Abstract
Peripheral nerve injuries result in disrupted cellular communication between the central nervous system and somatic distal end targets. The peripheral nervous system is capable of independent and extensive regeneration; however, meaningful target muscle reinnervation and functional recovery remain limited and may result in chronic neuropathic pain and diminished quality of life. Macrophages, the primary innate immune cells of the body, are critical contributors to regeneration of the injured peripheral nervous system. However, in some clinical scenarios, macrophages may fail to provide adequate support with optimal timing, duration, and location. Here, we review the history of immunosuppressive and immunomodulatory strategies to treat nerve injuries. Thereafter, we enumerate the ways in which macrophages contribute to successful nerve regeneration. We argue that implementing macrophage-based immunomodulatory therapies is a promising treatment strategy for nerve injuries across a wide range of clinical presentations.
Keywords: peripheral nerve injury, immunoengineering, immunomodulation, macrophages, regeneration
Graphical Abstract
1. Introduction
Peripheral nerve injury (PNI) can affect individuals across sex, age, and socioeconomic status and can occur as the result of trauma, iatrogenic causes, and long-term irritation. Unlike neurons in the central nervous system (CNS), neurons in the injured peripheral nervous system (PNS) are able to spontaneously regenerate long-projecting axons and are capable of restoring complete physiological function. However, in many cases of PNI, incomplete or misguided regeneration results in degeneration of denervated tissue, prolonged or chronic pain, lifelong disability, and decreased quality of life (Wojtkiewicz et al., 2015). Meaningful functional recovery is often limited because of the slow regenerative pace of growing nerves (1-2 mm/day) and the diminishing window for successful reinnervation of the distal end targets (12-18 months for motor function) (Grinsell and Keating, 2014). Peripheral nerve recovery is affected by age (with younger individuals exhibiting more robust recovery); injury location (with sites close to the distal end targets recovering more fully); and injury severity (with stretch or compression injuries recovering better than transection or shredding injuries) (Grinsell and Keating, 2014; Menorca et al., 2013; Rosenberg et al., 2012).
It is difficult to accurately calculate the epidemiological burden of traumatic PNI because PNI diagnosis and treatment are not prioritized during trauma and PNI is frequently associated with other comorbidities (Ciaramitaro et al., 2010). For example, approximately 60% of individuals presenting with a PNI also present with a traumatic brain injury (Noble et al., 1998; Stone and Keenan, 1988). People with comorbidities including diabetes and vascular disease may be more susceptible to nerve injury because their nerves are less resistant to mechanical forces and because these comorbidities are associated with chronic or dysregulated inflammation (Tezcan, 2017; Tsalamandris et al., 2019). Furthermore, it can take up to 50 days after admission into a clinic or hospital to diagnose certain types of nerve injuries appropriately (Noble et al., 1998).
While traumatic PNIs are complex and may significantly reduce long term quality of life, they affect a relatively small population of individuals in comparison to other trauma outcomes. Of the approximately 3.5 million people who experienced upper limb trauma annually (Ootes et al., 2012), between 1-3% exhibit nerve injury (Padovano et al., 2020; Taylor et al., 2008). In line with these numbers, a comprehensive study of Swedish data from 1998-2006 estimated that 0.0139% of the general population experienced nerve injuries with the majority of these injuries occurring in the wrist and hand (Asplund et al., 2009). Indeed, many studies over the last several decades conclude that the vast majority of traumatic nerve injuries are crush injuries in the hand or wrist of males between the ages of 20-24 (Asplund et al., 2009; Kankaanpää and Bakalim, 1976; McAllister et al., 1996; Noble et al., 1998; Taylor et al., 2008).
Damage to the PNS affects efferent (signal transmission from the CNS) and afferent (signal transmission towards the CNS) nervous system communication with sensory organs, skeletal muscle, and autonomic systems. Treatment of PNS injuries has traditionally prioritized a hands-off approach with surgical intervention strategies being implemented in only the most severe and unrecoverable cases without repair. However, nascent research suggests that the immune system plays a leading role in tissue regeneration and recovery following PNI. Immune cells such as macrophages, the primary innate immune cells of the body, interact and influence resident Schwann cells, neurons, axons, fibroblasts, and myocytes to enhance functional recovery. Although previously underappreciated, macrophages are leading contributors to nerve homeostasis, regeneration, and also have an immense capacity to supplement and enhance current treatment strategies. In this paper, we will review the history of affecting the immune system after peripheral nerve injuries, enumerate the ways in which macrophages beneficially contribute to nerve regeneration, list current immunomodulatory techniques, and discuss the challenges of bringing immunoengineering therapies into clinical use.
2. Historic Attempts of Immunosuppression and Immunomodulation after PNI
In the early first century, the classic tetrad of inflammation was first described by Celsus, a Roman physician, as calor (warmth), dolor (pain), tumor (swelling), and rubor (redness/hyperemia) (Rosenthal, 1961). Broadly, these signs result from physiological changes necessary to protect injured tissue and facilitate regeneration. In the 1940s, early studies by Medawar expanded on these findings by investigating the role of inflammation and healing in a rabbit skin graft model (Medawar, 1944). He summarized that the evolution of normal healing following skin autograft repair starts with vascularization, followed by generalized hyperplasia or cell proliferation, and finally partial retrograde differentiation to allow for reformation of normal skin (Medawar, 1944). In contrast, allograft repair (termed “homografts” in older texts) resulted in acute vascularization and inflammation, but the foreign tissue was subsequently destroyed.
In line with these experiments, surgeons in the 1940s investigated if allograft nerve repairs could be utilized to enhance recovery following nerve injury. Disappointingly, mounting clinical evidence reporting poor functional recovery, especially following large segmental defects, led surgeons to consider that “the nerve [allo]graft should be regarded as an incontrovertible failure” (Barnes et al., 1946). Similar to skin grafts, the substrate provided by the allograft elicited an immune response, destroying the foreign tissue, and ultimately led to graft failure. To address these complications, researchers in the 1960s demonstrated frozen irradiated allografts for peripheral nerve repair mitigated some immune response and increased regeneration, suggesting that further immune suppression could stimulate greater functional recovery (Marmor, 1964a, 1964b).
Given the association between inflammation and graft failure, attention turned towards immunosuppressive agents to enhance the efficacy of allograft repair. These efforts exhibited varying degrees of success in early experimental and clinical studies (Ducker and Hayes, 1970; Marmor, 1970; Marmor et al., 1967; Pollard et al., 1973; Pollard and Fitzpatrick, 1973; Pollard and McLeod, 1971). For example, FK506 (tacrolimus) is a potent immunosuppression agent, which may also improve functional recovery following peripheral nerve repair (for review, see (Kuffler, 2009) and (Konofaos and Terzis, 2013)). During the mid-1990s, FK506 gained FDA-approval to prevent rejection/prolong survival of solid organ transplantation, including allotransplanted heart, kidney, skin, limbs, pancreas, and duodenum tissues (Arai et al., 1989; Inamura et al., 1988; Ochiai et al., 1987b, 1987a; S. Todo et al., 1988; Sato et al., 1989). In 2002, researchers tested FK506 efficacy using rodent models of chronic axotomy or chronic denervation. They demonstrated that FK506 administration enhanced axonal outgrowth after a chronic axotomy but exhibited minimal effects following prolonged denervation (Sulaiman et al., 2002).
While FK506 is a promising adjunctive strategy, its clinical efficacy remains unclear. In the U.K., phase II clinical trials have been completed studying FK506 tolerance after nerve repair and reported 5 patients who were treated within 7 days after nerve repair for up to 60 days post-operative. Although no adverse events were reported, no significant improvement was detected compared to expected clinical recovery (Phan and Schuind, 2012). To the best of our knowledge, no double-blind placebo-controlled randomized studies have been performed yet, with only one proposed in the U.S. in 2009 that was withdrawn in 2018 due to lack of funding (NCT00950391). Although FK506 is widely considered a neuroenhancer, as a potent immunosuppression agent, it may lead to unwarranted severe side effects such as increased risk of infections. Indeed, in the last several years, researchers at the University of Toronto have worked to develop methods to locally deliver FK506 to the injured nerve, thus avoiding systemic immunosuppression (Tajdaran et al., 2015). These studies suggest that local delivery of FK506 to the transection injury site promoted motor and sensory neuron regeneration and was associated with larger numbers of myelinated axons (Tajdaran et al., 2019). Indeed, this local FK506 delivery system also enhanced nerve regeneration through transection injuries repaired with allografts (Zuo et al., 2021). Together, these data suggest that control over FK506 administration and distribution could enhance the translatability of this treatment strategy.
Other immunosuppressive strategies have been administered after nerve injury, however, like FK506, many of these strategies simultaneously suppress healthy regeneration. For example, treatment with high doses of minocycline, an antibiotic with potent anti-inflammatory activity, was found to decrease macrophage recruitment and activation, reduce phagocytic Schwann cell activation, slow Wallerian degeneration, and inhibit nerve regeneration in a rat model of sciatic nerve injury (Keilhoff et al., 2006). Similarly, macrophages were found to be essential for ascending afferent fiber regeneration in the CNS following a conditioning lesion in the periphery (Aguilar Salegio et al., 2011). In this study, macrophage depletion via clodronate liposome treatment abolished the ability of dorsal root ganglion cells to regenerate afferent fibers after a combined sciatic nerve injury followed by dorsal column cut in rats. Likewise, eliminating macrophages with clodronate liposomes limited regrowth of injured nerves and reduced functional recovery of sensory and motor tasks (Chen et al., 2015b; Mokarram et al., 2017). These findings suggest that macrophages are essential for successful nerve regeneration after injury.
In line with these findings, attention has shifted away from immunosuppressive strategies for promoting injured nerve regeneration. Instead, strategies aimed to employ monocyte homing, phagocytosis, and phenotype could accelerate and/or improve the extent of tissue regeneration following disease and trauma. This shift can be attributed at least in part to a growing understanding of the complex role of inflammation in wound healing across multiple tissues and organs, including PNI, that will be extensively discussed in the following sections.
Monocyte-derived macrophages are key regulators of the innate immune response that have been long been appreciated for their role identifying pathogens and fighting disease. Circulating blood monocytes are derived from hematopoietic stem cells in the bone marrow and macrophages can either be tissue-resident macrophages or monocyte-derived macrophages. In healthy nerve development, tissue-resident macrophages come from macrophage infiltration in late embryonic development (Amann and Prinz, 2020). The tissue-resident macrophage population is replaced over time by infiltrating bone marrow-derived cells. The replacement rate of macrophages in healthy nerves is inversely correlated with age (Amann and Prinz, 2020). Upon sensing injury or disease through chemoattractant cues, monocytes leave the blood stream and infiltrate into damaged tissues. Indeed, monocytes home into all tissues, including the CNS and PNS, following injury (Klyachko et al., 2014; Tong et al., 2016). One of the most widely appreciated functions completed by macrophages is their ability to remove pathological and necrotic components. In the late 1800s, a Russian scientist, Elie Metchnikoff, observed and described that foreign material could be engulfed by migratory mesodermal cells (Metchnikoff, 1887). He termed these cells ‘phagocytes’ (meaning ‘eating cells’) and termed the process ‘phagocytosis’ (Kaufmann, 2008). These findings, along with other findings related to antibodies, helped found the field of immunology in the late nineteenth century (Kaufmann, 2008).
For over one hundred years these phagocytic macrophages were appreciated exclusively for their role in promoting inflammation and removing pathological material. However, in 1992, scientists began to learn that macrophages could exhibit a spectrum of behavioral phenotypes depending on the environmental cues that they were exposed to (Stein et al., 1992). Macrophages can secrete inflammatory factors, phagocytose apoptotic cells, act as antigen presenting cells, stimulate angiogenesis, guide extracellular matrix deposition, and influence stem cell development (Sunderkotter et al., 1994; Wynn et al., 2013; Wynn and Vannella, 2016). These ranges of behaviors are termed phenotypes in macrophages and are the subject of much research. Over the last couple decades a number of new behavioral phenotypes and functional profiles have been described for macrophages (Mosser and Edwards, 2008; Wynn and Vannella, 2016). Macrophage behavioral phenotype and function is now understood to be a highly plastic process that varies as a function of timing, location, and environmental context (Brown et al., 2014; Spiller et al., 2016, 2015; Wofford et al., 2019b). Careful regulation of macrophage phenotypes can be critical for tissue regeneration because these cells also oversee endogenous regeneration pathways (Brown et al., 2014).
Together these findings imply macrophages are major drivers of tissue regeneration across a multitude of organ systems. Here, we aim to review the major contributions of macrophages to PNI regeneration and to explore innovative strategies in immunoengineering (Figure 1). Immunoengineering is a new discipline that deals with design, fabrication, and redirection of the innate and adaptive immune systems in order to mitigate disease and promote tissue regeneration. Immunoengineering holds immense promise in developing therapeutics to redirect or enhance endogenous regeneration pathways. Indeed, immunoengineering strategies that can control macrophage infiltration, activation, and phenotype with spatial and temporal resolution could drastically improve PNI treatment. Future immunoengineering strategies require a comprehensive understanding of the various roles macrophages play in peripheral nerve repair. Below we will discuss several different ways in which macrophages contribute to regeneration following PNI.
