Abstract
Paraoxonase-2 (PON2) enhances mitochondria function and protects against oxidative stress. Stimulating its expression has therapeutic potential for diseases where oxidative stress plays a significant role in the pathology, such as Parkinson’s disease. Clinical and preclinical evidence suggest that the anti-diabetic drug pioglitazone may provide neuroprotection in Parkinson’s disease, Alzheimer’s disease, and stroke, but the biochemical pathway(s) responsible has not been fully elucidated. To determine the effect of pioglitazone on PON2 expression we treated male African green monkeys with oral pioglitazone (5 mg/kg/day) for 1 and 3 weeks. We found that pioglitazone increased PON2 mRNA and protein expression in brain following 1 week of treatment, however, by 3 weeks of treatment PON2 expression had returned to baseline. This transient increase was detected in substantia nigra, striatum, hippocampus, and dorsolateral prefrontal cortex The short-term impact of pioglitazone on PON2 expression in striatum may contribute to the discrepancy in the potency of the drug between short-term animal models and clinical trials for Parkinson’s disease. Both PON2 and pioglitazone’s receptor, peroxisome proliferator-activated receptor gamma (PPARγ), possess sex- and brain region-dependent expression, which may play a role in the short-term effect of pioglitazone and provide clues to extending the beneficial effects of PON2 activation.
Keywords: Pioglitazone, Paraoxonase-2, Peroxisome proliferator-activated receptor gamma, Parkinson’s disease, Striatum, Substantia nigra
Graphical Abstract
1. Introduction
Paraoxonase-2 (PON2) is a key defender against oxidative stress, which is major factor in the etiology of neurodegenerative disorders. Located in the inner mitochondrial membrane, PON2 enhances the function of coenzyme Q in the electron transport chain, and thereby reduces the production of reactive oxygen species that can lead to oxidative stress (1, 2). Particularly compelling is the link of PON2 with Parkinson’s disease (PD) as its expression is highest in dopamine-rich regions of the brain that are vulnerable in PD (1), and PON2 deficiency hypersensitizes neurons to oxidative stress induced by the parkinsonian protoxin, 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) (3, 4). Furthermore, PON2 expression peaks during development and has fallen by adulthood (5), which coincides with the typical onset of PD and the age-related susceptibility of dopamine neurons to MPTP and to methamphetamine in primates (6, 7). Therefore, PON2 is a promising neuroprotective target in PD and other neurological disorders where oxidative stress plays a significant role in the etiology.
Pioglitazone is an anti-diabetic drug that has received considerable interest as a neuroprotective agent in a range of neurological disorders that involve oxidative stress (4). Clinical trials, supported by animal studies, have demonstrated that pioglitazone has beneficial effects in Alzheimer’s disease, dementia, stroke, and schizophrenia, reviewed in (4). In PD research, pioglitazone has yielded promising preclinical results in both rodent and nonhuman primate models. In mice, pioglitazone attenuates microglia activation in the 6-hydroxydopamine model of PD (8), and in MPTP-treated monkeys pioglitazone treatment reduced inflammation and the loss of dopamine neurons (9). Moreover, several epidemiological studies report a lower incidence of PD in diabetic patients using pioglitazone, and a meta-analysis found that overall pioglitazone users have a 25% lower risk of developing PD (10). Despite this promising evidence, the recent clinical trial, ‘NIH Exploratory Trials in Parkinson Disease’ (NET-PD), found that pioglitazone did not alter the progression of early stage PD (11). However, like all clinical trials, this investigation had a number of limitations that could explain the lack of effect, including a small cohort and a potentially sub-threshold dose for neuroprotection as doses used were relevant to treatment of type 2 diabetes mellitus rather than doses equivalent to those used in a PD model that demonstrated neuroprotection (9). Currently pioglitazone mechanisms of neuroprotection are not fully understood and further studies are needed in order to realize its full potential as a neuroprotective agent.
