Abstract
Objective:
To explore oral ondansetron usage and impact on outcomes in clinical practice.
Methods:
This observational study was a planned secondary analysis of two trials conducted in ten United States (U.S.) and six Canadian institutions, between 2014 and 2017. Children 3-48 months old with gastroenteritis and ≥3 episodes of vomiting in the 24 hours preceding emergency department (ED) presentation were included. Oral ondansetron was administered at provider discretion. Principal outcomes were intravenous fluid administration and hospitalization at the index visit and during the subsequent 72 hours, and diarrhea and vomiting frequency during the 24-hours following the ED visit.
Results:
794 children were included: median age 16.0 months (IQR: 10.0-26.0), 50.1% (398/794) received oral ondansetron. In propensity-adjusted analysis (n=528), children administered oral ondansetron were less likely to receive intravenous fluids at the index visit (aOR 0.50; 95% CI: 0.29, 0.88), but there were no differences in the frequencies of intravenous fluid administration within the first 72 hours (aOR 0.65; 95%CI 0.39, 1.10), and hospitalization at the index visit (aOR 0.31; 95%CI 0.09, 1.10) or the subsequent 72 hours (aOR 0.52; 95%CI 0.21, 1.28). Episodes of vomiting (aOR 0.86; 95%CI 0.63, 1.19) and diarrhea (aOR 1.11; 0.93, 1.32) during the 24 hours following ED discharge also did not differ.
Conclusions:
Among preschool-aged children with gastroenteritis seeking ED care, oral ondansetron administration was associated with a reduction in index ED visit intravenous fluid administration, but not with intravenous fluids administered within 72 hours, hospitalization, or vomiting and diarrhea in the 24 hours following discharge.
Introduction
Background
Ondansetron is a widely used 5-hydroxytryptamine-3 receptor antagonist which has been demonstrated to reduce vomiting, intravenous fluid rehydration, and hospitalization in clinical trials of children with acute gastroenteritis (AGE).1-4 This has led to its routine administration to children who present for care with AGE to U.S. emergency departments (ED).5 Although some single center studies have observed associations between reductions in intravenous rehydration rates and increased ondansetron usage, multicenter ED administrative database studies have failed to demonstrate similar reductions in intravenous fluid administration and hospitalization rates.6,7
Importance
The aforementioned conflicting findings highlight the value of better understanding factors in the clinical ED environment related to ondansetron usage and clinical outcomes. Such assessments require prospectively collected, patient-level clinical data. The importance of understanding how disease severity influences beneficial effects was highlighted in two recent studies which differed only in the presence or absence of dehydration in participants. While ondansetron administration had no beneficial effect when administered to children without dehydration, among those with dehydration it reduced both vomiting and intravenous rehydration use.8,9
Additional factors that complicate the translation of ondansetron efficacy into clinical effectiveness relate to the timing, route of administration, and concomitant use of oral rehydration therapy.10 A Pediatric Health Information System analysis (2002-2011) showed a high rate of intravenous ondansetron administration among children administered intravenous fluids.7 Among those who received oral ondansetron and intravenous fluids, the sequence of ondansetron administration relative to the initiation of intravenous rehydration could not be determined and it was unknown if oral rehydration therapy was attempted following ondansetron administration.7 Lastly, the data did not allow for assessment of short term outcomes after disposition from the ED, including return visits or subsequent treatment with intravenous fluids.
Goals of This Investigation
Because of these knowledge gaps, a more comprehensive understanding of the use and utility of ondansetron in routine clinical practice among children with AGE is needed.10 Thus, we performed an a priori planned secondary analysis of a merged dataset from two multicenter clinical trials that evaluated probiotics use in children with AGE.11-14 While the aim of the trials was to determine the effect of probiotic vs. placebo, the focus of this sub-analysis was oral ondansetron use and related outcomes in children with vomiting. Ondansetron was not part of the study protocols and was administered at the discretion of the treating physicians. We sought to evaluate, in routine clinical practice, the associations between oral ondansetron administration, oral and intravenous fluid use, hospitalization and short-term clinical outcomes. We also explore clinical factors associated with oral ondansetron use.
METHODS
Study Design and Setting
This was a planned secondary analysis of the Pediatric Emergency Care Applied Research Network (PECARN) Probiotic Study13,14 and the Pediatric Emergency Research Canada (PERC) Probiotic Regimen for Outpatient Gastroenteritis Utility of Treatment (PROGUT)11,12 randomized, placebo controlled trials of probiotics in children who presented for ED care with AGE-associated diarrhea. Participants were enrolled Nov 2013-June 2017 in ten U.S. (PECARN) and in six Canadian (PERC) EDs. The PECARN Probiotic study provided a 5-day course of Lactobacillus rhamnosus GG (LGG) or placebo, and the PROGUT trial a 5-day course of a combination probiotic (L. rhamnosus R0011 and L. helveticus R0052) or placebo. Both clinical trials were approved by the ethics committees at participating sites and written consent was obtained from a caregiver of each participant. All ED care, including use of ondansetron, treatment with oral or intravenous fluids, and hospitalization was at the discretion of the treating provider. After ED discharge, parents or guardians completed electronic or telephone follow-up surveys every 24 hours until both vomiting and diarrhea had ceased for 24 hours. Survey questions targeted clinical symptoms and health care utilization during the preceding 24-hour period.
