Abstract
Introduction
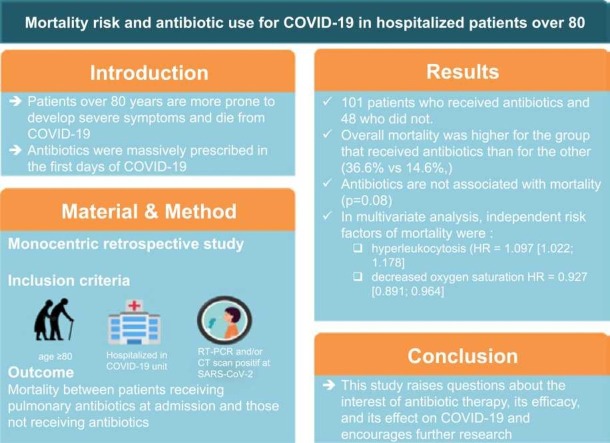
Patients over 80 years of age are more prone to develop severe symptoms and die from COVID-19. Antibiotics were massively prescribed in the first days of the pandemic without evidence of super infection. Antibiotics may increase the risk of mortality in cases of viral pneumonia. With age and antibiotic use, the microbiota becomes altered and less protective effect against lethal viral pneumonia. Thus we assessed whether it is safe to prescribe antibiotics for COVID-19 pneumonia to patients over 80 years of age.
Method
We conducted a retrospective monocentric study in a 1240-bed university hospital. Our inclusion criteria were patients aged ≥ 80 years, hospitalized in a COVID-19 unit, with either a positive SARS-CoV-2 RT-PCR from a nasopharyngeal swab or a CT scan within 72 h after or prior to hospitalization in the unit suggestive of infection.
Results
We included 101 patients who received antibiotics and 48 who did not. The demographics in the two groups were similar. Overall mortality was higher for the group that received antibiotics than for the other group (36.6% vs 14.6%,). According to univariate COX analysis, the risk of mortality was higher (HR = 1.98 [0.926; 4.23]) but non-significantly for the antibiotic group. In multivariate analysis, independent risk factors of mortality were an increased leukocyte count and decreased oxygen saturation (HR = 1.097 [1.022; 1.178] and HR = 0.927 [0.891; 0.964], respectively).
Conclusion
This study raises questions about the interest of antibiotic therapy, its efficacy, and its effect on COVID-19 and encourages further research.
Keywords: COVID-19, Mortality, Hospitalisation, Elderly, Antibiotics use
Graphical abstract
1. Introduction
The COVID-19 pandemic has spread rapidly, and hospitals worldwide have been overwhelmed by patients. Although the elderly do not appear to have a higher incidence of COVID-19, they are more likely to develop more severe symptoms [1], [2], [3], [4]. The mortality rate is 10- to 100-fold higher for patients older than 65 years than in the younger population [5].
Antibiotics have been massively prescribed because of the lack of knowledge on the virus and the lack of guidelines for its management [6]. In the first days of the pandemic, a study published in March 2020 showed antibiotic prescriptions for 95% of cases [7]. It is common knowledge that antibiotics do not increase survival of patients with viral infections, even though macrolides have antiviral activity against certain viruses in vitro, even SARS-Cov2 [8], [9], [10]. However several studies failed to demonstrate a significant benefit of azithromycin against SARS-Cov2 [11], [12], [13].
Hence, aside from rare cases of bacterial co-infection or super-infection (3.5–8%), antibiotics are useless against COVID-19 pneumonia [14], [15], [16]. The difficulty lies in the diagnosis of bacterial co-infection. Indeed, in most cases, there is no simple clinico-biological or radiological evidence to differentiate viral from viral plus bacterial infections [7], [17]. Moreover, antibiotics were widely prescribed without microbiological samples in COVID-19 or even despite negative samples [18], [19]. Prescribing useless antibiotics may not be harmless. Obviously, the emergence of resistance is a threat and studies in mice suggest a link between antibiotic therapy and a higher risk of mortality in viral pneumonia. This finding addresses the protective effect of the microbiota and the diversity of the microbiome against lethal viral pneumonia [20], [21], [22].
The fact that the elderly are at greater risk of having an altered microbiota, [23], [24], [25] have a greater risk of death from COVID-19, and that antibiotics may increase the risk of mortality in viral pneumonia, raises the question of whether it safe to prescribe antibiotics for COVID-19 pneumonia to patients older than 80 years. We aimed to determine whether antibiotics reduce the survival of COVID-19 patients older than 80 years.
2. Materials and methods
2.1. Setting
We conducted a retrospective monocentric study according to the STROBE criteria in a 1240-bed university hospital in Amiens, in the north of France, where the impact of the pandemic was particularly intense. During the study period, the pandemic led to the opening of nine COVID-19 units in medicine and two intensive care units. The number of beds dedicated to COVID-19 was 158 in medicine and 62 in intensive care.
