Abstract
Background:
Machine perfusion is gaining interest as an efficient method of tissue preservation of Vascularized Composite Allografts (VCA). The aim of this study was to develop a protocol for ex vivo subnormothermic oxygenated machine perfusion (SNMP) on rodent hindlimbs and to validate our protocol in a heterotopic hindlimb transplant model.
Methods:
In this optimization study we compared three different solutions during 6 hours of SNMP (n=4 per group). Ten control limbs were stored in a preservation solution on Static Cold Storage [SCS]). During SNMP we monitored arterial flowrate, lactate levels and edema. After SNMP, muscle biopsies were taken for histology examination and energy charge analysis. We validated the best perfusion protocol in a heterotopic limb transplantation model with 30-day follow up (n=13). As controls, we transplanted untreated limbs (n=5) and hindlimbs preserved with either 6 or 24 hours of SCS (n=4 and n=5).
Results:
During SNMP, arterial outflow increased, and lactate clearance decreased in all groups. Total edema was significantly lower in the HBOC-201 group compared to the BSA group (p=0.005), 4.9 (4.3–6.1) vs. 48.8 (39.1–53.2) percentage, but not to the BSA + PEG group (p=0.19). Energy charge levels of SCS controls decreased 4-fold compared to limbs perfused with acellular oxygen carrier HBOC-201, 0.10 (0.07–0.17) vs. 0.46 (0.42–0.49) respectively (p=0.002).
Conclusion:
Six hours ex vivo SNMP of rodent hindlimbs using an acellular oxygen carrier HBOC-201 results in superior tissue preservation compared to conventional SCS.
Keywords: oxygenated machine perfusion, preservation, plastic surgery, limb transplantation, face transplantation, energy charge, free flap, oxygen carrier, vascularized composite allotransplantation
INTRODUCTION
Vascularized composite allotransplantation (VCA) remains the most advanced treatment option to restore motor function and aesthetics in patients living with devastating disfigurements. To date, worldwide more than 200 patients have benefited from VCA, the majority receiving hand/upper extremity or face transplants (1, 2). Since in all fields of transplantation, graft viability prior to transplantation is inextricably linked to post-transplant success, minimization of graft injury prior to transplantation is also key to improve outcomes in VCA (3).
The current gold standard of graft preservation is based on cooling the graft in a cold preservation solution (4° C) on ice, referred to as static cold storage (SCS). The significant drop in temperature lowers the metabolic rate of the tissue, which enable the graft to temporarily cope with the absence of oxygen and nutrients. Muscle cells (the dominant tissue type as per quantity in most VCA grafts) are, however, highly metabolically active which allows only for an extremely limited ischemia time; irreversible cell damage already occurs after as little as 4 hours of ischemia (4). Moreover, upon reperfusion, the sudden abundance of oxygen will aggravate cell damage even more, by initiating reactive oxygen species (ROS) formation and intracellular calcium influx leading to mitochondrial dysfunction and eventually cell death. Apoptotic and necrotic muscle cells ultimately trigger the immune system, affecting both early and long-term graft function (5–7).
Ex vivo machine perfusion is gaining increasing attention as an alternative method of VCA graft preservation. During ex vivo machine perfusion, the oxygenated perfusate allows maintenance of aerobic metabolism, thereby limiting tissue damage and allowing for quality assessment and possibly viability improvement. Other groups have reported favorable results of both hypothermic and normothermic machine perfusion of VCA grafts compared to SCS (8–10). Oxygenated subnormothermic machine perfusion (SNMP) is a perfusion modality performed at room temperature (21°C) and aims to enable energy metabolism while keeping tissue metabolic demands easy to fulfill. SNMP of both rat and human livers prior to transplantation has been shown to improve the quality of the liver by reducing ischemia-induced damage (11–13).
In this study, we develop a protocol for 6 hours of SNMP on VCA grafts. First, we compare three media options in terms of perfusion characteristics as well as energy status after perfusion. In all cases we use a muscle culture media as the base, which has not been considered previously in VCA perfusion literature. Differentiating components in the three perfusion solutions are i) polyethylene glycol (PEG) and ii) acellular oxygen carrier HBOC-201 (Hemopure®, HbO2, Therapeutics LLC) with prostaglandin. PEG is a water-soluble nontoxic polymer with multiple beneficial effects. Addition of large PEG molecules in vivo is associated protective effects against I/R injury in both rat hearts and livers (14, 15). The protective effects were associated with decreased vascular permeability, decreased oxidative stress, and inhibition of cell death (16). HBOC-201 is a hemoglobin-based oxygen carrier polymer (250 kDa) that has the capacity to unload oxygen in peripheral tissues at sub-physiological temperatures (17). Finally, for the perfusion protocol with best ex vivo results, we test in a heterotopic hindlimb transplant model.
MATERIALS & METHODS
Animals & Housing.
For perfusion optimization, twelve Lewis rats (250–300g) were used as hindlimb donors and muscle biopsies of another 3 rats were used to set reference values (Charles Rivers Laboratories, Wilmington, MA, USA). For transplant studies, thirty-seven male Lewis rats (250–300g) were used as donors and thirty-seven male Lewis rats (300–350g) were used as recipients. Male rats easily facilitate systemic heparinization via the penile vein during the limb procurement, as detailed in the next section. Animals were housed and maintained in accordance with the National Research Council guidelines and the experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of the Massachusetts General Hospital (Boston, MA, USA).
Limb Procurement
A heterotopic model as previously described by Ulusal and colleagues was used (18). Our protocol for limb procurement can be found in the supplementary data section.
Perfusion Solutions
In first part of this study, 3 different perfusion solutions were tested ex vivo for 6 hours of subnormothermic machine perfusion (SNMP) on rodent partial hindlimbs. A detailed overview of all perfusion solutions is summarized in Table 1. In all groups, skeletal muscle media with basic epidermal and fibroblast growth factors (PromoCell, C-23160, Heidelberg, Germany) provided the base of the solution. Bovine serum albumin (BSA) was the base colloid component in all groups. Also, additional supplements such as insulin, heparin, dexamethasone, hydrocortisone and antibiotics were same between groups. The main differences between these perfusion solutions were based on the presence or absence of these 2 components:
Table 1.
Overview machine perfusion solutions
| Group 1 BSA n=4 | Group 2 BSA + PEG n=4 | Group 3 HBOC-201 n=4 | |
|---|---|---|---|
|
| |||
| Solution base | |||
| - PromoCell muscle media (mL) | 500 | 500 | 375 |
| - HBOC-201 (mL) | - | - | 125 |
|
| |||
| Differentiating additives | |||
| - Bovine serum albumin (BSA) (g) | 10 | 10 | 10 |
| - Polyethylene glycol (PEG) (g) | - | 15 | 15 |
| - Prostaglandin1 (μL/min) | - | - | 0.2 |
|
| |||
| Additional supplements | |||
| - Penicillin-Streptomycin (mL) | 2 | 2 | 2 |
| - L-glutamine (mL) | 5 | 5 | 5 |
| - Insulin (μL) | 100 | 100 | 100 |
| - Heparin (mL) | 1 | 1 | 1 |
| - Hydrocortisone (μL) | 100 | 100 | 100 |
| - Dexamethasone (μg) | 8 | 8 | 8 |
Overview of the different perfusion solutions. Abbreviations used; BSA = bovine serum albumin, PEG = polyethylene glycol and HBOC-201 = hemoglobin based oxygen carrier-201.