Participants
Children eligible for enrollment in either clinical trial were aged 3 to 48 months, had ≥3 episodes of diarrhea in the preceding 24 hours and were diagnosed clinically as having AGE. Exclusion criteria were as outlined in the parent studies.12,14 Children eligible for this secondary analysis were those who in addition to diarrhea had ≥3 episodes of vomiting in the 24 hours preceding ED presentation. We excluded those children treated with oral ondansetron and intravenous fluids within 30 minutes (as decision made to administer intravenous fluids likely made irrespective of effect of ondansetron) as well as those treated with intravenous ondansetron.
Outcomes
The principal objectives were to determine if oral ondansetron administration at the discretion of the treating physician was associated with 1) a reduction in intravenous fluid administration or hospitalization at the index visit and within 72 hours, and 2) alterations in the frequency of vomiting and diarrhea episodes during the 24 hours following study enrollment. The secondary objective was to describe demographic, clinical, and ED factors associated with oral ondansetron administration at the index (i.e. enrollment) ED visit.
Measurements
The oral ondansetron cohort included those who received oral ondansetron and were managed for at minimum 30 minutes without intravenous fluids, allowing time for oral fluid administration. A minimum 30-minute oral rehydration window was pre-specified to avoid classifying as treatment failures children for whom the initial treatment plan included the intention to administer intravenous fluids. We report the level of dehydration using the Clinical Dehydration Scale (CDS) score which classifies children into three severity groups - no dehydration (score = 0), some dehydration (scores = 1 to 4), and moderate-to-severe dehydration (scores = 5 to 8)15; Appendix 1. Illness severity at the time of study enrollment was classified using the Modified Vesikari Scale (MVS) score a composite measure that includes fever, and duration and severity of vomiting and diarrhea, and ranges from 0 to 20, with higher scores indicating more severe disease;16,17 Appendix 1.
Analysis
All analyses were specified a priori. We included data from all participants in the parent trials who met eligibility criteria for this secondary analysis supplemented by multiple imputation to account for missing data as per the parent studies.11,13 Imputation was performed using chained equations separately for each parent trial, and assumed that data were missing at random. Imputation models included key baseline characteristics, treatment allocation, and all outcomes. Our analytic approach, further detailed below, first identified factors associated with oral ondansetron administration. We then evaluated the association between oral ondansetron administration and the outcomes of interest through unadjusted analyses followed by adjusted analyses using propensity score methods.
Factors associated with oral ondansetron administration:
We estimated country- and site- specific use of oral ondansetron and estimated the difference between countries with a 95% confidence interval. To identify factors associated with oral ondansetron administration, we fit logistic regression models to calculate the unadjusted and adjusted odds-ratios and 95% confidence intervals (CI) for the likelihood of receiving oral ondansetron for the following a priori identified independent variables: age, sex, duration of vomiting and diarrhea prior to randomization, number of vomits and diarrheal episodes in the 24 hours prior to randomization, baseline CDS and MVS scores, and country (i.e. U.S. vs. Canada). Baseline MVS score was removed from the adjusted model due to collinearity with diarrhea and vomiting frequency measures, which are components of the composite MVS score.
Association between oral ondansetron administration and outcomes of interest:
Logistic regression models were used to evaluate unadjusted associations between oral ondansetron administration and the outcomes of intravenous fluid administration and hospitalization. Negative binomial models were used to evaluate the association between oral ondansetron and frequency of vomiting and diarrhea episodes during the 24 hours following study enrollment. All unadjusted analyses were repeated as stratified models to estimate separate odds-ratios for dehydrated (including some and moderate/severe dehydration combined) and not dehydrated patients.
