Abstract
System xc- (Sxc-) is a heteromeric antiporter (L-cystine/L-glutamate exchanger) expressed predominately on astrocytes in the central nervous system. Its activity contributes importantly to the maintenance of the ambient extracellular glutamate levels, as well as, to cellular redox homeostasis. Since alterations in glutamate levels and redox modifications could cause structural changes, we analyzed gross regional morphology of thionin-stained brain sections and cellular and subcellular morphology of Golgi-Cox stained layer V pyramidal neurons in the primary motor cortex (PM1) of mice naturally null for SLC7A11 (SLC7A11sut/sut) – the gene that encodes the substrate specific light-chain (xCT) for Sxc-. Intriguingly, in comparison to age and sex-matched wild-type (SLC7A11+/+) littermate controls, we found morphologic changes — including increased dendritic complexity and mushroom spine area in males and reduced corpus callosum and soma size in females — that have previously been described, in each case, as morphological correlates of excitability. Consistent with this, we found that both male and female SLC7A11sut/sut mice had lower convulsive seizure thresholds and greater seizure severity than their sex-matched wild-type (SLC7A11+/+) littermates after acute challenge with two pharmacologically distinct chemoconvulsants: the Glu receptor agonist, kainic acid (KA), or the GABAA receptor antagonist, pentylenetetrazole (PTZ). These results suggest that loss of Sxc- signaling in males and females perturbs excitatory/inhibitory (E/I) balance in vivo, potentially through its regulation of cellular and subcellular morphology.
Keywords: xCT, SLC7A11, Excitatory/inhibitory balance
Graphical Abstract
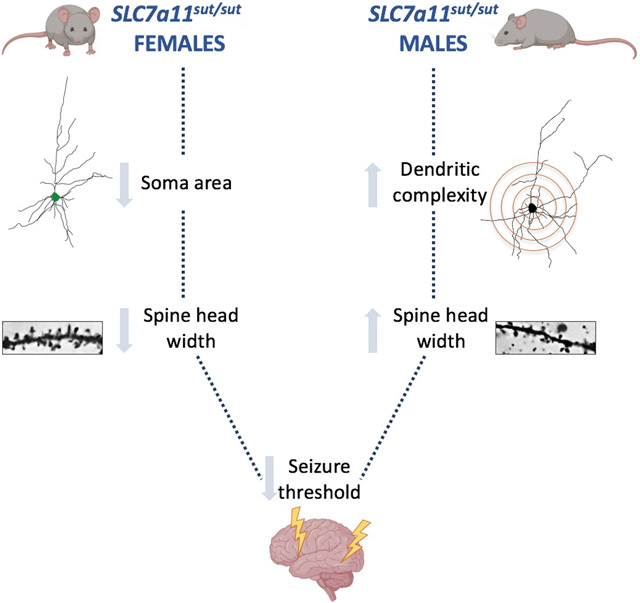
Male (M) and female (F) mice null for the cystine/glutamate antiporter, system xc-, have neuronal structural alterations including enhanced dendritic complexity (M), decreased soma size (F), and alterations in spine head widths (M,F). These cellular morphological alterations may explain the reduction in convulsive seizure threshold demonstrated in both sexes.
INTRODUCTION
Proper growth and function of the central nervous system (CNS) depends on numerous cellular and molecular processes that tune and shape the brain's circuitry. Astrocytes, a major glial cell population in mammalian brain, crucially regulate synaptic connectivity during post-natal development both through direct physical contact and secretion of molecules and transmitters [for review see (Eroglu & Barres, 2010) ]. For example, the structural assembly of excitatory synapses is dependent upon astrocyte-secreted factors such as thrombospondin 1 and 2 (Christopherson et al., 2005), cholesterol (Mauch et al., 2001), and hevin (Kucukdereli et al., 2011), whereas synapse maturation and maintenance are promoted by astrocyte-derived glypicans (Allen et al., 2012). Physical interaction with astrocytic processes and/or the release of neurochemicals, including glutamate (Glu), are known to shape neuronal dendritic arbor outgrowth and spinogenesis (Kwon & Sabatini, 2011; Withers, Farley, Sterritt, Crane, & Wallace, 2017).
System xc- (Sxc-) — a heterodimeric transporter (Sato, Tamba, Ishii, & Bannai, 1999) expressed mainly in astrocytes (Ottestad-Hansen et al., 2018; Zhang et al., 2014) and best known for exporting intracellular L-Glu in exchange for extracellular L-cystine in a 1:1 stoichiometry (Bannai, 1986) — is a major source of ambient extracellular Glu that bathes the CNS in vivo (Baker, Xi, Shen, Swanson, & Kalivas, 2002; De Bundel et al., 2011b). It also contributes to maintaining the intracellular/extracellular redox homeostasis and thus, redox-sensitive cellular processes. In vitro studies demonstrate that cystine import through Sxc- is important for the synthesis and maintenance of the thiol antioxidant glutathione (GSH) (Sato et al., 1998), and it facilitates the cysteine/cystine redox cycle across the cell plasma membrane (Banjac et al., 2008). Importantly, shifts in redox equilibrium can lead to abnormalities in the developing brain (Bowers et al., 2011; Coffman & Su, 2019).
Given that astrocytes, Glu, and redox homeostasis are important for production and maintenance of regional, cellular, and subcellular brain morphology, it seems reasonable to speculate that loss of Sxc- activity could alter the development of mouse brain. To test this hypothesis, we utilized a mouse – first analyzed by Chintala and colleagues because of a subtle gray (sut) coat – with a large deletion in exon 12 of the SLC7A11 gene, which encodes for xCT – the substrate specific light chain of Sxc- (Chintala et al., 2005), rendering them null for xCT protein and thus Sxc- activity (McCullagh & Featherstone, 2014). Previous work by Shih and colleagues demonstrated that 3-4 month old male SLC7A11sut/sut mice display signs of brain atrophy — including cortical thinning, ventricular enlargement and shrinkage of striatum — which the group surmised may have begun early in development (Shih et al., 2006). Female mice were never analyzed. Thus, in this study we sought to confirm the original gross morphological changes in the different brain structures of SLC7A11sut/sut male mice, to similarly analyze the female SLC7A11sut/sut brain, and to extend the studies in both male and female brain to the cellular and subcellular level.
Present results are the first to demonstrate that Sxc- contributes to mouse brain morphology in male and female mice differentially, at the gross, cellular, and subcellular level. The changes in each have been described as morphological correlates of excitability, and in keeping with this idea, chemoconvulsant challenge revealed an intriguing increase in excitability in both sexes.
MATERIALS AND METHODS
Animals.
Experiments were carried out on female and male mice at 8-12 weeks of age in accordance with the National Institutes of Health guidelines for the use of experimental animals as approved by the Institutional Animal Care and Use Committee of Syracuse University. Mice were maintained on a 12 hr light/dark schedule (7am/7pm) with standard mouse chow and water provided ad libitum. Mice that harbor a natural null mutation in the SLC7A11 gene (SLC7A11sut/sut) having no xCT mRNA (Chintala et al., 2005) or protein (McCullagh & Featherstone, 2014) in brain and their wild-type littermates (SLC7A11+/+) were derived from F1 heterozygous (SLC7A11+/sut) breeding units obtained by crossing SLC7A11sut/sut C3H/HeSnJ male mice [Jackson Laboratories (JAX) Stock #001310] with SLC7A11+/+ C3H/HeSnJ female mice (JAX, Stock #000661). Male and female F2 SLC7A11+/sut progeny were also used as breeding units. At weaning, pups were separated into cages by sex and marked for identification by ear punch. Genotyping was performed via PCR analysis of tail genomic DNA samples: +/+ primers, 5'- GAA GTG CTC CGT GAA GAA GG -3' (forward), 5'- ATC TCA ATC CTG GGC AGA TG -3' (reverse); sut/sut primers, 5'- CCA CTG TTG TAG GTC AGC TTA GG -3’ (forward), 5'- CAG GAC CTG TGA ATA TGA TAG GG −3' (reverse). Experimental littermate mice were then arranged two to four per cage such that at least one mouse of each genotype was represented. The breeding and housing strategies employed control for environmental differences, genetic background influences, and genetic drift (Barnwell et al., 2009; Pick & Little, 1965; Wolfer, Crusio, & Lipp, 2002).
Gross Brain Morphological Measurements.
Brains were harvested from naïve 12 week old SLC7A11+/+ and SLC7A11sut/sut littermates and snap-frozen on dry ice in Optimal Cutting Temperature (O.C.T) compound (Tissue-Tek, Torrance, CA). A subset of brains were weighed and photographed prior to snap freezing (Supplementary Fig. 1). Frozen sections (coronal, 40 μm) were cut on a cryostat at 80 μm intervals, mounted on SuperFrost Plus slides (Fisher Scientific, Houston, TX), and thionin-stained as we previously described (Chowdhury, Allen, Thorn, He, & Hewett, 2018). Experimenters blind to genotype used ImageJ Software (version 1.47v or 1.51n; National Institutes of Health, Bethesda, MD, RRID: SCR_003070) to make gross brain morphological measurements on scanned images (Epson 3170; 720 dpi) of slices at +1.1, −0.1, −0.94, −1.46, and −2.54 mm relative to bregma. Mean cortical width from each individual was obtained by averaging six non-overlapping, straight tool measurements taken from the apex of the corpus callosum to the pial layer in the somatosensory cortex (three each bilaterally). Mean corpus callosum width from each individual was obtained by averaging three non-overlapping, straight tool measurements taken from the base to the apex of the corpus callosum at the midline. Mean ventricular, striatal, hemispheric, hippocampal and corpus callosum cross-sectional areas were determined by averaging user-defined pixel volumes taken of the respective regions measured three times bilaterally (six in total) or three times total for structures decussating the midline (corpus callosum) using the free-hand tool. With the exception of the hippocampus, measurements were performed by three individuals with data expressed as the mean ± SD of averaged data from all individuals.
Cellular Morphological Measurements.