Prostaglandin is Alprostadil 500mcg/mL vial is diluted in 50mL of saline according to manufacturing instructions. This mixture was added to the solution via a syringe pump at a flow rate of 0.2 μL/min.
Addition of polyethylene glycol (PEG) with a molecular weight of 35 kDa.
Addition of an acellular oxygen carrier, HBOC-201 (Hemopure®, HbO2, Therapeutics LLC) in combination with vasodilator prostaglandin
The total volume of the perfusion solution was 500 mL in all groups.
Machine Perfusion
For 6 hours of SNMP, we used a custom-made machine perfusion system as displayed in Figure 1. Key components for our system were a rotating pump (07522–20 DRIVE MFLEX L/S 600RPM 115/230, Cole-Parmer, Vernon Hills, IL), tubing (Masterflex platinum-cured silicone tubing, L/S 16, Cole-Parmer, Vernon Hills, IL) and a membrane oxygenator, bubble trap chamber and tissue bath (catalog numbers 130144, 130149 and 158400 respectively, Radnoti LTD, Dublin, Ireland). Vascular pressure was measured via a pressure transducer (PT-F, Living Systems Instrumentation, St Albans City, VT) and read by a portable pressure monitor (PM-P-1, Catamount Research and Development, St Albans, VT). Prior to connecting the limb, pressures of the system without the limb were noted at different flow rates (Pressurewithout). During perfusion, pressures with the limb were observed (Pressurewith) and flows were adjusted accordingly to aim for a vascular pressure between 30–40 mmHg. The vascular pressure was calculated as Pressurewith - Pressurewithout. Vascular resistance was calculated as by dividing the vascular pressure by the flow rate.
Figure 1. Ex vivo subnormothermic machine perfusion set up with HBOC-201 perfusion solution.
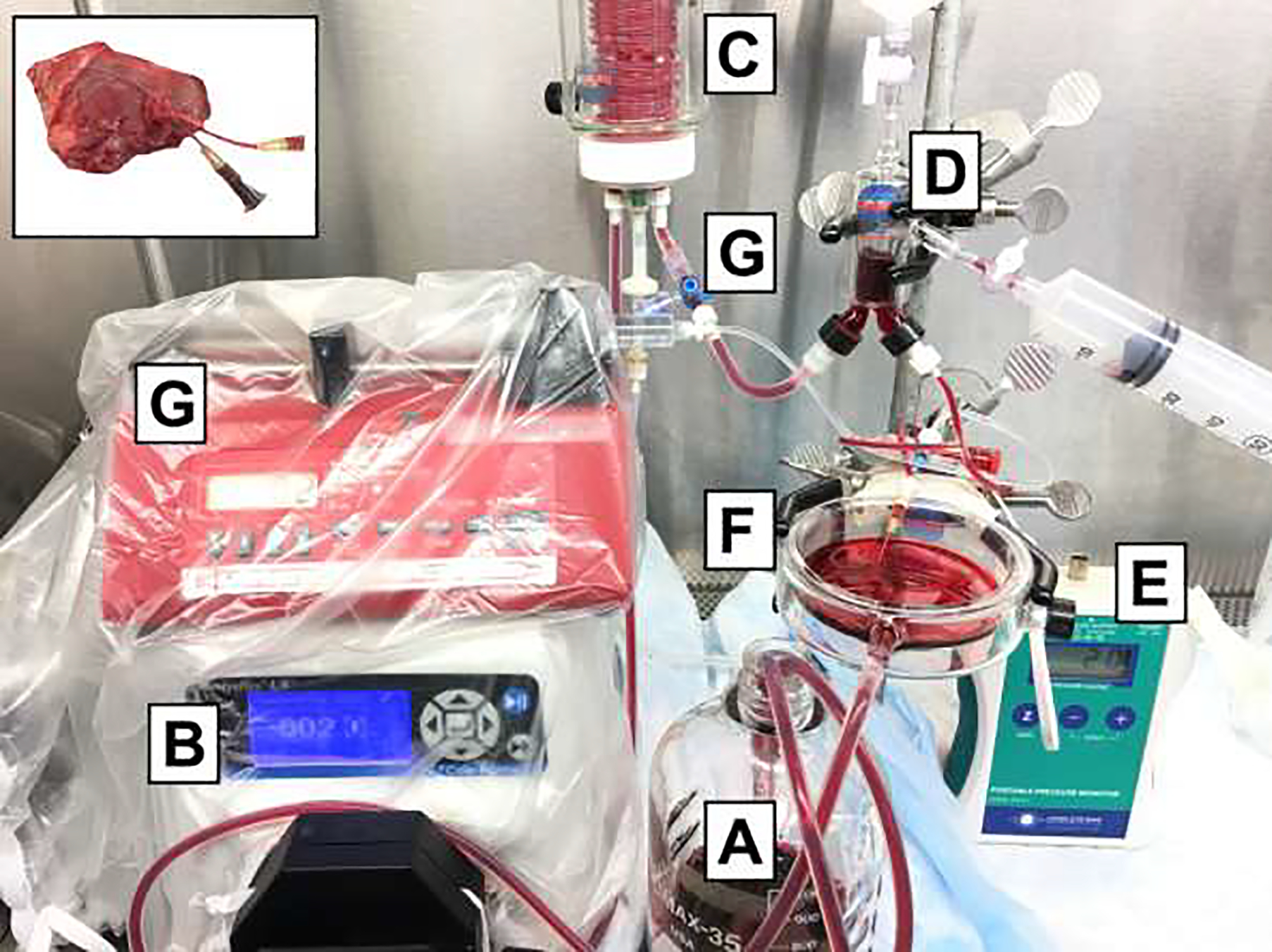
The circuit consists of perfusion solution (A) that is pumped via a roller pump (B) to the oxygenator (C), that is oxygenated with a carbogen mixture (5% CO2 and 95% oxygen). The solution then goes through the bubble trap (D) to prevent air bubbles going into the limb. The pressure is measured (E) at the level of the limb that is laying the basin (F). Inflow samples are measure at the inflow valve (G) with outflow samples are measured directly from the venous outflow canula (as shown in upper left panel).
During 6 hours of perfusion, perfusion samples were collected from both the arterial inflow and venous outflow. An i-STAT analyzer (Albott, Princeton, NJ) was used to measure perfusate levels of potassium and lactate as well as oxygen tension and saturation. At the end of 6 hours of SNMP, biopsies form the rectus femoris muscle were taken. Biopsies were snap-frozen in liquid nitrogen and stored in a −80 °C freezer for mass spectrometry or stored in formalin for histological analysis.
Transplantation
In total, 13 right partial hindlimbs were transplanted were transplanted after 6 hours of SNMP (HBOC-201 group). Transplant controls included hindlimbs that were preserved for 6 hours of SCS (n=4), 24 hours of SCS (n=5) or hindlimbs that were transplanted directly after harvest referred to as fresh controls (n=5). Heterotopic hindlimb transplantation was performed as described previously by Ulusal and colleagues (18). Post-operative follow-up was 30 days in all study groups. Viability of the graft was assessed by physical examination: temperature (cold or body temperature), color (pale or blue) and turgor (swelling).
Perfusate Injury Markers
Lactate clearance (μmol/min) was calculated by the difference between the arterial and venous lactate concentration (mmol/L) and corrected for flow (mL/min). Potassium release (μmol/min) was calculated as differences in concentration (mmol/L) between the arterial inflow and venous outflow and corrected for flow (mL/min).