To account for confounding by indication and other factors related to both the administration of oral ondansetron and the outcomes of interest, we performed adjusted analyses using propensity score methods.18,19 This helped remove bias and permitted comparisons between children with similar baseline characteristics who were administered oral ondansetron and those who were not.20,21
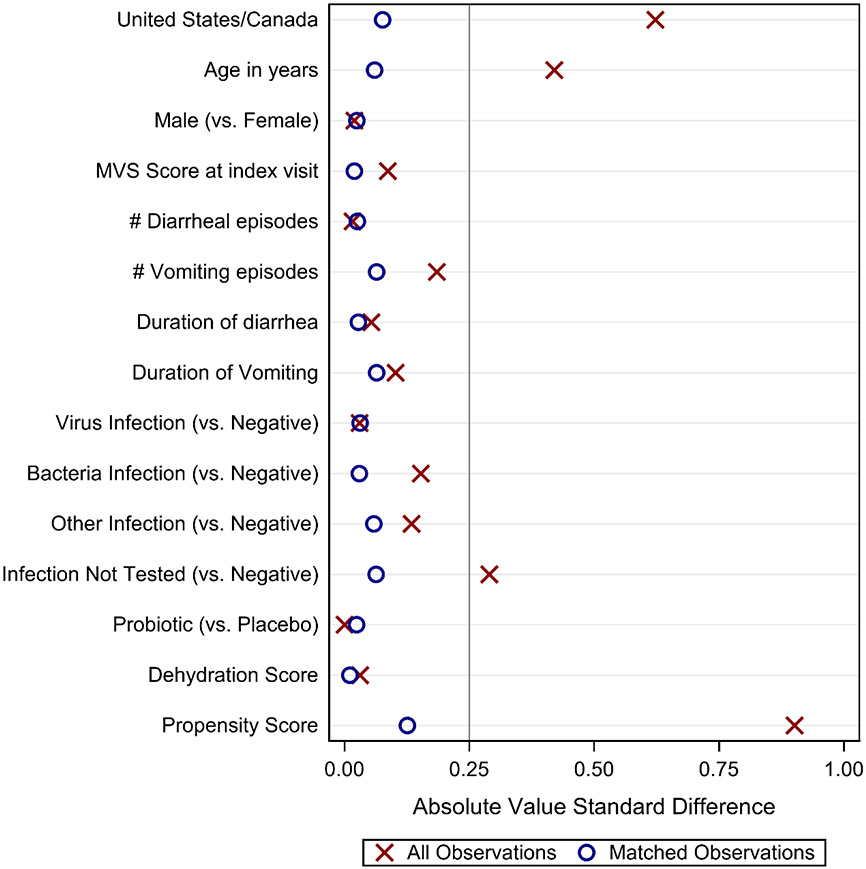
A log-odds propensity score was estimated for the likelihood that an individual participant would have received oral ondansetron (dependent variable) during the index ED visit given an observed set of characteristics.21,22 Logistic regression was used to calculate propensity scores to match children who received oral ondansetron with those who did not. All independent variables from logistic regression models detailed above were included in the propensity score model, including baseline MVS score. Additionally, organism, randomized treatment (probiotic or placebo) and the continuous CDS were included. Patients who received oral ondansetron were sequentially matched with those who did not 1:1 in descending order of propensity score and without replacement. Matches were forced based on the CDS score dehydration severity category to ensure similar levels of dehydration and to facilitate analyses stratified by the CDS score category. If there was no candidate match within a 0.25 standard deviation of the propensity score, the patient was excluded from the matched analysis. Baseline characteristics of matched pairs were compared to characteristics of unmatched data using absolute standardized differences to assess the success of this approach.23 Absolute standardized differences were plotted and compared to a value of 0.25, which represents a common threshold for optimized balance.24
The association between oral ondansetron and the outcomes of interest were estimated as odds-ratios from logistic regression models applied to binary outcomes, and as rate-ratios from negative binomial regression models applied to frequency outcomes. Ondansetron group was included as the independent variable, and matched pairs were treated as clusters using generalized estimating equation (GEE) methods.25 We performed sub-analyses stratified by dehydration to describe the associations between oral ondansetron administration, use of intravenous fluids at the index ED visit, hospitalization and vomiting and diarrhea frequency for dehydrated (including some and moderate/severe) and non-dehydrated patients. We repeated the analysis using a more stringent difference of ≤0.10 standard deviations as criteria for propensity score matching as a sensitivity analysis.
All analyses, including propensity score estimations, were repeated for each of 10 multiple imputations and results combined according to standard methods.26 Two sided P values of less than 0.05 were considered statistically significant. No adjustments were made for multiple comparisons. Analyses were performed using SAS Software (version 9.4, SAS Institute Inc. Cary, NC, USA). Propensity score estimation was performed using the PSMatch Procedure in SAS.
RESULTS
Characteristics of study subjects
In total, 1,857 children were enrolled in the two trials and 794 (PECARN, N=405; PERC, N=389; Figure 1) met sub-study eligibility criteria: median age was 16.0 months (IQR: 10.0, 26.0) and 56% (445/794) were male; Table 1. Baseline MVS and durations of diarrhea and vomiting were missing for 9%, 11% and 9% of children respectively. Other variables, including IV and hospitalization outcomes, ondansetron use, age, sex, number of diarrhea and vomiting episodes, and CDS score were never missing in the analyzed set of patients. The proportion of participants administered ondansetron varied by site (range: 17% - 81%) and was less frequent in Canada than in the U.S. (Supplemental Figure 1).
Figure 1. Patient flow diagram.
