that are integral to the systemic and neuroinflammatory cascade of acute chorioamnionitis and brain injury (Gupta & Pulliam, 2014; Monsivais et al., 2020; Pillay et al., 2017; Salomon & Rice, 2017).
2. Chorioamnionitis
Chorioamnionitis refers to inflammation of the chorioamniotic membranes (Kim et al., 2015). Though inflammation can occur under sterile conditions (Roberts et al., 2012; Romero et al., 2014), infectious pathogenesis predominantly occurs via an ascending infection from the lower genital tract during pregnancy (Kim et al., 2015). Acute chorioamnionitis has a broad clinical diagnosis as well as a more definitive histopathologic diagnosis. For the purposes of this review, we will refer to both clinical chorioamnionitis and histologic chorioamnionitis separately. Clinical chorioamnionitis was recently redefined as “Intrauterine Inflammation and/or Infection” (Triple I) to more uniformly define the variable clinical manifestations (Higgins et al., 2016). This definition delineates between “isolated maternal fever”, “suspected Triple I” (maternal fever plus fetal tachycardia, maternal leukocytosis, and/or purulent cervical discharge), and “confirmed Triple I” (maternal fever, symptoms, and microbiologic or histopathologic evidence of infection/inflammation of the amniotic fluid or placenta (Higgins et al., 2016). Histologic chorioamnionitis is defined as neutrophil infiltration of any element of the choriodecidual space (Redline et al., 2003). These are maternally derived neutrophils that migrated from the decidua in response to inflammation and are not usually present in the chorioamniotic membranes (Kim et al., 2015). This is in contrast to funisitis (inflammation of the umbilical cord), in which the infiltrating neutrophils are of fetal origin and is associated with more severe CNS injury and neurodevelopmental impairment (Salas et al., 2013; Kim et al., 2015). Intra-amniotic inflammation is associated with significantly higher expression of both pro- and anti-inflammatory cytokines and chemokines (Romero et al., 2016). The resulting inflammation increases the risk of preterm labor, spontaneous preterm delivery, and premature rupture of membranes (PROM), as well as a fetal inflammatory response syndrome that can transition to postnatal systemic inflammatory response syndrome or early-onset sepsis (Cappelletti et al., 2020; Kallapur et al., 2014; Tang et al., 2019; Romero et al., 2007; Lee et al., 2013). All of which are significant sequelae of chorioamnionitis as prematurity, FIRS, and early-onset sepsis are all associated with increased risk of perinatal brain injury and neurodevelopmental impairment (Tang et al., 2019; Mukhopadhyay et al., 2020; Volpe, 2009).
3. Association between chorioamnionitis and brain injury
Chorioamnionitis has been associated with increased risk for CP, intraventricular hemorrhage, periventricular leukomalacia, perinatal arterial ischemic stroke, autism spectrum disorder, and epilepsy (Anblagan et al., 2016; Dammann & Leviton, 1997; Venkatesh et al., 2020; Leviton et al., 2010; Sorg et al., 2020; Redline & O’Riordan, 2000). CP is a lifelong motor and neurodevelopmental disorder with a wide variety of clinical manifestations including disorders of movement and posture, chronic pain, epilepsy, as well as cognitive and behavioral disorders (Aisen et al., 2011). The Extremely Low Gestational Age Newborn (ELGAN) study demonstrated that isolation of microorganisms from the placenta increased risk for the development of white matter injury and CP at 2 year follow up evaluation (Leviton et al., 2010). Additionally, evidence of chorioamniotic membrane inflammation and sustained postnatal elevation of inflammation-related proteins, correlated with neonatal ventriculomegaly as well as CP (Leviton et al., 2016; O’Shea et al., 2012; Leviton et al., 2010). At 10 years of life, the investigators found that histologic chorioamnionitis was associated with increased risk of CP, autism spectrum disorder, and epilepsy (Venkatesh et al., 2020).
ELGANs also had elevated concentrations of inflammation-related proteins including interleukin-1β (IL-1β), tumor necrosis factor alpha (TNF-α), and C-reactive protein (CRP), proportional to the severity of histologic chorioamnionitis (Hecht et al., 2011; Leviton et al., 2011a). Similarly, a recent study showed that higher elevation of CRP in the immediate postnatal period was significantly associated with histologic chorioamnionitis and correlated with the degree of severity (Ryan et al., 2020). In further studies, the investigators also found that ELGANs with elevated inflammatory cytokine levels of IL-6 and TNF-α on day of life 1, were nearly twice as likely to demonstrate sustained cytokine elevation through the end of the first month of life (Dammann et al., 2016). Sustained elevation throughout the first postnatal month, placed newborns at increased risk for developing ventriculomegaly, intraventricular hemorrhage, and/or white matter damage, as well as neurodevelopmental impairment (O’Shea et al., 2012; Leviton et al., 2013; Leviton et al., 2011b). Another study showed that premature infants exposed to histologic chorioamnionitis had increased risk for severe intraventricular hemorrhage and when compounded with an additional placental injury, there was increased risk of poor neurodevelopmental outcomes (Kaukola et al., 2006). The risk of developing cerebral palsy was associated with severe histologic chorioamnionitis and similarly increased with additional placental abnormalities (Redline & O’Riordan, 2000). Children with cerebral palsy were significantly associated with a history of histologic chorioamnionitis and were more likely to have spastic diplegic subtype as well as periventricular white matter injury (Shevell et al., 2014). Multiple meta-analyses show an increased risk in the development of CP in infants born to mothers with evidence of clinical and/or histologic chorioamnionitis (Ayubi et al., 2021; Shatrov et al., 2010; Shi et al., 2017a; Wu & Colford Jr., 2000). These data suggest that infants born in the setting of histologic chorioamnionitis produce a significant postnatal systemic inflammatory response that may be sustained for weeks and ultimately contribute to structural and functional brain injury (Kuban et al., 2015).
4. Mechanisms of inflammatory signal transduction between placenta and developing brain: cytokines
The fetal inflammatory response syndrome is defined as histopathologic funisitis and/or elevated acute phase reactant, IL-6, in umbilical cord plasma or serum (Jung et al., 2020; Gomez et al., 1998; Gotsch et al., 2007). The degree of fetal inflammatory response by histologic severity correlated with higher mortality and neurodevelopmental impairment (Salas et al., 2013). Transcriptome and proteosome analyses of FIRS demonstrated increased concentrations of pro-inflammatory chemokines and cytokines including IL-8, chemokine (C-X-C motif) ligand 6 (CXCL-6), CXCL-10, IL-1, TNF-α, IL-6, and chemokine (C-C motif) ligand 2 (CCL2) (Madsen-Bouterse et al., 2010). Pro-inflammatory chemokines such as IL-8 and CXCL-6 create a large chemotactic gradient, inducing neutrophil migration into the chorioamniotic membranes and amniotic fluid (Mittal et al., 2008). This promotes a type of feed-forward inflammatory cascade whereby recruited and activated neutrophils express the chemokine receptor CXCR2, a receptor for IL-8 and CXCL-6, which furthers neutrophil recruitment (Cappelletti et al., 2020; Mittal et al., 2008; Kolaczkowska & Kubes, 2013; Sadik et al., 2011; Tecchio et al., 2014; Yoon et al., 1997). Our previous work demonstrated excess CXCL1/CXCR2 signaling within both the placenta and developing brain in our model of CNS injury associated with chorioamnionitis in rats (Jantzie et al., 2014; Jantzie et al., 2015; Maxwell et al., 2015; Yellowhair et al., 2018). In addition to preterm labor, preterm PROM (PPROM), and preterm delivery (Romero et al., 2007; Keelan, 2018; Kemp, 2014), FIRS is associated with increased rates of neonatal morbidities and multisystemic involvement including neonatal sepsis, bronchopulmonary dysplasia (BPD), retinopathy of prematurity, necrotizing enterocolitis, as well as brain injury (Tang et al., 2019; Jung et al., 2020; Gomez et al., 1998; Gotsch et al., 2007).
