Abstract
Background:
Major depressive disorder (MDD) involves depressed mood (high negative affect, predominantly) and low interest/pleasure (low positive affect). In past research, negative affect has improved more than positive affect during acute-phase antidepressant medication or cognitive therapy (CT). We extended this literature by differentiating depressed mood and two dimensions of low interest (general and sexual), assessing persistence of symptom differences after acute-phase CT response, and testing whether continuation treatment acted differently on depressed mood versus low interest.
Methods:
We analyzed data from two randomized controlled trials. Patients with recurrent MDD first received acute-phase CT. Then, responders were randomized to 8-month continuation treatments and assessed for 16-24 additional months.
Results:
Depressed mood and low general interest improved more than low sexual interest during acute-phase CT. Among responders, these symptom differences persisted for at least 2 years and were not changed by continuation CT or antidepressant medication.
Limitations:
Generalization of findings to other patient populations and treatments is uncertain. Depressed mood and low interest scales were constructed from standard symptom measures and overlapped empirically.
Conclusions:
Less improvement during CT, and persistent low sexual interest despite continuation treatment, highlights the need for MDD treatments more effectively targeting this positive affective symptom.
Keywords: depression, cognitive therapy, anhedonia, positive affect, negative affect, continuation treatment
Defining symptoms of major depressive disorder (MDD) include diminished interest or pleasure (low positive affect) and depressed mood (predominantly high negative affect; American Psychiatric Association, 2013; Clark & Watson, 1991; Mineka, Watson, & Clark, 1998). Recent research suggests that negative affect improves more than does positive affect during acute-phase treatment of MDD with antidepressant medication or cognitive therapy (CT; Dunn et al., 2020). However, the relative stability or change in symptom profiles after response to CT for MDD, and whether continuation-phase treatment affects specific symptoms differently, are largely unknown. In the current study, we aimed to (1) replicate past research showing that depressed mood improves more than low interest during acute-phase CT; and (2) extend the literature by testing (a) to what extent differences in depressed mood and low interest persist after response to acute-phase CT and (b) whether continuation treatment changes these symptoms to different extents.
Two broad affective systems central to depression have been differentiated (Clark & Watson, 1991, 2021). Negative and positive affectivity align with positive and negative valence systems referenced in the National Institute of Mental Health’s Research Domain Criteria (Craske et al., 2016; Sanislow, 2016; but also see Watson, Stanton, & Clark, 2017) and encompass both stable temperament and changeable mood/affect states. Core aspects of positive and negative temperament include the propensity to experience corresponding affect. Negative affect includes emotional distress (e.g., fear, anger, anxiety) and positive affect includes pleasant activation (e.g., energy, enthusiasm, excitement). As a multi-symptom syndrome, MDD involves both high negative and low positive affectivity (Mineka et al., 1998).
Because MDD is often recurrent, treatment may be provided in phases (Bockting et al., 2015; Vittengl & Jarrett, 2014). Acute-phase treatment aims to decrease symptoms of a major depressive episode to produce response (e.g., no longer meet criteria for a major depressive episode) and remission (e.g., several weeks with minimal depressive symptoms). Continuation-or maintenance-phase treatment then promotes sustained remission and recovery (longer periods of no or few depressive symptoms) and helps prevent relapse or recurrence (return of symptoms at the intensity of a major depressive episode), particularly among patients at greater risk (e.g., due to early age of MDD onset, history of more depressive episodes, unstable response or remission; Bockting et al., 2015; Vittengl & Jarrett, 2014).
Efficacious acute-phase treatments for MDD include CT, with symptom-reduction similar to antidepressant medication and superior to pill placebo (Cuijpers et al., 2014). Although acute-phase CT and antidepressant medication have roughly equal effect sizes, CT provides greater protection against relapse/recurrence after acute-phase treatment ends (Cuijpers et al., 2013). Similarly, continuation CT or antidepressant medication each reduce relapse/recurrence risk substantively compared to control groups (Biesheuvel-Leliefeld et al., 2015).
During acute-phase CT or antidepressant medication for MDD, scores on general depressive symptom scales decrease considerably, on average perhaps 1-3 SD (Craighead et al., 2015; Vittengl & Jarrett, 2014). These depressive symptom scales include the Hamilton Rating Scale for Depression (HRSD; Hamilton, 1960), Beck Depressive Inventory (BDI; Beck et al., 1961), and Inventory of Depressive Symptomatology--Self-report (IDS-SR; Rush et al., 1996), all usually scored by summing ratings across a broad range of affective (e.g., depressed mood, low interest/pleasure), behavioral (e.g., changes in sleep and eating), and cognitive (e.g., difficulty concentrating, suicidal ideation) symptoms.
However, when positive and negative affective symptoms are differentiated, improvements during MDD treatment may differ. In randomized controlled trials comparing acute-phase CT to antidepressant medication, negative affect improved (decreased about 1.4 SD) more than positive affect (increased 1.1-1.2 SD; Dunn et al., 2020). Differences in symptom changes did not vary significantly with CT versus medication. After acute-phase treatment, mean negative affect was normative whereas positive affect remained low. To what extent and how long differences in negative and positive affective symptoms persist after acute-phase treatment is unclear.
The current study addressed this question using data from two, multi-phase, randomized controlled trials. In the primary dataset (Jarrett et al., 2013), adult outpatients with recurrent MDD received acute-phase CT. Overall symptom reduction was about 1.7-1.9 SD during acute-phase CT (Vittengl et al., 2013). Responders were assessed for 32 total months after acute-phase CT. During the first 8 months after acute-phase CT, lower risk responders were only assessed, whereas higher risk responders were randomized to continuation-phase CT, fluoxetine, or pill placebo with clinical management. During the continuation phase, CT (18% of patients relapsed) or fluoxetine (18%) reduced relapse compared to pill placebo (33%; Jarrett et al., 2013). However, lower risk responders (5%) relapsed even less (Jarrett et al., 2016).
