Abstract
Background and Purpose:
Cerebrovascular disease (CVD) pathologies including vessel disease (atherosclerosis, arteriolosclerosis, cerebral amyloid angiopathy) and tissue injury (macro-, micro-infarcts) each contribute to Alzheimer’s and other forms of dementia. CVD is often a complex mix of neuropathologies, with little known about the frequencies of differing combinations or their associations with cognition.
Methods:
We investigated 32 possible CVD combinations (3 types of vessel disease, 2 types of tissue injury) using autopsy data from 1,474 decedents (~88 years at death; 65% female) of Rush Alzheimer’s Disease Center studies. We determined frequencies of all 32 CVD combinations and their relationships with global and domain-specific cognitive decline using mixed effect models adjusted for demographics, neuropathologies, time before death, and interactions of these variables with time.
Results:
Of the 1,184 decedents with CVD neuropathology (80% of the total sample), 37% had a single CVD (67–148 decedents/group) while 63% had mixed CVD profiles (11–54 decedents/group). When considered as two distinct groups, the mixed CVD profile group (but not the single CVD profile group) showed faster cognitive decline across all domains assessed compared to decedents without CVD neuropathology. Most mixed CVD profiles, especially those involving both atherosclerosis and arteriolosclerosis, showed faster cognitive decline than any single CVD profile considered alone; specific mixed CVD profiles differentially associated with individual cognitive domains.
Conclusions:
Mixed CVD, more common than single CVD, is associated with cognitive decline, and distinct mixed CVD profiles show domain-specific associations with cognitive decline. CVD is not monolithic, but consists of heterogenous person-specific combinations with distinct contributions to cognitive decline.
Keywords: cerebrovascular disease, neuropathology, cognitive decline
Summary
This study sheds light on the contributions of the most prevalent CVD combinations with cognition. Results suggest that CVD is not monolithic, but consists of heterogeneous person-specific combinations that have distinct contributions to cognitive decline.
Introduction
Cerebrovascular disease (CVD) pathology increases with increasing age and is a common neuropathological comorbidity in neurodegenerative disorders including Alzheimer’s disease (AD). While reports suggest anywhere between 33–75% of decedents found to have AD-related neuropathology also exhibit CVD including vessel disease (arteriolosclerosis, atherosclerosis, and cerebral amyloid angiopathy, CAA) and/or tissue injury (macro- and micro-infarcts) [1–3], little is known about the most common frequencies and combinations of CVD. This is particularly important given that investigators are beginning to suggest that it is not simply the number of pathologies that is important to cognitive aging and dementia, but the type of pathologies that co-exist [1, 4–6].
While we and others [7–17] have outlined differential relationships between individual CVD-related neuropathologies and cognition, to our knowledge, few if any studies have systematically examined frequencies of 32 of the many possible CVD combinations of vessel disease and tissue damage pathology to determine the most common combinations prior to assessing for associations with cognition. Thus, the first aim of this study was to determine the most prevalent CVD pathology profiles by systematically examining the 32 possible CVD combinations of 3 types of vessel disease (arteriolosclerosis, atherosclerosis, and CAA) and 2 types of tissue injury (macro- and micro-infarcts) found in over 1,450 decedents of the Rush Alzheimer’s Disease Center’s community-based clinical-neuropathological cohort studies. Our second aim was to determine the relationships between resulting CVD combinations and change in global and domain-specific cognitive functions. Based on the literature relating individual CVD neuropathologies and cognition [7–17], we hypothesized that when considered in the same model, mixed CVD profiles would be those most often related to cognitive decline, particularly perceptual speed, with profiles involving arteriolosclerosis and/or atherosclerosis with additional gross tissue damage differentially associating with domain-specific declines in working memory and visuospatial abilities.
Materials and Methods
Data can be requested at https://www.radc.rush.edu
Participants
Described in detail elsewhere [18–21], subjects of this research were participants in prospective, community-based, clinical-pathologic cohort studies of aging, either the Religious Orders Study (1994-present), the Rush Memory and Aging Project (1997-present), or the Minority Aging Research Study (2004-present). An Institutional Review Board of Rush University Medical Center approved all studies and participants gave written informed consent for all aspects of the study in accordance with the Declaration of Helsinki. All participants included analyses for this project also signed an anatomic gift act for donation of the brain at the time of death. All studies follow the same recruitment, biospecimen and data collection as well as clinical and neuropathological processing and analysis. Additionally, STROBE guidelines for observational studies were followed.
Neuropathology
At the time of these analyses, postmortem samples were obtained from 1,528 decedents; details of brain autopsy procedures described elsewhere [15, 19, 22]. Briefly, the post-mortem neuropathological evaluation included a uniform structured assessment with procedures that include and extend those outlined by the pathologic dataset recommended by the National Alzheimer’s Disease Coordinating Center. Evaluations were performed blinded to clinical data and reviewed by a board-certified neuropathologist. Of the 1,528 decedents with available post-mortem samples, 1,474 had all relevant data as outlined below and were included in our analytic sample (Supplemental Table 1).
Cerebrovascular Disease Outcomes of Interest
Vessel Disease –
We quantified three types of vessel disease: arteriolosclerosis, atherosclerosis, and cerebral amyloid angiopathy (CAA). We used the term arteriolosclerosis to describe the histological changes commonly found in the small vessels of the brain in aging including intimal deterioration, smooth muscle degeneration, and fibrohyalinotic thickening of arterioles with consequent narrowing of the vascular lumen. We evaluated the vessels of the anterior basal ganglia with a semi-quantitative grading system, i.e., 0 (none) to 7 (occluded) and compressed these levels into none (0), mild (1), moderate (2), and severe (3) as previously reported [23–25]. The degree of intracranial atherosclerosis of the circle of Willis vessels was recorded on a semi-quantitative scale, i.e., none (0), mild (1), moderate (2), and severe (3), as previously described [26].
CAA was quantified in mid-frontal, mid-temporal, parietal, and calcarine cortices [9]. For each region, meningeal and parenchymal vessels were assessed for amyloid deposition and scored from 0 (no deposition), 1 (scattered segmental but no circumferential deposition), 2 (circumferential deposition up to 10 vessels), 3 (circumferential deposition up to 75% of the total vessels of the region), to 4 (circumferential deposition on more than 75% of the total vessels of the region). The CAA variable used for the current analyses grouped participants by no/mild or moderate/severe regional average of the maximum of the meningeal and parenchymal CAA scores as follows: no (average=0), mild (average<1.5), moderate (1.5≤average≤2.5), and severe (average>2.5).
Across all three types of vessel disease, moderate to severe categorizations were combined and considered “positive” for vessel disease whereas no/none to mild were combined and considered “negative” for vessel disease.
Tissue Injury –
We quantified two types of tissue damage: gross infarcts, i.e., macroinfarcts, and microinfarcts coding for none versus ≥1 chronic infarcts regardless of location and/or size, as relevant.
