Abstract
Objective:
Prediction of amputation wound healing is challenging due to the multifactorial nature of critical limb ischemia and lack of objective assessment tools. Up to one-third of amputations require revision to a more proximal level within one year. We tested a novel wound imaging system to predict amputation wound healing at initial evaluation.
Methods:
Patients planned to undergo amputation due to critical limb ischemia were prospectively enrolled. Clinicians evaluated the patients in traditional fashion and all clinical decisions for amputation level were determined by the clinician’s judgement. Multispectral images of the lower extremity were obtained preoperatively using a novel wound imaging system. Clinicians were blinded to the machine analysis. A standardized wound healing assessment was performed on postoperative day 30 by physical exam to determine whether the amputation site achieved complete healing. If operative revision or higher level of amputation was required, this was undertaken based solely upon the provider’s clinical judgement. A machine learning algorithm combining the multispectral imaging data with patient clinical risk factors was trained and tested using cross-validation to measure the wound imaging system’s accuracy of predicting amputation wound healing.
Results:
A total of 22 patients undergoing 25 amputations (10 toe, 5 transmetatarsal, 8 below- knee, and 2 above-knee amputations) were enrolled. Eleven amputations (44%) were non-healing after 30 days. The machine learning algorithm had 91% sensitivity and 86% specificity for prediction of non-healing amputation sites (area under curve 0.89).
Conclusions:
This pilot study suggests that a machine learning algorithm combining multispectral wound imaging with patient clinical risk factors may improve prediction of amputation wound healing and therefore decrease the need for re-operation and incidence of delayed healing. We propose that this in turn may offer significant cost savings to the patient and health system in addition to decreasing length of stay for patients.
Keywords: critical limb ischemia, lower extremity amputation, multispectral imaging, machine learning
Table of Contents Summary
A machine learning algorithm using multispectral imaging of superficial tissues and patient clinical risk factors was highly accurate for prediction of amputation wound healing in this prospective, pilot study including 25 lower extremity amputations. This algorithm may lend significant cost savings and decrease length of stay for patients undergoing lower extremity amputation.
Introduction
Over 150,000 patients undergo non-traumatic lower extremity amputations in the United States annually, with amputation rates increasing in recent years.1 Selection of amputation level is a complicated clinical decision. No single test is currently accepted as the gold-standard for predication of wound healing. The surgeon must balance the most functional level of amputation to maintain or improve quality of life against the likelihood for failure of healing at any given level of amputation (LOA). Surgeons determine appropriate LOA using clinical judgement, combining patient clinical risk factors, physical exam, and any available invasive or non-invasive vascular studies. Unfortunately, up to one-third of amputations fail to heal, requiring re-amputation to a more proximal level.2 Failure of primary amputation wound healing decreases patient quality of life and adds substantially to the costs of care for patients with critical limb ischemia.3
The lack of technology to aid the management of critical limb ischemia, including decisions regarding most appropriate amputation level, was recently highlighted by the American Heart Association Council on Peripheral Vascular Disease. 4 In particular, the Council called for development of technology that provides spatial information on tissue oxygenation because these variables factor heavily in determining wound healing potential. Multispectral imaging (MSI) is an imaging technology capable of providing relevant information, including tissue oxygenation, on factors influencing wound healing via systematic measurement of light reflectance patterns from the biological tissues including skin and wounds. Using specifically selected wavelengths of light, MSI is capable of measuring variation in important tissue characteristics that influence wound healing. Indeed, MSI has previously shown potential to predict the healing potential of burn wounds.5,6,7
Therefore, we sought to determine whether a wound imaging system that implements a machine-learning algorithm to evaluate multispectral images of superficial tissues at selected LOA combined with patient clinical risk factors can accurately predict amputation wound healing. Herein, we report the results of a pilot study designed to evaluate the performance of the wound imaging system.
Methods
Study Design and Patient Population
This prospective study enrolled subjects with critical limb threatening ischemia planned for lower extremity amputation (toe, transmetatarsal, below-knee, or above-knee) at two hospitals (NCT03611361). Institutional Review Board (IRB) approval was obtained prior to initiation of enrollment. Adult patients (age >17 years) were eligible for enrollment if they were planned for amputation due to critical limb threatening ischemia and had an anticipated life- expectancy exceeding 3 months. A history of prior revascularization on the affected limb was permissible, but all patients were deemed to have no further revascularization options (surgical or endovascular) at the time of enrollment in this study. Patients undergoing an amputation for a reason other than critical limb ischemia were not eligible for enrollment. Patients scheduled for any procedure other than primary amputation which may affect wound healing (including invasive peripheral angiography/intervention) prior to or within the 30-days following amputation were also not eligible for enrollment.