In the earlier dataset (Jarrett et al., 2001), used here for replication, adult outpatients with recurrent MDD received acute-phase CT, and mean symptom reduction was about 2.0-2.2 SD (Vittengl et al., 2005). Responders were assessed for 24 months after acute-phase CT, including 8 months continuation CT or assessment-only control. During the continuation phase, CT (10%) reduced relapse compared to only assessment (33%; Jarrett et al., 2001).
We used items from the HRSD, BDI, and IDS-SR to construct scales separating depressed mood from low interest. Although MDD diagnostic criteria do not differentiate facets of interest/pleasure (American Psychiatric Association, 2013), sexual interest may improve less than general interest during acute-phase treatment (Snippe et al., 2021). Accordingly, we analyzed general and sexual interest separately. In replication of past research, we hypothesized that depressed mood would improve more than low general and sexual interest during acute-phase CT. Extending past research, we explored the extent to which differences in depressed mood and low interest persisted after response to acute-phase CT, and whether continuation treatment acted differently on these symptoms. Because this post-CT analysis was novel, we considered it exploratory and did not test specific hypotheses.
Method
Data from two, separate randomized controlled trials were analyzed. We refer to the larger (Jarrett et al., 2013) and smaller (Jarrett et al., 2001) as the primary and replication datasets, respectively. Following we describe methods relevant to current analyses. Earlier publications contain additional detail (Jarrett et al., 2001; Jarrett et al., 2010; Jarrett et al., 2013). The trials were approved by institutional review boards at the University of Texas Southwestern Medical Center (both samples) and University of Pittsburgh Medical Center (primary sample).
Participants
Adult outpatients provided written informed consent for assessment and treatment, met criteria for recurrent MDD (American Psychiatric Association, 1994, 2000), and scored ≥ 14 (primary dataset) or ≥ 16 (replication dataset) on the 17-item HRSD (Hamilton, 1960). Diagnoses were made using the Structured Clinical Interview for DSM (primary dataset: First et al., 1996; replication dataset: Spitzer et al., 1989). Table 1 shows participant demographics.
Table 1.
Description of the Acute-phase Samples
| Primary dataset (N = 523) % or M (SD) |
Replication dataset (N = 156) % or M (SD) |
|
|---|---|---|
| Female | 67.5% | 74.4% |
| Age in years | 42.4 (12.1) | 41.2 (11.1) |
| Ethnicity | ||
| Asian | 5.2% | 0.0% |
| Black | 10.3% | 7.7% |
| Hispanic | 0.0% | 4.5% |
| White | 80.9% | 86.5% |
| Other | 3.6% | 1.3% |
Procedure
Acute phase.
All patients received CT (Beck et al., 1979) in a 12-week protocol. Psychotropic medication was not provided or permitted during the acute phase. Cognitive therapy targets both depressed mood (e.g., by identifying and modifying unrealistically negative thought processes) and low interest/ pleasure (e.g., with behavioral activation to facilitate engagement with sources of reinforcement). Therapists were doctoral-level clinicians who demonstrated competence in CT and received ongoing supervision. In the primary dataset (N = 523), patients received 16 or 20 CT sessions, with more sessions for patients with less early improvement. In the replication dataset (N = 156), patients received 20 CT sessions. Patients received two sessions/week for the first 4 or 8 weeks and then weekly sessions for the remainder of the 12-week acute phase, to total 16 or 20 sessions. Sessions lasted 50-60 minutes each.
Continuation phase.
Consenting acute-phase CT responders entered the 8-month continuation phase. In the primary sample, higher-risk responders (n = 241, no major depressive episode and the final acute-phase HRSD score ≤ 12, but one or more of the last seven acute-phase HRSD scores ≥ 7) were randomized to 8 months of continuation CT, fluoxetine, or pill placebo with clinical management, whereas lower-risk responders (n = 49, no major depressive episode and the last seven acute-phase HRSD scores all ≤ 6) were only assessed. In the replication sample, responders (n = 84, no major depressive episode and the final acute-phase HRSD score ≤ 9) were randomized to 8 months of continuation-phase CT or assessment-only.
In both samples, the continuation-phase CT protocol included 10 sessions (two sessions/month for 2 months, then one session/month for 6 months; Jarrett, 1989). Sessions were about 60 minutes each.
In the primary sample, lower risk responders were assessed at 4-month intervals. In the replication sample, assessment-only included evaluation on the same schedule as continuation CT.
In the primary sample only, the fluoxetine and matched pill-placebo plus clinical-management protocol (Fawcett et al., 1987) included 10 sessions provided by experienced pharmacotherapists on the same schedule as continuation CT. The pharmacotherapy protocol was double-blinded. Pharmacotherapists were prohibited from using specific CT methods.
Follow-up phase.
No protocol treatment was provided during follow-up. Independent evaluators, blind to continuation-phase assignment, assessed patients every 4 months for 24 (primary sample) or 16 (replication sample) months. Research personnel helped patients who relapsed or recurred find treatment outside the study protocol.
Measures
Depressive symptoms.
Clinicians completed the HRSD. Patients completed the BDI and IDS-SR. These scales have demonstrated high reliability and validity during CT (Vittengl et al., 2005; Vittengl et al., 2013). Depressive symptoms were measured before acute-phase CT, weekly during acute-phase CT, at a post-acute-phase CT assessment, and then every 4 months for 32 (primary dataset) or 24 (replication dataset) total months after acute-phase CT.
Items from the BDI, HRSD, and IDS-SR were used to score scales reflecting depressed mood, low general interest, and low sexual interest. The depressed mood scale included BDI items 1 (sadness) and 10 (crying), HRSD item 1 (depressed mood), and IDS-SR item 5 (feeling sad). The general interest scale included BDI items 4 (loss of enjoyment/satisfaction) and 12 (loss of interest), and IDS-SR items 19 (general disinterest) and 21 (anhedonia). The sexual interest scale included BDI item 21 (sexual disinterest), HRSD item 14 (loss of libido), and IDS-SR item 22 (sexual disinterest). Items were selected based on their face-valid content. Item ratings were divided by each item’s maximum possible score (i.e., BDI and IDS-SR items divided by 3, and HRSD items divided by 2 or 4). These standardized rating were then averaged to form the symptom scales. Higher scores indicated more severe symptomatology.