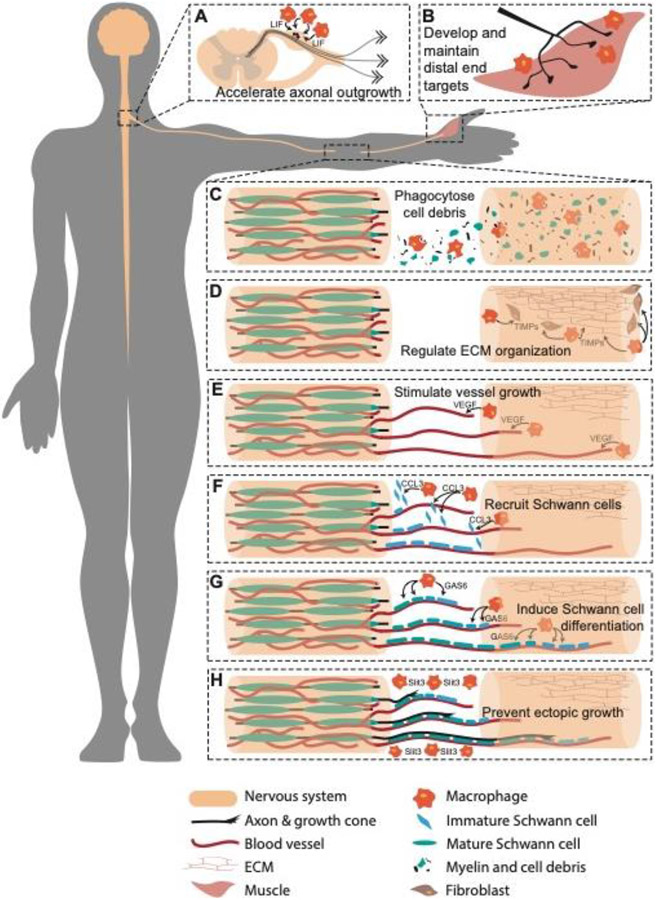
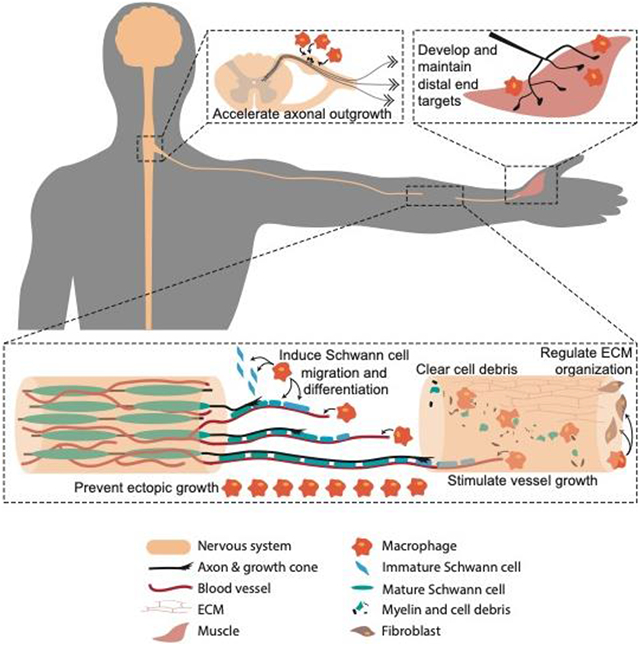
Figure 1. Macrophages contribute to peripheral nerve regeneration.
(A) Macrophages at the dorsal root ganglia release neurotrophic factors such as leukemia inhibitor factor (LIF) that stimulate axonal outgrowth. (B) Macrophages at the distal end target contribute to synapse formation and neurotransmitter regulation. (C-H) Macrophages at the injury site (C) clear myelin fragments and cellular debris, (D) regulate extracellular matrix (ECM) organization through secreting tissue inhibitors of metalloproteinase (TIMPs), (E) stimulate angiogenesis through secretion of vascular endothelial growth factor (VEGF), (F) recruit Schwann cells with chemokine ligand 3 (CCL3), (G) induce Schwann cell maturation through growth arrest specific 6 (GAS6), and (H) prevent ectopic growth with slit homolog 3 protein (Slit3) expression.
3. Macrophage Contributions to Regeneration Following PNI
3.1. Macrophages phagocytose myelin debris and axon fragments
Following mechanical trauma, axons distal to the injury site degenerate. This process of axonal degradation is termed Wallerian degeneration because it was first described in 1850 by an English physician, Augustus Waller (Waller, 1850). Mechanical trauma to the nerves; through either transection, crush, stretch, or irritation injury can damage the cytoskeletal structure in axons, which is essential for transporting proteins and nutrients from the neuronal soma to the distal synapses of the neuron (Smith and Meaney, 2000). Following injury, the damaged axon will undergo a neuron-autonomous process of rapid granular disintegration of the axonal cytoskeleton. This process occurs along the entire length of the distal axon and along the proximal axon up to the first node of Ranvier. The time to initiation of axonal fragmentation is dependent upon fiber size with thinner axons undergoing fragmentation more rapidly than thicker axons (Lubinska, 1977). A rapid influx of calcium into the injured axons activates calpains which go on to cleave neurofilament, spectrin, and tubulin (Wang et al., 2012). Degradation of the internal axonal structure through calpain-mediated cleavage and the ubiquitin-proteasome system, which facilitates microtubule disassembly, together can induce rapid axonal degradation (Wang et al., 2012).
Following injury but before the process of fragmentation, macrophages increase in numbers along the length of the nerve in a complement-dependent pathway (Dailey et al., 1998). Macrophage population size increases as a result of dividing resident endoneurial macrophages and infiltrating blood monocytes (Rosenberg et al., 2012; Ydens et al., 2020). Infiltrating monocytes typically arrive within 120 minutes of nerve transection (in a zebrafish model) and closely associate with damaged, intact axons, waiting and scanning the damaged axon until fragmentation is initiated. Immediately after axonal fragmentation occurs (typically 145-200 minutes following a transection in this zebrafish model), these scanning macrophages rush to phagocytose residual axonal debris and myelin fragments that remain in the wake of the degenerating axon (Figure 1C; Rosenberg et al., 2012; Vargas and Barres, 2007). In contrast to rapid Wallerian degeneration and myelin removal in the PNS, Wallerian degeneration and myelin removal in the CNS is exceptionally slow (Avellino et al., 1995). Indeed, myelin residuals in the CNS are not effectively removed and can remain in the wound environment for months to years and are associated with delayed or thwarted regeneration (Fawcett and Asher, 1999; Vargas and Barres, 2007).
In the past, Schwann cells have been widely believed to be responsible for monocyte recruitment to sites of nerve injury (Vargas and Barres, 2007). However, knock out studies in zebrafish have demonstrated that monocyte recruitment to the site of PNI can occur in the absence Schwann cells and their chemoattractant signals (Rosenberg et al., 2012). However, Schwann cells likely enhance the recruitment of these immune cells through paracrine signaling (Chen et al., 2015b; Martini et al., 2008; Mueller et al., 2003). Indeed, Schwann cells can release a number of chemoattractant signals including but not limited to CCL2 (monocyte-chemoattractant protein-1, MCP-1), leukemia inhibitory factor (LIF), interleukin (IL)-1α, IL-1β, and pancreatitis-associated protein III (PAP-III) (Chen et al., 2015b).
The extent and duration of macrophage infiltration varies across animal species and injury model. Following sciatic nerve injury crush in a rat, macrophage numbers peak in the injury site one day after injury and gradually decrease over the next two weeks (Qian et al., 2018). Macrophages that do persist in the nerve move distally along the degrading distal nerve stump at a rate similar to the growth of regenerating axon growth cones (Qian et al., 2018). In a similar model of rat sciatic nerve crush, bone-marrow derived macrophages infiltrated into the nerve injury site and peak in numbers two weeks after the injury (Stratton et al., 2018). Thereafter, the number of macrophages plateau from 2 to 3 weeks and could be detectable in the recovering nerve up to 16 weeks after injury (Stratton et al., 2018). In a murine model of crush PNI, resident macrophages increase in numbers up until 14 days after the injury reaching a maximum of five times the control levels (Mueller et al., 2003). However, infiltrating monocyte-derived macrophages comprise the bulk of the macrophages in the nerve after injury, vastly exceeding the number of resident macrophages (Amann and Prinz, 2020; Ydens et al., 2020). Infiltrating macrophages increase in numbers up until about 14 days after injury, reaching a maximum of twenty times the control level at this time point (Mueller et al., 2003; Stratton et al., 2018). In a murine model of optic nerve crush (technically a model of CNS injury that interacts with the peripheral immune system), macrophages were detectable 3 days after nerve injury but peaked in numbers 5 days after injury (Perry et al., 1987). In a zebrafish model of laser-induced nerve ablation, macrophages were recruited to the injury site within 1 hour and closely associate with the distal nerve stump, where they remain until axonal fragmentation of the distal transected nerve begins (Rosenberg et al., 2012). After human nerve injury, macrophages are found in and distal to the nerve site for up to five months post-injury, the longest timepoint considered in this study (Stratton et al., 2018). These studies suggest that while there is variability in macrophage infiltration across species and injury models, a massive influx of these cells is a conserved characteristic across several models of PNI. Because macrophage contributions vary across injury models, it will be important for future studies to investigate which macrophage characteristics and behaviors are conserved in clinical cases of human nerve injury.
Perhaps the most commonly appreciated and thoroughly discussed role of macrophages in PNI repair is their ability to phagocytose myelin fragments from the distal nerve segment during Wallerian degeneration. Indeed, since the 1980s (Perry et al., 1987), myelin fragment removal has been appreciated as a critical step in peripheral nerve regeneration. Myelin removal helps establish a permissive environment for the regenerating nerve because myelin fragments are notoriously inhibitory to axonal growth cones because they expresses inhibitory molecules such as NI250 (Nogo) and myelin-associated glycoprotein (MAG) (Akbik et al., 2012; Fawcett and Asher, 1999; Schnell and Schwab, 1990). Nogo prevents axonal regrowth by causing growth cone collapse. The antibody IN-1 binds to Nogo and blocks its expression, resulting in enhanced axonal regeneration and improved functional recovery (Chen et al., 2000; Fawcett and Asher, 1999). Likewise, MAG is expressed in myelin and can also inhibit some, but not all, axonal growth cones (Akbik et al., 2012; Fawcett and Asher, 1999).
During Wallerian degeneration, Schwann cells break down myelin during the first 5-7 days after injury and package myelin into discrete ovoid segments (Perry et al., 1995). Thereafter, recruited and resident macrophages accelerate myelin clearance in a complement-dependent pathway. Indeed, C3-deficient serum precludes macrophage infiltration and decreases the extent of myelin fragments phagocytosis (Brück and Friede, 1991). Schwann cells can work in concert with macrophages to complete myelin phagocytosis and clearance from the injury site. While macrophages complete most of the myelin clearance, their presence may not be critical for myelin removal. Schwann cells can complete selective autophagy of these myelin components, termed myelinophagy, in their lysosomes by an mTOR-independent pathway (Gomez-Sanchez et al., 2015). In a CCR2−/− mouse model of PNI, macrophages did not infiltrate into the lesion site but myelin removal was initiated in the absence of macrophages (Niemi et al., 2013). These data suggest that macrophages are the primary cells responsible for rapidly clearing myelin, but in their absence, Schwann cells are capable of acting as alternates to step in and complete this critical function for regeneration (Niemi et al., 2013; Perry et al., 1995).
Removal of inhibitory myelin debris is essential for axonal regrowth into the distal nerve and end target. However, phagocytosis of myelin debris can also promote other beneficial effects on the injury environment’s regenerative capacity. Macrophages that phagocytose myelin have been shown to secrete neurotrophic factors that can enhance neuronal survival (Hikawa and Takenaka, 1996; Kroner et al., 2014). Likewise, macrophage phagocytosis of apoptotic cells via the thrombospondin receptor (CD36) downregulates inflammatory factors including tumor necrosis factor α (TNFα), IL-1, and IL-12 while simultaneously promoting the immunoregulatory cytokine IL-10 (Voll et al., 1997). However, myelin loading in macrophages is associated with prolonged inflammation when iron and TNFα has accumulated in the local environment (Kroner et al., 2014). Thus, the balance between the number of infiltrating cells and the amount of debris they clear is important in fostering a regenerative environment.
3.2. Macrophages modulate fibroblast behavior and extracellular matrix organization
The extracellular matrix (ECM) of peripheral nerves is uniquely tuned to provide mechanical support and present trophic cues for the PNS to communicate between the CNS and distal end targets. The ECM of peripheral nerves plays a critical role in nerve injury by acting as the primary structural component to resist mechanical forces during trauma. Furthermore, the ECM forms trophic and molecular guides that can facilitate proliferative Schwann cells organization, blood vessel growth, and axonal growth cone navigation.
In healthy mature nerves, fibroblasts and Schwann cells synthesize an organized ECM comprised of type I collagen, type IV collagen, fibronectin, laminin, and proteoglycans (Gonzalez-Perez et al., 2013). Following trauma, restructuring of the disrupted ECM is a pre-requisite for complete regeneration. Matrix metalloproteinases (MMPs) are major drivers of ECM degradation and their levels must be carefully regulated to prevent indiscriminate tissue digestion. Tissue inhibitor of MMPs (TIMPs) play a critical role in balancing MMP-driven degradation. In a model of sciatic nerve crush, MMPs are released from Schwann cells acutely after injury (Chattopadhyay et al., 2007). At the same time, macrophages and Schwann cells upregulate TIMP-1 (La Fleur et al., 1996). TIMP-1 secreted by macrophages and Schwann cells modulates MMP activity and precludes collagen IV degradation in the sciatic nerve basement membrane (La Fleur et al., 1996). In this way, macrophages contribute to the preservation of the basement membrane, which is an important construct for growing blood vessels, Schwann cell organization, and navigating axons (Figure 1D).
Following a crush or stretch nerve injury, the intact basal lamina can express membrane-bound neurotrophic factors and adhesion molecules that guide regenerating nerves (Gonzalez-Perez et al., 2013). However, after a more severe transection injury, the basal lamina is disrupted. Surgical procedures can be implemented to directly suture proximal and distal nerve stumps together to prevent a gap from forming between the segments. However, in the absence of surgical intervention, axons that grow out from the proximal stump can grow toward distal end targets if the regenerating cells can locate the degenerating distal nerve segment. During successful regeneration a fibrin mesh tethers the proximal and distal nerve segments (Gonzalez-Perez et al., 2013). Thereafter, fibroblasts secrete collagen and Schwann cells produce laminin. These ECM molecules are oriented longitudinally to form a hollow bridge, which reunites the severed nerve stumps (Gonzalez-Perez et al., 2013). Thereafter, the lumen of the bridge can be filled with neurotrophic factors to promote blood vessel development, axonal growth, and cell survival (Gonzalez-Perez et al., 2013).