The pathogenesis of PBI and neurodevelopmental disorders in the setting of the fetal inflammatory response is actively being investigated. The prevailing notion is that an intermittent or sustained inflammatory response primes the fetus and neonate for a multi-hit model of brain injury with in utero and postnatal as well as acute and chronic inflammation and immune dysregulation (Leviton et al., 2016; Dammann & Leviton, 2014; Korzeniewski et al., 2014). Importantly, there is evidence that alterations in major white matter tracts from antenatal inflammation exposure originates in utero, independent of gestational age or other predictors of neurodevelopmental impairments such as postnatal sepsis or bronchopulmonary dysplasia (Anblagan et al., 2016).
Pro-inflammatory cytokines can cause both direct and indirect injury to the developing brain. Infants with both histologic chorioamnionitis and FIRS had a higher incidence of brain injury than those with histologic chorioamnionitis alone (Lu et al., 2016). Those infants with brain injury had significantly higher levels of umbilical cord cytokines; levels of which also correlated with higher grades and stages of histologic chorioamnionitis (Lu et al., 2016). In this setting of neuroinflammation, there is evidence of increased permeability of the blood-brain barrier to immune cells as well as further production of pro-inflammatory cytokines by astrocytes and microglia (Brochu et al., 2011; Girard et al., 2010; Kuypers et al., 2012; Gilles & Leviton, 2020). Activated microglia, sustain the inflammatory response by releasing cytokines and cytotoxic mediators such as reactive oxygen and nitrogen species, that cause direct white matter injury via apoptosis and loss of oligodendrocyte precursors (Yap & Perlman, 2020; Zhang et al., 2018; Volpe et al., 2011). The remaining oligodendrocyte precursors exhibit dysregulated maturation by failing to myelinate axons (Yap & Perlman, 2020; Buser et al., 2012). Additionally, the immature oligodendrocytes are more susceptible to further neuroinflammatory damage from free radicals and excitotoxicity (Kuypers et al., 2012; Yap & Perlman, 2020; Volpe et al., 2011; Chau et al., 2014). Though much work has been done to understand the pathophysiology of PBI in the setting of chorioamnionitis, the underlying molecular mechanisms eliciting inflammation through the placental-fetal-brain axis remain unknown and therefore lack therapies to mitigate CNS injury.
5. Mechanisms of inflammatory signal transduction between placenta and developing brain: Exosomes
Exosomes may be important mediators of perinatal inflammation and brain injury in the setting of chorioamnionitis. Originally investigated for their role in tumorigenesis, exosomes have since been found to assist in immune modulation, intercellular communication and signaling, and post-transcriptional modification (Atay et al., 2011; Kalluri & LeBleu, 2020; Record, 2014). Exosomes are a type of extracellular vesicle that are approximately 30–150 nm in size (Kalluri & LeBleu, 2020). They are endosome-derived vesicles that are stored with other intraluminal vesicles within multivesicular bodies (Kalluri & LeBleu, 2020). Exosomes undergo two sequential invaginations, thereby allowing the origin cell membrane’s receptors and proteins to remain external (Anand, 2010). This allows for identification of the origin cell, but also for the ability of a cell to signal or alter another cell at great distances, even between mother and fetus (Anand, 2010; Sheller-Miller et al., 2019a).
Exosomes are produced by multiple cell types, including neural cells, astrocytes, microglia, tumor cells, epithelial cells, immune cells, as well as the endometrium and placental trophoblasts (Jin & Menon, 2018; Andjus et al., 2020; Gharbi et al., 2020). They are created by various cells in response to different physiologic states (Sheller et al., 2016). As a result, exosomes encapsulate a unique composition of cellular proteins, lipids, messenger RNA (mRNA), and micro-RNA (miRNA), that are unique to that cell under specific conditions, such as oxidative stress or infection (Sheller et al., 2016; Delorme-Axford et al., 2013). This key aspect of an exosome’s modifiable and unique cargo makes them ideal as potential biomarkers, reflecting the physiologic state of the origin cell (Pillay et al., 2017; Jin & Menon, 2018). The mechanisms underlying cargo packaging are not yet fully understood (van Niel et al., 2018). Once exosomes are secreted, they exhibit both autocrine and paracrine signaling and can interact in several ways with local and distant cells, as well as cross the blood-brain barrier (Sheller-Miller et al., 2019a; Alvarez-Erviti et al., 2011). Exosomes may bind with the recipient cell either by receptor-ligand interaction, fusion, or internalization, thereby releasing their contents and enacting functional changes (Anand, 2010; Valadi et al., 2007; Luo et al., 2009).
6. Exosomes in pregnancy
Exosomes are integral to maternal-fetal communication and modulate the physiologic and pathologic inflammation of pregnancy. As pregnancy, parturition, and related complications such as preterm labor, preeclampsia, gestational diabetes, and chorioamnionitis are rooted in immune dysregulation and inflammation, the role of exosomes in these processes is being studied (Gomez-Lopez et al., 2019; Kohli et al., 2016; Radnaa et al., 2021; Salomon et al., 2016; Sheller-Miller et al., 2019b). Placental-derived exosomes have been shown to assist with embryo implantation, placental angiogenesis, and immune modulation, which allows the semi-allogenic fetus to survive and grow (Stenqvist et al., 2013; Hedlund et al., 2009; Sabapatha et al., 2006; Salomon et al., 2013). For example, placental trophoblast exosomes induce the pro-inflammatory phase of early pregnancy by recruiting monocytes and stimulating the production of pro-inflammatory cytokines including IL-1β, TNF-α, and monocyte chemoattractant factor-1 (MCP-1/CCL2) (Atay et al., 2011). These cytokines have significant overlap with the inflammatory molecules directly implicated in the pathophysiology of FIRS and PBI (Gilles & Leviton, 2020; Yap & Perlman, 2020). Conversely, during the immunosuppressive, maintenance phase of pregnancy placental exosomes expressed both Fas ligand and programmed death ligand-1, which suppress maternal T-cell signaling at the maternal-fetal interface, allowing for fetal tolerance (Sabapatha et al., 2006). In addition to acting locally at the maternal-fetal interface, exosomes can be trafficked back and forth between mother and fetus and induce functional changes (Sheller-Miller et al., 2019a). Human placental trophoblast cells are able to internalize maternal macrophage-derived exosomes, which stimulate placental release of pro-inflammatory cytokines such as IL-6 and IL-8 (Holder et al., 2016). In an animal model of gestational diabetes, maternal-derived exosomes were able to cross the maternal-fetal barrier into the fetus and elicit cardiac developmental deficiencies such as ventricular septal defect, myocardial hypertrophy, and ventricular hypoplasia (Shi et al., 2017b).
7. Exosomes in pathologic pregnancies
The function and composition of placental exosomes are modified by, and contributes to, pathologic inflammatory states of pregnancy (Burkova et al., 2021; Czernek & Düchler, 2020). For example, miR-210 and miR-155 expression are both elevated in placenta exosomes of preeclamptic pregnancies (Biró et al., 2019; Shen et al., 2018). miR-210 is associated with impairment of trophoblast invasion and miR-155 may inhibit endothelial nitric oxide synthase expression, both of which are pathological alterations of preeclampsia (Biro et al., 2019´ ; Shen et al., 2018). Of note, a recent study showed that the differential expression of exosomal miRNA isolated from plasma of mothers with preeclampsia was even different than the miRNA isolated from whole plasma, highlighting the selectivity of exosomal cargo trafficking (Li et al., 2021).
Under oxidative stress or hyperglycemia, placental exosomes increase and activate monocytes and macrophages, stimulating proinflammatory cytokine release and resulting in a maternal systemic inflammatory response that contributes to many pathologies of pregnancy including preeclampsia, gestational diabetes, and chorioamnionitis (Pillay et al., 2017; Familari et al., 2017). Placental exosomes isolated from pregnancies complicated by gestational diabetes demonstrated differentially expressed miRNA profiles (Zhang et al., 2021) as well as increased release of pro-inflammatory cytokines (IL-6, IL-8, IFN-γ, and TNF-α) by endothelial cells (Burkova et al., 2021; Czernek & Düchler, 2020). Human umbilical cord serum, human umbilical vein endothelial cells, and placenta exposed to in utero maternal diabetes, had altered amounts and types of exosomal miRNA that are associated with metabolic pathways (Shah et al., 2021). Amnion epithelial cell-derived exosomes increased secretion of pro-inflammatory cytokines (IL-6, IL-8, prostaglandin E2 (PGE2), and nuclear factor-κβ (NF-κβ), inducing labor-promoting changes in maternal myometrial and decidual cells in vitro (Hadley et al., 2018). These studies further highlight the breadth of maternal-fetal communication and how exosome trafficking and their cargo contribute to feed-forward maternal-fetal inflammatory cascades similarly seen in chorioamnionitis. They also emphasize a novel route of communication and trafficking of inflammatory cargo that may be integral to the pathophysiology of PBI.