Supporting the scales’ reliability, alpha internal consistency for the depressed mood and low interest scales was moderately high in both datasets (median = .90, range .84-.93) at the end of acute-phase CT. We also examined the scales’ correlations with well-established positive and negative temperament measures (Clark et al., 2014), given to a subset (n = 324) of patients during the first week of CT in the primary dataset only. Supporting the scales’ validity, baseline depressed mood correlated significantly (p < .05, two-tailed) with negative (.18) but not with positive temperament (−.06); whereas low general and sexual interest correlated significantly with positive (−.19, −.15) but not negative (.09, .05) temperament, respectively.
Statistical Analyses
Repeated-measures multilevel models tested changes in symptom means. Multilevel models allow analysis of all patients, including those with incomplete data (e.g., due to attrition), to increase generalizability of results (e.g., Schafer & Graham, 2002). The two trials’ data were analyzed separately because of differences in the trials’ designs and, further, to evaluate replicability of the findings. Models included random intercepts to account for repeated symptom measures within participants. In these models, we estimated and contrasted means to clarify significant main effects and interactions.
To test the hypothesis regarding symptom change during acute-phase CT, we predicted symptom scores from the fixed effects of time (14 assessments: baseline/pre-CT, 12 weeks during CT, post-CT), scale (depressed mood, general interest, sexual interest), and the time-by-scale interaction. Similarly, to explore symptom levels after acute-phase CT, we predicted responders’ symptom scores from the fixed effects of time (post-acute assessments every 4 months through 32 [primary dataset] or 24 [replication dataset] months), scale, continuation group, and their interactions. The primary dataset included four continuation groups (lower-risk responders were only assessed; higher-risk responders received CT, fluoxetine, or pill placebo), and the replication dataset included two continuation groups (assessment-only or CT).
Results
Descriptive statistics for the symptom scales during the acute phase appear in Table 2. In both datasets, mean symptom levels were moderately high before (51-59%), and notably lower after (14-30%), CT. (Percentages refer to proportions of the maximum possible scores.) Mostly moderate concurrent correlations among scales (median = .43) suggested that depressed mood, low general interest, and low sexual interest were related but not synonymous.
Table 2.
Descriptive Statistics and Correlations among Symptom Scales in Two Datasets
| Correlations | ||||||||
|---|---|---|---|---|---|---|---|---|
| Symptom scale | N | M | SD | 1 | 2 | 3 | 4 | 5 |
| Primary dataset | ||||||||
| 1. Depressed mood: Pre-CT | 523 | 58.7% | 18.0% | |||||
| 2. Low general interest/pleasure: Pre-CT | 499 | 53.8% | 20.3% | .45* | ||||
| 3. Low sexual interest: Pre-CT | 523 | 51.0% | 30.3% | .19* | .37* | |||
| 4. Depressed mood: Post-CT | 395 | 19.5% | 21.3% | .26* | .15* | .13* | ||
| 5. Low general interest/pleasure: Post-CT | 368 | 18.5% | 20.7% | .20* | .32* | .20* | .71* | |
| 6. Low sexual interest: Post-CT | 395 | 29.9% | 30.4% | .11* | .19* | .52* | .40* | .53* |
| Replication dataset | ||||||||
| 1. Depressed mood: Pre-CT | 156 | 58.9% | 16.5% | |||||
| 2. Low general interest/pleasure: Pre-CT | 155 | 52.5% | 18.8% | .40* | ||||
| 3. Low sexual interest: Pre-CT | 156 | 54.2% | 31.4% | .26* | .30* | |||
| 4. Depressed mood: Post-CT | 128 | 16.0% | 19.8% | .17 | −.06 | .02 | ||
| 5. Low general interest/pleasure: Post-CT | 126 | 14.4% | 17.5% | .04 | .10 | .01 | .74* | |
| 6. Low sexual interest: Post-CT | 128 | 27.8% | 33.0% | .14 | .05 | .35* | .45* | .48* |
Note. M and SD are given as percent of the maximum possible score. CT = acute-phase cognitive therapy.
p < .05, two-tailed.
In acute-phase symptom models, the main effects of scale, time, and the scale*time interaction were significant in both datasets (see Table 3 and Figure 1). The scale effect indicated that low sexual interest exceeded depressed mood and low general interest. The time effect indicated that mean symptom intensity decreased. The interaction indicated that decreases differed among symptom scales. In the primary dataset, improvements in depressed mood (38.3%), general interest (34.3%), and sexual interest (20.3%) differed significantly from one another, ps < .05, two-tailed. In the replication dataset, improvements in depressed mood (42.1%) and general interest (37.3%) did not differ significantly, but both exceeded improvement in sexual interest (25.6%).
Table 3.
Prediction of Symptom Levels during Acute-phase Cognitive Therapy
| Fixed effects | df | F | p |
|---|---|---|---|
| Primary dataset (N = 523; Jarrett et al., 2013) | |||
| Scale | 2, 18000 | 199.31 | < .001 |
| Time | 13, 18000 | 406.35 | < .001 |
| Scale * time | 26, 18000 | 14.48 | < .001 |
| Replication dataset (N = 156; Jarrett et al., 2001) | |||
| Scale | 2, 5629 | 124.18 | < .001 |
| Time | 13, 5629 | 160.09 | < .001 |
| Scale * time | 26, 5629 | 2.92 | < .001 |
Note. The scale effect involves depressed mood, low general interest/pleasure, and low sexual interest symptoms. The time effect is 14 assessments conducted approximately weekly. Random intercepts accounting for nesting of data within participants were included in each model but are not shown.
Figure 1.