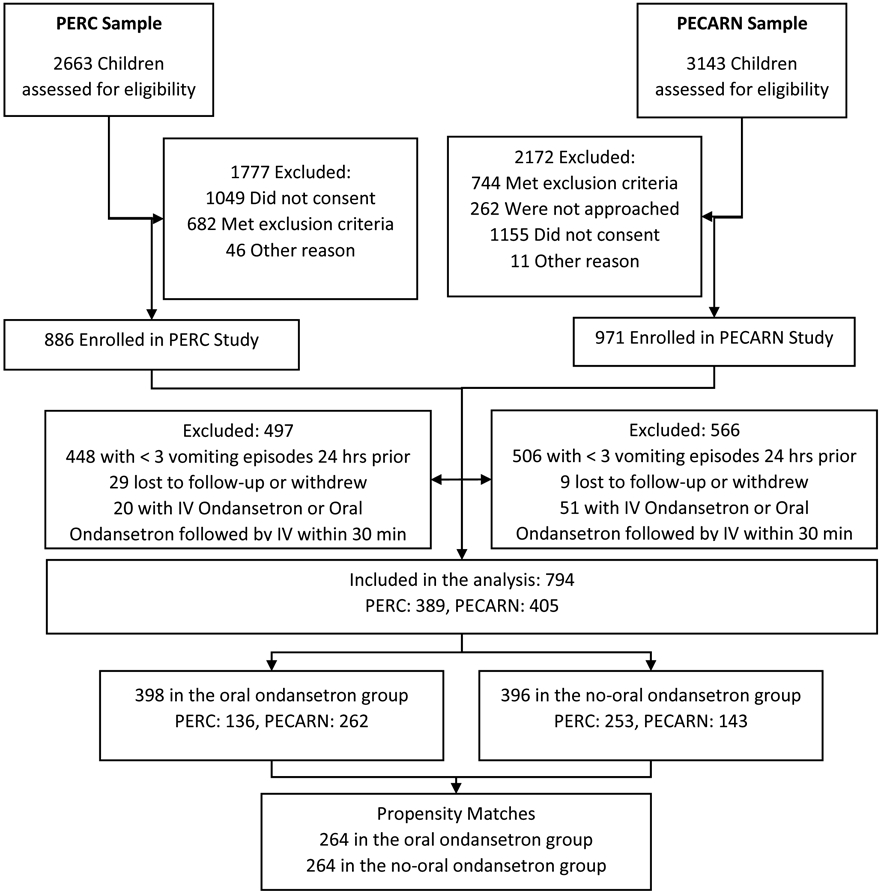
PERC, Pediatric Emergency Research Canada; PECARN, Pediatric Emergency Care Applied Research Network.
Table 1.
Characteristics of the sample by ondansetron groups. Number (% of row total) or median (interquartile range) shown.
| All Observations | Propensity Matched Observations | |||
|---|---|---|---|---|
| Received oral ondansetron3 (N = 398) |
Did not receive oral ondansetron3 (N = 396) |
Received oral ondansetron3 (N = 264) |
Did not receive oral ondansetron3 (N = 264) |
|
| Sex | ||||
| Male | 225 (50.6%) | 220 (49.4%) | 147 (49.6%) | 149 (50.4%) |
| Female | 173 (49.6%) | 176 (50.4%) | 117 (50.6%) | 115 (49.4%) |
| Age in Months | 18.8 [12.0 - 30.0] | 13.0 [8.5 - 23.0] | 16.4 [10.8 - 24.6] | 16.1 [9.0 - 25.3] |
| Number of diarrheal episodes in the 24 hours prior to randomization | 6.0 [4.0 - 8.0] | 5.0 [3.0 - 8.0] | 6.0 [4.0 - 8.0] | 5.1 [3.8 - 8.0] |
| Number of vomiting episodes in the 24 hours prior to randomization | 6.0 [4.0 - 8.0] | 5.0 [4.0 - 7.0] | 5.8 [4.0 - 8.0] | 5.0 [3.4 - 7.0] |
| Duration of diarrhea prior to randomization (hours) | 34.1 [18.0 - 54.9] | 36.9 [23.1 - 56.5] | 36.2 [19.5 - 56.9] | 34.7 [22.4 - 57.4] |
| Duration of vomiting prior to randomization (hours) | 32.2 [16.3 - 52.2] | 35.9 [22.1 - 53.7] | 34.4 [16.4 - 54.2] | 34.3 [21.4 - 56.4] |
| Baseline MVS Score 16 | 12.0 [11.0 - 14.0] | 12.0 [11.0 - 13.3] | 12.2 [11.0 - 14.0] | 12.3 [11.0 - 14.0] |
| Clinical Dehydration Scale Score 15 | ||||
| None (0) | 232 (51.6%) | 218 (48.4%) | 152 (50.0%) | 152 (50.0%) |
| Some (1-4) | 161 (49.7%) | 163 (50.3%) | 108 (50.0%) | 108 (50.0%) |
| Moderate/Severe (5-8) | 5 (25.0%) | 15 (75.0%) | 4 (50.0%) | 4 (50.0%) |
| Emergency Department Location | ||||
| Canada | 136 (35.0%) | 253 (65.0%) | 122 (48.1%) | 132 (51.9%) |
| United States | 262 (64.7%) | 143 (35.3%) | 141 (51.8%) | 132 (48.2%) |
Children administered oral ondansetron were older, had more vomiting episodes in the 24 hours preceding the index visit, and were more likely to be treated in a US-based ED vs. Canada (Table 1). Factors related to oral ondansetron administration in the multivariable logistic regression model are shown in Supplemental Table 1.
