Abstract
Objective:
To evaluate disease flare and post-vaccination reactions (reactogenicity) in patients with rheumatic and musculoskeletal diseases (RMD) following two-dose SARS-CoV-2 mRNA vaccination.
Methods:
1377 patients with RMD who received two-dose SARS-CoV-2-mRNA vaccination between 16 December 2020 and April 15, 2021 completed questionnaires detailing local and systemic reactions experienced within 7 days of each vaccine dose (D1, D2), and one month after D2 detailing flare of RMD. Associations between demographic/clinical characteristics and flare requiring treatment were evaluated using modified Poisson regression.
Results:
11 percent reported flare requiring treatment; there were no reports of severe flares. Flare was associated with prior SARS-CoV-2 infection (IRR 2.09, p=0.02), flare in the six months preceding vaccination (IRR 2.36, p<0.001) and use of combination immunomodulatory therapy (IRR 1.95, p<0.001). The most frequently reported local and systemic reactions included injection site pain (D1 87%, D2 86%) and fatigue (D1 60%, D2 80%); reactogenicity increased after D2, particularly for systemic reactions. No allergic reactions or SARS-CoV-2 diagnoses were reported.
Conclusion:
Flare of underlying RMD following SARS-CoV-2 vaccination was uncommon. There were no reports of severe flare. Local and systemic reactions typically did not interfere with daily activity. These early safety data can help address vaccine hesitancy in patients with RMD.
Keywords: SARS-CoV-2, COVID-19, mRNA vaccine, rheumatic disease, flare
INTRODUCTION
Since its emergence in December 2019, SARS-CoV-2 has spread worldwide, with an estimated 115 million infections and 5.6 million hospitalizations in the USA alone at the time of this report (1). In December 2020, two mRNA-based COVID-19 vaccines (mRNA-1273 and BNT162b2) were recommended for use by the Advisory Committee on Immunization Practices (2,3), with over 260 million doses administered in the USA to date (4). Predicted vaccination uptake of 70–80% is required to significantly reduce community transmission (5); addressing vaccine hesitancy is crucial in achieving this goal.
In clinical trials of the SARS-CoV-2 mRNA vaccines, participants reported local and systemic postvaccination reactions (reactogenicity), with greater reactogenicity reported following D2 (6,7). Patients with rheumatic and musculoskeletal diseases (RMD) were not well represented in these trials, and despite the significant morbidity and mortality associated with SARS-CoV-2 infection, studies have found that many patients with RMD remain hesitant about getting vaccinated due to concerns related to the risk of disease flare (8,9). We previously reported that local and systemic reactions following D1 were typically mild (10), but there is a paucity of data on reactions or flares following D2. Thus, we sought to evaluate disease flare and reactogenicity in patients with RMD following two-dose SARS-CoV-2 mRNA vaccination.
PATIENT AND METHODS
Study design and population
We conducted a prospective observational study of patients with RMD who received the SARS-CoV-2 mRNA vaccine between 16 December 2020 and 15 April 2021. Patients ≥18 years old on immunomodulatory therapy with a self-reported diagnosis of RMD were recruited to participate via social media postings by national RMD organizations and advocacy groups. Participants consented electronically and the study was approved by the Institutional Review Board at the Johns Hopkins School of Medicine (IRB00248540).
Surveys
Participants first completed an English-language enrollment form containing questions on demographics (age, sex, and race), RMD diagnosis, immunomodulatory regimen, and prior SARS-CoV-2 diagnosis. An online questionnaire was distributed 7 days after each vaccine (D1, D2) where participants answered solicited questions about local and systemic adverse events; the questionnaire also allowed participants to enter free-text information about their post-vaccination experience and adverse health events. Local symptoms such as pain, redness, and swelling, as well as systemic adverse events including fever, fatigue, headaches, chills, vomiting, diarrhea, and myalgia were captured using an ordinal scale; these were graded per their impact on daily activity including “no interference with daily activity,” “some interference with daily activity,” and “prevention of daily activity”. One month following D2, participants completed an online questionnaire pertaining to incidence and prior history of flare, incident flare as well as symptoms, duration, and treatment. Most questions were multiple choice format, and some allowed for an open-ended response if participants felt that no given choice was appropriate. Study data were collected and managed using the REDCap (Research Electronic Data Capture) tool, a secure, web-based software platform designed to support data capture for research studies (11).
