Abstract
Objective
To evaluate the effectiveness and safety of TAS-102 monotherapy and combination therapy with bevacizumab in the treatment of metastatic colorectal cancer.
Methods
The PubMed, Web of Science, MEDLINE, and Cochrane Library databases were searched for the literature on TAS-102 treatment of metastatic colorectal cancer. Extracted data include median overall survival (mOS), median progression-free survival (mPFS), and the incidence of adverse events for meta-analysis.
Results
Our study found that the mOS of patients treated with TAS-102 monotherapy was 6.95 (95% CI: 6.26-7.72) months and the mPFS was 2.53 (95% CI: 2.31-2.78) months. The mOS in patients treated by TAS-102 combined with bevacizumab was 10.41 (95% CI: 8.40-12.89) months, and the mPFS is 4.35 (95% CI: 3.05-6.20) months. In the control experiment, the patients' mOS and mPFS were improved. TAS-102+B vs. TAS-102 (OR = 0.41, 95% CI: 0.18-0.93; OR = 0.72, 95% CI: 0.63-0.83) and TAS-102 vs. placebo (OR = 0.44, 95% CI: 0.29-0.67; OR = 0.51, 95% CI: 0.42-0.62) were studied to actively prevent the occurrence of neutropenia, leukopenia, febrile neutropenia, anemia, and vomiting.
Conclusion
TAS-102 monotherapy and combination therapy with bevacizumab can significantly improve the survival of patients and prevent specific adverse events from happening.
1. Introduction
By 2020, it is estimated that colorectal cancer is the cause of 935,000 cancer-related deaths worldwide, accounting for 9% of all cancer deaths [1]. In the initial diagnosis, approximately 25% of colorectal cancer patients have concurrent metastatic disease, and more than half of the patients are diagnosed as metastases [2, 3]. Despite advances in the treatment of metastatic CRC (metastatic colorectal cancer), the survival rate is still poor. And the expected survival period without effective drug treatment is about 6 months [4–6].
TAS-102 (trifluridine/tipiracil) is an oral anticancer drug containing a thymidine analogue (trifluridine). It is composed of active cytotoxic component FTD and effective thymidine phosphorylase inhibitor TPI hydrochloride. The molar ratio is 1 : 0.5 [5]. FTD is the active cytotoxic component of the drug. TPI can prevent thymidine phosphorylase from rapidly degrading FTD into the inactive form [7, 8]. FTD/TPI is established as the third-line treatment for metastatic colorectal cancer. According to the results of the international phase III RECOURSE study, the study reported the significant benefits of FTD/TPI compared with placebo in terms of overall survival (OS) and acceptable security conditions [9, 10].
The efficacy and safety of FTD/TPI monotherapy in adults with refractory mCRC was first demonstrated in a Japanese phase II trial by Yoshino et al. [5] and later in the pivotal phase III RECOURSE trial [4]. In these two studies, TAS-102 showed good effectiveness, significantly improving median overall survival (mOS) and median progression-free survival (mPFS). TAS-102 combined with bevacizumab had good effectiveness in the treatment of metastatic colorectal cancer, while reducing the incidence of adverse events [11].
Regorafenib and TAS-102 are both considered new treatment options for salvage-line therapy. A meta-analysis showed similar effectiveness of the two drugs, but the occurrence of adverse events may be different [12]. The main goal of clinical trials is to establish the effectiveness and safety of the drug in a carefully selected group of patients. However, there are still differences from real-world applications. The actual application of TAS-102 needs more attention. This study conducted a meta-analysis of clinical trials in the practical application of TAS-102 and compared the safety and effectiveness of drugs in controlled trials and uncontrolled trials.
2. Materials and Methods
2.1. Search Strategy
PubMed, MEDLINE, Web of Science, and Cochrane databases were searched for eligible publications. The following keywords were used: “metastatic colorectal cancer” AND “TAS-102” OR “FTD/TPI.” There is no time limit for searching until the final search date on May 31, 2021. In addition, the reference list of applicable studies was manually checked for inclusion in other articles. Two researchers jointly completed this search process.
2.2. Inclusion and Exclusion
Inclusion criteria are as follows:
Patients who participated in the study and who were diagnosed with metastatic colorectal cancer
Clinical trials or prospective/retrospective cohort series studies
TAS-102 monotherapy or combined therapy with bevacizumab
Studies that reported the prognosis of patients after receiving treatment, with at least one of mOS and mPFS or objective response rate (ORR), disease control rate (DCR), and adverse drug reaction (ADR)
Exclusion criteria are as follows:
Negative diagnosis or diagnosis mixed with other influential diseases
Therapies that included other biological agents or chemotherapy
Inconsistent patient baseline data
Unobtained full-text articles or unavailable data
Animal experiments, reviews, abstracts, reviews, and reports
2.3. Data Extraction and Quality Assessment
Two researchers independently extracted relevant information from each study: first author, year of publication, demographic characteristics of participants including age and gender, ECOG performance status, (K) RAS status, grouping scheme, sample size, median OS, median PFS, HR, and the incidence of grade ≥ 3 AEs. We downloaded the full text. If in doubt, ask the original author for help. The Newcastle-Ottawa Quality Assessment Scale is used to evaluate the quality of the included controlled trials. The total score is 9 points, and scores above 5 are included in the meta-analysis. However, for the included one-arm experiment, the first 8 items of the MINORS item were selected for quality evaluation. Each item is 2 points, and the total score is 16 points, and studies with 10 points or more enter our research.
2.4. Statistical Analysis
Based on the recommendations of the Cochrane Collaboration, we conducted quantitative synthesis of the indicators included in the study. If I2 ≤ 50% and P > 0.01, heterogeneity was considered to exist, and then a fixed-effects model would be implemented; otherwise, a random-effects model would be performed [13]. If the data were accurate, subgroup analysis based on baseline, interventions, and comparators and/or sensitivity analysis that eliminated studies one by one would be conducted, if appropriate, to explore the source of heterogeneity. Otherwise, we would interpret the result carefully. The small sample effect size and publication bias were detected by funnel plots and statistical tests, respectively [14].
3. Results
3.1. The Characteristics of the Included Studies
855 studies were retrieved. Two investigators screened and included 25 studies [4, 5, 9–11, 15–34]. 14 controlled experiments and 11 single-arm experiments were included. The average age of 3780 participants was over 50 years old. The intervention methods are TAS-102 alone or combined with bevacizumab, and the control is regorafenib (REG) or placebo. The search and screening process is described in Figure 1. All studies included in this study were based on moderate- to high-quality evidence. Table 1 provides a brief description of these 25 studies. In the included studies, the score of the controlled experiment was above 5, and the score of the uncontrolled experiment was above 10. The quality of the literature can support the meta-analysis. Tables 2–5 and Figures 2 and Figure 3 summarize the literature quality evaluation situation. The registration number of this study in PROSPERO is CRD42021265697.
Figure 1.
Flow diagram showing the search and screening process.
Table 1.