Golgi-Cox Staining:
Brains from naïve SLC7A11+/+ and SLC7A11sut/sut littermates were Golgi-Cox stained using the FD Rapid Golgi Stain Kit (FD Neuro Technologies, Inc., Baltimore, MD) as per manufacturer’s instructions. Following removal from the cranium, brains were immediately rinsed in deionized (DI) water, placed in a proprietary impregnation solution (Solution A plus B) containing potassium dichromate, potassium chromate, and mercuric chloride, and stored at room temperature for 14 days in the dark. Brains were then transferred to a sucrose solution (Solution C) for three days followed by rapid freezing on dry ice. Frozen brains were cut serially (≈ +2.46 – −2.30 mm posterior to bregma) into 140 μm coronal sections, mounted on gelatin-coated slides (FD Neuro Technologies, Inc., Baltimore, MD), and air dried at room temperature for two days in the dark. Sections were Golgi-Cox stained as per manufacturer’s instructions (FD Rapid Golgi Stain Kit). Briefly, sections were initially rinsed 2x with DI water (four min each) then placed in a solution containing silver nitrate (Solution D plus E) for 10 min. Slides were rinsed in DI water 2x for four min each followed by serial dehydration in absolute ethanol (50%, 75%, 95%, 100%, 100%, 100%, 100%; four min each). Ethanol was cleared with xylenes (3x for four min each), after which coverslips were mounted using Permount mounting media.
Dendritic Morphology and Soma Area Analysis:
Photomicrographs of Golgi-Cox stained layer V pyramidal cells in the primary motor cortex (PM1) were obtained with a Nikon eclipse Ni-U upright microscope with motorized stage at 20x magnification. Layer V pyramidal cells in PM1 were chosen for analysis given that a subset of these neurons send direct projections to the spinal cord or striatum and are thus implicated in producing motor activity (Anderson, Sheets, Kiritani, & Shepherd, 2010), such as that observed in behavioral seizures. Moreover, Golgi-staining of these neurons revealed a population of cells that are sufficiently spatially isolated, allowing for morphometric analysis of dendritic processes. Neurons whose cell body and dendrites were completely impregnated and visible within the plane(s) of focus were selected for analysis. Neurons were reconstructed with Adobe Illustrator. The complexity of dendritic arborization in reconstructed neuronal drawings was quantified (Image J) using Sholl analysis as described (Sholl, 1953). Briefly, the number of dendrites intersecting concentric circles of a gradually increasing radius from the centroid of the soma were calculated. The soma cross-sectional area was quantified using the polygon tool in Image J. The number and length of primary, secondary, tertiary, apical, and basal dendrites were also determined using Neuron J. Experimenters blind to genotype performed the image acquisition and analyses. Six to 11 neurons from 3-5 mice/genotype/sex were analyzed; data are expressed as the mean ± SD of each group.
Spine Morphology and Density Analysis:
Photomicrographs of Golgi-Cox stained secondary apical dendrites on layer V pyramidal cells in the PM1 were obtained with a Nikon eclipse Ni-U upright microscope with motorized stage at 60x magnification. Neurons whose cell body and dendrites were completely impregnated and were visible within the plane(s) of focus were selected and all spines residing on the secondary apical neurites were included in the analysis. The average length of secondary apical neurite analyzed was 111.62 μm in males and 113.76 μm in females, with 63–71 dendrites/genotype/sex analyzed. Spine morphometric analysis was carried out as described (Risher, Ustunkaya, Alvarado, & Eroglu, 2014). In brief, Z-stack sections were analyzed using RECONSTRUCT software by measuring the length and head width of all protrusions (spines) along the secondary apical dendrite. The spine density was determined by calculating the number of protrusions/μm of dendrite. The length-to-width ratios (LWR) of individual spines were calculated in Microsoft Excel and used for hierarchical classification into the following categories: branched (entered manually by experimenter), filopodia (length > 2μm), mushroom (width > 0.6 μm), long thin (length > 1μm), thin (LWR > 1), and stubby (LWR ≤ 1). Image acquisition and subsequent analyses were performed by experimenters blinded to genotype. Analysis comprised a total of 5055 or 4763 spines from SLC7A11+/+ males or females, respectively, and 5355 or 4430 spines from SLC7A11sut/sut males or females, respectively, from secondary apical dendrites on eight or nine neurons from three mice/genotype/sex; data are expressed as the mean ± SD of each group.
Chemoconvulsant dosing paradigms.
Five days prior to each study, mice were acclimated to handling by performing mock daily intraperitoneal (i.p.) injections that consisted of inverting the mouse and rubbing its abdomen. Prior to each experiment, mice were brought into the procedure room, weighed, and allowed to acclimatize for at least one hour.
Pentylenetetrazole (PTZ, Sigma Chemical Co., St. Louis, MO) or kainic acid (KA, Abcam, Cambridge, U.K.) solutions were prepared fresh on day of use in saline or 0.05M phosphate buffered saline, respectively. All solutions were filter sterilized and administered i.p. in a volume of 10 ml/kg body weight. Male mice were injected with a single dose of 35 mg/kg PTZ or 12 mg/kg KA whereas female mice were injected with a single dose of 42 mg/kg PTZ or 15 mg/kg KA. These doses were chosen for each sex based on initial dose-ranging studies performed in wild-type mice only (not shown) as they produced a median maximal seizure score of 1-2 (PTZ) or 4 (KA) (scoring criteria described below), thus allowing us to assess for potentiation of seizures in Sxc- null mice should it occur. Following each injection, mice were observed for 30 min and the time and severity of behavioral seizures were scored and recorded by an experimenter blinded to genotype using a modified Racine scale. For PTZ-induced seizures, a 5-point behavioral scoring system was used (0 - 4: 0 = no behavioral change; 1 = hypoactivity; 2 = myoclonus; 3 = generalized convulsion with righting reflex; 4 = generalized convulsion with loss of righting reflex). For KA-induced seizures, we created a 9-point behavioral scoring system to account for strain dependent behaviors (0 – 8: 0 = no behavioral change; 1 = hypoactivity; 2 = hyperactivity; 3 = myoclonus; 4 = kyphosis; 5 = kyphosis with forelimb clonus; 6 = generalized convulsion with righting reflex; 7 = generalized convulsion with loss of righting reflex and/or violent jumping; 8 = status epilepticus i.e. a sustained score of 5, 6, or 7 for ≥ 20 min) (Ferraro et al., 1999; Pitkanen, 2006; Racine, 1972). Data represents the maximal seizure score obtained by a single mouse over the 30 min observation period. The percentage of animals exhibiting clonic/convulsive seizures was determined by dividing the number of animals with a maximum seizure score ≥ 3 (PTZ) or ≥ 5 (KA) by the total number of animals injected. The latency to first seizure (any score) or the latency to convulsive seizure (score ≥ 3 [PTZ] or ≥ 5 [KA]) was determined by calculating the time between chemoconvulsant injection and seizure onset. Data were pooled from 16 (PTZ) or 6 (KA) independent experiments performed over 9 (PTZ) or 2 (KA) months.
Statistical analysis.
All statistical analyses were performed using GraphPad Prism (Version 8.0.1 or 9.0.0 Graphpad Software, Inc., La Jolla, CA). Brain morphological parameters, including areas, lengths, and widths, were assessed for normality (Kolmogorov-Smirnov) prior to comparison using an unpaired t test, two-way ANOVA, or a mixed effects model. Prior to parametric analysis, counted or percentage data were log (y = log(y)) or arcsine (y = arcsine[sqrt(y/100)] transformed, respectively. Seizure severity was compared using a Mann-Whitney U test, whereas proportions indicating the percent of mice convulsing were compared using a Fisher’s exact test. Exact p values are included in text and/or described in figure legends as appropriate. Prior to data collection or using preliminary data, a power analysis was performed using GraphPad StatMate to determine the minimum number of samples required for adequate power to detect differences between groups.
RESULTS
SLC7A11 null mutation changes the gross anatomical structure of male and female brain.
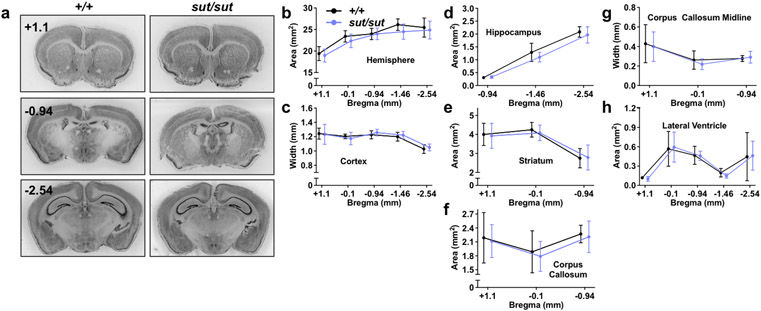
Gross brain morphological alterations, including cortical thinning and striatal shrinkage, have been reported previously in male SLC7A11sut/sut mice (Shih et al., 2006). Herein, we repeated the measurements in male mice and similarly analyzed the female SLC7A11sut/sut brain to determine whether there might be any sex differences. In our colony, SLC7A11sut/sut mice are born at the expected Mendelian ratio and appear to develop normally. Overall, brain and body weights of young adult mice (three months of age) are not different from sex-matched SLC7A11+/+ littermate controls (Supp. Fig. 1). Surprisingly, at a gross brain morphological level, every brain region analyzed — hemisphere [Fig. 1B; genotype, F(1,13) = 1.240, p = 0.2857; distance from bregma, F(4,52) = 73.63, p < 0.0001; interaction, F(4,52) = 1.158, p = 0.3399], cortex [Fig. 1C; genotype, F(1,13) = 0.1620, p = 0.6938; distance from bregma, F(4,52) = 20.15, p < 0.0001; interaction, F(4,52) = 0.4884, p = 0.7442], hippocampus [Fig. 1D; genotype, F(1,28) = 2.622, p = 0.1166; distance from bregma, F(2,50) = 480.9, p < 0.0001; interaction, F(2,50) = 1.966, p = 0.1507] striatum [Fig. 1E; genotype, F(1,13) = 0.1541, p = 0.7010; distance from bregma, F(2,26) = 30.45, p < 0.0001; interaction, F(2,26) = 0.1395, p = 0.8704], and lateral ventricle [Fig. 1H; genotype, F(1,13) = 0.0102, p = 0.9211; distance from bregma, F(4,52) = 28.15, p < 0.0001; interaction, F(4,52) = 0.1548, p = 0.9600] — in male SLC7A11sut/sut mouse brain was indistinguishable in size from their male SLC7A11+/+ age-matched littermate controls (mixed effects model).
Figure 1. Gross morphological comparison between male SLC7A11+/+ and SLC7A11sut/sut littermate mice.
a-h) Gross brain morphological measurements were made on thionin-stained coronal brain sections from naïve 12 week old male SLC7A11+/+ (+/+; n = 7) and SLC7A11sut/sut (sut/sut; n = 8) littermates. Sections of 40 μm thickness were imaged using an Epson Digital Scanner at 720 dpi. Digitized images were processed and quantified using NIH Image J software. Representative thionin-stained coronal sections spanning +1.1 to −2.54 anterior to posterior from bregma from male SLC7A11+/+ and SLC7A11sut/sut mice derived from SLC7A11+/sut breeders are depicted in (a). Quantification of hemispheric area (b), cortical width (c), hippocampal area (d), striatal area (e), corpus callosum area (f), corpus callosum width (g), or lateral ventricle area (h) was performed in sections derived from SLC7A11+/+ and SLC7A11sut/sut mice over 3-5 coronal sections as described in Materials and Methods. Data are expressed as the mean ± SD width (mm) or area (mm2). Upon editor request, jitter was intentionally added to the x-axis of Figures 1B-F to aid in the visual presentation of the error bars. No significant genotype differences were observed (mixed-effects model with Šidák’s multiple comparisons).
