Abstract
Worldwide, many vaccines have been developed in response to the COVID-19 pandemic. Unilateral reactive axillary adenopathy related to the COVID-19 vaccine is a well-known occurrence. In addition, axillary edema has also been observed following COVID-19 vaccinations in patients undergoing breast MRI, and radiologists need to be aware of this possibility to avoid performing unnecessary work-up that can be costly to the health care system and be stressful for patients.
Introduction
Although unilateral axillary adenopathy has been well-recognized after administration of the COVID-19 vaccine and its management has been previously discussed and recently reviewed with a polled data [1], a new phenomenon has emerged, specifically as an imaging finding in women undergoing breast MRI. It refers to an acute axillary edema, which can be seen alone or in association with lymphadenopathy. This article aims to discuss 2 high-risk patients with histories of recent ipsilateral upper extremity COVID-19 vaccination who presented with imaging detected ipsilateral unilateral axillary edema with associated adenopathy.
Case 1
A 55-year-old high-risk woman with a family history of breast cancer and a lifetime risk of breast cancer estimated at 28% presented for a screening breast MRI. The patient had received the second dose of the Pfizer-BioNTech vaccine in the left upper arm 3 days before the MRI. On MRI, the breasts had minimal background enhancement and were unremarkable; in the left axilla, lymphadenopathy with associated edema was detected. The patient was recalled for further evaluation of her left axilla. On a subsequent ultrasound., 6 days after the vaccination, a prominent lymph node was identified, without associated edema (Fig. 1).
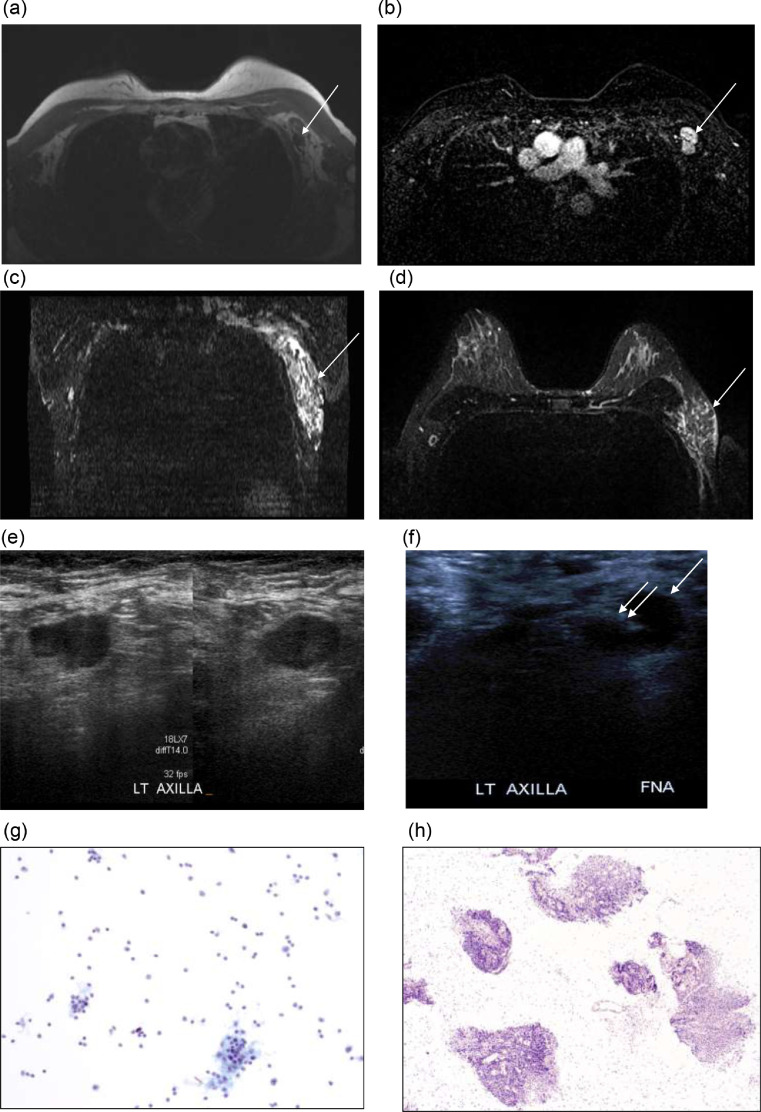
Fig. 1.