Main Propensity Score-Matched Analysis
Oral ondansetron administration was not associated with the 6 principal outcomes in unadjusted analyses (Table 2). A high level of covariate balance was achieved, and all compared characteristics had an absolute standardized difference of less than 0.25 after matching (Figure 2). Propensity score distributions of ondansetron groups showed broad overlap before matching and were very similar after matching (Supplemental Figure 2). The mean standardized difference in propensity scores between children receiving and not receiving oral ondansetron was 0.13 and the mean propensity score (i.e. log odds) of receiving oral ondansetron was 0.51 and 0.49 for matched children who received and did not receive ondansetron, respectively.
Table 2.
Association between oral ondansetron administration and clinical outcomes. Unadjusted and Propensity-Adjusted odds- and rate-ratios represent the increase in odds or rate associated with oral ondansetron received in the emergency department compared to no oral ondansetron.
| Unadjusted Analysis | Propensity-Matched Analysis | |||||
|---|---|---|---|---|---|---|
| Binary Outcomes: N (%) | Received oral ondansetron (N = 398) |
Did not receive oral ondansetron (N = 396) |
Odds-Ratio (95% CI) |
Received oral ondansetron (N = 264) |
Did not receive oral ondansetron (N = 264) |
Adjusted Odds-Ratio (95% CI) |
| IV fluids at the index visit | 37 (9.3%) | 47 (11.9%) | 0.76 (0.48, 1.20) | 20 (7.6%) | 37 (14.0%) | 0.50 (0.29, 0.88) |
| IV fluids following the index visit (up to 72 hours) | 12 (3.0%) | 11 (2.9%) | 1.05 (0.46, 2.40) | 8 (3.1%) | 10 (3.9%) | 0.76 (0.27, 2.17) |
| IV fluids at the index visit and/or up 72 hours post-index visit | 48 (12.1%) | 50 (12.7%) | 0.94 (0.62, 1.44) | 27 (10.4%) | 40 (15.1%) | 0.65 (0.39, 1.10) |
| Hospitalization at the index visit | 9 (2.3%) | 16 (4.0%) | 0.55 (0.24, 1.26) | 4 (1.5%) | 12 (4.6%) | 0.31 (0.09, 1.10) |
| Hospitalization following the index visit (up to 72 hours) | 6 (1.5%) | 6 (1.6%) | *** | 5 (1.8%) | 6 (2.3%) | *** |
| Hospitalization at the index visit and/or up 72 hours post-index visit | 15 (3.8%) | 20 (5.1%) | 0.73 (0.37, 1.44) | 9 (3.3%) | 16 (6.1%) | 0.52 (0.21, 1.28) |
| Count Outcomes: Median (Interquartile Range) |
Rate-Ratio (95% CI) |
Adjusted Rate-Ratio (95% CI) |
||||
| Number of vomiting episodes during the 24 hours following the index ED visit | 0.0 (0.0, 1.0) | 0.0 (0.0, 2.0) | 0.82 (0.62, 1.08) | 0.0 (0.0, 1.1) | 0.0 (0.0, 2.0) | 0.86 (0.63, 1.19) |
| Number of diarrhea episodes during the 24 hours following the index ED visit | 3.0 (1.0, 4.0) | 3.0 (1.0, 5.0) | 1.06 (0.94, 1.21) | 3.0 (1.2, 5.0) | 3.0 (1.0, 4.4) | 1.11 (0.93, 1.32) |
IV, Intravenous; ED, Emergency Department.
Models were not able to be fit to this outcome due to the small number of patients who experienced the outcome.
Figure 2. Results of propensity score matching: absolute standardized differences between demographic and clinical characteristics prior to matching and among matched pairs.
Absolute standardized differences were calculated among all 794 observations (denoted by ‘X’) and among 528 propensity-matched matched observations (denoted by ‘O’). A vertical reference line is plotted at the threshold value of 0.25 standard units. Absolute value standard differences are the absolute differences between the mean values for the oral ondansetron relative to the no oral ondansetron groups of all observations (‘X’s) or matched observations (‘O’s) divided by the pooled standard deviation calculated using all observations. For binary characteristics, the group means are taken to be the proportion p, and the variance as p(1-p).
* Results are combined over 10 imputed datasets. Number of matched pairs varied from 262 pairs/524 patients to 265 pairs/530 patients.
