Abstract
Some individuals’ understanding of informed consent (IC) information may improve with electronic delivery, but others may benefit from face-to-face (F2F). This randomized, multisite study explores how individuals from diverse backgrounds understand electronic IC documents versus F2F, their confidence in understanding, and enrollment in research. A total of 501 patients at two U.S. biobanks with diverse populations participated. There were no overall differences between electronic and F2F understanding, but F2F predicted higher confidence in understanding and enrollment. Ethnicity and a higher educational level predicted higher understanding and confidence. Study findings suggest that electronic consent may lead to better understanding for non-Hispanic patients of higher socioeconomic status. F2F processes may lead to better understanding and higher enrollment of patients from Hispanic and lower socioeconomic levels. Researchers should carefully consider how they implement electronic IC processes and whether to maintain an F2F process to better address the needs and limitations of some populations.
Keywords: electronic, interactive, multimedia, informed consent, biobanking
INTRODUCTION
Background
Obtaining informed consent (IC process) is an ethically and legally important communication and decision-making process for the purposes of enabling informed, voluntary participation in research (Beauchamp & Childress, 1979; Berg et al., 2001). Presently, there are several different means by which IC processes are conducted: face-to-face (F2F) interactions, by mail (ground), or electronically, using digital delivery platforms (e.g., smartphones, tablets, desktop computers), the internet, different formats (PDF documents, HTML, video, multimedia, cartoons, PowerPoint slides), and a broad array of digital tools that encourage interactivity, which can include quizzes, pull-down menus, hyperlinks, vignettes, and simulations (De Sutter, et al., 2020; Francesco et al., 2019; Nishimura et al., 2013). Today, researchers theoretically have the option of consenting individuals to their studies through exclusive use of an electronic IC (eIC) process, or by using an eIC process in combination with other consenting means such as F2F interactions and/or consent mailings.
It is not known to what extent, if at all, researchers are blending the use of different consent modalities in an effort to recruit individuals into research. At most, there is some evidence suggesting that eIC processes are becoming increasingly popular owing in part to the efficiency of electronic platforms over more traditional consenting means (De Sutter et al., 2020; Simon et al., 2014). Other factors that have undoubtedly increased interest in eIC have been increased access to broadband internet and availability of development tools and developers. Studies have remarked on the high costs of informed consent since the early 1990s, and efforts to automate the IC process have been repeatedly viewed as a useful cost containment step for hospitals and research centers (Schuck, 1994).
On average, biobanks recruit relatively large numbers of participants (Henderson et al., 2013) and when recruitment and obtaining informed consent are closely connected, both stand to benefit considerably from the use of electronic processes. Among some biobanks, F2F interactions are associated with a higher rate of consent than eIC processes (e.g., Boutin et al., 2016). However, many more individuals can be reached electronically than through F2F interactions, translating into significant gains in recruitment. Investigators at the Partners HealthCare Biobank, for example, a large biospecimen and data repository at Massachusetts General Hospital, found that their recruitment rate increased tenfold when shifting their recruitment process from F2F interactions to a recruiting and eIC process in which people were emailed and then directed to a secure internet platform (Boutin et al., 2016). Other investigators have identified similarly dramatic recruitment gains using digital technologies (e.g., Wilbanks, 2018).
It is conceivable that the capacity to efficiently access and recruit large numbers of individuals using eIC may lead researchers and research organizations away from other, more traditional IC pathways, including F2F interactions. However, before they make this transition, researchers should carefully consider the complex dynamics surrounding participant understanding in the IC process, and the possible need to maintain an alternative to electronic means of consent.
Participant understanding in the IC process
It is widely expected, although not proven, that eIC processes can improve individuals’ understanding of information that needs to be legally and ethically conveyed to them before they are enrolled into research (Boutin et al., 2016; Branch, 2017; Simon et al., 2014; Wilbanks, 2018). Long, complicated, and non-interactive IC processes have been repeatedly associated with inadequate understanding or misunderstanding of critical research information following individuals’ participation in IC processes generally (Beskow et al., 2015; Joffe et al., 2001; Simon et al., 2015). Across 25 quantitative studies examined by Eisenhauer et al. (2019), understanding of IC elements was measured at less than 80%. In the biobanking arena, commonly misunderstood elements of IC included the scope of biobank research, the potential risks and benefits of participation, biospecimen ownership, privacy and confidentiality, and the return of individual-level results (Allen & McNamara, 2011; Lemke et al., 2010; Ormond et al., 2009).
Understanding of the IC document is a larger issue for participants in populations underrepresented in research. In the US, individuals with less education performed more poorly on IC understanding measures compared to those with college educations (Agre & Rapkin, 2003; Flory et al., 2007; Goddard et al., 2009). Lower literacy and low health literacy have had an effect on IC understanding (Hughson et al., 2016). Lower levels of income have correlated with lower understanding of consent documents (Eisenhauer et al., 2019; Goddard et al., 2009). Race and ethnicity may also play a role. In the US, African Americans, Hispanics, and individuals with limited English proficiency have displayed gaps in understanding IC information for genomic and clinical research (Griffin et al., 2006; Kaphingst et al., 2012; Simon et al., 2006). In sum, inadequate understanding of IC information is associated with a range of factors, notably education, literacy, and income, and is a persistent challenge in ensuring that people’s (non)participation in research is informed and voluntary.
Electronic and interactive multimedia: An effective response?
One challenge for researchers is how to improve understanding of the IC document, particularly for underrepresented groups, and eIC has been posited as a possible way to improve on traditional IC. Transferring the traditional F2F IC process to digital/electronic opens up the possibility for integration of multimedia materials (words with supporting audio and visual information) into the IC process. Despite a mix of supportive evidence in informed consent research, multimedia tools have been theorized to improve understanding of traditional IC materials (Tait, Voepel-Lewis & Levine, 2015; Palmer, Lanouette, & Jeste, 2012). An IC process is in part a learning task, and multimedia may enhance participant learning. A large body of research in the educational literature has reliably shown improvements in understanding and memory of information when multimedia instruction is compared to information presented in words alone (Cherry et al., 1996; Clark & Mayer, 2008; Mayer, 2005, 2009; Sadoski & Paivio, 2001). Beyond applications of multimedia in education, it has also been used effectively in clinical settings, where it has been extensively used to obtain informed consent for medical procedures (i.e., not research) (Chiou & Chung, 2012; Schenker et al., 2011; Tait & Voepel-Lewis, 2015).
One advantage of F2F consent processes is the presence of the researcher, who can interact with prospective participants to help them understand research concepts and procedures. So besides multimedia, electronic technologies such as those used for eIC processes can also include interactivity, to help participants understand research while maintaining the advantages of eIC, such as scalability. People learn better through active use of new information, including repetition (practice) and connecting information to prior knowledge (Anderson et al., 2017). In contrast to passive learning such as reading text or watching a video, including interactivity in eIC can correct misconceptions and help participants remember information. One approach is to embed quizzes, such as multiple-choice questions, into the IC process, whether face to face or digital. For example, IC studies using interactive “test/feedback” techniques, including some using multimedia, have showed improvement in patient knowledge of informed consent (Nishimura et al., 2013; Schenker et al., 2011).
Given the capacity of eIC processes such as interactive multimedia to standardize information delivery, use empirically validated methods of instruction, and enhance user engagement with the use of graphics, audio, video, pull-down menus, and other capabilities, eIC tools are widely expected to positively affect IC understanding (e.g., Grady, 2017; Shenoy, 2015). Wilbanks comments that eIC “provides an opportunity to truly inform research participants about clinical protocols [to] provide a meaningful choice architecture to support a potential participant’s decision making about whether or not to enroll” (2018, p. 110).
However, studies exploring how eIC processes affect understanding compared to other consent methods have had mixed results. Meta-analyses suggest that eIC processes are no better at promoting understanding of research studies than traditional consent methods (Nishimura et al., 2013; Lunt et al., 2019), although one meta-analysis recommends multimedia as a way to improve short-term understanding of information (Farrell et al., 2014), and some studies have shown an improvement in longterm retention (Hughson et al., 2016; Cornoiu et al., 2011; Rowbotham et al., 2013).
In 2013, we conducted a prospective randomized study of the informed consent process for a biobank (Simon et al., 2015). The study used a 2 × 2 design exploring traditional face-to-face informed consent as opposed to enhanced interactive and multimedia approaches to delivering the informed consent process. Participants were randomly assigned to one of four groups:
F2F Standard Interactivity – paper consent document with standard researcher-participant discussion (Control)
F2F Enhanced Interactivity – paper consent document, researcher-participant discussion, and 13 targeted, interactive questions on paper
Multimedia Standard Interactivity – electronic consent document text, graphics, and verbatim narration of text with participants able to ask questions at any time to a researcher present in the room
Multimedia Enhanced Interactivity – electronic, multimedia consent procedure as above and 13 targeted, interactive questions delivered electronically
The study was conducted at a single site among a well-educated population. The results showed independent effects for Multimedia (p = 0.04) and Enhanced Interactivity (p = 0.007) in improving participants’ understanding of the consent document. Interactivity also showed an increase in participant confidence in their understanding (p = 0.01). In this study, 79% of participants reported at least a college degree. Almost one in four (24%) reported a household income of $100,000 or more. And they were predominantly Caucasian (96%). Because of the robust effects for Multimedia and Enhanced Interactivity in this prior study, we were interested in exploring these approaches with more diverse populations, particularly people from underrepresented groups, including nonCaucasians, people from lower sociodemographic backgrounds, and people with less education.
eIC among diverse research populations
To our knowledge, few studies compare electronic to other consent methods for understanding in diverse populations where people are likely to have lower levels of education and face health literacy and technology barriers. Among studies that explore diversity, Griffin et al. (2006) investigated recall of basic information at the end of a trial among male U.S. military Veterans and showed an effect for age and race/ethnicity. The authors suggest that differences may be related to education, health literacy, and/or patients’ exposure to scientific terminology. A few studies have focused on participants with mental illness (Dunn et al., 2002; Moser et al., 2006), showing some benefit.
Individuals’ understanding of IC materials is commonly expected to improve with electronic tools (Boutin et al., 2016; Tait & Voepel-Lewis, 2015; Frelich et al., 2015; Lentz et al., 2016). Yet real-world data to support this expectation are limited. For biobanks, our prior studies (e.g., Simon et al., 2015) have shown that an interactive multimedia consent document illustrated with graphics and using interactive questions resulted in better understanding of key elements of biobank participation when compared to a conventional F2F process. However, our latest, single-site study, a prototype to this study, was carried out with a well-educated and racially and ethnically homogenous sample. Because of the robustness of the results in our previous study, we hypothesized that enhanced interactivity with multimedia would improve understanding and confidence of understanding among different populations in different settings. In the current study, what was called “F2F Standard Interactivity” in our prior study is called F2F IC in this study. Similarly, what was called “Enhanced Interactivity with Multimedia” is defined as Electronic Informed Consent (eIC). How effectively a similar multimedia presentation that uses similar enhanced interactivity would promote understanding in other biobanks and among diverse populations, including among individuals with limited formal education, was the question driving the current study.
METHODS
Study Design
This randomized, multisite study (Consent Study) was conducted at three biobanks located in the US Midwest, Northeast, and Southeast. We were seeking sites that were unlike our pilot study: possibly based in urban settings, drawing from populations of diverse races and/or ethnicities and from different sociodemographic and educational backgrounds. These sites offered good contrasts for our prior study as well as between each other. In addition, the Northeast site included two study groups based on the language preferred by the participant: English and Spanish. All sites were linked to academic research centers and were in urban areas. The Northeast site was part of a Federally Qualified Health Center.