Our study focused on people over 80 years of age with COVID-19 hospitalised in a dedicated unit in our university hospital. We followed the patients during their hospitalisation in a COVID-19 unit.
The inclusion period was from February 29, 2020 (opening of the first COVID-19 unit in our centre) to June 7, 2020 (closure of most COVID-19 units).
Our inclusion criteria were patients aged ≥ 80 years, hospitalized in a COVID-19 unit with either a positive SARS-CoV-2 RT-PCR (reverse transcriptase‐polymerase chain reaction assay) from a nasopharyngeal swab or a CT scan within 72 h after or prior to hospitalization in the unit suggestive of infection, as determined by the radiologist.
Our exclusion criteria were the absence of a positive SARS-CoV-2 RT-PCR from a nasopharyngeal swab or a CT scan suggestive of infection, as determined by the radiologist, and positive results more than 72 h after or prior to hospitalisation.
The primary outcome was mortality between patients receiving pulmonary antibiotics at admission and those not receiving antibiotics during their stay. We also assessed whether the use of antibiotics was discussed by analysing the clinico-biological characteristics between the two groups.
2.2. Data source
The study was registered at the French National Data Protection Commission (Commission nationale de l’informatique et des libertés; reference: PI2020_843_0097). Computerized extractions from the patient electronic health record of our university hospital were provided by the establishment's IT department after receiving regulatory approval, according to the French legislation on retrospective analysis and routine clinical practice. One reader was assigned to review the entirety of all medical records to have the most clinical details.
2.3. Variables assessed
The data collected for each patient included demographic information, such as age, gender, place of residence (home, nursing home), number of medications, and the Charlson’s comorbidity score; biological data (an interval of more or less than 72 h was accepted), including creatinine clearance, according to the MDRD (modification of diet in renal disease), albumin, CRP (C-reactive protein), haemoglobin, and total bilirubin levels and leukocyte, platelet, and absolute neutrophil counts (ANC); and clinical data, such as SaO2 (oxygen saturation), oxygen flow and temperature, and systolic blood pressure. The prescription of thromboprophylaxis was also analysed.
Patients were divided into two groups based on whether or not they received antibiotics. The characteristics of the antibiotics were collected along with the name, the beginning date and duration of antibiotic therapy. Patients who received only antiretrovirals were classified in the antibiotic-free group.
An interval of 72 h was accepted for initiation before hospitalization.
2.4. Statistics
Quantitative variables are expressed as means and standard deviations when the distribution was normal and medians and interquartile ranges otherwise. Normality was measured by Student’s t-test. Qualitative variables are expressed as percentages.
The outcome was the time between the beginning of hospitalization and death or the end of hospitalization. Survival curves were generated by group using the Kaplan-Meier estimator and compared using a log-rank test. A semi-parametric Cox model allowed analysis of the outcome. Thus, univariate analyses were performed to allow variable selection at the level of 5%. The selected variables and the group (with /without antibiotics) were subsequently put in a multivariable model. The selected quantitative variables were checked for log-linearity. The proportional hazard (PH) assumption was assessed using a statistical test based on scaled Schoenfeld residuals. Multicollinearity was detected using the variance inflation factor (VIF) and variables showing multicollinearity removed from the multivariable model. All tests were two-sided, with a level of significance of 5%. Statistical analyses were performed using R software, version 4.0.1 ©2021 with tableone, survey, survival, and car packages.
3. Results
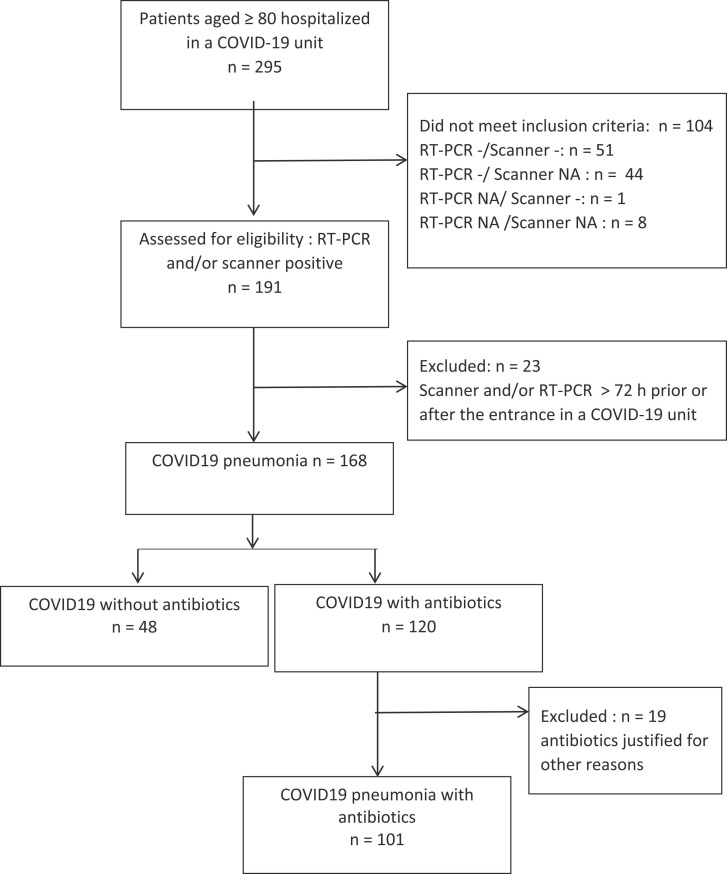
During the study period, 295 patients aged ≥ 80 were hospitalized in COVID-19 units ( Fig. 1). Among them, 104 were excluded because they did not meet the inclusion criteria for a positive SARS-CoV-2 RT-PCR or CT scan in favour of COVID-19 infection. Twenty-three more patients were excluded because the time of diagnosis was not within 72 h before or after hospitalization in the unit. Among the included patients, 120 (71.4%) received antibiotics and 48 (28.6%) did not. Among the patients who received antibiotics, 19 were excluded because the antibiotics were justified by a documented non-pulmonary infection. The average length of stay was 8.4 days and the median 7 days [0 days - 30 days].