Study Procedures
After providing informed consent, the subjects underwent imaging of the lower extremity planned for amputation with a multispectral imaging device. Prior to wound imaging, the subject’s surgeon selected and declared the intended LOA using their clinical judgement including patient history, physical exam, and any available perfusion studies such as invasive or non-invasive angiography, arterial ultrasound, ankle-brachial indices, or toe pressures. Multispectral images of the superficial tissues at the intended LOA were obtained circumferentially to ensure inclusion of the planned amputation wound skin flap (Figure 1). Each image acquisition required approximately 30 seconds. Subject clothing and any wound dressings were removed prior to imaging. Imaging was performed no more than 14 days prior to the planned amputation. The surgeons performing amputations were blinded to the multispectral wound imaging data. Subject past medical history including commonly accepted risk factors affecting wound healing were prospectively recorded during the wound imaging session.
Figure 1.
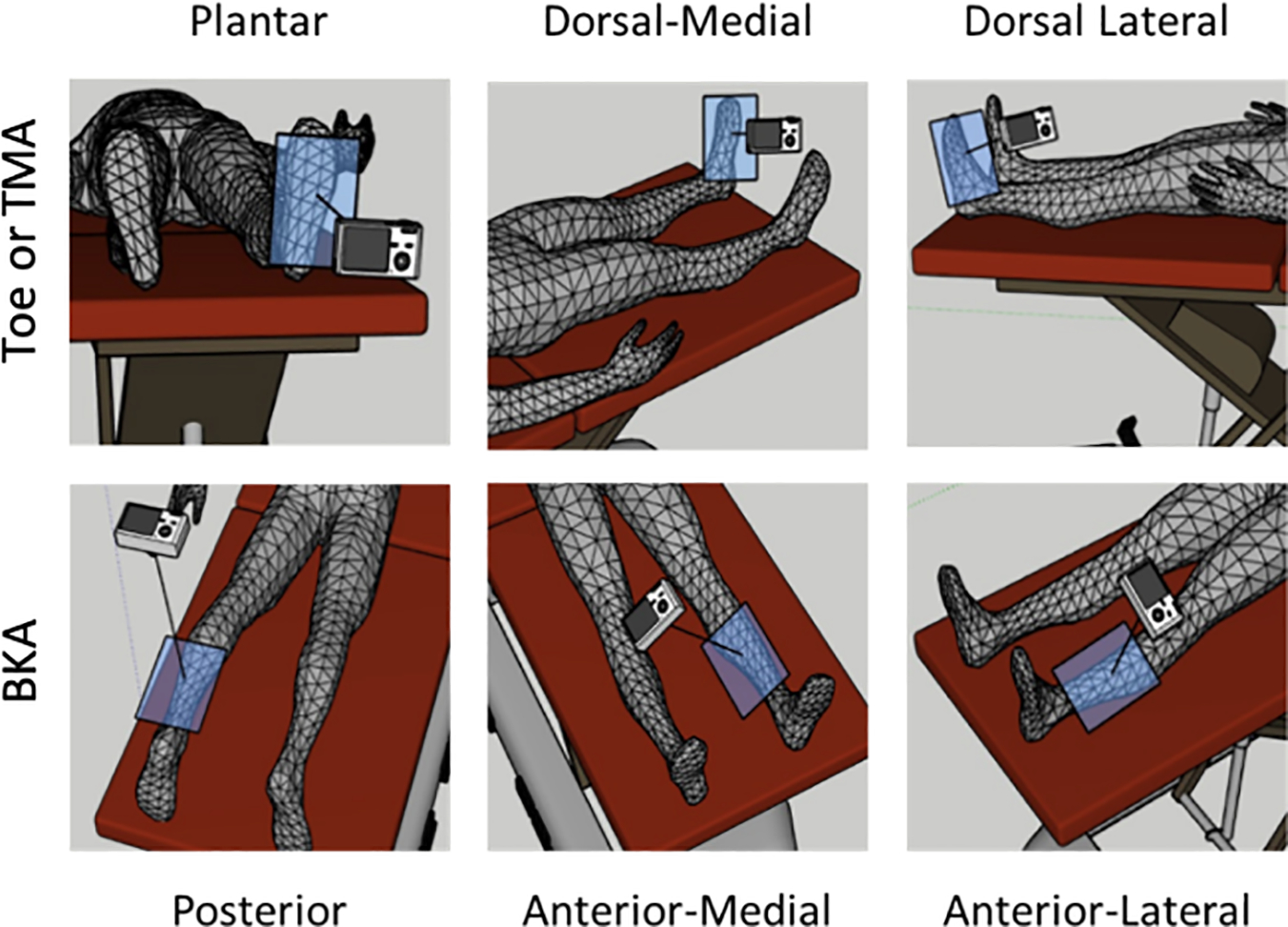
Schematic of circumferential multispectral imaging obtained prior to amputation.