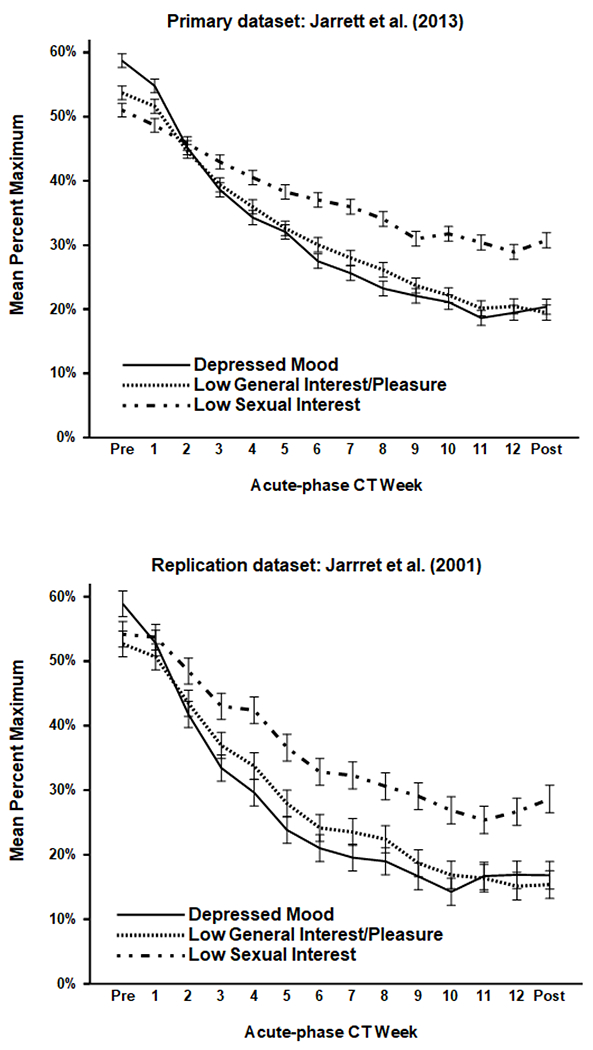
Mean symptom levels (± 1 SE) during acute-phase cognitive therapy (CT) for recurrent major depressive disorder. The top (N = 523) and bottom (N = 156) panels depict data from the Jarrett et al. (2013) and Jarrett et al. (2001) clinical trials, respectively.
In post-acute symptom models, the main effects of scale and scale*group interactions were relevant to the exploratory research question about symptom profiles (see Table 4 and Figure 2). In both datasets, the scale effect indicated that low sexual interest exceeded both depressed mood and low general interest, ps < .05, two-tailed, but the latter two symptom means did not differ significantly. Estimated means for depressed mood, low general interest, and low sexual interest, respectively, were 12.8%, 11.7%, and 22.9% in the primary dataset and 8.4%, 8.5%, and 15.2% in the replication dataset.
Table 4.
Prediction of Symptom Levels after Response to Acute-phase Cognitive Therapy
| Fixed effects | df | F | p |
|---|---|---|---|
| Primary dataset (N = 290; Jarrett et al., 2013) | |||
| Group | 3, 4587 | 3.78 | .010 |
| Scale | 2, 4587 | 180.29 | <.001 |
| Time | 8, 4587 | 1.66 | .103 |
| Group * scale | 6, 4587 | 9.77 | <.001 |
| Group * time | 24, 4587 | 2.06 | .002 |
| Scale * time | 16, 4587 | 0.43 | .975 |
| Group * scale * time | 48, 4587 | 0.89 | .689 |
| Replication dataset (N = 84; Jarrett et al., 2001) | |||
| Group | 1, 1323 | 0.86 | .353 |
| Scale | 2, 1323 | 26.99 | <.001 |
| Time | 6, 1323 | 2.21 | .040 |
| Group * scale | 2, 1323 | 0.46 | .633 |
| Group * time | 6, 1323 | 2.72 | .013 |
| Scale * time | 12, 1323 | 0.86 | .587 |
| Group * scale * time | 12, 1323 | 0.71 | .745 |
Note. The group effect refers to the eight-month continuation phase treatment assignment—in the primary dataset, lower-risk patients were only assessed, whereas higher-risk patients were randomized to continuation CT, fluoxetine, or pill placebo with clinical management; in the replication dataset, patients were randomized to continuation CT or assessment-only. The scale effect involves depressed mood, low general interest/pleasure, and low sexual interest symptoms. The time effect is 9 (primary dataset) or 7 (replicated dataset) assessments conducted approximately every 4 months after acute-phase CT. Random intercepts accounting for nesting of data withing participants were included in each model but are not shown.
Figure 2.
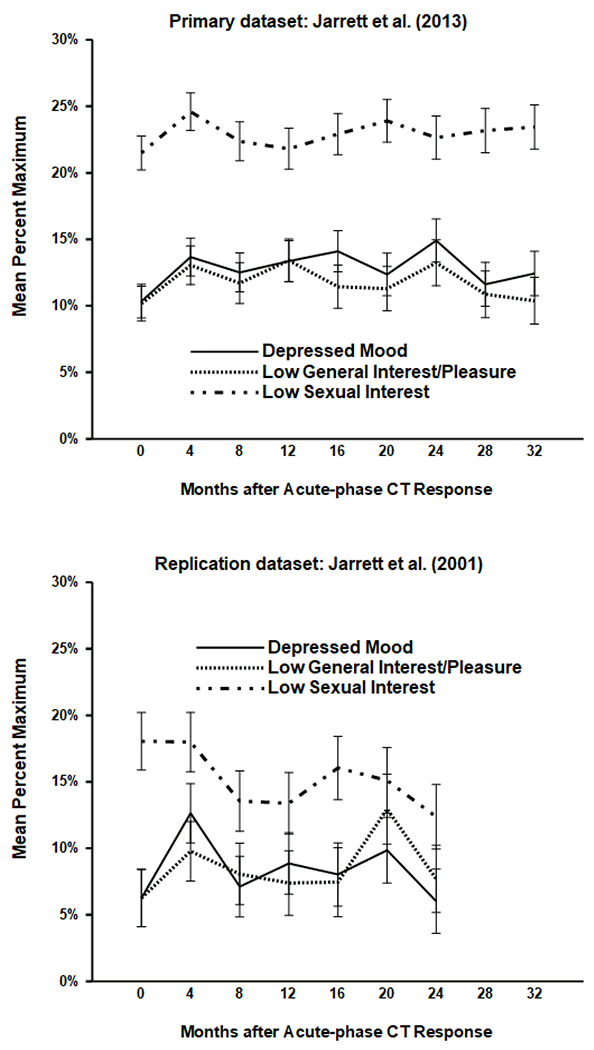
Mean symptom levels (± 1 SE) after response to acute-phase cognitive therapy (CT) for recurrent major depressive disorder. The top (N = 290) and bottom (N = 84) panels depict data from the Jarrett et al. (2013) and Jarrett et al. (2001) clinical trials, respectively.