Pioglitazone is a peroxisome proliferator-activated receptor gamma (PPARγ) agonist, which is a nuclear receptor that initiates transcription of genes involved in anti-inflammatory responses, mitochondrial biogenesis, and oxidative stress defence (4). Therefore, PPARγ has emerged as a neuroprotective target and is pertinent to PD due to its expression in dopamine neurons. Most of the preclinical studies on pioglitazone focus on Nrf2 and anti-inflammatory pathways, but PPARγ agonists may target multiple pathways and little is known about the precise molecular mechanisms of PPARγ that lead to neuroprotective effects. PON2 is another target of PPARγ that has not yet received adequate attention. In fact, elevated PON2 expression has been linked with PPARγ (12), and we recently showed that pioglitazone stimulates PON2 expression in male mouse striatum following one week treatment (13). Thus we suspect that the pioglitazone-induced increase in the expression of PON2 in striatum likely contributes to the neuroprotective effects of the drug observed in preclinical models of PD (8, 9). However, it is not yet known whether pioglitazone stimulates PON2 expression in nonhuman primate brain and if so, whether this effect persists with continued treatment. This is crucial information, as nonhuman primates share greater homology to humans than rodents in all respects including genomics, biochemistry, neuroanatomy, and pharmacology.
2. Methods
2.1. Animals
Male African green monkeys (chlorocebus sabaeus) weighing 5–7 KG were housed at St. Kitts Biomedical Research Foundation (SKBRF), an AAALAC accredited facility. All procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by SKBRF’s Institutional Animal Care and Use Committee. Euthanasia was carried out after injection of an overdose of pentobarbital. Brains were perfused with cold saline, dissected and flash-frozen in liquid nitrogen.
2.2. Pioglitazone treatment and tissue collection
Monkeys received daily oral doses of pioglitazone HCl (BOC Sciences, Shirley, NY) at 5 mg/kg/day, mixed with jam for 7 days (n = 6) or 21 days (n = 6); this dose and route were selected based on the positive effects of pioglitazone in the monkey MPTP model, which did not alter clinical parameters, including glucose levels (9). Control group monkeys received jam (vehicle) only for 7 days (n = 2) or 21 days (n = 3). Treatment start dates were staggered so that 3 weeks and 1 week treatment ended within 3 days of each other. On the day of the last dose, animals were euthanized by an injected overdose of sodium pentobarbital. Brains were perfused in situ with cold saline, then removed and sectioned into 4-mm slabs using a brain matrix. Several samples of striatum, substantia nigra, dorsolateral prefrontal cortex, and hippocampus were dissected on a refrigerated surface (4 °C) and flash-frozen in liquid nitrogen.
2.3. Circulating levels of pioglitazone
Pioglitazone (PIO) (>99% purity) was purchased from_BOC Sciences (Shirley, NY). Pioglitazone-d4 (PIO-d4) was purchased from Cayman Chemical (Ann Arbor, Michigan). Water, 0.1% formic acid, acetonitrile, 0.1% formic acid and methanol (LCMS grade) were purchased from Fisher Chemical (Hampton, NH). The standards were prepared in control monkey plasma at the concentration range of 0.5–500 ng/mL, quality controls (QCs) were prepared in same matrix at the concentration of 1.75, 35 and 350 ng/mL. The extraction procedure was briefly described as, 50μL of samples or standards and QCs were extracted with 200μL 50% methanol and 50% acetonitrile with 0.1% formic acid spiked with 3ng/mL PIO-d4, centrifuged then 50μL water, 0.1% formic acid was added to 50μL of supernatant, mixed and 5μL was injected onto LCMS system for analysis.
LCMS analysis was performed on an API 4000 QTrap® mass spectrometer (Applied Biosystems Sciex, Toronto, Canada) coupled with Agilent HP1200 HPLC system (Agilent Technologies, Santa Clara, CA). Analyst 1.7 software was used for data acquisition and analysis. A targeted Multiple Reaction Monitoring (MRM) method was utilized to quantify the level of PIO, MIII and MIV. An Agilent Zorbax SB-C18 column (5 micros, 2.1 × 50 mm, P.N.860975–902) coupled with Agilent C18 guard column was utilized for the liquid chromatography separation at 50°C. The isocratic gradient started with 70% of 0.1% formic acid in water (A) and 30% of 0.1% formic acid in acetonitrile (B), maintained for 5 minute.
API 4000 Qtrap mass spectrometer was operated using an electrospray ionization (ESI) source in positive ion mode. MRM transition monitored for PIO and PIO-d4 were 357.3/134.1 and 361.2/134.1 respectively. The declustering, entrance, collision cell exit potentials and collision energy were 95 eV, 10 eV and 38 eV respectively. The ion spray voltage was 5000 eV, source temperature was 400°C and ion source gases 1 and 2 pressures were both 50 psi, curtain gas and CAD gas was set as 15 psi and high mode.
