Abstract
Background:
Animal and epidemiologic studies indicate that air pollution may adversely affect fertility. Epidemiologic studies have been restricted largely to couples undergoing fertility treatment or have retrospectively ascertained time-to-pregnancy among pregnant women.
Objectives:
We examined the association between residential ambient air pollution and fecundability, the per-cycle probability of conception, in a large preconception cohort of Danish pregnancy planners.
Methods:
During 2007–2018, we used the Internet to recruit and follow women who were trying to conceive without the use of fertility treatment. Participants completed an online baseline questionnaire eliciting socio-demographic characteristics, lifestyle factors, and medical and reproductive histories and follow-up questionnaires every 8 weeks to ascertain pregnancy status. We determined concentrations of ambient nitrogen oxides (NOx), nitrogen dioxide (NO2), carbon monoxide (CO), ozone (O3), particulate matter <2.5 μm (PM2.5) and <10 μm (PM10), and sulfur dioxide (SO2) at each participant’s residential address. We calculated average exposure during the year before baseline, during each menstrual cycle over follow-up, and during the entire pregnancy attempt time. We used proportional probabilities regression models to estimate fecundability ratios (FRs) and 95% confidence intervals (CIs), adjusting for potential confounders and co-pollutants. The analysis was restricted to the 10,183 women who were trying to conceive for <12 cycles at study entry whose addresses could be geocoded.
Results:
During 12 months of follow-up, 73% of participants conceived. Higher concentrations of PM2.5 and PM10 were associated with small reductions in fecundability. For example, the FRs for a one interquartile range (IQR) increase in PM2.5 (IQR=3.2 μg/m3) and PM10 (IQR=5.3 μg/m3) during each menstrual cycle were 0.93 (95% CI: 0.87, 0.99) and 0.91 (95% CI: 0.84, 0.99), respectively. Other air pollutants were not appreciably associated with fecundability.
Conclusions:
In this preconception cohort study of Danish women, residential exposures to PM2.5 and PM10 were associated with reduced fecundability.
Keywords: Air pollution, fecundability, particulate matter, preconception cohort, time-to-pregnancy, traffic
BACKGROUND
Ambient air pollution is an important environmental threat. The Global Burden of Disease Study estimates that over 4 million deaths are attributable to ambient air pollution every year,1 and epidemiologic studies demonstrate that air pollution exposure is associated with premature mortality2–5 and increased risks of cardiovascular disease,6 respiratory disease,7–9 cancer,10 and adverse birth outcomes.11, 12 These risks are present even at low exposure levels, indicating that current air quality regulations may be insufficient to protect human health.2, 13, 14
Although infertility, defined as the inability to conceive during 12 months of unprotected intercourse, affects 10–15% of couples,15–17 few risk factors have been identified. Some mechanisms through which air pollution is hypothesized to affect other health outcomes may also play a role in the etiology of infertility.18–20 Laboratory studies demonstrate that diesel exhaust, particulate matter, and other combustion-related pollutants have hormone-like activity.21–23 In mice, chronic exposure to urban pollution in Sao Paolo, Brazil reduced the number of antral follicles and caused longer estrus cycles, higher risk of implantation failure, longer time to pregnancy, and fewer live births.24, 25
The epidemiologic literature also indicates a potential role for air pollution in human fertility. Three ecological studies in Spain,26 China,27 and the United States28 found correlations between fertility rates and concentrations of fine particulate matter (PM2.5) and nitrogen oxides (NOx) at the census tract or county levels. Studies of couples from the United States29–31 and Korea32 who were undergoing fertility treatment consistently reported associations between increasing concentrations of air pollutants at patients’ home address and poorer treatment outcomes. Four epidemiologic studies have evaluated the association of air pollution with fertility among couples trying to conceive spontaneously. Although results varied in magnitude and by pollutant, all four studies found that higher levels of air pollution were associated with reduced fecundability or higher infertility risk.33–36 Some of these studies were limited by retrospective assessment of time-to-pregnancy, restriction to couples who had a live birth, or measurement of binary infertility rather than continuous time-to-pregnancy. Here, we examine residential concentrations of ambient air pollution in relation to fecundability, the per cycle probability of conception, in a large cohort of pregnancy planners in Denmark.
METHODS
Cohort selection
Snart-Gravid.dk (SG; translates to ‘soon pregnant’) is an Internet-based preconception cohort of female pregnancy planners, designed to study risk factors for subfertility.37, 38 Enrollment began in 2007 and continued until 2011, at which point the study began enrolling male partners and was renamed Snart-Foraeldre.dk (SF; translates to ‘soon parents’). Enrollment in SF is ongoing. Recruitment originally took place through advertisements on netdoktor.dk, min-mave.dk, Facebook, and blogs. Beginning in 2018, we primarily recruit participants through a governmental e-mail box (e-Boks). Eligible female participants are aged 18–49 years, residents of Denmark, and attempting to conceive without the use of fertility treatment. Female participation involves the completion of a baseline questionnaire on socio-demographic characteristics, behavioral factors, and reproductive and medical histories. Participants subsequently complete bi-monthly follow-up questionnaires to ascertain pregnancy status. All participants are required to provide a valid e-mail address and their Civil Personal Registration (CPR) number.
Between June 2007 and November 2018, 12,063 eligible women completed the baseline questionnaire. In the current study, we excluded 385 women with an implausible date of last menstrual period (LMP) data, 1,274 women who had been trying to conceive for ≥12 cycles at study entry, and 221 women whose reported residential addresses could not be geocoded. The analytic sample included 10,183 women. For analysis of air pollution during follow-up (see exposure assessment below), we excluded 125 women with air pollution data available during the year before baseline but not during follow-up, for an analytic sample of 10,058 women.
Outcome assessment
At baseline, we collected information on attempt time at study entry, date of the first day of the LMP, cycle regularity, and typical cycle length (for women with regular cycles) or number of periods per year (for women with irregular cycles). On follow-up questionnaires, we asked women for the date of their LMP, whether their cycles had been regular since their previous questionnaire, and the length of their most recent cycle (for women with regular cycles). We also asked women if they were currently pregnant, if they had experienced a pregnancy loss since their previous questionnaire, and if they had initiated fertility treatment. Women who were pregnant reported the LMP date for their pregnancy and how their pregnancy was confirmed (e.g., urine test, blood test, ultrasound). We asked women who were not pregnant if they were still trying to conceive.