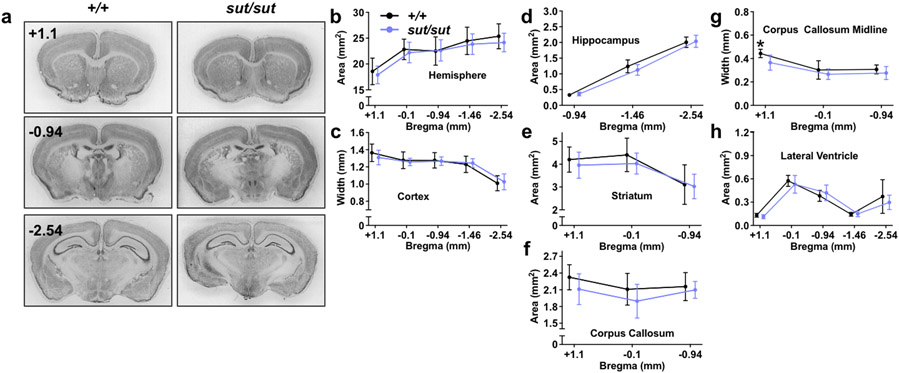
When comparing female SLC7A11sut/sut to female SLC7A11+/+ age-matched littermate controls we found the hemisphere [Fig. 2B; genotype, F(1,15) = 0.3519, p = 0.5619; distance from bregma, F(4,60) = 132.4, p < 0.0001; interaction, F(4,60) = 1.163, p = 0.3362], cortex [Fig. 2C; genotype, F(1,15) = 0.1045, p = 0.7510; distance from bregma, F(4,60) = 86.8, p < 0.0001; interaction, F(4,60) = 1.24, p = 0.3038], and hippocampal [Fig. 2D; genotype, F(1,14) = 0.0976, p = 0.7593; distance from bregma, F(2,28) = 595.2, p < 0.0001; interaction, F(2,28) = 1.499, p = 0.2407] brain regions were similar in size, although we note that the striatal area of female SLC7A11sut/sut mice is, on average, ≈6% smaller than their sex-matched SLC7A11+/+ littermate controls [Fig. 2E; genotype, F(1,15) = 0.7843, p = 0.3898; distance from bregma, F(2,30) = 38.94, p < 0.0001; interaction, F(2,30) = 0.544, p = 0.5860; mixed effects model]. Further changes were noted in female brains with respect to white matter tracts; the area and width of the corpus callosum of female SLC7A11sut/sut mice is reduced as compared to SLC7A11+/+ sex- and age-matched littermate controls [Fig. 2F; genotype, F(1,15) = 3.237, p = 0.0921; distance from bregma, F(2,30) = 4.41, p = 0.0209; interaction, F(2,30) = 0.7505, p = 0.4808 and Fig. 2G; genotype, F(1,15) = 6.918, p = 0.0189; distance from bregma, F(2,30) = 32.47, p < 0.0001; interaction, F(2,30) = 1.216, p = 0.3106; mixed effects model with Šidák’s multiple comparisons]. These changes in corpus callosum were not noted in male SLC7A11sut/sut mice [Fig. 1F; genotype, F(1,13) = 0.2044, p = 0.6587; distance from bregma, F(2,26) = 12.63, p = 0.0001; interaction, F(2,26) = 0.0277, p = 0.9727 and Fig. 1G; genotype, F(1,13) = 0.2403, p = 0.6321; distance from bregma, F(2,26) = 12.62, p = 0.0001; interaction, F(2,26) = 0.3398, p = 0.7150; mixed effects model]. The lack of both pyknosis and ventricular enlargement [Fig. 2H; genotype, F(1,15) = 0.5398, p = 0.4738; distance from bregma, F(1.9,29) = 67.32, p < 0.0001; interaction, F(4,60) = 1.02, p = 0.4042; mixed effects model] indicates that the changes in female SLC7A11sut/sut mice are unlikely to be due to neurodegeneration, but instead, likely represent a neurodevelopmental change.
Figure 2. Gross morphological comparison between female SLC7A11+/+ and SLC7A11sut/sut littermate mice.
a-h) Gross brain morphological measurements were made on thionin-stained coronal brain sections from naïve 12 week old female SLC7A11+/+ (+/+; n = 9) and SLC7A11sut/sut (sut/sut; n = 8) littermates. Sections of 40 μm thickness were imaged using an Epson Digital Scanner at 720 dpi. Digitized images were processed and quantified using NIH Image J software. Representative thionin-stained coronal sections spanning +1.1 to −2.54 anterior to posterior from bregma from female SLC7A11+/+ and SLC7A11sut/sut mice derived from SLC7A11+/sut breeders are depicted in (a). Quantification of hemispheric area (b), cortical width (c), hippocampal area (d), striatal area (e), corpus callosum area (f), corpus callosum width (g), or lateral ventricle area (h) was performed in sections derived from SLC7A11+/+ and SLC7A11sut/sut mice over 3-5 coronal sections as described in Materials and Methods. Data are expressed as the mean ± SD width (mm) or area (mm2). Upon editor request, jitter was intentionally added to the x-axis of Figures 2B-F to aid in the visual presentation of the error bars. An asterisk (*) represents a significant between group difference (*p = 0.0189 mixed-effects model with Šidák’s multiple comparisons).
SLC7A11 null mutation changes the cellular and subcellular anatomical structure of male and female brain.
Next, we extended the gross morphological analyses of both male and female brain to the cellular and subcellular level. At the cellular level, Golgi microscopic studies of primary motor cortex layer V pyramidal neurons from PM1 of young adult mice show interesting sex and genotype differences in cellular structure.
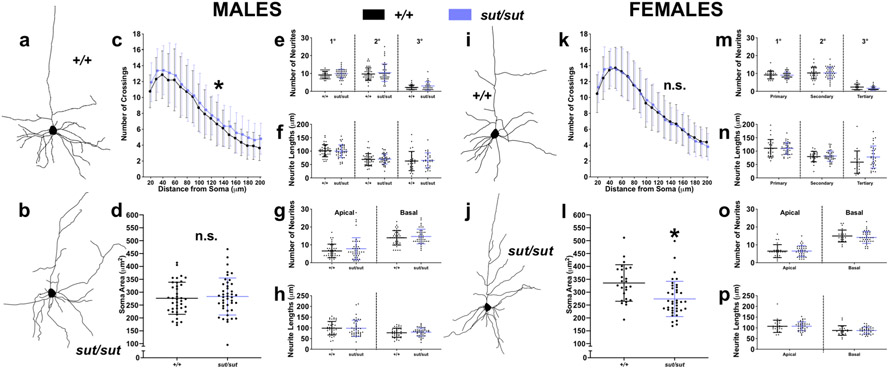
The dendritic complexity of layer V pyramidal neurons of male SLC7A11sut/sut mice — as determined via Sholl analysis – is significantly increased when compared to age- and sex-matched SLC7A11+/+ controls [Fig. 3C; genotype, F(1,1420) = 18.78, p < 0.0001; distance from soma, F(18,1420) = 91.21, p < 0.0001; interaction, F(18,1420) = 0.2490, p = 0.9994; two-way ANOVA]. No genotypic differences in neuronal soma size between male SLC7A11+/+ and SLC7A11sut/sut mice was found [Fig. 3D; t(80) = 0.4583, p = 0.6480; unpaired t test]. We next examined whether the increased dendritic complexity in males was accompanied by changes in length and/or number of primary neurites as well as the secondary and tertiary branches associated with these primary neurites. Surprisingly, it was not, as the number of neurites [Fig. 3E; genotype, F(1,213) = 3.258, p = 0.0725; branch order, F(2,213) = 113.3, p < 0.0001; interaction, F(2,213) = 0.0041, p = 0.9958; two-way ANOVA] as well as their lengths [Fig. 3F; genotype, F(1,213) = 0.0339, p = 0.8542; branch order, F(2,213) = 46.63, p < 0.0001; interaction, F(2,213) = 0.1333, p = 0.8753; two-way ANOVA] were similar between males of either genotype. Furthermore, no genotype differences were observed in the number or length of apical or basal neurites [Fig. 3G; genotype, F(1,160) = 1.781, p = 0.1839; neurite polarity, F(1,160) = 95.19, p < 0.0001; interaction, F(1,160) = 0.1170, p = 0.7328; and Fig. 3H; genotype, F(1,160) = 0.0858, p = 0.7700; neurite polarity, F(1,160) = 19.04, p < 0.0001; interaction, F(1,160) = 0.1418, p = 0.7070; two-way ANOVA]. Further multiple comparison analyses of differences between neurite branch orders and polarities demonstrated identical trends in neurite number and length within each genotype, the results of which are detailed in Appendix A. In sum, our results indicate that within SLC7A11+/+ and SLC7A11sut/sut male mice, there were more primary or secondary neurites as compared to tertiary (Fig. 3E), and primary neurites were longer as compared to both secondary or tertiary neurites (Fig. 3F). With respect to branch order, in both genotypes we found that there were more basal as compared to apical neurites (Fig. 3G), and that apical neurites were longer as compared to basal neurites (Fig 3H). These results indicate that SLC7A11+/+ and SLC7A11sut/sut male mice demonstrate similar trends in neurite outgrowth and frequency with respect to branch order and polarity of primary motor cortex layer V pyramidal neurons.
Figure 3. Dendritic complexity and soma size is altered in SLC7A11sut/sut pyramidal cells.