Statistical analysis
Baseline demographics and clinical characteristics were evaluated with descriptive statistics, stratified by disease flare after vaccination requiring treatment. Binary and categorical variables were expressed as number (percentage) and continuous variables with median (interquartile range). Associations between flare requiring treatment and participant characteristics were evaluated with modified Poisson regression with a robust variance estimator, generating incidence rate ratios (IRR). Associations were not calculated for variables with a frequency <10. Changes in denominators of questions due to participants selecting “prefer not to answer” or not responding are noted in the footnotes of the corresponding tables, as are questions with a multiple-select format where the sum of responses may exceed the total N. All tests were two-sided with an α level of 0.05. We conducted analysis using Stata software, version 16.1 (StataCorp LLC).
RESULTS
Baseline characteristics
A total of 1377 participants with RMD underwent BNT162b2 (55%) or mRNA-1273 (45%) vaccination (Table 1). Median (IQR) age was 47 (36–59), with 92% female, and 10% non-white. The most common RMD diagnoses included inflammatory arthritis (47%), systemic lupus erythematous (SLE, 20%), and overlap connective tissue disease (20%). The most common therapeutic regimens included combination therapy (50%), conventional disease modifying anti-rheumatic drugs (cDMARDs) (26%) and biologic therapy (22%). Combination therapy was defined as treatment with cDMARD and biologic agent with/without glucocorticoid, or biologic agent and glucocorticoid therapy. 3% reported a prior SARS-CoV-2 infection.
Table 1.
Demographic and clinical characteristics of 1377 patients with RMD, stratified by disease flare requiring treatment or no flare from D1 SARS-CoV-2 mRNA vaccine up to 1 month after D2.
| Overall (n=1377) | Disease flare (n=151) | No disease flare (n=1226) | p-value1 | |
|---|---|---|---|---|
| Age, median (IQR) | 47 (37, 59) | 46 (36, 57) | 47 (37, 60) | 0.2 |
| Female sex, no. (%)2 | 1266 (92) | 144 (95) | 1122 (92) | 0.1 |
| Non-white, no. (%)2 | 130 (10) | 8 (5) | 122 (10) | 0.1 |
| Diagnosis, no. (%) | ||||
| Inflammatory arthritis3 | 647 (47) | 73 (48) | 574 (47) | 0.7 |
| Systemic lupus erythematosus | 273 (20) | 30 (20) | 243 (20) | >0.9 |
| Sjogren’s syndrome | 65 (5) | 7 (5) | 58 (5) | >0.9 |
| Myositis | 66 (5) | 6 (4) | 60 (5) | 0.8 |
| Vasculitis | 41 (3) | 7 (5) | 34 (3) | 0.2 |
| Scleroderma | 14 (1) | 2 (1) | 12 (1) | 0.7 |
| Overlap connective tissue disease4 | 271 (20) | 26 (17) | 245 (20) | 0.5 |
| Vaccine, no. (%) | ||||
| BNT162b2 | 755 (55) | 82 (54) | 673 (55) | 0.9 |
| mRNA-1273 | 622 (45) | 69 (46) | 553 (45) | |
| Therapy, no. (%) | ||||
| Conventional DMARD5 | 352 (26) | 23 (15) | 329 (27) | 0.002 |
| Biologic 6 | 303 (22) | 22 (15) | 281 (23) | 0.02 |
| Glucocorticoid monotherapy7 | 35 (3) | 6 (4) | 29 (2) | 0.3 |
| Immunomodulatory monotherapy8 | 6 (0.4) | 1 (1) | 5 (0.4) | - |
| Combination9 | 681 (50) | 99 (66) | 582 (48) | <0.001 |
| Flare in 6 months prior to vaccine, no. (%) | 767 (56) | 113 (75) | 654 (53) | <0.001 |
| Prior COVID-19 diagnosis, no. (%) | 50 (3) | 11 (7) | 39 (3) | 0.02 |
Comparisons between disease flare and no disease flare groups, categories with an overall n<10 were not analyzed