We followed women from their baseline LMP date until pregnancy or one of the following censoring events: 12 cycles of follow-up, initiation of fertility treatment, cessation of pregnancy attempt, or loss to follow-up. We estimated the dates of each cycle that occurred during follow-up using information on self-reported LMP dates and cycle length. We defined the first day of each cycle as the first day of menses. Since follow-up questionnaires were completed bi-monthly, we estimated LMP dates between questionnaires by subtracting cycle length from the later reported LMP date, making sure that the time difference between the earlier reported and the estimated LMP dates was not smaller than half of the cycle length.
Exposure assessment
By linking participants’ CPR numbers to the Danish Central Registration System,39 we collected geographical coordinates of all residential addresses from one year before enrollment through the end of follow-up. We obtained daily estimates of individual air pollutant concentrations at each participants’ address(es) using the Danish Eulerian Hemispheric Model (DEHM)/Urban Background Model (UBM)/AirGIS modeling system.40 This validated system comprises three air pollution models,41 including the DEHM, which accounts for long-range transport of air pollution down to a resolution of 5.6 km x 5.6 km;42, 43 the UBM, which determines the local background level of pollutants on a 1-km2 grid resolution for all of Denmark;44–46 and the Operational Street Pollution Model (OSPM), which estimates air pollutant concentrations at the residential level.42, 43, 47 When comparing annual averages from the 2018 DEHM/UBM/AirGIS models with air pollutant measurements from the 17 stations included in the Danish routine modeling program, correlation coefficients ranged from 0.7–0.9, depending on the pollutant, site, and time period.40, 48 For three permanent monitoring stations at street/urban/rural locations, and using 14–18 years of measured data, the correlation with modelled data for the pollutants NOx, PM2.5 were in the range 0.61–0.94 (NOx) 0.72–0.89 (PM2.5) depending on the applied time resolutions (annual, month, hour).49
We linked participant data with daily estimates between 2006 through 2018 of nitrogen dioxide (NO2), nitrogen oxides (NOx), carbon monoxide (CO), ozone (O3), particulate matter <2.5 μm (PM2.5), particulate matter <10 μm (PM10) and sulfur dioxide (SO2). We averaged daily estimates across three different time periods relative to the pregnancy attempt to identify the most relevant window of exposure. First, we calculated average daily exposure during the year before enrollment as a measure of long-term exposure. Second, we calculated average daily concentrations during each menstrual cycle, which is more temporally granular, reflecting variability across the year. Third, we calculated a “cumulative average” preconception exposure variable, which we defined as the average daily concentrations from baseline LMP date through the last day of a given menstrual cycle. This measure reflects exposure throughout the preconception period.
Covariate assessment
We collected covariate data on the baseline questionnaire, including socio-demographic information (age, educational attainment, annual household income), anthropometrics (height, weight), lifestyle factors (cigarette smoking, alcohol intake, physical activity, sugar-sweetened soda intake, daily multivitamin or folic acid use), reproductive history (gravidity, parity), and intensity of trying to conceive (intercourse frequency, timing of intercourse).
Statistical analysis
We examined associations between individual air pollutants and fecundability by fitting proportional probabilities regression models to estimate fecundability ratios (FRs) and 95% confidence intervals (CIs). The FR estimates the per-cycle probability of conception in exposed compared with unexposed women. We calculated FRs for an increase in air pollutant concentrations equivalent to the interquartile range (IQR). We used the Anderson-Gill data structure, with one observation per observed menstrual cycle at risk, to account for left truncation due to delayed entry into the risk set.50, 51 We included indicator variables for cycle at risk to account for the decline in baseline fecundability with increasing attempt time. We fitted restricted cubic splines to examine non-linear continuous associations between exposure and outcome.
We selected covariates for adjustment using a directed acyclic graph. Final models adjusted for age (<25, 25–29, 30–34, ≥35 years), educational attainment (≤12, 13–15, 16, ≥17 years), monthly household income (<25,000, 25,000–39,999, 40,000–64,999, ≥65,000 DKK), parity conditional on gravidity (nulligravid, gravid but not parous, parous), month and year of each cycle, and monthly average ambient temperature (<0, 0–4, 5–9, 10–14, and ≥15°C). We also adjusted for co-pollutants, including SO2, NO2 (to represent NOx, NO2, CO, and O3) and PM2.5 (to represent the two sizes of PM (PM2.5 and PM10)). We stratified final models by gravidity and parity (nulligravid, gravid but nulliparous, parous), age (<30 vs. ≥30 years) and BMI (<25 vs. ≥25 kg/m2). Several studies,52, 53 including one of fertility treatment outcomes,54 have shown that associations between air pollution and adverse health outcomes may be stronger among individuals with low folate intake. Therefore, we stratified final models by daily multivitamin or folic acid intake. Finally, we conducted a sensitivity analysis restricted to women with <3 cycles of attempt time at study entry, among whom attempt time at study entry is less likely to be misclassified.
Missing data
We used multiple imputation to deal with missing values for covariates and outcome. We used fully conditional specification methods with 400 iterations to generate 5 imputed data sets. Covariate missingness was generally low, ranging from 0% (age) to 9% (income). For women who did not complete any follow-up questionnaires (14%), we assigned them one cycle of follow-up and imputed their pregnancy status at the end of that cycle.
Ethics approval
The study was registered at Aarhus University to comply with Danish law on data protection and approved by the institutional review board at the Boston University Medical Campus. All participants provided online informed consent.
RESULTS
We followed 10,183 women for 35,004 menstrual cycles. During the 12-month study period, 57% of participants conceived (73% after accounting for censoring using life-table methods). Eight percent of women attempted pregnancy for 12 cycles without conception, 10% initiated fertility treatment, 3% stopped trying to conceive, and 22% were lost to follow-up. Loss to follow-up was inversely associated with air pollution exposure, educational attainment and household income.