The study compared an electronic version of each biobank’s IRB-approved consent document, including interactive questions, to the F2F consent processes already used at these biobanks. The consent documents differed in length, readability, and content. These documents ranged from less than 2 pages to 7 pages, not including the signature pages (see Table 1). The readability scores ranged from a grade level of 7 to 11. Readability score results were calculated using the Fry Readability Index (Fry, 1977), the only validated scoring formula currently available that provides a grade level and which can be theoretically compared between English and Spanish. Syllable and word count were calculated at https://www.webfx.com/tools/readable/. Syllables per 100 words and words per sentence were calculated for each consent document (not including the signature pages) and the results were used in the Fry readability graph to determine a grade level estimate. The Spanish score was calculated by subtracting 67 from the syllables per 100 count (Gilliam, Peña, & Mountain, 1980).
Table 1.
Key Consent Document Characteristics.
| Consent document characteristics | |||||
|---|---|---|---|---|---|
|
| |||||
| Study group | Word count | Readability (grade level)a | Total number of slides | Number of interactive questions | Question/slide ratio |
|
| |||||
| Midwest | 1,692 | 11 | 62 | 11 | 0.177 |
| Northeast-E | 939 | 10 | 43 | 9 | 0.209 |
| Northeast-S | 997 | 7 | 43 | 9 | 0.209 |
| Southeast | 3,477 | 11 | 127 | 19 | 0.150 |
Note. Northeast-E = English-speaking Northeast; Northeast-S = Spanish-speaking Northeast.
Readability score results were calculated using the Fry readability index.
Study Sites
The Midwest biobank focused on blood-based specimens, where participants agreed to provide an extra sample during their next blood draw. The biobank group identified patients who were asked to return to their clinic for a blood draw and directed participants to a special office specifically reserved for the biobank consenting process. Many of the participants at this site had already participated in an IC process for another biobank project prior to their participation in this study. The staff at this site were also career researchers and research assistants who have been recruiting for this biobank for months to years prior to this study.
The Northeast biobank emphasized collecting leftover blood, tissue, or fluids. Patients at this site were recruited from clinics where patients were waiting for appointments, and the consenting process was carried out in a private location in the clinic. Occasionally, consenting was interrupted while patients attended their appointments and completed after appointments were done. Research staff at this site conducting the IC process were recently hired graduate students from Premed programs who were fluent in both English and Spanish. Training on recruitment and F2F IC for this biobank was done by a site biobank administrator.
The Southeast biobank procured leftover tissue from breast cancer surgery as well as blood and saliva. Initially, they recruited patients at appointments prior to surgery and performed the consenting process after appointments. Later, because of low enrollment, recruitment was moved to a cancer infusion clinic, where the consenting process was conducted while patients were undergoing infusion. Because this change converted the study at this site to a hypothetical design, we have excluded the site’s data from our analysis in this paper (see the rationale below).
Site Procedures
The researchers in this study were particularly interested in the eIC process in authentic settings for biobanks. Thus, this Consent Study used the existing consent documents for each site, including the version in Spanish translated at the Northeast site. In addition, the Consent Study was embedded in the standard procedures and staff assignments used at each site. The main differences this study imposed on each biobank’s consent process included a brief consent to the Consent Study, the study’s data collection instruments, and the interactive, multimedia intervention in the randomly assigned eIC group.
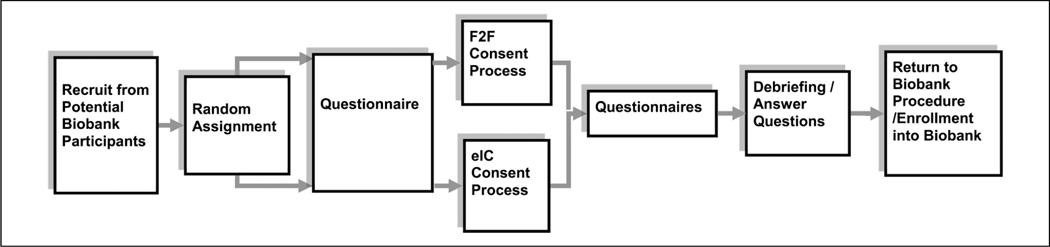
Prior to the start of the study, staff who conducted the study were trained in the procedures of the study in detail. Because this study was inserted into the regular site biobank recruiting and consent process, the staff were asked to maintain the site’s normal recruitment and consent process, with the exception of recruiting for this study, the eIC condition, and the questionnaires used in this study (Figure 1). The steps for training the study staff included the following: what to say to recruit participants from individuals being recruited for the biobank, how and when to assign participants to conditions, how to log timestamps, what to say to prepare participants for their assigned consent process, including explaining the tablet interface, how to conduct the electronically delivered questionnaires, and how to debrief participants and end the study. Once the Consent Study was completed, staff transitioned participants to the regular site biobank procedure for enrolling participants. Thus, except for differences in the consent document, environmental differences in sites, and experience levels of staff, the procedures for this study were controlled between sites.
Figure 1.
Study procedure within site biobank consent process.
Because of logistical issues from researcher schedules, site differences, and funding restrictions, individual staff had to conduct both eIC and F2F conditions for this study. Participants received $35 incentive to help offset parking or transportation and their time and inconvenience in participating in the study.
Standard Consent Process
For the F2F condition, each site used their standard face-to-face IC process that they had normally used for enrolling participants to their respective biobanks. At the Midwest site, staff took participants to a private office used exclusively for the IC process. At the Northeast site, staff recruited participants in clinic waiting rooms and settled in private locations in or near the clinics to complete the IC process. In all cases, staff discussed the salient concepts and procedures from their consent document and answered questions as needed. (This is equivalent to what we called “F2F Standard Interactivity” in our previous study.)
eIC Intervention
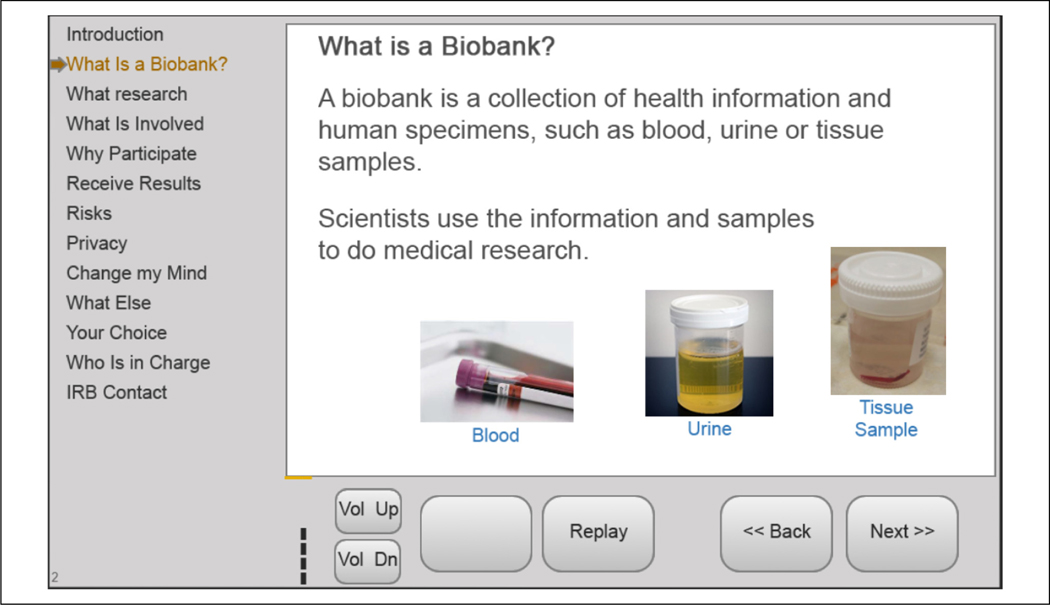
The eIC intervention was conducted in place of the F2F IC process. The design of the eIC intervention in this study closely followed the researchers’ previous study (Simon et al., 2014). An online eIC version of each biobank’s consent document used a PowerPoint-style slideshow developed in an XHTML-based application and delivered using a tablet computer. Exact text from the consent document was segmented in slides based on roughly one concept per slide (usually 1–2 sentences), with some slides containing relevant graphics (see Figure 2). When possible, the same graphics were used across sites. Interactive, multiple-choice questions were placed throughout each slideshow to reinforce crucial concepts. The number of interactive questions varied based on the consent document, and questions addressed concepts that were congruent across sites. (This is equivalent to what we called “Multimedia Enhanced Interactivity” in our previous study.) The Spanish version of the slideshow at the Northeast site was identical to the English version, except for the translation and the audio narration. The narration was performed by a native speaker from the site’s most common dialect group (Dominican).
Figure 2.
Sample electronic informed consent (eIC) screen using exact wording from the biobank consent document with added graphics.
At the Midwest site, like the F2F IC process, the eIC intervention was conducted at a private office reserved for the IC process. At the Northeast site, again like their F2F process, the intervention was conducted at a private location in or near the clinic where participants were recruited. Staff explained how to navigate the multimedia interface, such as how to go forward and back in the slideshow, until participants were comfortable with the interface. Then participants were allowed to complete the eIC process on their own. Staff remained close by to help with any technical questions and to answer any questions about the biobank. Across all sites the eIC slideshow used the same interface, all delivered via tablet computers, with research assistants trained to conduct the procedures for this study consistently across all sites.
Anecdotally based on our prior research, we anticipated that the eIC intervention would add some elements to the F2F process. Participants who had no experience with touchscreens, or tablet touchscreens specifically, might have needed some time to get used to touching buttons on the screen. Wireless networks had not always provided optimal bandwidth for multimedia content in tablets, so there may have been delays in moving from slide to slide. Staff would take some extra time training participants on the interface. On the other hand, the text and narration of the document content was tested with the target population, and delivery of eIC content would be more standardized than delivery of the IC document during typical F2F interactions. The social interactions of a F2F process would also not be as much a part of the content delivery in the eIC process.
Because we were investigating how an interactive multimedia presentation of IC would improve understanding and confidence of understanding among people of different races, ethnicities, and sociodemographic and educational backgrounds, we planned to use regression analyses so that we could not only identify effects, but we could also learn the relative contributions of these factors to understanding and confidence.
Procedure
Eligibility for this study (the Consent Study) was determined by the inclusion criteria of each of the biobanks. Eligible individuals were recruited to the Consent Study under a waiver of elements of consent. On enrollment into the Consent Study, participants were assigned a study ID, randomized to receive either the F2F or eIC condition, and asked to fill out a brief Computer Self-Efficacy Assessment. Biobank staff then either conducted the F2F consent process as they would normally or introduced participants to the e-tablet. For eIC participants, the researchers showed how to navigate the slideshow using the tablet; then participants were asked to complete the slideshow on their own, though researchers remained available for assistance. In both conditions, researchers were available to answer questions anytime during the consent process. After completing the F2F or eIC condition, participants were asked if they intended to enroll in the biobank (i.e., Intent to Enroll). Participants then completed several assessments (see below) and a demographic questionnaire. Staff then invited further questions and ended the Consent Study, at which point participants could enroll in the biobank (Actual Enrollment). All instruments were translated into Spanish for the Spanish-speaking participants at the Northeast site by translators fluent in the site’s dominant dialect.
Outcome measures
Understanding
One advantage of biobank informed consent research across different sites is that biobank consent documents contain largely the same concepts. For this study, a single assessment (Appendix A) focusing on key elements of biobank information (Kaye et al., 2015) for the participating study sites was developed based on the Common Rule (General requirements, 2009) and those elements recommended in Beskow and Weinfurt (2019). The measuring and scoring system used in the assessment was drawn from the validated QuIC measure used in Ormond et al. (2009) and Joffe et al. (2001). This assessment included 29 “site-accurate” statements with which participants could respond Agree, Disagree, or Unsure. Statements were scored (100=correct response; 50=unsure response; 0=incorrect response). Response scores were then averaged. Higher average scores indicated better understanding.
Confidence in Understanding
Confidence in Understanding, also used in Joffe et al.’s QuIC instrument (2001), was adapted for this study as a measure of participants’ confidence in their understanding of key information provided in the biobank consent document (Appendix B). In this study, the Confidence assessment included 15 statements related to basic biobank concepts rated on a 5-point Likert scale (“I didn’t understand this at all” to “I understood this very well”).