Post-amputation wound care was performed according to the preferences of the operating surgeon. Subjects underwent a standardized wound healing assessment on postoperative day 30 (±5 days) during routine clinical follow-up, and no deviations from this timeframe occurred in patients surviving to at least postoperative day 25. The surgeon who performed the initial amputation, still blinded to any multispectral imaging data, evaluated the amputation wound and graded the wound as “healing” or “non-healing” according to prespecified criteria (Table 1).
Table 1.
Prespecified criteria for standardized primary amputation wound healing assessment on postoperative day 30 (±5 days).
| Category | Criteria |
|---|---|
|
| |
| Non-Healing | • Development of necrosis |
| • Development of infection, including gangrene or abscess | |
| • Ulceration occurring within or adjacent to the surgical wound | |
| • Disruption or dehiscence of suture line | |
| • Drainage or exudate expressed from the suture line | |
| • Evidence of inflammatory response including swelling, cellulitis, or skin discoloration | |
| • Hematoma formation | |
| • Revision of the amputation to a more proximal level | |
|
| |
| Healing | • Re-epithelialization of tissue within the incision site |
| • Absence of all criteria for non-healing wounds | |
Multispectral Imaging
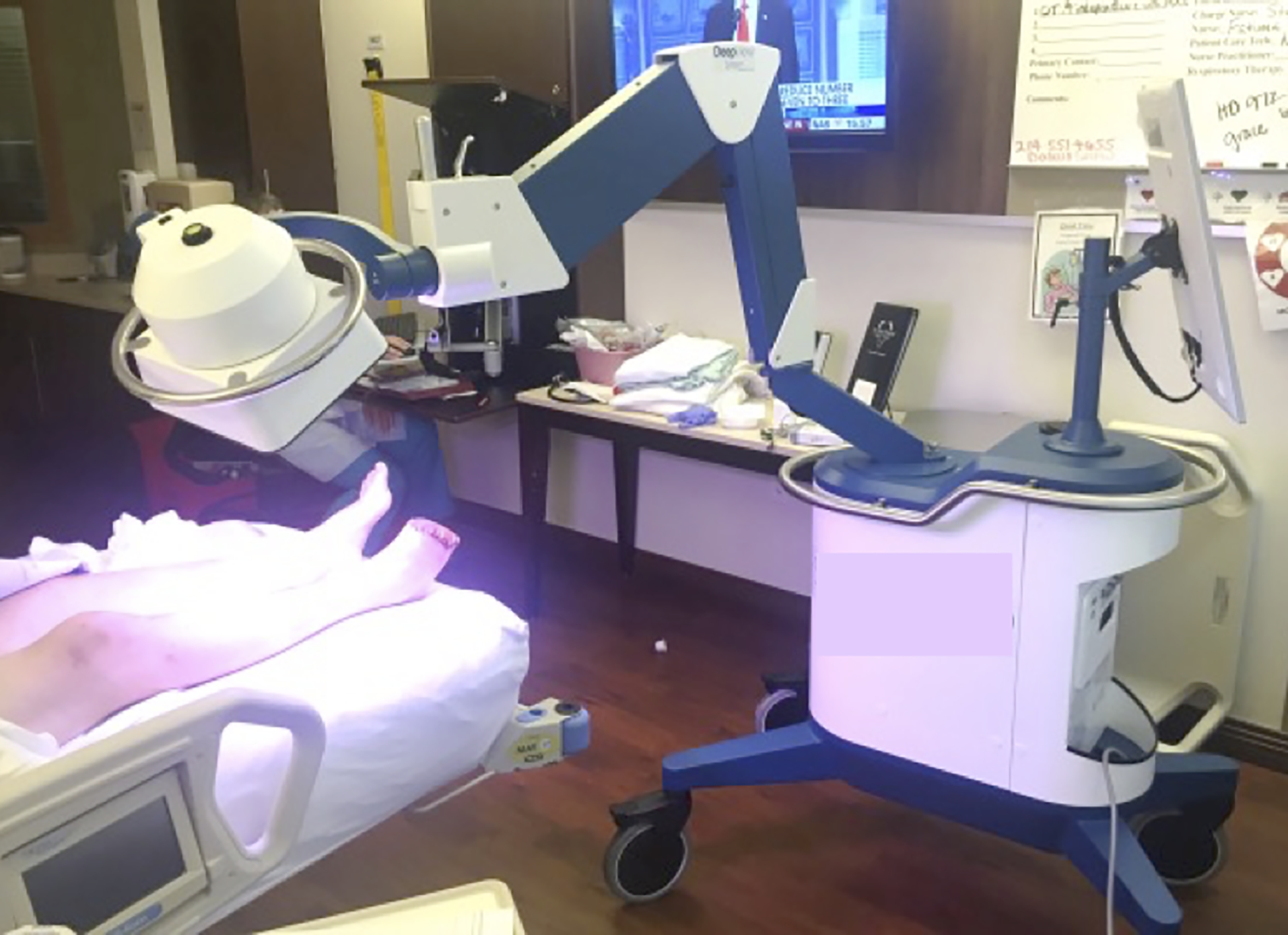
The multispectral wound imaging device used in this study is commercially available with an approved indication for use involving wound imaging (DeepView Wound Imaging System 2.0; Spectral MD, Dallas, TX) (Figure 2). However, the device does not have an approved indication specific to aiding the selection of level of amputation, so it was classified as an investigational device pertaining to its use in this study. Per CFR 812.2(b), the device met criteria for classification as a “non-significant risk” device, and therefore an investigational device exception for this study was granted by the local IRB.