Macroinfarct examination documented age (acute/subacute/chronic), size, and location (side/region) of macroinfarcts visible to the naked eye on fixed slabs. All grossly visualized and suspected infarcts were dissected for histologic confirmation [14, 15].
Microinfarcts were defined as any chronic infarct seen by microscopic examination on 6μm paraffin-embedded sections, stained with hematoxylin/eosin and not identified by gross inspection. A minimum of nine regions in one hemisphere were examined (six cortical regions: midfrontal, middle temporal, entorhinal, hippocampal, inferior parietal, and anterior cingulate cortices; two subcortical regions: anterior basal ganglia and thalamus; and midbrain) [13].
Cognitive Functioning
Details of the annual cognitive evaluation have been described in numerous publications [19, 21, 27]. Briefly, a global composite cognitive function score was derived from performance on 19 cognitive tests including tests of episodic, semantic, and working memory as well as perceptual organization/visuospatial abilities (hereafter referred to as visuospatial abilities) and perceptual speed. The score was created by converting raw scores on all cognitive tests to standard (z) scores using the mean and standard deviation for each from the baseline evaluation. A participant’s standard scores were then averaged across all tests to yield a single composite score summarizing level of global cognitive function. We used similar procedures to create five domain-specific cognitive composite scores. The use of summary measures decreased ceiling effects and other sources of measurement error, and have been used in many previous studies [18].
Covariates
Date of birth, sex, and years of education were obtained from the baseline interview; date of death was obtained at autopsy. NIA AD-Reagan criteria relying on AD-related plaque and tangle burden [28] were used to determine the high/intermediate (1) or low/no (0) likelihood of a postmortem diagnosis of AD. Hippocampal sclerosis was evaluated unilaterally in a coronal section of the midhippocampus at the level of the lateral geniculate body, and graded as absent or present based on severe neuronal loss and gliosis in CA1 and/or subiculum [26]. TDP-43 immunohistochemistry was performed on six sections of the brain: amygdala, hippocampus (CA1 and dentate), and midfrontal, midtemporal, and entorhinal cortices using a rat phosphorylated monoclonal TAR5P-1D3 (pS409/410; 1:100; Ascenion, Munich, Germany) TDP-43 antibody. Three stages of TDP-43 distribution were recognized (stage 1, localized to amygdala; stage 2, extension to hippocampus and/or entorhinal cortex; stage 3, extension to the neocortex), and the severity of the TDP-43 cytoplasmic inclusions in neurons and glia were rated on a 6-point scale [26, 29]. Lastly, neocortical Lewy bodies (LB) were identified on sections stained with alpha-synuclein including the midfrontal, midtemporal, and inferior parietal cortices.
Statistical Analyses
All analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC). Characteristics of the final analytic sample were determined, and those with CVD neuropathology (single or mixed) were compared to those with little to no CVD neuropathology using parametric and non-parametric statistics as indicated across demographic, cognitive, AD-related and other neuropathologic variables.
To assess associations of CVD profiles and cognitive decline, we employed linear mixed effects regression models of longitudinal global and domain-specific cognition (separately) with fixed and random effects for level and time, with years before death defining the time scale. Given that CVD profiles are mutually exclusive, we did not run separate mixed effects regression models per profile; instead, we examined the association of each CVD profile, together in the same model, with change in cognition by employing dummy variables for each CVD profile (or group of CVD profiles) and interactions with time. Individuals without any CVD neuropathology, i.e., little to no vessel disease and no tissue damage at autopsy, were the reference group across all analyses. Models also included terms for age, sex, education, years before death, and the interaction of the time scale (years before death) with all demographic variables, as well as AD-related neuropathology and other neuropathologies including hippocampal sclerosis, TDP43, and LB and their interactions with time.
All p-values are two-sided and the statistical significance was set at p<0.05 for all demographic comparisons; we corrected for multiple comparisons when assessing between-group differences in cognitive domain scores (n=5) as well as other neuropathologies (n=4) resulting in p-values≤0.01. When assessing associations of CVD profiles and global cognitive decline, p<0.05 was used in addition to 95% confidence intervals (CI); however, in addition to 95% CI, we corrected for multiple comparisons when assessing associations of CVD profiles and cognitive domain scores resulting in a p≤0.01.
Results
A total of 1,474 persons had all available data at the time of these analyses; age at death (mean=89.0) ranged between 65.9 and 108.2 years. Participants were highly educated, averaging 16.2 years of education. We created a matrix of all possible CVD combinations of the three forms of vessel disease and the two forms of tissue damage. This resulted in a total of 32 CVD combinations or profiles: 5 single CVD neuropathology profiles, 26 profiles representing the various combinations of mixed CVD neuropathologies, and 1 profile with no CVD neuropathology, i.e., little to no vessel disease and no tissue damage at autopsy, which served as the reference group. The 32 combinations are shown in Table 1.
Table 1.
Decedent frequency for all possible CVD combinations (N=1,474)
| Tissue Damage (4 combinations) | |||||
|---|---|---|---|---|---|
| NONE | Micro | Gross | Micro+Gross | ||
| Vessel Disease (8 combinations) | NONE | 290 | 67 | 83 | 43 |
| Arteriolosclerosis (ART) | 69 | 26 | 26 | 28 | |
| Atherosclerosis (ATH) | 75 | 22 | 30 | 27 | |
| Cerebral Amyloid Angiopathy (CAA) | 148 | 42 | 41 | 30 | |
| ART+ATH | 52 | 13 | 54 | 46 | |
| ART+CAA | 52 | 10 | 12 | 18 | |
| ATH+CAA | 31 | 11 | 26 | 14 | |
| ART+ATH+CAA | 22 | 16 | 28 | 22 | |
NOTE: Micro=microinfarcts, Gross=gross infarcts. Black cell=reference group; Dark Grey cells=specific profiles representing CVD combinations of ≥30 decedents; Light Grey cells =aggregated less common mixed profiles with limited representation, i.e., 10 to 28 decedents/group.
When individuals with any CVD neuropathology were combined into one group (n=1,184; 80% of the total sample) and compared to individuals with little to no CVD neuropathology (i.e., the reference group; n=290), individuals with CVD neuropathology were on average 2 years older at death [t(1472)=−5.84, p<0.0001]; although the age distributions overlap. There were no other differences in participant characteristics (all p-values≥0.075) between those with and without CVD neuropathology (Table 2); however, those with CVD neuropathology scored lower on global cognition (p<0.0001) and all composite measures of cognition compared to those without CVD neuropathology – all cognitive domains survived multiple comparison correction (all p-values≤0.01) except visuospatial ability (p=0.04). After adjusting for age at death, those with CVD neuropathology also had a higher burden of neocortical Lewy bodies compared to those without CVD neuropathology (p=0.034, although this did not survive multiple comparison correction).