Oxygen Consumption
Total oxygen consumption was calculated by the difference between the arterial and venous oxygen content and corrected for flow. The following formula was used for calculations:
Total oxygen consumption (μL O2/min) = cO2 * (pO2art-ven * flow) + (Hb + cHb + (SO2art-ven * flow)), where cO2 is the oxygen solubility coefficient (3.14 * 10−5 mLO2/mmHg O2/mL), pO2art-ven is the difference in partial oxygen pressure between in artery inflow and venous outflow (mmHg), flow is the arterial inflow (mL/min), Hb is the hemoglobin concentration (g/mL) and cHb is the oxygen binding capacity of Hb (1.26 for HBOC-201).
Energy Charge Analysis
All muscle biopsies were analyzed with liquid chromatography-mass spectrometry for energy cofactors adenosine triphosphate [ATP], adenosine diphosphate [ADP], adenosine monophosphate [AMP], which are previously demonstrated as indicators of viability for liver perfusion and transplant (19).
All frozen tissue biopsies were pulverized, weighted (averaging ~25 mg) and analyzed for energy cofactors using a liquid chromatography-mass spectrometry system (AB Sciex, Foster City, CA), as previously described (19).
Histology Analysis of Muscle Biopsies
Muscle biopsies were fixated in formalin, paraffin embedded, and cross-sectioned. Slides were stained with hematoxylin and eosin (H&E) and apoptosis marker TUNEL by the pathology department.
Statistical Analysis
Continuous data are reported as medians with interquartile range, categorial variables as absolute numbers. Differences between groups were analyzed using a Kruskal-Wallis H test with a Dunn’s post-test or Mann-Whitney test when applicable. All statistical analysis was performed using Prism 5.0a for Mac OSX (GraphPad Software, La Jolla, CA). P values less than 0.05 were considered to be significant.
RESULTS
Procurement
Average procurement time was 20 minutes (+/− 5 min) with an average warm ischemia time until machine perfusion of 10–15 minutes.
Optimization of Perfusion Solution
Hemodynamic Parameters
Arterial flow increased in all groups during the first half of perfusion and remained stable thereafter (Figure 2A). After 1 hour of SNMP, median flows were significantly higher in the BSA group compared to the HBOC-201 group (p=0.01), 1.4 (1–2.1) vs. 0.4 (0.2–0.4) mL/min respectively. Median flows continued to be higher in the BSA group compared to the HBOC-201 (p=0.27), but not the BSA + PEG (p=0.43), group until the end of 6 hours perfusion.
Figure 2. Overview perfusion parameters.
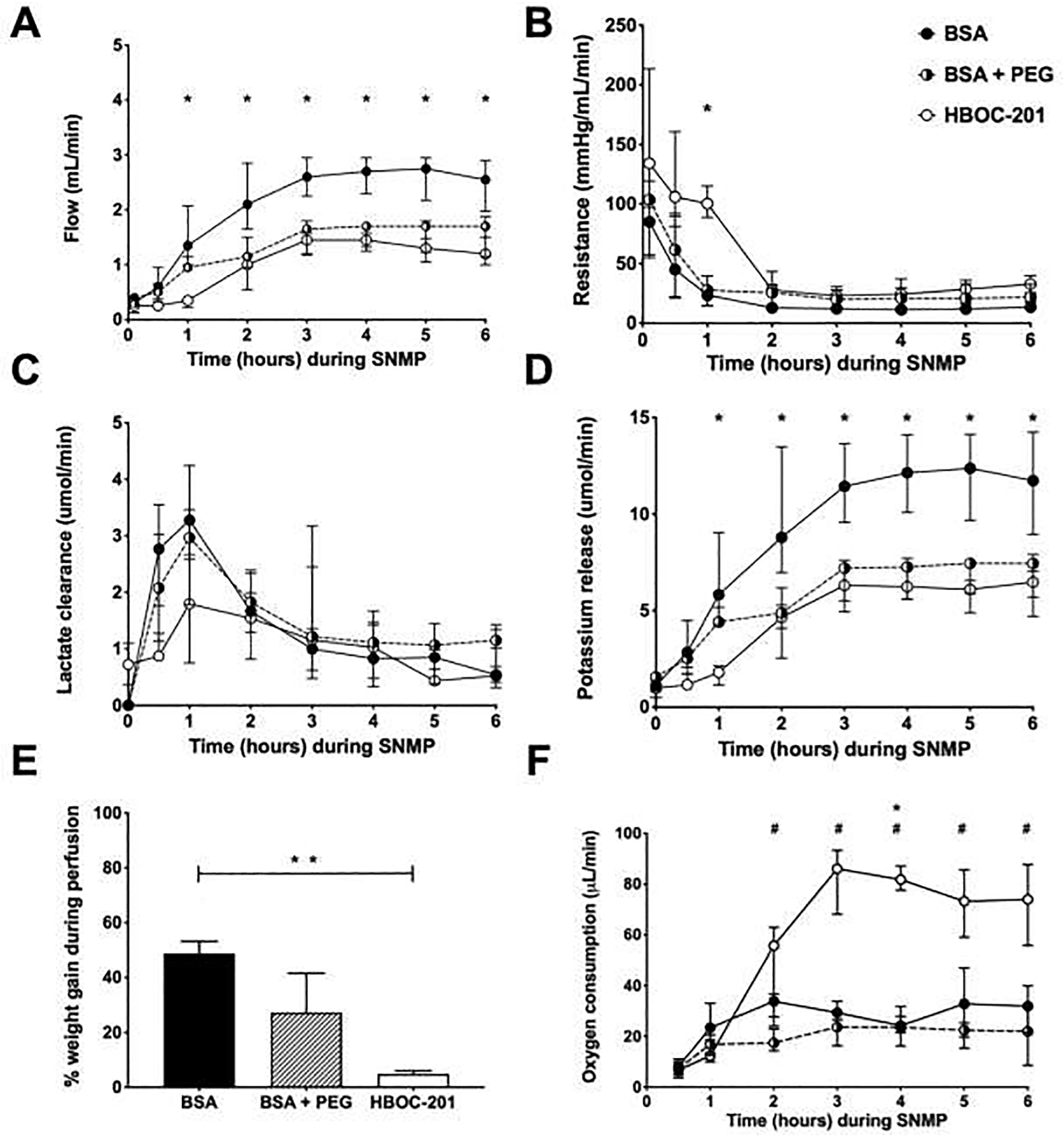
In all groups, arterial flow increased while vascular resistance decreased over the course of perfusion (Panel A&B). Lactate levels peaked during the first hour of perfusion and decreased thereafter (Panel C). After 2 hours of SNMP, potassium levels were significantly higher in the BSA group compared to HBOC-201 group, but not compared to the BSA + PEG group (Panel D). Weight gain was calculated as the difference compared to baseline and was significantly higher in the BSA group compared to HBOC-201 group (p=0.005) (Panel E). After 2 hours of SNMP, oxygen consumption was significantly higher in the HBOC-201 group compared to the BSA (Panel F). Abbreviations used: Abbreviations used; BSA = bovine serum albumin, PEG = polyethylene glycol and HBOC-201 = haemoglobin-based oxygen carrier-201. Asterix (*) indicates significance between HBOC-201 and BSA, hashtag (#) indicates significance between HCOC-201 and BSA + PEG.
Vascular resistance decreased in all groups during the first hour of perfusion and remained stable thereafter (Figure 2B). After 1 hour of SNMP, median vascular resistance was significantly higher in the HBOC-201 compared to the BSA (p=0.01), but not in the BSA + PEG group (p=0.10).