Figure 2.
Picture of the wound imaging device under use in a clinical setting.
The multispectral wound imaging device measured reflectance of 8 select wavelengths of light in the visible and near-infrared spectrum (400 – 1,000 nanometers). The measurements create a spectral signature that can quantify key superficial tissue properties relevant to the microcirculation. These spectral signatures are sensitive to the volume fraction of oxygenated and deoxygenated hemoglobin, capillary density, fat content, water content, and collagen denaturation among other skin tissue components.8 Spectral signatures of skin tissue were generated on a pixel-by-pixel basis from the multispectral wound images. These MSI signatures were included as data points analyzed in the machine learning algorithm as described below. The output of the algorithm was solely a binary prediction for “healing” or “non-healing” at the imaged level of amputation—no visual output from the algorithm was generated by the algorithm for clinician review.
Machine Learning Analysis
A commonly used machine learning architecture for image segmentation (“Very Deep Convolutional Networks for Large-Scale Image Recognition” from the Visual Geometry Group at Oxford9) was modified with a Feature-wise Linear Modulation technique10 to integrate MSI data with patient clinical risk factors in order to train a machine learning algorithm that predicts primary amputation wound healing. Patient clinical risks factors included in algorithm training were determined by ranking absolute correlation coefficients between the prespecified clinical risk factors and 30-day wound healing outcome. Any clinical risk factor with an absolute correlation greater than 0.25 (> 0.25 or < −0.25) was implemented in the machine learning algorithm.
The algorithm was trained and tested using a leave-one-out cross validation technique.11 In essence, the algorithm was trained using all available amputations except one, and then tested on the amputation that had been left out. This process was repeated until each amputation had been left out, so that each amputation was used to measure the accuracy of the algorithm. The primary outcome was the overall performance of the machine learning algorithm using multispectral imaging and patient clinical risk factors as inputs to predict primary amputation wound healing on postoperative day 30. Secondary outcomes of interest were algorithm performance using only multispectral imaging data and only patient clinical risk factor data as inputs. Algorithm performance was assessed with measurements of accuracy, sensitivity, specificity, and area under the curve (AUC).
Results
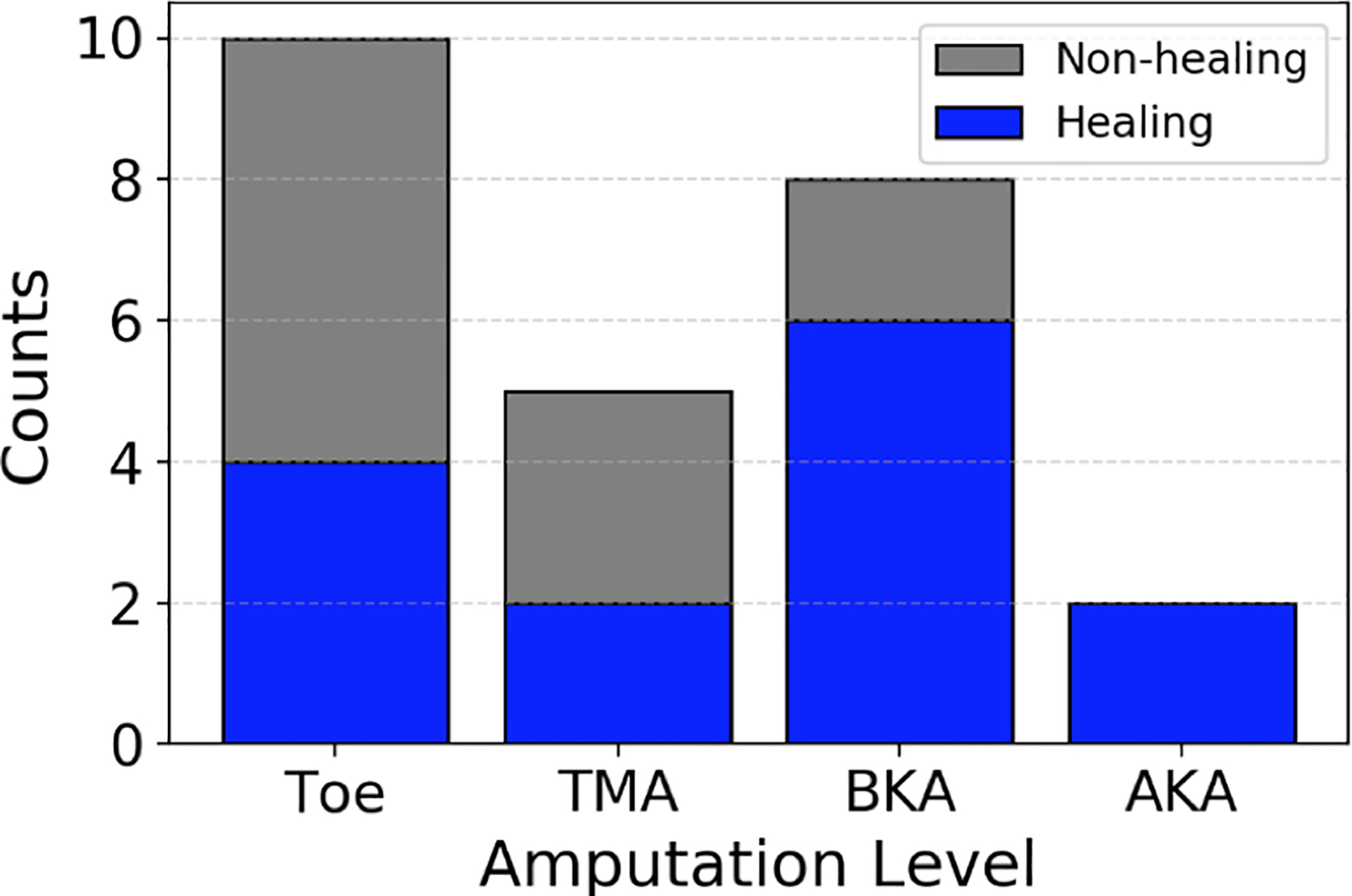