The denominators for these categories differ from the total N as 1 participant selected “prefer not to answer” for sex and 23 selected “prefer not to answer” for race
Rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, reactive arthritis and inflammatory bowel disease associated arthritis
Overlap denotes a combination of two or more of the above conditions
Azathioprine, hydroxychloroquine, leflunomide, methotrexate, mycophenolate, sulfasalazine, and tacrolimus
Abatacept, adalimumab, anakinra, baricitinib, belimumab, certolizumab, etanercept, golimumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, tofacitinib, upadacitinib, and ustekinumab
Prednisone and prednisone equivalents
Intravenous immunoglobulin (IVIg) or subcutaneous immunoglobulin (SCIg), a p-value was not calculated as sample too small
Denotes cDMARD and biologic and/or glucocorticoid, or biologic and glucocorticoid
Flare requiring treatment : incidence and characteristics
767 (56%) of participants reported at least one flare of their underlying RMD in the six months preceding D1. 151 (11%) of participants reported flare requiring treatment following vaccination, of which the majority (60%) occurred after D2 (Supplementary Table 1). The rate of flare requiring treatment was similar in both vaccine types. Most (91%) reported worsening of pre-existing symptoms, while 72% reported onset of a new symptom (Supplementary Figure 1). Patients with inflammatory arthritis more commonly reported symptoms of worsening joint pain, swelling and stiffness while patients with SLE reported worsening joint pain, fatigue, and myalgia (Supplemental Table 2). Flares that required treatment typically lasted 10 days (IQR 6–22) and were most commonly treated with oral corticosteroids (75%), while 23% of participants reported up-titration of their baseline immunomodulatory therapy. No participant required hospital or ICU admission. Factors found to be associated with flare requiring treatment included a prior SARS-CoV-2 diagnosis (IRR 2.09 95%, CI 1.21–3.60), a history of flare in the 6 months prior to D1 (IRR 2.36, 95% CI 1.66–3.36) and use of combination therapy (IRR 1.95 95% CI 1.41–2.68). Participants on cDMARD (IRR 0.52, 95% CI 0.34–0.80) or biologic therapy (IRR 0.60,95% CI 0.39–0.93) had lower incidence of flare (Table 2).
Table 2.
Adjusted incident rate ratios of having a flare requiring treatment by demographic and clinical characteristics
| aIRR (95% CI) | p-value | |
|---|---|---|
| Age, >55 | 0.79 (0.57–1.11) | 0.2 |
| Sex, female | 1.79 (0.86–3.72) | 0.1 |
| Race, white | 1.86 (0.93–3.7) | 0.1 |
| Vaccine, Pfizer | 0.98 (0.72–1.32) | 0.9 |
| Flare in 6 months prior to vaccine | 2.36 (1.66–3.36) | <0.001 |
| Prior SARS-CoV-2 diagnosis | 2.09 (1.21–3.60) | 0.008 |
| Diagnoses | ||
| Inflammatory arthritis1 | 1.06 (0.78–1.43) | 0.7 |
| Systemic lupus erythematosus | 1.00 (0.69–1.46) | >0.9 |
| Sjogren’s syndrome | 0.98 (0.48–2.00) | >0.9 |
| Myositis | 0.82 (0.38–1.79) | 0.6 |
| Vasculitis | 1.58 (0.79–3.17) | 0.2 |
| Systemic sclerosis | 1.31 (0.36–4.76) | 0.7 |
| Overlap connective tissue disease2 | 0.85 (0.57–1.27) | 0.4 |
| Therapy | ||
| Conventional DMARD3 | 0.52 (0.34–0.80) | 0.003 |
| Biologic 4 | 0.60 (0.39–0.93) | 0.02 |
| Glucocorticoid monotherapy5 | 1.59 (0.75–3.34) | 0.2 |
| Immunoregulatory monotherapy6 | - | - |