Perfusate Injury Markers
In all groups, lactate clearance increased within the first hour of perfusion and declined thereafter, as presented in Figure 2C. During 6 hours of perfusion, there was no statistical difference in lactate clearance between the groups. It should be noted that the HBOC-201 perfusion fluid had a median lactate concentration of 2.9 mmol/L (2.9–3.0) prior to perfusion while the BSA and BSA + PEG had unmeasurable concentration of lactate prior to perfusion. This is due to the presence of sodium lactate (27 mmol/L) in the HBOC-201 solution as described in the product sheet by the manufacturer (Hemopure, HbO2, Therapeutics LLC). During the 3 hours of SNMP, potassium concentration increased in all groups but levels stabilized thereafter, as presented in Figure 2D. After 1 hour of SNMP, median potassium release was significantly higher in the HBOC-201 group compared to the BSA group (p=0.006), 5.8 (4.3–9.0) vs. 1.8 (1.1–2.1), but not compared to the BSA + PEG group, 5.8 (4.3–9.0) vs. 4.4 (4.2–5.2) (p=0.27). While potassium levels normalized in all groups and after 6 hours of SNMP, median potassium release did not significantly differ between groups (p=0.55).
Weight Gain due to Edema
Limbs were weighed prior to and after 6 hours of SNMP. Median start weight of all limbs prior to perfusion was 19 (17–21) grams and did not differ between groups (p=0.11). Median weight gain (as a percentage of baseline) was significantly lower in the HBOC-201 group compared to the BSA alone group (p=0.005), median increase of 4.9 (4.3–6.1) vs. 48.8 (39.1–53.2), but not compared to the BSA + PEG group (p=0.19), median increase of 27.3 (20.5–41.6) respectively (Figure 2E).
Total Oxygen Consumption
After 2 hours of SNMP, total oxygen consumption was significantly higher in the HBOC-201 group compared to the BSA + PEG group (p=0.03), 55.8 (27.7–63.0) vs. 17.5 (14.3–23.0) μL/min, but not to BSA alone (p=0.87), 55.8 (27.7–63.0) vs. 33.9 (24.0–36.6) μL/min respectively. While oxygen consumption stabilized after the first 2 hours in the BSA and BSA + PEG groups, oxygen consumption in the HBOC-201 continued to increase during the first 4 hours of SNMP before it stabilized for the remainder of SNMP (Figure 2F). After 4 hours of SNMP, total oxygen consumption was significantly higher in the HBOC-201 group compared to both the BSA group (p=0.05), 86.1 (68.1–93.3) vs. 29.4 (26.5–33.8), and the BSA + PEG group (p=0.03), 86.1 (68.1–93.3) vs. 23.7 (16.3–26.6). At the end of 6 hours SNMP, total oxygen consumption continued to be significantly higher in the HBOC-201 group compared to theBSA + PEG group (p=0.03), 74.0 (55.8–87.8) vs. 22.0 (8.6–31.5), but not compared to the BSA alone group (p=0.13), 74.0 (55.8–87.8) vs. 31.9 (21.5–40.0) respectively.
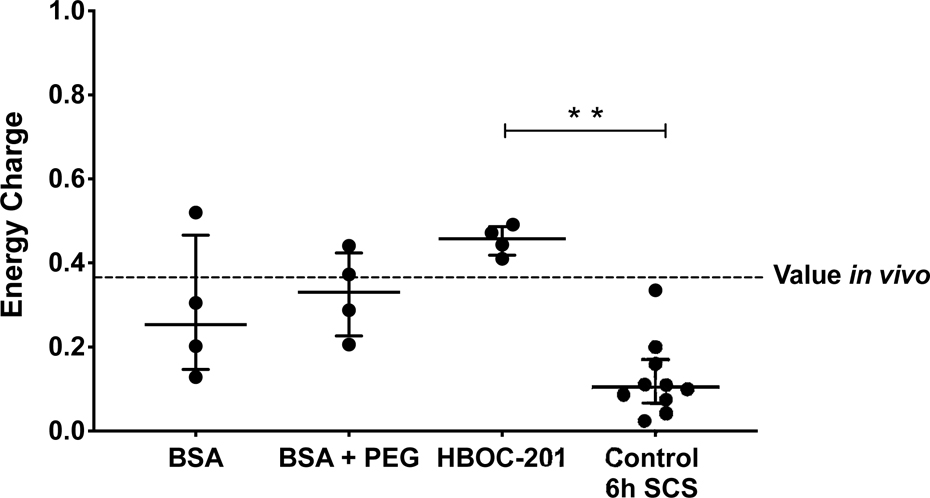
Energy Charge
Energy charge values are displayed in Figure 3. At the end of 6 hours of perfusion, median energy charge levels were comparable between the BSA, BSA + PEG and HBOC-201 groups, 0.25 (0.15–0.47) vs. 0.33 (0.23–0.42) vs. 0.46 (0.42–0.49) (p=0.20) respectively. Interestingly, all energy charge levels of all groups were comparable to the energy charge ratio of in vivo controls (median ratio 0.37 (0.19–0.58)), as indicated by the red dotted line in Figure 3. However, energy charge ratios of SCS control limbs were significantly lower compared to HBOC-201 perfused limbs, 0.10 (0.07–0.17) vs. 0.46 (0.42–0.49) (p=0.002), but not to BSA (p=0.15) and BSA + PEG (p=0.08) limbs.
Figure 3. Energy charge values.
Energy charge ratios of SCS control limbs were significantly lower compared to HBOC-201 perfused limbs, but not BSA and BSA + PEG limbs (p=0.002) respectively. The red dotted line indicates median energy charge levels in vivo. Abbreviations used: Abbreviations used; BSA = bovine serum albumin, PEG = polyethylene glycol and HBOC-201 = hemoglobin-based oxygen carrier-201.
Perfused Limb Histology Assessment
None of the muscle biopsies showed myocyte injury or degeneration after perfusion. Furthermore, none of the muscle biopsies showed apoptotic cell death. Biopsies of BSA perfused limbs showed, however, more signs of interstitial edema compared to HBOC-201 perfused limbs (Figure 4).
Figure 4. Representative muscle histology of limbs after 6 hours of SNMP with BSA, BSA + PEG and HBOC-201 respectively.