Enrollment
Intent to Enroll:
Immediately after participants underwent either the eIC or F2F consent process, they were asked whether they planned to participate in the biobank. This variable was used to gauge the effect on participant intention to enroll in the biobank at the point immediately following the consent condition.
Actual Enrollment:
Once the Consent Study was completed (including the assessments), participants were given the opportunity to enroll in the biobank.
Demographics
Among demographic factors, some studies have linked level of education of participants as a factor related to ability to understand a consent document (Flory, Wendler, & Emanuel, 2007; El-Wakeel, Taylor, & Tate, 2006). A few studies have associated age with understanding, particularly with older populations (Flory, Wendler, & Emanuel; Dunn et al., 2007; Griffin et al., 2006). Gender has been associated with recruitment and attitudes about participation (Coakley et al., 2012; Farmer et al., 2007; Lewis et al., 1998). Race may be a factor in participants’ understanding of research and biobanking terminology as well as in trust in research and researchers (Davis et al., 2019; Kim & Milliken, 2019; George, Duran, & Norris, 2014). Ethnicity may also play a role in how some populations respond to the IC process (McFarlane et al., 2019; Quinn, G., et al., 2012; Quinn, S., et al., 2013; Skinner et al., 2019; Rangel et al., 2018). And ethnicity can be a factor when the IC document is translated into Spanish (Wells et al., 2013). Income can affect recruitment and informed consent success in several ways, including practical matters such as ability to take time to participate and ability to afford travel and parking needed for participation. Also, income can reflect historical or experiential influences, such as access to neighborhoods and employment (Grunfeld et al., 2002; Brintnall-Karabelas et al., 2011; Braveman et al., 2005). Therefore, collected demographic information included education, age, gender, race, ethnicity, and income. Participants were asked their highest level of education using nine categories. To simplify analysis, the Education responses were converted into a dichotomous variable, those whose education was a high school diploma or less and those who had education beyond high school.
Data Management and Analysis
Data were collected through Qualtrics (Provo, UT) internet-based survey software, delivered by the e-tablets. The data were analyzed using R, version 3.6.0. For analysis, the data were separated into three study groups we are calling “Group” to avoid confusion with the term “site,” which refers to the physical locations of the study Groups. The Groups for analysis included Midwest, English-speaking Northeast (Northeast-E), and Spanish-speaking Northeast (Northeast-S) Groups.
At the Southeast site initially the Consent Study experienced significant recruitment difficulties likely related to the timing of the biobank’s recruitment process, challenges integrating the Consent Study into clinic workflows, and the length of the biobank’s consent document. Only seven participants were recruited over the first six-month recruitment period and none for the eIC condition. So the decision was made to convert the study to a hypothetical one that included patients other than those eligible for the biobank described in the consent document. Although the study participants at this site were cancer patients, the consent document did not necessarily reflect their diagnosis nor did the document describe procedures they were likely to experience. Further, the procedures at this site no longer followed the authentic consent process we sought in this study. Importantly, the new procedures did not allow for participants to enroll in the biobank, making their Intent to Enroll hypothetical and leaving no data for Actual Enrollment. Therefore, we chose not to include the Southeast site in our analysis for this paper. The results for this site are however included in a separate table (see Table 2).
Table 2.
Southeast Site Data and Results.
| Consent document characteristics | ||||
|---|---|---|---|---|
|
| ||||
| Word count | Readability (grade level)a | Total number of slides | Number of interactive questions | Question/slide ratio |
|
| ||||
| 3,477 | 11 | 127 | 19 | 0.150 |
| Participant demographics | ||||
| Race | ||||
| American Indian/Alaskan native | 1 (0.5%) | |||
| Asian | 20 (9.8%) | |||
| Native Hawaiian/Pacific Islander | 0 (0.0%) | |||
| African American | 32 (15.7%) | |||
| Caucasian | 136 (66.7%) | |||
| More than one race | 6 (2.9%) | |||
| Other | 6 (2.9%) | |||
| Not reported | 3 (1.5%) | |||
| Ethnicity | ||||
| Hispanic | 18 (8.8%) | |||
| Not Hispanic | 181 (88.7%) | |||
| Not reported | 5 (2.5% | |||
| Age | ||||
| Mean | 49.6 | |||
| Median | 53 | |||
| Range | 18–84 | |||
| Income | ||||
| <$50,000 | 69 (33.8%) | |||
| ≥$50,000 | 124 (60.8%) | |||
| Not reported | 11 (5.4%) | |||
| Education | ||||
| High school or less | 15 (7.4%) | |||
| Beyond high school | 186 (91.2%) | |||
| Understanding and confidence in understanding scores | ||||||
|---|---|---|---|---|---|---|
| eIC | F2F | |||||
|
|
|
|||||
| Mean | SD | Mean | SD | Difference | p | |
|
| ||||||
| Mean understanding score (out of 100) | 82.65 | 9.79 | 80.07 | 9.03 | 2.58 | .090 |
| Mean confidence in understanding score (out of 100) | 91.63 | 8.87 | 89.58 | 13.79 | 2.05 | .340 |
| Intent to enroll in the biobank by condition | ||||||
| eIC | F2F | |||||
|
|
|
|||||
| Yes | % | Yes | % | Difference | p | |
|
| ||||||
| Intent to enroll | 81 | 76.4 | 110 | 98.2 | −29 | 0.000*** |
Note. eIC= electronic informed consent; F2F =face-to-face; Northeast-E = English-speaking Northeast; Northeast-S = Spanish-speaking Northeast;
<0.001.
Readability score results were calculated using the Fry readability index.
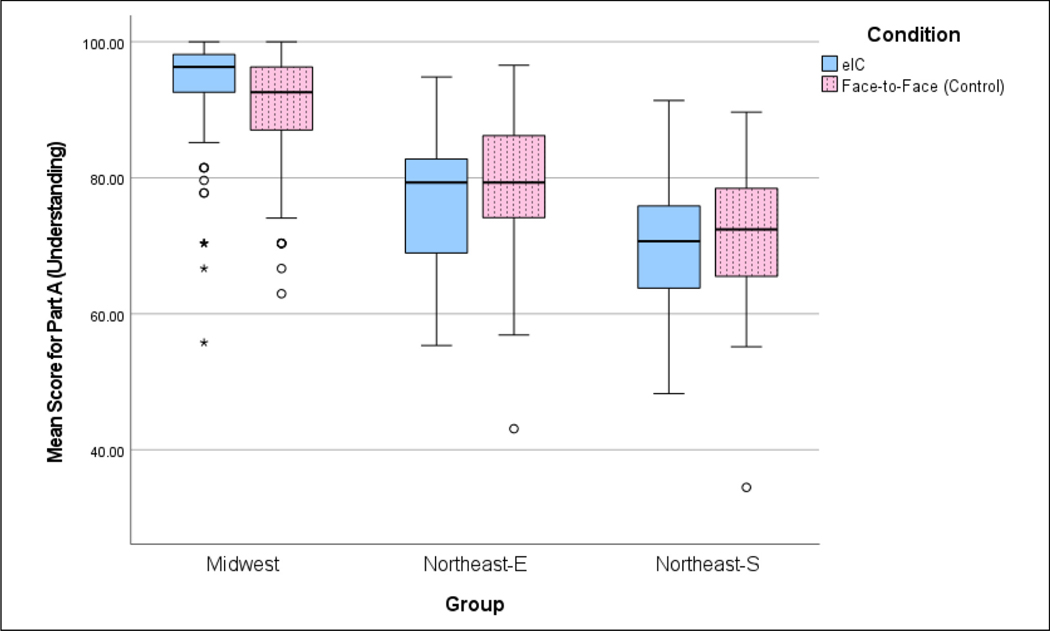
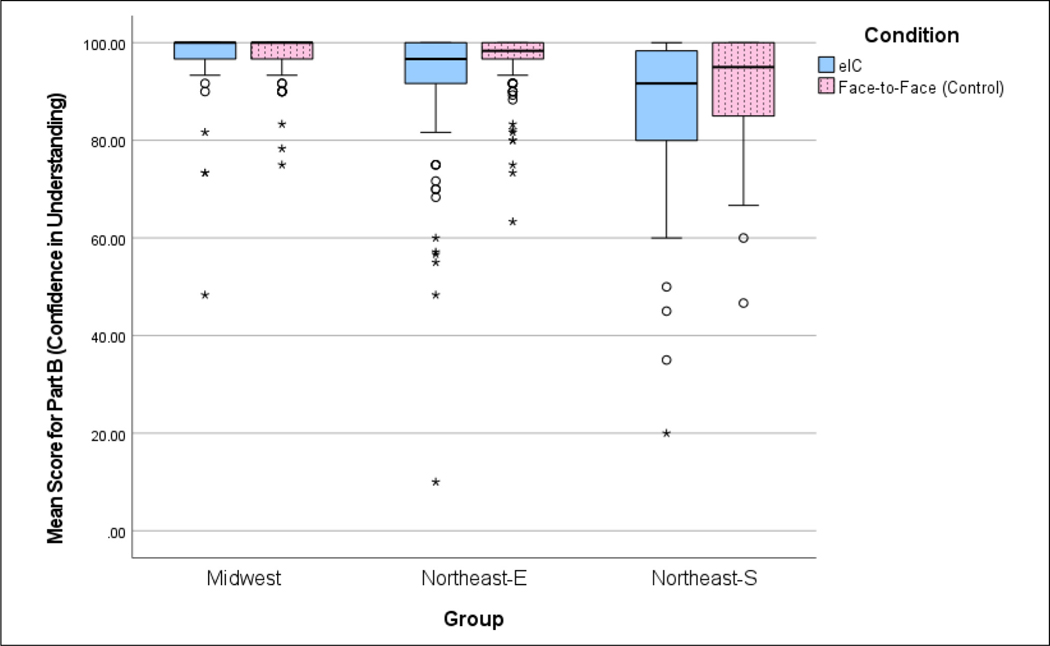
Tobit regression is used to model the Understanding score and the score on Confidence in Understanding in order to take care of the ceiling effect on these scores. Both scores are capped at 100 (See Figures 3 and 4). In addition to Condition (eIC vs. F2F), Group, Age, Gender, Education, Race, and Ethnicity are considered as covariates. Since Income was highly associated with Education (Pearson chi-squared test, p < 0.001), it is not considered as a covariate in order to avoid error inflation from multicollinearity (Wilbanks, 2018). The final model is built using the purposeful selection process proposed by Hosmer et al. (2013, Chapter 4). Distributions of these scores are visually checked via Figures 3 and 4. Association between two categorical variables is tested using Pearson’s chi-square test.
Figure 3.
Box plot comparison of mean understanding scores for the group by condition (electronic informed consent [eIC] vs. face-to-face [F2F]).
Figure 4.
Box plot comparison of mean confidence in understanding scores for the group by condition (electronic informed consent [eIC] vs. face-to-face [F2F]).
RESULTS
Between August 2017 and November 2018, a total of 501 adults were randomly assigned to either a F2F (n = 251) or eIC (n = 250) consent process for the three study groups: Midwest (n = 204), Northeast-E (n = 196), and Northeast-S (n = 101), leaving out the Southeast site’s participants.
Of the total participants enrolled, 154 (30.7%) identified as Caucasian, 79 (15.8%) as African American, and 164 (32.7%) reported themselves as Other (see Table 3), meaning they do not identify among any of the listed races (when asked to comment, many signifying “Other” reported that they identify as “Hispanic” or “Latino” under this Race category). Half of them (n = 244, 48.7%) identified as Hispanic for Ethnicity, 92.6% of whom participated in the Northeast-E and Northeast-S Groups. Almost three-quarters (n = 362, 72.8%) reported as female. The average age was 47.4 (range 18–84). Household income at or above $50,000 (an approximate median for the US at the time of the study) was reported by 60.8% in the Midwest Group, 13.3% in the Northeast-E Group, and 5.0% in the Northeast-S Group. Note that 36 (35.6%) did not report income in the Northeast-S group. Education beyond high school was reported by 91.2% in the Midwest Group, 59.2% in the Northeast-E Group, and 31.7% in the Northeast-S Group.