2.4. Western blot analysis
Tissue was sonicated in lysis buffer (Cell Signaling Technology, Danvers, MA) with a Complete™ Mini ethylenediaminetetraacetic acid (EDTA)-free protease inhibitor (Roche Diagnostics, Laval, QC). Protein concentration was determined using BCA assay (PierceTM BCA protein assay kit, Thermo Scientific, Rockford, IL, USA). The Western blot protocol was carried out using Bio-Rad (Hercules, CA) equipment and consumables for stain-free protein quantification, as per manufacturer’s instructions. Samples were diluted in in 4x Laemmli loading buffer, heated, and separated on stain-free mini- Protean TGX gels (4–15%) using gel electrophoresis. Proteins were then transferred to nitrocellulose membranes and imaged with a ChemiDoc XRS+ (Bio-Rad Laboratories, Hercules, CA) so that blots could be normalized to the total protein per lane. Membranes were blocked for 1 h at room temperature with 5% nonfat dry milk in a Tris buffered saline (TBS) wash buffer containing 0.1% Tween 20. Membranes were incubated overnight in blocking buffer at 4°C with a PON2 antibody (1:1000; ab183710, Abcam, Cambridge, MA). Membranes were washed and then incubated for 2 h at room temperature with an HRP-conjugated secondary antibody (1:10000; #7074, Cell Signaling Technology, USA) in blocking buffer. After washing, the antibody complex was visualized by Clarity chemiluminescence (Bio-Rad Laboratories) and imaged with the ChemiDoc. PON2 expression was normalized to total lane protein using ImageLab software (Bio-Rad Laboratories) within ChemiDoc.
2.5. RT-PCR
Tissue was homogenized with an RNase-free disposable pellet pestle (Kimble® Kontes) and by passage through 19-gauge needle. Total RNA was isolated using TRI Reagent® (Sigma). RNA concentration and purity was assessed with a Nanodrop 2000 (Thermo Scientific). cDNA was synthesized using QuantiTect® Reverse Transcription Kit (Qiagen) according to manufacturer’s Protocol. RT-PCR was performed using iTaq™ Universal SYBRR Green Supermix (Bio-Rad). Primers (Supplementary Table 1) were added to a final concentration of 300 nM. Primers were designed using NCBI primer-BLAST (32) and synthesized at the W.M. Keck Foundation Oligo Synthesis Resource (Yale University). Primer specificity was assessed empirically by electrophoresis.
RT-PCR was performed on a Light Cycler 480 (Roche) with the following cycling conditions: Reactions began with 2 min at 95 °C, followed by 55 cycles of 15 s at 95 °C, 60 s at 60 °C. cDNAfree ‘no template’ controls were included for each amplicon and correct target amplification was confirmed by melting-temperature analysis (Tm) and agarose gel electrophoresis. Raw fluorescent data was analysed by LinRegPCR, to obtain an initial amount (N0) of target cDNA in terms of arbitrary fluorescence units (AFU), while accounting for differences in amplification efficiencies between amplicons. Amplification efficiencies estimated by linear regression analysis were in the range 1.8–2.0. Six ‘house-keeping’ genes were analysed as candidate reference genes using the software packages Normfinder and BestKeeper. The genes were chosen from different functional groups: ACTB, TUBB (structure); PPIA (protein chaperone); ATP5B (metabolism); ALU-SEQ (repetitive sequence). A ‘normalization factor’ was determined as the geometric mean of the three most stably-expressed genes: TUBB, ATP5B, and ALU-SEQ.
2.6. Statistical analysis
Statistical analysis was performed in Prism 8 (Graphpad, La Jolla, CA). Normality was assessed by the Shapiro-Wilk test. The effect of treatment was assessed by one-way ANOVA (α = 0.05) (for normally distributed data) or by Kruskal-Wallis (α = 0.05; non-normally distributed data (PON2 expression in DFC and HIP; UCP2 mRNA expression). Correction for multiple comparisons in normally distributed data was determined by Dunnett’s multiple comparison test and by Dunn’s multiple comparison test in non-normally distributed data. mRNA correlation was determined by Pearson’s correlattion coefficient (r). Data are expressed as the mean ± SEM.