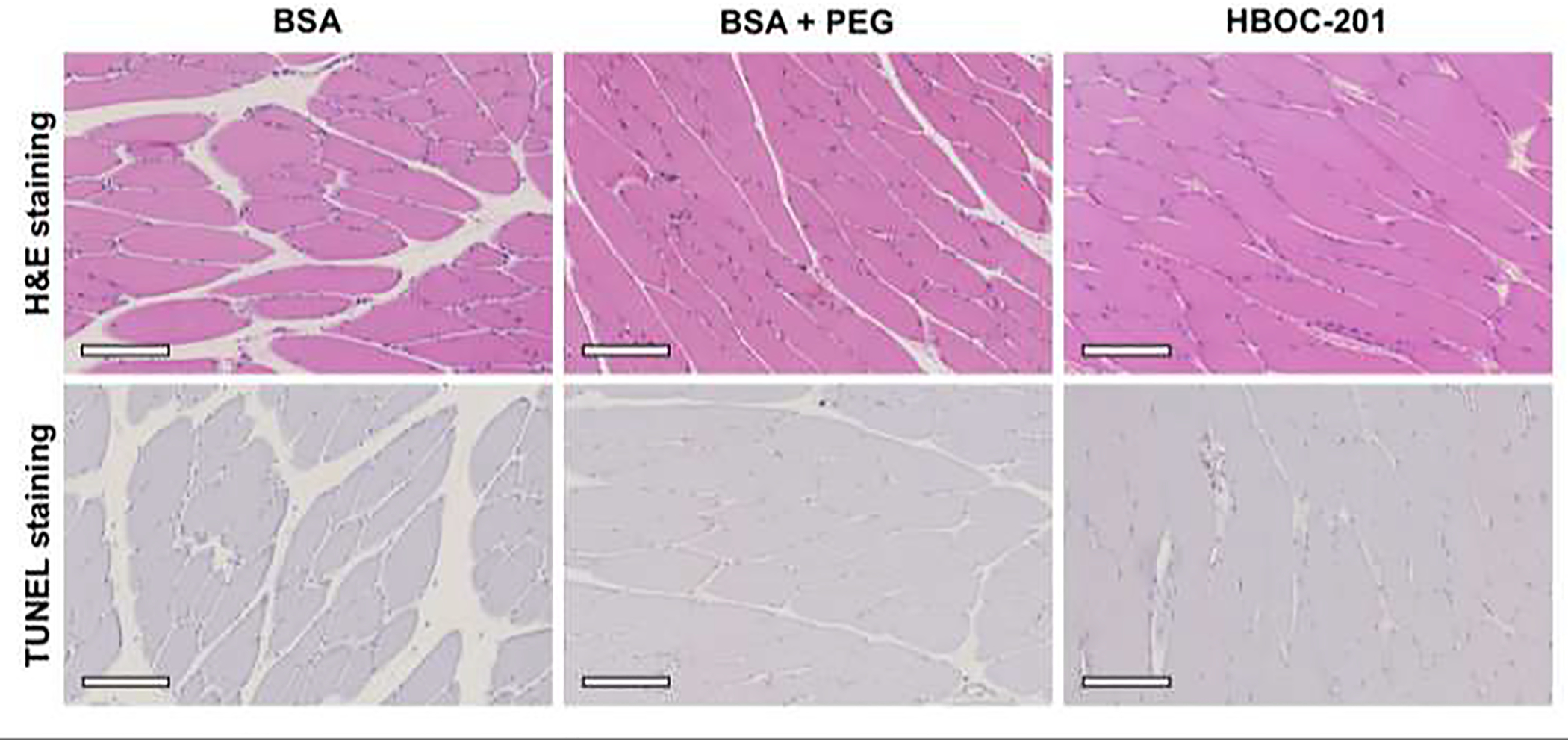
Upper panels represent H&E stained biopsies, lower panels show TUNEL stained biopsies. All biopsies show a normal polygonal structure with no signs of apoptosis. All slides are shown at 10x magnification, and the white ox indicates 200 mm. Abbreviations used: H&E = hematoxylin & eosin, BSA = bovine serum albumin, PEG = polyethylene glycol and HBOC-201 = hemoglobin-based oxygen carrier – 201.
Transplant Survival
Grafts were transplanted and followed for 30 days post-operatively (Figure 5). Mortality rates due to graft failure were 20% in the fresh control group (i.e. untreated control grafts), 15% in the HBOC-201 group and 25% in the SCS group (Figure 6). In the negative control group (24 hours SCS) all animals died as a consequence of graft failure (100%). Automutilation was a significant factor and counted for 50% of the failed experiments in the fresh control group, 20% of the failed experiments in the 24 hours SCS group and 23% of the failed experiments in the HBOC-201 group. Survival was therefore plotted in two separate graphs: first displaying all transplant results (Figure 6A) latter where automutilation is censored (Figure 6B).
Figure 5. Heterotopic hind limb transplant grafts on post-operative days 0, 7, 15, 21 and 30.
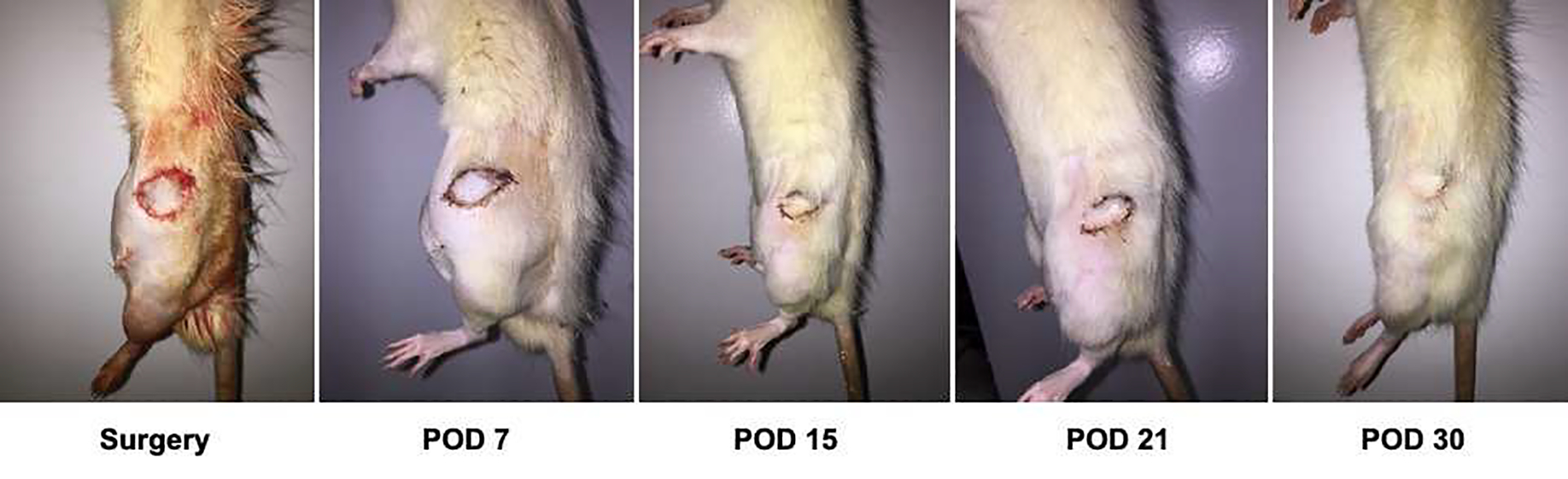
Post-operative follow-up of heterotopic hind limb transplant grafts.
Figure 6. Transplant survival rates after 30 day follow up.