Photomicrographs of Golgi-Cox stained primary motor cortex (PM1) layer V pyramidal cells from male (a-h) or female (i-p) naïve SLC7A11+/+ (+/+; n = 38-39 neurons from 4 males or n = 25 neurons from 3 females) and SLC7A11sut/sut (sut/sut; n = 43 neurons from 5 males or n = 35-39 neurons from 4 females) littermates were reconstructed using Adobe Illustrator as depicted by representative tracings from SLC7A11+/+ (a, i) or SLC7A11sut/sut (b, j) mice.
c, k) The complexity of dendritic arborization of reconstructed neurons was explored by Sholl analysis. Each data point represents the mean number of crossings ± SD in neurons derived from male (c) or female (k) mice, to which a jitter to the x-axis was added to aid in the visual presentation of the error bars An asterisk (*) represents a significant between genotype difference (p < 0.0001, two-way ANOVA with Bonferroni’s multiple comparisons).
d, l) The cross-sectional soma areas of reconstructed neurons were quantified using NIH Image J software. Data points represent the soma area of individual neurons while the horizontal bar represents the mean soma area ± SD for the group derived from male (d) or female (l) mice [black (+/+) and blue (sut/sut)]. An asterisk (*) represents a significant between group difference (p = 0.0009, unpaired t test).
e-h, m-p) Neurite processes on reconstructed neurons were quantified using NIH Image J software. The horizontal lines [black (+/+) and blue (sut/sut)] represent the mean ± SD number or length of primary, secondary, or tertiary (e, f, m, n) or apical or basal (g, h, o, p) neurites on neurons derived from male (e-h) or female (m-p) mice. No significant between genotype differences in males or females (two-way ANOVA with Bonferroni’s multiple comparisons).
No genotype differences in the dendritic complexity of layer V pyramidal neurons of female SLC7A11sut/sut mice was evident [Fig. 3K; genotype, F(1,1082) = 0.0310, p = 0.8603; distance from soma, F(18,1082) = 103.4, p < 0.0001; interaction, F(18,1082) = 0.4637, p = 0.9725; two-way ANOVA] whereas quantification of somata cross-sectional area in neurons derived from female SLC7A11sut/sut brains revealed an 18.5% reduction as compared to those analyzed from sex-matched SLC7A11+/+ littermate control brains (Fig. 3L; 273.7 ± 68.58 μm2 vs. 335.6 ± 70.89 μm2 [mean ± SD]; t(62) = 3.475, p = 0.0009; unpaired t test). No between-genotype differences in neurite processes with respect to overall neurite number [Fig. 3M; genotype, F(1,173) = 0.7400, p = 0.3908, branch order, F(2,173) = 183, p < 0.0001; interaction, F(2, 173) = 0.3070, p = 0.7360; or length [Fig. 3N; genotype, F(1,173) = 2.145, p = 0.1449, branch order, F(2,173) = 29.52, p < 0.0001, interaction, F(2,173) = 1.776, p = 0.1724] were found (two way ANOVA). Likewise, no differences in the number of neurites in either the apical or basal dendrites [Fig 3O; genotype, F(1,124) = 0.4057, p = 0.5253; neurite polarity, F(1,124) = 0.179.5, p < 0.0001; genotype, F(1,124) = 0.5051, p = 0.4786] or their length [Fig. 3P; genotype, F(1,124) = 0.0025, p = 0.9604; neurite polarity, F(1,124) = 25.73, p < 0.0001; interaction, F(1,124) = 0.0066, p = 0.9353] were found when comparing female SLC7A11sut/sut and SLC7A11+/+ brains. Further multiple comparison analyses of differences between neurite branch orders and polarities demonstrated identical trends in neurite number and length within females of either genotype; these effects are further summarized in Appendix A.
Next, we quantitatively analyzed dendritic spines on secondary apical dendrites of cortical layer V pyramidal cells to determine whether there are genotype-dependent alterations. Our results indicate that the density of spines/μm is similar between genotypes of both male (Fig. 4A; t(15) = 0.3363, p = 0.7413) and female (Fig. 4C; t(16) = 0.9504, p = 0.3560) littermates. We next investigated the proportion of spines hierarchically classified as nascent (filopodia, long thin), immature (thin), or mature (stubby, mushroom, branched) (Risher et al., 2014). In both males and females, no between-genotype differences were observed in the proportion of mushroom (males; Fig 4b; t(15) = 0.5348, p = 0.6006, females; Fig 4d; t(16) = 0.6113, p = 0.5496), filopodia (males; Fig 4b; t(15) = 1.547, p = 0.1427, females; Fig 4d; t(16) = 2.096, p = 0.0523), stubby (males; Fig 4b; t(15) = 0.7563, p = 0.4612, females; Fig 4d; t(16) = 0.7956, p = 0.4379), branched (males; Fig 4b; t(15) = 0.7714, p = 0.4524, females; Fig 4d; t(16) = 1.461, p = 0.1633), or thin (males; Fig 4b; t(15) = 1.615, p = 0.1272, females; Fig 4d; t(16) = 0.2332, p = 0.8186) spines. However, a small increase in the proportion of long thin spines was observed in SLC7A11sut/sut females (Fig 4d; t(16) = 2.299, p = 0.0353) but not males (Fig 4b; t(15) = 2.087, p = 0.0544) as compared to sex-matched SLC7A11+/+ littermates (unpaired t test on arcsine transformed data).
Figure 4. Comparison of dendritic spine density and morphology between SLC7A11+/+ and SLC7A11sut/sut mice.
Golgi-Cox stained dendritic spines located on secondary apical dendrites of primary motor cortex layer V pyramidal cells from male (a-b) or female (c-d) naïve SLC7A11+/+ (+/+; n = 9 neurons from 4 males or n = 9 neurons from 3 females) and SLC7A11sut/sut (sut/sut; n = 8 neurons from 3 males or n = 9 neurons from 3 females) littermates were analyzed using the Risher et al. method as described in Materials and Methods.
a, c) The horizontal lines represent the mean number of spines/10 μm dendritic length ± SD derived from individual neurons from (a) male or (c) female SLC7A11+/+ [black circles] and SLC7A11sut/sut [blue circles] mice. Inset: representative photomicrographs (60x) of secondary apical dendritic spines from Golgi-Cox stained tissue. Both male and female SLC7A11sut/sut mice have similar dendritic spine densities on layer V pyramidal cells as compared to sex-matched SLC7A11+/+ controls (unpaired t test on log-transformed data).
b, d) The horizontal lines represent the mean percentage of spines ± SD derived from individual neurons of (b) male or (d) female SLC7A11+/+ (black circles, n = 5056 spines from 4 males or n = 4763 spines from 3 females) or SLC7A11sut/sut (blue circles, n = 5355 spines from 3 males or n = 4430 spines from 3 females) mice categorized as either mushroom, filopodia, stubby, branched, thin, or long thin. An asterisk (*) represents a significant between-genotype difference (p = 0.0353, unpaired t test on arcsine transformed data, n = 8-9 neurons/genotype).
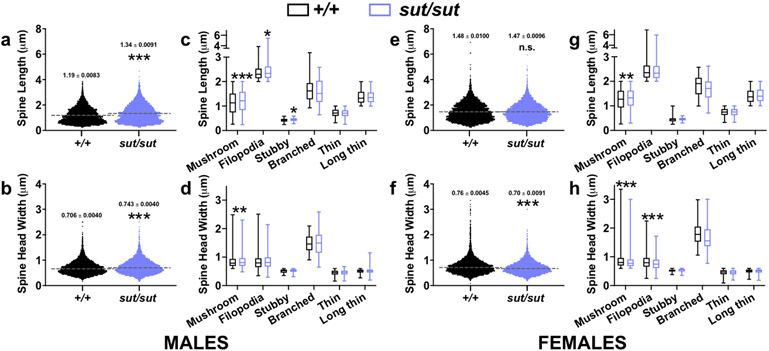
Further analysis of all spines used to derive the aforementioned classifications reveal additional genotype differences in spine head widths and lengths. In males, SLC7A11sut/sut mice have an increase in mean spine length (Fig. 5A; t(15) = 12.1, p < 0.0001; unpaired t test) and head width (Fig. 5B; t(15) = 7.122, p < 0.0001; unpaired t test) as compared to sex-matched SLC7A11+/+ littermates. These changes are driven by increases in mushroom (t(15) = 5.855, p < 0.0001), filopodia (t(15) = 2.806, p = 0.0133), and stubby (t(15) = 2.268, p = 0.0385) spine lengths (Fig. 5C; unpaired t test) and mushroom spine head widths (Fig. 5D; t(15) = 2.730, p = 0.0077; unpaired t test), respectively. In females, the mean length of all spines combined is similar between genotypes (Fig. 5E, t(16) = 0.0719, p = 0.9436; unpaired t test), however, stratification of this parameter by spine type reveals that SLC7A11sut/sut mushroom spines are longer as compared to spines found on SLC7A11+/+ littermate pyramidal cells (Fig. 5G, t(16) = 3.496, p = 0.0015; unpaired t test). Loss of Sxc- in female mice results in decreased spine head widths as compared to sex-matched SLC7A11+/+ littermate controls (Fig. 5F, t(16) = 10.14, p < 0.0001; unpaired t test), an effect driven by decreases in mushroom (t(16) = 8.034, p < 0.0001) and filopodia (t(16) = 5.664, p < 0.0001) type spines (Fig. 5H; unpaired t test). Together, these results provide morphological evidence that dendritic spines, the locus of the vast majority of fast synaptic excitatory neurotransmission (Colonnier, 1968), may be weakened in SLC7A11sut/sut females and strengthened in SLC7A11sut/sut males, as compared to sex- and age-matched SLC7A11+/+ mice.
Figure 5. Comparison of spine lengths and spine head widths between male or female SLC7A11+/+ and SLC7A11sut/sut mice.
a, e, b, f) Scatterplots represent the distribution of spine length (a,e) or spine head width (b, f) variables in males (a-b) or females (e-f) used to derive length-to-width ratios in Fig. 4b or 4d, respectively, with the mean (dashed line) denoted. An asterisk (*) represents a significant between group difference (p < 0.0001, unpaired t test, n = 8-9 neurons/genotype in males, n = 9 neurons/genotype in females).
c, d, g, h) Box-and-whisker plots represent the distribution of male (c, d) or female (g, h) spine lengths (c, g) or spine head widths (d, h) from spines categorized as mushroom, filopodia, stubby, branched, thin, or long thin in Fig. 4b (males) or 4d (females). The lower, middle, and upper margins of the boxes represent the 25th percentile, the median, and the 75th percentile, respectively, whereas the whiskers represent the minimum and maximum values. An asterisk (*) represents a significant between group difference (*p < 0.05, **p < 0.01, ***p < 0.0001 unpaired t test, n = 8-9 neurons/genotype in males, n = 9 neurons/genotype in females).
Changes in excitability uncovered by chemoconvulsant challenge in male and female SLC7A11sut/sut mice
Previous studies have demonstrated that the cellular morphological alterations described above occur in association with increased network excitability, either tipping the balance toward excitation directly (Chen et al., 2009; Klenowski et al., 2016; Mainen & Sejnowski, 1996; Zito, Scheuss, Knott, Hill, & Svoboda, 2009) or occurring at the expense of inhibition (Hsu et al., 2012; Ye et al., 2015). Hence, we next explored whether there is an E/I imbalance in either male or female SLC7A11sut/sut mice by comparing their seizure scores and convulsive seizure thresholds to that of age- and sex-matched SLC7A11+/+ control mice following systemic administration of the chemoconvulsants pentylenetetrazole (PTZ) or kainic acid (KA).