Currently, there is a paucity of research linking macrophage function to fibroblast activity and ECM deposition in the nerve. However, macrophages have been strongly implicated in ECM organization in a number of other tissues (Snyder et al., 2016; Witherel et al., 2019). Indeed, recent research illustrates that secreted factors from macrophages polarized to differing phenotypes can have differential effects on fibroblast gene expression and ECM organization. Specifically, macrophages polarized to an inflammatory phenotype with lipopolysaccharide (LPS) and interferon (IFN)γ caused fibroblasts to upregulate the ECM genes COL3A1, COL5A1, DCN, HSPG2, and PDGFRA relative to fibroblasts treated with unstimulated macrophages (Witherel et al., 2021). Macrophages polarized to an anti-inflammatory phenotype with IL-4 and IL-13 caused fibroblasts to upregulated the ECM genes COL3A1, COL5A1, FN1, HSPG2, and VCAN relative to fibroblasts treated with unstimulated macrophages (Witherel et al., 2021). While the specific factors secreted from the polarized macrophage populations that regulate fibroblast activity have yet to be elucidated, gene analyses suggest that some combination of ECM-promoting and angiogenesis-promoting cues may guide this response. These findings suggest that macrophages in PNI can play roles in regulating basement membrane degradation, but we suspect that future research will identify more mechanisms by which macrophages affect nerve ECM organization.
3.3. Macrophages initiate vascularization in the injury microenvironment
During PNI, vasculature that supplies nutrients, oxygen, and waste removal to nerve may be directly damaged. Macrophages play a key role in regrowth of functional vessels by signaling for endothelial cells to proliferate and migrate and releasing key angiogenic growth factors (Graney et al., 2020; Sunderkotter et al., 1994). Damaged vasculature causes the injury microenvironment to become hypoxic. Macrophages are uniquely equipped to sense and respond to localized vessel damage because they respond to hypoxia and can initiate regeneration across a transected nerve gap (Cattin et al., 2015). Decreased oxygen levels cause the transcription factor, hypoxia-inducible factor 1-alpha (HIF-1α), to initiate transcription of vascular endothelial growth factor (VEGF)-A in macrophages. VEGF-A is a critical driver of angiogenesis and vascular development by coordinating endothelial cell differentiation and proliferation (Mackenzie and Ruhrberg, 2012; Spiller et al., 2014). After a nerve injury, macrophages release VEGF, causing endothelial cells to form functional and polarized blood vessels that span the hypoxic space between two nerve segments (Figure 1E; Cattin et al., 2015). Infiltration of endothelial cells and subsequent blood vessel formation decreases the hypoxia of the gap microenvironment (Cattin et al., 2015). Following systematic blood vessel development in the hypoxic nerve injury environment, Schwann cells, in both the proximal and distal nerve stump migrate along the blood vessels to create pathways for the regenerating axons (Cattin et al., 2015).
Additionally, it has been suggested that VEGF-A can promote neurogenesis, migration, and axon guidance (Mackenzie and Ruhrberg, 2012). Indeed, VEGF-A increases the rate and extent of axonal outgrowth from sympathetic ganglia independent of its effects on vasculogenesis and angiogenesis (Long et al., 2009; Marko and Damon, 2008).
3.4. Macrophages stimulate Schwann cell migration and maturation in injury sites
In a healthy nerve, Schwann cells myelinate functional axons, and comprise approximately 60% of the total cell population. After a crush injury to the peripheral nerve, the number of S100+ Schwann cells within the injured nerve distal to the crush drops to half of their healthy numbers within one day after injury (Qian et al., 2018). Over time, the S100+ Schwann cell population in the nerve increase in a logarithmic fashion, reaching baseline numbers two weeks after injury (Qian et al., 2018). To aid in the recruitment of Schwann cells to the injury, hypoxic macrophages upregulate the expression of CCL3, which is known to induce Schwann cell migration and thereby, increase the numbers of immature Schwann cells (SOX2+) in the nerve injury site (Figure 1F; Stratton et al., 2018; van Emmenis, 2018).
In addition to affecting Schwann cell recruitment, macrophages can also regulate Schwann cell myelination. As the primary glial support cell in the PNS, Schwann cells play a lead role in nerve homeostasis and regeneration after injury (Boliívar et al., 2020). Following Wallerian degeneration, Schwann cells dedifferentiate to transiently adopt an immature phenotype that is associated with the early stages of nerve repair (Bolívar et al., 2020). Macrophages regulate Schwann cell maturation, which is necessary for remyelination, by releasing the factor growth arrest-specific 6 (Gas6) (Figure 1G; Stratton et al., 2018). Gas6 binds with high affinity to the Schwann cell tyrosine kinase receptor, Tyro3, cueing Schwann cell maturation and subsequent myelination of axons that express the membrane marker neuregulin (NRG)-1 type III (Michailov et al., 2004; Miyamoto et al., 2015). Gas6 secreted from macrophages increases the number of mature SOX10+ cells and their subsequent myelination of regenerating axons. Thereby, Gas6 increases the conduction velocity of action potentials in the regenerating nerves indirectly (Stratton et al., 2018). Indeed, genetic deletion of GAS6 after PNI resulted in impaired remyelination (Stratton et al., 2018). Together, these data implicate macrophages as a key regulator of Schwann cell recruitment, maturation, and myelination following injury.
3.5. Macrophages prevent ectopic axon growth and cell migration
Following nerve injury, axons must navigate across any gap between proximal and distal nerve stumps, and thereafter, through the denervated distal nerve stump in order to reach an appropriate end target. One mechanism for PNI regenerative failure is through axonal misdirection, or the aberrant growth of axons into ectopic locations as first described by Ramón y Cajal (Ramon y Cajal, 1928). Axonal misdirection after PNI is difficult to correct and is strongly associated with neuroma formation and chronic neuropathic pain that is commonly experienced by patients with PNI (Lobato, 2008). Indeed, neuromas and chronic pain are some of the most impactful determinants of reduced quality of life following nerve injury (Wojtkiewicz et al., 2015), as discussed below.
Macrophages play a critical role in preventing ectopic nerve growth following trauma by presenting chemical guardrails to guide the distribution of Schwann cells in an injured nerve. It has been well described that after nerve injury, Schwann cells organize themselves into Bands of Bungner, forming regeneration tracks for regenerating axons across any transection gap and into the distal nerve segment (Jessen and Mirsky, 2016). Because Schwann cells direct axonal growth and targeting, macrophage control over Schwann cell distribution can indirectly prevent axonal misdirection. Following transection injury in a mouse, macrophages organize themselves to form a perimeter around the nerve injury site (Dun et al., 2019). These perimeter macrophages secrete high levels of the glycoprotein Slit3, a ligand that binds to Robo receptors. When Slit3, secreted by macrophages, binds to Robo1 receptors on the surface of Schwann cells, fibroblasts and axons, it locally halts cell migration and regeneration. In this way, macrophages make the outer barrier around the regenerating gap and prevent Schwann cells, fibroblasts, and axons from growing in the wrong direction (Figure 1H; Dun et al., 2019). Knocking out Slit3 and/or Robo1 resulted in ectopic cell migration and subsequent ectopic nerve growth (Dun et al., 2019).
3.6. Macrophages accelerate axonal outgrowth
While leukocyte infiltration has long been described in the injury environment after PNI (Ramon y Cajal, 1928), it was not until 1972 when Smith and Adrian observed that macrophages infiltration was not confined to the PNI site, but that a large number of macrophages also localized with the nerve ganglia after PNI (Smith and Adrian, 1972). In the early 1990s, Lu and Richardson built on this observation and found that generating an inflammatory response around the dorsal root ganglia after nerve injury – either by injecting Corynebacterium parvum or isogenous macrophages – accelerated axonal regrowth (Lu and Richardson, 1991). Indeed, in exploring the function of these immune cells, researchers have observed that macrophage-induced inflammation near the neuronal cell bodies is associated with increased neurite outgrowth and regeneration after PNI (Hikawa and Takenaka, 1996; Jager et al., 2020; Niemi et al., 2016). Environmental stimuli generated by the injury may prime macrophages to alter their behavioral profile and enhance neuronal regeneration (Lavin et al., 2014). Research suggests that inflammatory macrophages that are allowed to phagocytose myelin will release factors into the conditioned media that increase dorsal root ganglion neurite outgrowth length and branching complexity (Boven et al., 2006; Kroner et al., 2014). Furthermore, phagocytosis of apoptotic cells can lead to downregulation of inflammation and secretion of IL-10 (Voll et al., 1997).
Research over the last couple decades has strengthened our understanding of specific ways that macrophages contribute to axonal regeneration. Following nerve injury, CCL2, a chemoattractant for infiltrating monocytes, is expressed in the distal nerve segment and on neuronal cell bodies in the dorsal root of the nerve. As a result, monocytes are recruited to both areas (Niemi et al., 2013). CCL2 expression on dorsal root ganglia (DRG) neurons is correlated with macrophage infiltration into the ganglia and with DRG neurite outgrowth (Niemi et al., 2016). After a sciatic nerve injury, transected neurons upregulate CCL2 expression and cause CCR2 expressing macrophages to upregulate both inflammatory (CD86) and anti-inflammatory (ARG1, CD206, and YM1) genes (Niemi et al., 2016). Crosstalk between CCL2-expressing neurons and CCR2+ macrophages, causes injured neurons to upregulate regeneration-associated genes (RAGs) including IL6, GAP43, JUN, ATF3, GAL, SMAD1, SOX11, and SPRR1a (Niemi et al., 2016). Furthermore, crosstalk between CCL2-expressing neurons and CCR2+ macrophages caused upregulation of these RAGs and subsequently increased neurite outgrowth. Interestingly, stimulating neurons with recombinant CCL2 or overexpression of CCL2 on neurons in the absence of CCR2 macrophages prevents the amplified neurite outgrowth from DRGs. Together, these data suggest that macrophages can respond to injured neurons in the DRG and can stimulate these injured cells to promote regenerative behaviors after PNI.
Leukemia Inhibitory Factor (LIF) is a pleiotropic cytokine that has be implicated as a key factor in neuronal injury and axonal outgrowth (Nicola and Babon, 2015). LIF expression is increased in neurons after nerve injury and is inversely correlated with sensory and motor neuron death after injury (Cheema et al., 1994; Curtis et al., 1994). Knockout studies removing LIF reduced sensory neuron growth capacity, which was rescued when exogenous LIF was reintroduced (Cafferty et al., 2001). LIF can also affect macrophage behavior and phenotype. Interestingly, LIF receptors are missing on almost all immune cells except for macrophages (Nicola and Babon, 2015). LIF expression can act as a chemoattractant to recruit macrophages to sites of nervous system injury (Sugiura et al., 2000). Macrophages stimulated with inflammatory cues cause macrophages to produce and release LIF, amplifying the amount of LIF in the injury microenvironment. Macrophages recruited to the ganglia via CCL2 can help stimulate the biogenic response (via LIF/STAT3) that amplifies axonal regrowth (Figure 1A; Cattin and Lloyd, 2016; Niemi et al., 2016). Furthermore, elevated LIF levels cause macrophages to reduce reactive oxygen species production and to also reduce TNFα production (Hendriks et al., 2008). Simultaneously, LIF-treated macrophages significantly increase myelin phagocytosis relative to untreated cells (Hendriks et al., 2008). Interestingly, myelin uptake by macrophages suppressed macrophage LIF production and secretion in a dose-dependent fashion (Hendriks et al., 2008). These data suggest that neurons, macrophages, and other support cells can produce LIF after PNI to promote neuronal survival, axonal outgrowth, and transient myelin clearance.
Oncomodulin is another potent factor released by macrophages that is responsible for outgrowth of retinal ganglion axons (Yin et al., 2006, 2003). Oncomodulin signals through calmodulin-dependent protein kinase II (CaMKII) to induce transcriptional changes within damaged neurons (Yin et al., 2006). Studies that have characterized oncomodulin release have been completed in models of optic nerve injury. Because the optic nerve injury environment can be quite distinct from other sites of nerve injury (Ydens et al., 2020), it will be interesting to learn whether oncomodulin is a secreted growth factor in other sites of nerve injury.
Other macrophage-derived neurotrophic factors likely contribute to healthy nerve regeneration after trauma. For example, macrophages stimulated with IL-1β are the driving source of secreted nerve growth factor (NGF) after PNI (Lindholm et al., 1987). Other neurotrophic factors have also been reported in models of PNI including brain-derived neurotrophic factor (BDNF), glial-derived neurotrophic factor (GDNF), and neurotrophin (NT)-3 (Liu and Wang, 2020). While macrophages have been associated with the production of each of these, it remains unclear if macrophages are the primary source of these factors after nerve injury (Snyder et al., 2016).
3.7. Macrophages help develop and maintain distal end targets
Development, maturation, and upkeep of the distal end targets are crucial steps in establishing and maintaining functional PNS connectivity, as well as reestablishing connectivity after PNI. Peripheral nerves can innervate distal end targets as afferent (sensory) or efferent (motor) axons of the somatic nervous system or they can integrate with distal end targets as part of the autonomic nervous system (either sympathetic or parasympathetic). There is emerging evidence that macrophages are involved in the development and maintenance of these neuro-somatic interfaces following PNI (Rios et al., 2021).
Recent research efforts out of the Snyder-Warwick laboratory have identified macrophages as important contributors to reinnervation and neuromuscular junction (NMJ) development after injury (Jablonka-Shariff et al., 2020; Lu et al., 2020). Their research suggests that macrophages act in concert with terminal Schwann cells, nonmyelinating cells in the muscle that are involved in NMJ maintenance and innervation. They observed that changing terminal Schwann cell behavior (through conditional Gpr126 knockout mice) resulted in reduced macrophage recruitment, reduced innervation, and motor deficits after nerve injury (Jablonka-Shariff et al., 2020). Additionally, they observed that preventing monocyte and macrophage recruitment to the injury site delayed innervation and decreased functional recovery (Lu et al., 2020). When VEGF-A was conditionally knocked out from macrophages, they observed that the extent of innervation was decreased and functional recovery was diminished (Lu et al., 2020). Together these data suggest that macrophages may play a significant role in re-establishing the NMJ after PNI (Figure 1B).