In the primary dataset only, the scale*group interaction was statistically significant (Table 4). Consistent with visual inspection of Figure 3, follow-up tests showed that sexual interest, but not depressed mood or general interest, varied significantly across continuation-phase groups. Low sexual interest was more severe among higher risk responders receiving continuation-phase CT (25.7%), fluoxetine (22.8%), or pill placebo (29.4%) than lower-risk responders who were only assessed (13.8%), ps < .05, two-tailed. In addition, the contrast between fluoxetine and pill placebo was significant for low sexual interest. However, the non-significant group*scale*time interaction suggested that this pattern of symptom differences did not clearly change as continuation-phase treatment progressed or ended. No interactions of scale with continuation-phase group or time were significant in the replication dataset.
Figure 3.
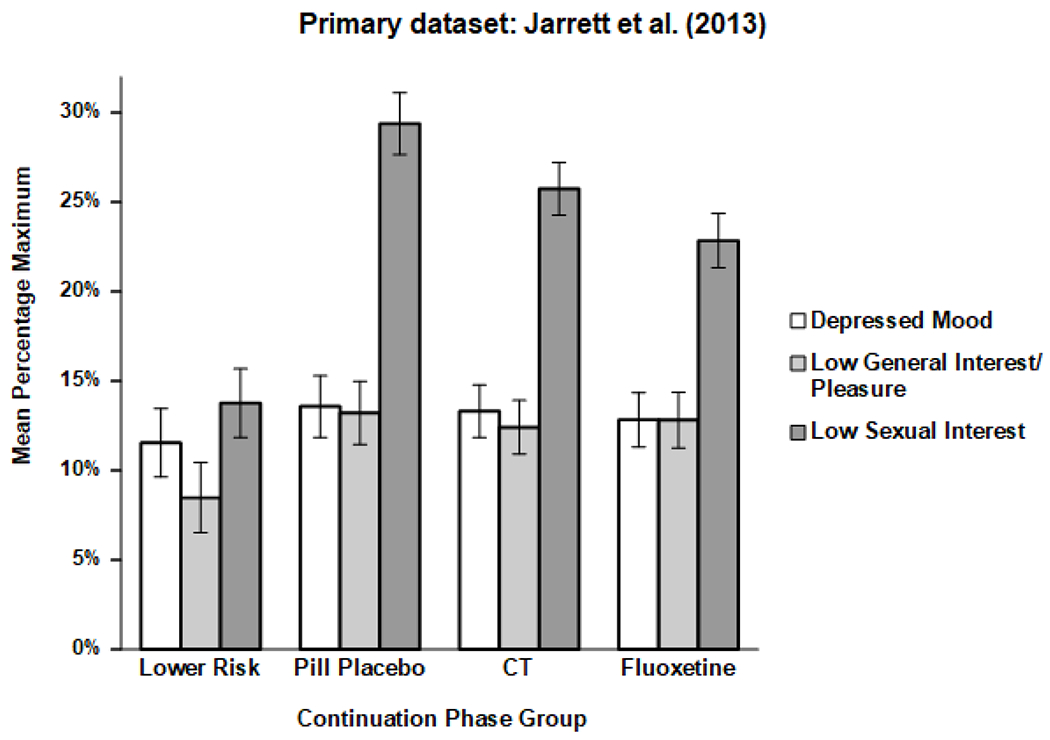
Mean symptoms levels (± 1 SE) for 32 months after response to acute-phase cognitive therapy for recurrent major depressive disorder in the Jarrett et al. (2013; N = 290) dataset, separated by continuation treatment group. CT = cognitive therapy.
To clarify the clinical significance of residual depressed mood, low general interest, and low sexual interest among acute-phase CT responders, we predicted time to MDD relapse or recurrence in Cox regression models (see Table 5). Because the symptom scales were substantively inter-correlated (see Table 2), rather than focusing on the scales’ regression coefficients individually, we computed two planned contrasts: The main effect of average symptom intensity considering all three scales, and the interaction reflecting differences among the scales, in predicting relapse/recurrence. Greater average residual symptoms predicted relapse/recurrence substantively (hazard ratios) in the primary (2.08) and replication (4.12) datasets, and the three symptoms did not differ significantly in predicting relapse/recurrence.
Table 5.
Prediction of Relapse/Recurrence from Residual Symptoms at the End of Acute-phase CT
| Primary dataset (N = 274; Jarrett et al., 2013) | |||
|---|---|---|---|
| Symptom predictors | B | SE | p |
| Depressed mood | 1.92 | 0.96 | .046 |
| Low general interest | −0.54 | 0.93 | .565 |
| Low sexual interest | 0.81 | 0.47 | .080 |
| Planned contrasts | |||
| Main effect: Average symptom intensity | 0.73 | 0.31 | .019 |
| Interaction: Differences among symptoms | χ2(2) = 2.25 | .324 | |
| Replication dataset (N = 84; Jarrett et al., 2001) | |||
| Symptom predictors | B | SE | p |
| Depressed mood | 2.87 | 1.64 | .079 |
| Low general interest | 0.89 | 1.73 | .608 |
| Low sexual interest | 0.48 | 0.60 | .420 |
| Planned contrasts | |||
| Main effect: Average symptom intensity | 1.41 | 0.58 | .016 |
| Interaction: Differences among symptoms | χ2(2) = 1.93 | .381 | |
Note. Analyzed samples were responders to acute-phase cognitive therapy (CT). Tabled results are from Cox regression models conducted separately in the two datasets. The models predicted major depressive relapse or recurrence over 32 (primary dataset) or 24 (replication dataset) months after acute-phase CT. The symptom scale predictors were entered in the models simultaneously. Planned contrasts utilized the predictors’ tabled coefficients. Models controlled continuation phase group: In the primary dataset, lower-risk patients were only assessed, whereas higher-risk patients were randomized to continuation CT, fluoxetine, or pill placebo with clinical management; in the replication dataset, patients were randomized to continuation CT or assessment-only
Discussion
The current analyses clarified changes in depressed mood (predominantly negative affect) and low interest/pleasure (low positive affect) during and after acute-phase CT for MDD. Replicating and extending past research (Dunn et al., 2020), we found that depressed mood, general interest, and sexual interest improved substantively. However, depressed mood and general interest improved significantly more than did sexual interest. Moreover, patients continued to report greater difficulty with low sexual interest than with depressed mood or low general interest for at least 2 years after response to acute-phase CT. Differential effects of continuation-phase CT or fluoxetine on these symptom profiles were not evident. This pattern of findings was replicated in two randomized controlled trial datasets.