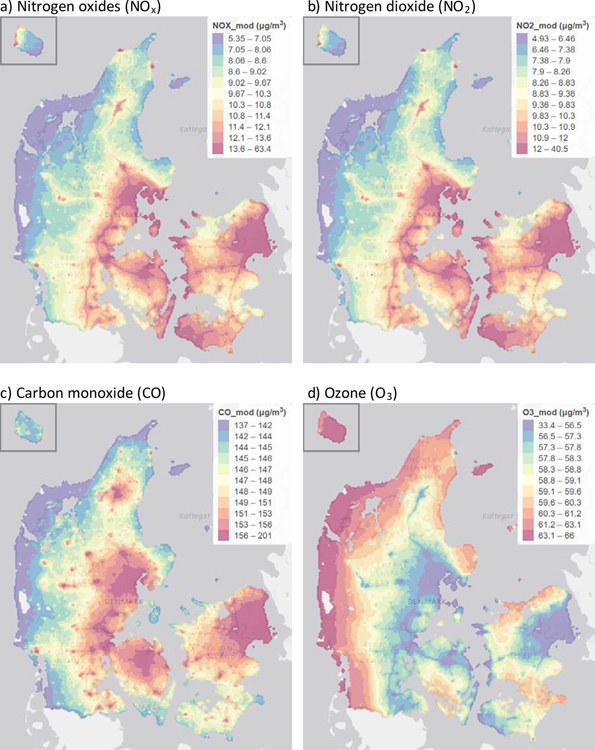
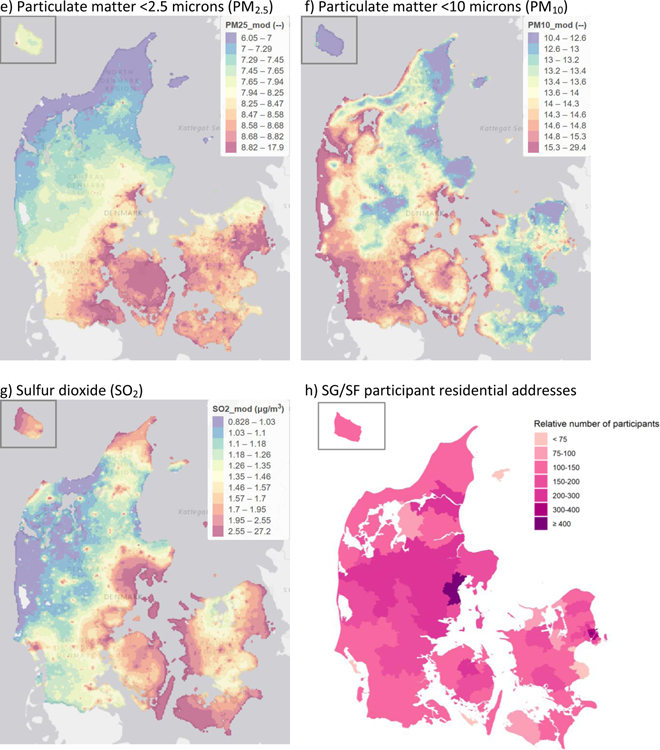
eTable 1 shows the distribution of pollutants during the year before baseline among SG/SF participants as well as the European Union Air Quality Standard limits. Most participants were exposed to air pollution at levels well below the limit values set by the European Union. Pollutants were highly correlated; for instance, Spearman correlation coefficients for NOx, NO2, and CO ranged from 0.91 to 0.96, and O3 was strongly inversely correlated with these pollutants (Spearman correlation coefficients ranged from −0.83 to −0.90). The correlation coefficient between PM2.5 and PM10 was 0.85. Other correlations between pollutants were moderate. Figure 1a–g shows the spatial distribution of each pollutant across Denmark, and Figure 1h shows the locations of SG/SF participant residences.
Figure 1.
Maps showing spatial distributions across Denmark of a) NOx, b) NO2, c) CO, d) O3, e) PM2.5, f) PM10, g) SO2 and h) SG/SF participants (number of participants per 100,000 inhabitants). Pollutant maps are annual averages of each pollutant during 2012, modelled with DEHM/UBM at 1km x 1km spatial resolution. Only areas with Danish residential addresses are displayed. Empty cells are unpopulated (i.e., water or forest). The data is displayed in quantile scale, with variable ranges in the color classes, arranged so that each color covers a similar total area in the map. Units for all pollutants are μg/m3.
Table 1 presents baseline characteristics of participants across categories of NOx and PM2.5 concentrations. Air pollution concentrations declined over time and exhibited strong seasonal variation, with highest concentrations in the summer. Participants with higher exposure to NOx were less likely to have low educational attainment compared with lower exposed participants, whereas the opposite was true for PM2.5. For both pollutants, participants with higher exposures were more likely to have lower household income, BMI, and physical activity. They were also less likely to have a previous live birth. Finally, higher exposed participants were less likely to be timing intercourse to the fertile window, but were also less likely to report intercourse <1 time/week.
Table 1.
Distribution of baseline characteristics by NOx and PM2.5 levels in the year before baseline, Snart Gravid and Snart Foraeldre
| NOx (μg/m3) |
PM2.5 (μg/m3) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristicsa | Q1: <12.8 | Q2: 12.8–16.5 | Q3: 16.6–21.0 | Q4: 21.1–28.6 | Q5: ≥28.7 | Q1: <8.5 | Q2: 8.5–9.1 | Q3: 9.2–9.9 | Q4: 10.0–10.7 | Q5: ≥10.8 |
| Number | 2012 | 2077 | 2003 | 2053 | 2038 | 2115 | 1843 | 2247 | 1932 | 2046 |
| Age (years), mean | 28.7 | 28.6 | 28.8 | 28.5 | 28.7 | 29.2 | 28.5 | 28.4 | 28.5 | 28.7 |
| Month of enrollment, % | ||||||||||
| December – February | 25.9 | 27.9 | 24.4 | 23.4 | 25.8 | 32.7 | 24.1 | 23.4 | 24.0 | 20.5 |
| March – May | 26.9 | 25.5 | 27.3 | 23.2 | 22.4 | 36.8 | 28.6 | 24.5 | 21.3 | 14.2 |
| June – August | 25.6 | 24.8 | 26.2 | 26.2 | 31.7 | 13.0 | 22.0 | 25.0 | 28.6 | 52.4 |