In addition to regulating innervation of the efferent nerves, macrophages can influence regeneration and function of the autonomic nervous system. The autonomic PNS innervates virtually all organ systems, including adipose tissue. Sympathetic nervous system innervation of adipose tissue in necessary to regulate energy expenditure and metabolic rate throughout the body. Resident macrophages in adipose tissue contribute to successful innervation (Wolf et al., 2017). Indeed, deleting MECP2, a nuclear transcription regulator, in brown adipose tissue macrophages reduced the extent of sympathetic innervation which, thereafter, caused metabolic imbalances and spontaneous obesity in the animal subjects (Wolf et al., 2017).
Furthermore, research within the last couple years has found that macrophages play a distinct and critical role in maintenance of distal end target homeostasis. Sympathetic neuron-associated macrophages (SAMs) have been described as a phylogenetically distinct group of macrophages that are involved in sympathetic innervation and function (Pirzgalska et al., 2017). SAMs are involved in the clearance of norepinephrine from peripheral synapses and varicosities through expression of the norepinephrine transport protein solute carrier family 6 member 2 (SLC6A2) and through expression of the degradation enzyme monoamine oxidase A (MAOA) (Pirzgalska et al., 2017). Interestingly, when macrophages collected and processed excessive norepinephrine from the synapse, the macrophages changed their phenotypic profile to upregulate the inflammatory genes TNF and IL1 and decreased the anti-inflammatory genes IL4RA and ARG1 (Pirzgalska et al., 2017). These findings suggest that in addition to initiating innervation of the regenerating PNS, macrophages can act as detectors of excessive neurotransmitters and respond to neuron signals by adapting their behavioral phenotype.
Together, these data implicate macrophages in reinnervation and synaptic homeostasis, but it is feasible to speculate that macrophages could contribute to end-processes in other ways. It is well established that microglia, the innate immune cells of the CNS, systematically prune the synapses of billions of neurons to develop functional circuitry in the healthy brain (Hong et al., 2016). Furthermore, microglia prune dysfunctional synapses of motor neurons inside the spinal cord after peripheral nerve injury (Salvany et al., 2021). Although research has enumerated the ways in which nerve macrophages are distinct from microglia (Ydens et al., 2020), it is tempting to theorize that macrophages could play a role pruning the NMJs and/or sensory receptor endings at the distal end targets. However, studies investigating macrophages in this role have yet to be completed.
4. Immunoengineering Macrophages as a Strategy to Repair Injured Nerves
Clearly, as macrophages play a critical role in tissue regeneration, several immunoengineering strategies have emerged to harness their regenerative potential across a number of organ systems and diseases. Indeed, each characteristic and function executed by macrophages offers exciting opportunities for innovative tissue repair (Lee et al., 2016). Because monocytes circulate in the blood stream, they are an exceptionally convenient source of cells that could be collected and exogenously reprogramed as a mechanism for autologous cell therapy. Because monocytes home to sites of injury, they could be used to deliver cargo, such as drugs, to inaccessible or delicate tissue. Because macrophages phagocytose debris and pathogens, they could be employed to clear up sites of necrosis, cancer, or infection. Because macrophages exhibit a wide spectrum of phenotypes, they could be utilized to modulate local inflammation, antigen presentation, or adaptive immune cell activation. Alternatively, phenotype manipulation could enhance vascularization, ECM deposition, cytokine signaling, or cellular regrowth rates.
In addition to the spectrum of phenotypes and functions that macrophages can provide to regenerating tissues, macrophages also offer unique advantages that could increase their utility in repairing different types of nerve injuries. Indeed, clinical nerve injuries can be caused due to transection, crush, stretch, or irritation. Peripheral nerve regeneration research over the last several decades has focused on developing implantable nerve conduits and grafts that can supplement or replace autologous nerve grafts collected from sacrificial or redundant donor nerves (the current clinical gold standard) (Pfister et al., 2011). These strategies have made significant progress in enhancing regeneration of transected nerves; however, they are not appropriate treatments for many crush, stretch, or irritation injuries. Macrophages could supplement nerve scaffolds and autografts, but they can also be delivered as an injectable cell therapy (Chan and Viswanathan, 2019; Raheja et al., 2012). Indeed, completed and ongoing clinical trials have administered monocytes or macrophages through direct injection, intrathecal injection, or intramuscular injection to treat diseases and disorders including spinal cord injury, cerebral palsy, and critical limb ischemia, respectively (Chan and Viswanathan, 2019). These trials have had mixed results, but they offer a glimpse into a promise of injectable cell-based therapies in the future. Indeed, another pre-clinical study investigated if bone marrow-derived mononuclear cells (which include both immune and progenitor cell populations) could enhance regeneration after a sciatic nerve transection in rats. They observed a dose-dependent positive correlation between the number of bone marrow-derived cells administered and nerve regeneration (Raheja et al., 2012). Like these studies suggest, macrophage-based therapies could increase the percentage of treatable nerve injuries by promoting collateral branching of intact axons (the primary mechanism for nerve regeneration (Menorca et al., 2013)), accelerating axonal outgrowth, supporting a regenerative injury environment, minimizing axonal misdirection, and preserving the re-innervation capacity of distal end targets. Therapeutics aimed to enhance, guide, and accelerate regeneration that are applicable for crush, stretch, irritation, and transection nerve injuries are most needed to enhance clinical treatment.
5. Current Immunoengineering Strategies
Recent research efforts have shifted from immunosuppressive techniques to instead focusing on strategies of immunomodulation as a potential means of augmenting nerve regeneration after injury. To date, a handful of studies have been conducted that attempt to employ biomaterial and tissue engineering technologies to control aspects of the immune response to promote nerve regeneration.
Engineered immunomodulatory strategies that are applicable for nerve injuries can generally be categorized as scaffold-based techniques, cellular therapy techniques, or a combinatorial approach. Scaffold-based techniques were originally inspired as a way to enhance the efficacy of nerve conduits (Pfister et al., 2011). Scaffold-based techniques focus on building biomimetic implantable architecture that encourages or facilitates regeneration (Pfister et al., 2011). These implants are most applicable for addressing transection injuries. Cellular therapies are a newer genre of immunomodulation largely inspired by progress in the stem cell and cancer research fields. Cellular therapies aim to hijack or direct endogenous or exogenously reprogrammed cells in the body to enhance regeneration. Cellular therapies are generally injectable, and thus, can be employed as either local or systemic therapeutics (Lee et al., 2016).
Within scaffold-based techniques, the composition, structure, degradation, and drug-loading characteristics of the implant can all be altered, as each of these can modulate the regenerative potential. Indeed, structural materials that comprise the nerve graft can affect macrophage adhesion and phenotype. For example, researchers employing a chitosan nerve graft without drug loaded reported that the chitosan itself was immunomodulatory and was associated with nerve regeneration (Stenberg et al., 2017). Additionally, many other materials including hyaluronic acid, alginate, and dextran have been shown to affect macrophage phenotype (Li and Bratlie, 2021; Meng et al., 2015). Furthermore, the structural organization of nerve scaffolds can also regulate immune cell functions. Indeed, nerve guidance channels made from aligned nanofibers have been used to promote pro-healing macrophage phenotypes, yielding increases in Schwann cell infiltration and axon regeneration in rats, although fiber alignment also provides physical guidance cues to regenerating cells (Jia et al., 2019). Constructing nerve grafts with some combination of these materials with controlled graft architecture could help regulate immune cell behavior in the injury environment.
Additionally, other researchers attempted to increase the regenerative efficacy of nerve grafts by incorporating immunomodulatory drugs. In the Bellamkonda laboratory, for instance, local delivery of IFN-γ or IL-4 from nerve guidance channels was used to bias macrophage populations towards either pro-inflammatory or pro-regenerative phenotypes (Mokarram et al., 2012). Drug-releasing nerve guidance channels were implanted in a 15 mm rat tibial nerve gap injury model for 3 weeks. While little differences were observed from local delivery of IFN-γ, IL-4 delivery dramatically enhanced Schwann cell infiltration and axon regeneration as compared with controls. Within this model, a higher ratio of pro-healing to pro-inflammatory macrophages was correlated with increased numbers of regenerating axons. Similarly, nerve guidance channels capable of local delivery of collagen VI, an ECM with demonstrated immunomodulatory activity (Chen et al., 2015a), affected macrophage phenotype and promoted axon regeneration and maturation as compared with non-drug loaded conduits when implanted in a 15 mm sciatic nerve gap in rats (Lv et al., 2017). Finally, the Bellamkonda group has also targeted pro-healing monocyte recruitment via local delivery of the CX3CR1 receptor ligand fractalkine (Mokarram et al., 2017). Notably, fractalkine delivery targeted monocyte/macrophage numbers and phenotype, dramatically increasing axonal growth into nerve guidance channels spanning a 15 mm rat sciatic nerve gap even as compared to IL-4-releasing channels.
In contrast to scaffold-based strategies, cellular therapies generally aim to directly reprogram a specific cell population. Importantly, cellular therapies do not require surgical intervention at a nerve lesion site and can be injected systemically or locally at the site of crush, irritation, stretch, or transection nerve injuries. Because macrophages play a leading role in regeneration and pathology, researchers have worked to control these cells after disease or damage.
For cellular therapies that are administered systemically, the engineered cells first need to home to sites of injury and regulate cellular behavior thereafter. Several researchers have utilized the innate homing ability of monocytes to their advantage by loading or adhering drug-loaded particles to monocytes in other models of injury and disease. Homing monocytes loaded with particles increased the localization of the particles to the sites of injury (Anselmo et al., 2015a). For example, engineered nanoparticle backpacks can adhere to the surface of immune cells. The unique geometry and mechanical properties of these backpacks prevented phagocytic internalization but allowed particle adhesion to immune cells (Anselmo et al., 2015b; Anselmo and Mitragotri, 2014). Thereafter, these “backpack-loaded” monocytes could home to sites of inflammation (the skin, lungs, and brain in their experiments) and increase the percentage of particles in the injured tissue relative to non-adhered particles (Anselmo et al., 2015a; Anselmo and Mitragotri, 2014). Likewise, other researchers have utilized intracellular magnetic particles to target chemotherapeutics to cancer sites (Nguyen et al., 2017). They found that the chemotherapeutic paclitaxel could be released from intracellular microparticles inside macrophages at detectable levels into the extracellular space (Nguyen et al., 2017). Because monocytes can infiltrate tumors, the authors theorized that particle-loaded macrophages could effectively deliver chemotherapeutic drugs (Nguyen et al., 2017). Finally, particle-loaded monocyte can be employed to deliver the natural redox enzyme, catalase, into the injured brain by intracellularly loading the catalase-loaded particles into homing monocytes (Brynskikh et al., 2010; Klyachko et al., 2014). In these experiments, intracellular catalase-loaded particles were able to infiltrate into the CNS and reduced neuroinflammation in a model of Parkinson’s disease (Brynskikh et al., 2010; Klyachko et al., 2014). Together, these studies exemplify the utility of employing the homing capabilities of monocytes. Building on this work, our laboratory has collaborated to engineer a strategy to intracellularly control macrophage phagocytosis and behavioral phenotypes in inflammatory environments (Wofford et al., 2020; Wofford et al., 2019a). This was the first attempt to intracellularly reprogram macrophage phenotype with a biomaterial platform (Wofford et al., 2020; Wofford et al., 2019a). Indeed, these intracellular drug-loaded microparticles were able to preserve an anti-inflammatory macrophage phenotype even when the cells were cultured in inflammatory conditions (Wofford et al., 2020). We speculate that these platforms, if implemented into a model of PNI, could guide macrophage behaviors and enhance regeneration and functional recovery.
Separately, scientists have utilized new genetic engineering strategies to try to modulate macrophage phenotype (Burke et al., 2002). For example, Forbes & Peppas utilized a polycationic nanocarrier with bound siRNA to facilitate phagocytic uptake and transfection in a murine macrophage cell line (Forbes and Peppas, 2014). Others have found that cellular phenotype can be modulated by transfecting or knocking down specific genes within macrophages (Cao et al., 2019; Gong et al., 2017; Kimura et al., 2016; Kitamoto et al., 2013; Kranz et al., 2016; Ma et al., 2013). However, genetically engineering macrophage phenotypes still presents many challenges because transfection is generally non-specific, has low transfection efficiency, and increases the immunogenicity of genetically manipulated cells (Burke et al., 2002). Therefore, genetic engineering strategies have yet to reach their full translational potential. Clearly, there is a need to develop a strategy (genetic, biomaterial, or otherwise) to modulate and maintain macrophage phenotype over time in order to employ these reprogrammed cells as a translational cell therapy for regenerative medicine.
Importantly, these studies represent the beginnings of a foray into immunomodulation as a therapeutic strategy following injury. Because the immune response to injury is a dynamic process, investigations into a single macrophage phenotype, cytokine, or drug may ultimately lead to more complex approaches capable of directing variable immune reactions optimized for nerve regeneration. In addition, as understanding of immune involvement in nerve regeneration deepens, higher-level immunomodulatory strategies are likely to emerge as excellent opportunities for improving nerve regeneration.
6. Limitations of Immunoengineering
As we have discussed, macrophages contribute to peripheral nerve regeneration through a number of pathways that can accelerate regeneration and ultimately improve functional recovery. However, many unknowns still remain about the precise timing and phenotype of macrophages after injury. As previously mentioned, macrophages can express a number of different behavioral phenotypes and execute a wide range of functions. Careful synchronized coordination across infiltrating and resident macrophages is essential for successful tissue regeneration. One can imagine that pathology could easily be exacerbated if a pro-inflammatory phenotype, phagocytic behaviors, chronic vascularization, excessive ECM deposition, or inhibition of axonal regeneration were cued at the wrong time or location after trauma. Indeed, many gaps still remain in our knowledge about the desirable phenotypes across spatial and temporal resolution. Furthermore, engineering strategies need to be developed in order to regulate changing phenotypes across challenging long-gap defects and along the length of proximally injured nerves. Neither of these (often co-occurring) clinical needs are trivial. Indeed, the immense potential of macrophage immunotherapy is fundamentally linked with the enormous complexity of controlling the immune system.