Fig. 1.
Flow chart describing the selection the patients.
The characteristics of the patients are presented in Table 1. The demographics were similar between the two groups. Most of the patients lived at home. Charlson's co-morbidity score was low for both groups (median = 6, IQR 5–7). Most patients received antibiotics (67.8% with antibiotics vs 32.2% without).
Table 1.
Comparison of clinico-biological characteristics of antibiotic and non-antibiotic groups at admission.
| Total | Without antibiotics | With antibiotics | p | |
|---|---|---|---|---|
| N (%) | 149 | 48 (32.2) | 101 (67.8) | |
| Age (years), median [IQR] | 85.50 [82.00, 89.00] | 86.00 [83.00, 90.00] | 85.00 [82.00, 88.00] | 0.560 |
| Sex, N (%) | 0.428 | |||
| Male | 66 (44.3) | 19 (39.6) | 47 (46.5) | |
| Female | 83 (55.7) | 29 (60.4) | 54 (53.5) | |
| Living place, N (%) | 0.619 | |||
| Nursing Home | 38 (25.5) | 11 (22.9) | 27 (26.7) | |
| Home | 111 (74.5) | 37 (77.1) | 74 (73.3) | |
| Number of Medications before hospitalization, mean (SD) | 7.38 (3.33) | 6.73 (2.83) | 7.69 (3.51) | 0.074 |
| Charlson Comorbidity Index, median [Q1, Q3] | 6.00 [5.00, 7.00] | 6.00 [5.00, 7.00] | 6.00 [5.00, 7.00] | 0.306 |
| Infection COVID-19, N (%) | 0.659 | |||
| Nosocomiala | 25 (16.8) | 9 (18.8) | 16 (15.8) | |
| Community | 124 (83.2) | 39 (81.2) | 85 (84.2) | |
| MDRDb at admission, median [Q1, Q3] | 62.00 [42.25, 94.00] | 73.00 [53.00, 96.00] | 57.00 [40.00, 91.25] | 0.057 |
| Corticoids, N (%) | 25 (16.8) | 2 (4.2) | 23 (22.8) | 0.005 |
| Thromboprophylaxis (%) | 111 (74.5) | 33 (68.8) | 78 (77.2) | 0.271 |
| CRPc at admission, median [Q1, Q3] | 86.95 [35.77, 154.47] | 48.80 [16.20, 83.40] | 107.65 [55.88, 166.93] | < 0.001 |
| Haemoglobin at admission, mean (SD) | 12.42 (1.85) | 11.99 (2.02) | 12.62 (1.74) | 0.062 |
| Leucocytes at admission, median [Q1, Q3] | 6.92 [5.10, 10.00] | 6.10 [4.90, 8.50] | 7.15 [5.30, 10.38] | 0.169 |
| Saturation at admission, median [Q1, Q3] | 95.00 [93.00, 97.00] | 95.00 [94.00, 97.00] | 95.00 [92.25, 97.00] | 0.342 |
| Oxygen at admission, median [Q1, Q3] | 2.00 [0.00, 4.00] | 0.00 [0.00, 3.00] | 3.00 [1.00, 4.00] | < 0.001 |
| Temperature (°C), mean (SD) | 37.03 (0.88) | 36.97 (0.73) | 37.06 (0.95) | 0.557 |
IQR: interquartile range, SD: standard deviation.
The NA were imputed by simple imputation to not exclude patients in the following analyses
Maximum of 3 non applicable for a variable.
Binary variables: imputation by the most frequent modality.
Continuous variables: imputation by the median.
nosocomial infection: infection occurring in a patient during the process of care in a hospital or other health care facility, which was not present or incubating at the time of admission [36].
MDRD: modification of diet in renal disease.
CRP: C-reactive protein.