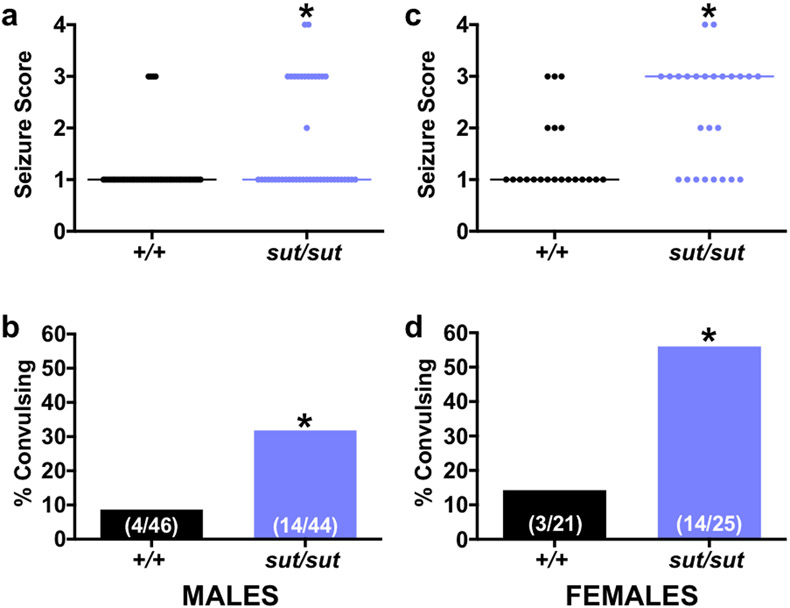
Overall, we found that both male and female SLC7A11sut/sut mice are more excitable than their SLC7A11+/+ littermates after acute challenge with the GABAA receptor antagonist, PTZ (Fig 6). In response to 35 mg/kg PTZ, the median maximal seizure score (MMSS) of male mice was identical in both genotypes (Stage 1), however, there was a significant increase in SLC7A11sut/sut seizure severity overall (Fig. 6A; p = 0.0031; Mann-Whitney U test). Additionally, the incidence of convulsions — that is the percentage of mice exhibiting a score ≥ 3 — was significantly higher for male SLC7A11sut/sut mice as compared to SLC7A11+/+ controls [Fig. 6B; 31.8% (14/44) vs 8.7% (4/46)], respectively; p = 0.0081; Fisher’s exact test). In response to 42 mg/kg, female SLC7A11sut/sut mice showed a similar reduction in convulsive seizure threshold with 56% (14/25) demonstrating a seizure score ≥ 3 as compared to just 14% (3/21) of female SLC7A11+/+ mice (Fig 6D; p = 0.0054; Fisher’s exact test). Female SLC7A11sut/sut mice also displayed a higher MMSS than their SLC7A11+/+ counterparts (Fig. 6C; stage 3 vs. stage 1, respectively, p = 0.0030; Mann-Whitney U test). Of note, the mean latency to first seizure (any score) or first convulsive seizure (seizure score ≥ 3) did not significantly differ between SLC7A11+/+ and SLC7A11sut/sut mice of either sex (Table 1).
Figure 6. SLC7A11sut/sut mice are hyperexcitable following PTZ-administration.
Male (a-b) or female (c-d) naïve SLC7A11+/+ (+/+; n = 46 males or n = 21 females) and SLC7A11sut/sut (sut/sut; n = 44 males or n = 25 females) littermates were administered a single dose of 35 mg/kg (males) or 42 mg/kg (females) PTZ (i.p.). Seizure behavior was scored using a 5-point scale as described in Materials and Methods.
a, c) Individual seizure scores: Each data point [black circles (+/+) or blue circles (sut/sut)] represents the maximal seizure score obtained by an individual male (a) or female (c) mouse administered PTZ during a 30 min observation period. Horizontal lines represent the median seizure score for each genotype. An asterisk (*) represents a significant between group difference (a; p = 0.0031, c; p = 0.0030, Mann-Whitney U Test).
b, d) Convulsive index: Bars [black bar (+/+) or blue bar (sut/sut)] represent the proportion (fraction within bars) of male (b) or female (d) mice that experienced a convulsive seizure (seizure score ≥ 3) in a (b) or c (d) expressed as a % of total mice exposed to PTZ. An asterisk (*) represents a significant between group difference (b; p = 0.0081, d; p = 0.0054, Fisher’s Exact Test).
Table 1.
Latency to clonic/convulsive seizure in mice administered PTZ or KA in Figures 6 and 7, respectively. Data are expressed as mean ± SD. Unpaired t test with Welch’s correction.
| Chemoconvulsant | Genotype | Sex | Clonic/convulsive seizure latency (sec) |
p value |
|---|---|---|---|---|
| PTZ | SLC7A11+/+ | M | 632.3 ± 122.2 | 0.1104 |
| SLC7A11sut/sut | 444.3 ± 93.07 | |||
| PTZ | SLC7A11+/+ | F | 190.7 ± 61.57 | 0.0643 |
| SLC7A11sut/sut | 377.4 ± 52.71 | |||
| KA | SLC7A11+/+ | M | 1711 ± 37.5 | n/a |
| SLC7A11sut/sut | 1444 ± 122.6 | |||
| KA | SLC7A11+/+ | F | 1493 ± 171 | n/a |
| SLC7A11sut/sut | 1044 ± 148.5 | |||
| nonconvulsive seizure (PTZ | p value | |||
| PTZ | SLC7A11 +/+ | M | 92.07 + 7.154 | 0.4979 |
| SLC7A11sut/sut | 101.6 + 12.05 | |||
| PTZ | SLC7A11+/+ | F | 111.4 + 7.209 | 0.9715 |
| SLC7A11sut/sut | 111.8 + 7.706 |
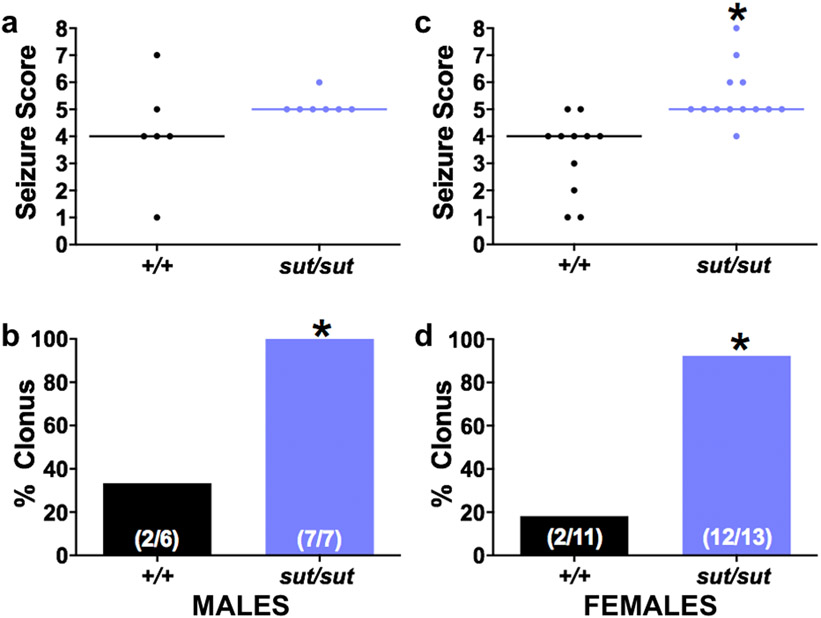
Whether this finding was model dependent was explored via analyzing responses to systemic administration of KA, a glutamate receptor agonist that directly elicits excitation. Once again, our results indicate that both male and female SLC7A11sut/sut mice have a lower convulsive seizure threshold (Fig 7). Specifically, 100% [7/7] of male and 92.3% [12/13] of female SLC7A11sut/sut mice exhibited behavioral clonic or convulsive seizures manifest by kyphosis with forelimb clonus (seizure score = 5), a generalized convulsion (seizure score = 6), a generalized convulsion with loss of righting reflex and/or violent jumping (seizure score = 7), or status epilepticus (seizure score = 8) as compared to a small subset of their male (33.3% [2/6]) or female (18.3% [2/11]) SLC7A11+/+ littermates (Fig. 7B; p = 0.0210 [males]; Fig. 7D; p = 0.0005 [females]; Fisher’s exact test). Overall, the MMSS of both male and female SLC7A11sut/sut mice was stage 5 vs. stage 4 for SLC7A11+/+ mice of either sex (Fig. 7A; p = 0.1142 [males]; Fig. 7C; p = 0.0002 [females]; Mann-Whitney U test). Finally, it is noteworthy that the latency to clonic seizure was reduced in both male and female SLC7A11sut/sut versus sex-matched SLC7A11+/+ mice (Table 1), although the sample sizes preclude a sufficiently powered statistical comparison given that only two male and two female SLC7A11+/+ mice exhibited clonic/convulsive seizures in response to KA. Taken together, the decrease in SLC7A11sut/sut convulsive seizure threshold, unmasked by challenge with two pharmacologically distinct chemoconvulsants, suggests that both male and female SLC7A11sut/sut mice have a shift in their E/I balance towards excitation.
Figure 7. SLC7A11sut/sut mice are hyperexcitable following KA-administration.
Male (a-b) or female (c-d) naïve SLC7A11+/+ (+/+; n = 6 males or n = 11 females) and SLC7A11sut/sut (sut/sut; n = 7 males or n = 13 females) littermates were treated with a single dose of 12 mg/kg (males) or 15 mg/kg (females) KA (i.p.). Seizure behavior was scored using a 9-point scale as described in Materials and Methods.
a, c) Individual seizure scores: Each data point [black circles (+/+) or blue circles (sut/sut)] represents the maximal seizure score obtained by an individual male (a) or female (c) mouse administered KA during a 30 min observation period. SLC7A11sut/sut males have a non-significant increase in KA-induced seizure severity as compared to SLC7A11+/+ littermates (p = 0.1142, Mann-Whitney U test). An asterisk (*) represents a significant between group difference (*p = 0.0002, Mann-Whitney U test).
b, d) Convulsive index: Bars [black bar (+/+) or blue bar (sut/sut)] represent the proportion (fraction within bars) of male (b) or female (d) mice that experienced a clonic/convulsive seizure (seizure score ≥ 5) in a (b) or c (d) expressed as a % of total mice exposed to KA. An asterisk (*) represents a significant between group difference (b; p = 0.0210, d; p = 0.0005, Fisher’s Exact Test).