Characteristics of included studies.
| Study | Age (years) | Sex (male/female) | ECOG performance status (0/≥1) | KRAS status (wild/mutated) | Methods | Sample | mOS (months) | mPFS (months) |
|---|---|---|---|---|---|---|---|---|
| Mayer et al. 2015 [4] | 63 (27-82) | 326/208 | 301/233 | 272/262 | TAS-102 | 534 | 7.1 (6.5-7.8) | 2 (1.9-2.1) |
| 63 (27-82) | 165/101 | 147/119 | 131/135 | Placebo | 266 | 5.3 (4.6-6.0) | 1.7 (1.7-1.8) | |
| Pfeiffer et al. 2020 [15] | 64 (57-69) | 24/22 | 23/23 | 19/27 | TAS-102+B | 46 | NA | 4.6 (3.5-6.5) |
| 67 (58-72) | 30/17 | 15/32 | 18/29 | TAS-102 | 47 | NA | 2.6 (1.6-3.5) | |
| Sueda et al. 2016 [16] | 66 (44-80) | 10/4 | 1/13 | 9/5 | TAS-102 | 14 | 6.3 (3.21-9.93) | 1 (0.92-6.39) |
| 59 (37-83) | 12/11 | 10/13 | 12/11 | REG | 23 | 5.8 (3.7-11.7) | 0 (1.64-4.52) | |
| Masuishi et al. 2017 [17] | NA | 30/24 | NA | 21/32 | TAS-102 | 54 | 6.5 (5.3-8.3) | 2.1 (1.8-3.1) |
| NA | 90/56 | NA | 78/67 | REG | 146 | 6.7 (5.8-7.6) | 2.1 (1.8-2.5) | |
| Makiyama et al. 2018 [18] | 66 (39-82) | 6/5 | 5/6 | NA | TAS-102+B | 11 | Not reached | 5.8 |
| 69 (47-82) | 20/13 | 11/22 | NA | TAS-102 | 33 | 6.4 | 1.8 | |
| Yoshino et al. 2012 [5] | 63 (28-80) | 64/48 | 72/40 | 54/45 | TAS-102 | 112 | 9.0 (7.3-11.3) | NA |
| 62 (39-79) | 28/29 | 35/22 | 24/26 | Placebo | 57 | 6.6 (4.9-8.0) | NA | |
| Cutsem et al. 2017 [9] | 60.2 (11.86) | 31/33 | 28/36 | 35/29 | TAS-102 | 64 | 6.5 | NA |
| 58.5 (11.02) | 18/17 | 13/22 | 17/18 | Placebo | 35 | 4.3 | NA | |
| 61.8 (9.98) | 167/104 | 138/133 | 123/148 | TAS-102 | 271 | NA | NA | |
| 62.1 (10.42) | 82/50 | 68/64 | 68/64 | Placebo | 132 | NA | NA | |
| 61.9 (10.09) | 113/65 | 128/50 | 94/84 | TAS-102 | 178 | 7.8 | NA | |
| 62.1 (10.40) | 58/30 | 60/28 | 40/48 | Placebo | 88 | 6.7 | NA | |
| Xu et al. 2017 [10] | 58 (26-81) | 170/101 | 64/207 | 172/99 | TAS-102 | 271 | 7.8 (7.1-8.8) | NA |
| 56 (24-80) | 84/51 | 30/105 | 85/50 | Placebo | 135 | 7.1 (5.9-8.2) | NA | |
| Longo-Muñoz et al. 2016 [19] | 5 (27-81) | 48/32 | 24/56 | 35/45 | TAS-102 | 80 | 6.8 | 2 |
| 5 (39-78) | 21/11 | 11/21 | 17/15 | Placebo | 32 | 4.6 | 1.7 | |
| Moriwaki et al. 2018 [20] | 64 (29-86) | 197/130 | 128/199 | 160/161 | TAS-102 | 327 | 7.4 (6.6-8.3) | NA |
| 64 (31-84) | 126/97 | 95/128 | 88/109 | REG | 223 | 7.9 (6.8-9.2) | NA | |
| Kotani et al. 2019 [21] | 60 (23-79) | 35/25 | 35/25 | 28/32 | TAS-102+B | 60 | 8.6 (6.9-10.3) | 3.7 (2.3-5.1) |
| 65 (30-80) | 42/24 | 42/24 | 30/36 | TAS-102 | 66 | 8.0 (6.7-9.4) | 2.2 (1.8-2.6) | |
| Fujii et al. 2020 [11] | 67 (50-74) | 13/8 | NA | 10/11 | TAS-102+B | 21 | 14.4 (7.9-NA) | NA |
| 67.5 (59.8-71.2) | 16/20 | NA | 16/20 | TAS-102 | 36 | 4.5 (3.2-6.5) | NA | |
| Ogata et al. 2020 [22] | 68 (40-85) | 38/39 | 35/42 | 53/24 | TAS-102 | 77 | 11.4 | 3.3 |
| 66 (41-81) | 30/27 | 30/27 | 36/21 | REG | 57 | 9.9 | 2 | |
| Nose et al. 2020 [23] | 73 (49-90) | 16/16 | 12/20 | 14/17 | TAS-102+B | 32 | 11.7 | 4.7 |
| 70.5 (43-88) | 15/9 | 7/17 | 14/10 | TAS-102 | 24 | 6.3 | 1.8 | |
| Cicero et al. 2020 [24] | 78 (70-86) | 28/22 | 18/32 | 18/22 | TAS-102 | 50 | 6.7 (5.7-11.3) | 2.1 (1.2-3.2) |
| Cecchini et al. 2021 [25] | NA | NA | NA | NA | TAS-102 | 41 | 6.8 (5.7-10) | 2.7 (2.4-4.8) |
| Sforza et al. 2017 [26] | 65 (48-82) | 31/12 | 27/16 | 16/27 | TAS-102 | 43 | 6.6 (2.8-10.4) | 2.8 (2.5-3.1) |
| Montes et al. 2020 [27] | 63 (37-83) | 108/52 | 18/142 | 57/103 | TAS-102 | 160 | 7.64 (6.15-9.13) | 2.75 (2.57-2.94) |
| Takahashi et al. 2021 [28] | 73 (65-81) | 21/9 | NA | NA | TAS-102 | 30 | 5.7 (3.7-8.9) | 2.3 (1.9-4.3) |
| Kwakman et al. 2018 [29] | 62 (30-88) | 92/44 | 46/90 | 53/83 | TAS-102 | 136 | 5.4 (4.0-6.9) | 2.1 (1.8-2.3) |
| Moehler et al. 2021 [30] | 60 (35-78) | 6/6 | 6/6 | NA | TAS-102 | 12 | 11.1 (2.3-18.2) | 3.81 (1.51-5.29) |
| Yoshida et al. 2020 [31] | 67 (45-78) | 20/12 | 23/9 | 14/18 | TAS-102+B | 32 | 9.2 (5.5-12.8) | 4.5 (1.8-7.1) |
| Wallander et al. 2020 [32] | 65 (38-78) | 28/20 | 13/34 | 17/29 | TAS-102 | 48 | 6.4 (4.4-8.4) | 2.3 (1.8-2.7) |
| Satake et al. 2020 [33] | 69 (33-82) | 24/20 | 25/19 | 25/19 | TAS-102+B | 44 | 10.86 (8.32-13.68) | 4.29 (2.54-5.83) |
| Carries et al. 2019 [34] | 65.29 (40-88) | 49/35 | 13/71 | 31/53 | TAS-102 | 84 | 8.3 (6.23-9.87) | 2.62 (2.32-3.05) |
Table 2.
The Newcastle-Ottawa Quality Assessment Scale for included controlled studies.
| Study | Selection of the study groups | Comparability of the groups | Outcome | Total score |
|---|---|---|---|---|
| Mayer et al. 2015 [4] | ⭐⭐⭐⭐ | ⭐ | ⭐⭐ | 7 |
| Pfeiffer et al. 2020 [15] | ⭐⭐⭐⭐ | ⭐ | ⭐⭐ | 7 |
| Sueda et al. 2016 [16] | ⭐⭐⭐⭐ | ⭐ | ⭐⭐ | 7 |
| Masuishi et al. 2017 [17] | ⭐⭐⭐⭐ | ⭐ | ⭐⭐ | 7 |
| Makiyama et al. 2018 [18] | ⭐⭐⭐⭐ | ⭐ | ⭐⭐ | 7 |
| Yoshino et al. 2012 [5] | ⭐⭐⭐⭐ | ⭐ | ⭐⭐ | 7 |
| Cutsem et al. 2017 [9] | ⭐⭐⭐⭐ | ⭐ | ⭐⭐ | 7 |
| Xu et al. 2017 [10] | ⭐⭐⭐⭐ | ⭐⭐ | ⭐⭐ | 8 |
| Longo-Muñoz et al. 2016 [19] | ⭐⭐⭐⭐ | ⭐⭐ | ⭐⭐ | 8 |
| Moriwaki et al. 2018 [20] | ⭐⭐⭐⭐ | ⭐⭐ | ⭐⭐ | 8 |
| Kotani et al. 2019 [21] | ⭐⭐⭐⭐ | ⭐⭐ | ⭐⭐ | 8 |
| Fujii et al. 2020 [11] | ⭐⭐⭐⭐ | ⭐ | ⭐⭐ | 7 |
| Ogata et al. 2020 [22] | ⭐⭐⭐⭐ | ⭐ | ⭐⭐ | 7 |
| Nose et al. 2020 [23] | ⭐⭐⭐⭐ | ⭐⭐ | ⭐⭐ | 8 |
Table 3.