SARS-CoV-2 RT-PCR was available in 99.3% of cases (i.e., SARS-CoV-2 RT-PCR was not performed for one patient). A CT scan was performed in 32 (78.5%) cases.
Corticosteroids were also more highly prescribed in the group receiving antibiotics (23.0% versus 4.2%).
Antibiotics were introduced during the hospitalization in 77 (76.2%) cases. For 57 (56.4%) patients, antibiotics were introduced during the first day of hospitalisation. Blood cultures were frequently performed in the antibiotic group (53.5% vs 33.3%). Two blood cultures came back positive (Staphylococcus coagulase-negative), both in the antibiotics group and none in the other. In the antibiotics group, 26 (25.7%) patients had a sputum examination, whereas only 3 (6.25%) had a sputum examination in the non-antibiotics group. The duration of antibiotic treatment was seven days. Antibiotics were not stopped, even if cultures or sputum examination were negative.
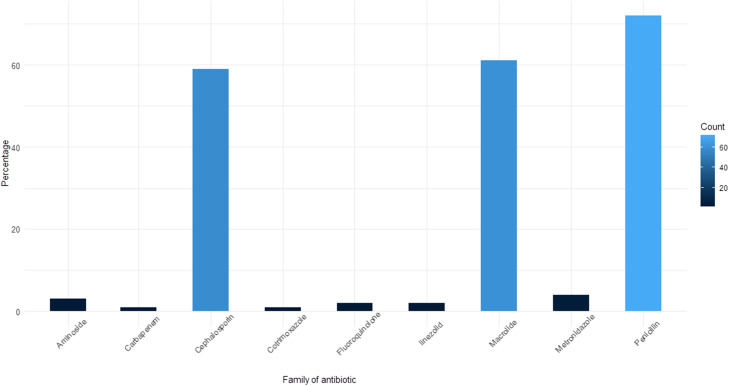
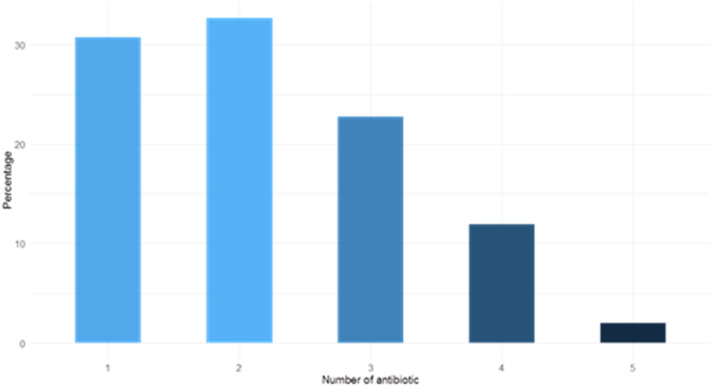
In total, 205 antibiotic prescriptions were analysed for 101 patients. The most commonly administered was penicillin, comprising 72 prescriptions (35.1%), macrolides comprising 61 (29.8%), and cephalosporins comprising 59 (28.8%) ( Fig. 2). Thirty-one (30.7%) patients received one antibiotic, most frequently amoxicillin plus clavulanic acid, which was prescribed 15 times (48.4% of monotherapy). Dual therapy was prescribed for 33 patients (32.7%), combining a macrolide with cephalosporin in 19 prescriptions (57.6% of dual therapy) ( Fig. 3). Piperacillin plus tazobactam, a broad-spectrum antibiotic, was prescribed 26 times (11.7% of prescriptions).
Fig. 2.
Antibiotic classes used by percentage during the stay concerning 149 patients. The most prescribed antibiotics were penicillins at 35.1% (amoxicillin and piperacillin in most cases associated with a β-lactamase inhibitor), macrolides at 29.8% (spiramycin and azithromycin) and cephalosporins at 28.8% (ceftriaxone, cefotaxime, cefepime and cefuroxime).
Fig. 3.
Number of different antibiotics during the stay used per patient by percentage. 149 patients were included. Most of them had 1 or 2 antibiotics. The number of antibiotics does not correspond to the number of therapeutic lines but to the number of antibiotics administered during the stay, either simultaneously or separately.
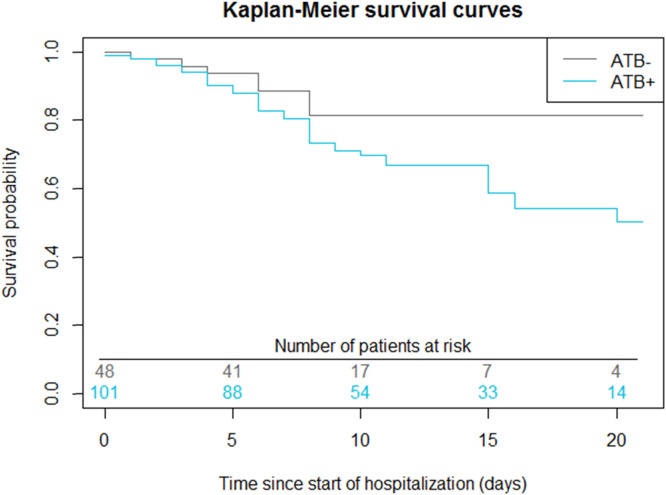
The overall mortality of our cohort was higher in the group receiving antibiotics than in the other group (37 patients (36.6%) vs 7 patients (14.6%), p = 0.07). According to the model resulting from the univariate COX analysis ( Table 2), the risk of mortality was higher (HR = 1.98 [0.926; 4.23]) but non-significant in the antibiotic group. By day 10, 65% of patients in the non-antibiotic group had been discharged from hospital ( Fig. 4).