It is important to note that human amnion epithelial cells and their exosomes also exhibit anti-inflammatory effects and are currently being studied as invivo therapies for a multitude of disease states (Papagianis et al., 2021; Siahanidou & Spiliopoulou, 2020; Thomi et al., 2019). Their ability to regulate inflammation likely stems from the pluripotency of human amnion epithelial cells, their ability to beneficially regulate cellular microenvironments with secretion of beneficial trophic factors, and because therapeutic cells and their exosomes are harvested from placentas of women with uncomplicated pregnancies (Zhang & Lai, 2020). These studies further suggest that the physiologic state of the cell of origin contributes significantly to exosomal function. Undoubtedly, exosomes as novel, anti-inflammatory therapeutics are an important topic for future review.
Similarly, placental exosomes can be involved in placental angiogenesis or endothelial dysfunction depending on the underlying physiologic state of the pregnancy. Hypoxic conditions led to increased release of placental mesenchymal stem cell-derived exosomes (Salomon et al., 2013). These isolated exosomes promoted endothelial cell migration and tube formation in an in vitro study using human placental microvascular endothelial cells (Salomon et al., 2013). Similarly, placental mesenchymal stem cell exosomes were shown to contain angiogenesis-related growth factors and demonstrated pro-angiogenic activity of endothelial cells in vitro and in vivo (Komaki et al., 2017). The abnormal placentation that underlies preeclampsia, increases oxidative stress, thereby increasing the release of placental exosomes (Pillay et al., 2017; Salomon et al., 2013). Placental exosomes isolated from patients with preeclampsia were shown to release anti-angiogenic factors endoglin and FMS-like tyrosine kinase 1 (Flt-1), which are suspected to play a role in the development of preeclampsia by reducing the proliferation and tube formation of endothelial cells (Chang et al., 2018; Tannetta et al., 2013). Those isolated exosomes were injected into pregnant mice, which induced vascular dysfunction and a preeclampsia-like phenotype consisting of maternal hypertension, decreased birth weights, and decreased surviving embryos per litter (Chang et al., 2018; Tannetta et al., 2013). These studies highlight the unique ability of exosomes to promote maternal-fetal crosstalk and intercellular signaling to enact significant functional changes as well as immunomodulation locally and at distant organs including the brain.
8. Exosomes in perinatal brain injury
Exosomes have been implicated in neuroinflammation and may contribute to perinatal brain injury via the placental-fetal-brain axis. There is a paucity of research on the impact of placental exosomes in chorioamnionitis. However, based upon recent and related literature, there is biological plausibility that placental exosomes may be integral to the pathophysiology of perinatal brain injury. A single study recently investigated potential biomarkers from fetal membrane-derived exosomes in the setting of inflammation and infection to better assess risk of preterm labor or PPROM (Monsivais et al., 2020). The study demonstrated increased and differential expression of unique exosomal proteins in response to either infectious (lipopolysaccharide (LPS)) or inflammatory (TNF-α) stimuli, further suggesting exosomes’ role within placental inflammatory pathologies (Monsivais et al., 2020). Based upon prior work (Yellowhair et al., 2018) our lab also explored whether exosome trafficking, as opposed to cytokine production, might account for the transmission of inflammatory signaling within the placental-fetal-brain axis. As described earlier, CXCL1 levels were highly elevated within the placentae and developing brain in our model of CNS injury associated with chorioamnionitis (CHORIO) (Jantzie et al., 2014; Jantzie et al., 2015; Maxwell et al., 2015). Sham or CHORIO placental explants were cultured on inserts and placental conditioned media (PCM) assayed for toxic mediators and inflammatory drivers. CHORIO placentas secreted more IL-1β, TNF-α, and CXCL1 compared to sham placentas (Figs. 1A-B). To determine the effect of CHORIO PCM on the developing brain, PCM from naive or CHORIO placental explants was cultured with postnatal day 2 (P2) brain slices. PCM from CHORIO placental explants induced high CXCL1 levels in brain slices but did not alter brain levels of TNF-α or IL-1β (Fig. 1C). To confirm whether cytokines themselves or placental-derived exosomes were able to induce brain CXCL1 expression, exosomes were isolated from CHORIO PCM. Exosomes from CHORIO placentas elevated CXCL1 mRNA in P2 brain slices. Brain CXCL1 mRNA levels are reduced by blockade of exosome release using the exosome generation inhibitor GW4869, indicating dampened CHORIO-induced inflammation (Fig. 2A). Accordingly, blockade of exosome generation improved neural cell health and preserved developmental trajectory as evidenced by increased gamma-amino-butyric acid A receptor (GABAAR) subunit ɣ2 mRNA in P2 brain slices cultured with exosome depleted PCM compared to exosome containing PCM (Fig. 2B). Taken together, these data demonstrated that blockade of placental exosome generation dampens chorioamnionitis-induced increases in brain CXCL1 and may improve the trajectory of neural cell development (Fig. 3).
Fig. 1.
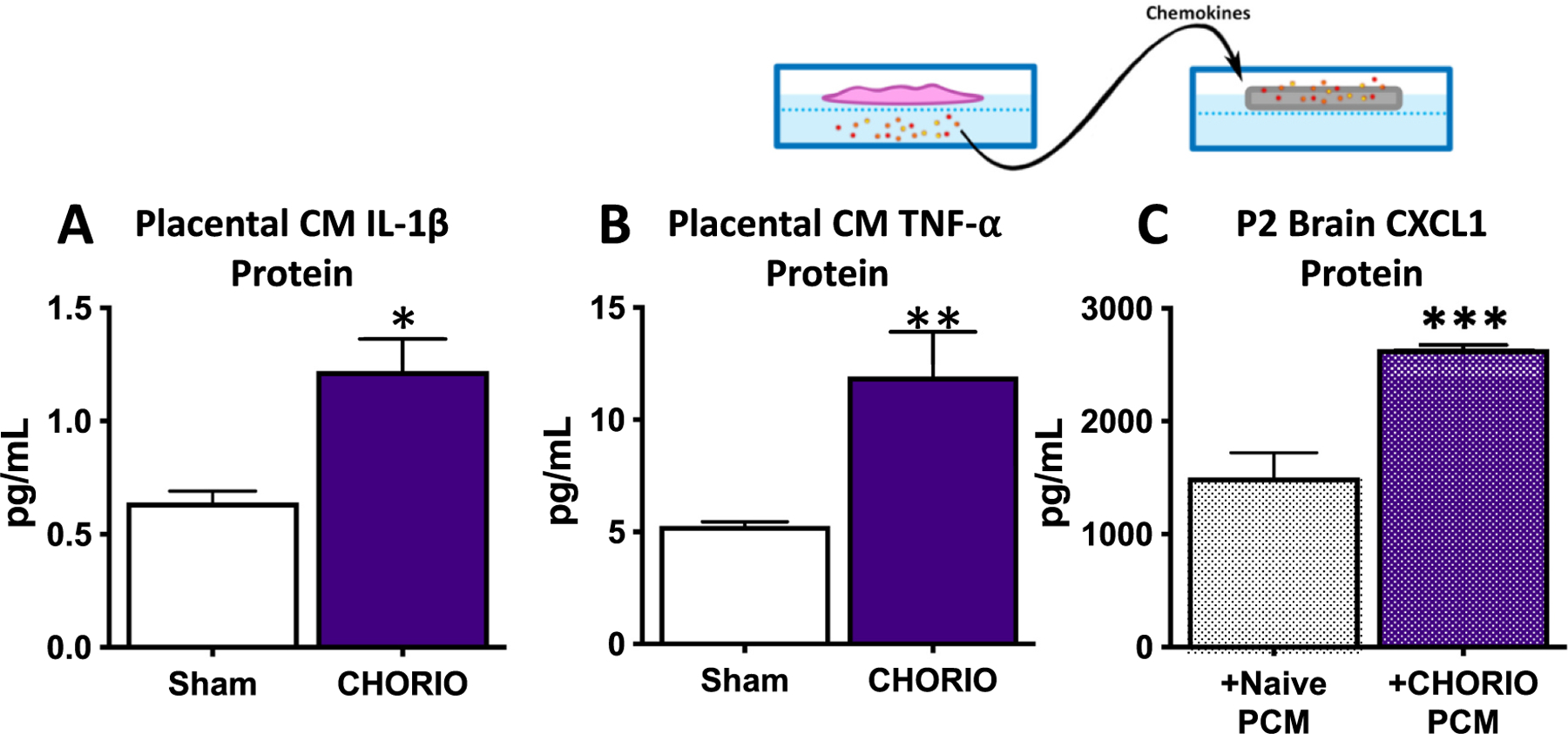
Placental-derived mediators drive neural injury in CHORIO. Sham or CHORIO placental explants were cultured on inserts and placental conditioned media (PCM) assayed for toxic mediators and inflammatory drivers (A-B). CHORIO placentas secrete more IL-1β (A), and TNF-α (B) compared to sham placentas. To determine the effect of CHORIO PCM on the developing brain, PCM from naive or CHORIO placental explants was cultured with postnatal day 2 (P2) brain slices (C). PCM from CHORIO placental explants induced high levels of CXCL1 in P2 brain slices. (n = 4–5, two independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001).