Commensurate with patients’ goals (Demyttenaere et al., 2015), our findings reinforce efforts to enhance CT’s effects on positive affective symptoms. Traditional CT targets both high negative affect (e.g., cognitive restructuring) and low positive affect (e.g., behavioral activation; Beck et al., 1979). However, basic research shows that negative affect is more changeable than is positive affect (e.g., Peeters et al., 2006), which may be reflected in reduced improvement in positive affective symptoms of MDD during treatment. Consequently, augmenting CT with well-being and positive affect interventions may improve patient outcomes (Dunn et al., 2019; Geschwind et al., 2019). For example, psychotherapy focused on increasing positive affect (e.g., with behavioral activation and other interventions to boost reward sensitivity) versus reducing negative affect (similar to traditional CT minus behavioral activation) has produced larger gains in positive affect and larger deceases in negative affect (Craske et al., 2019). Such outcomes are important because residual symptoms, including depressed mood, low general interest, and low sexual interest, were broadly predictive of MDD relapse/recurrence among acute-phase CT responders in the current samples.
Neither continuation-phase CT nor antidepressant medication changed symptom profiles in the analyzed datasets. It possible that most of the potential improvements in general and sexual interest occurred in the acute phase, leaving less room for additional gains among continuation phase among CT responders. Perhaps more likely, continuation-phase fluoxetine and CT may not have “targeted” sexual interest sufficiently and/or this symptom may take longer to normalize, even though sexual interest is clearly tied to general positive affect (Mehta et al., 2014).
For partnered patients, adding elements of couple therapy for depression (Whisman & Beach, 2012) might improve targeting of sexual interest during CT. For example, including partners in some CT sessions, identifying and reducing stress generation in relationships, and increasing caring behaviors (e.g., demonstration of affection, respect, and concern) may improve the quality of relationships in general and sexual interest in particular (cf. Debrot et al., 2017). For single patients who want romantic relationships, improvements in sexual interest might require emphasis on behavioral activation, perhaps augmented with social-skills training (e.g., Becker et al., 1987), aimed toward successful interactions with potential partners.
The current analyses had both strengths and limitations. Strengths included relatively large samples of carefully diagnosed patients receiving treatment from well-trained and supervised clinicians. Further, patients were assessed repeatedly before, during, and after treatment using well-established depressive symptom measures. Finally, the major findings replicated across independent datasets, allaying concerns about scientific reproducibility (Open Science Collaboration, 2015).
Limitations included uncertain generalizability of the results to other patient populations (e.g., depressive disorders other than recurrent MDD) and treatment environments (e.g., routine clinical practice). Further, the depressed mood and low interest scales were constructed from standard depressive symptoms measures and overlapped empirically. Future research using measures designed to differentiate facets of depression and related disorders (e.g., Watson et al., 2012) might provide greater insight into how and when these symptoms change.
Future research might also explore relations between improvements in positive affective symptoms, including low general and sexual interest, and psychosocial functioning with treatment of MDD. Past research indicates that psychosocial functioning improves more slowly, and less overall, than overall depressive symptoms during acute-phase treatment (Vittengl & Jarrett, 2014). Further, low interest and depressed mood have shown unique, substantive relations with psychosocial functioning (Fried & Nesse, 2014). Consequently, clarifying potentially causal connections between increases in positive affective symptoms and psychosocial functioning during treatment would be valuable from theoretical and clinical standpoints.
Enhancing adults’ positive affect is more difficult than dampening negative affect when treating MDD with antidepressant medication or CT (Craske et al., 2016; Dunn et al., 2020; Soskin et al., 2012). In the current analyses, the persistence of low sexual interest relative to depressed mood and general interest for years after response to acute-phase CT, even with continuation CT or antidepressant medication, highlights the need for better targeting of this positive affective symptom. Modifying and augmenting CT hold potential to target positive affective symptoms more effectively, decrease relapse/recurrence of MDD, and improve patients’ quality of life.
Highlights.
Major depressive disorder (MDD) involves depressed mood and low interest/pleasure.
We measured these symptoms during and after cognitive therapy (CT) for MDD.
Depressed mood and general interest improved more than sexual interest during CT.
These symptom differences persisted for at least two years after CT response.
Continuation CT or antidepressant medication did not change the symptom differences.
Financial Support.
This report was supported by grants R01 MH38238, K02 MH01571, K24 MH001571, R01 MH58397, R01 MH69619 (to Robin B. Jarrett, Ph.D.) and R01 MH58356 and R01 MH69618 (to Michael E. Thase, M.D.) from the National Institute of Mental Health (NIMH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIMH or the National Institutes of Health.
Conflict of Interest Statement.