One of the most deleterious outcomes of dysregulated nerve regeneration is the development of chronic pain. Previous studies have associated the immune system with maladaptive neuroma and pathological pain (Davies et al., 2020). Studies in the skin, synovium, muscle, and deep fascia among others have reported a link between macrophages and regeneration of afferent nerve endings. Specifically, researchers reported a positive correlation between the extent of innervation after injury and local inflammation (Barry et al., 2019). These findings suggest that excessive or chronic inflammation in the tissue can cause pathological innervation, termed hyperinnervation. The resulting hyperinnervation can cause nociception and hypersensitivity and is correlated with an abundance of macrophages in the local environment (Barry et al., 2019).
Administration of the inflammatory agent Freund’s Complete Adjuvant in the absence of injury can induce robust cutaneous hyperinnervation in the rodent hind paw (Chakrabarty et al., 2013). It was reported that macrophages, along with other adaptive immune cells (T cells), were responsible for this increased PNS response. These immune cells generated angiotensin II-synthesizing proteins, renin and angiotensinogen, which were critical for sensory end target innervation and the resulting hypersensitivity (Chakrabarty et al., 2013). Cutaneous hyperinnervation was prevented after the inflammatory challenge by administering an angiotensin receptor II antagonist (Chakrabarty et al., 2013).
Likewise, vestibulodynia, chronic pain in and around the vaginal opening, is characterized by hyperinnervation. In a rat model of vestibulodynia, researchers reported that macrophages and adaptive immune cells were responsible for producing renin-angiotensin-associated proteins, which were critical for the resulting hyperinnervation (Chakrabarty et al., 2018). These data implicate macrophages in sensory nerve innervation and development of functional sensory end targets. However, it also suggests that a carefully timed and regulated inflammatory phase is critical to balance innervation and prevent hypersensitivity or pain.
Finally, immune system reactivity and contributions to regeneration can vary considerably across species. Indeed, previous research suggests that the immune systems of mice (one of the most common animal models) and humans can be quite different from one another in the cell quantity, signaling pathways, and reactivity (Mestas and Hughes, 2004; Spiller et al., 2016). Studies confirming that positive macrophage effects observed in lower order animal models are conserved in clinical cases will be critical for successful translation. However, differences in immune system functionality across species could also yield clues into how to enhance human peripheral nervous system regeneration to mimic the robust recovery of other animal after nerve injury.
7. Future Directions
Innovative discoveries in the last several years suggest that immunomodulation capabilities will greatly expand in the years to come. One area for improvement in scaffold-based nerve therapies is fabricating nerve grafts with bioresponsive materials. Much progress has been made in the last several decades at generating materials that change in response to local environmental or cellular stimuli. These ‘smart’ biomaterials can change their physical organization or chemical composition in response to pH, purinergic, enzymatic, hypoxic, redox, temperature or mechanical stimuli (Lu et al., 2016). Depending on the chemical composition of the material, stimuli can cause scaffolds to swell, assemble, change charge, crosslink, complete a phase transition, dissociate, or release drugs. Because immunomodulation will most likely be successful if there is both spatial and temporal resolution, bioresponsive materials may hold the key for immunomodulatory scaffold success. For example, researchers in the Burdick laboratory developed an injectable, bioresponsive material that releases MMP-regulating factors (TIMPs) only after MMP levels become elevated (Purcell et al., 2014). After being injected into the injury environment, these hydrogels locally release TIMPs depending on the amount of MMP-generated degradation. In this way, the hydrogel can regulate the extent of local tissue degradation after injury by inhibiting matrix degradation factors at the time they become most elevated (Purcell et al., 2014). We suspect that introduction of similarly responsive biomaterials around transected nerves could also regulate the extent of basement membrane degradation after nerve injury by regulating the extent of MMP degradation.
Furthermore, one can speculate that enzyme-dependent drug/protein release could have other exciting applications in nerve injury. Perhaps hypoxia-sensitive biomaterials could release macrophage chemoattractant factors in order to recruit macrophages to lesion sites. In this way, macrophage recruitment would diminish as the lesion site becomes vascularized. Perhaps scaffolds could release factors that push macrophages toward a GAS6-generating phenotype in response to elevated CCL3 levels. In this way, Schwann cells maturation would be initiated after Schwann cell migration has peaked. Genetic engineering through CRISPR-Cas9 has unlocked the potential of cells to be precisely modified. We imagine that genetically engineered macrophages could be injected into distal end target sites. These genetically engineered macrophages could be programmed to transcribe and secrete anti-inflammatory factors when they sense increasing levels of inflammation in the environment. In this way, these engineered macrophage sentinels could halt regenerative inflammation before it generates hyperinnervation and chronic pain.
In addition to bioresponsive materials, cellular reprogramming strategies hold immense potential for future immunoengineering endeavors. Indeed, cells can be permanently or transiently perturbed to generate a desired behavior or phenotype. One area of exciting innovation is in modeling cells as Boolean Logic devices. We have previously reported that microparticles stored in the intracellular spaces of macrophages can influence their gene expression and protein production (Wofford et al., 2020). However, these polymeric drug-releasing microparticles could be replaced with more advanced engineered materials. For example, work from the DeForest laboratory has fabricated materials that respond to the environment with Boolean logic responsiveness (Badeau et al., 2018). These materials can respond to light, enzymes, or reductant stimuli to generate YES/OR/AND logic outputs. For example, if stimulus A and stimulus B are both present, then factor X will be released. Alternatively, if either stimulus A or stimulus B are present, then factor Y will be released. Boolean logic material gates can also be linked together so that AND/OR combinations work with a number of precise combinations. For example, if both stimulus A and stimulus B are present or if stimulus C alone is present, then factor Z will be released.
Using this technology, one could envision administering particles that are crosslinked with Boolean logic materials (sensitive to light and enzymes) inside or onto monocytes. These loaded monocytes could then be administered to patients with a crush, transection, irritation, or stretch nerve injury. Perhaps longwave light sources placed above the damaged nerve (at wavelengths known to penetrate the epidermis), would allow monocytes to release their drug payload only if they are in the region of the nerve injury and if there are enzymatic cues in the area. While these ideas are futuristic, we believe they are feasible strategies to modulate immune and other cell behaviors and provide a level of control that is not currently attainable, and would directly contribute to the safety, efficacy, and consistency of these approaches. Efforts aimed at combining advanced materials science, immunology, neuroscience, and regenerative medicine hold immense potential for developing translational and impactful therapies for nerve injury.
We have discussed several ways that macrophages contribute to healthy nerve regeneration. With thoughtful deployment, we anticipate that immunoengineering strategies will be capable of accelerating and improving nerve regeneration after trauma. Indeed, it is feasible to imagine utilizing immunomodulatory biomaterials to supplement existing nerve conduits and grafts. Instead of avoiding or suppressing the immune system, like previous efforts in the 20th century, nerve therapies that mimic desirable macrophage behaviors, control macrophage recruitment to injury sites, or regulate macrophage phenotype could increase the efficacy of many nerve treatment strategies.
8. Conclusions
In summary, after many decades of suppressing or avoiding the immune system, therapeutic strategies aimed at employing innate immune cells to enhance regeneration after PNI are on the horizon. Here, we have enumerated several ways that macrophages contribute to tissue homeostasis, healthy regeneration, or pathology in the PNS. We suspect that this list is not comprehensive, and that future research will identify other mechanisms of macrophage contributions to nerve degeneration and regeneration. We posit that translational therapeutics that can harness the regenerative potential of macrophages could have profound implications for the field of regenerative medicine.
Supplementary Material
HIGHLIGHTS.
Immunosuppressive treatments generally reduce nerve regeneration after injury
Macrophages exhibit diverse phenotypes to guide successful nerve regeneration
Immunomodulatory therapies offer promise for treating peripheral nerve injuries
ACKNOWLEDGEMENTS
This work was made possible through financial support provided by the National Institutes of Health [NINDS T32-NS043126 (Wofford); NINDS F32-NS116205 (Wofford); NINDS T32-EB005583 (Shultz); NINDS R43-NS108869 (Cullen)], the Department of Defense [W81XWH-16-1-0796 (Cullen); W81XWH-19-1-0867 (Cullen)], and the Department of Veterans Affairs [I01-BX003748 (Cullen)]. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Institutes of Health, Department of Defense, or Department of Veterans Affairs.
ABBREVIATIONS:
- BDNF
brain-derived neurotrophic factor
- CaMKII
calmodulin-dependent protein kinase II
- CCL
chemokine ligand
- CD
cluster of differentiation
- CNS
central nervous system
- DRG
dorsal root ganglia
- ECM
extracellular matrix
- FK506
Tacrolimus
- Gas6
growth arrest-specific 6
- GDNF
glial-derived neurotrophic factor
- HIF-1α
hypoxia-inducible factor 1-alpha
- IFN
interferon
- IL
interleukin
- LIF
leukemia inhibitory factor
- LPS
lipopolysaccharide
- MAG
myelin-associated glycoprotein
- MAOA
monoamine oxidase A
- MCP-1
monocyte-chemoattractant protein-1
- MMPs
Matrix metalloproteinases
- NGF
nerve growth factor
- NMJ
neuromuscular junction
- NRG
neuregulin
- NT
neurotrophin
- PAP-III
pancreatitis-associated protein III
- PNI
Peripheral nerve injury
- PNS
peripheral nervous system
- RAGs
regeneration-associated genes
- SAMs
Sympathetic neuron-associated macrophages
- SLC6A2
solute carrier family 6 member 2
- TIMPs
Tissue inhibitor of MMPs
- TNFα
tumor necrosis factor α
- VEGF
vascular endothelial growth factor
Footnotes
DIVERSITY STATEMENT
Because paper citations are considered a metric of scientific impact and because recent work found that citation practices in neuroscience fields can disproportionally cite papers authored by men in first and last author positions (Dworkin et al., 2020), we strove to balance citations within this paper across man (M) and woman (W) first and last authors in proportion to available manuscripts in the nerve injury field. After excluding citations of authors included in this manuscript, 47% were authored by MM (man first author and man last author), 14% were WM, 7% were MW, and 17% were WW. 16% of the citations in the manuscript were not factored into this calculation because author first names were unisex or only initials were provided. These citation rates are approximately proportional to the available citable papers in this field.