Fig. 2.
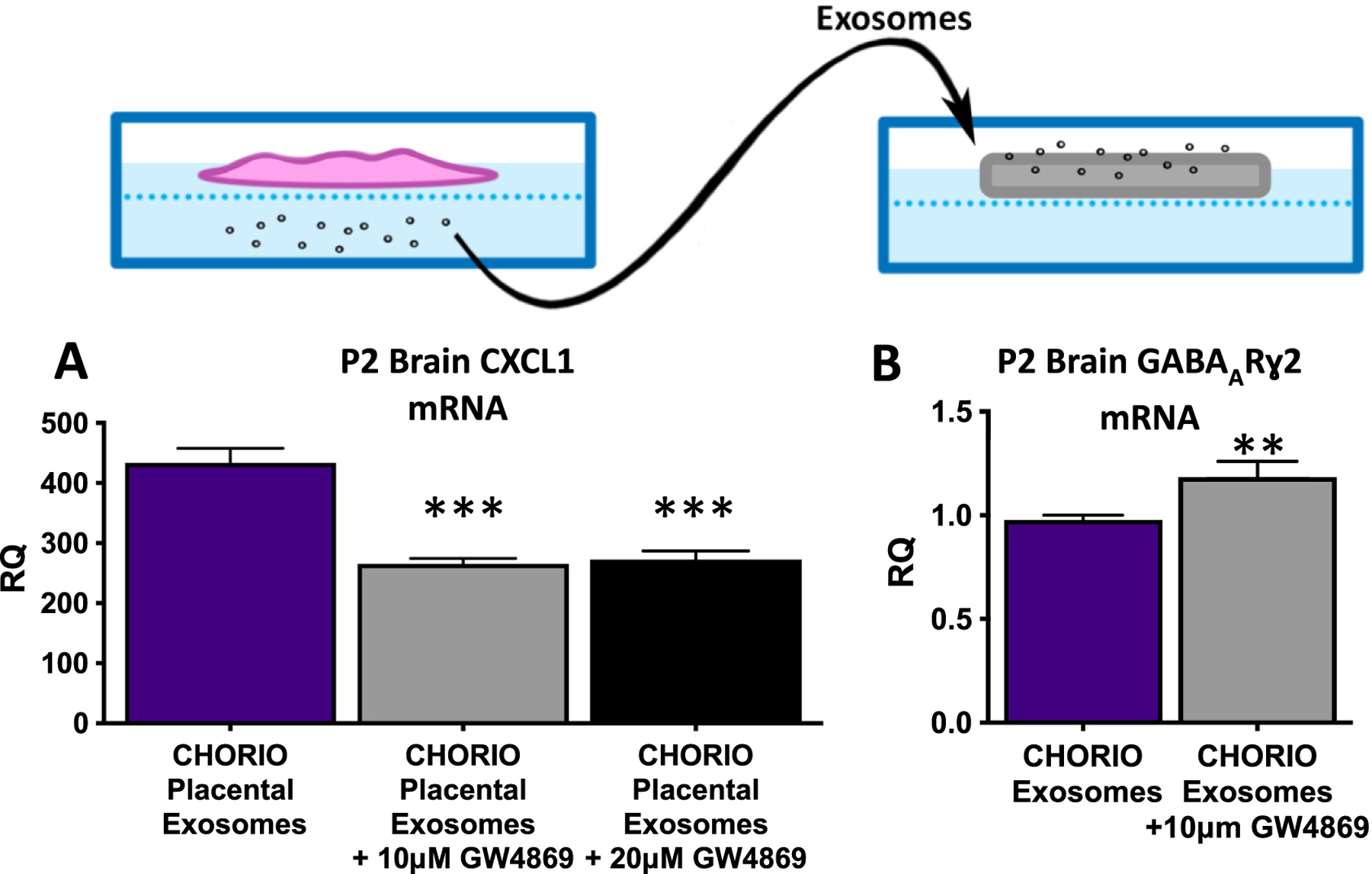
Placental-derived exosomes drive neural injury in CHORIO. To confirm whether cytokines themselves or placental-derived exosomes were able to induce brain CXCL1 expression, exosomes were isolated from CHORIO PCM (A). Exosomes from CHORIO placentae elevated CXCL1 mRNA in P2 brain slices (A - purple bar). Notably, cerebral CXCL1 mRNA levels were reduced by blockade of exosome release using the exosome generation inhibitor GW4869 (A - gray and black bars). Blockade of placental exosome generation also improved neural cell health and preserved developmental trajectory (B). Specifically, GABAARɣ2 mRNA was increased in P2 brain slices cultured with exosome depleted PCM compared to exosome containing PCM (B). (n = 4–5, two independent experiments, *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
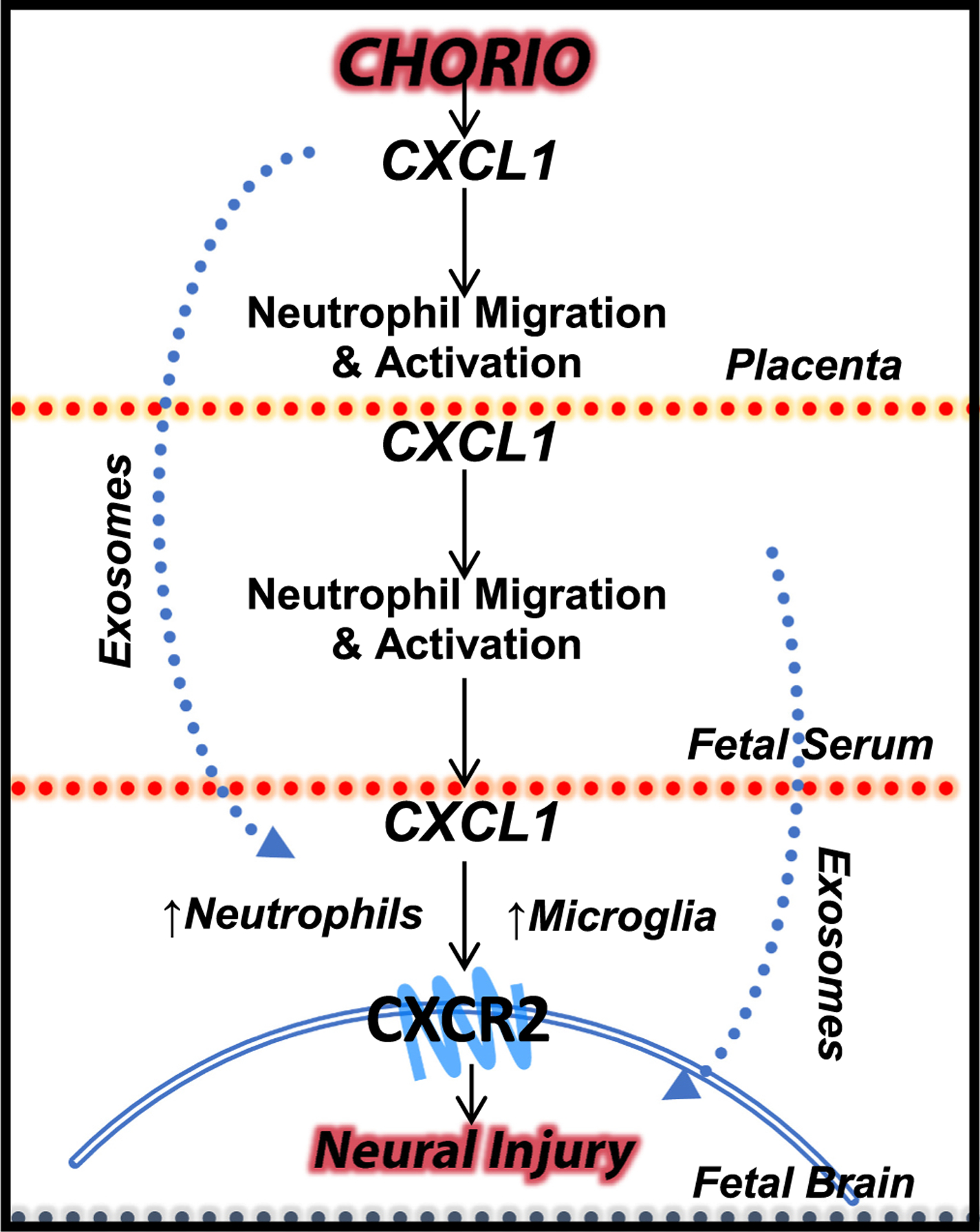
Placental-derived exosomes as a plausible pathophysiological mechanism in perinatal brain injury.