3. Results and Discussion
Here we assess whether pioglitazone stimulates PON2 expression in different regions of African green monkey brain. We discovered that the effect of pioglitazone on PON2 expression was both brain region and duration dependent (Figure 1). In four regions examined, PON2 expression was significantly higher than control following 1 week treatment but not following three weeks treatment: substantia nigra (SN; One-way ANOVA: F (2, 14) = 26.73, p < 0.0001); striatum (STR; One-way ANOVA: F (2, 14) = 14.7, p=0.0004); dorsolateral prefrontal cortex (DFC; One-way ANOVA: F (2, 14) = 4.32, p = 0.0345); and hippocampus (HIP); One-way ANOVA: F (2, 14) = 4.01, p = 0.0421). The largest increase in mean PON2 expression following 1 week treatment compared to vehicle-treated monkeys occurred in the DFC (388.4 ± 110.4 %), whereas STR, SN, and HIP had similar increases (82.4 ± 11.5 %, 64.9 ± 9.2%, and 72.0 ± 22.1%, respectively). PON2 mRNA expression was quantified in STR, the region with most relevance to PD, and like PON2 protein, it was also only increased following 1 week of pioglitazone treatment, determined by Dunnett’s multiple comparison (p = 0.048) (Figure 2A). The lack of effect of pioglitazone on PON2 expression at three weeks was not due to difference in pioglitazone absorption following oral administration, as we found plasma and cerebrospinal fluid concentration at time of sacrifice were not different between 1 and 3 week treatment groups (Table 1).
Figure 1. PON2 protein expression in brain regions of monkeys treated with pioglitazone (PIO).
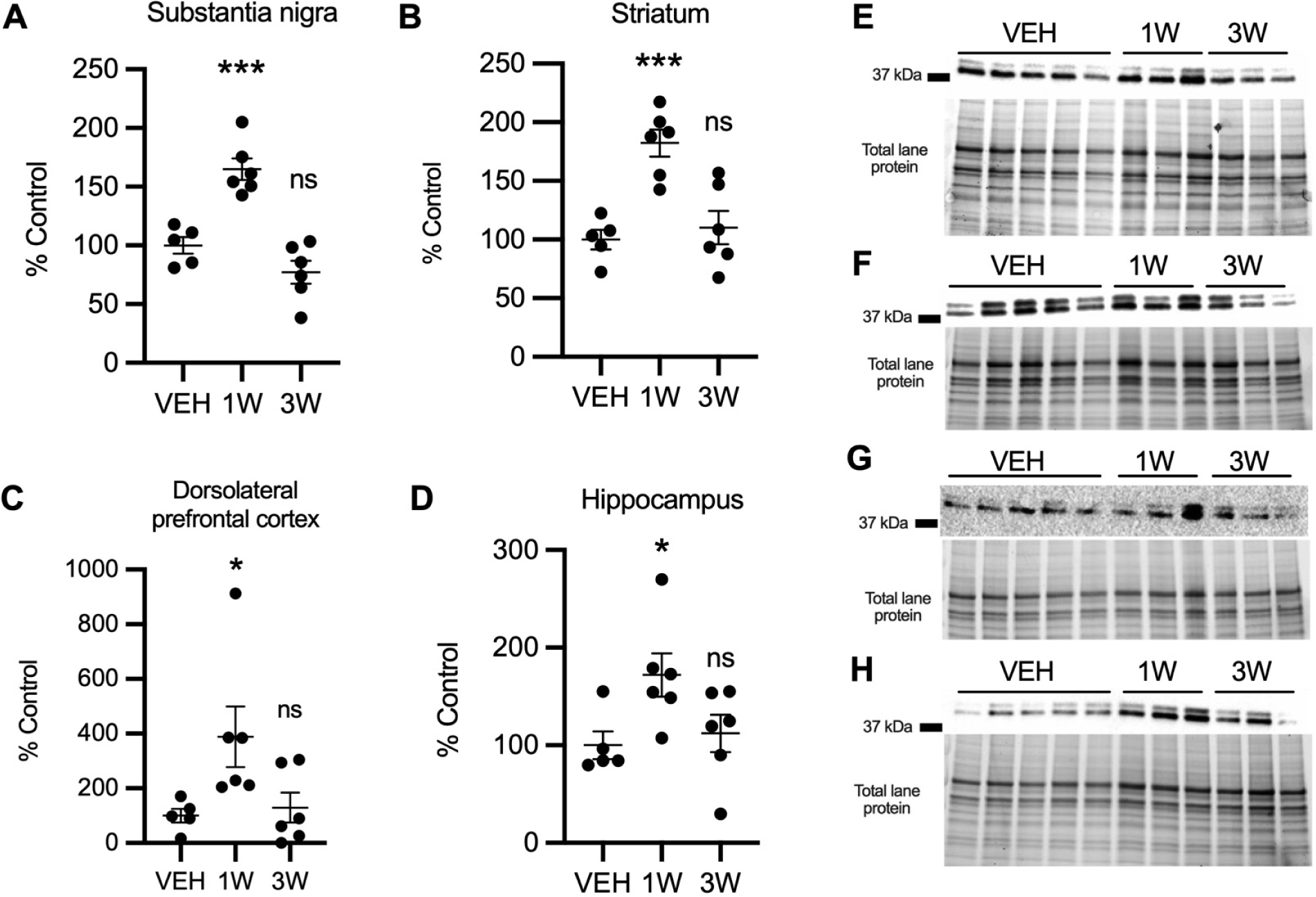