A total of 26 subjects were enrolled, and 22 completed the study. The other four patients were lost to mortality before a standardized wound healing assessment was completed. Twenty- five primary amputations were performed on the 22 patients who completed the study. The amputations included 10 toe, 5 transmetatarsal, 8 below-knee, and 2 above-knee amputations. Of these, 11 (44%) were non-healing on postoperative day 30 (Figure 3). Surgeon judgement was measured to be 56% accurate in predicting primary amputation wound healing; surgeons had 40% accuracy in predicting healing of minor amputations (toe and TMA) and 80% accuracy in predicting healing of major amputations (BKA and AKA). Patient demographics and clinical risk factors are listed in Table 2; the 11 bolded entries had absolute correlation with wound healing exceeding 0.25 and were included in the machine learning algorithm.
Figure 3.
Healing and non-healing amputations by level of amputation.
Table 2.
Subject demographics and clinical risk factors with correlation to 30-day primary amputation wound healing.
| Subject Demographics & Clinical Risk Factors | Value* | Correlation |
|---|---|---|
| Female | 6 (23) | 0.12 |
| Age, years | 66 ± 11 | 0.03 |
| Race/Ethnicity | ||
| Black | 6 (23) | −0.12 |
| Hispanic | 4 (15) | 0.05 |
| Non-Hispanic White | 15 (58) | 0.07 |
| BMI, kg/m2 | 31 ± 7 | 0.29 |
| Diabetes, Type II | 25 (96) | 0.31 |
| HbA1c, % | 8.0 ± 1.9 | 0.33 |
| Diabetic neuropathy | 8 (31) | 0.08 |
| Current tobacco use | 4 (15) | 0.05 |
| History of tobacco use | 11 (42) | 0.03 |
| Level of Amputation | ||
| Toe | 10 (38) | 0.26 |
| TMA | 6 (23) | 0.16 |
| BKA | 14 (54) | −0.26 |
| AKA | 4 (15) | −0.26 |
| Prior amputation | 14 (54) | −0.51 |
| Prior revascularization of ipsilateral extremity | 14 (54) | 0.14 |
| Prior myocardial infarction | 4 (15) | 0.27 |
| Prior cerebrovascular accident | 3 (12) | −0.08 |
| Taking anticoagulation | 17 (65) | 0.19 |
| Creatinine, mg/d | 2.2 ± 2.4 | 0.27 |
| Chronic kidney disease | 10 (38) | 0.43 |
| Currently on hemodialysis | 4 (15) | 0.27 |
Continuous variables reported as mean ± std. deviation; categorical variables reported as N (%)
AKA – above knee amputation; BKA – below knee amputation; TMA – transmetatarsal amputation
Machine learning algorithms were trained to evaluate the prespecified primary and secondary outcomes. When multispectral imaging data and patient clinical risk factors were included in algorithm training, its performance was 88% accuracy for all amputations with 91% sensitivity and 86% specificity (AUC 0.89). The algorithms trained on multispectral imaging data alone (71% accuracy, 66% sensitivity, and 75% specificity, AUC 0.70) and clinical risk factors alone (70% accuracy, 46% sensitivity, 84% specificity, AUC 0.65) underperformed as compared to the algorithm combining both data sets. These results are summarized in Table 3.