| September – November | 21.7 | 21.8 | 22.1 | 21.6 | 20.1 | 17.4 | 25.3 | 27.1 | 26.2 | 12.9 |
| Year of enrollment, % | ||||||||||
| 2007 – 2009 | 30.5 | 41.3 | 38.2 | 54.1 | 60.9 | 8.4 | 20.8 | 38.1 | 70.5 | 88.8 |
| 2010 – 2012 | 16.2 | 13.6 | 15.3 | 13.9 | 10.9 | 8.6 | 15.3 | 20.2 | 18.6 | 7.7 |
| 2013 – 2015 | 23.6 | 19.5 | 19.0 | 16.9 | 12.5 | 19.8 | 28.6 | 32.7 | 7.9 | 3.0 |
| 2016 – 2018 | 29.8 | 25.6 | 27.5 | 15.1 | 15.8 | 63.2 | 35.3 | 9.0 | 3.1 | 0.5 |
| ≤12 years education, % | 11.3 | 12.3 | 10.3 | 10.8 | 9.8 | 8.6 | 8.5 | 10.7 | 13.6 | 12.6 |
| Income <25,000/month DKK, % | 11.6 | 14.0 | 13.0 | 15.8 | 16.6 | 11.5 | 13.7 | 16.0 | 14.8 | 14.9 |
| BMI (kg/m2), mean | 25.7 | 24.9 | 24.0 | 23.8 | 23.2 | 25.2 | 24.4 | 24.2 | 24.4 | 23.4 |
| Physical activity (MET-hr/wk), mean | 56.5 | 46.7 | 47.5 | 41.5 | 40.0 | 62.8 | 54.1 | 46.6 | 36.0 | 32.0 |
| Current regular smoker, % | 13.0 | 12.9 | 10.0 | 9.4 | 8.8 | 10.7 | 9.0 | 10.9 | 13.2 | 10.6 |
| Alcohol (drinks/week), mean | 2.0 | 2.1 | 2.4 | 2.6 | 3.0 | 2.2 | 2.3 | 2.3 | 2.4 | 2.9 |
| Sugar-sweetened soda (dr/wk), mean | 1.3 | 1.1 | 1.0 | 0.9 | 0.8 | 1.1 | 0.9 | 1.1 | 1.1 | 0.9 |
| Daily multivitamins/folic acid use, % | 61.5 | 61.5 | 66.0 | 65.2 | 65.5 | 64.5 | 65.9 | 65.1 | 60.7 | 62.9 |
| Nulligravid, % | 40.9 | 43.3 | 51.4 | 56.7 | 63.3 | 47.2 | 49.3 | 48.5 | 51.3 | 59.7 |
| Gravid, but nulliparous, % | 12.5 | 14.5 | 14.1 | 16.2 | 17.4 | 14.1 | 15.5 | 14.4 | 15.7 | 15.1 |
| Parous, % | 46.6 | 42.2 | 34.5 | 27.1 | 19.4 | 38.8 | 35.2 | 37.1 | 33.0 | 25.2 |
| Intercourse <1 time/week, % | 20.1 | 19.4 | 17.8 | 15.5 | 15.4 | 21.6 | 17.4 | 18.5 | 14.9 | 15.1 |
| Hormonal last contraception, % | 63.8 | 62.0 | 59.3 | 62.2 | 61.6 | 59.1 | 62.2 | 62.8 | 62.9 | 61.6 |
| History of infertility, % | 17.3 | 13.4 | 9.7 | 9.6 | 7.2 | 12.2 | 12.3 | 11.9 | 11.7 | 9.3 |
| Doing something to improve chances, % | 67.4 | 62.4 | 63.9 | 59.4 | 57.3 | 71.7 | 68.9 | 65.8 | 53.6 | 49.9 |
Characteristics are standardized to the age distribution of the cohort at baseline.
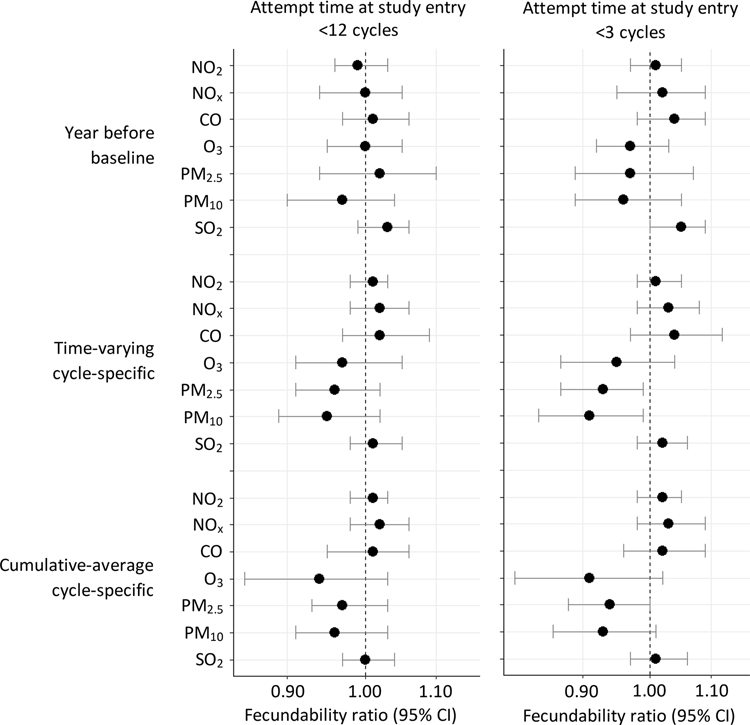
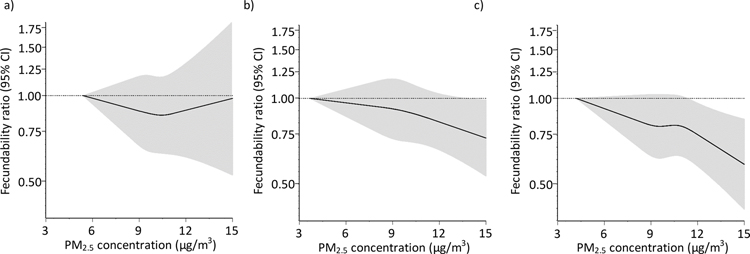
We found no appreciable association between average air pollution exposure during the year before baseline and fecundability (Figure 2). Results were similar when restricted to participants with <3 cycles of attempt time at study entry. Restricted cubic spline analyses confirmed that there were no strong associations between air pollution during the year before baseline and fecundability (Figure 2a; Figure 3a; eFigures 1a, 2a, 3a, 4a, and 5a).
Figure 2.
Association between residential ambient concentrations of air pollutants and fecundability, Snart Gravid and Snart Foraeldre, 2007–2019. Fecundability ratios are for a one-interquartile range increase in exposure, and are adjusted for age, education, income, parity conditional on gravidity, month of enrollment, year of enrollment, and average monthly ambient temperature. Models for NOx, NO2, CO and O3 are adjusted for PM2.5 and SO2. Models for PM2.5 and PM10 are adjusted for NOx and SO2. Models for SO2 are adjusted for NOx and PM2.5
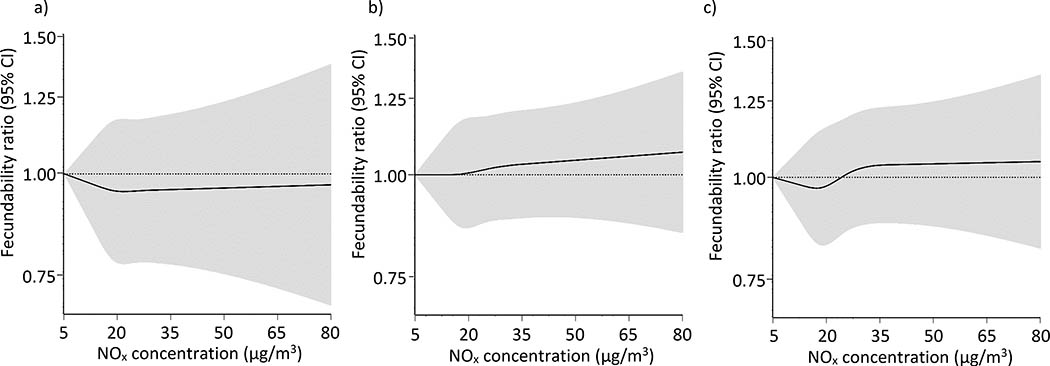
Figure 3.