DISCUSSION
Herein, we demonstrate genotype-dependent alterations in gross brain morphology, dendritic complexity, soma size, and dendritic spine morphology occur between SLC7A11sut/sut and SLC7A11+/+ mice, changes that in each sex represent morphological correlates of excitability. In keeping with this idea, we find reductions in the seizure threshold in SLC7A11sut/sut mice of both sexes as compared to sex-matched SLC7A11+/+ littermate controls in response to an acute dose of two pharmacologically distinct chemoconvulsants, PTZ and KA.
Neuronal hyperexcitability was uncovered in SLC7A11sut/sut mice by systemic administration of PTZ, a competitive GABAA receptor antagonist (Huang et al., 2001), or the AMPA and KA type glutamate receptor agonist KA (Vincent & Mulle, 2009). Seizure severity was more severe and the convulsive seizure threshold reduced in SLC7A11sut/sut mice of either sex as compared to SLC7A11+/+ (Fig 6,7). It should be noted that the dose of the chemoconvulsant required to uncover SLC7A11sut/sut hyperexcitability is sexually dimorphic as reflected by the need to administer a lower dose of either PTZ (35 vs. 42 mg/kg b.w.) or KA (12 vs. 15 mg/kg b.w) to male vs. female mice in order to uncover changes in excitability. This is not unusual as sex-dependent differences in susceptibility to PTZ- (Kokka, Sapp, Witte, & Olsen, 1992; Medina, Manhaes, & Schmidt, 2001) and KA-induced (Li & Liu, 2019; Mejias-Aponte, Jimenez-Rivera, & Segarra, 2002) seizures in rodents has been previously reported.
Some transgenic mouse lines that show enhanced seizure susceptibility (such as we see here; Fig 6,7) have spontaneous and/or handling-induced seizures (Liang & Patel, 2004). Others do not, yet EEG recordings detect subclinical seizures reflecting abnormal excitability (Corradini et al., 2014). We have not observed any handling-induced or spontaneous seizures in SLC7A11sut/sut mice of either sex. The possibility that these mice may have silent seizures is something, however, that needs to be explored given the hyperexcitable behavioral phenotype uncovered by chemoconvulsant challenge and our results showing female mice of the SLC7A11sut/sut genotype have significantly smaller corpus callosum width and soma size than those found in female SLC7A11+/+ controls (Fig 2,3), while male SLC7A11sut/sut mice have increased dendritic complexity (Fig 3) and larger mushroom spines (Fig 5). In either sex, we found no changes in dendritic spine density (spines/μm) on secondary apical dendrites (Fig 4), though it should be noted that we did not measure spine density on other branch orders nor on basal dendrites.
Previous studies have demonstrated that the cellular changes we describe represent morphological correlates of increased network excitability, either by tipping the balance toward excitation directly or by mitigating inhibitory tone. For instance, dendritic structure is critical for information integration and relay, and dendritic tree complexity influences the firing pattern of neurons (Chen et al., 2009; Klenowski et al., 2016; Mainen & Sejnowski, 1996), with size and geometry of the spine acting as the determining factors in synaptic signal strength and specificity (Nimchinsky, Sabatini, & Svoboda, 2002). To wit, distribution of functional AMPA receptors is highest in large, thick spines, with sparse distribution in thin spines and filopodia (Kasai, Matsuzaki, Noguchi, Yasumatsu, & Nakahara, 2003; Matsuzaki et al., 2001). This correlates precisely with their respective glutamate sensitivities (Matsuzaki et al., 2001), suggesting that cortical pyramidal cells in male SLC7A11sut/sut mice have an overall strengthening of synaptic efficacy. This conclusion is strengthened by evidence demonstrating male xCT null mutant mice have enhanced AMPA receptor immunoreactivity and spontaneous and evoked excitatory postsynaptic currents (EPSCs) as compared to wild-type control slices in the CA1 hippocampal subregion (Williams & Featherstone, 2014). Cellular morphological changes such as described herein have not yet been investigated in the transgenic xCT null mice.
Since tonic inhibition arises predominately from synapses on or near the soma (Andersen, Eccles, & Loyning, 1964; Soltesz, Smetters, & Mody, 1995), a small soma size, such as that found in SLC7A11sut/sut females (Fig 3), could result in diminished inhibitory input. This is a change that – despite a reduction in SLC7A11sut/sut female spine head widths (Fig 5) that could be compensatory perhaps in effort to maintain E/I balance in the face of normal transmission – has been demonstrated to occur in association with hyperexcitability. For instance, a small soma size is a phenotype of several mouse models of autism, including Rett syndrome (Kishi & Macklis, 2004; Wang, Reyes, & Zhou, 2013), all of which show increased seizure susceptibility (Chao et al., 2010; McLeod et al., 2013) ascribed to a reduction in inhibitory tone (Chao et al., 2010; Ito-Ishida, Ure, Chen, Swann, & Zoghbi, 2015). Finally, a reduction in corpus callosum size has been demonstrated in autism (Egaas, Courchesne, & Saitoh, 1995; Hardan, Minshew, & Keshavan, 2000), which frequently coexists in the same individual with epilepsy [for review see (Bozzi, Provenzano, & Casarosa, 2018)]. This phenotypic change has also been found in human temporal lobe epilepsy (TLE) patients via diffusion tensor imaging with changes correlating with the age at onset of epilepsy but not its duration, leading the authors to speculate that this change reflects a primary myelin defect (Arfanakis et al., 2002). A more recent study confirmed these white matter changes in human TLE, but also reported greater loss in patients with sclerosis and hippocampal atrophy vs those without, leading the authors to hypothesize that white matter damage may occur secondary to excitotoxic neuronal cell death (Scanlon et al., 2013). Whether the change in corpus callosum size found in female SLC7A11sut/sut mice results from a deficit of myelin or is indicative of a less tightly packed neuronal network remains to be determined.
With respect to the changes found in males, dendritic complexity and dendritic spine head width are maintained in part by levels of extracellular Glu (Kwon & Sabatini, 2011; Mattson, Dou, & Kater, 1988). Of note, hippocampus and striatum of male transgenic xCT deficient mice have reductions in Glu of up to 50% (Massie et al., 2011; McCullagh & Featherstone, 2014), whereas female xCT null striatal levels — which was the only region tested — are comparable to wild-type controls (Borra et al., 2014). Whether this extends to cortex, and whether it differentially alters neuronal morphology therein, requires further investigation.
The mechanism by which Sxc- contributes to the development and/or maintenance of soma size in female mice also requires further investigation. Given that ambient Glu concentrations are unaffected in female xCT null mice (Borra et al., 2014), it is tempting to speculate that differences in Sxc--mediated cystine import rather than Glu export underlie the change. Of interest, it has been previously shown that a reduction in protein synthesis results in decreased neuron soma size (Franklin & Johnson, 1998). Hence, decreased oxidized cystine influx, which is supported by Sxc-, could plausibly facilitate shunting of reduced cystine (aka cysteine) from protein building to cellular glutathione (GSH) production in an effort to maintain cellular redox balance (Ratan, Murphy, & Baraban, 1994). Additionally, the capacity to maintain GSH levels under conditions of oxidative stress is greater in females than in males (Du et al., 2004), suggesting that females may employ alternate mechanisms to sustain redox balance in response to perturbations, including loss of Sxc--mediated cystine import.
It should be noted that our gross morphology results are seemingly at odds with those of Shih and colleagues who previously reported that SLC7A11sut/sut male mice (female mice were not analyzed) at ≈13-15 weeks of age show enlarged lateral ventricles and striatal and cortical “thinning” (Shih et al., 2006). However, we find no morphological differences with regards to cortical width or striatal, hippocampal, lateral ventricle, or total hemispheric areas of 12 week old mice as compared to SLC7A11+/+ littermate controls in either SLC7A11sut/sut male or female mice (Fig 1,2). Of note, preliminary analysis of 14-16 week old male and female SLC7A11+/+ and SLC7A11sut/sut mouse brains also do not show signs of atrophy (SMSS and SJH, unpublished observations). Indeed, as mentioned earlier, the only gross morphological change we found is in the corpus callosum of female SLC7A11sut/sut mice (Fig. 2). We attribute these differential findings to different breeding strategies. In the former study, independent homozygous breeder lines generated experimental animals (Shih et al., 2006), whereas we breed heterozygotes and restrict the use of mice to F2 and F3 generations, thereby preventing genetic drift (Wolfer et al., 2002; Wolfer & Lipp, 2000). Additionally, our findings of hyperexcitability were not recapitulated in male transgenic xCT null (−/−) mice (female mice were not assessed), where it appears that elevated doses of the chemoconvulsants pilocarpine and KA, administered via continuous intravenous infusion (150μL/min), were required to elicit behavioral seizures as compared to wild-type littermates (De Bundel et al., 2011a). In the same study it was shown that male xCT−/− mice have decreased seizure severity and mortality in response to an acute intraperitoneal dose of NMDA (De Bundel et al., 2011a). The reason for these discrepancies in findings is not immediately clear. However, it may be accounted for by differences in how the Sxc- null was created [natural mutation (Chintala et al., 2005) vs targeted deletion (Sato et al., 2005)], the mouse strain (C3H/HeSnJ vs C57BL/6) (Loscher, Ferland, & Ferraro, 2017), or by the chemoconvulsants used, as well as their method of administration. Finally, these acute studies are different from previous work by ourselves and others demonstrating that loss of Sxc- signaling in male mice – either via mutation, targeted deletion, or pharmacological block – reduced epileptogenesis (i.e., the process of acquiring epilepsy) in several different electrical and chemoconvulsant models (Leclercq et al., 2019; Sears, Hewett, & Hewett, 2019). These studies suggest inhibition of Sxc- signaling may be a therapeutic option to thwart the development of epilepsy in predisposed individuals, which is fundamentally different from modulation of the acute convulsive seizure threshold as described herein. Still, it will be important to determine whether and at what doses pharmacological inhibition of Sxc- phenocopies what we find in SLC7A11sut/sut mice in both paradigms.
Overall, our results indicate that Sxc- contributes to gross, regional, cellular and subcellular mouse brain morphology in both male and female mice. This, coupled with the manifestation enhanced excitability, expressed by an increase in seizure severity and a reduction in convulsive seizure threshold following acute challenge with chemoconvulsants in the SLC7A11sut/sut C3H/HeSnJ mice irrespective of sex, raises the intriguing possibility that Sxc- signaling contributes to the endogenous network activity that maintains excitatory/inhibitory (E/I) balance in vivo, potentially through its regulation of cellular morphology.