Table 2.
Analytic sample characteristics stratified by presence or absence of CVD
| Analytic Sample (N=1474) | CVD Groups (n=1184) | Reference Group (n=290) | |
|---|---|---|---|
| Age at death (years)*** | 89.0±6.7 | 89.5±6.6 | 86.9±6.9 |
| Sex (% female) | 67.3% | 66.8% | 69.3% |
| Education (years) | 16.2±3.6 | 16.1±3.6 | 16.5±3.5 |
| Race (% white) | 94.9% | 95.5% | 92.7% |
| CVD-Related Neuropathology | |||
| Arteriolosclerosis (mod to severe, %) | 33.5% | 41.7% | 0% |
| Atherosclerosis (mod to severe, %) | 33.1% | 41.3% | 0% |
| CAA (mod to severe, %) | 35.4% | 44.1% | 0% |
| Macroinfarcts (one or more, %) | 35.9% | 44.6% | 0% |
| Microinfarcts (one or more, %) | 29.4% | 36.6% | 0% |
| AD-Related and Other Neuropathology | |||
| NIA-Reagan Diagnosis of AD (high or intermediate, %) | 64.8% | 66.6% | 57.2% |
| Hippocampal Sclerosis (present with CA1 region affected, %) | 8.1% | 8.8% | 5.2% |
| TDP-43 (neocortical, %) | 13.5% | 14.7% | 8.8% |
| Neocortical Lewy Bodies (neocortical, %)* | 12.3% | 11.5% | 15.8% |
| Cognitive Scores | |||
| Global Cognition*** | −0.12±0.7 | −0.16±0.7 | 0.04±0.6 |
| Episodic Memory*** | −0.17±0.8 | −0.21±0.8 | 0.02±0.7 |
| Semantic Memory*** | −0.15±0.9 | −0.18±0.9 | −0.01±0.9 |
| Working Memory** | −0.03±0.8 | −0.05±0.8 | 0.08±0.8 |
| Visuospatial Ability* | 0.02±0.8 | −0.002±0.8 | 0.10±0.7 |
| Perceptual speed*** | −0.18±0.9 | −0.23±0.9 | 0.007±0.9 |
NOTE: all values equal mean±standard deviation unless otherwise noted
p<0.05,
p≤0.01,
p<0.0001 for comparisons between CVD and Reference groups; comparisons involving AD-Related and Other Neuropathology were adjusted for age at death.
Single versus Mixed Cerebrovascular Disease
Eighty percent of decedents had CVD neuropathology; of these 1,184 decedents, 742 (63%) had mixed CVD neuropathology compared to 442 (37%) with only one CVD neuropathology. The 5 single CVD profiles included between 67 and 148 decedents per group with the most common being CAA; the 26 mixed CVD profiles included between 11 and 54 decedents per group with the most common combination being arteriolosclerosis combined with atherosclerosis and macroinfarcts. When individuals with either a single CVD neuropathology or a mixed CVD profile were combined into two separate groups (single and mixed, respectively) and compared (separately) to the reference group, the single CVD neuropathology group only differed from the reference on age at death (p=0.01). By contrast, the mixed CVD neuropathology group differed from the reference group on all participant (p-values≤0.03) and cognitive (p-values≤0.01) characteristics with the exception of sex (p=0.60); furthermore, after adjusting for age at death, this group also showed a higher likelihood of NIA-Reagan AD diagnosis compared to the reference group (p=0.005; Supplemental Table 2).
When the single CVD neuropathology group was compared to the mixed CVD neuropathology group, age at death was the only significant participant characteristic difference [t(861)=4.82, p<0.0001] (Supplemental Table 2). The mixed CVD neuropathology group scored lower than the single CVD neuropathology group on all cognitive composite measures (all p-values≤0.0009) with the exception of visuospatial ability (p=0.08), and, after adjusting for age at death, had a higher likelihood of an NIA-Reagan AD diagnosis (p=0.004), but no significant differences in hippocampal sclerosis, TDP-43, or neocortical LB burden (p-values≥0.12).
Single versus Mixed CVD Groups and Cognition
When individuals with single and mixed CVD profiles were entered as two separate groups into the same comprehensive model with global (Figure 1) and domain-specific cognition (Supplemental Table 3) as separate outcomes, only the mixed CVD profile group showed significant and negative associations with cognitive decline, regardless of the score assessed. Thus, decedents with evidence of mixed CVD pathology had faster rates of decline before death in global cognition, episodic and non-episodic memory, as well as visuospatial abilities and processing speed. Additionally, while all four neuropathology covariates associated with declines in global cognition (p-values<0.001), differential involvement was seen for some cognitive domains (Supplemental Table 3).
Figure 1.
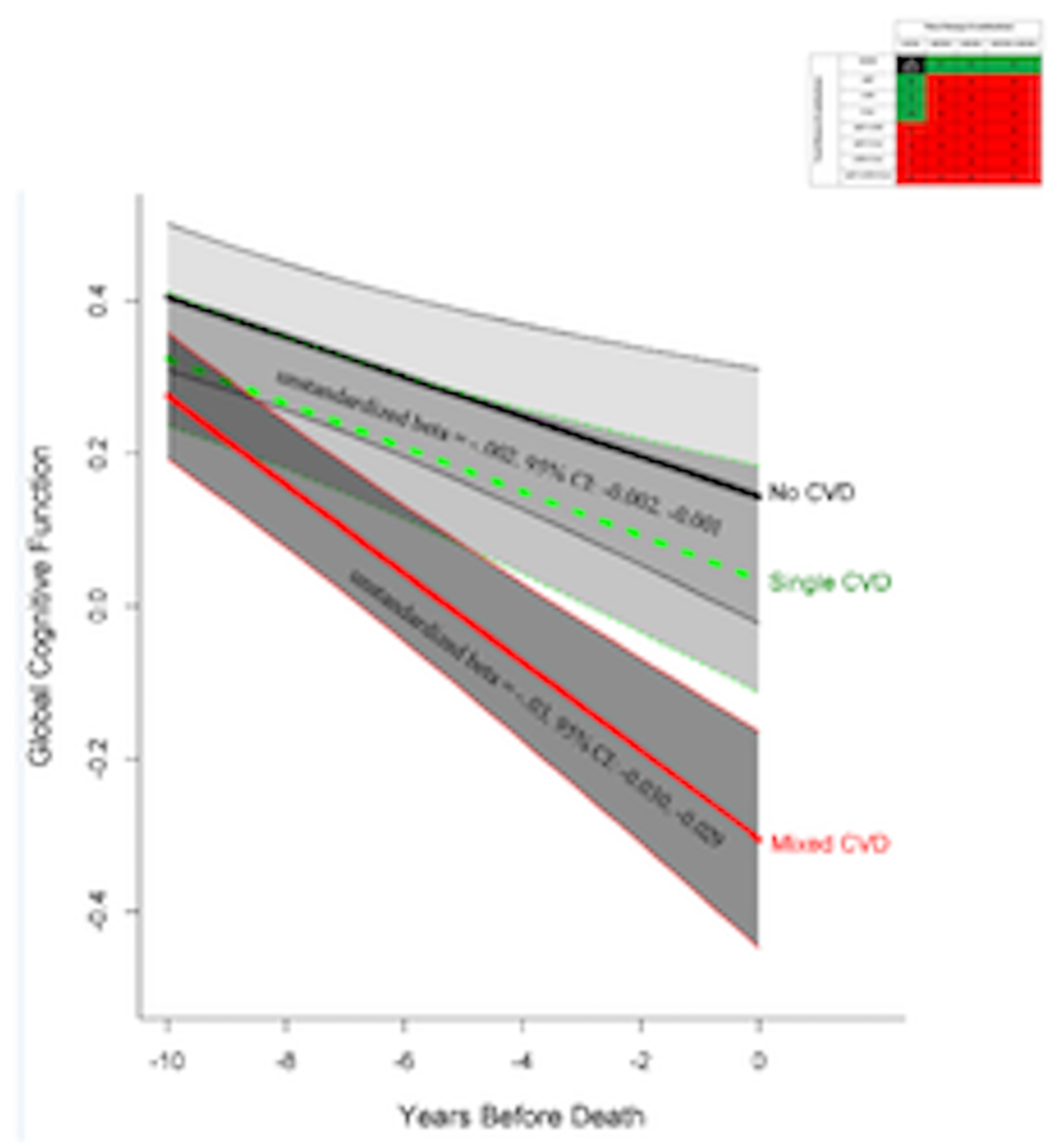
The association of single (green) and mixed (red) CVD profile groups with global cognitive decline compared to the reference group (black). The model also included terms for age, sex, education, time (years before death), AD-Reagan diagnosis, hippocampal sclerosis, TDP43, Lewy bodies, and their interactions with time. Unstandardized beta coefficients and 95% confidence intervals are provided with significance represented by the solid line for terms of interest; all lines display 95% confidence intervals around point estimates.