Table 3.
Performance of primary amputation wound healing prediction by surgeons and machine learning algorithms.
| Method | Accuracy | Sensitivity | Specificity | AUC | Accuracy Minor Amps | Accuracy Major Amps |
|---|---|---|---|---|---|---|
| Surgeon Judgement | 56% | n/a | n/a | n/a | 40% | 80% |
| Clinical Risk Factors Only | 70% | 46% | 84% | 0.65 | ... | ... |
| MSI only | 71% | 66% | 75% | 0.70 | ... | ... |
| MSI + Clinical Risk Factors | 88% | 91% | 86% | 0.89 | 87% | 90% |
AUC – area under curve; MSI – multispectral imaging
Minor amputations: toe and transmetatarsal amputations
Major amputations: above-knee and below-knee amputations
Discussion
In this pilot study, we have demonstrated the potential utility of a novel wound imaging system that combines advanced multispectral imaging, patient clinical risk factors, and machine learning analysis to predict primary amputation wound healing. The multispectral wound imaging system outperformed surgeon judgment (88% vs 56% accuracy) for prediction of primary amputation wound healing by postoperative day 30.
Amputations due to critical limb threatening ischemia place a significant burden on the United States healthcare system, and re-amputation/re-operation that occurs after failure to heal the primary amputation wound significantly increase this cost and hinder patient quality of life.3 Surgeons must use their best judgement, considering patient clinical risk factors, physical exam, and potentially a variety of invasive and non-invasive vascular studies to select optimal LOA. In order to offer the best functional outcome, surgeons must also balance a desire for maximum limb-salvage against the increased likelihood that primary amputation wounds will fail to heal at more distal LOAs. Unfortunately, there is currently no available technology that is widely accepted and validated to select optimal LOA. This information and technology gap was recently highlighted by the American Heart Association Council on Peripheral Vascular Disease, which released a scientific statement summarizing the limitations of current technology and advocating for the development of improved imaging technologies.4
Historically, ankle-brachial indices (ABIs) have been measured to guide therapy in patients with critical limb ischemia, including selection of LOA when amputation is necessary.12 However, the limitations of ABI for prediction of amputation wound healing have been repeatedly demonstrated.13,14 Furthermore, nearly 20% of patients will have non-compressible vessels, rendering ABIs non-diagnostic.15 Other wound and perfusion imaging technologies have been studied for predication of amputation wound healing. Two of the most thoroughly studied technologies are transcutaneous oxygen measurement (TCOM) and laser Doppler imaging (LDI).16,17 Neither, however, has become widely adopted due to important limitations including high intra-operator variability, sensitivity to motion artifact and ambient room temperature, and uncertainty about interpretation of data output thresholds to predict healing.4,18,19
This study was motivated by our hypothesis that multispectral imaging has to the potential to overcome some of the limitations of other proposed technologies. First developed for military reconnaissance applications, MSI technology measures reflectance patterns of several (generally no more than a dozen) key wavelengths of light selected for a particular application.5 MSI devices are relatively simple to implement using light-emitting diodes that cover the range of selected wavelengths and a camera with filters that isolate individual wavelengths of interest for measurement. Importantly multispectral images can be obtained rapidly, without patient contact, and while using non-X-ray wavelengths of light. Thus, motion artifact and potential harm to patient are minimized. Several of the authors have previous experience using MSI technology to predict healing potential of burn wounds. For that application and the current one, the selected wavelengths of light were chosen based on their ability to measure variation in local tissue characteristics that are likely to influence wound healing, including tissue oxygenation, fat content, and collagen denaturation.6 In controlled experiments using a burn animal model, MSI was 80–89% accurate in differentiating superficial from deep burn injuries, an essential determination when assessing whether a burn wound is likely to heal.7
However, there is a key distinction between potential applications of MSI technology in the evaluation of patients with burn wounds versus those with critical limb threatening ischemia. Burn patients are commonly young (even pediatric), burdened with relatively few comorbidities, and rarely have peripheral vascular disease; therefore local tissue factors (specifically, burn depth) can be considered in isolation when trying to predict healing potential.20 In contradistinction, patients with critical limb threatening ischemia are typically older, have many comorbidities, and by definition have anatomical vascular disease, all of which heavily influence healing potential beyond local tissue conditions. This concept has been previously conceptualized as a triple matrix for assessment of the threatened limb that must account for not only local tissue factors but also patient risk factors and anatomical disease patterns.21 Therefore, the algorithm reported in this study was designed to account for local tissue conditions as measured by the MSI technology and global patient risk factors that collectively influence the likelihood of amputation wound healing. Importantly, the algorithm was also trained to provide an easily-interpretable, binary output – healing versus non-healing – rather than complex imaging maps or indices that may be subject to variable interpretation.