MINORS quality evaluation for included uncontrolled studies.
| Study | Clear purpose | Patient continuity | Data collection | Appropriate endpoint | Objective evaluation endpoint | Adequate follow-up time | Low lost to follow-up rate | Sample size estimation | Total score |
|---|---|---|---|---|---|---|---|---|---|
| Cicero et al. 2020 [24] | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 12 |
| Cecchini et al. 2021 [25] | 2 | 2 | 2 | 2 | 1 | 2 | 0 | 0 | 11 |
| Sforza et al. 2017 [26] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 13 |
| Montes et al. 2020 [27] | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 12 |
| Takahashi et al. 2021 [28] | 2 | 2 | 1 | 2 | 1 | 2 | 1 | 0 | 11 |
| Kwakman et al. 2018 [29] | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 0 | 13 |
| Moehler et al. 2021 [30] | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 0 | 11 |
| Yoshida et al. 2020 [31] | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 15 |
| Wallander et al. 2020 [32] | 2 | 2 | 2 | 2 | 1 | 2 | 2 | 0 | 13 |
| Satake et al. 2020 [33] | 2 | 2 | 2 | 2 | 2 | 2 | 1 | 2 | 15 |
| Carries et al. 2019 [34] | 2 | 2 | 2 | 2 | 1 | 2 | 1 | 0 | 12 |
Table 4.
Meta-analysis results for the occurrence of adverse events in uncontrolled experiments.
| Outcomes | Any grade | Grade > 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Methods | Trials | Rate (95% CI) | I 2 | P | Methods | Trials | Rate (95% CI) | I 2 | P | |
| Nonhematological | ||||||||||
| Vomiting | TAS-102 | 5 | 0.10 (0.04-0.16) | 57.60% | <0.001 | TAS-102 | 3 | 0.02 (-0.00-0.05) | 0% | 0.059 |
| TAS-102+B | 2 | 0.21 (0.12-0.30) | 0% | <0.001 | ||||||
| Nausea | TAS-102 | 6 | 0.27 (0.17-0.38) | 81.00% | <0.001 | TAS-102 | 2 | 0.01 (-0.01-0.04) | 0% | 0.251 |
| TAS-102+B | 2 | 0.58 (0.47-0.69) | 0% | <0.001 | TAS-102+B | 2 | 0.07 (0.01-0.12) | 0% | 0.021 | |
| Asthenia | TAS-102 | 8 | 0.36 (0.25-0.47) | 86.20% | <0.001 | TAS-102 | 6 | 0.05 (0.03-0.08) | 0% | <0.001 |
| TAS-102+B | 2 | 0.56 (0.40-0.72) | 53.60% | <0.001 | TAS-102+B | 1 | 0.03 (-0.03-0.09) | 0.31 | ||
| Decreased appetite | TAS-102 | 4 | 0.25 (0.13-0.37) | 75.90% | <0.001 | TAS-102 | 1 | 0.10 (-0.01-0.21) | NA | 0.068 |
| TAS-102+B | 1 | 0.66 (0.49-0.82) | NA | <0.001 | TAS-102+B | 1 | 0.06 (-0.02-0.15) | NA | 0.144 | |
| Diarrhea | TAS-102 | 7 | 0.13 (0.07-0.19) | 76.00% | <0.001 | TAS-102 | 5 | 0.06 (0.01-0.12) | 75.30% | 0.023 |
| TAS-102+B | 2 | 0.22 (0.12-0.31) | 1.90% | <0.001 | TAS-102+B | 1 | 0.03 (0.00-0.05) | NA | 0.043 | |
| Abdominal pain | TAS-102 | 3 | 0.17 (0.04-0.30) | 72.30% | 0.012 | |||||
| Fever | TAS-102 | 4 | 0.06 (0.02-0.10) | 0% | 0.001 | TAS-102 | 1 | 0.10 (-0.01-0.21) | NA | 0.068 |
| TAS-102+B | 1 | 0.18 (0.07-0.30) | NA | 0.002 | TAS-102+B | 1 | 0.05 (-0.02-0.11) | NA | 0.148 | |
| Hematological | ||||||||||
| Neutropenia | TAS-102 | 7 | 0.55 (0.43-0.67) | 84.70% | <0.001 | TAS-102 | 8 | 0.30 (0.26-0.35) | 26.50% | <0.001 |
| TAS-102+B | 2 | 0.67 (0.57-0.78) | 0% | <0.001 | TAS-102+B | 2 | 0.10 (0.01-0.20) | 47.80% | 0.029 | |
| Anemia | TAS-102 | 6 | 0.49 (0.18-0.80) | 98.70% | 0.002 | TAS-102 | 8 | 0.07 (0.05-0.09) | 4.50% | <0.001 |
| TAS-102+B | 2 | 0.89 (0.82-0.96) | 0% | <0.001 | TAS-102+B | 2 | 0.09 (0.03-0.16) | 0% | 0.005 | |
| Leukopenia | TAS-102 | 2 | 0.66 (0.58-0.74) | 0% | <0.001 | TAS-102 | 2 | 0.06 (0.03-0.09) | 0% | <0.001 |
| TAS-102+B | 1 | 0.72 (0.56-0.87) | NA | <0.001 | TAS-102+B | 1 | 0.47 (0.30-0.64) | NA | <0.001 | |
| Febrile neutropenia | TAS-102 | 2 | 0.09 (-0.02-0.21) | 74.90% | 0.113 | TAS-102 | 3 | 0.08 (0.02-0.14) | 49.70% | 0.012 |
| Thrombocytopenia | TAS-102 | 6 | 0.26 (0.12-0.39) | 93.7% | <0.001 | TAS-102 | 5 | 0.01 (0.00-0.02) | 8.60% | 0.014 |
| TAS-102+B | 2 | 0.37 (0.21-0.53) | 0% | <0.001 | TAS-102+B | 2 | 0.06 (0.01-0.12) | 30.40% | 0.022 | |
Table 5.
Meta-analysis results for the occurrence of adverse events in controlled experiments.