Cerebrovascular Disease Combinations
Of the 26 mixed CVD combinations, 10 had sufficient numbers (≥30 decedents represented per group) for consideration as specific mixed CVD profiles while the remaining 16 had fewer numbers (≤28 decedents) and were aggregated into six less common mixed CVD profiles as outlined in Table 3.
Table 3.
Associations between cerebrovascular disease profiles and declines in global cognition
| Global Cognition unstandardized beta coefficient of interaction of profile group with time (95% CI) |
||
|---|---|---|
| Single CVD profiles | Cerebral Amyloid Angiopathy (CAA) | −0.02 (−0.020,−0.019) |
| GROSS infarcts | −0.01 (−0.010,−0.009) | |
| Atherosclerosis (ATH) | −0.003 (−0.003,−0.002) | |
| Arteriolosclerosis (ART) | 0.006 (0.005,0.006) | |
| MICRO infarcts | 0.03 (0.02,0.03) | |
| Specific mixed CVD profiles | ATH+ART+GROSS | −0.05 (−0.050,−0.049) |
| ATH+ART | −0.04 (−0.040,−0.039) | |
| ART+CAA | −0.02 (−0.020,−0.019) | |
| ATH+ART+GROSS+MICRO | −0.04 (−0.040,−0.039) | |
| GROSS+MICRO | −0.005 (−0.005,−0.004) | |
| CAA+GROSS | −0.005 (−0.005,−0.004) | |
| CAA+MICRO | −0.01 (−0.010,−0.009) | |
| ATH+CAA | 0.007 (0.006,0.007) | |
| ATH+GROSS | −0.05 (−0.051,−0.048) | |
| CAA+GROSS+MICRO | −0.007 (−0.007,−0.006) | |
| Aggregated less common mixed CVD profiles | ART+any tissue damage combination | −0.03 (−0.030,−0.029) |
| ALL VESSEL only, +GROSS or +GROSS+MICRO | −0.07 (−0.07,−0.06) | |
| ATH+CAA+any tissue damage combination | −0.06 (−0.06,−0.05) | |
| ATH+MICRO with or w/o GROSS | −0.03 (−0.030,−0.029) | |
| ART+CAA+any tissue damage combination | −0.03 (−0.030,0.029) | |
| MICRO+ART+ATH alone or +CAA | −0.02 (−0.020,−0.019) |
NOTE: Specific mixed CVD profiles listed in order of descending sample size. The model included terms for each of the 21 profiles listed in the table and their interactions with time (years before death), adjusting for age, sex, education, NIA-Reagan AD diagnosis, hippocampal sclerosis, TDP-43, neocortical Lewy bodies and their interactions with time. Bolded entries signify significance based on 95% confidence intervals (CI) and p-values≤0.05.
Cerebrovascular Disease Combinations and Global Cognition
When single, specific, and the less common (aggregated) CVD profiles were entered into one mixed effects regression model, results revealed eight mixed profiles (but no single CVD profiles) independently associated with global cognitive decline (see Table 3 for estimate details). Of the eight mixed CVD profiles significantly related to global cognitive decline, half (four) involved both atherosclerosis and arteriolosclerosis with or without tissue injury; the remaining four profiles included decedents with either atherosclerosis and tissue injury (2 groups) or arteriolosclerosis with tissue injury (2 groups). All four neuropathology covariates associated with declines in global cognition (unstandardized beta coefficients ranged from −0.06 to −0.03; p-values<0.001).
Specific CVD Profile Combinations and Cognitive Domain Scores
While Table 4 provides specific model details, we outline a few notable findings of mixed effects regression analyses in which all CVD profiles were entered into a single model with each cognitive domain as a separate outcome. First, similar to global cognitive decline, decedents having profiles including atherosclerosis and arteriolosclerosis with or without tissue injury most consistently had declines across multiple domains (i.e., four or five of the five domains assessed) although only those related to working memory and perceptual speed (Figure 2) survived multiple comparison correction. Second, decedents with single CVD pathologies (either vessel disease or tissue injury) had little overall decline in domain-specific cognitive functions, with the exception of persons with CAA alone who had significant decline in semantic memory and visuospatial ability survive multiple comparison correction. Third, those with profiles including all 3 vessel diseases with or without tissue injury have among the greatest magnitude of decline across both episodic and non-episodic domains. Finally, and perhaps most importantly, there is abundant heterogeneity not only in the CVD pathology profiles, but in their associations with domain-specific cognitive decline. All four neuropathology covariates associated with declines in episodic and semantic memory (p-values<0.001); all but hippocampal sclerosis (p-values≥0.06) associated with working memory and perceptual speed (p-values≤0.004) while only NIA-Reagan AD diagnosis and neocortical Lewy Bodies associated with visuospatial ability (p-values≤0.001).