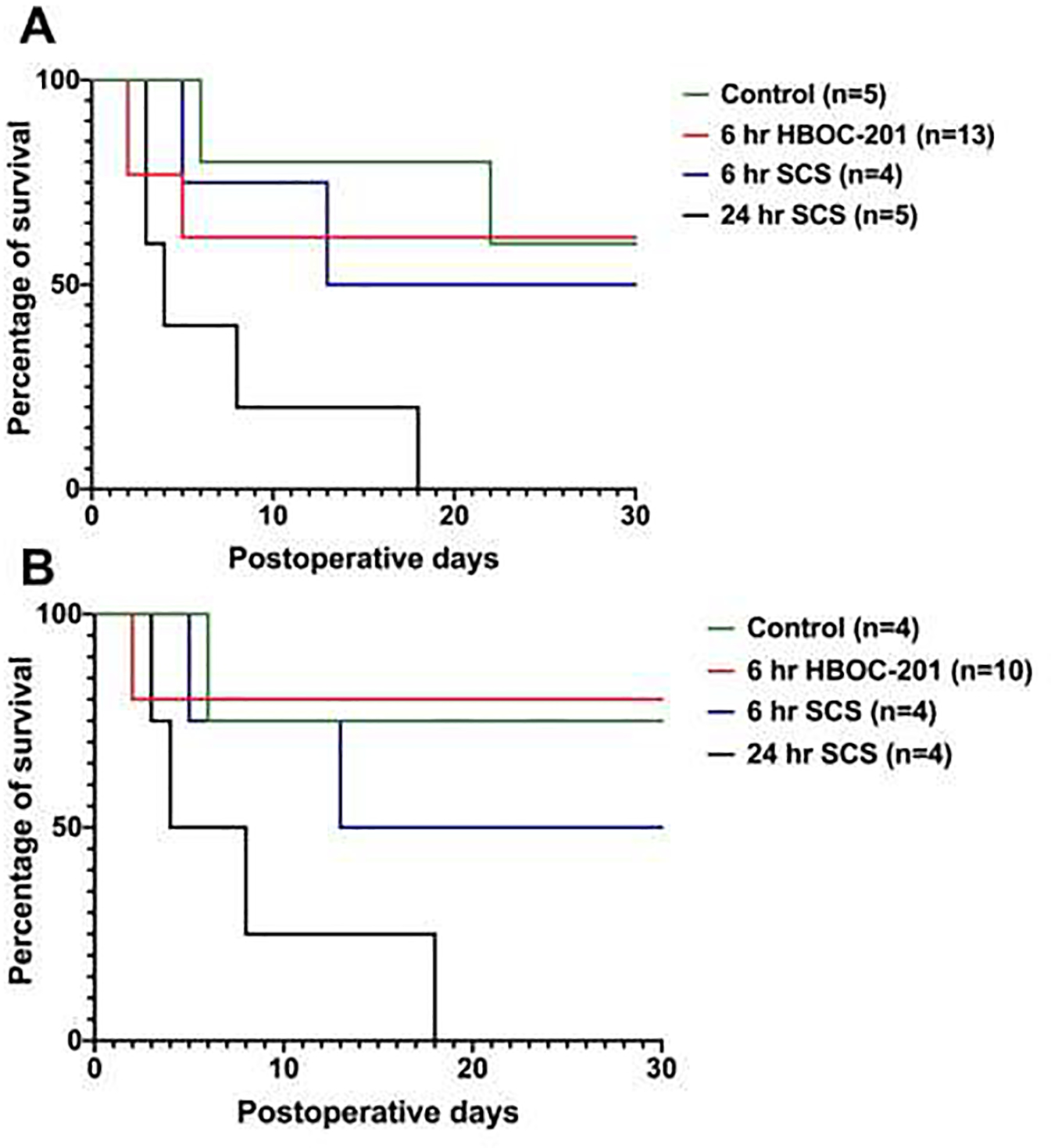
Figure 6A shows survival rates including the failed experiments due to automutilation while these are excluded in Figure 6B. Overall survival in the 6 hours HBOC-201 group is 80% excluding the failed experiments due to automutilation (Figure 6B) and 62% including the failed experiments due to automutilation (Figure 6A). The survival of the fresh controls is 75% excluding the failed experiments due to automutilation (Figure 6B) and 60% including the failed experiments due to automutilation (Figure 6A). Overall survival of the 6 hours SCS and 24 hours of SCS are 50% and 0% respectively in both graphs (Figure 6A and B).
Overall survival of the animals that received a graft that was perfused for 6 hours with the HBOC-201 was slightly higher compared to the fresh control group; 80% vs. 75% excluding automutilation (Figure 6B) and 62% versus 60% including automutilation (Figure 6A). Animals that received a graft that was preserved with 6 hours of SCS had a survival of 50% after 30 days while none of the negative controls that received a graft that was preserved using 24 hours of SCS completed the follow up (0% survival) (both Figure 6A and 6B).
DISCUSSION
Rapid decay of graft viability using SCS forms a logistic barrier for expanding the use of VCA transplantation, which has unique matching requirements compared to solid organs. Further, the severe time limit hinders new developments in the field of VCA transplant, in particular because the time is not sufficient for tolerance protocols currently in trials (3). Here we report the development of a protocol to successfully preserve VCA grafts for up to 6 hours ex vivo using SNMP. Our key findings are 1) that 6 hour ex vivo SNMP is able to maintain energy charge levels comparable to in vivo controls, while energy charge levels dropped significantly in grafts preserved with SCS; 2) addition of HBOC-201 to the preservation solution significantly decreases edema and increases peak oxygen extraction; and 3) transplantation of grafts perfused after 6 hours of SNMP are successful.
In solid organ transplantation, energy charge levels prior to transplantation significantly correlate with post-operative outcome (19). It is also well known that during cold ischemia, cellular energy levels rapidly decline (20, 21). In the field of VCA, cold ischemia has been frequently described as a contributor to immune activation, rejection and inhibition of tolerance (22). Previous studies have reported histopathological changes of both muscle cells and nerves during cold ischemia (23). To our knowledge this is the first to report to energy charge levels during cold ischemic preservation and machine perfusion on VCA grafts.
Edema is frequently observed upon revascularization of a VCA graft in vivo (24). During VCA transplantation, important graft edema may reflect an obstructed venous outflow, inadequate lymphatic drainage, or allograft rejection (25, 26). During ex vivo perfusion, graft edema is, however, more likely to be caused by the diffusion of perfusion solution components into the interstitial space (i.e. rationale of colloids in static cold perfusion solutions) (27). Such interstitial expansion may result in inadequate tissue perfusion and even cell death, due to compression of delicate, thin-walled capillaries (27). Other groups have reported reduced edema during ex vivo perfusion of porcine limbs, upon addition of the colloid dextrose to their modified phosphate buffered saline solution (28).
In this study, weight gain due to edema was significantly lower in the PEG and HBOC-201 group, compared to the BSA alone group. In previous studies, PEG has shown to reduce endothelial leakage and HBOC-201 is a large molecule not likely to extravasate (16). The addition of PEG and HBOC-201 might thus increase viscosity of the solution, thereby compromising vascular flow rates as observed in this study, yet still lead to better tissue perfusion as reflected by increased peak oxygen extraction. Thus, in our opinion high flow vascular flow rates alone do not indicate adequate tissue perfusion.
Potassium and lactate levels are well-known ‘real time’ parameters of tissue damage during ex vivo organ prefusion. In this study, median potassium release was significantly higher in the BSA group compared to BSA + PEG and HBOC-201 perfused limbs. High potassium release can be an early sign of tissue necrosis and are common signs of hypoxia observed during extra corporal perfusion (29–31). Blood lactate levels are a balance between lactate production and elimination. Lactate production is a result of anaerobic glucose metabolism, most commonly caused by tissue hypoxia and hypoperfusion. Lactate can be produced by most cells but is mainly produced by tissues with a high metabolic rate such as muscles (32). In vivo, the majority of the lactate is cleared from the blood by the liver (~80%) and to a lesser degree by the kidneys and muscles (32). In this study we calculated the lactate clearance of an isolated, inactive limb. Studies have shown that inactive muscles actively participate in lactate clearance although it is highly depended on blood flow, arterial lactate concentration and muscle metabolism (33, 34). Our study showed that in all groups, lactate clearance increased during the first hours of perfusion and decreased thereafter.