Table 2.
Risk factors of mortality (univariate model).
| HR brut | IC95% | p | |
|---|---|---|---|
| ATBa | 1.98 | [0.926; 4.23] | 0.08 |
| Age (years) | 1.009 | [0.954; 1.067] | 0.7 |
| Sex | 0.5 | ||
| Female (ref) | 1 | ||
| Male | 1.205 | [0.690; 2.104] | |
| Living place | 0.2 | ||
| Home(ref) | 1 | ||
| Nursing home | 1.549 | [0.838; 2.862] | |
| Medications before hospitalization | 1.005 | [0.9209; 1.096] | 0.9 |
| Charlson Comorbidity Index | 0.974 | [0.826; 1.148] | 0.7 |
| Infection | 0.4 | ||
| Community(ref) | 1 | ||
| Nosocomial | 0.751 | [0.362; 1.556] | |
| MDRDb at admission | 0.991 | [0.983; 0.999] | 0.02 |
| Corticoids | 1.449 | [0.790; 2.658] | 0.2 |
| Thromboprophylaxis | 0.605 | [0.330; 1.111] | 0.1 |
| CRP at admission | 1.005 | [1.002; 1.008] | < 0.001 |
| Haemoglobin at admission | 1.118 | [0.956; 1.309] | 0.2 |
| Leucocytes at admission | 1.115 | [1.046; 1.190] | < 0.001 |
| Saturation at admission | 0.9162 | [0.882; 0.952] | < 0.001 |
| O2c at entrance | 1.153 | [1.094; 1.216] | < 0.001 |
| Temperature at admission (°C) | 1.324 | [0.975; 1.80] | 0.07 |
p: Wald test
ATB: Antibiotics.
MDRD: modification of diet in renal disease.
O2: Oxygen.
Fig. 4.
Survival in the antibiotic group versus the antibiotic-free group. Log-rank test for comparison of survival curves: p = 0.07. At the 5% threshold level, there was no significant difference between the survival curves of the ATB- and ATB+ groups.
In multivariate analysis ( Table 3), independent risk factors of mortality were an elevated leukocyte count and reduced oxygen saturation (HR = 1.097 [1.022; 1.178] and HR = 0.927 [0.891; 0.964], respectively).
Table 3.
Multivariate model of mortality.
| Adjusted HR | 95% CI | p | |
|---|---|---|---|
| ATB | 1.539 | [0.706; 3.354] | 0.278 |
| MDRD at admission | 0.994 | [0.986; 1.002] | 0.163 |
| Leucocyte count at admission | 1.097 | [1.022; 1.178] | 0.010 |
| Saturation at admission | 0.927 | [0.891; 0.964] | 0.0001 |
Adjustment for the variables linked to the primary endpoint at the 5% threshold in univariate analysis (previous table).
Note: there was no adjustment for CRP or O2 because these variables are linked to antibiotic use and increase the variance of the estimators by more than 20% (VIF test).
4. Discussion
As expected in our study, the overall mortality was 29.5% in subjects aged ≥ 80 years hospitalized in COVID-19 units. This value is lower than that in nursing homes but higher than that for hospitalized patients overall [3], [26], [27], [28], [29]. We found a clear trend towards higher mortality in the antibiotic group based on univariate and multivariate COX survival but it is non-significant. Only an elevated leukocyte count and reduced oxygen saturation at entrance could predict mortality during the stay. Antibiotic prescription does not appear to worsen nor improve the vital prognosis.
During the first wave of COVID-19, antibiotics were massively prescribed [6], [7]. In our study, antibacterial agents were prescribed to 67.8% of our patients. By contrast, the estimated rate of co-infection in the literature is approximately 3–8% [14], [15], [16]. Evidence that could have encouraged introducing antibiotics was limited, as sputum examinations were prescribed in only 12% of cases. This percentage was higher in the antibiotics group (25.7%) but still low. Blood cultures were prescribed more often, 47% of patients overall, increasing to 53.5% for those who received antibiotic therapy. However, only two blood cultures were positive, one for Staphylococcus capitis and one for Staphylococcus hominis. These bacteria make up part of the commensal skin flora and it is possible that they come from contamination. The introduction of antibiotics was highly associated with elevated CRP levels and the need for oxygen at admission. An early rise in CRP levels can predict respiratory failure and intubation in COVID-19 patients who are stable at admission but it is not a sign of pulmonary co-infection [30], [31].