Table 4.
Associations between specific CVD profiles and decline in domain-specific cognitive functioning
| Domain-specific Cognitive Composite Scores unstandardized beta coefficient of interaction of profile group with time (95% CI) |
||||||
|---|---|---|---|---|---|---|
| Episodic Memory | Semantic Memory | Working Memory | Visuospatial Ability | Perceptual Speed | ||
| Single CVD profiles | Cerebral Amyloid Angiopathy (CAA) | −0.01 (−0.010,−0.009) | −0.03 (−0.030,−0.029) | −0.02 (−0.020,−0.019) | −0.02 (−0.020,−0.019) | −0.02 (−0.020,−0.019) |
| GROSS infarcts | −0.01 (−0.010,−0.009) | −0.02 (−0.020,−0.019) | −0.02 (−0.020,−0.019) | −0.005 (−0.005,−0.004) | −0.01 (−0.010,−0.009) | |
| Atherosclerosis (ATH) | 0.005 (0.004,0.005) | −0.01 (−0.010,−0.009) | −0.01 (−0.010,−0.009) | −0.01 (−0.010,−0.009) | −0.02 (−0.020,−0.019) | |
| Arteriolosclerosis (ART) | 0.01 (0.009,0.010) | −0.004 (−0.004,−0.003) | −0.0005 (−0.005,−0.004) | −0.001 (−0.001,−0.0009) | −0.007 (−0.007,−0.006) | |
| MICRO infarcts | 0.04 (0.039,0.040) | 0.02 (0.019,0.020) | 0.02 (0.019,0.010) | −0.003 (−0.003,−0.002) | 0.002 (0.0019,0.0010) | |
| Specific mixed CVD profiles | ATH+ART+GROSS | −0.04 (−0.040,−0.039) | −0.04 (−0.041,−0.038) | −0.05 (−0.050,−0.049) | −0.03 (−0.030,−0.029) | −0.04 (−0.040,−0.039) |
| ATH+ART | −0.04 (−0.041,−0.038) | −0.05 (−0.051,−0.048) | −0.02 (−0.020,−0.019) | −0.03 (−0.030,−0.029) | −0.05 (−0.051,−0.048) | |
| ART+CAA | −0.03 (−0.030,−0.029) | −0.03 (−0.031,−0.028) | −0.02 (−0.020,−0.019) | −0.01 (−0.010,−0.009) | −0.05 (−0.050,−0.049) | |
| ATH+ART+GROSS+MICRO | −0.05 (−0.051,−0.048) | −0.03 (−0.031,−0.028) | −0.02 (−0.020,−0.019) | −0.03 (−0.030,−0.029) | −0.04 (−0.041,−0.038) | |
| GROSS+MICRO | −0.007 (−0.007,−0.006) | −0.01 (−0.010,−0.009) | −0.02 (−0.020,−0.019) | 0.0001 (0.00009,0.0001) | −0.02 (−0.020,−0.019) | |
| CAA+GROSS | 0.008 (0.007,0.008) | 0.008 (0.007,0.008) | −0.02 (−0.020,−0.019) | −0.02 (−0.020,−0.019) | −0.006 (−0.006,−0.005) | |
| CAA+Micro | −0.01 (−0.010,−0.009) | −0.03 (−0.031,−0.028) | 0.009 (0.008,0.009) | −0.008 (−0.008,−0.007) | −0.0009 (−0.0009,−0.0008) | |
| ATH+CAA | 0.01 (−0.010,−0.009) | −0.01 (−0.010,−0.009) | 0.006 (0.005,0.006) | −0.002 (−0.0020,−0.0019) | −0.02 (−0.020,−0.019) | |
| ATH+GROSS | −0.06 (−0.06,−0.05) | −0.04 (−0.042,−0.037) | −0.008 (−0.008,−0.007) | −0.04 (−0.041,−0.038) | −0.07 (−0.07,−0.06) | |
| CAA+GROSS+MICRO | −0.007 (−0.007,−0.006) | −0.03 (−0.031,−0.028) | −0.009 (0.01,0.59) | 0.0006 (0.01,0.97) | −0.01 (−0.010,−0.009) | |
| Aggregated less common mixed CVD profiles | ART+any tissue damage | −0.02 (−0.020,−0.019) | −0.05 (−0.050,−0.049) | −0.03 (−0.030,−0.029) | −0.03 (−0.030,−0.029) | −0.02 (−0.020,−0.019) |
| ALL VESSEL only, +GROSS or +GROSS+MICRO | −0.07 (−0.071,−0.068) | −0.09 (−0.09,−0.08) | −0.06 (−0.06,−0.05) | −0.03 (−0.030,−0.029) | −0.07 (−0.071,−0.068) | |
| ATH+CAA+any tissue damage | −0.05 (−0.051,−0.048) | −0.09 (−0.09,−0.08) | −0.04 (−0.040,−0.039) | −0.03 (−0.030,−0.029) | −0.07 (−0.072,−0.067) | |
| ATH+MICRO with or w/o GROSS | −0.04 (−0.040,−0.039) | −0.03 (−0.031,−0.028) | −0.01 (−0.010,−0.009) | −0.03 (−0.030,−0.029) | −0.02 (−0.020,−0.019) | |
| ART+CAA+any tissue damage | −0.03 (−0.031,−0.028) | −0.03 (−0.031,−0.028) | −0.03 (−0.030,−0.029) | −0.04 (−0.040,−0.039) | −0.04 (−0.041,−0.038) | |
| MICRO+ART+ATH alone or +CAA | 0.008 (0.007,0.008) | −0.07 (−0.074,−0.065) | −0.01 (−0.010,−0.009) | −0.07 (−0.072,−0.067) | −0.07 (−0.072,−0.067) | |
NOTE: Specific mixed CVD profiles listed in order of descending sample size. The model included terms for each of the 21 profiles listed in the table and their interactions with time (years before death), adjusting for age, sex, education, NIA-Reagan diagnosis, hippocampal sclerosis, TDP-43, neocortical Lewy bodies and their interactions with time. Bolded entries represent significance based on 95% confidence intervals (CI) combined with multiple comparison correction p-value≤0.01
Figure 2.