9. Exosomes in neuroinflammation
Exosomes have also been implicated in other contexts of systemic and neuroinflammation that would suggest a possible role in perinatal brain injury. During pathological states, peripheral exosomes, selectively containing aberrant miRNA and neurotoxic cargo, can travel to neural cells and repress or dysregulate specific gene expression (Gupta & Pulliam, 2014). Trafficked exosomes may also induce neuroinflammation by the stimulation of recipient cells to secrete pro-inflammatory cytokines. For example, the increased exosomes isolated from serum of children with autism spectrum disorder, stimulated microglia to produce the proinflammatory cytokine IL-1β (Tsilioni & Theoharides, 2018). In an animal model of BPD, the alveolar-derived exosomes from neonatal rats exposed to hyperoxia not only induced the pathological lung changes of BPD, they also were found to cross the blood-brain barrier and induce neuroinflammatory damage (Ali et al., 2021). Locally, activated monocytes and macrophages can cross the blood brain barrier and secrete exosomes, which are internalized by neural cells such as astrocytes. Exosomes then cause neuronal dysfunction via the release of dysregulated proteins and mRNA (Gupta & Pulliam, 2014).
Exosomes are also involved in neural development, regeneration, and disease. LPS-induced neutrophil exosomes secreted increased miRNA-122, which reduced occludin expression leading to decreased integrity and increased permeability of the blood brain barrier (Li et al., 2021). In an in vitro and in vivo model of glaucoma, exosomes from retinal microglia exposed to elevated hydrostatic pressure were shown to sustain inflammatory activation of other retinal microglia and induce retinal neurodegeneration (Aires et al., 2020). Healthy mice injected with exosomes from serum of LPS-challenged mice induced microgliosis and astrogliosis in response to elevated pro-inflammatory cytokine and miRNA expression (Li et al., 2018). Microglia and astrocytes themselves also release their own exosomes and have been implicated in neuroinflammation and neurodegeneration (Andjus et al., 2020; Gharbi et al., 2020). Astrocyte-derived exosomes have been shown to induce neuroinflammation via toll-like receptor 4 (TLR4) activation (Ibáñez et al., 2019). Exosomes isolated from activated microglia induced dopaminergic neurodegeneration, akin to the pathophysiology of Parkinson’s disease (Tsutsumi et al., 2019). Another study showed that exosomes released from microglia exposed to inflammatory stimuli transiently fuse with neurons and transfect miRNA, leading to alterations of synaptic structure and decreased dendritic spines (Prada et al., 2018). This demonstrates a mechanism by which exosome-mediated, intercellular neuroinflammation and neurodegeneration propagates. Additionally, microglia-derived exosomes have been implicated in Alzheimer’s disease and amyotrophic lateral sclerosis, by storing and transmitting pathogenic proteins including amyloid β peptides and protein Tau, as well as mutated superoxide dismutase 1, respectively (Andjus et al., 2020; Kim et al., 2020). In veterans with a remote history of traumatic brain injury and cognitive impairment, CNS-derived exosomes demonstrated increased concentrations of inflammatory cytokines IL-6 and TNF-α, decades from the original injury (Peltz et al., 2020). Similarly, a recent study demonstrated that exposure to prenatal inflammation using a preclinical model of CP led to a chronically hyper-reactive and maladaptive immune system that persisted into adulthood (Kitase et al., 2021). Immune dysfunction consisted of elevated pro-inflammatory cytokines including IL-1β, TNF-α, IL-6, MCP-1 and CXCL1 that continued until juvenile equivalent ages, while primed peripheral blood mononuclear cells remained hyper-reactive into adulthood with concomitant pro-inflammatory secretome (Kitase et al., 2021). This study suggests that inutero inflammation not only leads to acute perinatal brain injury but may continue lifelong in those with CP by exacerbating present damage and sensitizing the brain to persistent injury (Kitase et al., 2021). Importantly these data match studies in humans showing altered inflammatory responses that persist for at least 6–14 years. Like the data supporting that sustained changes in immune response, and long-term alterations in molecular and cellular neuroinflammation exist in survivors of perinatal brain injury, future work should similarly address sustained changes in exosome function, composition, and cargo in children with CP and other forms of perinatal brain injury, especially those with prominent inflammatory pathophysiology.
10. Conclusion
Placental-driven mediators such as chemokines, cytokines, and exosomes have been implicated in neuroinflammation and may contribute to perinatal brain injury in the setting of chorioamnionitis. Exosomes are integral to intercellular communication within the maternal-placental-fetal axis and have been shown to traffic cargo that promotes systemic and neuroinflammation. Evidence from pathological processes such as preeclampsia, gestational diabetes, and traumatic brain injury suggest that exosomes propagate and sustain the inflammatory cascade well beyond the acute state. This suggests that the brain injuries and neurodevelopmental disorders with a component of exosome-mediated inflammation as part of their pathophysiology may have chronic adverse implications in disorders such as cerebral palsy. The evidence reviewed here highlights the need to investigate exosomes isolated pre- and postnatally, as well as in older children with known brain injuries or neurodevelopmental disorders to identify new diagnostics, biomarkers, and potential therapeutic targets.
Acknowledgement
This work was supported by the National Institutes of Health R01HL139492 to LLJ.