Table 3.
Participant Demographics.
| Participant demographics | ||||
|---|---|---|---|---|
|
| ||||
| Midwest | Northeast-E | Northeast-S | All Study Groups | |
|
| ||||
| Race | ||||
| American Indian/Alaskan native | 1 (0.5%) | 3 (1.5%) | 2 (2.0%) | 6 (1.2%) |
| Asian | 20 (9.8%) | 5 (2.6%) | 0 (0.0%) | 25 (5.0%) |
| Native Hawaiian/Pacific Islander | 0 (0.0%) | 1 (0.5%) | 0 (0.0%) | 1 (0.2%) |
| African American | 32 (15.7%) | 47 (24.0%) | 0 (0.0%) | 79 (15.8%) |
| Caucasian | 136 (66.7%) | 14 (7.1%) | 4 (4.0%) | 154 (30.7%) |
| More than one race | 6 (2.9%) | 30 (15.3%) | 20 (19.8%) | 56 (11.2%) |
| Other | 6 (2.9%) | 89 (45.4%) | 69 (68.3%) | 164 (32.7%) |
| Not reported | 3 (1.5%) | 7 (3.6%) | 6 (5.9%) | 16 (3.2%) |
| Ethnicity | ||||
| Hispanic | 18 (8.8%) | 127 (64.8%) | 99 (98.0%) | 244 (48.7%) |
| Not Hispanic | 181 (88.7%) | 59 (30.1%) | 1 (1.0%) | 241 (48.1%) |
| Not reported | 5 (2.5% | 10 (5.1%) | 1 (1.0%) | 16 (3.2%) |
| Age | ||||
| Mean | 49.6 | 42.9 | 51.4 | 47.4 |
| Median | 53 | 43 | 53 | 49 |
| Range | 18–84 | 18–79 | 20–80 | 18–84 |
| Income | ||||
| <$50,000 | 69 (33.8%) | 153 (78.1%) | 60 (59.4%) | 282 (56.3%) |
| ≥$50,000 | 124 (60.8%) | 26 (13.3%) | 5 (5.0%) | 155 (30.9%) |
| Not reported | 11 (5.4%) | 17 (8.7%) | 36 (35.6%) | 64 (12.8%) |
| Education | ||||
| High school or less | 15 (7.4%) | 79 (40.3%) | 68 (67.3%) | 162 (32.3%) |
| Beyond high school | 186 (91.2%) | 116 (59.2%) | 32 (31.7%) | 339 (67.7%) |
Note. Northeast-E = English-speaking Northeast; Northeast-S = Spanish-speaking Northeast.
Primary Outcomes
Understanding
Overall, the mean F2F score for Understanding was 83.16 (SD = 13.08) and eIC mean score was 82.08 (SD = 11.33), with a difference of 0.08 (See Table 4).
Table 4.
Mean Understanding and Confidence in Understanding Scores by Condition and Study Group.
| Understanding and confidence in understanding scores | |||||
|---|---|---|---|---|---|
|
| |||||
| eIC | F2F | ||||
|
|
|
||||
| Study group | Mean | SD | Mean | SD | Difference |
|
| |||||
| Mean understanding score (out of 100) | |||||
| Midwest | 92.87 | 8.23 | 89.96 | 7.57 | 2.91 |
| Northeast-E | 77.03 | 9.50 | 79.40 | 9.33 | −2.37 |
| Northeast-S | 70.34 | 10.83 | 71.46 | 10.06 | −1.12 |
| Overall | 82.16 | 13.08 | 82.08 | 11.33 | 0.08 |
| Mean confidence in understanding score (out of 100) | |||||
| Midwest | 97.65 | 6.61 | 97.66 | 4.35 | −0.01 |
| Northeast-E | 91.82 | 14.26 | 96.12 | 6.61 | −4.30 |
| Northeast-S | 85.97 | 18.18 | 90.76 | 11.98 | −4.80 |
| Overall | 93.03 | 13.46 | 95.66 | 7.74 | −2.63 |
Note. eIC = electronic informed consent; F2F = face-to-face; Northeast-E = English-speaking Northeast; Northeast-S = Spanish-speaking Northeast.
To understand the relationship between variables of interest and the Understanding scores, we started with a Tobit regression that includes Condition (F2F vs. eIC), Education (high school vs. post high school), Group (Midwest, Northeast-E, and Northeast-S), Age, Gender, Race, and Ethnicity as predictors. This model also includes the interaction between Condition and Group and the interaction between Education and Group. All terms are significant except Age (p = 0.307), Gender (p = 0.736), and Ethnicity (p = 0.299). In particular, the significant interaction effect between Condition and Group (p < 0.001) and between Education and Group (p = 0.005) suggests the need to evaluate the effect of Condition within each Group.
In the Midwest Group the eIC condition scored higher in Understanding (p = 0.001) than the F2F condition. Conversely, Understanding scores for the eIC condition were lower than those for F2F in both Northeast Groups. Also, mean Understanding scores for each Group show a clear difference between Groups (Midwest Group 91.4 (SD = 8.0); Northeast-E 78.2 (SD = 9.5); Northeast-S 70.9 (SD = 10.4)). The mean for the combined Northeast Groups was 75.7 (SD = 10.4).
Midwest Group.
For the Midwest Group, a Tobit regression is conducted, which includes Condition, Education, Age, Gender, Race, and Ethnicity as predictors. Age, Gender, and Ethnicity are not significant (p = 0.519, 0.620, and 0.939, respectively). After removing these three variables and refitting the model, the results show that eIC has a significant higher mean Understanding score than F2F (estimated coefficient = 4.026 with SE = 1.107, p < 0.001; see Table 5).
Table 5.
Tobit Regression Results on the Effect of eIC Versus F2F. See the Text for Covariates that are Controlled for.
| Response | Study group | Estimate (F2F as reference) | SE | p |
|---|---|---|---|---|
|
| ||||
| Understanding | Midwest | 4.026 | 1.107 | <.001*** |
| Northeast-E | −2.987 | 1.351 | .028* | |
| Northeast-S | −0.512 | 2.197 | .816 | |
| Confidence in understanding | Overall | −4.025 | 1.665 | .016* |
Note. There is no significant study group–condition interaction for confidence in understanding score. There are no study group-specific results. eIC = electronic informed consent; F2F = face-to-face; Northeast-E = English-speaking Northeast; Northeast-S = Spanish-speaking Northeast;
< 0.05;
< 0.001.
Northeast Groups.
For the Northeast-E and Northeast-S Groups, there is no ceiling effect on the Understanding score (see Figure 3). A linear regression model is used rather than a Tobit model. For the Northeast-E Group, Age, Gender, and Ethnicity are not significant (p = 0.645, 0.161, and 0.130, respectively). After removing these three variables and refitting the model, eIC has a significant lower mean Understanding score than F2F (estimated coefficient = −2.987 with SE = 1.351. p = 0.028; see Table 5). For the Northeast-S Group, Education, Race, Gender, and Ethnicity are not significant (p = 0.140, 0.376, 0.098, and 0.657, respectively). After removing these variables and refitting the model, the fitted mean of eIC is lower than that of F2F and is not significant (estimated coefficient = −0.512 with SE = 2.197. p = 0.816; see Table 5).
Confidence in Understanding
Overall, the mean F2F score for Confidence in Understanding was 95.66 (SD = 7.74) and eIC mean score was 93.03 (SD = 13.46), with a difference of −2.63 (See Table 4).
A Tobit model is used for the score on Confidence in Understanding. The final model contains Group and Education as predictors while there is no significant interaction between Group and Condition or Group and Education (p = 0.102 and 0.686, respectively). There is a difference (estimated coefficient = −4.025 with SE = 1.665. p = 0.016) in the overall Confidence scores between eIC (Score = 93.03) and F2F (Score = 95.66) conditions after adjusting for Group and Education (see Table 5). The Confidence score trended higher in the F2F condition in all individual Groups and was significantly higher at Northeast-E (p = 0.016, controlling for Education) (see Figure 4).
Secondary Outcomes
Education
As a dichotomous variable, Education beyond high school was a predictor for higher Understanding scores (p < 0.001) and higher Confidence in Understanding (p < 0.001).
Intent to Enroll
For Intent to Enroll in the Biobank, 442 (88.4%) participants responded “Yes” to the question, “Based on the information you have now, do you plan to agree to take part in the [local biobank]?” immediately after completing the IC process (See Table 6). Of these respondents, 226 (51.1%) were in the F2F and 216 (48.9%) were in the eIC condition. Based on a Pearson chi-square test, there is no significant difference in expressing intent to enroll in the biobank between the F2F and the eIC condition (p = 0.313).
Table 6.
Intent to Enroll in the Biobank and Actual Enrollment.
| Enrollment in the biobank by condition and study group | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| eIC | F2F | |||||
|
|
|
|||||
| Study group | Yes | % | Yes | % | Difference | p |
|
| ||||||
| Intent to enroll | ||||||
| Midwest | 102 | 100 | 102 | 100 | 0 | 1.000 |
| Northeast-E | 81 | 82.7 | 82 | 83.7 | −1 | .975 |
| Northeast-S | 33 | 66.0 | 42 | 82.4 | −9 | .061 |
| Overall | 216 | 86.4 | 226 | 90.0 | −10 | .313 |
| Actual enrollment | ||||||
| Midwest | 102 | 100 | 102 | 100 | 0 | 1.000 |
| Northeast-E | 40 | 40.8 | 54 | 55.1 | −14 | .046* |
| Northeast-S | 13 | 26.0 | 30 | 58.8 | −17 | .001*** |
| Overall | 155 | 62.0 | 186 | 74.1 | −31 | .004** |
| Overall | 155 | 62.0 | 186 | 74.1 | −31 | .004** |
Actual Enrollment
Actual enrollment in the biobanks was 68.1% (n = 341), with 62.0% (n = 155) of eIC participants and 74.1% (n = 186) of F2F participants enrolling (See Table 6). A logistic regression for enrollment was conducted using Condition, Understanding, and Income (Confidence in Understanding, Satisfaction, Education were not associated with enrollment so not included). The results show that F2F consent (p = 0.004), understanding the consent document (p < 0.001), and income above the median (p = 0.002) were predictive of enrollment in this study. It should be noted that 100% of participants at the Midwest site enrolled.
DISCUSSION
Analysis of Results
Understanding.
Contrary to our earlier findings (Simon et al., 2015), results of this study, conducted with diverse participants in two regions of the US, do not provide clear evidence that an eIC process is more effective than a F2F process at promoting understanding of biobank consent documents. On the other hand, this study does provide evidence that a F2F IC process may be more effective at improving potential participants’ confidence in their understanding of the consent document as compared to eIC. This latter result is contrary to our pilot study.
However, effects in this study were likely masked by a Group interaction. The Midwest group scored higher in Understanding for the eIC condition, whereas both Northeast Groups scored higher in the F2F condition. Also, notably the Midwest Group scored higher in Understanding (91.4) than the Northeast Groups combined (75.7). This 15-point difference in Understanding scores between sites suggests that a further explanation is needed.
At the Midwest site – a well-educated, predominantly Caucasian population with few individuals of Hispanic ethnicity – for most individuals the consent process followed consent for a larger, unrelated biobank project, the consent process was conducted in a space separated from the clinic, and the research staff were seasoned consenters. On the other hand, at the Northeast site – with a population with less education, below the national median in income, racially diverse, and most identifying as Hispanic – the participants were recruited while waiting for clinic appointments, the process took place in ad hoc private areas throughout the clinics, and the staff were relatively new at the consenting process, though trained to do so. The Midwest site more closely mirrored the site for our pilot study both in population and consenting environment, so it is not surprising that the Midwest’s results were similar to the pilot. However, the differences in results between the Northeastern English and Spanish Groups and the Midwest require more reflection. Factors that can be identified from prior research include educational differences, socioeconomic differences, racial and ethnic backgrounds, and differences in the research environments.