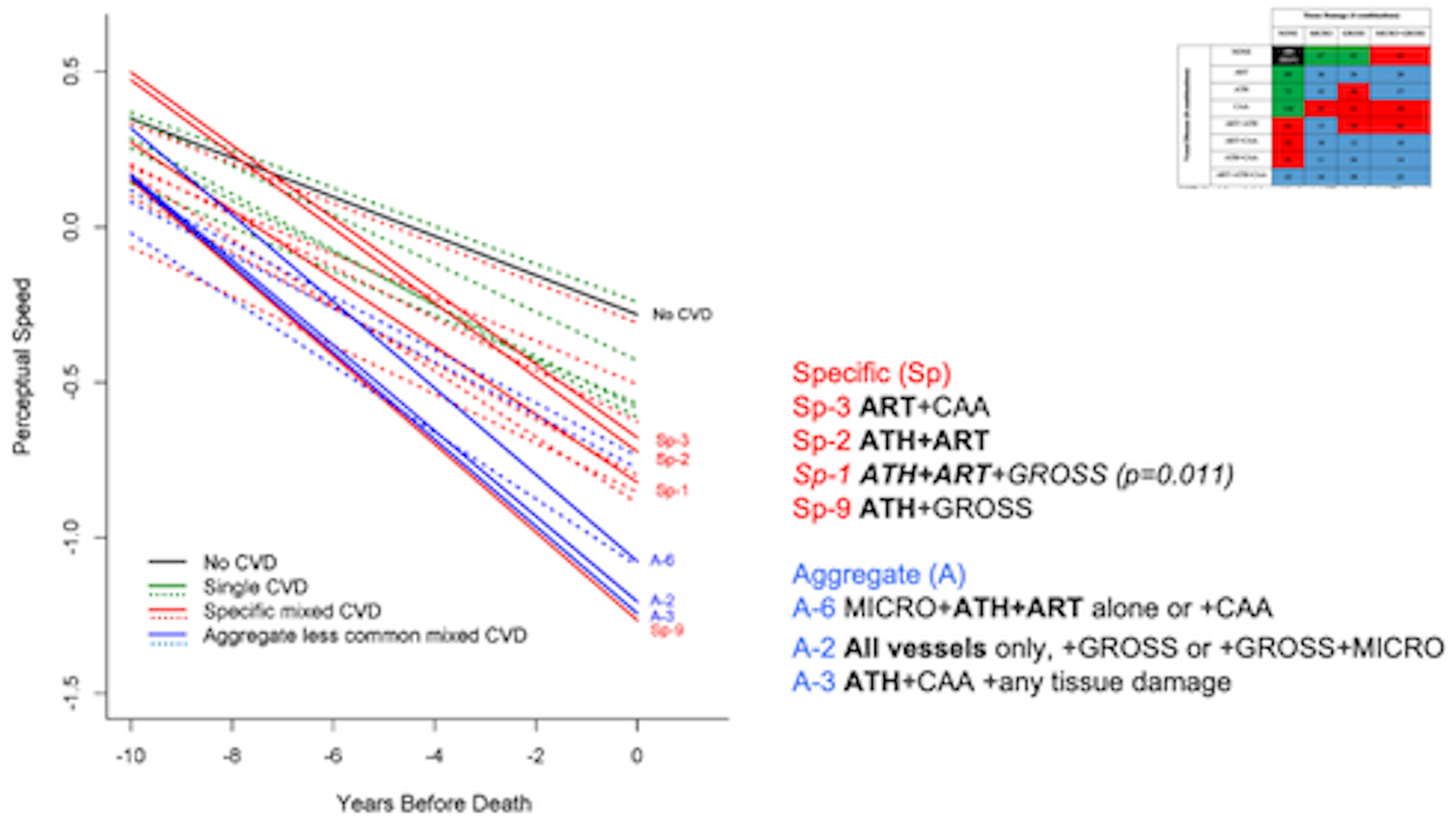
The association of all Single CVD profiles (green), Specific mixed CVD profiles (in red), and Aggregated less common mixed CVD profiles (blue) with change in perceptual speed over time compared to the reference group (black). The model also included terms for age, sex, education, time (years before death), AD-Reagan diagnosis, hippocampal sclerosis, TDP43, and Lewy bodies, and their interactions with time. Significance, determined via 95% confidence intervals and a multiple comparison corrected p-value<.01, is indicated by solid red and/or blue lines and detailed to the right of the figure.
Cerebrovascular Disease and Clinical Alzheimer’s dementia
Proximate to death, 625 (42.4%) of participants were diagnosed with clinical Alzheimer’s dementia using NINCDS/ADRDA criteria [30] using methods previously described [31]. We investigated whether single and/or mixed CVD profile groups were associated with this clinical diagnosis using a Cox proportional hazard model that adjusted for age (at death), sex, education, NIA-Reagan AD diagnosis, hippocampal sclerosis, TDP-43, and neocortical Lewy bodies. The mixed CVD profile group had a significantly higher odds ratio of having clinical Alzheimer’s dementia (OR=2.58, 95% CI: 1.80,3.71; p<0.0001) when compared to the reference group. The single CVD profile group did not (OR=1.24, 95% CI: 0.84,1.84; p=0.26). All AD-related and other neuropathology covariates were associated with a higher odds ratio of clinical Alzheimer’s dementia (OR ranged from 1.37 to 4.08; all p-values≤0.0001).
Discussion
To our knowledge, this is one of the largest (N=1,474) studies to examine the frequencies and most common person-specific CVD combinations of 3 types of vessel diseases (arteriolosclerosis, atherosclerosis, CAA) and 2 types of tissue damage (macro-, micro-infarcts) and how they relate to cognitive decline and clinical Alzheimer’s dementia. We found that all 32 possible combinations occurred in older community dwelling individuals. Overall, mixed CVD profiles were more common than single CVD profiles, with over half of the positive CVD neuropathology sample found to have some form of mixed CVD at death. Given the heterogeneity of these mixed CVD profiles, the number having any specific combination of mixed CVD was consequently small, whereas the groups with only a single CVD at death tended to be larger. Nonetheless, when considering CVD profiles as related to cognitive decline and dementia, results revealed that mixed CVD profiles associated with both more often than single CVD profiles, and regardless of the cognitive domain examined. Furthermore, different mixed CVD profiles contributed to distinct cognitive domains with these profiles oftentimes involving atherosclerosis and arteriolosclerosis; however, the addition of other CVD neuropathologies changed the pattern of associates for specific cognitive domains. Moreover, despite single CVD pathologies having higher numbers of individuals per group, it was the mixed CVD profiles with heterogeneous and smaller groups of person-specific combinations that had the most notable and differential associations with cognitive decline. Overall, these data suggest that CVD is not a monolithic disease, but is a more complex neuropathological milieu as it relates to brain behavior relationships.
Results of this study contribute to the literature in several ways. First, previous research investigating associations between CVD-related neuropathology and cognition as well as clinical Alzheimer’s dementia have tended to look at these relationships based on individual vessel disease [3, 7–12] or tissue damage [3, 11, 13–17, 32, 33]. We extended this work to include a comprehensive investigation of all possible combinations of 3 types of vessel disease and 2 types of tissue injury and included decedents that presented with little to no CVD neuropathology as our reference, all while simultaneously controlling for other neuropathologies. In doing so, we found mixed CVD pathologies were more common than single CVD pathologies and also more common in older age groups. When parsed in this way, we found that decedents with mixed CVD profiles had a higher likelihood of NIA-Reagan AD diagnosis compared to decedents with single CVD or little to no CVD neuropathology regardless of covariates including age. More work is needed to delve deeper into this finding given much, albeit not all, of past neuropathology research suggests that CVD is independent of neurodegenerative pathologies. It is important to note that CAA was included as a CVD pathology; it is possible that CAA was partially driving the relationship between mixed CVD and AD, however, this study was not designed to address this question specifically. It will be important to investigate multiple CVD and AD pathologies in future work.