| Combination therapy7 | 1.95 (1.41–2.68) | <0.001 |
Rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis, reactive arthritis and inflammatory bowel disease associated arthritis
Overlap denotes a combination of two or more of the above conditions
Azathioprine, hydroxychloroquine, leflunomide, methotrexate, mycophenolate, sulfasalazine, and tacrolimus
Abatacept, adalimumab, anakinra, baricitinib, belimumab, certolizumab, etanercept, golimumab, infliximab, ixekizumab, rituximab, secukinumab, tocilizumab, tofacitinib, upadacitinib, and ustekinumab
Prednisone and prednisone equivalents
Intravenous immunoglobulin (IVIg) or subcutaneous immunoglobulin (SCIg), a p-value was not calculated as sample too small
Denotes cDMARD and biologic and/or glucocorticoid, or biologic and glucocorticoid
Local and systemic reactogenicity
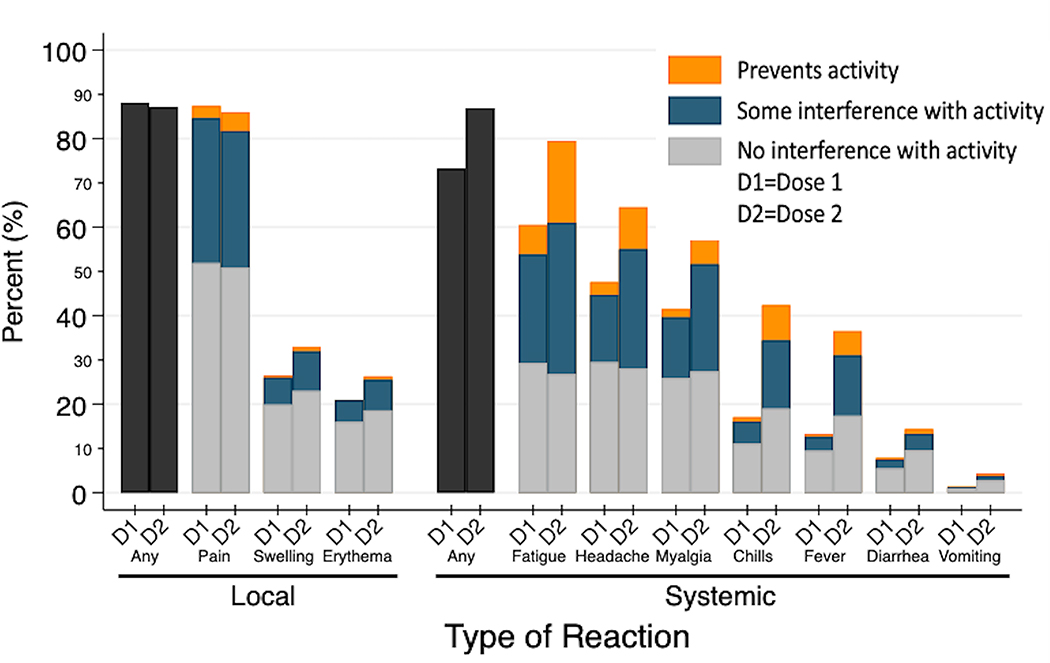
The most frequently reported local and systemic reactions included injection site pain (D1 87%, D2 86%) and fatigue (D1 60%, D2 80%) (Figure 1). Reactogenicity increased after D2, particularly for systemic reactions, including fatigue (80%), headache (65%), myalgia (63%) and chills (42%). Reports of reactions that prevented daily activities were uncommon, with myalgia (11%) and fatigue (19%) most frequently reported. One patient (0.07%) reported hospital admission for management of diarrhea following D2.
Figure 1.
Local site and systemic adverse reactions in 1377 patients with RMD within 7 days after D1 and D2 of SARS-CoV-2 mRNA vaccination.
DISCUSSION
We studied 1377 patients with RMD who received two-dose SARS-CoV-2 mRNA vaccination and did not identify any major safety concerns. There were no reports of severe disease flare. Local and systemic reactions were common, but consistent with expected vaccine reactogenicity.
Eleven percent of participants reported flare of their underlying disease requiring treatment. No participant required intravenous therapy or hospitalization. Use of combination therapy, as well as report of flare in the six months preceding vaccination were associated with flare; these factors may be a surrogate for more refractory disease at baseline and thus the relationship with vaccination is unclear. There was a positive association between prior SARS-CoV-2 infection and flare, which may suggest immunological priming.
Local and systemic reactions were common, but reassuringly, very few patients reported symptoms that prevented daily activities. Participants reported systemic events (particularly myalgia and fatigue) at higher frequency than clinical trials; similar to the trials reactogenicity increased after D2 (6,7). One patient reported hospitalization for management of systemic event. There were no reported cases of anaphylaxis requiring epinephrine or newly diagnosed SARS-CoV-2 infection.
Limitations of this study include a lack of data on immunomodulatory timing and dosing. We did not assess baseline disease activity and background rate of flare was not quantified. Most participants were female and white, which may limit generalizability.
The strengths of this study include a national sample with early, novel information about adverse reactions after two-dose BNT162b2 and mRNA-1273 vaccination. All data was patient-reported; this is a strength, but given that recruitment was by convenience sampling, the data is also susceptible to responder bias.