In the propensity-matched analysis, oral ondansetron administration was associated with a reduction in intravenous fluid administration at the index ED visit, just 1 of our 6 principal outcomes (Table 2).
In subset analyses, among children with evidence of dehydration oral ondansetron was associated with 2 of the 6 principal outcomes: intravenous fluids at index ED visit and hospitalization within 72 hours (Supplemental Table 2). Among children without evidence of dehydration, oral ondansetron was not associated with any of the 6 principal outcomes (Supplemental Table 3).
In sensitivity analyses with a stricter matching criterion of 0.1 standard deviation caliper, and a resultant 243 matched pairs, results were unchanged except that ondansetron was associated with reduced overall hospitalizations at the index visit (aOR 0.18; 95% CI 0.03, 0.96) and among dehydrated patients (aOR 0.12; 95% CI 0.02, 0.84).
LIMITATIONS
The data presented are observational and informative to understanding outcomes of routine clinical practice, but unlike clinical trials, must be interpreted as describing associations. The clinical trials from which this planned sub-analysis derived data included only those families who consented for trial participation. There is the potential for recall errors in the number of episodes of vomiting and diarrhea reported by parents before and after the ED visit. Although an objective dehydration scale score was employed, all clinical dehydration scales have suboptimal accuracy.27 We were unable to quantify oral rehydration therapy volumes and home ondansetron administration is unknown. Study participants discharged home could have been admitted to other hospitals and such visits may not have been reported by caregivers on their daily surveys. We did not collect information about nausea or overall satisfaction; both are potentially associated with ondansetron administration and independent of dehydration. While propensity score matching helps approximate randomization, it is possible the groups compared remained unbalanced because of unmeasured characteristics.
DISCUSSION
In this secondary analysis of prospective data from multiple institutions, we observed wide variation between EDs in oral ondansetron use and an association between oral ondansetron administration and improved outcomes and decreased resource use. Overall, ondansetron was administered to nearly half of children with AGE and its use was associated with older age, more vomiting episodes, and treatment in the U.S. In these “real world” settings, treatment with oral ondansetron was associated with the clinically important outcome of reduced intravenous rehydration.
The data we analyzed and reported are detailed, allowing a patient-level description of ED use and outcomes related to oral ondansetron in clinical practice. The administration of ondansetron in the U.S. to greater than 50% of children is consistent with other reports which describe the widespread use of ondansetron in children with AGE.4,28 Despite its broad use, the data regarding the effectiveness in children with AGE outside of clinical trials has been limited. In a study that included 804,000 children assigned an AGE ICD code who were treated in 18 US-based institutions over a ten-year period, despite a considerable increase in ondansetron use, there were no associated reductions in intravenous rehydration use or hospitalizations.7 However, in that study, most children who received intravenous rehydration also received intravenous ondansetron and did not have a period of ondansetron-assisted oral rehydration. In contrast, the extensive patient-level data available in the current study, allowed an evaluation of oral ondansetron use followed by a time interval to ensure an oral rehydration period occurred prior to the decision to administer intravenous fluids, if required. Furthermore, as ondansetron is most likely administered to children with more severe symptoms, we adjusted for clinical features and employed a propensity analysis to account for confounding. This approach allowed us to determine that oral ondansetron administration is associated with a reduction in the frequency of intravenous fluid administration.
Given the high rate of ondansetron use in EDs, particularly in the U.S., it is helpful to better understand which children are most likely to benefit from oral ondansetron. The outcome of the unadjusted analyses revealed no association between oral ondansetron and decreased resource use; propensity score matching allowed more precise estimates of associations. While our data showed oral ondansetron administration was associated with decreased intravenous fluid use at the index visit, it did not show an association with our 5 other principal outcomes: IV fluids within 72 hours, hospitalization at either index visit or within 72 hours, or the frequency of vomiting or diarrhea within 24 hours. Of note, the wide 95%CIs reflect our limited power to assess these outcomes in our propensity model.
In summary, oral ondansetron administration was associated with reduction in use of intravenous fluids at the index ED visit administration. It was not associated with reduced hospitalization, reduced intravenous fluid use or hospitalization within 72 hours, or with increases in vomiting or diarrheal episodes. Ondansetron was widely used, more so in the U.S. compared to Canada, to treat children with AGE and frequent vomiting. Our data imply that oral ondansetron administration followed by oral rehydration therapy appears associated with a reduction in the frequency of index ED visit intravenous fluid administration, but not other analyzed clinical outcomes. To optimize outcomes and resource use, these findings can inform current use. Future research should strive to identify those children most likely to benefit from a dose of oral ondansetron.
Supplementary Material
Funding:
The current sub analysis was unfunded.