Studies have shown education to be associated with better understanding of IC materials (Eisenhauer et al., 2019; Agre & Rapkin, 2003; Montalvo & Larson, 2014; Tait et al., 2010a; 2010b). In the current study, the Group with more education (Midwest) tended to do better with eIC. On the other hand, in the Groups with less education (Northeast-E and -S), the site’s scores were lower than those at the Midwest site, and understanding trended higher for F2F participants.
For culturally underrepresented groups, in a review of approaches to the IC process and recruitment for research (Hughson, 2016), results suggest underrepresented groups may prefer electronic presentations, even when the eIC process takes longer, and interactive presentations, such as quizzes, were promising, though there may be difficulties for older participants in using the technologies. The results from this study do not support that optimism. Other factors may have had an overriding effect on the results. One study (Quinn et al., 2012) suggests that preferred methods of conducting informed consent processes may not coincide with methods preferred by minority participants. For example, researchers tend to prefer one-on-one consent, reading the consent document, conducted in one meeting. On the other hand minorities may prefer having family members present, using pictures and illustrations with a brief summary at the end of each segment, and conducted in multiple meetings. Another study suggested that Hispanic participants tended to favor extended family involvement and the inclusion of the patient’s doctor in decision making (Quinn et al., 2013). In this study, consent processes at the Northeast site were conducted in one meeting without family members present, unless they happened to have come to the healthcare appointment. Under the eIC condition, the consent document was basically read to participants, though graphics and illustrations were included. Thus, the F2F condition at the Northeast site may have conformed more to participants’ preferences, though neither condition would have been ideal.
In this study, the decision was made to drop Income from the analysis because it correlated so highly with Education. However, it should be noted that we should not take this lightly, as education and income are not interchangeable. Either of these variables can independently indicate differences in experiences, such as childhood poverty, dramatic loss of income for some reason, trauma, or neighborhood socioeconomic conditions, based on economic factors more related to wealth than education (Braveman, 2005; Kim, 2005). In relation to this study, at the county level for the Northeast site, the median income was below the national median. It is possible this reflects a population of immigrants or first-generation citizens who would have very different experiences, such as encounters with authorities, access to healthcare, opportunities for employment, than the population at the Midwest site, which was more affluent.
Confidence in Understanding.
Confidence in Understanding scores overall showed a main effect for the F2F Group. At the Group level, the Midwest Group showed no difference between the eIC and F2F conditions in their confidence. However, the Northeast-E Group demonstrated higher scores for the F2F condition and the Northeast-S Group showed a trend toward higher scores for the F2F condition, suggesting that F2F may be more effective than eIC at promoting confidence in these Groups’ understanding of biobank consent information. Again, this is somewhat contrary to the findings from our pilot study, where Confidence in Understanding showed an effect for Interactivity, though it did not show an effect for Multimedia. Thus, it appears that multimedia alone may have little effect on confidence in participants’ understanding of a consent document. And although adding questions to an eIC (enhanced interactivity in our prior study) may help participants feel more confident in their understanding, questions alone did not appear to override other effects that may benefit a F2F encounter in the IC process for the Northeast site population.
This study did not collect data on the quality of the IC process from the perspective of participants. One can imagine that several factors can affect the confidence they felt in their understanding of what they learned during the process. For one, if participants feel a connection with a knowledgeable researcher, they may be more comfortable listening to explanations and asking questions when they are not clear. Research staff may be more comfortable or be more effective at answering questions when they are part of continuing dialog. Further, interruptions in the process during the use of technology, such as awkwardness managing an unfamiliar interface or slow-loading graphics, may undermine the credibility of content. On the other hand, researchers could be intimidating to some participants so that they are reluctant to ask questions, and they may put effort into managing how they appear to the researcher (e.g., not to appear ignorant) at the expense of effort at understanding the content being presented. It appears that the benefits of an F2F IC process may be more important than those for eIC for giving participants subjective confidence in what they have learned.
Intent to Enroll and Actual Enrollment.
All participants at the Midwest site expressed an intent to enroll in the biobank and actually completed enrollment. The reason for this could reasonably be attributed to their prior exposure to another biobank recruitment effort, which may either have created a sampling bias or predisposed them to look favorably on enrolling in research. It is also possible that education, race, or other factors, such as a more organized IC approach (in a separate office with experienced, professional consenters) also contributed to a more positive perception of research participation (Fam & Ferrante, 2018; Ford, et al., 2008; Gabriel, Cohen, & Sun, 2014; Kim & Milliken, 2019). Prior research has shown an association between prosocial behavior (such as individuals who contribute to charity, are blood or organ donors, or have priorities about health) and participation in biobanks and genetic research (Ridgeway et al., 2013; Broekstra et al., 2021). In addition, prior engagement with biobanks and familiarity with DNA, genetics, and genomics are also associated with greater likelihood of participation (Gaskell et al., 2013; Middleton et al. 2019). Regardless, any difference in the F2F or eIC condition was apparently not enough to discourage any individuals from enrolling.
Northeast participants, on the other hand, were less likely than the Midwest Group to express intent to enroll and to actually enroll in the biobank, with 80.1% of all participants at the Northeast site (English and Spanish) expressing an intent to enroll. For English speakers at the Northeast site (Northeast-E Group), there was no difference in Intent to Enroll, but for Spanish speakers (Northeast-S), F2F participants trended toward Intent to Enroll. At the point of the actual enrollment decision at the Northeast site, fewer participants chose to enroll (46.1%), compared to their intent, and those in the F2F condition were more likely to actually enroll (56.4% vs. 35.8%, p = 0.010), both for English (55.1% vs, 40.8%, p = 0.046) and Spanish (58.8% vs. 26.0%, p < 0.001). This drop from intent to actual enrollment could be in part attributable to the lengthy questionnaire they completed for the study, after which it may have left them tired or feeling out of time to complete the enrollment. Another factor in the Northeast’s lower enrollment rate could have been the nature of the consent form. The Northeast site used a shortened, simplified consent form. Ridgeway et al. (2013) showed an association between a simplified form and nonresponse for recruitment efforts to a biobank. Further, individuals from racial or ethnic minorities may be less likely to participate in research for historical reasons, as they are aware of past abuses by researchers to participants (Heredia et al., 2017; Hildebrand et al. 2018; Kim & Milliken, 2019; Rangel et al., 2018).
But this does not explain the difference between conditions. Hispanic culture is not monolithic, coming from different regional backgrounds, in how they respond to medicine, research, authority, and other factors, and nonMexican-Americans, as the Northeast site population, are less likely to participate in research (Gabriel et al., 2014). Many regard a connection with their primary care physicians as primary and authoritative to their decisions about healthcare and participation in research (Hildebrand et al., 2018; Quinn et al., 2012). Many prefer recruitment for research to be integrated into their healthcare so they can hear from trusted providers about the safety and benefits of research (Quinn et al., 2013). They may also need more than a single contact with researchers to establish trust (Quinn et al., 2012). It is possible that the introduction of technology to deliver the informed consent information interrupted the human connection needed by some to feel comfortable enough to enroll in the biobank, even though they may have understood the value of the research. It is also possible that other factors, such as a F2F consent process may encourage asking questions and concomitant statements of reassurance needed to nurture trust. It is important to note, however, that a link of rate of enrollment to trust has been questioned. Studies that have controlled for factors that limit minorities from enrolling, such as amount of recruitment effort and individual health status related to socioeconomic issues, have suggested that minorities are not less likely to enroll in research regardless of their levels of trust in research and the researchers (Hagiwara et al., 2013; Wendler et al., 2005)
Length and readability of consent:
Consent documents at the sites differed in length and readability. Document length can be logically expected to affect how well participants understand it; however, recent research does not appear to support this (Beskow et al., 2015; Eisenhauer et al., 2019). On the other hand, readability has generally been shown to affect participants’ comprehension of consent documents (Ley & Florio, 1996). Here, the Midwest consent document was 79.4% longer than the Northeast-E document and had a higher readability score (11.5 vs. 9.0). Yet the Midwest Group scored significantly higher than the Northeast-E Group in Understanding. We assume other factors such as education likely overrode any advantage of length and readability.
Conclusion
The IC process is complex, affecting individuals differently, likely in part because of individuals’ differing experiences given their culture, education, childhood, income, community values, and other factors. Lakes et al. (2012) using data from the National Children’s Study assert that 1) an IC document may not be sufficient for a shared understanding between participant and researcher, 2) decisions to participate may go beyond a strict risk-benefit analysis, 3) decisions to participate in research may not be purely individual, and 4) IC documents may not provide sufficient and appropriate information. Indeed, interviews with participants and nonparticipants (or ex-participants) suggests that decisions to participate may rely heavily on individuals’ pre-existing values and beliefs, such as optimism, sense of duty, or view that society is fundamentally benevolent (Nobile et al., 2016). In addition, trust may be dominantly influenced by individuals’ feelings about the institution, such as the biobank, and by the institution’s representatives. Therefore, the informed consent process may not be so much a rational process as about the individual’s feelings of trust in general, the research context (e.g., a public biobank vs. commercial entity), and the reputation of the organization (Broekstra et al., 2020).
Thus, researchers should consider the importance of trusting relationships, and, among Spanish speakers, of building a meaningful relationship with multiple visits. Here, the Northeast Groups were predominantly Hispanic, but also shared characteristics that put them at one end of a spectrum in terms of income and education. These factors may have played an important role in how these populations understand information about health and medical research, how they perceive the IC process, and what they expect from the relationship between themselves and the researchers.
If expected participants do not generally have basic health literacy, a one-size-fits-all eIC may not be adequate to fully inform individuals about research. Some of the difference is inherent in the delivery of digital information, where today’s technology does not provide the flexibility or accessibility of person-to-person communication (e.g., the ability to answer unexpected questions). If populations are likely to ask questions of the researcher during F2F settings, interacting with an electronic device may suppress reaching out to have questions answered, even if the researcher is present. Further, as indicated by Fam & Ferrante (2018), the environment within which a consent process takes place can create barriers to understanding and enrollment. At the Northeast site, patients may have been distracted by the atmosphere of a waiting room. Some had their IC process interrupted by their appointment, and this may have affected their engagement in the IC and interest in enrolling.
Hispanic populations place particular importance on their relationships with researchers (Hildebrand et al., 2018). Many Hispanic individuals want to establish a connection with researchers at several levels. However, using an electronic device may interrupt relationship building. Relationship building can be enhanced by the presence and participation of healthcare providers with whom patients generally have established trust. But at this Northeastern site, this dynamic may have been at odds with the patients working the consent process around their appointment, where their physician and our researchers were unconnected, the biobank was not presented as a part of their care, and the biobank recruitment did not involve multiple visits.
The divergence in the effect of socioeconomics and ethnicity on informed consent understanding in this study suggests that an electronic approach to IC may be more appropriate for better educated, higher income individuals, while a F2F approach may result in better understanding, confidence in understanding, and enrollment for less educated, lower income individuals of Hispanic origin.
Study Limitations
Site differences such as the readability of consent documents, how the site consent processes were conducted, clinic environments, recruitment strategies, and other factors were most likely determinative of site differences in the results. However, we undertook processes to mitigate study-related differences between sites. A systematic approach was taken to the development of the electronic tool at all sites, including heuristic analyses, focus groups, and usability studies, to ensure that each eIC module was usable for its target population. However, one factor in particular may have affected usability of the tablets. The research staff at all sites reported that wireless connectivity for the e-tablets was not always optimal, and consequently tablet functions sometimes did not respond to touch robustly, which could result in frustration among some users.
Further, for budgetary and logistical reasons blinding of study staff was not possible; each research staff member conducted both F2F and eIC consent conditions. To minimize carryover effects, staff were trained for consistency in their approach to participants, and early-phase data were monitored. However, lack of blinding could have affected results, particularly if staff unconsciously adjusted their behaviors based on what they observed or learned when facilitating one or the other study condition.