CONFLICT OF INTEREST
DKC is a co-founder and RBS is an employee of Axonova Medical, LLC, which is a University of Pennsylvania spin-out company focused on translation of advanced regenerative therapies to treat nervous system disorders. KLW has filed a patent application related to control of macrophage phenotype via intracellular microparticles (U.S. Patent App. PCT/US18/34906). No other author has declared a potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aguilar Salegio EA, Pollard AN, Smith M, Zhou XF, 2011. Macrophage presence is essential for the regeneration of ascending afferent fibres following a conditioning sciatic nerve lesion in adult rats. BMC Neurosci. 12. doi: 10.1186/1471-2202-12-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbik F, Cafferty WBJ, Strittmatter SM, 2012. Myelin Associated Inhibitors: A Link Between Injury-Induced and Experience-Dependent Plasticity. Exp. Neurol 235, 43–52. doi: 10.1016/j.expneurol.2011.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann L, Prinz M, 2020. The origin, fate and function of macrophages in the peripheral nervous system-an update. Int. Immunol 32, 709–717. doi: 10.1093/intimm/dxaa030 [DOI] [PubMed] [Google Scholar]
- Anselmo, Gilbert JB, Kumar S, Gupta V, Cohen RE, Rubner MF, Mitragotri S, 2015a. Monocyte-mediated delivery of polymeric backpacks to inflamed tissues: a generalized strategy to deliver drugs to treat inflammation. J. Control. Release 199, 29–36. doi: 10.1016/j.jconrel.2014.11.027 [DOI] [PubMed] [Google Scholar]
- Anselmo, Mitragotri S, 2014. Cell-Mediated Delivery of Nanoparticles: Taking Advantage of Circulatory Cells to Target Nanoparticles. J Control Release 531–541. doi: 10.1016/j.micinf.2011.07.011.Innate [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmo, Zhang M, Kumar S, Vogus DR, Menegatti S, Helgeson ME, Mitragotri S, 2015b. Elasticity of nanoparticles influences their blood circulation, phagocytosis, endocytosis and targeting. ACS Nano 9, 3169–3177. doi: 10.1021/acsnano.5b00147 [DOI] [PubMed] [Google Scholar]
- Arai K, Hotokebuchi T, Miyahara H, Arita C, Mohtai M, Sugioka Y, Kaibara N, 1989. Limb allografts in rats immunosuppressed with FK506. Transplantation 48, 782–786. [DOI] [PubMed] [Google Scholar]
- Asplund M, Nilsson M, Jacobsson A, Von Holst H, 2009. Incidence of traumatic peripheral nerve injuries and amputations in Sweden between 1998 and 2006. Neuroepidemiology 32, 217–228. doi: 10.1159/000197900 [DOI] [PubMed] [Google Scholar]
- Avellino AM, Hart D, Dailey AT, MacKinnon M, Ellegala D, Kliot M, 1995. Differential Macrophage Responses in the Peripheral and Central Nervous System during Wallerian Degeneration of Axons. Exp. Neurol 136, 183–198. doi: 10.1006/exnr.1995.1095. [DOI] [PubMed] [Google Scholar]
- Badeau BA, Comerford MP, Arakawa CK, Shadish JA, DeForest CA, 2018. Engineered modular biomaterial logic gates for environmentally triggered therapeutic delivery. Nat. Chem doi: 10.1038/nchem.2917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes R, Bacsich P, Wyburn GM, Kerr AS, 1946. A study of the fate of nerve homografts in man. Br. J. Surg 34, 34–41. [DOI] [PubMed] [Google Scholar]
- Barry CM, Matusica D, Haberberger RV, 2019. Emerging Evidence of Macrophage Contribution to Hyperinnervation and Nociceptor Sensitization in Vulvodynia. Front. Mol. Neurosci 12, 1–7. doi: 10.3389/fnmol.2019.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolívar S, Navarro X, Udina E, 2020. Schwann Cell Role in Selectivity of Nerve Regeneration. Cells 9. doi: 10.3390/cells9092131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boven LA, Van Meurs M, Van Zwam M, Wierenga-Wolf A, Hintzen RQ, Boot RG, Aerts JM, Amor S, Nieuwenhuis EE, Laman JD, 2006. Myelin-laden macrophages are anti-inflammatory, consistent with foam cells in multiple sclerosis. Brain 129, 517–526. doi: 10.1093/brain/awh707 [DOI] [PubMed] [Google Scholar]
- Brown BN, Sicari BM, Badylak SF, 2014. Rethinking regenerative medicine: A macrophage-centered approach. Front. Immunol. 5, 1–11. doi: 10.3389/fimmu.2014.00510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brück W, Friede RL, 1991. The role of complement in myelin phagocytosis during PNS wallerian degeneration. J. Neurol. Sci 103, 182–187. doi: 10.1016/0022-510X(91)90162-Z [DOI] [PubMed] [Google Scholar]
- Brynskikh AM, Zhao Y, Mosley RL, Li S, Boska MD, Klyachko NL, Kabanov AV, Gendelman HE, Batrakova EV, 2010. Macrophage Delivery of Therapeutic Nanozymes in a Murine Model of Parkinson’s Disease. Nanomedicine 5, 379–396. doi: 10.2217/nnm.10.7.Macrophage [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke B, Sumner S, Maitland N, Lewis CE, 2002. Macrophages in gene therapy: cellular delivery vehicles and in vivo targets. J. Leukoc. Biol 72, 417–28. [PubMed] [Google Scholar]
- Cafferty WBJ, Gardiner NJ, Gavazzi I, Powell J, McMahon SB, Heath JK, Munson J, Cohen J, Thompson SWN, 2001. Leukemia inhibitory factor determines the growth status of injured adult sensory neurons. J. Neurosci 21, 7161–7170. doi: 10.1523/jneurosci.21-18-07161.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J, Dong R, Jiang L, Gong Y, Yuan M, You J, Meng W, Chen Z, Zhang N, Weng Q, Zhu H, He Q, Ying M, Yang B, 2019. LncRNA-MM2P Identified as a Modulator of Macrophage M2 Polarization. Cancer Immunol. Res 7, 292–305. doi: 10.1158/2326-6066.cir-18-0145 [DOI] [PubMed] [Google Scholar]
- Cattin A-L, Burden JJ, Van Emmenis L, Mackenzie FE, Hoving JJA, Garcia Calavia N, Guo Y, McLaughlin M, Rosenberg LH, Quereda V, Jamecna D, Napoli I, Parrinello S, Enver T, Ruhrberg C, Lloyd AC, 2015. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 162, 1127–1139. doi: 10.1016/j.cell.2015.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattin AL, Lloyd AC, 2016. The multicellular complexity of peripheral nerve regeneration. Curr. Opin. Neurobiol 39, 38–46. doi: 10.1016/j.conb.2016.04.005 [DOI] [PubMed] [Google Scholar]
- Chakrabarty A, Liao Z, Mu Y, Smith PG, 2018. Inflammatory renin-angiotensin system disruption attenuates sensory hyperinnervation and mechanical hypersensitivity in a rat model of provoked vestibulodynia. J. Pain 19, 264–277. doi: 10.1016/j.jpain.2017.10.006.Inflammatory [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty A, Liao Z, Smith PG, 2013. Angiotensin II receptor type 2 activation is required for cutaneous sensory hyperinnervation and hypersensitivity in a rat hind paw model of inflammatory Pain. J. Pain 14, 1053–1065. doi: 10.1016/j.jpain.2013.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MWY, Viswanathan S, 2019. Recent progress on developing exogenous monocyte/macrophage-based therapies for inflammatory and degenerative diseases. Cytotherapy 21, 393–415. doi: 10.1016/j.jcyt.2019.02.002 [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S, Myers RR, Janesa J, Shubayev V, 2007. Cytokine regulation of MMP-9 in peripheral glia: Implications for pathological processes and pain in injured nerve. Brain Behavioir Immun. 21, 561–568. doi: 10.1016/j.bbi.2006.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheema SS, Richards LJ, Murphy M, Bartlett PF, 1994. Leukaemia inhibitory factor rescues motorneurones from axotomy-induced cell death. Neuroreport 5, 989–992. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar MED, Frank M, Schnell L, Spillmann AA, Christ F, Schwab ME, 2000. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature 403, 434–439. doi: 10.1038/35000219 [DOI] [PubMed] [Google Scholar]
- Chen P, Cescon M, Zuccolotto G, Nobbio L, Colombelli C, Filaferro M, Vitale G, Feltri ML, Bonaldo P, 2015a. Collagen VI regulates peripheral nerve regeneration by modulating macrophage recruitment and polarization. Acta Neuropathol. 129, 97–113. doi: 10.1007/s00401-014-1369-9 [DOI] [PubMed] [Google Scholar]
- Chen P, Piao X, Bonaldo P, 2015b. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 130, 605–618. doi: 10.1007/s00401-015-1482-4 [DOI] [PubMed] [Google Scholar]
- Ciaramitaro P, Mondelli M, Logullo F, Grimaldi S, Battiston B, Sard A, Scarinzi C, Migliaretti G, Faccani G, Cocito D, 2010. Traumatic peripheral nerve injuries: Epidemiological findings, neuropathic pain and quality of life in 158 patients. J. Peripher. Nerv. Syst 15, 120–127. doi: 10.1111/j.1529-8027.2010.00260.x [DOI] [PubMed] [Google Scholar]
- Curtis R, Scherer SS, Somogyi R, Adryan KM, Ip NY, Zhu Y, Lindsay RM, DiStefano PS, 1994. Retrograde axonal transport of LIF is increased by peripheral nerve injury: Correlation with increased LIF expression in distal nerve. Neuron 12, 191–204. doi: 10.1016/0896-6273(94)90163-5 [DOI] [PubMed] [Google Scholar]
- Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M, 1998. Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J. Neurosci 18, 6713–6722. doi: 10.1523/jneurosci.18-17-06713.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies AJ, Rinaldi S, Costigan M, Oh SB, 2020. Cytotoxic Immunity in Peripheral Nerve Injury and Pain. Front. Neurosci 14, 1–20. doi: 10.3389/fnins.2020.00142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducker TB, Hayes GJ, 1970. Peripheral nerve grafts: experimental studies in the dog and chimpanzee to define homograft limitations. J. Neurosurg 32, 236–243. doi: 10.3171/jns.1970.32.2.0236 [DOI] [PubMed] [Google Scholar]
- Dun X. peng, Carr L, Woodley PK, Barry RW, Drake LK, Mindos T, Roberts SL, Lloyd AC, Parkinson DB, 2019. Macrophage-Derived Slit3 Controls Cell Migration and Axon Pathfinding in the Peripheral Nerve Bridge. Cell Rep. 26, 1458–1472.e4. doi: 10.1016/j.celrep.2018.12.081 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dworkin JD, Linn KA, Teich EG, Zurn P, Shinohara RT, Bassett DS, 2020. The extent and drivers of gender imblance in neuroscience reference lists. Nat. Neurosci 23. [DOI] [PubMed] [Google Scholar]
- Fawcett JW, Asher R. a., 1999. The glial scar and central nervous system repair. Brain Res. Bull 49, 377–391. doi: 10.1016/S0361-9230(99)00072-6 [DOI] [PubMed] [Google Scholar]
- Forbes DC, Peppas NA, 2014. Polymeric nanocarriers for siRNA delivery to murine macrophages. Macromol. Biosci 14, 1096–1105. doi: 10.1002/mabi.201400027 [DOI] [PubMed] [Google Scholar]
- Gomez-Sanchez JA, Carty L, Iruarrizaga-Lejarreta M, Palomo-Irigoyen M, Varela-Rey M, Griffith M, Hantke J, Macias-Camara N, Azkargorta M, Aurrekoetxea I, De Juan VG, Jefferies HBJ, Aspichueta P, Elortza F, Aransay AM, Martínez-Chantar ML, Baas F, Mato JM, Mirsky R, Woodhoo A, Jessen KR, 2015. Schwann cell autophagy, myelinophagy, initiates myelin clearance from injured nerves. J. Cell Biol 210, 153–168. doi: 10.1083/jcb.201503019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong M, Zhuo X, Ma A, 2017. STAT6 Upregulation Promotes M2 Macrophage Polarization to Suppress Atherosclerosis. Med. Sci. Monit. Basic Res 23, 240–249. doi: 10.12659/MSMBR.904014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Perez F, Udina E, Navarro X, 2013. Extracellular matrix components in peripheral nerve regeneration, 1st ed, International Review of Neurobiology. Elsevier Inc. doi: 10.1016/B978-0-12-410499-0.00010-1 [DOI] [PubMed] [Google Scholar]
- Graney PL, Ben-Shaul S, Landau S, Bajpai A, Singh B, Eager J, Cohen A, Levenberg S, Spiller KL, 2020. Macrophages of diverse phenotypes drive vascularization of engineered tissues. Sci. Adv 6. doi: 10.1126/sciadv.aay6391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinsell D, Keating CP, 2014. Peripheral Nerve Reconstruction after Injury: A Review of Clinical and Experimental Therapies. Biomed Res. Int 2014. doi: 10.1155/2014/698256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks JJA, Slaets H, Carmans S, de Vries HE, Dijkstra CD, Stinissen P, Hellings N, 2008. Leukemia inhibitory factor modulates production of inflammatory mediators and myelin phagocytosis by macrophages. J. Neuroimmunol 204, 52–57. doi: 10.1016/j.jneuroim.2008.07.015 [DOI] [PubMed] [Google Scholar]
- Hikawa N, Takenaka T, 1996. Myelin-stimulated macrophages release neurotrophic factors for adult dorsal root ganglion neurons in culture. Cell. Mol. Neurobiol 16, 517–528. doi: 10.1007/BF02150231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S, Dissing-Olesen L, Stevens B, 2016. New insights on the role of microglia in synaptic pruning in health and disease. Curr. Opin. Neurobiol 36, 128–134. doi: 10.1016/j.conb.2015.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura N, Nakahara K, Kino T, Goto T, Aoki H, Yamaguchi I, Kohsaka M, Ochiai T, 1988. Prolongation of skin allograft survival in rats by a novel immunosuppressive agent, FK506. Transplantation 45, 206–209. [DOI] [PubMed] [Google Scholar]
- Jablonka-Shariff A, Lu CY, Campbell K, Monk KR, Snyder-Warwick AK, 2020. Gpr126/Adgrg6 contributes to the terminal Schwann cell response at the neuromuscular junction following peripheral nerve injury. Glia 68, 1182–1200. doi: 10.1002/glia.23769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager SE, Pallesen LT, Richner M, Harley P, Hore Z, McMahon S, Denk F, Vægter CB, 2020. Changes in the transcriptional fingerprint of satellite glial cells following peripheral nerve injury. Glia 68, 1375–1395. doi: 10.1002/glia.23785 [DOI] [PubMed] [Google Scholar]
- Jessen KR, Mirsky R, 2016. The repair Schwann cell and its function in regenerating nerves. J. Physiol 594, 3521–3531. doi: 10.1113/JP270874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Yang W, Zhang K, Qiu S, Xu J, Wang C, Chai Y, 2019. Nanofiber arrangement regulates peripheral nerve regeneration through differential modulation of macrophage phenotypes. Acta Biomater. 83, 291–301. doi: 10.1016/j.actbio.2018.10.040 [DOI] [PubMed] [Google Scholar]