A second contribution of our work is that it informs and extends previous reports that all three forms of vessel disease and both forms of tissue injury, when considered as singular pathologies, are significantly associated with episodic memory [7, 9, 10, 12–14] and processing speed [7, 9, 10, 13, 15] to suggest that when considered along with their various combinations, it may be their mixed profiles that are more strongly associated with declines in these and other cognitive domains. Consistent with prior research [3, 34] however, the relative contributions of AD-related and other neuropathologies remained consistently higher than those seen for CVD neuropathologies regardless of the cognitive and/or clinical Alzheimer’s dementia outcome investigated. Third, we took a data driven approach to determine how combinations should be investigated as they related to cognition. Specifically, CVD profiles with at least 30 decedents per group were entered into regression models as distinct groups while CVD profiles with limited representation were aggregated to create larger, neuropathologically similar CVD profiles for inclusion. Thus, whereas previous study of CVD combinations have focused on a priori composite indices comprised mainly of tissue injury [35], our results suggest that vessel disease in the form of arteriolosclerosis and atherosclerosis (and their various combinations with other CVD pathologies) may be equally, if not more important when considering the role of ‘non-AD’ neuropathology in cognitive decline. Lastly, this study in conjunction with a UK-based study of 14 different vessel and parenchymal pathologies in 113 decedents [36] provides empirical support for considering not simply the number of pathologies present as it relates to cognitive aging, but the type of damage that co-exists [1, 4–6].
Despite being absent from the diagnostic formulation for clinical Alzheimer’s dementia [30, 37], decedents with a mixed CVD profile were more likely to be diagnosed with Alzheimer’s dementia proximate to death when compared to decedents with little to no CVD neuropathology. This suggests the potential role of mixed CVD in the clinical diagnosis of Alzheimer’s dementia. Not only did the mixed CVD profile group have a significantly higher odds ratio of having clinical Alzheimer’s dementia, cognitive associates resulting from our breakdown of the mixed CVD profiles may help shed light on specific vessel and/or tissue injury combinations most important for the expression of age-related cognitive decline. While a large number of mixed CVD profiles associated with faster declines in perceptual speed after correcting for multiple comparisons, fewer mixed CVD profiles associated with faster declines in visuospatial ability, episodic, semantic and/or working memory. Specifically, having a CVD profile that included atherosclerosis and arteriolosclerosis with or without tissue injury contributed to multiple cognitive domains including both episodic and non-episodic memory. Together with the fact that the only single pathology that consistently associated with cognitive decline (except episodic memory) was CAA and profiles including all 3 vessel diseases had the greatest magnitude of domain-specific cognitive decline suggests that vessel disease is a key contributor to cognitive aging.
We believe these results will facilitate the development of vascular biomarkers, may enable more refined clinical trials, and ultimately offer relevant targets for ensuing intervention studies in vivo, particularly when taken within the context of the proposed timeline that CVD neuropathology may exert its effect on cognition [3, 11]. We recognize that determining the full panel of the CVD biomarkers investigated in this study may not be possible at this time; however, our work identified a few CVD neuropathologies present in the majority of CVD combinations that related to cognitive decline. These CVD neuropathologies, if studied as ‘early’ CVD biomarkers, might reflect a final common pathway for multiple mixed CVD-related profiles. For example, recent studies by our group [3, 11] have described atherosclerosis and arteriolosclerosis (key players in many of our significant mixed CVD profile and cognition relationships) as potent associates of increasingly severe cognitive decline over the course of aging, but named CAA (the only single pathology associated with decline across multiple cognitive domains) as a less robust and more stable contributor to cognition over time. While beyond the scope of this study, our results combined with results reported in the larger literature (e.g., [38]) would suggest that future vascular biomarker development and targeted interventions may wish to focus on the development of in vivo markers of arteriolosclerosis and atherosclerosis and intervene on underlying mechanisms associated with these forms of CVD neuropathology given they may be more detrimental to cognition over the lifespan.
This study has strengths and limitations. To our knowledge, this is the largest community-based study that examined postmortem human brain for the frequency of CVD combinations of 3 types of vessel disease and 2 types of tissue injury and their relationship with change in cognition. Additionally, uniform clinical and pathologic evaluation protocols as applied across our cohorts by the same group of investigators, made it efficient to merge the data for combined analysis. A limitation is that this is an association study, thus, our results cannot establish a causal relationship between CVD profiles and cognition. Furthermore, the extent of CVD is likely under-reported in our study given the neuropathological examination of our select pathologies were restricted both in terms of quantification as well as brain areas, e.g., our semi-quantitative levels of arteriolosclerosis were restricted to one section of the anterior basal ganglia. Admittedly, combining our neuropathological approach with neuroimaging may have resulted in even higher prevalence of CVD than already detected. While we adjusted for other neuropathologies including AD-related neuropathology, we may have omitted other less apparent confounders. Furthermore, we did omit other forms of CVD not well measured by neuropathology [36] including microbleeds and white matter hyperintensities as well as more molecular-based markers of cerebrovascular dysfunction including disruptions of blood brain barrier/endothelium likely critical for cognitive decline. Lastly, the CVD profiles studied were not investigated in terms of regional involvement (a consideration that we felt was beyond the scope of this study but nonetheless important for cognitive decline), and focused exclusively on moderate to severe levels of vessel disease saying nothing about minimal or mild forms of atherosclerosis, arteriolosclerosis, or CAA.
Supplementary Material
Funding Sources
This work was supported by the NIH grants R01-AG043379, P30-AG10161, RF1-AG22018, R01-AG15819, R01-AG24480, R01-AG17917, R01-AG042210, R01-AG064233, R01-AG34374, UH2-NS100599.
Non-standard Abbreviations and Acronyms
- CVD
cerebrovascular disease
- AD
Alzheimer’s disease
- CAA
cerebral amyloid angiopathy
- LB
Lewy bodies
Footnotes
Disclosures
None
List of Supplemental Material
Flow chart for analytic sample.