Data on vaccine safety in patients with RMD has been lacking, which has contributed to vaccine refusal and hesitancy. It has been shown that patients with RMD are more willing to reconsider vaccination if provided with more medical education and recommended by a physician (10). While there are case reports of flare following mRNA vaccination (12,13), this is the first large scale study evaluating flare and reactogenicity in patients with RMD. Local and systemic reactions should be anticipated and setting expectations with patients may alleviate anxiety. This is particularly relevant given periodical revaccination may be required in future (14). These early, reassuring results may ameliorate concern among patients and inform critical discussions regarding vaccine hesitancy or refusal.
CONCLUSION
In this observational study of 1377 patients with RMD, there were no reports of severe disease flare. Local and systemic reactions were common but typically did not interfere with daily activity. There were no findings that warranted concern about the safety of SARS-CoV-2 mRNA vaccination in patients with RMD. These early data can continue to address vaccine hesitancy in this patient population.
Supplementary Material
FUNDING
This research was made possible with generous support of the Ben-Dov family. This work was supported by grant number F32DK124941 (Boyarsky), and K23DK115908 (Garonzik-Wang) from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), K24AI144954 (Segev) from National Institute of Allergy and Infectious Diseases (NIAID), K23AR073927 (Paik) from National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAIM). The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government.
Footnotes
CONFLICT OF INTEREST
Dorry L. Segev, MD PhD has the following financial disclosures: consulting and speaking honoraria from Sanofi, Novartis, CSL Behring, Jazz Pharmaceuticals, Veloxis, Mallinckrodt, Thermo Fisher Scientific.
Lisa Christopher-Stine has the following financial disclosures: consultant fees from Janssen, Boehringer-Ingelheim, Mallinckrodt, EMD-Serono, Allogene, and ArgenX.
Duvuru Geetha has the following disclosures: consultant to ChemoCentryx and Aurinia
The other authors of this manuscript have no financial disclosures or conflicts of interest to disclose as described by Arthritis & Rheumatology
REFERENCES
- 1.Estimated Disease Burden of COVID-19. Centers for Disease Control and Prevention. Accessed May 21st 2021. https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/burden.html [Google Scholar]
- 2.Oliver SE, Gargano JW, Marin M, Wallace M, Curran K, Chamberland M et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine: United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69(5152):1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver SE, Gargano JW, Marin M, Wallace M, Curran K, Chamberland M et al. The Advisory Committee on Immunization Practices’ interim recommendation for use of Moderna COVID-19 vaccine: United States, December 2020. MMWR Morb Mortal Wkly Rep 2021;69(5152):1653–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trends in number of COVID-19 vaccinations in the US. Centers for Disease Control and Prevention. Accessed May 15, 2021. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends [Google Scholar]
- 5.Anderson RM, Vegvari C, Truscott J, Collyer BS. Challenges in creating herd immunity to SARS-CoV-2 infection by mass vaccination. 2020. November 21;396(10263):1614–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Polack F, Thomas S, Kitchin N, Absalon J, Gurtman J, Lockhart S et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. New England Journal of Medicine 2020;383(27), pp.2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baden L, El Sahly H, Essink B, Kotloff K, Frey S, Novak R et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. New England Journal of Medicine 2021;384(5), pp.403–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Priori R, Pellegrino G, Colafrancesco S, Alessandri C, Ceccarelli F, Di France M et al. SARS-CoV-2 vaccine hesitancy among patients with rheumatic and musculoskeletal diseases: a message for rheumatologists. Annals of the Rheumatic Diseases 2021. February 23:annrheumdis-2021–220059 [DOI] [PubMed] [Google Scholar]
- 9.Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H et al. Vaccination against COVID-19: Expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol. 2021. April;3(4):e243–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly CM, Ruddy JA, Boyarsky BJ, Avery RK, Werbel WA, Segev DL et al. Safety of the first dose of mRNA SARS-CoV-2 vaccines in patients with rheumatic and musculoskeletal diseases. Annals of the Rheumatic Diseases 2021. March 19:annrheumdis-2021–220231. [Google Scholar]
- 11.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009. April;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terracina KA, Tan FK. Flare of rheumatoid arthritis after COVID-19 vaccination. Lancet Rheumatol. 2021. March 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrot L, Hemon M, Busnel JM, Muis-Pistor O, Picard C, Zandotti C et al.First flare of ACPA-positive rheumatoid arthritis after SARS-CoV-2 infection. Lancet Rheumatol. 2021. January;3(1):e6–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skegg D, Gluckman P, Boulton G, Hackmann H, Karim SSA, Piot P, et al. Future scenarios for the COVID-19 pandemic. Lancet. 2021. February 27;397(10276):777–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.