Supplementary Material
Supplementary Figure 1. Brain and body weight comparison between SLC7A11+/+ and SLC7A11sut/sut littermate mice
a-d) Brain and body weights of male (a-b) or female (c-d) naïve 12 week old SLC7A11+/+ (+/+; n = 6 males or n = 4-6 females) and SLC7A11sut/sut (sut/sut; n = 7 males or n = 6-7 females) littermates were recorded. Data points represent individual mice whereas the horizontal line [black (+/+) and blue (sut/sut)] represent the mean ± SD brain (a, c) or body (b, d) weight for each group. Inset: representative whole brains derived from male or female SLC7A11+/+ and SLC7A11sut/sut littermates. No significant within-sex genotype differences were observed (a; p = 0.2073, b; p = 0.8061, c; p = 0.2525, d; p = 0.4352; unpaired t test).
STATEMENT OF SIGNIFICANCE:
Alterations in excitatory/inhibitory (E/I) balance in brain contribute to the pathobiology of neurological, neurodegenerative, and neurodevelopmental disorders. A deeper understanding of the cellular and molecular mechanisms regulating physiological balance is needed to improve current clinical strategies for managing E/I perturbations. Herein, we provide evidence that the signaling of system xc-, a cystine/glutamate antiporter found predominately expressed on astrocytes, contributes to the endogenous network activity that regulates excitability in brains of both male and female mice, likely through regulation of cellular morphology.
Other acknowledgements:
The authors would like to thank Dr. James Hewett for insightful comments regarding the interpretation of data, as well as Rhea Saini and Diem Ho for technical assistance. This work was supported by grants from the US National Institutes of Health (NIH) – R01 NS051445 and R01 NS105767.
Footnotes
Conflict of interest: The authors declare no competing financial interests.
Data accessibility statement: The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Allen NJ, Bennett ML, Foo LC, Wang GX, Chakraborty C, Smith SJ, & Barres BA (2012). Astrocyte glypicans 4 and 6 promote formation of excitatory synapses via GluA1 AMPA receptors. Nature, 486(7403), 410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P, Eccles JC, & Loyning Y (1964). Location of Postsynaptic Inhibitory Synapses on Hippocampal Pyramids. J Neurophysiol, 27, 592–607. doi: 10.1152/jn.1964.27.4.592 [DOI] [PubMed] [Google Scholar]
- Anderson CT, Sheets PL, Kiritani T, & Shepherd GM (2010). Sublayer-specific microcircuits of corticospinal and corticostriatal neurons in motor cortex. Nature Neuroscience, 13(6), 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Hermann BP, Rogers BP, Carew JD, Seidenberg M, & Meyerand ME (2002). Diffusion tensor MRI in temporal lobe epilepsy. Magnetic resonance imaging, 20(7), 511–519. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, & Kalivas PW (2002). The origin and neuronal function of in vivo nonsynaptic glutamate. J Neurosci, 22(20), 9134–9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banjac A, Perisic T, Sato H, Seiler A, Bannai S, Weiss N, … Daniel P (2008). The cystine/cysteine cycle: a redox cycle regulating susceptibility versus resistance to cell death. Oncogene, 27(11), 1618–1628. [DOI] [PubMed] [Google Scholar]
- Bannai S (1986). Exchange of cystine and glutamate across plasma membrane of human fibroblasts. Journal of Biological Chemistry, 261(5), 2256–2263. [PubMed] [Google Scholar]
- Barnwell LF, Lugo JN, Lee WL, Willis SE, Gertz SJ, Hrachovy RA, & Anderson AE (2009). Kv4.2 knockout mice demonstrate increased susceptibility to convulsant stimulation. Epilepsia, 50(7), 1741–1751. doi: 10.1111/j.1528-1167.2009.02086.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borra S, McCullagh EA, Featherstone DE, Baker PM, Ragozzino ME, & Shippy SA (2014). Determining striatal extracellular glutamate levels in xCT mutant mice using LFPS CE-LIF. Analytical Methods, 6(9), 2916–2922. doi: 10.1039/C4AY00392F [DOI] [Google Scholar]
- Bowers K, Li Q, Bressler J, Avramopoulos D, Newschaffer C, & Fallin MD (2011). Glutathione pathway gene variation and risk of autism spectrum disorders. J Neurodev Disord, 3(2), 132–143. doi: 10.1007/s11689-011-9077-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozzi Y, Provenzano G, & Casarosa S (2018). Neurobiological bases of autism-epilepsy comorbidity: a focus on excitation/inhibition imbalance. Eur J Neurosci, 47(6), 534–548. doi: 10.1111/ejn.13595 [DOI] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, … Zoghbi HY (2010). Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature, 468(7321), 263–269. doi: 10.1038/nature09582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Prithviraj R, Mahnke AH, McGloin KE, Tan JW, Gooch AK, & Inglis FM (2009). AMPA glutamate receptor subunits 1 and 2 regulate dendrite complexity and spine motility in neurons of the developing neocortex. Neuroscience, 159(1), 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintala S, Li W, Lamoreux ML, Ito S, Wakamatsu K, Sviderskaya EV, … Swank RT (2005). Slc7a11 gene controls production of pheomelanin pigment and proliferation of cultured cells. Proceedings of the National Academy of Sciences of the United States of America, 102(31), 10964–10969. doi: 10.1073/pnas.0502856102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury T, Allen M, Thorn T, He Y, & Hewett S (2018). Interleukin-1β protects neurons against oxidant-induced injury via the promotion of astrocyte glutathione production. Antioxidants, 7(8), 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson KS, Ullian EM, Stokes CC, Mullowney CE, Hell JW, Agah A, … Barres BA (2005). Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell, 120(3), 421–433. [DOI] [PubMed] [Google Scholar]
- Coffman JA, & Su YH (2019). Redox regulation of development and regeneration. Curr Opin Genet Dev, 57, 9–15. doi: 10.1016/j.gde.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Colonnier M (1968). Synaptic patterns on different cell types in the different laminae of the cat visual cortex. An electron microscope study. Brain Research, 9(2), 268–287. [DOI] [PubMed] [Google Scholar]
- Corradini I, Donzelli A, Antonucci F, Welzl H, Loos M, Martucci R, … Matteoli M (2014). Epileptiform activity and cognitive deficits in SNAP-25(+/−) mice are normalized by antiepileptic drugs. Cereb Cortex, 24(2), 364–376. doi: 10.1093/cercor/bhs316 [DOI] [PubMed] [Google Scholar]
- De Bundel D, Schallier A, Loyens E, Fernando R, Miyashita H, Van Liefferinge J, … Massie A (2011a). Loss of system x(c)(−) does not induce oxidative stress but decreases extracellular glutamate in hippocampus and influences spatial working memory and limbic seizure susceptibility. Journal of Neuroscience, 31(15), 5792–5803. doi: 10.1523/jneurosci.5465-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bundel D, Schallier A, Loyens E, Fernando R, Miyashita H, Van Liefferinge J, … Massie A (2011b). Loss of System xc- Does Not Induce Oxidative Stress But Decreases Extracellular Glutamate in Hippocampus and Influences Spatial Working Memory and Limbic Seizure Susceptibility. J Neurosci, 31(15), 5792–5803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du L, Bayir H, Lai Y, Zhang X, Kochanek PM, Watkins SC, … Clark RS (2004). Innate gender-based proclivity in response to cytotoxicity and programmed cell death pathway. Journal of Biological Chemistry, 279(37), 38563–38570. [DOI] [PubMed] [Google Scholar]
- Egaas B, Courchesne E, & Saitoh O (1995). Reduced size of corpus callosum in autism. Arch Neurol, 52(8), 794–801. doi: 10.1001/archneur.1995.00540320070014 [DOI] [PubMed] [Google Scholar]
- Eroglu C, & Barres BA (2010). Regulation of synaptic connectivity by glia. Nature, 468(7321), 223–231. doi: 10.1038/nature09612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, St Jean P, Schork NJ, Mulholland N, … Berrettini WH (1999). Mapping loci for pentylenetetrazol-induced seizure susceptibility in mice. J Neurosci, 19(16), 6733–6739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JL, & Johnson EM (1998). Control of neuronal size homeostasis by trophic factor–mediated coupling of protein degradation to protein synthesis. The Journal of cell biology, 142(5), 1313–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardan AY, Minshew NJ, & Keshavan MS (2000). Corpus callosum size in autism. Neurology, 55(7), 1033–1036. doi: 10.1212/wnl.55.7.1033 [DOI] [PubMed] [Google Scholar]
- Hsu R, Schofield CM, Cruz CGD, Jones-Davis DM, Blelloch R, & Ullian EM (2012). Loss of microRNAs in pyramidal neurons leads to specific changes in inhibitory synaptic transmission in the prefrontal cortex. Molecular and Cellular Neuroscience, 50(3), 283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R-Q, Bell-Horner CL, Dibas MI, Covey DF, Drewe JA, & Dillon GH (2001). Pentylenetetrazole-induced inhibition of recombinant γ-aminobutyric acid type A (GABAA) receptors: mechanism and site of action. Journal of Pharmacology and Experimental Therapeutics, 298(3), 986–995. [PubMed] [Google Scholar]
- Ito-Ishida A, Ure K, Chen H, Swann JW, & Zoghbi HY (2015). Loss of MeCP2 in Parvalbumin-and Somatostatin-Expressing Neurons in Mice Leads to Distinct Rett Syndrome-like Phenotypes. Neuron, 88(4), 651–658. doi: 10.1016/j.neuron.2015.10.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Matsuzaki M, Noguchi J, Yasumatsu N, & Nakahara H (2003). Structure–stability–function relationships of dendritic spines. Trends in neurosciences, 26(7), 360–368. [DOI] [PubMed] [Google Scholar]
- Kishi N, & Macklis JD (2004). MECP2 is progressively expressed in post-migratory neurons and is involved in neuronal maturation rather than cell fate decisions. Mol Cell Neurosci, 27(3), 306–321. doi: 10.1016/j.mcn.2004.07.006 [DOI] [PubMed] [Google Scholar]