Before the COVID-19 pandemic, one of the main objectives of the WHO and other global organisations was to reduce and prevent antimicrobial resistance [32]. With the first wave of COVID-19, prescribers were confronted with a new threat and prescribed antibiotics, even without evidence of its utility.
Numerous studies have addressed the protective effect of the microbiota and the diversity of the microbiome against lethal viral pneumonia [20], [21], [22]. The microbiome is altered in the elderly and by antibiotics [23], [24], [25]. Lactobacillus sp. can help and modulate the inflammatory response and protect against lethal viral infections. However, this bacteria is sensitive to many of the antibiotics used in our patients [33], [34], [35].
This study had several limitations, including the fact that it was a monocentric study, retrospective, on a small number of participants, and part of the data came from unstructured text collections and not a standardized health record, with a possible bias of subjectivity. The date of contamination and onset of symptoms were not known and therefore the date of admission to the COVID-19 unit does not necessarily reflect the same duration of the disease before admission for all patients. Moreover, the antibiotics prescribed were very heterogeneous in terms of the molecules, combinations, duration of exposure, and route of administration. Finally, there was no microbiota analysis in this cohort.
Nevertheless, this study is one of the first to focus on the use of antibiotics against COVID-19, especially on a frail population aged 80 years or older. Our results are consistent with those of previous studies on high mortality and antibiotic exposure, suggesting a similar phenomenon in our population. This study could be complemented by an analysis of the microbiota and its relationship with mortality in elderly COVID-19 patients, as well as a randomised clinical trial measuring the benefit-risk ratio of the most commonly used antibiotic therapies.
Clinicians should be cautious about antibiotic prescriptions in the absence of strong evidence of mixed lung infection, especially because their prevalence is not high, a certain diagnosis is not accessible, and the benefit/risk ratio is not clear.
5. Conclusion
Mortality trended towards being higher in the antibiotics group. Our study highlights the massive use of antibiotics against COVID-19 in very frail elderly patients. Prescribers relied on little evidence to introduce them. This study raises questions about the interest of antibiotic therapy, its efficacy, and its effect on COVID-19 and merits further research.
Funding
This work was not supported. (grant number PI2020_843_0097).
The funding bodies didn’t play any role in the design of the study and collection, analysis, and interpretation of data, nor in writing the manuscript.
CRediT authorship contribution statement
Andreea Rosca: Conceptualization, Methodology, Investigation, Writing – original draft, Visualization. Thibault Balcaen: Formal analysis, Investigation, Software, Data curation, Writing – review & editing. Jean-Philippe Lanoix: Writing – review & editing. Audrey Michaud: Formal analysis, Writing - original draft. Julien Moyet: Writing – review & editing. Ingrid Marcq: Writing – review & editing. Jean-Luc Schmit: Writing – review & editing. Frederic Bloch: Writing – review & editing. Guillaume Deschasse: Conceptualization, Methodology, Writing – review & editing, Supervision.
Conflict of interest statement
None.
Acknowledgments
We thank all the healthcare staff who helped fighting against COVID-19 in our hospital. And specially Abdallah Al-Salameh, Claire Andrejak, Hervé Dupont, Vincent Goeb, Maité Jaureguy, Sylvie Lion, Julien Maizel, Benoit Vaysse, Rachel Desailloud1, Olivier Ganry and Jean-Daniel Lalau.
Lastly, we are grateful to Alex Edelman & Associates for his English checking manuscript.