The eIC delivery platform, a slideshow format, is not the only way that an IC document may be delivered to prospective participants. Other approaches, such as use of video, animations, text with links to external information, vignettes, and combinations of these methods can be used to help potential participants understand a research study. These approaches may have varying levels of success for different studies, in varying environments and populations.
Best Practices
Based on the results of this study, it appears that site, recruitment environment, and population characteristics, such as levels of education, socioeconomic status, and ethnicity may be factors in the capacity of interactive multimedia to promote IC understanding, confidence in understanding, and enrollment. While we cannot be sure which of these factors affected the differences between sites because of the study design, we can see that the sites had clear differences in populations and recruitment practices. It is likely, therefore, to conclude that one or more factors reduced the effectiveness of the eIC process to promote understanding, confidence in understanding, and enrollment in this study. These findings suggest that biobanks should be cautious about how eIC is implemented and to whom, and should not abandon F2F informed consent without considering its potential value to certain populations.
Researchers should consider their target population’s demographics to determine whether groups are likely to have issues with an eIC approach. Feedback from prospective research participants into a study’s eIC processes prior to deployment of the eIC could help identify potential barriers to understanding, confidence in understanding, and enrollment. Potential research participants may benefit from a choice – either a F2F or an electronic IC process. In addition, a systematic design and development approach of eIC materials, such as recommended in https://www.usability.gov/ and the FDA guidance on eIC (Food and Drug Administration, 2015), could reduce possible drawbacks of eIC. Finally, researchers should consider how participants are recruited and where the IC process is delivered, with sensitivity to the target population. Importantly, disrupting the dynamics and advantages of the F2F consent process by converting it to electronic delivery could present challenges to informed consent and enrollment for biobanks.
Research Agenda
The current study was designed to determine whether interactive multimedia is a viable alternative to traditional face-to-face informed consent processes in authentic contexts. The results of this study suggest that eIC may be superior to F2F for certain populations—most likely participants who have some post-high school education, and who have a higher socioeconomic, nonHispanic background. It is also possible that certain recruitment practices, such as in-clinic approaches that do not include communication and integration with patients’ healthcare system, could be factors. Because this study was not designed to determine the factors that affected the differences in understanding and confidence in understanding among the different site populations, further research should explore cultural factors, economic factors, and educational factors that influence individuals in how they respond to eIC, particularly among Hispanic populations whose income is below the national median and who have not had education beyond high school. Such research may need to explore how and where people are recruited and how that affects IC effectiveness.
Further, the relationship between the researchers and the target population can be crucial to improving the eIC process and study enrollment. The results of enrollment in this study show that different approaches to the IC process can have different effects on specific populations. In particular, participants at the Midwest site appeared to benefit in understanding and engagement from eIC, but at the Northeast site participants appeared to benefit more from face-to-face contact with researchers. There needs to be further investigation into how differing populations, particularly those in underrepresented groups such as certain ethnic groups and those in lower socioeconomic groups, are affected by an environment that might undermine relationships. And because eIC is likely going to be used at greater levels, and more likely in the larger studies, we should investigate factors in the recruitment and consent environment that enhance the relationship between researchers and their target population.
Educational Implications
In general, healthcare researchers are not widely familiar with technology or, particularly, multimedia delivery of information, especially as it relates to recruitment and informed consent. Thus, having some familiarity with how cognitive processes are affected by multimedia, such as that described in Mayer (2009), can help researchers develop effective multimedia. More importantly, familiarity with user-centered design (see https://www.usability.gov/what-and-why/user-centered-design.html) can inform researchers of best practices for creating multimedia applications that maximize the effectiveness of interactive multimedia to target populations. Most important is the use of usability studies (De Sutter, et al., 2020; digital.gov, 2021; Krug, 2014) to inform developers of how effectively their multimedia design works with target users. Without usability data, researchers risk deploying their interactive multimedia in ways that are ineffective, or worse, discouraging to participation.
In addition, the role of trust can have implications for the IC process. As biobanks consider the use of eIC in recruitment and consent, they should also consider environmental and population factors that can interact to affect the relationship between potential participants and researchers. Thus, researchers should be familiar in particular with the needs of underrepresented populations so that they can prepare for possible ways that person-to-person connections can be undermined by intermediaries, such as technology (email, digital delivery of information, online video chat). Particularly when an eIC process is a necessity, such as in large trials, researchers should consider ways of mitigating interruptions in fostering trust, such as connecting recruitment strategies with primary healthcare providers or multiple contacts.
APPENDIX
Appendix A: Knowledge and Understanding Assessment
Below, you will find several statements about the (biobank name) that was just described to you. Thinking about the information you received about the (biobank name) please read each statement carefully. Then tell us whether you agree with the statement, you disagree with the statement, or you are unsure about the statement by circling the appropriate response. Please respond to each statement as best you can.
Appendix A:
| Domain construct | Statement | Your response | ||
|---|---|---|---|---|
|
| ||||
| Nature of the research | 1. I am being asked to take part in genetic research. | Agree correct for all three biobanks | Disagree | Unsure |
| 2. The research will be made possible by the (biobank name) I was just informed about. | Agree correct for all three biobanks | Disagree | Unsure | |
| 3. I am being asked to take part in the (biobank name) because I am a patient at the medical center I am currently visiting. | Agree correct for all three biobanks | Disagree | Unsure | |
| 4. The (biobank name) provides medical care. | Agree | Disagree correct for all three biobanks | Unsure | |
| Purpose of research | 5. The (biobank name) supports research using biological samples (e.g., blood or tissue) as well as access to my health information. | Agree correct for all three biobanks | Disagree | Unsure |
| 6. The (biobank name) supports research to better understand and find treatments for diseases linked to genetics. | Agree correct for all three biobanks | Disagree | Unsure | |
| 7. The purpose of the (biobank name) is to profit from selling my sample and health information to researchers. | Agree | Disagree correct for all three biobanks | Unsure | |
| Duration of the research | 8. My sample could be used in research for many years to come. | Agree correct for all three biobanks | Disagree | Unsure |
| 9. There is no specific end date for my participation in the (biobank name) unless I ask the (biobank name) to end my participation. | Agree correct for all three biobanks | Disagree | Unsure | |
| Research Procedures | 10. I am being asked to donate a sample from which researchers can get my genetic information. | Agree correct for all three biobanks | Disagree | Unsure |
| 11. I will also be asked to complete one or more questionnaires if I choose to take part in the (biobank name). | Agree correct for Southeast and Midwest | Disagree correct for Northeast | Unsure | |
| 12. If I choose to take part, the (biobank name) can get information from my medical records. | Agree correct for all three biobanks | Disagree | Unsure | |
| 13. Approved researchers may NOT use information from my medical records. | Agree | Disagree correct for all three biobanks | Unsure | |
| 14. Only tissue, blood, or body fluids leftover from my medical tests or procedures will be used for the (biobank name). | Agree correct for Northeast | Disagree correct for Southeast and Midwest | Unsure | |
| 15. The (biobank name) will contact me every time my sample or health information is used for research. | Agree | Disagree correct for Northeast and Southeast | Unsure | |
| 1. | N/A for Midwest | |||
| 16. If I take part, I can choose whether the (biobank name) contacts me in the future. | Agree correct for Southeast | Disagree correct for Northeast | Unsure | |
| 1. | N/A for Midwest | |||
| Confidentiality | 17. My sample will be given a research code to help protect my privacy and confidentiality. | Agree correct for all three biobanks | Disagree | Unsure |
| Agree | Disagree | Unsure | ||
| 18. Only researchers at this medical center will be given access to my sample. | correct for all three biobanks | |||
| Risks of the research | 19. There is a small risk that people without permission may find out information about me if I take part in the (biobank name). | Agree correct for all three biobanks | Disagree | Unsure |
| 20. There is a small risk that taking part in the (biobank name) may make it harder for me to obtain some types of insurance. | Agree correct for all three biobanks | Disagree | Unsure | |
| Benefits | 21. I should not expect any direct benefits if I choose to take part in the (biobank name). | Agree correct for all three biobanks | Disagree | Unsure |
| 22. I should expect to receive personal research results if I take part in the (biobank name). | Agree | Disagree correct for all three biobanks | Unsure | |
| Statement that research is voluntary | 23. If I take part in the (biobank name), I will receive better medical care now. | Agree | Disagree correct for all three biobanks | Unsure |
| 24. I can decide whether or not to take part in the (biobank name). | Agree correct for all three biobanks | Disagree | Unsure | |
| Right to withdraw | 25. By withdrawing from the (biobank name), I can stop my samples and health information from being given to researchers in the future. | Agree correct for all three biobanks | Disagree | Unsure |
| Study Contacts | 26. The information presented to me lists the name(s) of the person or persons I can contact if I have questions about the (biobank name). | Agree correct for all three biobanks | Disagree | Unsure |
| Costs and Compensation | 27. There will be no cost to me or my insurance company if I take part in the (biobank name). | Agree correct for all three biobanks | Disagree | Unsure |
| 28. I will get paid for taking part in the (biobank name). | Agree | Disagree correct for all three biobanks | Unsure | |
| Study Authorization | 29. If I sign this consent, I will give the (biobank name) permission to store my sample and make it available to researchers who have been given permission. | Agree correct for all three biobanks | Disagree | Unsure |
Appendix B: Confidence in Understanding of the Consent Document
When you went through the IC process, how well did you understand the following aspects of the (biobank name)? If you did not understand the item at all, please select 1. If you understood it very well, please select 5. If you understood it somewhat, please select a number between 1 and 5.