References
- Aires ID, Ribeiro-Rodrigues T, Boia R, et al. , 2020. Exosomes derived from microglia exposed to elevated pressure amplify the neuroinflammatory response in retinal cells. Glia 68, 2705–2724. [DOI] [PubMed] [Google Scholar]
- Aisen ML, Kerkovich D, Mast J, et al. , 2011. Cerebral palsy: clinical care and neurological rehabilitation. Lancet Neurol 10, 844–852. [DOI] [PubMed] [Google Scholar]
- Ali A, Zambrano R, Duncan MR, et al. , 2021. Hyperoxia-activated circulating extracellular vesicles induce lung and brain injury in neonatal rats. Sci. Rep 11, 8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ, 2011. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol 29, 341–345. [DOI] [PubMed] [Google Scholar]
- Anand PK, 2010. Exosomal membrane molecules are potent immune response modulators. Commun Integr Biol 3, 405–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anblagan D, Pataky R, Evans MJ, et al. , 2016. Association between preterm brain injury and exposure to chorioamnionitis during fetal life. Sci. Rep 6, 37932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andjus P, Kosanović M, Milićević K, et al. , 2020. Extracellular vesicles as innovative tool for diagnosis, regeneration and protection against neurological damage. Int. J. Mol. Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atay S, Gercel-Taylor C, Suttles J, Mor G, Taylor DD, 2011. Trophoblast-derived exosomes mediate monocyte recruitment and differentiation. Am. J. Reprod. Immunol 65, 65–77. [DOI] [PubMed] [Google Scholar]
- Ayubi E, Sarhadi S, Mansori K, 2021. Maternal infection during pregnancy and risk of cerebral palsy in children: a systematic review and meta-analysis. J. Child Neurol 36, 385–402. [DOI] [PubMed] [Google Scholar]
- Biró O, Fóthi Á, Alasztics B, Nagy B, Orbán TI, Rigó J Jr., 2019. Circulating exosomal and argonaute-bound microRNAs in preeclampsia. Gene 692, 138–144. [DOI] [PubMed] [Google Scholar]
- Brochu ME, Girard S, Lavoie K, Sébire G, 2011. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: an experimental study. J. Neuroinflammation 8, 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkova EE, Sedykh SE, Nevinsky GA, 2021. Human placenta exosomes: biogenesis, isolation, composition, and prospects for use in diagnostics. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buser JR, Maire J, Riddle A, et al. , 2012. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann. Neurol 71, 93–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappelletti M, Presicce P, Kallapur SG, 2020. Immunobiology of acute chorioamnionitis. Front. Immunol 11, 649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang X, Yao J, He Q, Liu M, Duan T, Wang K, 2018. Exosomes from women with preeclampsia induced vascular dysfunction by delivering sFlt (soluble Fms-like tyrosine kinase)-1 and sEng (soluble endoglin) to endothelial cells. Hypertension 72, 1381–1390. [DOI] [PubMed] [Google Scholar]
- Chau V, McFadden DE, Poskitt KJ, Miller SP, 2014. Chorioamnionitis in the pathogenesis of brain injury in preterm infants. Clin. Perinatol 41, 83–103. [DOI] [PubMed] [Google Scholar]
- Czernek L, Düchler M, 2020. Exosomes as messengers between mother and fetus in pregnancy. Int. J. Mol. Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann O, Leviton A, 1997. Maternal intrauterine infection, cytokines, and brain damage in the preterm newborn. Pediatr. Res 42, 1–8. [DOI] [PubMed] [Google Scholar]
- Dammann O, Leviton A, 2014. Intermittent or sustained systemic inflammation and the preterm brain. Pediatr. Res 75, 376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammann O, Allred EN, Fichorova RN, Kuban K, O’Shea TM, Leviton A, 2016. Duration of systemic inflammation in the first postnatal month among infants born before the 28th week of gestation. Inflammation 39, 672–677. [DOI] [PubMed] [Google Scholar]
- Delorme-Axford E, Donker RB, Mouillet JF, et al. , 2013. Human placental trophoblasts confer viral resistance to recipient cells. Proc. Natl. Acad. Sci. U. S. A 110, 12048–12053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Familari M, Cronqvist T, Masoumi Z, Hansson SR, 2017. Placenta-derived extracellular vesicles: their cargo and possible functions. Reprod. Fertil. Dev 29, 433–447. [DOI] [PubMed] [Google Scholar]
- Gharbi T, Zhang Z, Yang GY, 2020. The function of astrocyte mediated extracellular vesicles in central nervous system diseases. Front. Cell Dev. Biol 8, 568889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles FH, Leviton A, 2020. Neonatal white matter damage and the fetal inflammatory response. Semin. Fetal Neonatal Med 25, 101111. [DOI] [PubMed] [Google Scholar]
- Girard S, Sébire G, Kadhim H, 2010. Proinflammatory orientation of the interleukin 1 system and downstream induction of matrix metalloproteinase 9 in the pathophysiology of human perinatal white matter damage. J. Neuropathol. Exp. Neurol 69, 1116–1129. [DOI] [PubMed] [Google Scholar]
- Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM, 1998. The fetal inflammatory response syndrome. Am. J. Obstet. Gynecol 179, 194–202. [DOI] [PubMed] [Google Scholar]
- Gomez-Lopez N, Motomura K, Miller D, Garcia-Flores V, Galaz J, Romero R, 2019. Inflammasomes: their role in normal and complicated pregnancies. J. Immunol 203, 2757–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotsch F, Romero R, Kusanovic JP, et al. , 2007. The fetal inflammatory response syndrome. Clin. Obstet. Gynecol 50, 652–683. [DOI] [PubMed] [Google Scholar]
- Gupta A, Pulliam L, 2014. Exosomes as mediators of neuroinflammation. J. Neuroinflammation 11, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley EE, Sheller-Miller S, Saade G, et al. , 2018. Amnion epithelial cell-derived exosomes induce inflammatory changes in uterine cells. Am. J. Obstet. Gynecol 219, 478.e1-.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hecht JL, Fichorova RN, Tang VF, Allred EN, McElrath TF, Leviton A, 2011. Relationship between neonatal blood protein concentrations and placenta histologic characteristics in extremely low GA newborns. Pediatr. Res 69, 68–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedlund M, Stenqvist AC, Nagaeva O, et al. , 2009. Human placenta expresses and secretes NKG2D ligands via exosomes that down-modulate the cognate receptor expression: evidence for immunosuppressive function. J. Immunol 183, 340–351. [DOI] [PubMed] [Google Scholar]
- Higgins RD, Saade G, Polin RA, et al. , 2016. Evaluation and management of women and newborns with a maternal diagnosis of chorioamnionitis: summary of a workshop. Obstet. Gynecol 127, 426–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holder B, Jones T, Sancho Shimizu V, et al. , 2016. Macrophage exosomes induce placental inflammatory cytokines: a novel mode of maternal-placental messaging. Traffic 17, 168–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibáñez F, Montesinos J, Ureña-Peralta JR, Guerri C, Pascual M, 2019. TLR4 participates in the transmission of ethanol-induced neuroinflammation via astrocyte-derived extracellular vesicles. J. Neuroinflammation 16, 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Corbett CJ, Berglass J, et al. , 2014. Complex pattern of interaction between in utero hypoxia-ischemia and intra-amniotic inflammation disrupts brain development and motor function. J. Neuroinflammation 11, 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzie LL, Winer JL, Maxwell JR, Chan LA, Robinson S, 2015. Modeling encephalopathy of prematurity using prenatal hypoxia-ischemia with intra-amniotic lipopolysaccharide in rats. J. Vis. Exp (105), 53196 10.3791/53196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Menon R, 2018. Placental exosomes: a proxy to understand pregnancy complications. Am. J. Reprod. Immunol 79, e12788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung E, Romero R, Yeo L, et al. , 2020. The fetal inflammatory response syndrome: the origins of a concept, pathophysiology, diagnosis, and obstetrical implications. Semin. Fetal Neonatal Med 25, 101146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallapur SG, Presicce P, Rueda CM, Jobe AH, Chougnet CA, 2014. Fetal immune response to chorioamnionitis. Semin. Reprod. Med 32, 56–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R, LeBleu VS, 2020. The biology, function, and biomedical applications of exosomes. Science 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaukola T, Herva R, Perhomaa M, et al. , 2006. Population cohort associating chorioamnionitis, cord inflammatory cytokines and neurologic outcome in very preterm, extremely low birth weight infants. Pediatr. Res 59, 478–483. [DOI] [PubMed] [Google Scholar]