- Klenowski PM, Fogarty MJ, Shariff M, Belmer A, Bellingham MC, & Bartlett SE (2016). Increased synaptic excitation and abnormal dendritic structure of prefrontal cortex layer V pyramidal neurons following prolonged binge-like consumption of ethanol. eNeuro, 0248-0216.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokka N, Sapp DW, Witte U, & Olsen RW (1992). Sex differences in sensitivity to pentylenetetrazol but not in GABAA receptor binding. Pharmacol Biochem Behav, 43(2), 441–447. doi: 10.1016/0091-3057(92)90174-e [DOI] [PubMed] [Google Scholar]
- Kucukdereli H, Allen NJ, Lee AT, Feng A, Ozlu MI, Conatser LM, … Sage EH (2011). Control of excitatory CNS synaptogenesis by astrocyte-secreted proteins Hevin and SPARC. Proceedings of the National Academy of Sciences, 108(32), E440–E449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H-B, & Sabatini BL (2011). Glutamate induces de novo growth of functional spines in developing cortex. Nature, 474(7349), 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclercq K, Liefferinge JV, Albertini G, Neveux M, Dardenne S, Mairet-Coello G, … Kaminski RM (2019). Anticonvulsant and antiepileptogenic effects of system x c - inactivation in chronic epilepsy models. Epilepsia, 60(7), 1412–1423. doi: 10.1111/epi.16055 [DOI] [PubMed] [Google Scholar]
- Li F, & Liu L (2019). Comparison of kainate-induced seizures, cognitive impairment and hippocampal damage in male and female mice. Life Sci, 232, 116621. doi: 10.1016/j.lfs.2019.116621 [DOI] [PubMed] [Google Scholar]
- Liang L-P, & Patel M (2004). Mitochondrial oxidative stress and increased seizure susceptibility in Sod2−/+ mice. Free Radical Biology and Medicine, 36(5), 542–554. [DOI] [PubMed] [Google Scholar]
- Loscher W, Ferland RJ, & Ferraro TN (2017). The relevance of inter- and intrastrain differences in mice and rats and their implications for models of seizures and epilepsy. Epilepsy Behav, 73, 214–235. doi: 10.1016/j.yebeh.2017.05.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainen ZF, & Sejnowski TJ (1996). Influence of dendritic structure on firing pattern in model neocortical neurons. Nature, 382(6589), 363–366. [DOI] [PubMed] [Google Scholar]
- Massie A, Schallier A, Kim SW, Fernando R, Kobayashi S, Beck H, … Michotte Y (2011). Dopaminergic neurons of system x(c)(−)-deficient mice are highly protected against 6-hydroxydopamine-induced toxicity. Faseb J, 25(4), 1359–1369. doi: 10.1096/fj.10-177212 [DOI] [PubMed] [Google Scholar]
- Matsuzaki M, Ellis-Davies GC, Nemoto T, Miyashita Y, Iino M, & Kasai H (2001). Dendritic spine geometry is critical for AMPA receptor expression in hippocampal CA1 pyramidal neurons. Nature Neuroscience, 4(11), 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M, Dou P, & Kater S (1988). Outgrowth-regulating actions of glutamate in isolated hippocampal pyramidal neurons. Journal of Neuroscience, 8(6), 2087–2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauch DH, Nägler K, Schumacher S, Göritz C, Müller E-C, Otto A, & Pfrieger FW (2001). CNS synaptogenesis promoted by glia-derived cholesterol. Science, 294(5545), 1354–1357. [DOI] [PubMed] [Google Scholar]
- McCullagh EA, & Featherstone DE (2014). Behavioral characterization of system xc-mutant mice. Behavioural Brain Research, 265, 1–11. doi: 10.1016/j.bbr.2014.02.010 [DOI] [PubMed] [Google Scholar]
- McLeod F, Ganley R, Williams L, Selfridge J, Bird A, & Cobb SR (2013). Reduced seizure threshold and altered network oscillatory properties in a mouse model of Rett syndrome. Neuroscience, 231, 195–205. doi: 10.1016/j.neuroscience.2012.11.058 [DOI] [PubMed] [Google Scholar]
- Medina AE, Manhaes AC, & Schmidt SL (2001). Sex differences in sensitivity to seizures elicited by pentylenetetrazol in mice. Pharmacol Biochem Behav, 68(3), 591–596. doi: 10.1016/s0091-3057(01)00466-x [DOI] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Jimenez-Rivera CA, & Segarra AC (2002). Sex differences in models of temporal lobe epilepsy: role of testosterone. Brain Res, 944(1–2), 210–218. doi: 10.1016/s0006-8993(02)02691-4 [DOI] [PubMed] [Google Scholar]
- Nimchinsky EA, Sabatini BL, & Svoboda K (2002). Structure and function of dendritic spines. Annu Rev Physiol, 64, 313–353. doi: 10.1146/annurev.physiol.64.081501.160008 [DOI] [PubMed] [Google Scholar]
- Ottestad-Hansen S, Hu QX, Follin-Arbelet VV, Bentea E, Sato H, Massie A, … Danbolt NC (2018). The cystine-glutamate exchanger (xCT, Slc7a11) is expressed in significant concentrations in a subpopulation of astrocytes in the mouse brain. Glia, 66(5), 951–970. doi: 10.1002/glia.23294 [DOI] [PubMed] [Google Scholar]
- Pick JR, & Little JM (1965). Effect of Type of Bedding Material on Thresholds of Pentylenetetrazol Convulsions in Mice. Lab Anim Care, 15, 29–33. [PubMed] [Google Scholar]
- Pitkanen A (2006). Models of Seizures and Epilepsy. In. Boston, MA: Elsevier Academic Press. [Google Scholar]
- Racine RJ (1972). Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol, 32(3), 281–294. [DOI] [PubMed] [Google Scholar]
- Ratan RR, Murphy TH, & Baraban JM (1994). Macromolecular synthesis inhibitors prevent oxidative stress-induced apoptosis in embryonic cortical neurons by shunting cysteine from protein synthesis to glutathione. Journal of Neuroscience, 14(7), 4385–4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risher WC, Ustunkaya T, Alvarado JS, & Eroglu C (2014). Rapid Golgi analysis method for efficient and unbiased classification of dendritic spines. PLoS One, 9(9), e107591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, Kuriyama-Matsumura K, Siow R, Ishii T, Bannai S, & Mann GE (1998). Induction of cystine transport via system xc− and maintenance of intracellular glutathione levels in pancreatic acinar and islet cell lines. Biochimica et Biophysica Acta (BBA)-Biomembranes, 1414(1-2), 85–94. [DOI] [PubMed] [Google Scholar]
- Sato H, Shiiya A, Kimata M, Maebara K, Tamba M, Sakakura Y, … Bannai S (2005). Redox imbalance in cystine/glutamate transporter-deficient mice. Journal of Biological Chemistry, 280(45), 37423–37429. doi: 10.1074/jbc.M506439200 [DOI] [PubMed] [Google Scholar]
- Sato H, Tamba M, Ishii T, & Bannai S (1999). Cloning and expression of a plasma membrane cystine/glutamate exchange transporter composed of two distinct proteins. Journal of Biological Chemistry, 274(17), 11455–11458. [DOI] [PubMed] [Google Scholar]
- Scanlon C, Mueller SG, Cheong I, Hartig M, Weiner MW, & Laxer KD (2013). Grey and white matter abnormalities in temporal lobe epilepsy with and without mesial temporal sclerosis. Journal of Neurology, 260(9), 2320–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears SMS, Hewett JA, & Hewett SJ (2019). Decreased epileptogenesis in mice lacking the System xc (−) transporter occurs in association with a reduction in AMPA receptor subunit GluA1. Epilepsia Open, 4(1), 133–143. doi: 10.1002/epi4.12307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, Erb H, Sun X, Toda S, Kalivas PW, & Murphy TH (2006). Cystine/glutamate exchange modulates glutathione supply for neuroprotection from oxidative stress and cell proliferation. The Journal of neuroscience, 26(41), 10514–10523. doi: 10.1523/jneurosci.3178-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl D (1953). Dendritic organization in the neurons of the visual and motor cortices of the cat. Journal of anatomy, 87(Pt 4), 387. [PMC free article] [PubMed] [Google Scholar]
- Soltesz I, Smetters DK, & Mody I (1995). Tonic inhibition originates from synapses close to the soma. Neuron, 14(6), 1273–1283. doi: 10.1016/0896-6273(95)90274-0 [DOI] [PubMed] [Google Scholar]
- Vincent P, & Mulle C (2009). Kainate receptors in epilepsy and excitotoxicity. Neuroscience, 158(1), 309–323. [DOI] [PubMed] [Google Scholar]
- Wang IT, Reyes AR, & Zhou Z (2013). Neuronal morphology in MeCP2 mouse models is intrinsically variable and depends on age, cell type, and Mecp2 mutation. Neurobiol Dis, 58, 3–12. doi: 10.1016/j.nbd.2013.04.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LE, & Featherstone DE (2014). Regulation of hippocampal synaptic strength by glial xCT. The Journal of neuroscience, 34(48), 16093–16102. doi: 10.1523/jneurosci.1267-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withers GS, Farley JR, Sterritt JR, Crane AB, & Wallace CS (2017). Interactions with astroglia influence the shape of the developing dendritic arbor and restrict dendrite growth independent of promoting synaptic contacts. PLoS One, 12(1), e0169792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfer DP, Crusio WE, & Lipp HP (2002). Knockout mice: simple solutions to the problems of genetic background and flanking genes. Trends Neurosci, 25(7), 336–340. [DOI] [PubMed] [Google Scholar]
- Wolfer DP, & Lipp HP (2000). Dissecting the behaviour of transgenic mice: is it the mutation, the genetic background, or the environment? Exp Physiol, 85(6), 627–634. [PubMed] [Google Scholar]
- Ye Z, Mostajo-Radji MA, Brown JR, Rouaux C, Tomassy GS, Hensch TK, & Arlotta P (2015). Instructing perisomatic inhibition by direct lineage reprogramming of neocortical projection neurons. Neuron, 88(3), 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, … Ruderisch N (2014). An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. Journal of Neuroscience, 34(36), 11929–11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zito K, Scheuss V, Knott G, Hill T, & Svoboda K (2009). Rapid functional maturation of nascent dendritic spines. Neuron, 61(2), 247–258. doi: 10.1016/j.neuron.2008.10.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Brain and body weight comparison between SLC7A11+/+ and SLC7A11sut/sut littermate mice
a-d) Brain and body weights of male (a-b) or female (c-d) naïve 12 week old SLC7A11+/+ (+/+; n = 6 males or n = 4-6 females) and SLC7A11sut/sut (sut/sut; n = 7 males or n = 6-7 females) littermates were recorded. Data points represent individual mice whereas the horizontal line [black (+/+) and blue (sut/sut)] represent the mean ± SD brain (a, c) or body (b, d) weight for each group. Inset: representative whole brains derived from male or female SLC7A11+/+ and SLC7A11sut/sut littermates. No significant within-sex genotype differences were observed (a; p = 0.2073, b; p = 0.8061, c; p = 0.2525, d; p = 0.4352; unpaired t test).