The data analyzed were from The Pediatric Emergency Care Applied Research Network (PECARN) Probiotic Study, funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and from the Probiotic Regimen for Outpatient Gastroenteritis Utility of Treatment (PROGUT) study, funded by the Canadian Institutes of Health Research. Dr. Freedman is supported by the Alberta Children’s Hospital Foundation Professorship in Child Health and Wellness.
Footnotes
Conflicts of Interest: Dr. Stephen Freedman has served as a consultant to Redhill Biopharma and has received in kind study drug and placebo from GlaxoSmithKline and Institut Rosell Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Cindy G. Roskind, Department of Emergency Medicine, Columbia University Vagelos College of Physicians & Surgeons, New York, NY, USA..
David Schnadower, Division of Emergency Medicine, Cincinnati Children’s Hospital Medical Center, Cincinnati, and Department of Pediatrics, University of Cincinnati College of Medicine, OHIO, USA.
Cody S. Olsen, Department of Pediatrics, University of Utah, Salt Lake City, UT, USA.
T. Charles Casper, Department of Pediatrics, University of Utah, Salt Lake City, UT, USA.
Phillip I. Tarr, Division of Gastroenterology, Hepatology, & Nutrition, Department of Pediatrics, Washington University in St. Louis School of Medicine, St. Louis, MO, USA.
Karen J. O’Connell, Division of Emergency Medicine, Children's National Health System, Department of Pediatrics, The George Washington School of Medicine and Health Sciences, Washington, DC, USA.
Adam C. Levine, Department of Emergency Medicine, Rhode Island Hospital/Hasbro Children's Hospital and Brown University, Providence, RI, USA..
Naveen Poonai, Department of Pediatrics, Internal Medicine, Epidemiology & Biostatistics, Schulich School of Medicine and Dentistry, London, Canada.
Suzanne Schuh, Division of Pediatric Emergency Medicine, Department of Pediatrics, Hospital for Sick Children, University of Toronto and Research Institute, Hospital for Sick Children, Toronto, Canada.
Alexander J. Rogers, Departments of Emergency Medicine and Pediatrics, University of Michigan, Ann Arbor, MI, USA.
Seema R. Bhatt, Division of Emergency Medicine, Cincinnati Children's Hospital Medical Center, Department of Pediatrics, University of Cincinnati College of Medicine, Cincinnati, OH, USA..
Serge Gouin, Department of Pediatric Emergency Medicine, Centre Hospital Universitaire (CHU) Ste-Justine, Université de Montréal, Montreal, Canada.
Prashant Mahajan, Departments of Emergency Medicine and Pediatrics, University of Michigan, Ann Arbor, MI, USA, and Division of Emergency Medicine, Department of Pediatrics, Children’s Hospital of Michigan, Wayne State University, Detroit, MI, USA.
Cheryl Vance, Departments of Pediatrics and Emergency Medicine, University of California, Davis, School of Medicine, Sacramento, CA, USA..
Katrina Hurley, Division of Pediatric Emergency Medicine, IWK Health Center, Halifax, NS, Canada.
Ken J. Farion, Departments of Pediatrics and Emergency Medicine, Children’s Hospital of Eastern Ontario, University of Ottawa, Ottawa, Canada.
Robert E. Sapien, Department of Emergency Medicine, University of New Mexico, Albuquerque, NM, USA.
Stephen B. Freedman, Sections of Pediatric Emergency Medicine and Gastroenterology, Departments of Pediatrics and Emergency Medicine, Alberta Children's Hospital, Alberta Children's Hospital Research Institute, Cumming School of Medicine, University of Calgary, Calgary, AB, Canada.