| Outcomes | Any grade | Grade > 3 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Intervention | Trials | OR (95% CI) | I 2 | P | Intervention | Trials | OR (95% CI) | I 2 | P | |
| Nonhematological | ||||||||||
| Vomiting | TAS-102 | 5 | 2.99 (2.17-4.13) | 16.70% | <0.001 | TAS-102 | 5 | 3.72 (1.21-11.43) | 0% | 0.022 |
| TAS-102+B | 1 | 0.53 (0.09-3.03) | 0.479 | |||||||
| Nausea | TAS-102 | 3 | 3.32 (1.31-4.44) | 0% | <0.001 | TAS-102 | 2 | 1.79 (0.54-5.90) | 0% | 0.338 |
| TAS-102+B | 2 | 0.80 (0.36-1.78) | 0% | 0.59 | TAS-102+B | |||||
| Asthenia | TAS-102 | 5 | 1.45 (1.08-121.96) | 55.40% | 0.015 | TAS-102 | 8 | 0.85 (0.58-1.25) | 0% | 0.4 |
| TAS-102+B | 2 | 1.43 (0.76-2.66) | 0% | 0.265 | TAS-102+B | 2 | 0.59 (0.11-3.17) | 0% | 0.534 | |
| Decreased appetite | TAS-102 | 6 | 1.43 (0.90-2.26) | 61.90% | 0.127 | TAS-102 | 7 | 0.88 (0.58-1.32) | 0% | 0.527 |
| TAS-102+B | 1 | 0.54 (0.13-2.29) | NA | 0.405 | TAS-102+B | 2 | 0.17 (0.02-1.42) | 0% | 0.1 | |
| Diarrhea | TAS-102 | 4 | 1.63 (0.79-3.37) | 55.70% | 0.043 | TAS-102 | 3 | 1.30 (0.13-12.59) | 60.60% | 0.82 |
| TAS-102+B | 2 | 0.73 (0.10.5.62) | 0% | 0.453 | TAS-102+B | 1 | 0.14 (0.01-3.02) | NA | 0.209 | |
| Abdominal pain | TAS-102 | 2 | 1.23 (0.86-1.76) | 0% | 0.256 | TAS-102 | 4 | 0.57 (0.30-1.06) | 0% | 0.075 |
| Fever | TAS-102 | 3 | 0.42 (0.09-2.02) | 86.60% | 0.277 | TAS-102 | 2 | 3.14 (0.54-18.10) | 0% | 0.201 |
| Hematological | ||||||||||
| Neutropenia | TAS-102 | 4 | 28.21 (1.40-568.32) | 96.60% | 0.029 | TAS-102 | 9 | 32.40 (12.88-81.52) | 31.00% | <0.001 |
| Anemia | TAS-102+B | 1 | 3.33 (1.10-10.12) | NA | 0.034 | TAS-102+B | 3 | 2.37 (1.17-4.77) | 34.20% | 0.016 |
| TAS-102 | 3 | 4.94 (3.11-7.85) | 63.50% | <0.001 | TAS-102 | 8 | 4.38 (2.78-6.89) | 26.70% | <0.001 | |
| TAS-102+B | 2 | 0.58 (0.20-1.69) | 0% | 0.321 | TAS-102+B | 2 | 0.61 (0.25-1.48) | 0% | 0.272 | |
| Leukopenia | TAS-102 | 2 | 72.00 (42.51-121.95) | 0% | <0.001 | TAS-102 | 5 | 24.16 (6.12-95.34) | 14.10% | <0.001 |
| TAS-102+B | 1 | 1.80 (0.77-4.19) | NA | 0.172 | TAS-102+B | 1 | 1.54 (0.73-3.24) | NA | 0.258 | |
| Febrile neutropenia | TAS-102 | 2 | 7.83 (0.75-81.26) | 17.70% | 0.085 | TAS-102 | 5 | 7.71 (2.11-28.16) | 0% | 0.002 |
| TAS-102+B | 1 | 0.42 (0.08-2.25) | NA | 0.312 | TAS-102+B | 1 | 2.24 (0.20-25.37) | NA | 0.514 | |
| Thrombocytopenia | TAS-102 | 4 | 2.27 (0.51-10.22) | 93.10% | 0.284 | TAS-102 | 6 | 1.21 (0.38-3.80) | 64.30% | 0.749 |
| TAS-102+B | 2 | 2.17 (0.39-11.91) | 41.90% | 0.374 | TAS-102+B | 1 | 0.74 (0.04-12.49) | NA | 0.836 | |
Figure 2.
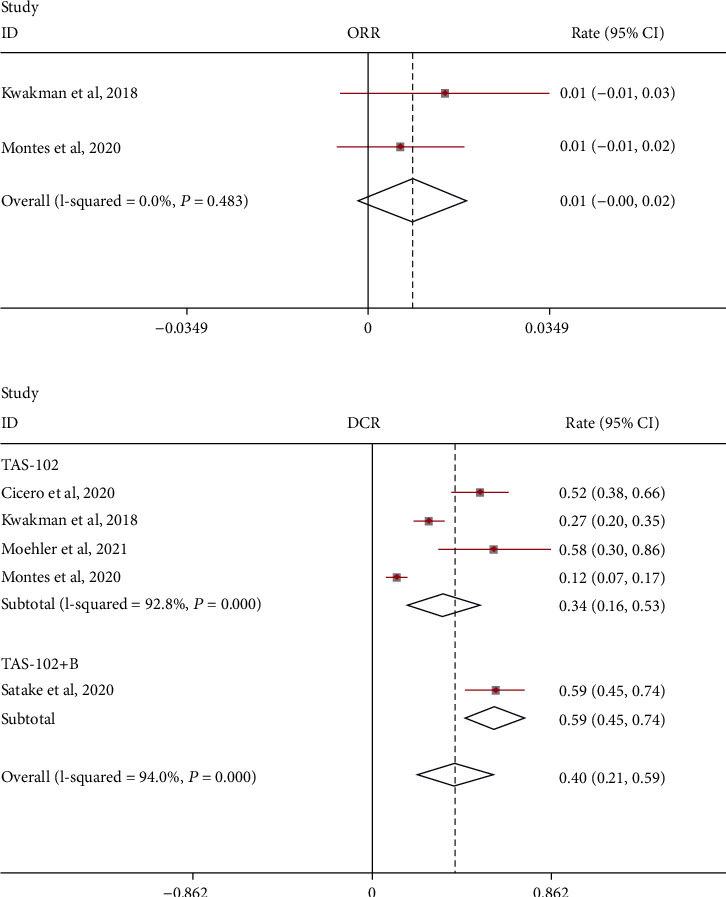
Objective response rate (ORR) and disease control rate (DCR) of TAS-102 monotherapy or combination therapy with bevacizumab for metastatic colorectal cancer.
Figure 3.
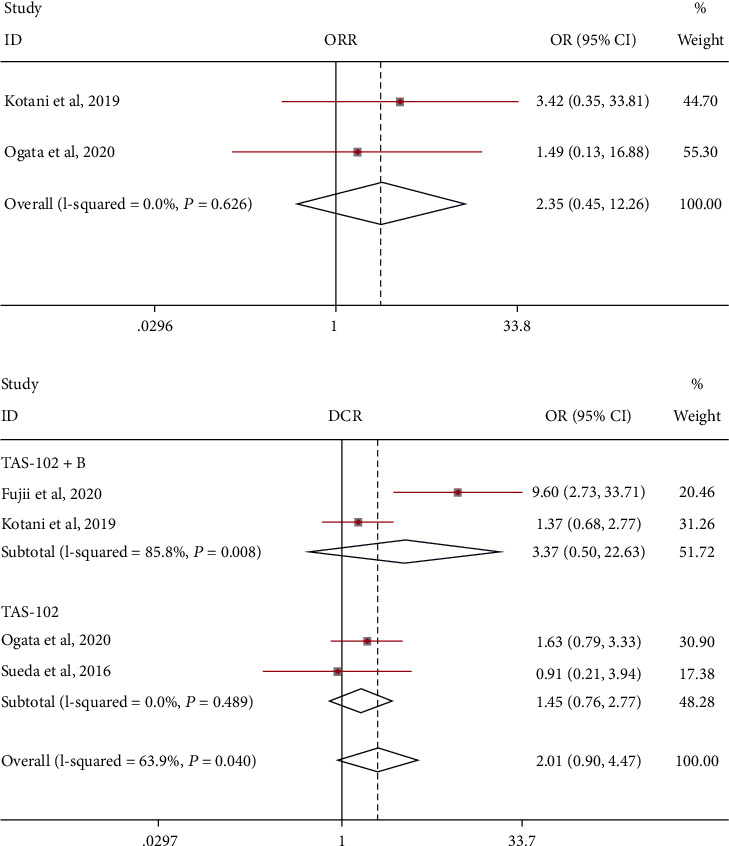
Objective response rate (ORR) and disease control rate (DCR) of those treated with TAS-102 monotherapy or combination therapy with bevacizumab for metastatic colorectal cancer.