Association between residential concentrations of particulate matter <2.5 μm (PM2.5) and fecundability, fit using restricted cubic splines. PM2.5 is modeled as a) the average during the year before baseline, b) average concentration over each menstrual cycle, and c) cumulative-average exposures. The reference value is the lowest observed value in the cohort, and there are three knots located at the 10th, 50th, and 90th percentiles. The x-axis ranges from the minimum value to the 95th percentile. Spline curves are adjusted for age, education, income, parity conditional on gravidity, month of enrollment, year of enrollment, average monthly ambient temperature, and concentrations of NOx and SO2.
Assessment of average daily exposures during each menstrual cycle showed that NOx, NO2, CO, O3, and SO2 were not strongly related to fecundability (Figure 2). PM2.5 and PM10 concentrations were associated with small reductions in fecundability, particularly when restricting to participants with <3 cycles of attempt time at study entry (FRs for a one IQR increase in PM2.5 (IQR=3.2 μg/m3) and PM10 (IQR=5.3 μg/m3) were 0.93 [95% CI: 0.87, 0.99] and 0.91 [95% CI: 0.84, 0.99], respectively). In other words, for every one IQR increase in PM2.5 and PM10, participants have 7% and 9% lower probabilities of conceiving in a given menstrual cycle, respectively. In restricted cubic splines analyses, average daily concentrations of PM2.5 during each menstrual cycle (Figure 3b) were associated with reduced fecundability. Reductions in fecundability were also observed for O3 (eFigure 4b) and PM10 (eFigure 5b). Average daily concentrations of other pollutants during each menstrual cycle were not meaningfully associated with fecundability (Figure 4b; eFigures 2b, 3b, 6b).
Figure 4.
Association between residential concentrations of nitrogen oxides (NOx) and fecundability, fit using restricted cubic splines. NOx is modeled as a) the average during the year before baseline, b) average concentration over each menstrual cycle, and c) cumulative-average exposures. The reference value is the lowest observed value in the cohort, and there are three knots located at the 10th, 50th, and 90th percentiles. The x-axis ranges from the minimum value to the 95th percentile. Spline curves are adjusted for age, education, income, parity conditional on gravidity, month of enrollment, year of enrollment, average monthly ambient temperature, and concentrations of PM2.5 and SO2.
Associations for cumulative average concentrations during the entire preconception period were similar to those during each menstrual cycle (Figure 2). For example, among participants with <3 cycles of attempt time at study entry, FRs for a one IQR increase in PM2.5 (IQR=2.4 μg/m3) and PM10 (IQR=4.2 μg/m3) concentrations were 0.94 [95% CI: 0.88, 1.00] and 0.93 [95% CI: 0.86, 1.01], respectively. Ozone (IQR=19.8 μg/m3) was also associated with slightly reduced fecundability (FR for a one IQR increase=0.91 [95% CI: 0.81, 1.02]). In restricted cubic spline analyses, preconception concentrations of both PM2.5 (Figure 3c) and PM10 (eFigure 5c) were associated with reduced fecundability. Cumulative average concentrations of other pollutants were not associated with fecundability (Figure 4c; eFigures 2c, 3c, 4c, 6c).
Associations were slightly stronger among nulliparous women (eTable 3), but did not differ appreciably across strata of age (eTable 4), BMI (eTable 5), or multivitamin or folic acid supplementation (eTable 6), with the exception of ozone, which was associated with reduced fecundability only among women with no multivitamin or folic acid intake.
COMMENT
Principal findings
In this prospective preconception cohort study of female pregnancy planners residing in Denmark, we found that increasing estimated residential ambient concentrations of PM2.5 and PM10 were associated with slightly reduced fecundability. Associations were stronger when examining exposures more proximal to the pregnancy attempt (i.e., cycle-specific exposures that varied over follow-up) than for longer-term exposure windows (i.e., the year before study enrollment). Notably, these associations were observed at relatively low concentrations. NOx, NO2, CO, O3, and SO2 concentrations were largely unrelated to fecundability. Our results add to the growing literature demonstrating that air pollution, and especially particulate matter, may be related to lower fertility.
Strengths of the study
Our study has several strengths. First, we estimated time-to-pregnancy prospectively in a large cohort of women actively trying to conceive. This is a key advantage over previous studies that have measured time-to-pregnancy retrospectively in early pregnancy,36 restricted to women with a live birth,33 or ascertained dichotomous measures of infertility.34 Second, we used a validated air pollution modeling approach that has shown a strong correlation with measurements taken at monitoring stations.41–43 Linkage to Danish registries allowed us to precisely account for residential mobility during the study period, as residents are required to report address changes within five days of the move. Finally, we collected data on a wide range of confounders. As in any epidemiologic study, unmeasured confounding is possible. We do not suspect strong unmeasured confounding by neighborhood-level socioeconomic status, given the spatial patterns of exposure across Denmark. However, confounding by other environmental exposures such as noise, green space, or other hazardous pollutants is possible.
Limitations of the study
As we based our exposure assessment solely on participants’ residential addresses, we were not able to account for air pollution exposure indoors or in non-residential locations. We also did not consider time-activity patterns. In addition, air pollution concentrations at each residential address were modeled, rather than directly measured. Due to the prospective nature of the study, we expect that exposure misclassification was non-differential with respect to the outcome, which would tend to bias results toward the null in the extreme exposure categories.
We assessed both long- and short-term exposures to try to identify the etiologically relevant window of exposure. Because we did not have sufficiently granular data on the numerous biologic processes that occur leading up to pregnancy (i.e., folliculogenesis, ovulation, implantation), we were unable to measure exposure in finer windows that may be more biologically relevant, as studies of couples undergoing IVF have done.29–32 In addition, because the vast majority of couples reside at the same address and exposure was residence-based, we were not able to separate out effects of air pollution on female vs. male partners.