- Kankaanpää U, Bakalim G, 1976. Peripheral nerve injuries of the upper extremity: Sensory return of 137 neurorrhaphies. Acta Orthop. 47, 41–45. doi: 10.3109/17453677608998970 [DOI] [PubMed] [Google Scholar]
- Kaufmann SHE, 2008. Immunology’s foundation: The 100-year anniversary of the Nobel Prize to Paul Ehrlich and Elie Metchnikoff. Nat. Immunol 9, 705–712. doi: 10.1038/ni0708-705 [DOI] [PubMed] [Google Scholar]
- Keilhoff G, Langnaese K, Wolf G, Fansa H, 2006. Inhibiting Effect of Minocycline on the Regeneration of Peripheral Nerves. Dev. Neurobiol 67, 1382–1395. doi: 10.1002/dneu.20384 [DOI] [PubMed] [Google Scholar]
- Kimura T, Nada S, Takegahara N, Okuno T, Nojima S, Kang S, Ito D, Morimoto K, Hosokawa T, Hayama Y, Mitsui Y, Sakurai N, Sarashina-Kida H, Nishide M, Maeda Y, Takamatsu H, Okuzaki D, Yamada M, Okada M, Kumanogoh A, 2016. Polarization of M2 macrophages requires Lamtor1 that integrates cytokine and amino-acid signals. Nat. Commun 7, 1–15. doi: 10.1038/ncomms13130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamoto S, Egashira K, Ichiki T, Han X, McCurdy S, Sakuda S, Sunagawa K, Boisvert WA, 2013. Chitinase inhibition promotes atherosclerosis in hyperlipidemic mice. Am. J. Pathol 183, 313–325. doi: 10.1016/j.ajpath.2013.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klyachko NL, Haney MJ, Zhao Y, Manickam DS, Mahajan V, Suresh P, Hingtgen SD, Mosley RL, Gendelman HE, Kabanov AV, Batrakova EV, 2014. Macrophages offer a paradigm switch for CNS delivery of therapeutic proteins. Nanomedicine 9, 1403–1422. doi: 10.2217/nnm.13.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konofaos P, Terzis JK, 2013. FK506 and nerve regeneration: Past, present, and future. J. Reconstr. Microsurg 29, 141–148. doi: 10.1055/s-0032-1333314 [DOI] [PubMed] [Google Scholar]
- Kranz LM, Diken M, Haas H, Kreiter S, Loquai C, Reuter KC, Meng M, Fritz D, Vascotto F, Hefesha H, Grunwitz C, Vormehr M, Hüsemann Y, Selmi A, Kuhn AN, Buck J, Derhovanessian E, Rae R, Attig S, Diekmann J, Jabulowsky RA, Heesch S, Hassel J, Langguth P, Grabbe S, Huber C, Türeci Ö, Sahin U, 2016. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 534, 396–401. doi: 10.1038/nature18300 [DOI] [PubMed] [Google Scholar]
- Kroner A, Greenhalgh AD, Zarruk JG, Passos dos Santos R, Gaestel M, David S, 2014. TNF and Increased Intracellular Iron Alter Macrophage Polarization to a Detrimental M1 Phenotype in the Injured Spinal Cord. Neuron 83, 1098–1116. doi: 10.1016/j.neuron.2014.07.027 [DOI] [PubMed] [Google Scholar]
- Kuffler DP, 2009. Chapter 18 Enhancement of Nerve Regeneration and Recovery by Immunosuppressive Agents. Int. Rev. Neurobiol 87, 347–362. doi: 10.1016/S0074-7742(09)87018-9 [DOI] [PubMed] [Google Scholar]
- La Fleur M, Underwood JL, Rappolee DA, Werb Z, 1996. Basement membrane and repair of injury to peripheral nerve: Defining a potential role for macrophages, matrix metalloproteinases, and tissue inhibitor of metalloproteinases-1. J. Exp. Med 184, 2311–2326. doi: 10.1084/jem.184.6.2311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I, 2014. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326. doi: 10.1016/j.cell.2014.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Kivimäe S, Dolor A, Szoka FC, 2016. Macrophage-based cell therapies: The long and winding road. J. Control. Release 240, 527–540. doi: 10.1016/j.jconrel.2016.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Bratlie KM, 2021. The Influence of Polysaccharides-Based Material on Macrophage Phenotypes. Macromol. Biosci doi: 10.1002/mabi.202100031 [DOI] [PubMed] [Google Scholar]
- Lindholm D, Heumann R, Meyer M, Thoenen H, 1987. Interleukin-1 regulates synthesis of nerve growth factor in non-neuronal cells of rat sciatic nerve. Nature 330, 658–659. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang H, 2020. Peripheral nerve injury induced changes in the spinal cord and strategies to counteract/enhance the changes to promote nerve regeneration. Neural Regen. Res 15, 189–198. doi: 10.4103/1673-5374.265540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobato RD, 2008. Historical vignette of Cajal’s work “Degeneration and regeneration of the nervous system” with a reflection of the author. Neurocirugia 19, 456–468. doi: 10.1016/S1130-1473(08)70215-X [DOI] [PubMed] [Google Scholar]
- Long JB, Jay SM, Segal SS, Madri JA, 2009. VEGF-A and Semaphorin3A: Modulators of vascular sympathetic innervation. Dev Biol 334, 119–132. doi: 10.1016/j.ydbio.2009.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu CY, Santosa KB, Jablonka-Shariff A, Vannucci B, Fuchs A, Turnbull I, Pan D, Wood MD, Snyder-Warwick AK, 2020. Macrophage-Derived Vascular Endothelial Growth Factor-A Is Integral to Neuromuscular Junction Reinnervation after Nerve Injury. J. Neurosci 40, 9602–9616. doi: 10.1523/JNEUROSCI.1736-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Richardson PM, 1991. Inflammation near the nerve cell body enhances axonal regeneration. J. Neurosci 11, 972–978. doi: 10.1523/jneurosci.11-04-00972.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Aimetti AA, Langer R, Gu Z, 2016. Bioresponsive Materials. Nat. Rev. Mater 2, 1–19. doi: 10.1038/natrevmats.2016.75 [DOI] [Google Scholar]
- Lubinska L, 1977. Early course of Wallerian degeneration in myelinated fibres of the rat phrenic nerve. Brain Res. 130, 47–63. [DOI] [PubMed] [Google Scholar]
- Lv D, Zhou L, Zheng X, Hu Y, 2017. Sustained release of collagen VI potentiates sciatic nerve regeneration by modulating macrophage phenotype. Eur. J. Neurosci 45, 1258–1267. doi: 10.1111/ejn.13558 [DOI] [PubMed] [Google Scholar]
- Ma H, Zhong W, Jiang Y, Fontaine C, Li S, Fu J, Olkkonen VM, Staels B, Yan D, 2013. Increased atherosclerotic lesions in LDL receptor deficient mice with hematopoietic nuclear receptor Rev-erbα knock- down. J. Am. Heart Assoc 2, 1–10. doi: 10.1161/JAHA.113.000235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackenzie F, Ruhrberg C, 2012. Diverse roles for VEGF-A in the nervous system. Development 139, 1371–1380. doi: 10.1242/dev.072348 [DOI] [PubMed] [Google Scholar]
- Marko SB, Damon DH, 2008. VEGF promotes vascular sympathetic innervation. Am. J. Physiol. - Hear. Circ. Physiol 294, 2646–2652. doi: 10.1152/ajpheart.00291.2008 [DOI] [PubMed] [Google Scholar]
- Marmor L, 1970. Peripheral Nerve Grafts. Clin. Neurosurg 17, 126–141. doi: 10.1093/neurosurgery/17.cn_suppl_1.126 [DOI] [PubMed] [Google Scholar]
- Marmor L, 1964a. Regeneration of peripheral nerves by irradiated homografts 46-A, 383–395. [PubMed] [Google Scholar]
- Marmor L, 1964b. The repair of peripheral nerves by irradiated homografts. Clin. Orthop. Relat. Res 34, 161–169. [PubMed] [Google Scholar]
- Marmor L, Miner R, Foster J, 1967. Experimental prevention of nerve homograft rejection by use of immuosuppresive drugs. J. Neurosurg 27, 415–418. [DOI] [PubMed] [Google Scholar]
- Martini R, Fischer S, López-Vales R, David S, 2008. Interactions between schwann cells and macrophages in injury and inherited demyelinating disease. Glia 56, 1566–1577. doi: 10.1002/glia.20766 [DOI] [PubMed] [Google Scholar]
- McAllister RMR, Gilbert SEA, Calder JS, Smith PJ, 1996. The epidemiology and management of upper limb peripheral nerve injuries in modern practice. J. Hand Surg. Am 21, 4–13. [DOI] [PubMed] [Google Scholar]
- Medawar PB, 1944. the Behaviour and Fate of Skin Autografts and Skin Homografts in Rabbits. J. Anat 78, 176–196. [PMC free article] [PubMed] [Google Scholar]
- Meng FW, Slivka PF, Dearth CL, Badylak SF, 2015. Solubilized extracellular matrix from brain and urinary bladder elicits distinct functional and phenotypic responses in macrophages. Biomaterials 46, 131–140. doi: 10.1016/j.biomaterials.2014.12.044 [DOI] [PubMed] [Google Scholar]
- Menorca RMG, Fussell TS, Elfar JC, 2013. Peripheral Nerve Trauma: Mechanisms of Injury and Recovery. Hand Clin. 29, 317–330. doi: 10.1016/j.hcl.2013.04.002.Peripheral [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestas J, Hughes CCW, 2004. Of mice and not men: differences between mouse and human immunology. J. Immunol 72, 2731–2738. doi: 10.4049/jimmunol.172.5.2731 [DOI] [PubMed] [Google Scholar]
- Metchnikoff E, 1887. Sur la lutte des cellules de l’organisme contre l’invasion des microbes. Ann. Inst. Pasteur 1, 321–345. [Google Scholar]
- Michailov GV, Sereda MW, Brinkmann BG, Fischer TH, Haug B, Birchmeier C, Role L, Lai C, Schwab MH, Nave KA, 2004. Axonal Neuregulin-1 Regulates Myelin Sheath Thickness. Science (80-. ). 304, 700–703. doi: 10.1126/science.1095862 [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Torii T, Takada S, Ohno N, Saitoh Y, Nakamura K, Ito A, Ogata T, Terada N, Tanoue A, Yamauchi J, 2015. Involvement of the Tyro3 receptor and its intracellular partner Fyn signaling in Schwann cell myelination. Mol. Biol. Cell 26, 3489–3503. doi: 10.1091/mbc.E14-05-1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokarram N, Dymanusb K, Srinivasan A, Lyon JG, Tiptonb J, Chu J, English AW, Bellamkonda RV, 2017. Immunoengineering nerve repair. Proc. Natl. Acad. Sci 114, E5077–E5084. doi: 10.1073/pnas.1705757114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokarram N, Merchant A, Mukhatyar V, Patel G, Bellamkonda RV, 2012. Effect of modulating macrophage phenotype on peripheral nerve repair. Biomaterials 33, 8793–8801. doi: 10.1016/j.biomaterials.2012.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosser DM, Edwards JP, 2008. Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8, 958–969. doi: 10.1038/nri2448.Exploring [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M, Leonhard C, Wacker K, Ringelstein EB, Okabe M, Hickey WF, Kiefer R, 2003. Macrophage response to peripheral nerve injury: The quantitative contribution of resident and hematogenous macrophages. Lab. Investig 83, 175–185. doi: 10.1097/01.LAB.0000056993.28149.BF [DOI] [PubMed] [Google Scholar]
- Nguyen V. Du, Han J, Go G, Zhen J, Zheng S, Le VH, Park J, Park S, 2017. Feasibility study of dual-targeting paclitaxel-loaded magnetic liposomes using electromagnetic actuation and macrophages. Sensors Actuators B Chem. 240, 1226–1236. [Google Scholar]
- Nicola NA, Babon JJ, 2015. Leukemia Inhibitory Factor (LIF). Cytokine Growth Factor Rev 25, 533–544. doi: 10.1016/j.cytogfr.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi JP, DeFrancesco-Lisowitz A, Cregg J, Howarth M, Zigmond RE, 2016. Overexpression of the Monocyte Chemokine CCL2 in Dorsal Root Ganglion Neurons Causes a Conditioning-Like Increase in Neurite Outgrowth and Does So via a STAT3 Dependent Mechanism. Exp. Neurol 275, 25–37. doi: 10.1016/j.physbeh.2017.03.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemi JP, Defrancesco-Lisowitz A, Roldan-Hernandez L, Lindborg JA, Mandell D, Zigmond RE, 2013. A critical role for macrophages near axotomized neuronal cell bodies in stimulating nerve regeneration. J. Neurosci 33, 16236–16248. doi: 10.1523/JNEUROSCI.3319-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J, Munro CA, Prasad VSSV, Midha R, 1998. Analysis of upper and lower extremity peripheral nerve injuries in a population of patients with multiple injuries. J. Trauma - Inj. Infect. Crit. Care 45, 116–122. doi: 10.1097/00005373-199807000-00025 [DOI] [PubMed] [Google Scholar]
- Ochiai T, Nagata M, Nakajima K, Suzuki T, Sakamoto K, Enomoto K, Gunji Y, Uematsu T, Goto, Takesada, Hori S, Kenmochi T, Nakagouri T, Asano T, Isono K, Hamaguchi K, Tsuchida H, Nakahara K, Inamura N, Goto, Toshio, 1987a. Studies of the effects of FK506 on renal allografting in the beagle dog. Transplantation 44, 729–733. [DOI] [PubMed] [Google Scholar]
- Ochiai T, Nakajima K, Nagata M, Hori S, Asano T, Isono K, 1987b. Studies of the induction and maintenance of long-term graft acceptance by treatment with FK506 in heterotopic cardiac allotransplantation in rats. Transplantation 44, 734–738. [DOI] [PubMed] [Google Scholar]
- Ootes D, Lambers KT, Ring DC, 2012. The epidemiology of upper extremity injuries presenting to the emergency department in the United States. Hand 7, 18–22. doi: 10.1007/s11552-011-9383-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padovano WM, Dengler J, Patterson MM, Yee A, Snyder-Warwick AK, Wood MD, Moore AM, Mackinnon SE, 2020. Incidence of Nerve Injury After Extremity Trauma in the United States. Hand 1–9. doi: 10.1177/1558944720963895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry VH, Tsao JW, Fearn S, Brown MC, 1995. Radiation-induced Reductions in Macrophage Recruitment Have Only Slight Effects on Myelin Degeneration in Sectioned Peripheral Nerves of Mice 7. [DOI] [PubMed] [Google Scholar]
- Perry VHH, Brown MCC, Gordon S, 1987. The macrophage response to central and peripheral nerve injury: A possible role for macrophages in regeneration. J. Exp. Med 165, 1218–1223. doi: 10.1084/jem.165.4.1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister BJ, Gordon T, Loverde JR, Kochar AS, Mackinnon SE, Kacy Cullen D, 2011. Biomedical engineering strategies for peripheral nerve repair: Surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng 39, 81–124. doi: 10.1615/CritRevBiomedEng.v39.i2.20 [DOI] [PubMed] [Google Scholar]
- Phan DQD, Schuind F, 2012. Tolerance and effects of FK506 (tacrolimus) on nerve regeneration: A pilot study. J. Hand Surg. Eur Vol. 37, 537–543. doi: 10.1177/1753193411427826 [DOI] [PubMed] [Google Scholar]