References
- 1.Kang S.-J., Jung S.I. Age-related morbidity and mortality among patients with COVID-19. Infect. Chemother. 2020;52:154–164. doi: 10.3947/ic.2020.52.2.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.C. Annweiler, et al., National French Survey of Coronavirus Disease (COVID-19) Symptoms in People Aged 70 and Over. Clin Infect Dis. doi: 10.1093/cid/ciaa792. [DOI] [PMC free article] [PubMed]
- 3.Su Z., McDonnell D., Li Y. Why is COVID-19 more deadly to nursing home residents? QJM. 2021 doi: 10.1093/qjmed/hcaa343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsatsakis A., Calina D., Falzone L., Petrakis D., Mitrut R., Siokas V., Pennisi M., Lanza G., Libra M., Doukas S.G., Doukas P.G., Kavali L., Bukhari A., Gadiparthi C., Vageli D.P., Kofteridis D.P., Spandidos D.A., Paoliello M., Aschner M., Docea A.O. SARS-CoV-2 pathophysiology and its clinical implications: an integrative overview of the pharmacotherapeutic management of COVID-19. Food Chem. Toxicol. 2020;146 doi: 10.1016/j.fct.2020.111769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ioannidis J.P.A., Axfors C., Contopoulos-Ioannidis D.G. Population-level COVID-19 mortality risk for non-elderly individuals overall and for non-elderly individuals without underlying diseases in pandemic epicenters. Environ. Res. 2020;188 doi: 10.1016/j.envres.2020.109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gonzalez-Zorn B. Antibiotic use in the COVID-19 crisis in Spain. Clin. Microbiol. Infect. 2020;27:646–647. doi: 10.1016/j.cmi.2020.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., Zhang Y., Song J., Wang S., Chao Y., Yang Z., Xu J., Zhou X., Chen D., Xiong W., Xu L., Zhou F., Jiang J., Bai C., Zheng J., Song Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madrid P.B., Panchal R.G., Warren T.K., Shurtleff A.C., Endsley A.N., Green C.E., Kolokoltsov A., Davey R., Manger I.D., Gilfillan L., Bavari S., Tanga M.J. Evaluation of Ebola virus inhibitors for drug repurposing. ACS Infect. Dis. 2015;1:317–326. doi: 10.1021/acsinfecdis.5b00030. [DOI] [PubMed] [Google Scholar]
- 9.Retallack H., Di Lullo E., Arias C., Knopp K.A., Laurie M.T., Sandoval-Espinosa C., Mancia Leon W.R., Krencik R., Ullian E.M., Spatazza J., Pollen A.A., Mandel-Brehm C., Nowakowski T.J., Kriegstein A.R., DeRisi J.L. Zika virus cell tropism in the developing human brain and inhibition by azithromycin. Proc. Natl. Acad. Sci. USA. 2016;113:14408–14413. doi: 10.1073/pnas.1618029113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Damle B., Vourvahis M., Wang E., Leaney J., Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID‐19. Clin. Pharmcol. Ther. 2020;108:201–211. doi: 10.1002/cpt.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavalcanti A.B., Zampieri F.G., Rosa R.G., Azevedo L.C.P., Veiga V.C., Avezum A., Damiani L.P., Marcadenti A., Kawano-Dourado L., Lisboa T., Junqueira D.L.M., de Barros e Silva P.G.M., Tramujas L., Abreu-Silva E.O., Laranjeira L.N., Soares A.T., Echenique L.S., Pereira A.J., Freitas F.G.R., Gebara O.C.E., Dantas V.C.S., Furtado R.H.M., Milan E.P., Golin N.A., Cardoso F.F., Maia I.S., Hoffmann Filho C.R., Kormann A.P.M., Amazonas R.B., Bocchi de Oliveira M.F., Serpa-Neto A., Falavigna M., Lopes R.D., Machado F.R., Berwanger O. Hydroxychloroquine with or without azithromycin in mild-to-moderate Covid-19. N. Engl. J. Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg E.S., Dufort E.M., Udo T., Wilberschied L.A., Kumar J., Tesoriero J., Weinberg P., Kirkwood J., Muse A., DeHovitz J., Blog D.S., Hutton B., Holtgrave D.R., Zucker H.A. Association of treatment with hydroxychloroquine or azithromycin with in-hospital mortality in patients With COVID-19 in New York State. JAMA. 2020;323:2493. doi: 10.1001/jama.2020.8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina J.M., Delaugerre C., Le Goff J., Mela-Lima B., Ponscarme D., Goldwirt L., de Castro N. No evidence of rapid antiviral clearance or clinical benefit with the combination of hydroxychloroquine and azithromycin in patients with severe COVID-19 infection. Med. Mal. Infect. 2020;50:384. doi: 10.1016/j.medmal.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langford B.J., So M., Raybardhan S., Leung V., Westwood D., MacFadden D.R., Soucy J.R., Daneman N. Bacterial co-infection and secondary infection in patients with COVID-19: a living rapid review and meta-analysis. Clin. Microbiol. Infect. 2020;26:1622–1629. doi: 10.1016/j.cmi.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and fungal co-infection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baskaran V., Lawrence H., Lansbury L.E., Webb K., Safavi S., Zainuddin N.I., Huq T., Eggleston C., Ellis J., Thakker C., Charles B., Boyd S., Williams T., Phillips C., Redmore E., Platt S., Hamilton E., Barr A., Venyo L., Wilson P., Bewick T., Daniel P., Dark P., Jeans A.R., McCanny J., Edgeworth J.D., Llewelyn M.J., Schmid M.L., McKeever T.M., Beed M., Lim W.S. Co-infection in critically ill patients with COVID-19: an observational cohort study from England. J. Med. Microbiol. 2021;70 doi: 10.1099/jmm.0.001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ruuskanen O., Lahti E., Jennings L.C., Murdoch D.R. Viral pneumonia. Lancet. 2011;377:1264–1275. doi: 10.1016/S0140-6736(10)61459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.A. Bhimraj, et al., COVID-19 Guideline, Part 1: Treatment and Management. 〈https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/〉.
- 19.Coronavirus SARS-CoV-2: Recommandations Thérapeutiques. 〈https://www.hcsp.fr/Explore.cgi/AvisRapportsDomaine?clefr=785〉.