We measured time-to-pregnancy using self-reported information on LMP dates, menstrual cycle length, attempt time at study entry, and pregnancy status during follow-up. These variables were measured retrospectively on bi-monthly follow-up questionnaires, rather than prospectively using daily diaries. To the extent that any of these variables were measured with error, outcome misclassification could result. Given the high use of home pregnancy tests in SG/SF (95%), and the high specificity and sensitivity of home pregnancy tests, we expect pregnancy status was measured with little error. Participants did not receive pregnancy tests as part of their participation in the study, nor did we have a systematic protocol for pregnancy testing; however, over 95% of participants reported using home pregnancy tests, and the median gestational weeks at first pregnancy test was 4 weeks. We were unable to identify losses that occurred before the pregnancy could be identified with home pregnancy tests, which could introduce misclassification of TTP. Because air pollution has been associated with early pregnancy loss in some studies,55 in the present work, unidentified early losses could be manifesting as longer TTP. In addition, infertility is a multi-factorial condition with multiple etiologies and we were unable to examine separately the association between air pollution and cause-specific infertility.
Our study population includes volunteer pregnancy planners who were enrolled through the internet. Internet-based recruitment, which has been used in many observational studies and randomized trials, should not bias etiologic associations based on internal comparisons, as we have previously shown in this cohort.56 Pregnancy planning is related to higher socioeconomic status. Therefore, air pollution could have a weaker effect among pregnancy planners, who may be more likely to have advantageous housing characteristics (e.g., better filtration57) or other built environment features that reduce personal exposure to ambient pollution. Thus, our results may not be generalizable to populations with lower socioeconomic status. In addition, air pollution levels in this cohort and in Denmark generally are low relative to other parts of the world. Our results thus may not be generalizable to regions with higher exposures. Finally, air pollution exposures were lower among participants who were lost to follow-up, indicating that differential loss to follow-up may have biased our results. If participants lost to follow-up also had lower probability of conception, this would result in upward bias; therefore, we suspect that our results are conservative.
Interpretation
Our findings are largely consistent with the literature on this topic. Specifically, four existing epidemiologic studies have estimated the association between air pollution and fertility in couples trying to conceive spontaneously. In a birth cohort of 1,916 couples from the Czech Republic, FRs for a 10 μg/m3 increase in PM2.5 and NO2 at a nearby air monitoring station were 0.78 (95% CI: 0.65, 0.94) and 0.72 (95% CI: 0.53, 0.97), respectively.33 In the Nurses’ Health Study II, a U.S.-based prospective study of nurses, a 10 μg/m3 increase in annual PM2.5–10 concentrations was associated with 1.10 times the risk of self-reported infertility (95% CI: 0.96, 1.27).34 Among 501 couples from Michigan and Texas, the FRs for daily ozone concentration the day before ovulation and NOx concentrations 8 days post-ovulation were 0.83 (95% CI: 0.72, 0.96) and 0.84 (95% CI: 0.71, 0.99), respectively.35 Cycle-specific averages of other pollutants (including CO, PM2.5, PM10, and SO2) were not related to fecundability. Finally, in a retrospective study of 10,211 couples living in China, average exposure to PM2.5 during 1-, 3, and 5-year intervals before the attempt was associated with 11% lower fecundability (95% CI: 14%, 8%) and 1.20 times the risk of infertility (95% CI: 1.13, 1.27).36
Several biologic mechanisms exist through which air pollution, and PM specifically, could influence fertility. Animal and in vitro studies demonstrate that PM can increase systemic inflammation and oxidative stress,58 both of which are relevant for fertility.18, 20 Epidemiologic studies have shown that air pollution exposure may diminish ovarian reserve.59–61 In addition, epidemiologic studies of couples undergoing in vitro fertilization can inform underlying mechanisms, as each step in the reproductive process is observed and measured. For instance, in a cohort of 357 women undergoing fertility treatment at a Massachusetts hospital, associations between traffic-related air pollutants were strongest early in the IVF cycle, specifically before embryo transfer.29 This indicates that air pollution may have adverse effects on folliculogenesis or early embryonic development.
CONCLUSIONS
Our finding of an association between ambient exposure to PM2.5 and PM10 and fecundability among Danish pregnancy planners adds to the growing body of literature suggesting an adverse effect of air pollution on human fecundity. Of note, these findings were observed at levels generally lower than the European Union Air Quality Standards, indicating that current air quality standards may be insufficient to protect against adverse reproductive health effects.
Supplementary Material
SYNOPSIS.
Study question:
To what extent is ambient air pollution associated with fecundability among couples trying to conceive spontaneously?
What’s already known:
Animal and epidemiologic studies support a potential role for air pollution in the etiology of infertility.
What this study adds:
In this preconception cohort of 10,183 female pregnancy planners in Denmark, we found that residential exposure to ambient particulate matter <2.5 μm and <10 μm during the preconception period was related to slightly reduced fecundability. Notably, air pollution in this cohort was low, and almost entirely at levels deemed safe by the European Union air quality standards.
Acknowledgments
FUNDING
This work was funded by the National Institute of Environmental Health Sciences (R01-ES028923).