Dr. Vittengl is a paid reviewer for UpToDate. Dr. Clark has no financial interest or conflict of interest in the research. During the past three years, Dr. Thase has consulted with and/or served on advisory boards for AbVie (including Allergan and Forest Laboratories), Acadia, Alkermes, AstraZeneca, Axsome, Clexio, Jazz Pharmaceuticals, Johnson & Johnson (includes Janssen), Lundbeck, Merck, Otsuka, Perception, Pfizer Pharmaceuticals, Sunovion, and Takeda; he has received grant support from Acadia, Axsome, Myriad (includes Assurerx), Johnson & Johnson (Janssen), Takeda, the Agency for Healthcare Research and Quality, Patient Centered Outcomes Research Institute and the NIMH. He has received royalties from American Psychiatric Publishing, Inc. (APPI), Guilford Publications, Herald House, UpToDate, and W.W. Norton & Company, Inc. Dr. Thase’s spouse is an employee of Peloton Advantage, which does business with several pharmaceutical companies. Dr. Jarrett is a paid consultant to the NIH, NIMH, and UpToDate. She has equity holdings in Amgen, Johnson and Johnson, and Procter and Gamble. Her medical center charges fees for the cognitive therapy she provides to patients.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Ethical Approval. This research was conducted in accordance with the ethical standards of the American Psychological Association, relevant institutional review boards, and Helsinki Declaration.
Informed Consent: All individual participants included in the study provided informed consent for assessment and treatment.
Contributor Information
Jeffrey R. Vittengl, Department of Psychology, Truman State University, Kirksville, Missouri, USA.
Lee Anna Clark, Department of Psychology, University of Notre Dame, Notre Dame, Indiana, USA.
Michael E. Thase, Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania, USA
Robin B Jarrett, Department of Psychiatry, The University of Texas Southwestern Medical Center, Dallas, Texas, USA.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Beck AT, Rush AJ, Shaw BF, & Emery G (1979). Cognitive Therapy of Depression. Guilford: New York. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, & Erbaugh J (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Becker RE, Heimberg RG; Belleck AS (1987). Social skills training treatment for depression. New York: Pergamon. [Google Scholar]
- Biesheuvel-Leliefeld KE, Kok GD, Bockting CL, Cuijpers P, Hollon SD, van Marwijk HW, & Smit F (2015). Effectiveness of psychological interventions in preventing recurrence of depressive disorder: meta-analysis and meta-regression. Journal of Affective Disorders, 174, 400–410. 10.1016/j.jad.2014.12.016 [DOI] [PubMed] [Google Scholar]
- Bockting CL, Hollon SD, Jarrett RB, Kuyken W, & Dobson K (2015). A lifetime approach to major depressive disorder: The contributions of psychological interventions in preventing relapse and recurrence. Clinical Psychology Review, 41, 16–26. 10.1016/j.cpr.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Clark LA, Simms LJ, Wu KD, & Casillas A (2014). Schedule for Nonadapative and Adaptive Personality—2nd Edition (SNAP-2): Manual for Administration, Scoring, and Interpretation. University of Notre Dame, Notre Dame, IN. [Google Scholar]
- Clark LA, & Watson D (1991). Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology, 100(3), 316–336. 10.1037/0021-843X.100.3.316 [DOI] [PubMed] [Google Scholar]
- Clark LA, & Watson D (2021). Temperament: An organizing paradigm for trait psychology. In John OP, Robins RW, & Pervin LA (Eds.), Handbook of Personality: Theory and Research, 4th ed., (pp. 145–175). New York: Guilford Press. [Google Scholar]
- Craighead WE, Johnson BJ, Carey S, & Dunlop BW (2015). Psychosocial treatments for major depressive disorder. In Nathan PE & Gorman JM (Eds.), Treatments that work (4th ed.) (pp. 381–408). New York: Oxford University Press. [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, & Dour HJ (2016). Treatment for anhedonia: A neuroscience driven approach. Depression and Anxiety, 33(10), 927–938. 10.1002/da.22490 [DOI] [PubMed] [Google Scholar]
- Craske MG, Meuret AE, Ritz T, Treanor M, Dour H, & Rosenfield D (2019). Positive affect treatment for depression and anxiety: A randomized clinical trial for a core feature of anhedonia. Journal of Consulting and Clinical Psychology, 87(5), 457–471. 10.1037/ccpQ000396 [DOI] [PubMed] [Google Scholar]
- Cuijpers P, Hollon SD, van Straten A, Bockting C, Berking M, & Andersson G (2013). Does cognitive behaviour therapy have an enduring effect that is superior to keeping patients on continuation pharmacotherapy? A meta-analysis. BMJ open, 3(4), e002542. 10.1136/bmjopen-2012-002542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuijpers P, Turner EH, Mohr DC, Hofmann SG, Andersson G, Berking M, & Coyne J (2014). Comparison of psychotherapies for adult depression to pill placebo control groups: a meta-analysis. Psychological medicine, 44(4), 685–695. 10.1017/S003329171300Q457 [DOI] [PubMed] [Google Scholar]
- Debrot A, Meuwly N, Muise A, Impett EA, & Schoebi D (2017). More than just sex: Affection mediates the association between sexual activity and well-being. Personality & Social Psychology Bulletin, 43(3), 287–299. 10.1177/0146167216684124 [DOI] [PubMed] [Google Scholar]
- Demyttenaere K, Donneau A-F, Albert A, Ansseau M, Constant E, & van Heeringen K (2015). What is important in being cured from depression? Discordance between physicians and patients (1). Journal of Affective Disorders, 174, 390–396. 10.1016/j.jad.2014.12.004 [DOI] [PubMed] [Google Scholar]
- Dunn BD, German RE, Khazanov G, Xu C, Hollon SD, & DeRubeis RJ (2020). Changes in positive and negative affect during pharmacological treatment and cognitive therapy for major depressive disorder: A secondary analysis of two randomized controlled trials. Clinical Psychological Science, 8(1), 36–51. 10.1177/2167702619863427 [DOI] [Google Scholar]
- Dunn BD, Widnall E, Reed N, Owens C, Campbell J, & Kuyken W (2019). Bringing light into darkness: A multiple baseline mixed methods case series evaluation of Augmented Depression Therapy (ADepT). Behaviour Research and Therapy, 120. 10.1016/j.brat.2019.103418 [DOI] [PubMed] [Google Scholar]