3.2. Effectiveness and Safety of Uncontrolled Clinical Trials
Pooling the PFS data from 11 uncontrolled clinical trials revealed that the mOS of patients with metastatic colorectal cancer was 7.39 (95% CI: 6.43-8.49) months with a random-effects model (I2 = 49.4%, P = 0.031; Figure 4). A fixed-effects model was used, and the results were stable (mOS = 7.50, 95% CI: 6.84-8.22 months). Subgroup analysis showed that the mOS of TAS-102 combined with bevacizumab treatment may be higher: TAS-102+B: mOS = 10.41 (95% CI: 8.40-12.89) months and TAS-102: mOS = 6.95 (95% CI: 6.26-7.72) months. A sensitivity analysis that eliminated studies one by one did not detect abnormalities. The funnel chart and Begg's test (Egger's test) show that there is no publication bias.
Figure 4.
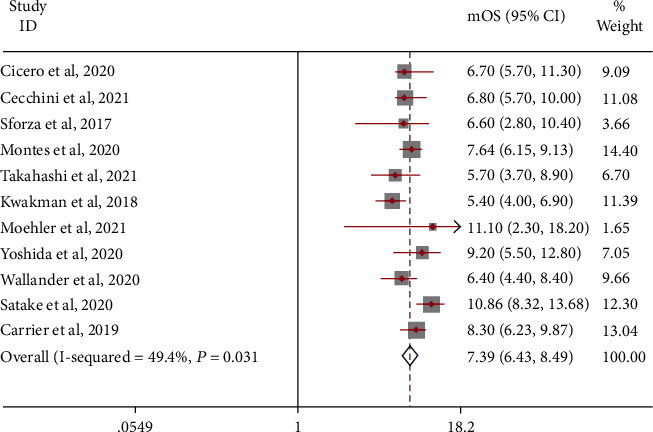
mOS in patients with metastatic colorectal cancer treated with TAS-102 monotherapy or combination therapy with bevacizumab.
Similarly, the mPFS of patients was 2.62 (95% CI: 2.37-2.90) months. A random-effects model was used (I2 = 64.2%; Figure 5). A fixed-effects model was used, and the results were stable (mPFS = 2.63, 95% CI: 2.51-2.75 months). Subgroup analysis showed that the mPFS of TAS-102 combined with bevacizumab treatment may be higher: TAS-102+B: mPFS = 4.35 (95% CI: 3.05-6.20) months and TAS-102: mPFS = 2.53 (95% CI: 2.31-2.78) months. A sensitivity analysis that precluded studies one by one did not detect abnormalities. The funnel chart and Begg's test (Egger's test) show that there is no publication bias.
Figure 5.
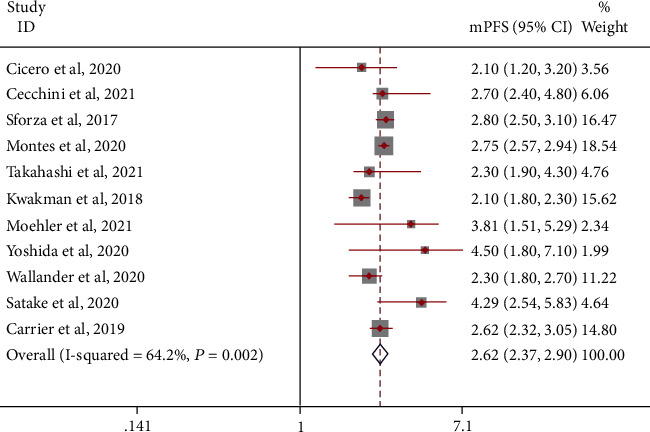
mPFS in patients with metastatic colorectal cancer treated with TAS-102 monotherapy or combination therapy with bevacizumab.
There is no description of the results of the combination of TAS-102 and bevacizumab in the treatment of metastatic colorectal cancer in this study. We use a random-effects model to analyze the objective response rate (ORR) and disease control rate (DCR) (Figure 2). The objective response rate does not seem to be significant and meaningful compared with the disease control rate:ORR = 0.01 (95% CI: -0.00-0.02) and DCR = 0.40 (95% CI: 0.21-0.59). Subgroup analysis showed that the DCR of TAS-102 combined with bevacizumab treatment may be higher: TAS-102+B: DCR = 0.59 (95% CI: 0.45-0.74) and TAS-102: DCR = 0.34 (95% CI: 0.16-0.53).
Grade ≥ 3 adverse events caused by TAS-102 monotherapy or combination therapy with bevacizumab are mainly leukopenia (0.06 and 0.47, respectively), neutropenia (0.30 and 0.10, respectively), decreased appetite (0.10 and 0.06, respectively), and fever (0.10 and 0.05, respectively). The incidence of other hematological or nonhematological adverse events did not reach 0.1. It was worth noting that the combination therapy of TAS-102 and bevacizumab led to multiple grades of adverse events including anemia, thrombocytopenia, vomiting, nausea, asthenia, decreased appetite, diarrhea, fever, and neutropenia.
3.3. Effectiveness and Safety of Controlled Clinical Trials
16 controlled clinical trials were included and divided into two designs (TAS-102+B vs. TAS-102 and TAS-102 vs. placebo). Under the first scheme, compared with the control group, the mOS was improved, and the risk ratio of death was 0.41 (95% CI: 0.18-0.93). A random-effects model was used (I2 = 73.0%; Figure 6). Similarly, the mOS death hazard ratio in the second scheme was 0.72 (95% CI: 0.63-0.83). A random-effects model was used (I2 = 58.7%; Figure 6). A fixed-effects model was used, and the results were stable. Sensitivity analysis that eliminated studies one by one did not detect abnormalities. The funnel chart and Begg's test (Egger's test) show that there is no publication bias.
Figure 6.
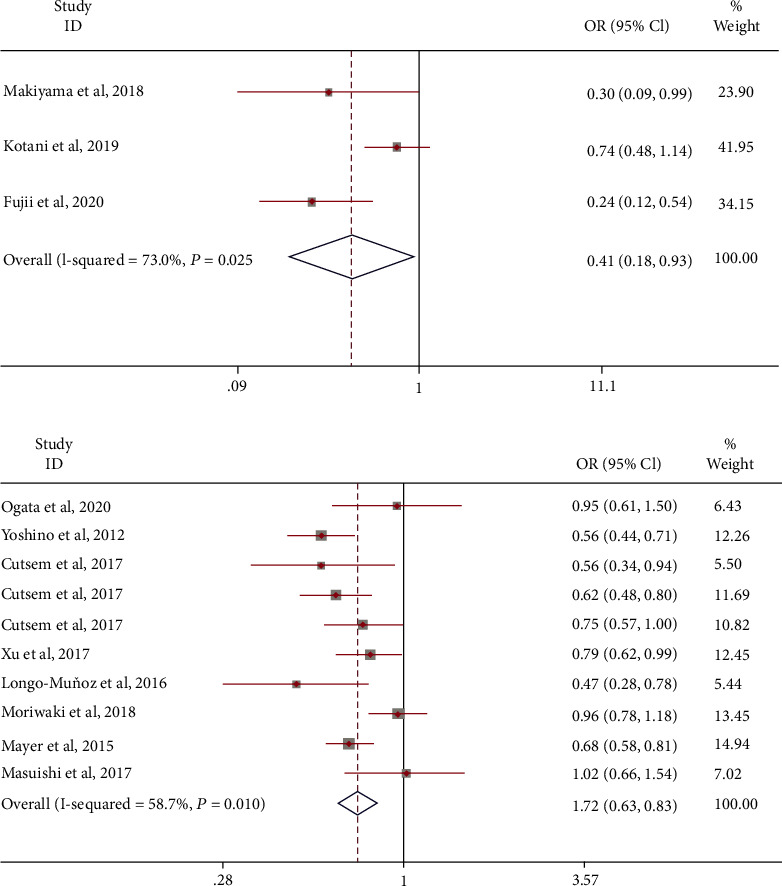
The odds ratio of mOS treated with combination therapy with bevacizumab or TAS-102 monotherapy as the experiment group.