As part of this study we show that limbs that are perfused for 6 hours with the HBOC-201 protocol can successfully be transplanted. Thirty days post-transplant survival rates in the HBOC-201 group were at least comparable and even slightly higher compared to survival rates of animals that received a limb that was preserved with SCS for an equal amount of time (80% vs 75% survival). None of the negative controls survived past day 18 (survival rate of 0%). Ulusal et al. reported a mortality rate for orthotopic hindlimb transplantation of 26.7% which is comparable to our findings. Regardless, this rat hindlimb transplant model remains in need of further improvements or alternatives in order to minimize the loss of animals especially due to automutilation. Future studies might include hind limb splints or head cones to prevent the animals form biting the graft.
Ozer et al. reported promising results of ex vivo preservation of porcine limbs using near normothermic machine perfusion with heparinized autologous blood (30). Only recently, the first ex vivo perfusion of a human limb was reported also using near normothermic machine perfusion with a plasma-based perfusate with packed red blood cells (with an average hemoglobin concentration of 4–6 g/dL) (9). While the use of acellular hemoglobin-based oxygen carriers such as HBOC-201 are gaining increasing attention as an alternative for red blood cells in ex vivo machine perfusion of solid organs (17, 35–37), only limited reports have been published about the use of HBOCs in VCA machine perfusion so far (37, 38).
The study has several limitations. In the HBOC-201 protocol, except from the addition of HBOC-201 we also added prostaglandin to the protocol. Addition of prostaglandin was necessary to overcome high vascular resistance, which was an immediate issue when we used HBOC-201 alone in initial testing. Testing the effect of prostaglandin alone on ex vivo SNMP of VCA grafts could be considered in the protocols we tested, here as well as others in literature. Due to the heterotopic model used and since we did not aim to study graft rejection, and we chose a follow-up duration of 30 days to be sufficient. Our results indicate that shorter periods could be considered sufficient, which may also reduce automutilation artifacts. Moreover, in this experimental set up we used male rats only since systemic heparinization during limb harvest was easily facilitated via the penile vein. While this might be an issue is small animals, we do not expect that our results of one sex only would potentially limit future VCA research as we expect to upsize the experimental subjects (i.e. swine and eventually human) in which vena puncture is not an issue.
CONCLUSION
This study demonstrates that 6 hours ex vivo SNMP of rodent hindlimbs is feasible using an acellular oxygen carrier, and results in superior tissue preservation compared with conventional cold preservation methods. Moreover, heterotopic transplantation of hindlimbs preserved with the HBOC-201 ex vivo SNMP protocol presented is feasible and shows promising results. Future studies may incorporate machine perfusion as part of a longer protocol to extend the preservation time even more or consider it in combination with mixed chimerism tolerance induction protocols.
HIGHLIGHTS.
Six hours of ex vivo subnormothermic machine perfusion (SNMP) of rodent vascularized composite allografts (VCA) is feasible using acellular oxygen carrier HBOC-201
SNMP results in superior tissue preservation compared to conventional static cold storage (SCS) preservation
Successful transplantation of VCA grafts preserved with 6 hours of SNMP is feasible and 30 day survival rates are comparable to SCS preserved graft
ACKNOWLEDGEMENTS
We would like to thank Stephanie E.J. Cronin for her assistance during the development of this protocol and the procurement of the limbs.
This material is partially based upon work supported by the National Science Foundation under Grant No. EEC 1941543.
Funding sources:
The U.S. Army Medical Research Acquisition Activity, 820 Chandler Street, Fort Detrick MD 21702-5014 is the awarding and administering acquisition office. This work was supported by the Office of Assistant Secretary of Defense for Health Affairs, through the Reconstructive Transplant Research Program, Technology Development Award under Award Nos. W81XWH1710680 and W81XWH1910440.
Funding from the U.S. National Institutes of Health (grants R44AI124835 and R44AI145782 in collaboration with Sylvatica Biotech Inc, and R01EB028782) and the Shriners Hospitals for Children (86200-BOS) is gratefully acknowledged. We thank the Shriners Boston Mass Spectrometry Core, and Morphology and Imaging Core for access to equipment and expertise.
Opinions, interpretations, conclusions and recommendations are those of the author and are not necessarily endorsed by the Department of Defense or the National Institutes of Health.
Abbreviations:
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- ATP
adenosine triphosphate
- BSA
bovine serum albumin
- Hb
hemoglobin
- HBOC-201
acellular oxygen carrier Hemopure®
- PEG
polyethylene glycol
- PO2
partial pressure of oxygen
- SO2
oxygen saturation
- SNMP
subnormothermic oxygenated machine perfusion
- UW
University of Wisconsin solution
- VCA
vascularized composite allotransplantation
APPENDIX
Limb procurement
Animals were anesthetized with isoflurane (Forane, Baxter, Deerfield, IL) using a Tech 4 vaporizer (Surgivet, Waukesha, WI). Animals were placed on a heating pad in a supine position and were shaved from the right ankle with the distal lower ribs as the proximal and midline as the medial landmarks. The animals were prepped in a sterile manner using povidone iodine and surgical drape. The line on medial on medial side of the hindlimb overlies the femoral vessels and the circular lines on mid-thigh and above the ankle, respectively, delineate the skin paddle (4 × 3 cm). We started by a circular skin incision at the location of the medial above the ankle. First, the anterior and posterior tibial pedicles were ligated first using 8/0 ethilon sutures. The Achilles tendon was then sectioned, and the tibial periosteum was exposed by pushing back all tendons using an Obwegeser periosteal elevator. At this point, animals were systemically heparinized (30 IU) via the dorsal penile vein. For the skin paddle, we incised the line on the inner thigh. The fat pad was then dissected out to identify the femoral vessels and all surrounding muscle were cut off. Subsequently, both the femoral artery and vein were skeletonized and cannulated with a 24-gauge intravenous catheter that was secured with 7/0 silk ligation. The graft was mobilized by cutting the bones above the ankle and under the inguinal ligament and flushed with 10mL heparinized saline (10 IU/mL) via the femoral artery till limpid outflow.