Axial T1-weighted sequence without fat saturation (A) and fat saturated, contrast enhanced MRI image (B) shows prominent left axillary lymph nodes with thickened cortex and absence of the fat in the hilum at level I (arrow). Bilateral T2-weighted short tau inversion recovery (STIR) reconstructed coronal (C) and axial (D) MRI demonstrate hyperintense T2 signal in the left axilla asymmetric compared to the right axilla, compatible with edema (arrow). Left axillary grayscale ultrasound, transverse plane (E,F) shows lymph node with thickened cortex, which underwent FNA (single arrow – lymph node; double arrow - needle). Magnified image of Pap-stained ThinPrep slide (G) showing polymorphous lymphoid population with follicular germinal center fragments. Magnified image of H&E-stained paraffin block (H) showing multiple fragments of benign lymphoid tissue with no evidence of metastatic carcinoma.
Case 2
A 52-year old high-risk woman, a BRCA2 gene mutation carrier, presented for screening breast MRI. The patient had received the second dose of the Pfizer-BioNTech vaccine in the left upper arm 2 days before the MRI. On MRI, lymphadenopathy with associated edema was detected in the left axilla. On the left axillary ultrasound, 5 days after vaccination, a slightly enlarged reactive lymph node with mild cortical thickening was seen without associated edema (Fig. 2).
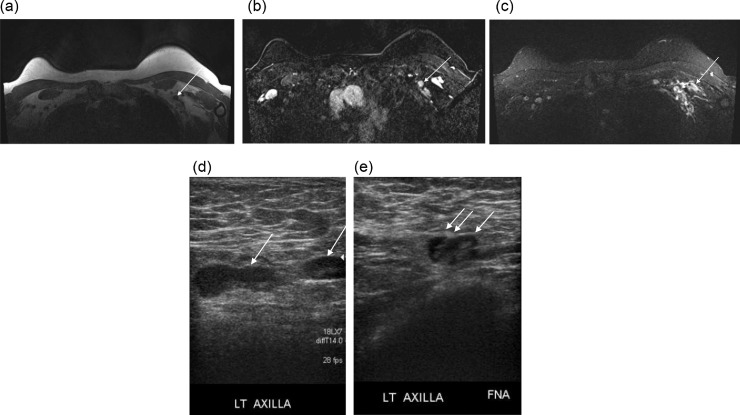
Fig. 2.
Axial T1-weighted sequence without fat saturation (A) and fat saturated, contrast enhanced MRI image (B) show a left axillary lymph node with lack of fat in the hilum, at level I (arrow). Bilateral T2-weighted short tau inversion recovery (STIR) MRI reconstructed axial (C) demonstrate hyperintense T2 signal in the left axilla asymmetric compared to the right axilla, compatible with edema. Bilateral axillary grayscale ultrasound, transverse plane (D,E) shows left-sided lymph node with thickened cortex but no edema is identified (D), which underwent FNA (single arrow – lymph node; double arrow - needle) (E). Magnified image of Pap-stained ThinPrep slide (F) showing polymorphous lymphoid population with follicular germinal center fragments.