Characteristics of single versus mixed CVD neuropathology groups
Associations between single and mixed CVD neuropathology groups (in the same model) and decline in domain-specific cognitive functioning
References
- 1.Kapasi A, DeCarli C, and Schneider JA, Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol, 2017;134:171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chui HC, Zheng L, Reed BR, Vinters HV and Mack WJ, Vascular risk factors and Alzheimer’s disease: are these risk factors for plaques and tangles or for concomitant vascular pathology that increases the likelihood of dementia? An evidence-based review. Alzheimers Res Ther, 2012;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyle PA, Yu L, Wilson RS, Leurgans SE, Schneider JA, and Bennett DA, Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol, 2018;83:74–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T and Corrada MM, Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology, 2015;85:535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brickman AM, Tosto G, Giutierrez J, Andrews H, Gu Y, Narkhede A, Rizvi B, Guzman V, Manly JJ, Vonsattel JP et al. , An MRI measure of degenerative and cerebrovascular pathology in Alzheimer disease. Neurology, 2018;91:e1402–e1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hachinski V Dementia: Paradigm shifting into high gear. Alzheimers Dement, 2019; 15:985–994. [DOI] [PubMed] [Google Scholar]
- 7.Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA and Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol, 2016;15:934–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA and Schneider JA, The Relationship of Cerebral Vessel Pathology to Brain Microinfarcts. Brain Pathol, 2017;27:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyle PA, Yu L, Nag S, Leurgans SE, Wilson RS, Bennett DA, and Schneider JA, Cerebral amyloid angiopathy and cognitive outcomes in community-based older persons. Neurology, 2015;85:1930–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ighodaro ET, Abner EL, Fardo DW, Lin AL, Katsumata Y, Schmitt FA, Kryscio RJ, Jicha GA, Neltner JH, Monsell SE, et al. , Risk factors and global cognitive status related to brain arteriolosclerosis in elderly individuals. J Cereb Blood Flow Metab, 2017;37:201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle PA, Yang J, Yu L, Leurgans SE, Capuano AW, Schneider JA, Wilson RS and Bennett DA, Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain, 2017;140:804–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfeifer LA, White LR, Ross GW, Petrovitch H, and Launer LJ. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology, 2002;58:1629–34. [DOI] [PubMed] [Google Scholar]
- 13.Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA and Schneider JA, Microinfarct pathology, dementia, and cognitive systems. Stroke, 2011;42:722–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL and Bennett DA. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol, 2007;62:59–66. [DOI] [PubMed] [Google Scholar]
- 15.Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA and Bennett DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology, 2003;60:1082–8. [DOI] [PubMed] [Google Scholar]
- 16.Jansen WJ, Wilson RS, Visser PJ, Nag S, Schneider JA, James BD, Leurgans SE, Capuano AW, Bennett DA and Boyle PA. Age and the association of dementia-related pathology with trajectories of cognitive decline. Neurobiol Aging, 2018;61:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Troncoso JC, Zonderman AB, Resnick SM, Crain B, Pletnikova O, and O’Brien RJ, Effect of infarcts on dementia in the Baltimore longitudinal study of aging. Ann Neurol, 2008;64:168–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS and Schneider JA, Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis, 2018;64:S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bennett DA, Schneider JA, Arvanitakis Z and Wilson RS, Overview and findings from the religious orders study. Curr Alzheimer Res, 2012;9:628–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA and Wilson RS, Overview and findings from the rush Memory and Aging Project. Curr Alzheimer Res, 2012;9:646–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barnes LL, Shah RC, Aggarwal NT, Bennett DA, and Schneider JA. The Minority Aging Research Study: ongoing efforts to obtain brain donation in African Americans without dementia. Curr Alzheimer Res, 2012;9:734–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bennett DA and Launer LJ. Longitudinal epidemiologic clinical-pathologic studies of aging and Alzheimer’s disease. Curr Alzheimer Res, 2012;9:617–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barnes LL, Leurgans SE, Aggarwal NT, Shah RC, Arvanitakis Z, James BD, Buchman AS, Bennett DA and Schneider JA. Mixed pathology is more likely in black than white decedents with Alzheimer dementia. Neurology, 2015;85:528–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schneider JA, Bienias JL, Wilson RS, Berry-Kravis E, Evans DA and Bennett DA, The apolipoprotein E epsilon4 allele increases the odds of chronic cerebral infarction [corrected] detected at autopsy in older persons. Stroke, 2005;36:954–9. [DOI] [PubMed] [Google Scholar]
- 25.Yu L, Boyle PA, Leurgans SE, Schneider JA and Bennett DA, Disentangling the effects of age and APOE on neuropathology and late life cognitive decline. Neurobiol Aging, 2014;35:819–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA and Schneider JA. Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol, 2015;77:942–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.James BD, Bennett DA, Boyle PA, Leurgans SE and Schneider JA. Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA, 2012;307:1798–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schneider JA, Aggarwal NT, Barnes LL, Boyle PA and Bennett DA, The neuropathology of older persons with and without dementia from community versus clinic cohorts. J Alzheimers Dis, 2009;18:691–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC and Parisi JE. Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol, 2008;116:215–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McKhann G, Drachman D, Folstein M, Katzman R, Price D and Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology, 1984;34:939–44. [DOI] [PubMed] [Google Scholar]
- 31.Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, Fox JH, Cochran EJ, Arends D, Treinkman AD et al. , Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology, 2006;27:169–76. [DOI] [PubMed] [Google Scholar]
- 32.Wang Z, van Veluw SJ, Wong A, Liu W, Shi L, Yang J, Xiong Y, Lau A, Biessels GJ and Mok VC, Risk Factors and Cognitive Relevance of Cortical Cerebral Microinfarcts in Patients With Ischemic Stroke or Transient Ischemic Attack. Stroke, 2016;47:2450–5. [DOI] [PubMed] [Google Scholar]
- 33.Kapasi A, Leurgans SE, James BD, Boyle PA, Arvanitakis Z, Nag S, Bennett DA, Buchman AS and Schneider JA, Watershed microinfarct pathology and cognition in older persons. Neurobiol Aging, 2018;70:10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boyle PA, Yu L, Leurgans SE, Wilson RS, Brookmeyer R, Schneider JA and Bennett DA, Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann Neurol, 2019;85:114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ezzati A, Wang C, Lipton RB, Altschul D, Katz MJ, Dickson DW and Derby CA. Association Between Vascular Pathology and Rate of Cognitive Decline Independent of Alzheimer’s Disease Pathology. J Am Geriatr Soc, 2017;65:1836–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skrobot OA, Attems J, Esiri M, Hortobagyi T, Ironside JW, Kalaria RN, King A, Lammie GA, Mann D, Neal J, et al. , Vascular cognitive impairment neuropathology guidelines (VCING): the contribution of cerebrovascular pathology to cognitive impairment. Brain, 2016;139:2957–2969. [DOI] [PubMed] [Google Scholar]
- 37.Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, Holtzman DM, Jagust W, Jessen F, Karlawish J et al. , NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimers Dement, 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chui HC and Ramirez-Gomez L. Clinical and imaging features of mixed Alzheimer and vascular pathologies. Alzheimers Res Ther, 2015;7:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