Footnotes
Conflict of interest statements:
K.U. has a financial interest in Organ Solutions, a company focused on developing organ preservation technology. K.U. and S.N.T. are on the scientific advisory board, are cofounders, and have financial interest in Sylvatica Biotech Inc. which aims to develop technology that stops or controls biological time. K.U, S.N.T, L. C. B., A.G.L. and C.L.C. have patent filings relevant to this manuscript. These interests are managed by the Mass General Brigham in accordance with their conflict of interest policies. C.B.T., P.T.F., M.A.R., R.J.P., L.A.L, have no conflicts of interest to declare in relation to this work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Mendenhall SD, Ginnetti MT, Sawyer JD, et al. Prevalence and Distribution of Potential Vascularized Composite Allotransplant Donors, Implications for Optimizing the Donor-recipient Match. Plast Reconstr Surg Glob Open 2018;6:e1833–e1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lantieri L, Cholley B, Lemogne C, et al. First human facial retransplantation: 30-month follow-up. Lancet 2020;396:1758–1765. [DOI] [PubMed] [Google Scholar]
- 3.Burlage LC, Tessier SN, Etra JW, Uygun K, Brandacher G. Advances in machine perfusion, organ preservation, and cryobiology: potential impact on vascularized composite allotransplantation. Curr Opin Organ Transplant 2018;23:561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: a review. Cardiovasc Surg 2002;10:620–630. [DOI] [PubMed] [Google Scholar]
- 5.Landin L, Cavadas PC, Garcia-Cosmes P, Thione A, Vera-Sempere F. Perioperative ischemic injury and fibrotic degeneration of muscle in a forearm allograft: functional follow-up at 32 months post transplantation. Ann Plast Surg 2011;66:202–209. [DOI] [PubMed] [Google Scholar]
- 6.Murata S, Miniati DN, Kown MH, et al. Superoxide dismutase mimetic m40401 reduces ischemia-reperfusion injury and graft coronary artery disease in rodent cardiac allografts. Transplantation 2004;78:1166–1171. [DOI] [PubMed] [Google Scholar]
- 7.Panizo A, Pardo FJ, Lozano MD, de Alava E, Sola I, Idoate MA. Ischemic injury in posttransplant endomyocardial biopsies: immunohistochemical study of fibronectin. Transplant Proc 1999;31:2550–2551. [DOI] [PubMed] [Google Scholar]
- 8.Ozer K, Rojas-Pena A, Mendias CL, Bryner BS, Toomasian C, Bartlett RH. The Effect of Ex Situ Perfusion in a Swine Limb Vascularized Composite Tissue Allograft on Survival up to 24 Hours. J Hand Surg Am 2016;41:3–12. [DOI] [PubMed] [Google Scholar]
- 9.Werner NL, Alghanem F, Rakestraw SL, et al. Ex Situ Perfusion of Human Limb Allografts for 24 Hours. Transplantation 2017;101:e68–e74. [DOI] [PubMed] [Google Scholar]
- 10.Kueckelhaus M, Fischer S, Sisk G, et al. A Mobile Extracorporeal Extremity Salvage System for Replantation and Transplantation. Ann Plast Surg 2016;76:355–360. [DOI] [PubMed] [Google Scholar]
- 11.Berendsen TA, Bruinsma BG, Lee J, et al. A simplified subnormothermic machine perfusion system restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant Res 2012;1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruinsma BG, Yeh H, Ozer S, et al. Subnormothermic machine perfusion for ex vivo preservation and recovery of the human liver for transplantation. Am J Transplant 2014;14:1400–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruinsma BG, Avruch JH, Weeder PD, et al. Functional Human Liver Preservation and Recovery by Means of Subnormothermic Machine Perfusion. J Vis Exp 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X, Philip JL, Razzaque MA, Lloyd JW, Muller CM, Akhter SA. High-molecular-weight polyethylene glycol inhibits myocardial ischemia-reperfusion injury in vivo. J Thorac Cardiovasc Surg 2015;149:588–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bejaoui M, Pantazi E, Folch-Puy E, et al. Protective Effect of Intravenous High Molecular Weight Polyethylene Glycol on Fatty Liver Preservation. Biomed Res Int 2015;2015:794287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bejaoui M, Pantazi E, Calvo M, et al. Polyethylene Glycol Preconditioning: An Effective Strategy to Prevent Liver Ischemia Reperfusion Injury. Oxid Med Cell Longev 2016;2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matton APM, Burlage LC, van Rijn R, et al. Normothermic machine perfusion of donor livers without the need for human blood products. Liver Transpl 2018;24:528–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ulusal AE, Ulusal BG, Hung L-M, Wei FC. Heterotopic hindlimb allotransplantation in rats: an alternative model for immunological research in composite-tissue allotransplantation. Microsurgery 2005;25:410–414. [DOI] [PubMed] [Google Scholar]
- 19.Bruinsma BG, Avruch JH, Sridharan GV, et al. Peritransplant energy changes and their correlation to outcome after human liver transplantation. Transplantation 2017;101:1637–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vajdová K, Graf R, Clavien P-A. ATP-supplies in the cold-preserved liver: A long-neglected factor of organ viability. Hepatology 2002;36:1543–1552. [DOI] [PubMed] [Google Scholar]
- 21.Berendsen TA, Izamis ML, Xu H, et al. Hepatocyte viability and adenosine triphosphate content decrease linearly over time during conventional cold storage of rat liver grafts. Transplantation proceedings 2011;43:1484–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kueckelhaus M, Fischer S, Seyda M, et al. Vascularized composite allotransplantation: current standards and novel approaches to prevent acute rejection and chronic allograft deterioration. Transpl Int 2016;29:655–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hautz T, Hickethier T, Blumer MJF, et al. Histomorphometric evaluation of ischemia-reperfusion injury and the effect of preservation solutions histidine-tryptophan-ketoglutarate and University of Wisconsin in limb transplantation. Transplantation 2014;98:713–720. [DOI] [PubMed] [Google Scholar]
- 24.Azari K, Imbriglia J, Goitz R, et al. Technical Aspects of the Recipient Operation in Hand Transplantation. Journal of Reconstructive Microsurgery 2012;28:27–34. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman CL, Marvin MR, Chilton PM, et al. Immunobiology in VCA. Transplant International 2016;29:644–654. [DOI] [PubMed] [Google Scholar]
- 26.Mundinger GS, Narushima M, Hui-Chou HG, et al. Infrared fluorescence imaging of lymphatic regeneration in nonhuman primate facial vascularized composite allografts. Ann Plast Surg 2012;68:314–319. [DOI] [PubMed] [Google Scholar]
- 27.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation 1988;45:673–676. [DOI] [PubMed] [Google Scholar]
- 28.Troutman EC. Hypothermic Machine Perfusion of Composite Tissues. :62. [Google Scholar]
- 29.Fichter AM, Ritschl LM, Borgmann A, et al. Development of an Extracorporeal Perfusion Device for Small Animal Free Flaps. PLoS One 2016;11:e0147755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muller S, Constantinescu MA, Kiermeir DM, et al. Ischemia/reperfusion injury of porcine limbs after extracorporeal perfusion. J Surg Res 2013;181:170–182. [DOI] [PubMed] [Google Scholar]
- 31.Constantinescu MA, Knall E, Xu X, et al. Preservation of amputated extremities by extracorporeal blood perfusion; a feasibility study in a porcine model. J Surg Res 2011;171:291–299. [DOI] [PubMed] [Google Scholar]
- 32.Consoli A, Nurjhan N, Reilly JJJ, Bier DM, Gerich JE. Contribution of liver and skeletal muscle to alanine and lactate metabolism in humans. Am J Physiol 1990;259:E677–684. [DOI] [PubMed] [Google Scholar]
- 33.Ahlborg G, Wahren J, Felig P. Splanchnic and peripheral glucose and lactate metabolism during and after prolonged arm exercise. J Clin Invest 1986;77:690–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bangsbo J, Aagaard T, Olsen M, Kiens B, Turcotte LP, Richter EA. Lactate and H+ uptake in inactive muscles during intense exercise in man. J Physiol 1995;488 ( Pt 1):219–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fontes P, Lopez R, van der Plaats A, et al. Liver preservation with machine perfusion and a newly developed cell-free oxygen carrier solution under subnormothermic conditions. Am J Transplant 2015;15:381–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laing RW, Bhogal RH, Wallace L, et al. The Use of an Acellular Oxygen Carrier in a Human Liver Model of Normothermic Machine Perfusion. Transplantation 2017;101:2746–2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.de Vries Y, van Leeuwen OB, Matton APM, Fujiyoshi M, de Meijer VE, Porte RJ. Ex situ normothermic machine perfusion of donor livers using a haemoglobin-based oxygen carrier: a viable alternative to red blood cells. Transplant International 2018;31:1283–1284. [DOI] [PubMed] [Google Scholar]
- 38.Said SA, Ordenana CX, Rezaei M, et al. Ex-Vivo Normothermic Limb Perfusion With a Hemoglobin-Based Oxygen Carrier Perfusate. Mil Med 2020;185:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]