PON2 expression in PIO-treated monkeys (n = 6) relative to vehicle (VEH; n = 5) in in striatum (A), substantia nigra (B), dorsolateral prefrontal cortex (C), and hippocampus (D). Significant increases were found in 1 week group versus vehicle in striatum (p = .0006), substantia nigra (p <.0004), dorsolateral prefrontal cortex (p = .034), and hippocampus (p = .043). (E-H) Representative blot showing PON2 expression and corresponding image of total protein in striatum (E), substantia nigra (F), dorsolateral prefrontal cortex (G), and hippocampus (H). Optical density of 37 kDa PON2 bands were first normalized to total protein per lane and then to vehicle group mean. Results are presented as mean ± SEM. Asterisks indicate statistical significance in comparison to vehicle group, *p < .05, *** p < .001.
Figure 2. Gene expression in striatum of monkeys treated with pioglitazone (PIO).
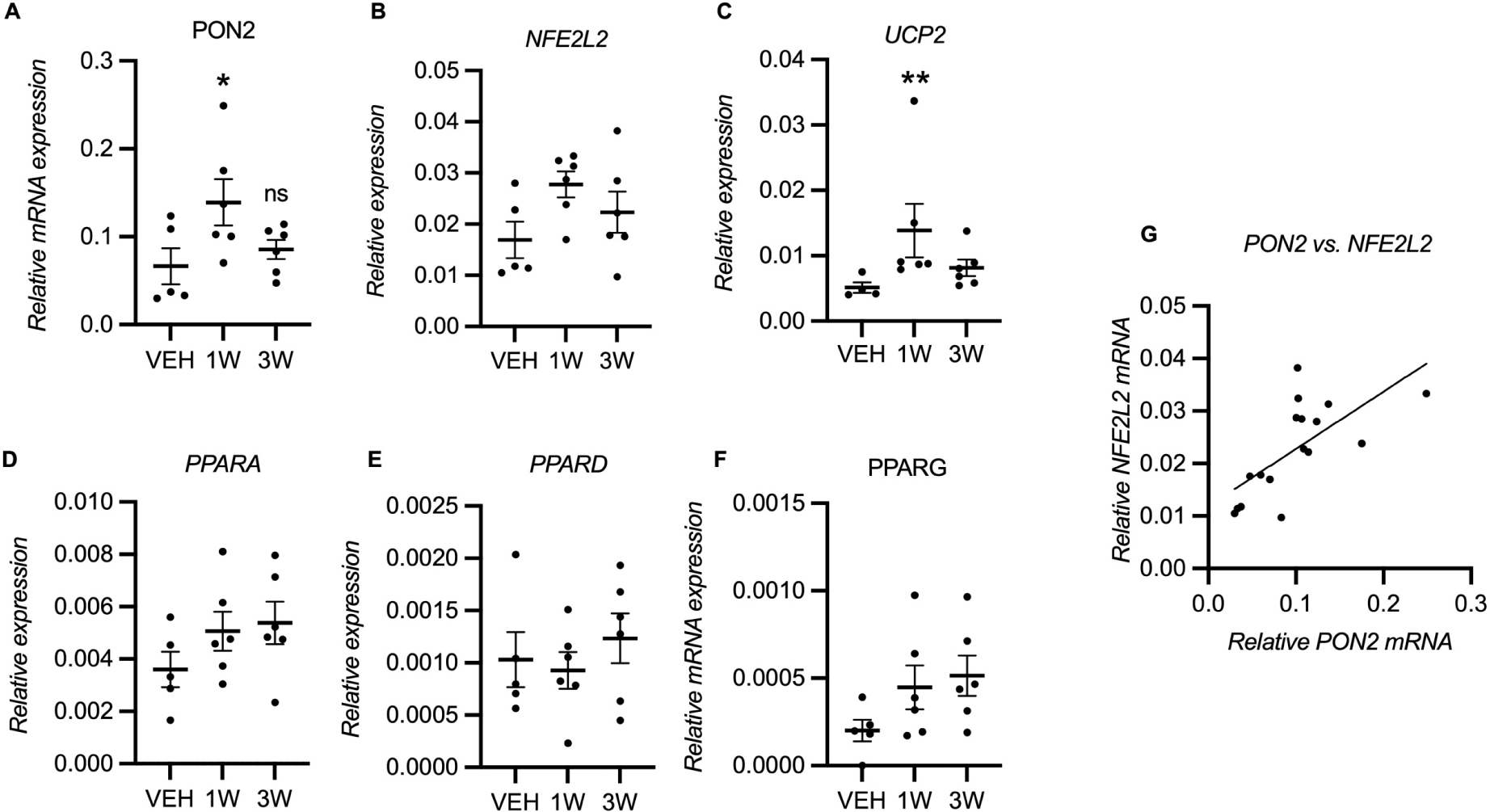
(A) PON2 mRNA was increased in 1 week PIO-treated monkeys compared to vehicle-treated monkeys (p =.048) but not in 3 week treatment group (n = 5–6). (C) UCP2 mRNA was also increased following 1 week but not 3 week PIO treatment (p = 0.007). (B, D, E, F) All other mRNA expression changes were not statistically significant. (G) PON2 mRNA was correlated with NFE2L2 mRNA (Pearson’s r = 0.68, adjusted p = 0.014). mRNA levels were normalized to ALU-SEQ, TUBB, and ATP5B. Results are presented as mean ± SEM. Asterisks indicate statistical significance in comparison to vehicle group, *p < .05. **p < .01. (C) One sample in vehicle group was lost during qPCR for UCP2.
Table 1:
Pioglitazone concentration in plasma and cerebrospinal fluid (CSF) at time of sacrifice (±SD)
| 1 week PIO (ng/mL) | 3 week PIO (ng/mL) | |
|---|---|---|
| plasma | 1174 ± 1192 | 2047 ± 1340 |