REFERENCES
- 1.Collaborators GBDRF. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018; 392:1923–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi L, Zanobetti A, Kloog I, Coull BA, Koutrakis P, Melly SJ, et al. Low-Concentration PM2.5 and Mortality: Estimating Acute and Chronic Effects in a Population-Based Study. Environ Health Perspect. 2016; 124:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu C, Chen R, Sera F, Vicedo-Cabrera AM, Guo Y, Tong S, et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N Engl J Med. 2019; 381:705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raaschou-Nielsen O, Thorsteinson E, Antonsen S, Holst GJ, Sigsgaard T, Geels C, et al. Long-term exposure to air pollution and mortality in the Danish population a nationwide study. EClinicalMedicine. 2020; 28:100605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Rodopoulou S, de Hoogh K, Strak M, Andersen ZJ, Atkinson R, et al. Long-Term Exposure to Fine Particle Elemental Components and Natural and Cause-Specific Mortality-a Pooled Analysis of Eight European Cohorts within the ELAPSE Project. Environ Health Perspect. 2021; 129:47009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brauer M, Casadei B, Harrington RA, Kovacs R, Sliwa K, Group WHFAPE. Taking a Stand Against Air Pollution-The Impact on Cardiovascular Disease: A Joint Opinion from the World Heart Federation, American College of Cardiology, American Heart Association, and the European Society of Cardiology. J Am Coll Cardiol. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park J, Kim HJ, Lee CH, Lee CH, Lee HW. Impact of long-term exposure to ambient air pollution on the incidence of chronic obstructive pulmonary disease: A systematic review and meta-analysis. Environ Res. 2021; 194:110703. [DOI] [PubMed] [Google Scholar]
- 8.Liu S, Jorgensen JT, Ljungman P, Pershagen G, Bellander T, Leander K, et al. Long-term exposure to low-level air pollution and incidence of asthma: the ELAPSE project. Eur Respir J. 2020. [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Jorgensen JT, Ljungman P, Pershagen G, Bellander T, Leander K, et al. Long-term exposure to low-level air pollution and incidence of chronic obstructive pulmonary disease: The ELAPSE project. Environ Int. 2021; 146:106267. [DOI] [PubMed] [Google Scholar]
- 10.Hvidtfeldt UA, Severi G, Andersen ZJ, Atkinson R, Bauwelinck M, Bellander T, et al. Long-term low-level ambient air pollution exposure and risk of lung cancer - A pooled analysis of 7 European cohorts. Environ Int. 2021; 146:106249. [DOI] [PubMed] [Google Scholar]
- 11.Stieb DM, Chen L, Eshoul M, Judek S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res. 2012; 117:100–111. [DOI] [PubMed] [Google Scholar]
- 12.Li X, Huang S, Jiao A, Yang X, Yun J, Wang Y, et al. Association between ambient fine particulate matter and preterm birth or term low birth weight: An updated systematic review and meta-analysis. Environ Pollut. 2017; 227:596–605. [DOI] [PubMed] [Google Scholar]
- 13.Papadogeorgou G, Kioumourtzoglou MA, Braun D, Zanobetti A. Low Levels of Air Pollution and Health: Effect Estimates, Methodological Challenges, and Future Directions. Curr Environ Health Rep. 2019; 6:105–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Di Q, Wang Y, Zanobetti A, Wang Y, Koutrakis P, Choirat C, et al. Air Pollution and Mortality in the Medicare Population. N Engl J Med. 2017; 376:2513–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith S, Pfeifer SM, Collins JA. Diagnosis and management of female infertility. JAMA. 2003; 290:1767–1770. [DOI] [PubMed] [Google Scholar]
- 16.Thoma ME, McLain AC, Louis JF, King RB, Trumble AC, Sundaram R, et al. Prevalence of infertility in the United States as estimated by the current duration approach and a traditional constructed approach. Fertil Steril. 2013; 99:1324–1331 e1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra A, Copen CE, Stephen EH. Infertility and impaired fecundity in the United States, 1982–2010: Data from the National Survey of Family Growth. National Health Statistics Reports. 2013:1–18, 11 p following 19. [PubMed] [Google Scholar]
- 18.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012; 10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal A, Rana M, Qiu E, AlBunni H, Bui AD, Henkel R. Role of oxidative stress, infection and inflammation in male infertility. Andrologia. 2018; 50:e13126. [DOI] [PubMed] [Google Scholar]
- 20.Maybin JA, Critchley HO, Jabbour HN. Inflammatory pathways in endometrial disorders. Mol Cell Endocrinol. 2011; 335:42–51. [DOI] [PubMed] [Google Scholar]
- 21.Oh SM, Ryu BT, Chung KH. Identification of estrogenic and antiestrogenic activities of respirable diesel exhaust particles by bioassay-directed fractionation. Arch Pharm Res. 2008; 31:75–82. [DOI] [PubMed] [Google Scholar]
- 22.Sidlova T, Novak J, Janosek J, Andel P, Giesy JP, Hilscherova K. Dioxin-like and endocrine disruptive activity of traffic-contaminated soil samples. Arch Environ Contam Toxicol. 2009; 57:639–650. [DOI] [PubMed] [Google Scholar]
- 23.Wang J, Xie P, Kettrup A, Schramm KW. Inhibition of progesterone receptor activity in recombinant yeast by soot from fossil fuel combustion emissions and air particulate materials. Sci Total Environ. 2005; 349:120–128. [DOI] [PubMed] [Google Scholar]
- 24.Mohallem SV, de Araujo Lobo DJ, Pesquero CR, Assuncao JV, de Andre PA, Saldiva PH, et al. Decreased fertility in mice exposed to environmental air pollution in the city of Sao Paulo. Environ Res. 2005; 98:196–202. [DOI] [PubMed] [Google Scholar]
- 25.Veras MM, Damaceno-Rodrigues NR, Guimaraes Silva RM, Scoriza JN, Saldiva PH, Caldini EG, et al. Chronic exposure to fine particulate matter emitted by traffic affects reproductive and fetal outcomes in mice. Environ Res. 2009; 109:536–543. [DOI] [PubMed] [Google Scholar]
- 26.Nieuwenhuijsen MJ, Basagana X, Dadvand P, Martinez D, Cirach M, Beelen R, et al. Air pollution and human fertility rates. Environ Int. 2014; 70:9–14. [DOI] [PubMed] [Google Scholar]
- 27.Xue T, Zhu T. Increment of ambient exposure to fine particles and the reduced human fertility rate in China, 2000–2010. Sci Total Environ. 2018; 642:497–504. [DOI] [PubMed] [Google Scholar]
- 28.Xue T, Zhu T. Association between fertility rate reduction and pre-gestational exposure to ambient fine particles in the United States, 2003–2011. Environ Int. 2018; 121:955–962. [DOI] [PubMed] [Google Scholar]
- 29.Gaskins AJ, Fong KC, Abu Awad Y, Di Q, Minguez-Alarcon L, Chavarro JE, et al. Time-Varying Exposure to Air Pollution and Outcomes of in Vitro Fertilization among Couples from a Fertility Clinic. Environ Health Perspect. 2019; 127:77002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Quraishi SM, Lin PC, Richter KS, Hinckley MD, Yee B, Neal-Perry G, et al. Ambient Air Pollution Exposure and Fecundability in Women Undergoing In Vitro Fertilization. Environ Epidemiol. 2019; 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Legro RS, Sauer MV, Mottla GL, Richter KS, Li X, Dodson WC, et al. Effect of air quality on assisted human reproduction. Hum Reprod. 2010; 25:1317–1324. [DOI] [PubMed] [Google Scholar]