Under the first scheme, compared with the control group, the mPFS was improved, and the risk ratio of death was 0.44 (95% CI: 0.29-0.67). A random-effects model was used (I2 = 60.9%; Figure 7). A fixed-effects model was used, and the results were stable. Similarly, the mPFS death hazard ratio in the second scheme was 0.51 (95% CI: 0.42-0.62). A random-effects model was used (I2 = 52.8%; Figure 7). A sensitivity analysis that eliminated studies one by one did not detect abnormalities. The funnel chart and Begg's test (Egger's test) show that there is no publication bias.
Figure 7.
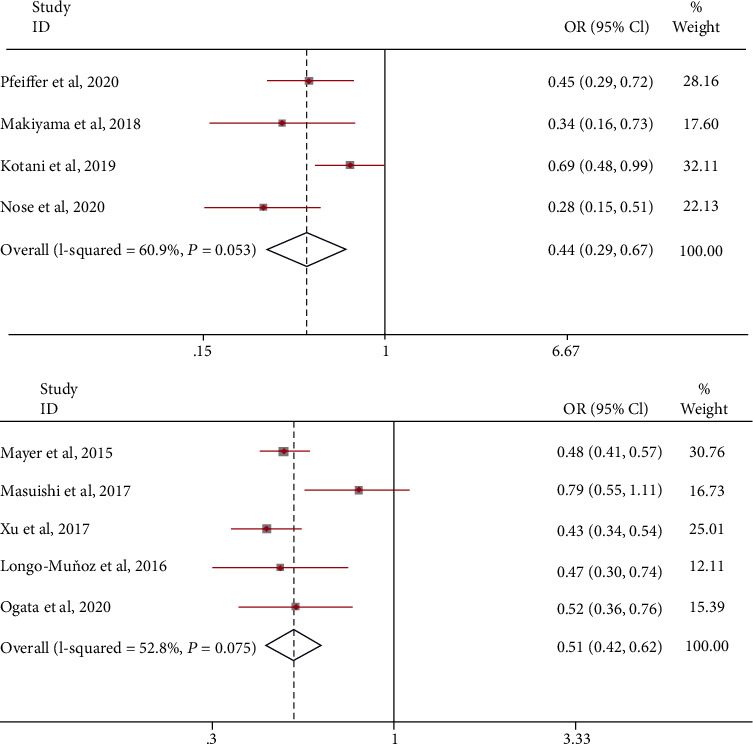
The odds ratio of mPFS treated with combination therapy with bevacizumab or TAS-102 monotherapy as the experiment group.
We separately analyzed the objective response rate (ORR) and disease control rate (DCR) of TAS-102 monotherapy versus placebo for metastatic colorectal cancer (Figure 3). However, ORR and DCR were, respectively, comparable in the TAS-102 monotherapy arm and placebo arm [OR = 2.35 (95% CI: 0.45-12.26), OR = 1.45 (95% CI: 0.76-2.77)]. Similarly, the combination of TAS-102 and bevacizumab was comparable with TAS-102 monotherapy in DCR [OR = 3.37 (95% CI: 0.50-22.63)].
Compared with placebo, grade ≥ 3 adverse events caused by TAS-102 may be more extensive and serious (Table 5), for instance, vomiting [OR = 3.72 (95% CI: 1.21-11.43)], neutropenia [OR = 32.40 (95% CI: 12.88-81.52)], anemia [OR = 4.38 (95% CI: 2.78-6.89)], leukopenia [OR = 24.16 (95% CI: 6.12-95.34)], and febrile neutropenia [OR = 7.71 (95% CI: 2.11-28.16)]. The same situation also occurred in any grade of adverse events. However, we only found that bevacizumab combination therapy can increase the occurrence of neutropenia [OR = 2.37 (95% CI: 1.17-4.77)].
4. Discussion
Almost 55% of colorectal cancer cases worldwide occur in more developed countries. Its incidence continues to rise in developing countries [35]. As with most cancer types, surgery is the main treatment method. For metastatic cancer, cytotoxic methods, such as neoadjuvant therapy and adjuvant therapy, are used before or after it. The main treatment options include fluoropyrimidine, oxaliplatin, and irinotecan. TAS-102 is an anticancer drug that has entered people's field of vision in recent years. Because of its excellent clinical efficacy and safety, it is often added to the treatment of colorectal cancer and gastric cancer in the middle and late stages and anticancer treatment programs for metastatic tumors.
Our study found that the mOS of patients treated with TAS-102 was 7.74 (95% CI: 6.09-9.85) months and the mPFS was 2.91 (95% CI: 2.38-3.57) months. The mOS in patients treated by TAS-102 combined with bevacizumab is 10.41 (95% CI: 8.40-12.89) months, and the mPFS is 4.35 (95% CI: 3.05-6.20) months. Combination therapy may have better effectiveness. As the current targeted drug for the treatment of metastatic colorectal cancer, it is a humanized monoclonal antibody against vascular endothelial growth factor (VEGF), which plays an antitumor effect by blocking the formation of tumor blood vessels and regulating the immune function of patients [36]. In 2004, the FDA approved bevacizumab combined with chemotherapy drugs as the first-line treatment for mCRC. A study showed that bevacizumab combined with first-line chemotherapy for metastatic colorectal cancer can significantly prolong the survival and PFS of patients with mCRC, improve the quality of life, increase the resectable rate of metastases, and improve the survival outcome of patients with mCRC [37, 38]. The number of adverse events has also been significantly reduced.
Although uncontrolled trials can observe the survival of patients, they cannot specify the improvement in survival. We included 16 studies that included two controlled protocols (TAS-102+B vs. TAS-102 and TAS-102 vs. placebo). In either scenario, we found a significant increase in mOS and mPFS. Surprisingly, we found that TAS-102 combined with bevacizumab will increase the incidence of grade ≥ 3 AEs (OR = 2.19, 95% CI: 1.40-3.44) compared to TAS-102 alone. The safety of bevacizumab is worthy of further consideration. This indicates that clinicians need to make careful decisions when making treatment options for patients with metastatic colorectal cancer, considering the patient's tolerance to anticancer drugs.
It is necessary to optimize the design plan when evaluating the efficacy of new drugs. Randomized controlled trials such as RECOURSE and TERRA are conducted in homogeneous populations, which can minimize the risk of bias [12]. In the current study, we have included real observational studies aimed at evaluating the effectiveness of a relatively small homogeneous population. These studies have the shortcomings of nonrandomized controlled studies. The studies we included have controlled and uncontrolled experiments. And the demographic characteristics and disease manifestations of the participants in the experiment are also quite different. This will actually affect the accuracy of our final results. Therefore, more rigorous and appropriate randomized controlled experiments need to be proposed. The published meta-analysis of TAS-102 involves the comparison of the effectiveness and safety of multiple therapeutic drugs [12, 39–43]. Regorafenib, TAS-102, fruquintinib, panitumumab, and cetuximab are recommended single-agent chemotherapy regimens for patients exhibiting disease progression. The safety of these drugs is difficult to assess. But the safety of the drug does affect the confidence of patients in the treatment plan. The most important thing is the improvement of symptoms and the management of side effects [44, 45].
In recent years, people have tried to develop a risk prognostic model for metastatic colorectal cancer [46, 47]. Although these analyses differ in methods and patient populations, ECOG PS, KRAS status, and the number of metastatic sites are common factors in many models. We observed that multiple stratification factors, including KRAS status, may affect the benefit of all patients from TAS-102 treatment but have no effect on the prognostic index. A better prognosis often puts higher requirements on the patient's body tolerance and survival status.