- Pirzgalska RM, Seixas E, Seidman JS, Link VM, Sánchez NM, Mahú I, Mendes R, Gres V, Kubasova N, Morris I, Arús BA, Larabee CM, Vasques M, Tortosa F, Sousa AL, Anandan S, Tranfield E, Hahn MK, Iannacone M, Spann NJ, Glass CK, Domingos AI, 2017. Sympathetic neuron-associated macrophages contribute to obesity by importing and metabolizing norepinephrine. Nat. Med 23, 1309–1318. doi: 10.1038/nm.4422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard JD, Fitzpatrick L, 1973. A comparison of the effects of irradiation and immunosuppressive agents on regeneration through peripheral nerve allografts: An ultrastructural study. Acta Neuropathol. 23, 166–180. doi: 10.1007/BF00685770 [DOI] [PubMed] [Google Scholar]
- Pollard JD, McLeod JG, 1971. An assessment of immunosuppressive agents in experimental peripheral nerve transplantation. Surgery, Gynecol. Obstet 132, 839–45. [PubMed] [Google Scholar]
- Pollard JD, McLeod JG, Gye RS, 1973. Regeneration Through Peripheral Nerve Allografts. Arch. Neurol 28, 31. doi: 10.1001/archneur.1973.00490190049005 [DOI] [PubMed] [Google Scholar]
- Purcell BP, Lobb D, Charati MB, Dorsey SM, Wade RJ, Zellars KN, Doviak H, Pettaway S, Logdon CB, Shuman J.a, Freels PD, Gorman Iii JH, Gorman RC, Spinale FG, Burdick J. a, 2014. Injectable and bioresponsive hydrogels for on-demand matrix metalloproteinase inhibition. Nat. Mater 13, 653–61. doi: 10.1038/nmat3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian T, Wang P, Chen Q, Yi S, Liu Q, Wang H, Wang S, Geng W, Liu Z, Li S, 2018. The dynamic changes of main cell types in the microenvironment of sciatic nerves following sciatic nerve injury and the influence of let-7 on their distribution. RSC Adv. 8, 41181–41191. doi: 10.1039/C8RA08298G [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raheja A, Suri V, Suri A, Sarkar C, Srivastava A, Mohanty S, Jain KG, Sharma MC, Mallick HN, Yadav PK, Kalaivani M, Pandey RM, 2012. Dose-dependent facilitation of peripheral nerve regeneration by bone marrow–derived mononuclear cells: a randomized controlled study. J. Neurosurg 117, 1170–1181. doi: 10.3171/2012.8.JNS111446 [DOI] [PubMed] [Google Scholar]
- Ramon y Cajal S, 1928. Degeneration & Regeneration of the Nervous System: Volume I.Hafner Publishing Co. [Google Scholar]
- Rios R, Jablonka-Shariff A, Broberg C, Snyder-Warwick AK, 2021. Macrophage roles in peripheral nervous system injury and pathology: Allies in neuromuscular junction recovery. Mol. Cell. Neurosci 111. doi: 10.1016/j.mcn.2021.103590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg AF, Wolman MA, Franzini-Armstrong C, Granato M, 2012. In vivo nerve-macrophage interactions following peripheral nerve injury. J. Neurosci 32, 3898–3909. doi: 10.1523/JNEUROSCI.5225-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal T, 1961. Aulus Cornelius: His Contributions to Dermatology. Arch. Dermatol 84, 613–618. [Google Scholar]
- Todo S, Ueda Y, Demetris JA, Imventarza O, Nalesnik M, Venkataramanan R, Makowka L, Starzl TE, 1988. Immunosuppression of canine, monkey, and baboon allografts by FK 506: with special reference to synergism with other drugs and to tolerance induction. Surgery 104, 239–249. [PMC free article] [PubMed] [Google Scholar]
- Salvany S, Casanovas A, Piedrafita L, Tarabal O, Hernández S, Calderó J, Esquerda JE, 2021. Microglial recruitment and mechanisms involved in the disruption of afferent synaptic terminals on spinal cord motor neurons after acute peripheral nerve injury. Glia 1216–1240. doi: 10.1002/glia.23959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K, Yamagishi K, Nakayama Y, Yokota K, Uchida H, Masaki Y, Watanabe K, Aso K, 1989. Pancreaticoduodenal Allotransplantation with Cyclosporine and FK506. Transplant. Proc 21, 1074–1075. [PubMed] [Google Scholar]
- Schnell L, Schwab ME, 1990. Axonal regeneration in the rat spinal cord produced by an antibody against myelin-associated neurite growth inhibitors. Nature 343, 269–272. doi: 10.1126/science.194.4266.718-a [DOI] [PubMed] [Google Scholar]
- Smith DH, Meaney DF, 2000. Axonal Damage in Traumatic Brain Injury. Neurosci. 6, 483–495. doi: 10.1177/107385840000600611 [DOI] [Google Scholar]
- Smith ML, Adrian EK, 1972. On the prescence of mononuclear leucocytes in dorsal root ganglia following transection of the sciatic nerve. Anat. Rec 172, 581–587. [DOI] [PubMed] [Google Scholar]
- Snyder RJ, Lantis J, Kirsner RS, Shah V, Molyneaux M, Carter MJ, 2016. Macrophages: A review of their role in wound healing and their therapeutic use. Wound Repair Regen. 24, 613–629. doi: 10.1111/wrr.12444 [DOI] [PubMed] [Google Scholar]
- Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G, 2014. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35, 4477–4488. doi: 10.1016/j.biomaterials.2014.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller KL, Nassiri S, Witherel CE, Anfang RR, Ng J, Nakazawa KR, Yu T, Vunjak-Novakovic G, 2015. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 37, 194–207. doi: 10.1016/j.biomaterials.2014.10.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller KL, Wrona EA, Romero-Torres S, Pallotta I, Graney PL, Witherel CE, Panicker LM, Feldman RA, Urbanska AM, Santambrogio L, Vunjak-Novakovic G, Freytes DO, 2016. Differential Gene Expression in Human, Murine, and Cell Line-derived Macrophages upon Polarization. Exp. Cell Res 347, 1–13. doi: 10.1016/j.yexcr.2015.10.017 [DOI] [PubMed] [Google Scholar]
- Stein M, Keshav S, Harris N, Gordon S, Stein BM, Keshav S, Harris N, Gordon S, Stein M, Keshav S, Harris N, Gordon S, 1992. Interleukin 4 Potently Enhances Murine Macrophage Mannose Receptor Activity: A Marker of Alternative Immunologic Macrophage Activation. J. Exp. Med 176, 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg L, Stößel M, Ronchi G, Geuna S, Yin Y, Mommert S, Mårtensson L, Metzen J, Grothe C, Dahlin LB, Haastert-Talini K, 2017. Regeneration of long-distance peripheral nerve defects after delayed reconstruction in healthy and diabetic rats is supported by immunomodulatory chitosan nerve guides. BMC Neurosci. 18, 1–27. doi: 10.1186/s12868-017-0374-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone L, Keenan MAE, 1988. Peripheral nerve injuries in the adult with traumatic brain injury. Clin. Orthop. Relat. Res doi: 10.1097/00003086-198808000-00017 [DOI] [PubMed] [Google Scholar]
- Stratton JA, Holmes A, Rosin NL, Trang T, Midha R, Biernaskie J, Stratton JA, Holmes A, Rosin NL, Sinha S, Vohra M, Burma NE, Trang T, Midha R, Biernaskie J, 2018. Macrophages Regulate Schwann Cell Maturation after Nerve Injury. Cell Rep. 24, 2561–2572.e6. doi: 10.1016/j.celrep.2018.08.004 [DOI] [PubMed] [Google Scholar]
- Sugiura S, Lahav R, Han J, Kou SY, Banner LR, de Pablo F, Patterson PH, 2000. Leukaemia inhibitory factor is required for normal inflammatory responses to injury in the peripheral and central nervous systems in vivo and is chemotactic for macrophages in vitro. Eur. J. Neurosci 12, 457–466. doi: 10.1046/j.1460-9568.2000.00922.x [DOI] [PubMed] [Google Scholar]
- Sulaiman OAR, Voda J, Gold BG, Gordon T, 2002. FK506 Increases Peripheral Nerve Regeneration after Chronic Axotomy but Not after Chronic Schwann Cell Denervation. Exp. Neurol 175, 127–137. doi: 10.1006/exnr.2002.7878 [DOI] [PubMed] [Google Scholar]
- Sunderkotter C, Steinbrink K, Goebeler M, Bhardwaj R, Sorg C, 1994. Macrophages and angiogenesis. J. Leukoc. Biol 55, 410–422. doi: 10.1002/jlb.55.3.410 [DOI] [PubMed] [Google Scholar]
- Tajdaran K, Chan K, Shoichet MS, Gordon T, Borschel GH, 2019. Local delivery of FK506 to injured peripheral nerve enhances axon regeneration after surgical nerve repair in rats. Acta Biomater. 96, 211–221. doi: 10.1016/j.actbio.2019.05.058 [DOI] [PubMed] [Google Scholar]
- Tajdaran K, Shoichet MS, Gordon T, Borschel GH, 2015. A novel polymeric drug delivery system for localized and sustained release of tacrolimus (FK506). Biotechnol. Bioeng 112, 1948–1953. doi: 10.1002/bit.25598 [DOI] [PubMed] [Google Scholar]
- Taylor CA, Braza D, Rice JB, Dillingham T, 2008. The incidence of peripheral nerve injury in extremity trauma. Am. J. Phys. Med. Rehabil 87, 381–385. doi: 10.1097/PHM.0b013e31815e6370 [DOI] [PubMed] [Google Scholar]
- Tezcan AH, 2017. Peripheral Nerve Injury and Current Treatment Strategies, in: Peripheral Nerve Regeneration - From Surgery to New Therapeutic Approaches Including Biomaterials and Cell-Based Therapies Development. pp. 3–31. doi: 10.5772/intechopen.68345 [DOI] [Google Scholar]
- Tong H, Kang W, Davy PMC, Shi Y, Sun S, Allsopp RC, Lu Y, 2016. Monocyte Trafficking, Engraftment, and Delivery of Nanoparticles and an Exogenous Gene into the Acutely Inflamed Brain Tissue – Evaluations on Monocyte-Based Delivery System for the Central Nervous System. PLoS One 11, 1–21. doi: 10.1371/journal.pone.0154022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsalamandris S, Antonopoulos AS, Oikonomou E, Papamikroulis GA, Vogiatzi G, Papaioannou S, Deftereos S, Tousoulis D, 2019. The role of inflammation in diabetes: Current concepts and future perspectives. Eur. Cardiol. Rev 14, 50–59. doi: 10.15420/ecr.2018.33.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Emmenis L, 2018. A novel role for macrophages in peripheral nerve regeneration. University College London. [Google Scholar]
- Vargas ME, Barres BA, 2007. Why Is Wallerian Degeneration in the CNS So Slow? Annu. Rev. Neurosci 30, 153–179. doi: 10.1146/annurev.neuro.30.051606.094354 [DOI] [PubMed] [Google Scholar]
- Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I, 1997. Immunosuppressive effects of apoptotic cells. Nature 390, 350–351. doi: 10.1038/37022 [DOI] [PubMed] [Google Scholar]
- Waller A, 1850. Experiments on the section of the glossopharyngeal and hypoglossal nerves of the frog, and observations of the alterations produced thereby in the structure of their primitive fibres. Philos. Trans. R. Soc. London 140, 423–429. doi: 10.1098/rspl.1843.0224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Medress ZA, Barres BA, 2012. Axon degeneration: Molecular mechanisms of a self-destruction pathway. J. Cell Biol 196, 7–18. doi: 10.1083/jcb.201108111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherel CE, Abebayehu D, Barker TH, Spiller KL, 2019. Macrophage and Fibroblast Interactions in Biomaterial-Mediated Fibrosis. Adv. Healthc. Mater 8, 1–16. doi: 10.1002/adhm.201801451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherel CE, Sao K, Brisson BK, Han B, Volk SW, Petrie RJ, Han L, Spiller KL, 2021. Regulation of extracellular matrix assembly and structure by hybrid M1/M2 macrophages. Biomaterials 269. doi: 10.1016/j.biomaterials.2021.120667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford KL, Cullen DK, Spiller KL, 2019a. Modulation of macrophage phenotype via phagocytosis of drug-loaded microparticles. J. Biomed. Mater. Res. Part A 107, 1–12. doi: 10.1002/jbm.a.36617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford KL, Loane DJ, Cullen DK, 2019b. Acute drivers of neuroinflammation in traumatic brain injury. Neural Regen. Res 14, 1–9. doi: 10.4103/1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford Kathryn L, Singh BS, Cullen DK, Spiller KL, 2020. Biomaterial-mediated Reprogramming of Monocytes via Microparticle Phagocytosis for Sustained Modulation of Macrophage Phenotype. Acta Biomater. 101, 237–248. doi: 10.1016/j.actbio.2019.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford KL, Singh BS, Cullen DK, Spiller KL, 2020. Biomaterial-mediated reprogramming of monocytes via microparticle phagocytosis for sustained modulation of macrophage phenotype. Acta Biomater. 101. doi: 10.1016/j.actbio.2019.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtkiewicz DM, Saunders J, Domeshek L, Novak CB, Kaskutas V, Mackinnon SE, 2015. Social impact of peripheral nerve injuries. Hand 10, 161–167. doi: 10.1007/s11552-014-9692-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf Y, Boura-Halfon S, Cortese N, Haimon Z, Sar Shalom H, Kuperman Y, Kalchenko V, Brandis A, David E, Segal-Hayoun Y, Chappell-Maor L, Yaron A, Jung S, 2017. Brown-adipose-tissue macrophages control tissue innervation and homeostatic energy expenditure. Nat. Immunol 18, 665–674. doi: 10.1038/ni.3746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Chawla A, Pollard JW, 2013. Macrophage biology in development, homeostasis and disease. Nature 496, 445–455. doi: 10.1038/nature12034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA, Vannella KM, 2016. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 44, 450–462. doi: 10.1016/j.immuni.2016.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ydens E, Amann L, Asselbergh B, Scott CL, Martens L, Sichien D, Mossad O, Blank T, De Prijck S, Low D, Masuda T, Saeys Y, Timmerman V, Stumm R, Ginhoux F, Prinz M, Janssens S, Guilliams M, 2020. Profiling peripheral nerve macrophages reveals two macrophage subsets with distinct localization, transcriptome and response to injury. Nat. Neurosci 23, 676–689. doi: 10.1038/s41593-020-0618-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Cui Q, Li Y, Irwin N, Fischer D, Harvey AR, Benowitz LI, 2003. Macrophage-derived factors stimulate optic nerve regeneration. J. Neurosci 23, 2284–2293. doi:23/6/2284 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Henzl MT, Lorber B, Nakazawa T, Thomas TT, Jiang F, Langer R, Benowitz LI, 2006. Oncomodulin is a macrophage-derived signal for axon regeneration in retinal ganglion cells. Nat. Neurosci 9, 843–852. doi: 10.1038/nn1701 [DOI] [PubMed] [Google Scholar]
- Zuo KJ, Shafa G, Chan K, Zhang J, Hawkins C, Tajdaran K, Gordon T, Borschel GH, 2021. Local FK506 drug delivery enhances nerve regeneration through fresh, unprocessed peripheral nerve allografts. Exp. Neurol 341. doi: 10.1016/j.expneurol.2021.113680 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.