There are several limitations of this study that are important to consider. First, this is a small pilot study with follow-up limited to 30 days. Formal algorithm training must be performed in a larger sample size of patients over a greater duration of follow-up to improve generalizability and clinical utility. Validation of the algorithm should occur in an independent data set rather than the cross-validation methods employed in our study before the wound imaging system is introduced into clinical practice as an aid in preoperative decision making. The performance of the wound imaging system may also be subject to other factors not considered in our study including ambient room temperature during image collection as well as patient skin tone. These factors will need to be evaluated in future investigations. Because surgeon prediction of wound healing was substantially better for major amputations, future studies with larger sample sizes should consider reporting algorithm performance specific to minor and major amputation levels to provide additional context regarding the potential of the device to meaningfully improve clinical decision-making in various clinical scenarios.
Finally, the Society of Vascular Surgeons developed the Wound characteristic, Ischemia, and foot Infection (WIfI) score as a facile clinical staging system to evaluate patients with critical limb ischemia.22 The WIfI score is well correlated with a variety of clinical outcomes relevant to patients with critical limb ischemia, and the score has recently been incorporated in international guidelines,23 landmark clinical trials,24,25 and the Vascular Quality Initiative registry.21 Although the current study did not prospectively collect data to facilitate calculation of the WIfI score in enrolled subjects, future studies should evaluate whether inclusion of the WIfI score, or any of its individual components, may improve algorithm performance.
Currently, up to one-third of primary amputations performed in patients with critical limb threatening ischemia require revision to a more proximal level.2 Unfortunately, there is no gold-standard test currently available to predict healing of an amputation wound with high reliability. In this pilot study, we have demonstrated proof-of-concept for a novel wound imaging system that combines multispectral superficial tissue imaging with patient clinical risk factors via machine learning analysis to predict primary amputation wound healing with a high degree of accuracy. Larger algorithm training and validation studies are planned to further evaluate this wound imaging system. If the validation studies confirm our findings, this imaging modality may offer significant savings in health care costs for patients with critical limb threatening ischemia undergoing lower extremity amputation.
Article Highlights.
Type of Research:
Prospective, multicenter, non-randomized cohort study.
Key Findings:
The machine learning algorithm analyzing multispectral imaging data of superficial tissues and patient clinical risk factors had 91% sensitivity and 86% specificity for prediction of non-healing amputation sites (area under curve 0.89).
Take Home Message:
Machine learning analysis of multispectral imaging and patient clinical risk factors may aid in selection of optimal level of amputation
Acknowledgements:
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R43HL142428.
Footnotes
Disclosure: JJS and RDB report consulting fees from Spectral MD; FY, PQ, SY, JET report salary from Spectral MD; JET, FY, PQ, SY, and JMD report equity in Spectral MD.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Geiss LS, Li Y, Hora I, Albright A, Rolka D, et al. Resurgence of diabetes-related nontraumatic lower-extremity amputation in the young and middle-aged adult U.S. population. Diabetes Care 2019;42:50–4. [DOI] [PubMed] [Google Scholar]
- 2.Kono Y, Muder R. Identifying the incidence of and risk factors for reamputation among patients who underwent foot amputation. Ann Vasc Surg 2012;26:1120–6. [DOI] [PubMed] [Google Scholar]
- 3.Dillingham T, Pezzin L, Shore A. Reamputation, mortality, and health care costs among persons with dysvascular lower-limb amputation. Arch Phys Med Rehabil 2005;86:480–6. [DOI] [PubMed] [Google Scholar]
- 4.Misra S, Shishehbor MH, Takahashi EA, Aronow HD, Brewster LP, Bunte MC, et al. Perfusion assessment in critical limb ischemia: Principles for understanding and the development of evidence and evaluation of devices. A scientific statement from the American Heart Association. Circulation 2019;140:e657–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thatcher JE, Squiers JJ, Kanick SC, King DR, Lu Y, Wang Y, et al. Imaging techniques for clinical burn assessment with a focus on multispectral imaging. Adv Wound Care 2016;5:360–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.King DR, Li W, Squiers JJ, Mohan R, Sellke E, Mo W, et al. Surgical wound debridement sequentially characterized in a porcine burn model with multispectral imaging. Burns 2015;41:1478–87. [DOI] [PubMed] [Google Scholar]