Discussion
Lymphadenopathy in the axillary and the neck regions is known to be related to COVID-19 vaccine injections as part of the immunological response [2]. This has been documented in the literature as occurring shortly after receiving a vaccine that elicits a strong immune reaction, such as Bacille Calmette-Guerin, smallpox, human papillomavirus, or H1N1 influenza [3], [4], [5], [6], and has been well described after the first and the second doses of 3 types of COVID-19 vaccines, including Pfizer-BioNTech, Moderna, and Oxford-AstraZeneca, commonly occurring within 2-4 days after vaccination with a median duration of 12 days and 5 days after the first and the second vaccinations, respectively [1]. Although the incidence of vaccine-induced axillary lymphadenopathy appears to vary according to patient's age and vaccine's type, axillary lymphadenopathy has been reported in 7% of patients aged 12-15 years and up to 64% in patients over 55 years with Pfizer -BioNTech (BNT162b2), and overall up to 1.1% with Modern (mRNA-1273) vaccine [7], [8], [9]. The true rate of vaccine-associated axillary lymphadenopathy may be higher than reported because axillary symptoms were registered as an incidental side effect, which may cause underestimation. However, as far as we know, there is only one case report describing COVID-19-related acute axillary edema as part of the immune reaction [10]. In line with what was previously reported [10], this phenomenon seems to be acute, self-limited, as it was imperceptible on subsequent ultrasound a few days after the vaccination. As the administration of COVID-19 vaccine has become widespread, it is important to recognize this acute vaccine-related reaction. In addition, a delay in scheduling screening MRI studies may be prudent to avoid identifying this phenomenon and to prevent any unnecessary work-ups.
Patient consent
While we understand that proper consent, permissions and releases must be obtained when authors wish to include case details or other personal information or images of patients and any other individuals in an Elsevier publication, specifically for this case report, an exemption is requested by the authors, since the patients’ and research subjects’ names, initials, hospital or social security numbers, dates of birth, or other personal or identifying information of the research subjects were not used.
Footnotes
Competing interests: All authors declare that there are no financial disclosures and personal relationships with other people or organizations that could inappropriately influence (bias) our work.
References
- 1.Keshavarz P, Yazdanpanah F, Rafiee F, Mizandari M. Lymphadenopathy following COVID-19 vaccination: imaging finding review. Acad Radiol. 2021;28(8):1058–1071. doi: 10.1016/j.acra.2021.04.007. Aug. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puledran B, Ahmed R. Immunological mechanisms of vaccination. Nat Immunol. 2011;12(6):509–517. doi: 10.1038/ni.2039. Jun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newfield L, Naschitz JE, Yeshurun D. BCG-induced axillary lymph-adenitis in the adult. Harefuah. 1990;119(7-8):199–200. [PubMed] [Google Scholar]
- 4.Studdiford J, Lamb K, Horvath K, Altshuler M, Stonehouse A. Development of unilateral cervical and supraclavicular lymphadenopathy after human papilloma virus vaccination. Pharmacotherapy. 2008;28(9):1194–1197. doi: 10.1592/phco.28.9.1194. [DOI] [PubMed] [Google Scholar]
- 5.Shirone N, Shinkai T, Yamane T, et al. Axillary lymph node accumulation on FDG-PET/CT after influenza vaccination. Ann Nucl Med. 2012;26(3):248–252. doi: 10.1007/s12149-011-0568-x. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J, Powderly WG, Opal SM. Vol. 2. Elsevier Health Sciences; Amsterdam: 2017. p. 145. (Infectious diseases). ISBN: 9780702079351. [Google Scholar]
- 7.Centers for Disease Control and Prevention; CDC - Atlanta. Georgia, United States: 2020. Local reactions, systemic reactions, adverse events, and serious adverse events: moderna COVID-19 vaccine.https://www.cdc.gov/vaccines/covid-19/info-by-product/moderna/reactogenicity.html Available from. Updated December 20. Accessed August 08, 2021. [Google Scholar]
- 8.Centers for Disease Control and Prevention; CDC - Atlanta. Georgia, United States: 2021. local reactions, systemic reactions, adverse events, and serious adverse events: Pfizer-BioNTech COVID-19 vaccine.https://www.cdc.gov/vaccines/covid-19/info-by-product/pfizer/reactogenicity.html Available from: Updated May 14. Accessed August 08, 2021. [Google Scholar]
- 9.Polack FP, Thomas SJ, Kichin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodard S, Zamora K. Axillary edema one day after COVID-19 vaccination. J Breast Imaging. 2021:1–2. doi: 10.1093/jbi/wbab037. [DOI] [Google Scholar]