- 32.Choe SA, Jun YB, Lee WS, Yoon TK, Kim SY. Association between ambient air pollution and pregnancy rate in women who underwent IVF. Hum Reprod. 2018; 33:1071–1078. [DOI] [PubMed] [Google Scholar]
- 33.Slama R, Bottagisi S, Solansky I, Lepeule J, Giorgis-Allemand L, Sram R. Short-term impact of atmospheric pollution on fecundability. Epidemiology. 2013; 24:871–879. [DOI] [PubMed] [Google Scholar]
- 34.Mahalingaiah S, Hart JE, Laden F, Farland LV, Hewlett MM, Chavarro J, et al. Adult air pollution exposure and risk of infertility in the Nurses’ Health Study II. Hum Reprod. 2016; 31:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nobles CJ, Schisterman EF, Ha S, Buck Louis GM, Sherman S, Mendola P. Time-varying cycle average and daily variation in ambient air pollution and fecundability. Hum Reprod. 2018; 33:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Q, Zheng D, Wang Y, Li R, Wu H, Xu S, et al. Association between exposure to airborne particulate matter less than 2.5 mum and human fecundity in China. Environ Int. 2021; 146:106231. [DOI] [PubMed] [Google Scholar]
- 37.Mikkelsen EM, Hatch EE, Wise LA, Rothman KJ, Riis A, Sorensen HT. Cohort Profile: The Danish Web-based Pregnancy Planning Study--’Snart-Gravid’. Int J Epidemiol. 2009; 38:938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huybrechts KF, Mikkelsen EM, Christensen T, Riis AH, Hatch EE, Wise LA, et al. A successful implementation of e-epidemiology: the Danish pregnancy planning study ‘Snart-Gravid’. Eur J Epidemiol. 2010; 25:297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt M, Pedersen L, Sorensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014; 29:541–549. [DOI] [PubMed] [Google Scholar]
- 40.Khan J, Kakosimos K, Raaschou-Nielsen O, Brandt J, Jensen SS, Ellermann T, et al. Development and performance evaluation of new AirGIS—a GIS based air posllution and human exposure modelling system. Atmos Environ. 2018; 198:102–121. [Google Scholar]
- 41.Brandt J, Christensen JH, Frohn LM, Palmgren F, Berkowicz R, Zlatev Z. Operational air pollution forecasts from European to local scale. Atmos Environ. 2001; 35:S91–S98. [Google Scholar]
- 42.Brandt J, Silver JD, Frohn LM, Geels C, Gross A, Hansen AB, et al. An integrated model study for Europe and North America using the Danish Eulerian Hemispheric Model with focus on intercontinental transport of air pollution. Atmos Environ. 2012; 53:156–176. [Google Scholar]
- 43.Frohn LM, Christensen JH, Brandt J. Development of a high-resolution nested air pollution model: the numerical approach. J Comput Phys. 2002; 179:68–94. [Google Scholar]
- 44.Brandt J, Christensen JH, Frohn LM, Berkowicz R. Operational air pollution forecast from regional scale to urban street scale. Part 1: system description. Phys Chem Earth (B). 2001; 26:781–786. [Google Scholar]
- 45.Brandt J, Christensen JH, Frohn LM, Berkowicz R. Operational air pollution forecast from regional scale to urban street scale. Part 2: performance evaluation. Phys Chem Earth (B). 2001; 26:825–830. [Google Scholar]
- 46.Brandt J, Christensen JH, Frohn LM, Berkowicz R. Air pollution forecasting from regional to urban street scale -- implementation and validation for two cities in Denmark. Phys Chem Earth. 2003; 28:335–344. [Google Scholar]
- 47.Berkowicz R OSPM-A parameterised street pollution model. Environ Monit Assess. 2000; 65:323–331. [Google Scholar]
- 48.Ellermann T, Nygaard J, Nojgaard JK, Nordstrom C, Brandt J, Christensen J, et al. The Danish Air Quality Monitoring Programme Annual Summary for 2018. Scientific Report from DCE--Danish Centre for Environment and Energy; 2020. [cited 2021 Jan 3]; Available from: https://dce2.au.dk/pub/SR360.pdf. [Google Scholar]
- 49.Ketzel M, Frohn LM, Christensen JH, Brandt J, Massling A, Andersen C, et al. Modelling ultrafine particle number concentrations at address resolution in Denmark from 1979 to 2018 -- Part 2: Local and street scale modelling and evulation. Atmos Environ. 2021; 264:118633. [Google Scholar]
- 50.Schisterman EF, Cole SR, Ye A, Platt RW. Accuracy loss due to selection bias in cohort studies with left truncation. Paediatr Perinat Epidemiol. 2013; 27:491–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howards PP, Hertz-Picciotto I, Poole C. Conditions for bias from differential left truncation. Am J Epidemiol. 2007; 165:444–452. [DOI] [PubMed] [Google Scholar]
- 52.Stingone JA, Luben TJ, Carmichael SL, Aylsworth AS, Botto LD, Correa A, et al. Maternal Exposure to Nitrogen Dioxide, Intake of Methyl Nutrients, and Congenital Heart Defects in Offspring. Am J Epidemiol. 2017; 186:719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Goodrich AJ, Volk HE, Tancredi DJ, McConnell R, Lurmann FW, Hansen RL, et al. Joint effects of prenatal air pollutant exposure and maternal folic acid supplementation on risk of autism spectrum disorder. Autism Res. 2018; 11:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gaskins AJ, Minguez-Alarcon L, Fong KC, Abu Awad Y, Di Q, Chavarro J, et al. Exposure to traffic-related air pollution, supplemental folate intake, and live birth among women undergoing assisted reproduction. Am J Epidemiol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ha S, Sundaram R, Buck Louis GM, Nobles C, Seeni I, Sherman S, et al. Ambient air pollution and the risk of pregnancy loss: a prospective cohort study. Fertil Steril. 2018; 109:148–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hatch EE, Hahn KA, Wise LA, Mikkelsen EM, Kumar R, Fox MP, et al. Evaluation of Selection Bias in an Internet-based Study of Pregnancy Planners. Epidemiology. 2016; 27:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chan WR, Joh J, Sherman MH. Analysis of air leakage measurements of US houses. Energy Build [Internet]. 2013; 66:616–625. [Google Scholar]
- 58.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004; 109:2655–2671. [DOI] [PubMed] [Google Scholar]
- 59.Kim H, Choe SA, Kim OJ, Kim SY, Kim S, Im C, et al. Outdoor air pollution and diminished ovarian reserve among infertile Korean women. Environ Health Prev Med. 2021; 26:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abareshi F, Sharifi Z, Hekmatshoar R, Fallahi M, Lari Najafi M, Ahmadi Asour A, et al. Association of exposure to air pollution and green space with ovarian reserve hormones levels. Environ Res. 2020; 184:109342. [DOI] [PubMed] [Google Scholar]
- 61.Gaskins AJ, Minguez-Alarcon L, Fong KC, Abdelmessih S, Coull BA, Chavarro JE, et al. Exposure to Fine Particulate Matter and Ovarian Reserve Among Women from a Fertility Clinic. Epidemiology. 2019; 30:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.