- 7.Thatcher JE, Li W, Rodriguez-Vaqueiro Y, Squiers JJ, Mo W, Lu Y, et al. Multispectral and photoplethysmography optical imaging techniques identify important tissue characteristics in an animal model of tangential burn excision. J Burn Care Res 2016;37:38–52. [DOI] [PubMed] [Google Scholar]
- 8.Zonios G, Bykowski J, Kollias N. Skin melanin, hemoglobin, and light scattering properties can be quantitatively assessed in vivo using diffuse reflectance spectroscopy. J Invest Derm 2001;117:1452–7. [DOI] [PubMed] [Google Scholar]
- 9.Simonyan K, Zisserman A. Very deep convolutional networks for large-scale image recognition. arXiv 2014;1409.1556v6: 1–14. [Google Scholar]
- 10.Perez E, Strub F, de Vries H, Dumoulin V, Courville A. FiLM: Visual reasoning with a general conditioning layer. arXiv 2017;1709.07871v2:1–13. [Google Scholar]
- 11.Hastie T, Tibshirani R, Friedman J. The elements of statistical learning. New York, NY: Spring Series in Statistics, Springer New York Inc, 2001. [Google Scholar]
- 12.Aboyans V, Criquie MH, Abraham P, Allison MA, Creager MA, Diehm C, et al. Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation 2012;126:2890–909. [DOI] [PubMed] [Google Scholar]
- 13.Sukul D, Grey SF, Henke PK, Gurm HS, Grossman PM. Heterogeneity of ankle-brachial indices in patients undergoing revasculariation for critical limb ischemia. JACC Cardiovasc Interv 2017;10:2307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bunte MC, Jacob J, Nudelman B, Shishehbor MH. Validation of the relationship between ankle-brachial and toe-brachial indices and infragenicular arterial patency in critical limb ischemia. Vasc Med 2015;20:23–9. [DOI] [PubMed] [Google Scholar]
- 15.Arain FA, Ye Z, Bailey KR, Chen Q, Liu G, Leibson CL, et al. Survival in patients with poorly compressible leg arteries. J Am Coll Cardiol 2012;59:400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keyzer-Dekker C, Moerman E, Leijdekkers V, Vahl A. Can transcutaneous oxygen tension measurement determine re-amputation levels? J Wound Care 2006;15:27–30. [DOI] [PubMed] [Google Scholar]
- 17.Mars M, McKune A, Robbs J. A comparison of laser Doppler fluximetry and transcutaneous oxygen pressure measurement in the dysvascular patient requiring amputation. Eur J Vasc Endovasc Surg 1998;16:53–8. [DOI] [PubMed] [Google Scholar]
- 18.Goodall R, Langridge B, Onida S, Davis A, Shalhoub J. Current status of noninvasive perfusion assessment in individuals with diabetic foot ulceration. J Vasc Surg 2019;69:315–7. [DOI] [PubMed] [Google Scholar]
- 19.Faglia E, Clerici G, Caminiti M, Quarantiello A, Curvi V, Morabito A. Predictive values of transcutaneous oxygen tension for above-the-ankle amputation in diabetic patients with critical limb ischemia. Eur J Vasc Endovasc Surg 2007;33:731–6. [DOI] [PubMed] [Google Scholar]
- 20.Monstrey S, Hoeksema H, Verbelen J, Pirayesh A, Blondeel P. Assessment of burn depth and burn wound healing potential. Burns 2008;34:761–9. [DOI] [PubMed] [Google Scholar]
- 21.Mills JL Sr. The application of the Society for Vascular Surgery Wound, Ischemia and foot Infection (WIfI) classification to stratify amputation risk. J Vasc Surg 2017;65:591–3. [DOI] [PubMed] [Google Scholar]
- 22.Mills JL, Conte MS, Armstrong DG, Pomposelli FB, Schanzer A, Sidawy AN, et al. The Society of Vascular Surgery lower extremity threatened limb classification system: risk stratification based on Wound, Ischemia, and foot Infection (WIfI). J Vasc Surg 2014;59:220–4. [DOI] [PubMed] [Google Scholar]
- 23.Conte MS, Bradbury AW, Kolh P, White JV, Dick F, Fitridge R, et al. Global Vascular Guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg 2019;S1–109.e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menard MT, Farber A, Assmann SF, Choudhry NK, Conte MS, Creager MA, et al. Design and rationale of the best endovascular versus best surgical therapy for patients with critical limb ischemia (BEST-CLI) Trial. J Am Heart Assoc 2016;5:e003219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Popplewell MA, Davies H, Jarrett H, Bate G, Grant M, Patel S, et al. Bypass versus angio plasty in severe ischaemia of the leg – 2 (BASIL-2) trial: study protocol for a randomised controlled trial. Trials 2016;17:11. [DOI] [PMC free article] [PubMed] [Google Scholar]