Heterogeneity is often an important factor in measuring the accuracy of meta-analysis results. It seems to be an unavoidable issue in evaluating the two important results of this study (mOS and mPFS). We use a random-effects model and fixed-effects model to mutually verify the final results and finally show that they are trustworthy. We have noticed that the highest proportion of women in the patient population in this study is 59.46% and the highest proportion of mutants in the KRAS status is 67.74%. Perhaps, it is because of this that a higher survival prognosis is obtained (mOS: 22.4 months, mPFS: 9.4 months). The choice of control drugs in controlled clinical trials to evaluate TAS-102 may be an important reason for the heterogeneity between studies. There is a significant difference in mOS between the placebo and the antitumor drug regorafenib. OR was 0.66 (95% CI: 0.59-0.74) and 0.97 (95% CI: 0.82-1.15), respectively. For another indicator (mPFS), there is no change. However, clinicians need to be cautious in their practical application.
This study has proved the good prognosis of TAS-102 monotherapy and combination therapy with bevacizumab for metastatic colorectal cancer. However, the occurrence of grade ≥ 3 AEs and any grade of adverse events is still worthy of attention. Even if it may be due to fewer experiments or a different patient population, it needs to be verified by more rigorous and randomized controlled clinical trials.
Contributor Information
Ping Shao, Email: 1048157125@qq.com.
Feng Zhang, Email: jonathan.cheung@foxmail.com.
Consent
Consent is not applicable.
Conflicts of Interest
The authors have declared that no conflict of interest exists.
Authors' Contributions
Cheng-Jiang Liu and Ting Hu wrote the main manuscript text; Ping Shao prepared the tables and figures; Wu-Yang Chu and Yu Cao helped prepare the figures; Feng Zhang reviewed, revised, and polished the manuscript.
References
- 1.World Health Organization. Global Cancer Observatory . 2021. https://gco.iarc.fr/GoogleScholar .
- 2.Lim H. J., Gill S., Speers C., et al. Impact of irinotecan and oxaliplatin on overall survival in patients with metastatic colorectal cancer: a population-based study. Journal of Oncology Practice/American Society of Clinical Oncology . 2009;5(4):153–158. doi: 10.1200/JOP.0942001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Cutsem E., Cervantes A., Nordlinger B., Arnold D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Annals of Oncology . 2014;25 doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 4.Mayer R. J., Van Cutsem E., Falcone A., et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. The New England Journal of Medicine . 2015;372(20):1909–1919. doi: 10.1056/NEJMoa1414325. [DOI] [PubMed] [Google Scholar]
- 5.Yoshino T., Mizunuma N., Yamazaki K., et al. TAS-102 monotherapy for pretreated metastatic colorectal cancer: a double- blind, randomised, placebo-controlled phase 2 trial. The Lancet Oncology . 2012;13(10):993–1001. doi: 10.1016/S1470-2045(12)70345-5. [DOI] [PubMed] [Google Scholar]
- 6.Hoyle M., Peters J., Crathorne L., et al. Cost-effectiveness of cetuximab, cetuximab plus irinotecan, and panitumumab for third and further lines of treatment for KRAS wild-type patients with metastatic colorectal cancer. Value in Health . 2013;16(2):288–296. doi: 10.1016/j.jval.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Emura T., Murakami Y., Nakagawa F., Fukushima M., Kitazato K. A novel antimetabolite, TAS-102 retains its effect on FU-related resistant cancer cells. International Journal of Molecular Medicine . 2004;13(4):545–549. doi: 10.3892/ijmm.13.4.545. [DOI] [PubMed] [Google Scholar]
- 8.Emura T., Suzuki N., Yamaguchi M., Ohshimo H., Fukushima M. A novel combination antimetabolite, TAS-102, exhibits antitumor activity in FU-resistant human cancer cells through a mechanism involving FTD incorporation in DNA. International Journal of Oncology . 2004;25(3):571–578. doi: 10.3892/ijo.25.3.571. [DOI] [PubMed] [Google Scholar]
- 9.Van Cutsem E., Mayer R. J., Laurent S., et al. The subgroups of the phase III RECOURSE trial of trifluridine/tipiracil (TAS-102) versus placebo with best supportive care in patients with metastatic colorectal cancer. European Journal of Cancer . 2018;90:63–72. doi: 10.1016/j.ejca.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J., Kim T. W., Shen L., et al. Results of a randomized, double-blind, placebo-controlled, phase III trial of trifluridine/tipiracil (TAS-102) monotherapy in Asian patients with previously treated metastatic colorectal cancer: the TERRA study. Journal of Clinical Oncology . 2018;36(4):350–358. doi: 10.1200/JCO.2017.74.3245. [DOI] [PubMed] [Google Scholar]
- 11.Fujii H., Matsuhashi N., Kitahora M., et al. Bevacizumab in combination with TAS-102 improves clinical outcomes in patients with refractory metastatic colorectal cancer: a retrospective study. The Oncologist . 2020;25(3):e469–e476. doi: 10.1634/theoncologist.2019-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen S. E., Andersen I. B., Jensen B. V., Pfeiffer P., Ota T., Larsen J. S. A systematic review of observational studies of trifluridine/tipiracil (TAS-102) for metastatic colorectal cancer. Acta Oncologica . 2019;58(8):1149–1157. doi: 10.1080/0284186X.2019.1605192. [DOI] [PubMed] [Google Scholar]
- 13.MANTEL N., HAENSZEL W. Statistical aspects of the analysis of data from retrospective studies of disease. Journal of the National Cancer Institute . 1959;22(4):719–748. [PubMed] [Google Scholar]
- 14.Stuck A. E., Rubenstein L. Z., Wieland D., et al. Bias in meta-analysis detected by a simple, graphical test. BMJ . 1998;316(7129):p. 469. doi: 10.1136/bmj.316.7129.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfeiffer P., Yilmaz M., Möller S., et al. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: an investigator-initiated, open-label, randomised, phase 2 trial. The Lancet Oncology . 2020;21(3):412–420. doi: 10.1016/S1470-2045(19)30827-7. [DOI] [PubMed] [Google Scholar]
- 16.Sueda T., Sakai D., Kudo T., et al. Efficacy and safety of regorafenib or TAS-102 in patients with metastatic colorectal cancer refractory to standard therapies. Anticancer Research . 2016;36(8):4299–4306. [PubMed] [Google Scholar]
- 17.Masuishi T., Taniguchi H., Hamauchi S., et al. Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: a retrospective comparison. Clinical Colorectal Cancer . 2017;16(2):e15–e22. doi: 10.1016/j.clcc.2016.07.019. [DOI] [PubMed] [Google Scholar]
- 18.Makiyama A., Yamaga S., Hirano G., Makiyama C., Muta T. A retrospective study to compare TAS-102 with TAS-102+BV in advanced colorectal cancer refractory to standard therapy. Annals of Oncology . 2018;29(7):vii75–vi096. doi: 10.1093/annonc/mdy375.041. [DOI] [Google Scholar]
- 19.Longo-Muñoz F., Argiles G., Tabernero J., et al. Efficacy of trifluridine and tipiracil (TAS-102) versus placebo, with supportive care, in a randomized, controlled trial of patients with metastatic colorectal cancer from Spain: results of a subgroup analysis of the phase 3 RECOURSE trial. Clinical & Translational Oncology . 2017;19(2):227–235. doi: 10.1007/s12094-016-1528-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moriwaki T., Fukuoka S., Taniguchi H., et al. Propensity score analysis of regorafenib versus trifluridine/tipiracil in patients with metastatic colorectal cancer refractory to standard chemotherapy (REGOTAS): a Japanese Society for Cancer of the Colon and Rectum multicenter observational study. The Oncologist . 2018;23(1):7–15. doi: 10.1634/theoncologist.2017-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kotani D., Kuboki Y., Horasawa S., et al. Retrospective cohort study of trifluridine/tipiracil (TAS-102) plus bevacizumab versus trifluridine/tipiracil monotherapy for metastatic colorectal cancer. BMC Cancer . 2019;19(1):p. 1253. doi: 10.1186/s12885-019-6475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogata M., Kotaka M., Ogata T., et al. Regorafenib vs trifluridine/tipiracil for metastatic colorectal cancer refractory to standard chemotherapies: a multicenter retrospective comparison study in Japan. PLoS One . 2020;15(6, article e0234314) doi: 10.1371/journal.pone.0234314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nose Y., Kagawa Y., Hata T., et al. Neutropenia is an indicator of outcomes in metastatic colorectal cancer patients treated with FTD/TPI plus bevacizumab: a retrospective study. Cancer Chemotherapy and Pharmacology . 2020;86(3):427–433. doi: 10.1007/s00280-020-04129-6. [DOI] [PubMed] [Google Scholar]