- Fawcett J, Epstein P, Fiester S, Elkin I, & Autry J (1987). Clinical management--imipramine/placebo administration manual. NIMH Treatment of Depression Collaborative Research Program. Psychopharmacology Bulletin, 23, 309–324. [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, & Williams JB (1996). Structured Clinical Interview for DSM-IV Axis I Disorders-Patient Edition (SCID-I/P, Version 2.0). New York, NY: New York State Psychiatric Institute, Biometrics Research Department. [Google Scholar]
- Fried EI, & Nesse RM (2014). The impact of individual depressive symptoms on impairment of psychosocial functioning. PloS One, 9(2), e90311. 10.1371/journal.pone.0090311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Arntz A, Bannink F, & Peeters F (2019). Positive cognitive behavior therapy in the treatment of depression: A randomized order within-subject comparison with traditional cognitive behavior therapy. Behaviour Research and Therapy, 116, 119–130. 10.1016/j.brat.2019.03.005 [DOI] [PubMed] [Google Scholar]
- Hamilton M (1960). A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry, 23, 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB (1989). Cognitive therapy for recurrent unipolar major depressive disorder. The continuation maintenance phase. Unpublished treatment manual. [Google Scholar]
- Jarrett RB, & Thase ME (2010). Comparative efficacy and durability of continuation phase cognitive therapy for preventing recurrent depression: design of a double-blinded, fluoxetine- and pill placebo-controlled, randomized trial with 2-year follow-up. Contemporary Clinical Trials, 31(4), 355–377. 10.1016/j.cct.2010.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, & Silver RC (2001). Preventing recurrent depression using cognitive therapy with and without a continuation phase. Archives of General Psychiatry, 58(4), 381–388. 10.1001/archpsvc.58.4.381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Gershenfeld H, Friedman ES, & Thase ME (2013). Preventing depressive relapse and recurrence in higher-risk cognitive therapy responders: A randomized trial of continuation phase cognitive therapy, fluoxetine, or matched pill placebo. JAMA Psychiatry, 70(11), 1152–1160. 10.1001/jamapsychiatry.2013.1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett RB, Minhajuddin A, Vittengl JR, Clark LA, & Thase ME (2016). Quantifying and qualifying the preventive effects of acute-phase cognitive therapy: Pathways to personalizing care. Journal of Consulting and Clinical Psychology, 84(4), 365–376. 10.1037/ccp0000069.supp (Supplemental) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta CM, Walls C, Blood EA, & Shrier LA (2014). Associations between affect, context, and sexual desire in depressed young women. Journal of Sex Research, 51(5), 577–585. 10.1080/00224499.2012.753026 [DOI] [PubMed] [Google Scholar]
- Mineka S, Watson D, & Clark LA (1998). Comorbidity of anxiety and unipolar mood disorders. Annual Review of Psychology, 49, 377–412. 10.1146/annurev.psych,49.1.377 [DOI] [PubMed] [Google Scholar]
- Open Science Collaboration (2015). Estimating the reproducibility of psychological science. Science, 349, 1–8. 10.1126/science.aac4716 [DOI] [PubMed] [Google Scholar]
- Rush AJ, Gullion CM, Basco MR, Jarrett RB, & Trivedi MH (1996). The Inventory of Depressive Symptomatology (IDS): Psychometric Properties. Psychological Medicine, 26, 477–486. 10.1017/s0033291700035558 [DOI] [PubMed] [Google Scholar]
- Peeters E, Berkhof J, Delespaul P, Rottenberg J, & Nicolson NA (2006). Diurnal mood variation in major depressive disorder. Emotion, 6(3), 383–391. 10.1037/1528-3542.6.3.383 [DOI] [PubMed] [Google Scholar]
- Sanislow CA (2016). Updating the Research Domain Criteria. World Psychiatry, 15(3), 222–223. 10.1002/wps.20374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7(2), 147–177. 10.1037/1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- Soskin DP, Carl JR, Alpert J, & Fava M (2012). Antidepressant effects on emotional temperament: Toward a biobehavioral research paradigm for major depressive disorder. CNS Neuroscience & Therapeutics, 18(6), 441–451. 10.1111/j.1755-5949.2012.00318.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snippe E, Doornbos B, Schoevers RA, Wardenaar KJ, & Wichers M (2021). Individual and common patterns in the order of symptom improvement during outpatient treatment for major depression. Journal of Affective Disorders, 290, 81–88. 10.1016/j.jad.2021.04.097 [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JBW, Gibbon M, & First MB (1989). Structured Clinical Interview for DSM-III-R-Outpatient Version (with Psychotic Screen). New York, NY: New York State Psychiatric Institute. [Google Scholar]
- Vittengl JR, & Jarrett RB (2014). Major depressive disorder. In Hofmann SG, Dozois DA, Rief W, Smits JJ (Eds.), The Wiley handbook of cognitive behavioral therapy (Vol. 3) (pp. 1131–1159). New York: Wiley-Blackwell. 10.1002/9781118528563.wbcbt48 [DOI] [Google Scholar]
- Vittengl JR, Clark LA, Kraft D, & Jarrett RB (2005). Multiple measures, methods, and moments: A factor-analytic investigation of change in depressive symptoms during acute phase cognitive therapy. Psychological Medicine, 35, 693–704. 10.1017/S00332917040Q4143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittengl JR, Clark LA, Thase ME, & Jarrett RB (2013). Nomothetic and idiographic symptom change trajectories in acute-phase cognitive therapy for recurrent depression. Journal of Consulting and Clinical Psychology, 81(4), 615–626. 10.1037/a0032879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, O’Hara MW, Naragon-Gainey K, Koffel E, Chmielewski M, Kotov R, … Ruggero CJ (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19, 399–420. 10.1177/1073191112449857 [DOI] [PubMed] [Google Scholar]
- Watson D, Stanton K, & Clark LA (2017). Self-report indicators of negative valence constructs within the research domain criteria (RDoC): A critical review. Journal of Affective Disorders, 216, 58–69. 10.1016/j.jad.2016.09.065 [DOI] [PubMed] [Google Scholar]
- Whisman MA, & Beach SR (2012). Couple therapy for depression. Journal of Clinical Psychology, 68, 526–535. 10.1002/jclp.21857 [DOI] [PubMed] [Google Scholar]