References
- 1.Fugetto F, Filice E, Biagi C, Pierantoni L, Gori D, Lanari M. Single-dose of ondansetron for vomiting in children and adolescents with acute gastroenteritis-an updated systematic review and meta-analysis. Eur J Pediatr. 2020;179(7):1007–1016. [DOI] [PubMed] [Google Scholar]
- 2.Rutman L, Klein EJ, Brown JC. Clinical Pathway Produces Sustained Improvement in Acute Gastroenteritis Care. Pediatrics. 2017;140(4). [DOI] [PubMed] [Google Scholar]
- 3.Freedman SB, Adler M, Seshadri R, Powell EC. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006;354(16):1698–1705. [DOI] [PubMed] [Google Scholar]
- 4.Benary D, Lozano JM, Higley R, Lowe D. Ondansetron Prescription Is Associated With Reduced Return Visits to the Pediatric Emergency Department for Children With Gastroenteritis. Ann Emerg Med. 2020;76(5):625–634. [DOI] [PubMed] [Google Scholar]
- 5.Kharbanda AB, Hall M, Shah SS, et al. Variation in resource utilization across a national sample of pediatric emergency departments. J Pediatr. 2013;163(1):230–236. [DOI] [PubMed] [Google Scholar]
- 6.Freedman SB, Tung C, Cho D, Rumantir M, Chan KJ. Time-series analysis of ondansetron use in pediatric gastroenteritis. J Pediatr Gastroenterol Nutr. 2012;54(3):381–386. [DOI] [PubMed] [Google Scholar]
- 7.Freedman SB, Hall M, Shah SS, et al. Impact of increasing ondansetron use on clinical outcomes in children with gastroenteritis. JAMA Pediatr. 2014;168(4):321–329. [DOI] [PubMed] [Google Scholar]
- 8.Freedman SB, Soofi SB, Willan AR, et al. Oral Ondansetron Administration to Nondehydrated Children With Diarrhea and Associated Vomiting in Emergency Departments in Pakistan: A Randomized Controlled Trial. Ann Emerg Med. 2019;73(3):255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freedman SB, Soofi SB, Willan AR, et al. Oral Ondansetron Administration to Dehydrated Children in Pakistan: A Randomized Clinical Trial. Pediatrics. 2019;144(6). [DOI] [PubMed] [Google Scholar]
- 10.Keren R Ondansetron for acute gastroenteritis: a failure of knowledge translation. JAMA Pediatr. 2014;168(4):308–309. [DOI] [PubMed] [Google Scholar]
- 11.Freedman SB, Williamson-Urquhart S, Farion KJ, et al. Multicenter Trial of a Combination Probiotic for Children with Gastroenteritis. N Engl J Med. 2018;379(21):2015–2026. [DOI] [PubMed] [Google Scholar]
- 12.Freedman SB, Williamson-Urquhart S, Schuh S, et al. Impact of emergency department probiotic treatment of pediatric gastroenteritis: study protocol for the PROGUT (Probiotic Regimen for Outpatient Gastroenteritis Utility of Treatment) randomized controlled trial. Trials. 2014;15:170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schnadower D, Tarr PI, Casper TC, et al. Lactobacillus rhamnosus GG versus Placebo for Acute Gastroenteritis in Children. N Engl J Med. 2018;379(21):2002–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnadower D, Tarr PI, Casper TC, et al. Randomised controlled trial of Lactobacillus rhamnosus (LGG) versus placebo in children presenting to the emergency department with acute gastroenteritis: the PECARN probiotic study protocol. BMJ Open. 2017;7(9):e018115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman JN, Goldman RD, Srivastava R, Parkin PC. Development of a clinical dehydration scale for use in children between 1 and 36 months of age. J Pediatr. 2004;145(2):201–207. [DOI] [PubMed] [Google Scholar]
- 16.Freedman SB, Eltorky M, Gorelick M, Pediatric Emergency Research Canada Gastroenteritis Study G. Evaluation of a gastroenteritis severity score for use in outpatient settings. Pediatrics. 2010;125(6):e1278–1285. [DOI] [PubMed] [Google Scholar]
- 17.Schnadower D, Tarr PI, Gorelick MH, et al. Validation of the modified Vesikari score in children with gastroenteritis in 5 US emergency departments. J Pediatr Gastroenterol Nutr. 2013;57(4):514–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haukoos JS, Lewis RJ. The Propensity Score. JAMA. 2015;314(15):1637–1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Austin PC. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivariate Behav Res. 2011;46(3):399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurth T, Walker AM, Glynn RJ, et al. Results of multivariable logistic regression, propensity matching, propensity adjustment, and propensity-based weighting under conditions of nonuniform effect. Am J Epidemiol. 2006;163(3):262–270. [DOI] [PubMed] [Google Scholar]
- 21.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williamson EJ, Forbes A. Introduction to propensity scores. Respirology. 2014;19(5):625–635. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC. Comparing paired vs non-paired statistical methods of analyses when making inferences about absolute risk reductions in propensity-score matched samples. Stat Med. 2011;30(11):1292–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rubin DB. Using Propensity Scores to Help Design Observational Studies: Application to the Tobacco Litigation. Health Services and Outcomes Research Methodology. 2001;2(3):169–188. [Google Scholar]
- 25.Austin PC. Propensity Scored Matching in the Cardiovascular Surgery Literature from 2004-2006: A systematic review and suggestions for Improvement. J Thor Cardiovasc Surg. 2007:134(5): 1128–1135.e3. [DOI] [PubMed] [Google Scholar]
- 26.Rubin D Multiple Imputation for Nonresponse in Surveys. New York: John Wiley & Sons; 1987. [Google Scholar]
- 27.Freedman SB, Vandermeer B, Milne A, Hartling L, Pediatric Emergency Research Canada Gastroenteritis Study Group. Diagnosing clinically significant dehydration in children with acute gastroenteritis using noninvasive methods: a meta-analysis. J Pediatr. 2015;166(4):908–916 e901-906. [DOI] [PubMed] [Google Scholar]
- 28.McLaren SH, Yim RB, Fleegler EW. Impact of Ondansetron Prescription on Return Emergency Department Visits Among Children with Acute Gastroenteritis. Pediatr Emerg Care. 2019. Epub Ahead of print Sep 12. doi: 10.1097/PEC.0000000000001907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.