- 20.T. Ichinohe, et al., Microbiota Regulates Immune Defense Against Respiratory Tract Influenza A Virus Infection, 6. [DOI] [PMC free article] [PubMed]
- 21.Percopo C.M., Rice T.A., Brenner T.A., Dyer K.D., Luo J.L., Kanakabandi K., Sturdevant D.E., Porcella S.F., Domachowske J.B., Keicher J.D., Rosenberg H.F. Immunobiotic Lactobacillus administered post-exposure averts the lethal sequelae of respiratory virus infection. Antivir. Res. 2015;121:109–119. doi: 10.1016/j.antiviral.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S., Jiang Z.Y., Sun Y.F., Yu B., Chen J., Dai C.Q., Wu X.L., Tang X.L., Chen X.Y. Microbiota regulates the TLR7 signaling pathway against respiratory tract influenza a virus infection. Curr. Microbiol. 2013;67:414–422. doi: 10.1007/s00284-013-0380-z. [DOI] [PubMed] [Google Scholar]
- 23.Kim S., Jazwinski S.M. The gut microbiota and healthy aging. Gerontology. 2018;64:513–520. doi: 10.1159/000490615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Claesson M.J., Jeffery I.B., Conde S., Power S.E., O'Connor E.M., Cusack S., Harris H.M., Coakley M., Lakshminarayanan B., O'Sullivan O., Fitzgerald G.F., Deane J., O'Connor M., Harnedy N., O'Connor K., O'Mahony D., van Sinderen D., Wallace M., Brennan L., Stanton C., Marchesi J.R., Fitzgerald A.P., Shanahan F., Hill C., Ross R.P., O'Toole P.W. Gut microbiota composition correlates with diet and health in the elderly. Nature. 2012;488:178–184. doi: 10.1038/nature11319. [DOI] [PubMed] [Google Scholar]
- 25.Angelucci F., Cechova K., Amlerova J., Hort J. Antibiotics, gut microbiota, and Alzheimer’s disease. J. Neuroinflamm. 2019;16:108. doi: 10.1186/s12974-019-1494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brouns S.H., Brüggemann R., Linkens A., Magdelijns F.J., Joosten H., Heijnen R., Ten Cate-Hoek A.J., Schols J., Ten Cate H., Spaetgens B. Mortality and the use of antithrombotic therapies among nursing home residents with COVID-19. J. Am. Geriatr. Soc. 2020;68:1647–1652. doi: 10.1111/jgs.16664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cangiano B., Fatti L.M., Danesi L., Gazzano G., Croci M., Vitale G., Gilardini L., Bonadonna S., Chiodini I., Caparello C.F., Conti A., Persani L., Stramba-Badiale M., Bonomi M. Mortality in an Italian nursing home during COVID-19 pandemic: correlation with gender, age, ADL, vitamin D supplementation, and limitations of the diagnostic tests. Aging. 2020;12:24522–24534. doi: 10.18632/aging.202307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M., Bonazzetti C., Covizzi A., Schiuma M., Passerini M., Piscaglia M., Coen M., Gubertini G., Rizzardini G., Cogliati C., Brambilla A.M., Colombo R., Castelli A., Rech R., Riva A., Torre A., Meroni L., Rusconi S., Antinori S., Galli M. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmcol. Res. 2020;158 doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., Zhao Y., Li Y., Wang X., Peng Z. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balmpouzis Z., Diamanti E., Kritikos A., Guery B., Nicod L.P. Systemic biomarkers in respiratory tract infections. Rev. Med. Suisse. 2018;14:2074–2078. [PubMed] [Google Scholar]
- 31.Mueller A.A., Tamura T., Crowley C.P., DeGrado J.R., Haider H., Jezmir J.L., Keras G., Penn E.H., Massaro A.F., Kim E.Y. Inflammatory biomarker trends predict respiratory decline in COVID-19 patients. CR Med. 2020;1 doi: 10.1016/j.xcrm.2020.100144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Antibiotic Resistance. 〈https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance〉.
- 33.Charteris W.P., Kelly P.M., Morelli L., Collins J.K. Antibiotic susceptibility of potentially probiotic Lactobacillus species. J. Food Prot. 1998;61:1636–1643. doi: 10.4315/0362-028x-61.12.1636. [DOI] [PubMed] [Google Scholar]
- 34.Salminen M.K., Rautelin H., Tynkkynen S., Poussa T., Saxelin M., Valtonen V., Järvinen A. Lactobacillus bacteremia, species identification, and antimicrobial susceptibility of 85 blood isolates. Clin. Infect. Dis. 2006;42:e35–e44. doi: 10.1086/500214. [DOI] [PubMed] [Google Scholar]
- 35.Lactobacillus | Johns Hopkins ABX Guide. 〈https://www.hopkinsguides.com/hopkins/view/Johns_Hopkins_ABX_Guide/540304/all/Lactobacillus?refer=true〉.
- 36.C. Landelle, D. Pittet, Definition, epidemiology, and general management of nosocomial infection. Oxford Textbook of Critical Care, Oxford University Press.