| Domain construct | How well did you understand… | I didn’t understand this at all | => | I understood this very well | ||
|---|---|---|---|---|---|---|
|
| ||||||
| Nature of the research | B1. That I am being asked to take part in the research. | 1 | 2 | 3 | 4 | 5 |
| Purpose of research | B2. The purpose of the (biobank name). | 1 | 2 | 3 | 4 | 5 |
| Duration of the research | B3. How long my sample and health information may be used for research. | 1 | 2 | 3 | 4 | 5 |
| Research procedures | B4. What I am being asked to donate. | 1 | 2 | 3 | 4 | 5 |
| B5. That my electronic medical record could be consulted by researchers. | 1 | 2 | 3 | 4 | 5 | |
| B6. Whether the (biobank name) may contact me in the future. | 1 | 2 | 3 | 4 | 5 | |
| B7. Whether I will be contacted when my sample or health information is used for research. | 1 | 2 | 3 | 4 | 5 | |
| Confidentiality | B8. How codes will be used to protect my confidentiality. | 1 | 2 | 3 | 4 | 5 |
| Risks of the research | B9. The possible risks of taking part in the (biobank name). | 1 | 2 | 3 | 4 | 5 |
| Statement that research is voluntary | B10. That taking part in the (biobank name) is voluntary. | 1 | 2 | 3 | 4 | 5 |
| Right to withdraw | B11. That I can withdraw from the (biobank name) at any time. | 1 | 2 | 3 | 4 | 5 |
| Study contacts | B12. Whom I should contact if I have questions or concerns about the (biobank name). | 1 | 2 | 3 | 4 | 5 |
| Costs and compensation | B13. How much it will cost me or my insurance company if I take part in the (biobank name). | 1 | 2 | 3 | 4 | 5 |
| Study authorization | B14. That by signing the consent form, I will give permission for the (biobank name) to store a sample from me and make it available to researchers who have permission. | 1 | 2 | 3 | 4 | 5 |
| Overall | B15. Overall, how well did you understand the purpose, the procedures, and the potential risks and benefits of taking part in the (biobank name)? | 1 | 2 | 3 | 4 | 5 |
REFERENCES
- Agre P, & Rapkin B. (2003). Improving informed consent: a comparison of four consent tools. IRB, 25(6), 1–7. [PubMed] [Google Scholar]
- Allen J, & McNamara B. (2011). Reconsidering the value of consent in biobank research. Bioethics, 25(3), 155–166. doi: 10.1111/j.1467-8519.2009.01749.x. [DOI] [PubMed] [Google Scholar]
- Allen MJ, Powers ML, Gronowski KS, & Gronowski AM (2010). Human tissue ownership and use in research: what laboratorians and researchers should know. Clin Chem, 56(11), 1675–1682. doi: 10.1373/clinchem.2010.150672. [DOI] [PubMed] [Google Scholar]
- Anderson EE, Newman SB, & Matthews AK (2017). Improving informed consent: stakeholder views. AJOB Empirical Bioethics, 8(3), 178–188. doi: 10.1080/23294515.2017.1362488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp TL, & Childress JF (1979). Principles of biomedical ethics. New York: Oxford University Press. [Google Scholar]
- Beleno v Texas Dept. of State Health Services (Tex WD. 2009). [Google Scholar]
- Berg JW, Appelbaum PS, Lidz CW, & Parker LS (2001). Informed consent: legal theory and clinical practice (2nd ed.). Oxford; New York: Oxford University Press. [Google Scholar]
- Beskow LM, & Weinfurt KP (2019). Exploring understanding of “understanding”: the paradigm case of biobank consent comprehension. The American Journal of Bioethics, 19(5), 6–18. doi: 10.1080/15265161.2019.1587031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beskow LM, Dombeck CB, Thompson CP, Watson-Ormond JK, & Weinfurt KP (2015). Informed consent for biobanking: consensus-based guidelines for adequate comprehension. Genet Med, 17(3), 226–233. doi: 10.1038/gim.2014.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutin NT, Mathieu K, Hoffnagle AG, Allen NL, Castro VM, Morash M, et al. (2016). Implementation of electronic consent at a biobank: an opportunity for precision medicine research. J Pers Med, 6(2). doi: 10.3390/jpm6020017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branch E. (2017, March 16). Advancing clinical trial efficiency with electronic informed consent, American Pharmaceutical Review. https://www.americanpharmaceuticalreview.com/Featured-Articles/335413-Advancing-Clinical-Trial-Efficiency-With-Electronic-Informed-Consent/ (accessed 2020 May 22). [Google Scholar]
- Braveman PA, Cubbin C, Egerter S, Chideya S, Marchi KS, Metzler M, & Posner S. (2005). Socioeconomic status in health research: one size does not fit all. JAMA, 294(22), 2879–2888. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- Brintnall-Karabelas J, Sung S, Cadman ME, Squires C, Whorton K, & Pao M. (2011). Improving recruitment in clinical trials: why eligible participants decline. Journal of Empirical Research on Human Research Ethics: An International Journal, 6(1), 69–74. doi: 10.1525/jer.2011.6.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekstra R, Aris-Meijer J, Maeckelberghe E, Stolk R, & Otten S. (2020). Trust in centralized large-scale data repository: a qualitative analysis. Journal of Empirical Research on Human Research Ethics, 15(4), 365–378. doi: 10.1177/1556264619888365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekstra R, Aris-Meijer J, Maeckelberghe E, Stolk R, & Otten S. (2021). Demographic and prosocial intrapersonal characteristics of biobank participants and refusers: the findings of a survey in the Netherlands. European Journal of Human Genetics, 29(1), 11–19. doi: 10.1038/s41431-020-0701-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry KE, Park DC, Frieske DA, & Smith AD (1996). Verbal and pictorial elaborations enhance memory in younger and older adults. Aging Neuropsychology and Cognition, 3(1), 15–29. doi: 10.1080/13825589608256609. [DOI] [Google Scholar]
- Chiou CP, & Chung YC (2012). Effectiveness of multimedia interactive patient education on knowledge, uncertainty and decision-making in patients with end-stage renal disease. J Clin Nurs, 21(9–10), 1223–1231. doi: 10.1111/j.1365-2702.2011.03793.x. [DOI] [PubMed] [Google Scholar]
- Clark RC, & Mayer RE. (2008). E-learning and the science of instruction: proven guidelines for consumers and designers of multimedia learning. San Francisco, CA: Pfeiffer. [Google Scholar]
- Coakley M, Fadiran EO, Parrish LJ, Griffith RA, Weiss E, & Carter C. (2012). Dialogues on diversifying clinical trials: successful strategies for engaging women and minorities in clinical trials. Journal of Women’s Health, 21(7), 713–716. doi: 10.1089/jwh.2012.3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornoiu A, Beischer AD, Donnan L, Graves S, & de Steiger R. (2011). Multimedia patient education to assist the informed consent process for knee arthroscopy. ANZ J Surg, 81(3), 176–180. doi: 10.1111/j.1445-2197.2010.05487.x. [DOI] [PubMed] [Google Scholar]
- Davis TC, Arnold CL, Mills G, & Miele L. (2019). A qualitative study exploring barriers and facilitators of enrolling underrepresented populations in clinical trials and biobanking. Frontiers in Cell and Developmental Biology, 7(74). doi: 10.3389/fcell.2019.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- digital.gov. (2021). Government usability case studies. Retrieved from https://digital.gov/resources/digitalgov-user-experience-resources/government-usability-case-studies/.
- De Sutter E, Zaçe D, Boccia S, Di Pietro ML, Geerts D, Borry P, Huys I. Implementation of electronic informed consent in biomedical research and stakeholders’ perspectives: Systematic review. J Med Internet Res. 2020. October 8;22(10):e19129. doi: 10.2196/19129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LB, Lindamer LA, Palmer BW, Golshan S, Schneiderman LJ, & Jeste DV (2002). Improving understanding of research consent in middle-aged and elderly patients with psychotic disorders. Am J Geriatr Psychiatry, 10(2), 142–150. [PubMed] [Google Scholar]
- Eisenhauer ER, Tait AR, Rieh SY, & Arslanian-Engoren CM (2019). Participants’ understanding of informed consent for biobanking: a systematic review. Clin Nurs Res, 28(1), 30–51. doi: 10.1177/1054773817722690. [DOI] [PubMed] [Google Scholar]
- El-Wakeel H, Taylor GJ, & Tate JJ (2006). What do patients really want to know in an informed consent procedure? a questionnaire-based survey of patients in the Bath area, UK. Journal of Medical Ethics, 32(10), 612. doi: 10.1136/jme.2005.013334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fam E, & Ferrante JM (2018). Lessons learned recruiting minority participants for research in urban community health centers. Journal of the National Medical Association, 110(1), 44–52. doi: 10.1016/j.jnma.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer DF, Jackson SA, Camacho F, & Hall MA (2007). Attitudes of African American and low socioeconomic status White women toward medical research. Journal of Health Care for the Poor and Underserved, 18(1), 85–99. Retrieved from https://muse.jhu.edu/article/210740. [DOI] [PubMed] [Google Scholar]
- Farrell EH, Whistance RN, Phillips K, Morgan B, Savage K, Lewis V, et al. (2014). Systematic review and meta-analysis of audio-visual information aids for informed consent for invasive healthcare procedures in clinical practice. Patient Educ Couns, 94(1), 20–32. doi: 10.1016/j.pec.2013.08.019. [DOI] [PubMed] [Google Scholar]
- Flory J, Wendler D, & Emanuel E. (2007). Informed consent for research. In Ashcroft RE, Dawson A, Draper H. & McMillan JR (Eds.), Principles of health care ethics (2nd ed., pp. 703–710). Chichester, UK: John Wiley & Sons Ltd. [Google Scholar]
- Food and Drug Administration. Use of an electronic informed consent in clinical investigations: questions and answers. Draft guidance for industry [fr doc. 2015–05377 filed 3–6–15; 8:45 am]. Rockville, MD: Food and Drug Administration; March 2015. [cited 2015 July 20]; Available from: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM436811.pdf. [Google Scholar]
- Ford JG, Howerton MW, Lai GY, Gary TL, Bolen S, Gibbons MC, … Bass EB (2008). Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer, 112(2), 228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- Frelich MJ, Bosler ME, & Gould JC (2015). Research electronic data capture (REDcap) electronic informed consent form (EICF) is compliant and feasible in a clinical research setting. International Journal, 2(3), 51. [Google Scholar]
- Fry E. (1977). Fry’s readability graph: clarifications, validity, and extension to Level 17. Journal of Reading. 1977;21(3), 242–252. Retrieved from http://www.jstor.org/stable/40018802. [Google Scholar]
- Gabriel A, Cohen CC, & Sun C. (2014). Consent to specimen storage and continuing studies by race and ethnicity: a large dataset analysis using the 2011–2012 National Health and Nutrition Examination Survey. The Scientific World Journal, 2014, 1–6. doi: 10.1155/2014/120891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskell G, Gottweis H, Starkbaum J, Gerber MM, Broerse J, Gottweis U, … Soulier A. (2013). Publics and biobanks: Pan-European diversity and the challenge of responsible innovation. European Journal of Human Genetics, 21(1), 14–20. doi: 10.1038/ejhg.2012.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- General requirements for informed consent (45CFR46.116). (2009). 45 C.F.R. Sect. 46.116. http://www.hhs.gov/ohrp/humansubjects/guidance/45cfr46.html (accessed 2020 May 22).