- 24.Cicero G., Addeo R., De Luca R., et al. TAS-102 in metastatic colorectal cancer (mCRC): efficacy, tolerability, and quality of life in heavily pretreated elderly patients: a real-life study. Context . 2020;9:1–8. doi: 10.7573/dic.2020-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cecchini M., Kortmansky J. S., Cui C., et al. A phase 1b expansion study of TAS-102 with oxaliplatin for refractory metastatic colorectal cancer. Cancer . 2021;127(9):1417–1424. doi: 10.1002/cncr.33379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sforza V., Martinelli E., Cardone C., et al. Clinical outcome of patients with chemorefractory metastatic colorectal cancer treated with trifluridine/tipiracil (TAS-102): a single Italian institution compassionate use programme. ESMO Open. . 2017;2(4, article e000229) doi: 10.1136/esmoopen-2017-000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montes A. F., Rivera F. V., Lago N. M., et al. Efficacy and safety of trifluridine/tipiracil in third-line and beyond for the treatment of patients with metastatic colorectal cancer in routine clinical practice: patterns of use and prognostic nomogram. Clinical & Translational Oncology . 2020;22(3):351–359. doi: 10.1007/s12094-019-02130-x. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi M., Sakamoto Y., Ohori H., et al. Phase II study of trifluridine/tipiracil (TAS-102) therapy in elderly patients with colorectal cancer (T-CORE1401): geriatric assessment tools and plasma drug concentrations as possible predictive biomarkers. Cancer Chemotherapy and Pharmacology . 2021;88(3):393–402. doi: 10.1007/s00280-021-04277-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwakman J. J. M., Vink G., Vestjens J. H., et al. Feasibility and effectiveness of trifluridine/tipiracil in metastatic colorectal cancer: real-life data from the Netherlands. International Journal of Clinical Oncology . 2018;23(3):482–489. doi: 10.1007/s10147-017-1220-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moehler M., Michel M., Stein A., et al. A phase I dose-escalation study of third-line regorafenib with trifluridine/tipiracil in metastatic colorectal cancer. Future Oncology . 2021;17(25):3309–3319. doi: 10.2217/fon-2021-0278. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida Y., Yamada T., Kamiyama H., et al. Combination of TAS-102 and bevacizumab as third-line treatment for metastatic colorectal cancer: TAS-CC3 study. International Journal of Clinical Oncology . 2021;26(1):111–117. doi: 10.1007/s10147-020-01794-8. [DOI] [PubMed] [Google Scholar]
- 32.Wallander M., Rolander B., Åvall-Lundqvist E., Elander N. O. Real world aspects of palliative trifluridine plus tiperacil (TAS-102) in refractory metastatic colorectal cancer. Journal of Gastrointestinal Oncology . 2020;11(4):616–625. doi: 10.21037/jgo-20-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satake H., Kato T., Oba K., et al. Phase Ib/II study of biweekly TAS-102 in combination with bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (BiTS study) The Oncologist . 2020;25(12):e1855–e1863. doi: 10.1634/theoncologist.2020-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carriles C., Jimenez-Fonseca P., Sánchez-Cánovas M., et al. Trifluridine/tipiracil (TAS-102) for refractory metastatic colorectal cancer in clinical practice: a feasible alternative for patients with good performance status. Clinical & Translational Oncology . 2019;21(12):1781–1785. doi: 10.1007/s12094-019-02154-3. [DOI] [PubMed] [Google Scholar]
- 35.Favoriti P., Carbone G., Greco M., Pirozzi F., Pirozzi R. E. M., Corcione F. Worldwide burden of colorectal cancer: a review. Updates in Surgery . 2016;68(1):7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 36.Goey K. K. H., Elias S. G., van Tinteren H., et al. Maintenance treatment with capecitabine and bevacizumab versus observation in metastatic colorectal cancer: updated results and molecular subgroup analyses of the phase 3 CAIRO3 study. Annals of Oncology . 2017;28(9):2128–2134. doi: 10.1093/annonc/mdx322. [DOI] [PubMed] [Google Scholar]
- 37.Xiong L., Lou Y., Wang L. Effect of bevacizumab combined with first-line chemotherapy on metastatic colorectal cancer. American Journal of Translational Research . 2021;13(4):3609–3617. [PMC free article] [PubMed] [Google Scholar]
- 38.Chida K., Kotani D., Nakamura Y., et al. Efficacy and safety of trifluridine/tipiracil plus bevacizumab and trifluridine/tipiracil or regorafenib monotherapy for chemorefractory metastatic colorectal cancer: a retrospective study. Therapeutic Advances in Medical Oncology . 2021;13(13) doi: 10.1177/17588359211009143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walter T., Hawkins N. S., Pollock R. F., Colaone F., Shergill S., Ross P. J. Systematic review and network meta-analyses of third-line treatments for metastatic colorectal cancer. Journal of Cancer Research and Clinical Oncology . 2020;146(10):2575–2587. doi: 10.1007/s00432-020-03315-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su G.-L., Wang Y.-Y., Wang J.-C., Liu H. A meta-analysis comparing regorafenib with TAS-102 for treating refractory metastatic colorectal cancer. The Journal of International Medical Research . 2020;48(7) doi: 10.1177/0300060520926408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao M., Zhou M., Zhang J. Comparison of efficacy and safety for patients with beyond second line treated metastatic colorectal cancer: a network meta-analysis of randomized controlled trials. Journal of Chemotherapy . 2020;32(4):163–170. doi: 10.1080/1120009X.2020.1728860. [DOI] [PubMed] [Google Scholar]
- 42.Zhang Q., Wang Q., Wang X., Li J., Shen L., Peng Z. Regorafenib, TAS-102, or fruquintinib for metastatic colorectal cancer: any difference in randomized trials? International Journal of Colorectal Disease . 2020;35(2):295–306. doi: 10.1007/s00384-019-03477-x. [DOI] [PubMed] [Google Scholar]
- 43.Chen J., Wang J., Lin H., Peng Y. Comparison of regorafenib, fruquintinib, and TAS-102 in previously treated patients with metastatic colorectal cancer: a systematic review and network meta-analysis of five clinical trials. Medical Science Monitor . 2019;25(25):9179–9191. doi: 10.12659/MSM.918411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strumberg D., Scheulen M. E., Schultheis B., et al. Regorafenib (BAY 73-4506) in advanced colorectal cancer: a phase I study. British Journal of Cancer . 2012;106(11):1722–1727. doi: 10.1038/bjc.2012.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshino T., Komatsu Y., Yamada Y., et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Investigational New Drugs . 2015;33(3):740–750. doi: 10.1007/s10637-014-0154-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.De Divitiis C. Prognostic and predictive response factors in colorectal cancer patients: between hope and reality. World Journal of Gastroenterology . 2014;20(41):15049–15059. doi: 10.3748/wjg.v20.i41.15049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrelli F., Coinu A., Cabiddu M., et al. Prognostic factors for survival with bevacizumab-based therapy in colorectal cancer patients: a systematic review and pooled analysis of 11, 585 patients. Medical Oncology . 2015;32(2):p. 456. doi: 10.1007/s12032-014-0456-z. [DOI] [PubMed] [Google Scholar]