- Keelan JA, 2018. Intrauterine inflammatory activation, functional progesterone withdrawal, and the timing of term and preterm birth. J. Reprod. Immunol 125, 89–99. [DOI] [PubMed] [Google Scholar]
- Kemp MW, 2014. Preterm birth, intrauterine infection, and fetal inflammation. Front. Immunol 5, 574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CJ, Romero R, Chaemsaithong P, Chaiyasit N, Yoon BH, Kim YM, 2015. Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance. Am. J. Obstet. Gynecol 213, S29–S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Otgontenger U, Jamsranjav A, Kim SS, 2020. Deleterious alteration of glia in the brain of Alzheimer’s disease. Int. J. Mol. Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitase Y, Chin EM, Ramachandra S, et al. , 2021. Sustained peripheral immune hyper-reactivity (SPIHR): an enduring biomarker of altered inflammatory responses in adult rats after perinatal brain injury. J. Neuroinflammation 18, 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli S, Ranjan S, Hoffmann J, et al. , 2016. Maternal extracellular vesicles and platelets promote preeclampsia via inflammasome activation in trophoblasts. Blood 128, 2153–2164. [DOI] [PubMed] [Google Scholar]
- Kolaczkowska E, Kubes P, 2013. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol 13, 159–175. [DOI] [PubMed] [Google Scholar]
- Komaki M, Numata Y, Morioka C, et al. , 2017. Exosomes of human placenta-derived mesenchymal stem cells stimulate angiogenesis. Stem Cell Res Ther 8, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewski SJ, Romero R, Cortez J, et al. , 2014. A “multi-hit” model of neonatal white matter injury: cumulative contributions of chronic placental inflammation, acute fetal inflammation and postnatal inflammatory events. J. Perinat. Med 42, 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuban KC, O’Shea TM, Allred EN, et al. , 2015. The breadth and type of systemic inflammation and the risk of adverse neurological outcomes in extremely low gestation newborns. Pediatr. Neurol 52, 42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuypers E, Ophelders D, Jellema RK, Kunzmann S, Gavilanes AW, Kramer BW, 2012. White matter injury following fetal inflammatory response syndrome induced by chorioamnionitis and fetal sepsis: lessons from experimental ovine models. Early Hum. Dev 88, 931–936. [DOI] [PubMed] [Google Scholar]
- Lee SM, Park JW, Kim BJ, et al. , 2013. Acute histologic chorioamnionitis is a risk factor for adverse neonatal outcome in late preterm birth after preterm premature rupture of membranes. PLoS One 8, e79941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A, Allred EN, Kuban KC, et al. , 2010. Microbiologic and histologic characteristics of the extremely preterm infant’s placenta predict white matter damage and later cerebral palsy. The ELGAN study. Pediatr. Res 67, 95–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A, Fichorova R, Yamamoto Y, et al. , 2011a. Inflammation-related proteins in the blood of extremely low gestational age newborns. The contribution of inflammation to the appearance of developmental regulation. Cytokine 53, 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A, Kuban K, O’Shea TM, et al. , 2011b. The relationship between early concentrations of 25 blood proteins and cerebral white matter injury in preterm newborns: the ELGAN study. J. Pediatr 158, 897–903.e1–5. [DOI] [PubMed] [Google Scholar]
- Leviton A, Allred EN, Dammann O, et al. , 2013. Systemic inflammation, intraventricular hemorrhage, and white matter injury. J. Child Neurol 28, 1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leviton A, Allred EN, Fichorova RN, Kuban KC, Michael O’Shea T, Dammann O, 2016. Systemic inflammation on postnatal days 21 and 28 and indicators of brain dysfunction 2years later among children born before the 28th week of gestation. Early Hum. Dev 93, 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JJ, Wang B, Kodali MC, et al. , 2018. In vivo evidence for the contribution of peripheral circulating inflammatory exosomes to neuroinflammation. J. Neuroinflammation 15, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Nong A, Huang Z, et al. , 2021. Exosomes containing miR-122–5p secreted by LPS-induced neutrophils regulate the apoptosis and permeability of brain microvascular endothelial cells by targeting OCLN. Am. J. Transl. Res 13, 4167–4181. [PMC free article] [PubMed] [Google Scholar]
- Lu HY, Zhang Q, Wang QX, Lu JY, 2016. Contribution of histologic chorioamnionitis and fetal inflammatory response syndrome to increased risk of brain injury in infants with preterm premature rupture of membranes. Pediatr. Neurol 61, 94–8.e1. [DOI] [PubMed] [Google Scholar]
- Luo SS, Ishibashi O, Ishikawa G, et al. , 2009. Human villous trophoblasts express and secrete placenta-specific microRNAs into maternal circulation via exosomes. Biol. Reprod 81, 717–729. [DOI] [PubMed] [Google Scholar]
- Madsen-Bouterse SA, Romero R, Tarca AL, et al. , 2010. The transcriptome of the fetal inflammatory response syndrome. Am. J. Reprod. Immunol 63, 73–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell JR, Denson JL, Joste NE, Robinson S, Jantzie LL, 2015. Combined in utero hypoxia-ischemia and lipopolysaccharide administration in rats induces chorioamnionitis and a fetal inflammatory response syndrome. Placenta 36, 1378–1384. [DOI] [PubMed] [Google Scholar]
- Mittal P, Romero R, Kusanovic JP, et al. , 2008. CXCL6 (granulocyte chemotactic protein-2): a novel chemokine involved in the innate immune response of the amniotic cavity. Am. J. Reprod. Immunol 60, 246–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsivais LA, Sheller-Miller S, Russell W, et al. , 2020. Fetal membrane extracellular vesicle profiling reveals distinct pathways induced by infection and inflammation in vitro. Am. J. Reprod. Immunol 84, e13282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay S, Puopolo KM, Hansen NI, et al. , 2020. Impact of early-onset sepsis and antibiotic use on death or survival with neurodevelopmental impairment at 2 years of age among extremely preterm infants. J. Pediatr 221, 39–46.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea TM, Allred EN, Kuban KC, et al. , 2012. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. J. Pediatr 160, 395–401.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papagianis PC, Ahmadi-Noorbakhsh S, Lim R, et al. , 2021. The effect of human amnion epithelial cells on lung development and inflammation in preterm lambs exposed to antenatal inflammation. PLoS One 16, e0253456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peltz CB, Kenney K, Gill J, Diaz-Arrastia R, Gardner RC, Yaffe K, 2020. Blood biomarkers of traumatic brain injury and cognitive impairment in older veterans. Neurology 95 e1126–e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay P, Moodley K, Moodley J, Mackraj I, 2017. Placenta-derived exosomes: potential biomarkers of preeclampsia. Int. J. Nanomedicine 12, 8009–8023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prada I, Gabrielli M, Turola E, et al. , 2018. Glia-to-neuron transfer of miRNAs via extracellular vesicles: a new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol 135, 529–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radnaa E, Richardson LS, Sheller-Miller S, et al. , 2021. Extracellular vesicle mediated feto-maternal HMGB1 signaling induces preterm birth. Lab Chip 21, 1956–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record M, 2014. Intercellular communication by exosomes in placenta: a possible role in cell fusion? Placenta 35, 297–302. [DOI] [PubMed] [Google Scholar]
- Redline RW, O’Riordan MA, 2000. Placental lesions associated with cerebral palsy and neurologic impairment following term birth. Arch. Pathol. Lab. Med 124, 1785–1791. [DOI] [PubMed] [Google Scholar]
- Redline RW, Faye-Petersen O, Heller D, Qureshi F, Savell V, Vogler C, 2003. Amniotic infection syndrome: nosology and reproducibility of placental reaction patterns. Pediatr. Dev. Pathol 6, 435–448. [DOI] [PubMed] [Google Scholar]