- George S, Duran N, & Norris K. (2014). A systematic review of barriers and facilitators to minority research participation among African Americans, Latinos, Asian Americans, and Pacific Islanders. American Journal of Public Health, 104(2), e16–e31. doi: 10.2105/ajph.2013.301706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesualdo F, Daverio M, Palazzani L, Dimitriou D, Diez-Domingo J, Fons-Martinez J, … Tozzi AE (2021). Digital tools in the informed consent process: a systematic review. BMC Medical Ethics, 22(1), 18. doi: 10.1186/s12910-021-00585-8. (Accession No. 33639926). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam B, Pena SC, Mountain L. (1980). The Fry graph applied to Spanish readability. The Reading Teacher, 33(4), 426–430. [Google Scholar]
- Goddard KAB, Smith KS, Chen C, McMullen C, & Johnson C. (2009). Biobank recruitment: motivations for nonparticipation. Biopreserv Biobank, 7(2), 119–121. doi: 10.1089/bio.2009.0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. (2017). Informed consent. N Engl J Med, 376(20), e43. doi: 10.1056/NEJMc1704010. [DOI] [PubMed] [Google Scholar]
- Griffin JM, Struve JK, Collins D, Liu A, Nelson DB, & Bloomfield HE (2006). Long term clinical trials: how much information do participants retain from the informed consent process? Contemp Clin Trials, 27(5), 441–448. doi: 10.1016/j.cct.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Grunfeld E, Zitzelsberger L, Coristine M, & Aspelund F. (2002). Barriers and facilitators to enrollment in cancer clinical trials. Cancer, 95(7), 1577. [DOI] [PubMed] [Google Scholar]
- Hagiwara N, Berry-Bobovski L, Francis C, Ramsey L, Chapman RA, & Albrecht TL (2014). Unexpected findings in the exploration of African American underrepresentation in biospecimen collection and biobanks. Journal of Cancer Education, 29(3), 580–587. doi: 10.1007/s13187-013-0586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon A. (2010, April 21). Indian tribe wins fight to limit research of its DNA, New York Times. http://www.nytimes.com/2010/04/22/us/22dna.html?pagewanted=all&_r=0 (accessed 2020, May 22). [Google Scholar]
- Havasupai tribe v. Arizona Board of Regents (2008). 220 Ariz. 214, 204 P.3d 1063 224. [Google Scholar]
- Henderson GE, Cadigan RJ, Edwards TP, Conlon I, Nelson AG, Evans JP, Davis AM, Zimmer C, Weiner BJ (2013). Characterizing biobank organizations in the U.S.: results from a national survey. Genome Med. 25;5(1):3. doi: 10.1186/gm407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heredia NI, Krasny S, Strong LL, Von Hatten L, Nguyen L, Reininger BM, … Fernández ME (2017). Community perceptions of biobanking participation: a qualitative study among Mexican-Americans in three Texas cities. Public Health Genomics, 20(1), 46–57. doi: 10.1159/000452093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hildebrand JA, Billimek J, Olshansky EF, Sorkin DH, Lee JA, & Evangelista LS (2018). Facilitators and barriers to research participation: perspectives of Latinos with type 2 diabetes. Eur J Cardiovasc Nurs, 17(8), 737–741. doi: 10.1177/1474515118780895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S, and Sturdivant RX. (2013). Applied Logistic Regression, 3ed. New York:Wiley. [Google Scholar]
- Hughson J. a., Woodward-Kron R, Parker A, Hajek J, Bresin A, Knoch U, et al. (2016). A review of approaches to improve participation of culturally and linguistically diverse populations in clinical trials. Trials, 17(1), 263. doi: 10.1186/s13063-016-1384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe S, Cook EF, Cleary PD, Clark JW, & Weeks JC (2001). Quality of informed consent: a new measure of understanding among research subjects. J Natl Cancer Inst, 93(2), 139–147. [DOI] [PubMed] [Google Scholar]
- Kaphingst KA, Facio FM, Cheng MR, Brooks S, Eidem H, Linn A, et al. (2012). Effects of informed consent for individual genome sequencing on relevant knowledge. Clin Genet, 82(5), 408–415. doi: 10.1111/j.1399-0004.2012.01909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye J, Whitley EA, Lund D, Morrison M, Teare H, & Melham K. (2015). Dynamic consent: a patient interface for twenty-first century research networks. Eur J Hum Genet, 23(2), 141–146. doi: 10.1038/ejhg.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, & Milliken EL (2019). Minority participation in biobanks: an essential key to progress. In Yong WH (Ed.), Biobanking: Methods and Protocols (pp. 43–50). Springer; New York. [DOI] [PubMed] [Google Scholar]
- Krug S. (2014). Don’t make me think: A common sense approach to web usability (Third Ed. ed.): New Riders. [Google Scholar]
- Lakes KD, Vaughan E, Jones M, Burke W, Baker D, & Swanson JM (2012). Diverse perceptions of the informed consent process: implications for the recruitment and participation of diverse communities in the national children’s study. American Journal of Community Psychology, 49(1), 215–232. doi: 10.1007/s10464-011-9450-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemke AA, Trinidad SB, Edwards KL, Starks H, Wiesner GL, & GRRIP Consortium. (2010). Attitudes toward genetic research review: results from a national survey of professionals involved in human subjects protection. J Empir Res Hum Res Ethics, 5(1), 83–91. doi: 10.1525/jer.2010.5.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz J, Kennett M, Perlmutter J, & Forrest A. (2016). Paving the way to a more effective informed consent process: recommendations from the clinical trials transformation initiative. Contemp Clin Trials, 49, 65–69. doi: 10.1016/j.cct.2016.06.005. [DOI] [PubMed] [Google Scholar]
- Ley P, & Florio T. (1996). The use of readability formulas in health care. Psychology, Health & Medicine, 1(1), 7–28. doi: 10.1080/13548509608400003. [DOI] [Google Scholar]
- Lewis CE, George V, Fouad M, Porter V, Bowen D, & Urban N. (1998). Recruitment strategies in the women’s health trial: feasibility study in minority populations. Controlled Clinical Trials, 19(5), 461–476. [DOI] [PubMed] [Google Scholar]
- Lunt H, Connor S, Skinner H, & Brogden G. (2019). Electronic informed consent: the need to redesign the consent process for the digital age. Intern Med J, 49(7), 923–929. doi: 10.1111/imj.14339. [DOI] [PubMed] [Google Scholar]
- Mayer RE. (2009). Multimedia learning (2nd ed.). Cambridge; New York: Cambridge University Press. [Google Scholar]
- Mayer R. (2005). Introduction to multimedia learning. In Mayer RE (Ed.), The cambridge handbook of multimedia learning (pp. 1–16). New York, NY: Cambridge University Press. [Google Scholar]
- McFarlane SJ, Morgan SE, Occa A, & Peng W. (2019). An evaluation of clinical trial multimedia to support Hispanic cancer patients’ informational and decision-making needs. Journal of Cancer Education. doi: 10.1007/s13187-019-01606-2. [DOI] [PubMed] [Google Scholar]
- Meehan A, Bundorf MK, Klimke R, Stults CD, Chan AS, Pun T, et al. (2020). Online consent enables a randomized, controlled trial testing a patient-centered online decision-aid for medicare beneficiaries to meet recruitment goal in short time frame. J Patient Exp, 7(1), 12–15. doi: 10.1177/2374373519827029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton A, Milne R, Thorogood A, Kleiderman E, Niemiec E, Prainsack B, … Morley KI (2019). Attitudes of publics who are unwilling to donate DNA data for research. European Journal of Medical Genetics, 62(5), 316–323. doi: 10.1016/j.ejmg.2018.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalvo W, & Larson E. (2014). Participant comprehension of research for which they volunteer: a systematic review. Journal of Nursing Scholarship, 46(6), 423–431. doi: 10.1111/jnu.12097. [DOI] [PubMed] [Google Scholar]
- Moser DJ, Reese RL, Hey CT, Schultz SK, Arndt S, Beglinger LJ, et al. (2006). Using a brief intervention to improve decisional capacity in schizophrenia research. Schizophrenia Bulletin, 32(1), 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura A, Carey J, Erwin PJ, Tilburt JC, Murad MH, & McCormick JB (2013). Improving understanding in the research informed consent process: a systematic review of 54 interventions tested in randomized control trials. BMC Med Ethics, 14, 28. doi: 10.1186/1472-6939-14-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobile H, Bergmann MM, Moldenhauer J, & Borry P. (2016). Participants’ accounts on their decision to join a cohort study with an attached biobank: a qualitative content analysis study within two German studies. Journal of Empirical Research on Human Research Ethics, 11(3), 237–249. doi: 10.1177/1556264616657463. [DOI] [PubMed] [Google Scholar]
- Ormond KE, Cirino AL, Helenowski IB, Chisholm RL, & Wolf WA (2009). Assessing the understanding of biobank participants. Am J Med Genet A, 149A(2), 188–198. doi: 10.1002/ajmg.a.32635. [DOI] [PubMed] [Google Scholar]
- Palmer BW, Lanouette NM, Jeste DV (2012). Effectiveness of multimedia aids to enhance comprehension of research consent information: a systematic review. IRB, 34(6):1–15. [PMC free article] [PubMed] [Google Scholar]
- Quinn SC, Garza MA, Butler J, Fryer CS, Casper ET, Thomas SB, … Kim KH (2012). Improving informed consent with minority participants: results from researcher and community surveys. Journal of Empirical Research on Human Research Ethics, 7(5), 44–55. doi: 10.1525/jer.2012.7.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn GP, McIntyre J, Gonzalez LE, Antonia TM, Antolino P, & Wells KJ (2013). Improving awareness of cancer clinical trials among Hispanic patients and families: audience segmentation decisions for a media intervention. Journal of Health Communication, 18(9), 1131–1147. doi: 10.1080/10810730.2013.768723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn SC, Garza MA, Butler J, Fryer CS, Casper ET, Thomas SB, … Kim KH (2012). Improving informed consent with minority participants: results from researcher and community surveys. Journal of Empirical Research on Human Research Ethics, 7(5), 44–55. doi: 10.1525/jer.2012.7.5.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel ML, Heredia NI, Reininger B, McNeill L, & Fernandez ME (2018). Educating Hispanics about clinical trials and biobanking. Journal of Cancer Education. doi: 10.1007/s13187-018-1417-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgeway JL, Han LC, Olson JE, Lackore KA, Koenig BA, Beebe TJ, & Ziegenfuss JY (2013). Potential bias in the bank: what distinguishes refusers, nonresponders and participants in a clinic-based biobank? Public Health Genomics, 16(3), 118–126. doi: 10.1159/000349924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowbotham MC, Astin J, Greene K, & Cummings SR (2013). Interactive informed consent: randomized comparison with paper consents. PLoS ONE, 8(3), e58603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadoski M, & Paivio A. (2001). Imagery and text: a dual coding theory of reading and writing. Mahwah, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Schenker Y, Fernandez A, Sudore R, & Schillinger D. (2011). Interventions to improve patient comprehension in informed consent for medical and surgical procedures: a systematic review. Med Decis Making, 31(1), 151–173. doi: 10.1177/0272989X10364247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuck PH. (1994). Rethinking informed consent. Yale Law J, 103(4):899–959. [PubMed] [Google Scholar]
- Shenoy P. (2015). Electronic informed consenting: a boon to modernize consenting process. Perspect Clin Res, 6(4), 173–174. doi: 10.4103/2229-3485.167091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon CM, Klein DW, & Schartz HA (2014). Traditional and electronic informed consent for biobanking: a survey of U.S. biobanks. Biopreserv Biobank, 12(6), 423–429. doi: DOI 10.1089/bio.2014.0045. [DOI] [PubMed] [Google Scholar]
- Simon CM, Klein DW, & Schartz HA (2015). Interactive multimedia consent for biobanking: a randomized trial. Genet Med, 18(1), 57–64. doi: 10.1038/gim.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon CM, Schartz HA, Rosenthal GE, Eisenstein EL, & Klein DW (2018). Perspectives on Electronic Informed Consent From Patients Underrepresented in Research in the United States: A Focus Group Study. Journal of Empirical Research on Human Research Ethics, 13(4), 338–348. doi: 10.1177/1556264618773883. [DOI] [PubMed] [Google Scholar]
- Simon CM, Zyzanski SJ, Durand E, Jimenez X, & Kodish ED (2006). Interpreter accuracy and informed consent among spanish-speaking families with cancer. J Health Commun, 11(5), 509–522. doi: 10.1080/10810730600752043. [DOI] [PubMed] [Google Scholar]
- Skinner JS, Fair AM, Holman AS, Boyer AP, & Wilkins CH (2019). The impact of an educational video on clinical trial enrollment and knowledge in ethnic minorities: a randomized control trial. Frontiers in Public Health, 7(104). doi: 10.3389/fpubh.2019.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. (2015). UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med, 12(3), e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait AR, & Voepel-Lewis T. (2015). Digital multimedia: a new approach for informed consent? JAMA, 313(5), 463–464. doi: 10.1001/jama.2014.17122. [DOI] [PubMed] [Google Scholar]
- Tait AR, Voepel-Lewis T, & Levine R. (2015). Using digital multimedia to improve parents’ and children’s understanding of clinical trials. Archives of Disease in Childhood, 100(6), 589–593. doi: 10.1136/archdischild-2014-308021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait AR, Voepel-Lewis T, Zikmund-Fisher BJ, & Fagerlin A. (2010a). The effect of format on parents’ understanding of the risks and benefits of clinical research: a comparison between text, tables, and graphics. Journal of Health Communication, 15(5), 487–501. doi: 10.1080/10810730.2010.492560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait AR, Voepel-Lewis T, Zikmund-Fisher BJ, & Fagerlin A. (2010b). Presenting research risks and benefits to parents: does format matter? Anesthesia and Analgesia, 111(3), 718–723. doi: 10.1213/ANE.0b013e3181e8570a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KJ, McIntyre J, Gonzalez LE, Lee J-H, Fisher KJ, Jacobsen PB, … Quinn GP (2013). Feasibility trial of a Spanish-language multimedia educational intervention. Clinical Trials, 10(5), 767–774. doi: 10.1177/1740774513495984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendler D, Kington R, Madans J, Wye GV, Christ-Schmidt H, Pratt LA, … Emanuel E. (2005). Are racial and ethnic minorities less willing to participate in health research? PLOS Medicine, 3(2), e19. doi: 10.1371/journal.pmed.0030019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilbanks J. (2018). Design issues in e-consent. J Law Med Ethic, 46(1), 110–118. doi: 10.1177/1073110518766025. [DOI] [PMC free article] [PubMed] [Google Scholar]