- Roberts DJ, Celi AC, Riley LE, et al. , 2012. Acute histologic chorioamnionitis at term: nearly always noninfectious. PLoS One 7, e31819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Espinoza J, Gonçalves LF, Kusanovic JP, Friel L, Hassan S, 2007. The role of inflammation and infection in preterm birth. Semin. Reprod. Med 25, 21–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Miranda J, Chaiworapongsa T, et al. , 2014. Prevalence and clinical significance of sterile intra-amniotic inflammation in patients with preterm labor and intact membranes. Am. J. Reprod. Immunol 72, 458–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero R, Chaemsaithong P, Korzeniewski SJ, et al. , 2016. Clinical chorioamnionitis at term II: the intra-amniotic inflammatory response. J. Perinat. Med 44, 5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan E, Eves D, Menon PJ, et al. , 2020. Histological chorioamnionitis is predicted by early infant C-reactive protein in preterm infants and correlates with neonatal outcomes. Acta Paediatr 109, 720–727. [DOI] [PubMed] [Google Scholar]
- Sabapatha A, Gercel-Taylor C, Taylor DD, 2006. Specific isolation of placenta-derived exosomes from the circulation of pregnant women and their immunoregulatory consequences. Am. J. Reprod. Immunol 56, 345–355. [DOI] [PubMed] [Google Scholar]
- Sadik CD, Kim ND, Luster AD, 2011. Neutrophils cascading their way to inflammation. Trends Immunol 32, 452–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas AA, Faye-Petersen OM, Sims B, et al. , 2013. Histological characteristics of the fetal inflammatory response associated with neurodevelopmental impairment and death in extremely preterm infants. J. Pediatr 163, 652–7.e1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon C, Rice GE, 2017. Role of exosomes in placental homeostasis and pregnancy disorders. Prog. Mol. Biol. Transl. Sci 145, 163–179. [DOI] [PubMed] [Google Scholar]
- Salomon C, Ryan J, Sobrevia L, et al. , 2013. Exosomal signaling during hypoxia mediates microvascular endothelial cell migration and vasculogenesis. PLoS One 8, e68451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon C, Scholz-Romero K, Sarker S, et al. , 2016. Gestational diabetes mellitus is associated with changes in the concentration and bioactivity of placenta-derived exosomes in maternal circulation across gestation. Diabetes 65, 598–609. [DOI] [PubMed] [Google Scholar]
- Shah KB, Chernausek SD, Teague AM, Bard DE, Tryggestad JB, 2021. Maternal diabetes alters microRNA expression in fetal exosomes, human umbilical vein endothelial cells and placenta. Pediatr. Res 89, 1157–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shatrov JG, Birch SCM, Lam LT, Quinlivan JA, McIntyre S, Mendz GL, 2010. Chorioamnionitis and cerebral palsy: a meta-analysis. Obstet. Gynecol 116, 387–392. [DOI] [PubMed] [Google Scholar]
- Sheller S, Papaconstantinou J, Urrabaz-Garza R, et al. , 2016. Amnion-epithelial-cell-derived exosomes demonstrate physiologic state of cell under oxidative stress. PLoS One 11, e0157614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheller-Miller S, Choi K, Choi C, Menon R, 2019a. Cyclic-recombinase-reporter mouse model to determine exosome communication and function during pregnancy. Am. J. Obstet. Gynecol 221, 502.e1-.e12. [DOI] [PubMed] [Google Scholar]
- Sheller-Miller S, Trivedi J, Yellon SM, Menon R, 2019b. Exosomes cause preterm birth in mice: evidence for paracrine signaling in pregnancy. Sci. Rep 9, 608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Li Y, Li R, et al. , 2018. Placenta-associated serum exosomal miR-155 derived from patients with preeclampsia inhibits eNOS expression in human umbilical vein endothelial cells. Int. J. Mol. Med 41, 1731–1739. [DOI] [PubMed] [Google Scholar]
- Shevell A, Wintermark P, Benini R, Shevell M, Oskoui M, 2014. Chorioamnionitis and cerebral palsy: lessons from a patient registry. Eur. J. Paediatr. Neurol 18, 301–307. [DOI] [PubMed] [Google Scholar]
- Shi Z, Ma L, Luo K, et al. , 2017a. Chorioamnionitis in the development of cerebral palsy: a meta-analysis and systematic review. Pediatrics 139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Zhao L, Cai W, et al. , 2017b. Maternal exosomes in diabetes contribute to the cardiac development deficiency. Biochem. Biophys. Res. Commun 483, 602–608. [DOI] [PubMed] [Google Scholar]
- Siahanidou T, Spiliopoulou C, 2020. Pharmacological neuroprotection of the preterm brain: current evidence and perspectives. Am. J. Perinatol 10.1055/s-0040-1716710. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Sorg AL, von Kries R, Klemme M, et al. , 2020. Risk factors for perinatal arterial ischaemic stroke: a large case-control study. Dev. Med. Child Neurol 62, 513–520. [DOI] [PubMed] [Google Scholar]
- Stenqvist AC, Nagaeva O, Baranov V, Mincheva-Nilsson L, 2013. Exosomes secreted by human placenta carry functional Fas ligand and TRAIL molecules and convey apoptosis in activated immune cells, suggesting exosome-mediated immune privilege of the fetus. J. Immunol 191, 5515–5523. [DOI] [PubMed] [Google Scholar]
- Tang Q, Zhang L, Li H, Shao Y, 2019. The fetal inflammation response syndrome and adverse neonatal outcomes: a meta-analysis. J. Matern. Fetal Neonatal Med 1–13. [DOI] [PubMed] [Google Scholar]
- Tannetta DS, Dragovic RA, Gardiner C, Redman CW, Sargent IL, 2013. Characterisation of syncytiotrophoblast vesicles in normal pregnancy and pre-eclampsia: expression of Flt-1 and endoglin. PLoS One 8, e56754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecchio C, Micheletti A, Cassatella MA, 2014. Neutrophil-derived cytokines: facts beyond expression. Front. Immunol 5, 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomi G, Surbek D, Haesler V, Joerger-Messerli M, Schoeberlein A, 2019. Exosomes derived from umbilical cord mesenchymal stem cells reduce microglia-mediated neuroinflammation in perinatal brain injury. Stem Cell Res Ther 10, 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilioni I, Theoharides TC, 2018. Extracellular vesicles are increased in the serum of children with autism spectrum disorder, contain mitochondrial DNA, and stimulate human microglia to secrete IL-1β. J. Neuroinflammation 15, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi R, Hori Y, Seki T, et al. , 2019. Involvement of exosomes in dopaminergic neurodegeneration by microglial activation in midbrain slice cultures. Biochem. Biophys. Res. Commun 511, 427–433. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lotvall JO, 2007. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol 9, 654–659. [DOI] [PubMed] [Google Scholar]
- van Niel G, D’Angelo G, Raposo G, 2018. Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol 19, 213–228. [DOI] [PubMed] [Google Scholar]
- Venkatesh KK, Leviton A, Hecht JL, et al. , 2020. Histologic chorioamnionitis and risk of neurodevelopmental impairment at age 10 years among extremely preterm infants born before 28 weeks of gestation. Am. J. Obstet. Gynecol 223, 745.e1-.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ, 2009. Brain injury in premature infants: a complex amalgam of destructive and developmental disturbances. Lancet Neurol 8, 110–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpe JJ, Kinney HC, Jensen FE, Rosenberg PA, 2011. The developing oligodendrocyte: key cellular target in brain injury in the premature infant. Int. J. Dev. Neurosci 29, 423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YW, Colford JM Jr., 2000. Chorioamnionitis as a risk factor for cerebral palsy: a meta-analysis. Jama 284, 1417–1424. [DOI] [PubMed] [Google Scholar]
- Yap V, Perlman JM, 2020. Mechanisms of brain injury in newborn infants associated with the fetal inflammatory response syndrome. Semin. Fetal Neonatal Med 25, 101110. [DOI] [PubMed] [Google Scholar]
- Yellowhair TR, Noor S, Maxwell JR, et al. , 2018. Preclinical chorioamnionitis dysregulates CXCL1/CXCR2 signaling throughout the placental-fetal-brain axis. Exp. Neurol 301, 110–119. [DOI] [PubMed] [Google Scholar]
- Yoon BH, Jun JK, Romero R, et al. , 1997. Amniotic fluid inflammatory cytokines (interleukin-6, interleukin-1beta, and tumor necrosis factor-alpha), neonatal brain white matter lesions, and cerebral palsy. Am. J. Obstet. Gynecol 177, 19–26. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lai D, 2020. Application of human amniotic epithelial cells in regenerative medicine: a systematic review. Stem Cell Res Ther 11, 439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Jyoti A, Balakrishnan B, et al. , 2018. Trajectory of inflammatory and microglial activation markers in the postnatal rabbit brain following intrauterine endotoxin exposure. Neurobiol. Dis 111, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Zhang T, Sun D, et al. , 2021. Diagnostic value of dysregulated microribonucleic acids in the placenta and circulating exosomes in gestational diabetes mellitus. J. Diabetes Investig 12, 1490–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]