| CSF | 24.43 ± 19.39 | 47.25 ± 57.29 |
We next assessed if two known pioglitazone-inducible genes, Nuclear factor erythroid 2-related factor 2 (NFE2L2; also known as NRF2), and uncoupling protein 2 (UCP2), were activated (Figure 2B, C). Only UCP2 mRNA was significantly increased (NFE2L2 One-way ANOVA: F (2, 14) = 2.386, p = 0.1283; UCP2 Kruskal-Wallis: H(3) = 8.43, p = 0.007). This transient stimulation of PON2 expression and variable activation of other target genes were unexpected and could be due to homeostatic mechanisms present in healthy animals. We then asked if pioglitazone induced changes in PPARγ and whether the other PPAR isoforms, PPARα and PPARβ/δ, were affected and may contribute to PON2 regulation. The PPARs act in two feedback loops: (1) a positive feedback loop between PPARγ and PPARβ/δ, and (2) a negative feedback loop between PPARα and PPARβ/δ (14). Thus, we hypothesized that pioglitazone would increase PPARγ expression, and cause a delaye increase in PPARα expression. We examined PPAR mRNAs in the striatum and found no significant difference in expression (post-hoc ‘test for trends’: PPARA p =0.13, PPARG p = 0.07). As it was possible we could not detect an effect due to lack of statistical power, we also assessed correlation between PON2 mRNA and other mRNAs. We found that NFE2L2 was correlated with PON2 (Pearson’s r = 0.68, Bonferonni-Sidak adjusted p = 0.014) (Figure 2G), indicating that PON2 and NFE2L2 are regulated by a shared mechanism. This correlation had a mid-range R2 value 0.46, which likely reflects that PON2 and NFE2L2 share regulatory mechanisms or function, considering that NFE2L2 expression is activated by other mechanisms not involving PPARγ pathway and that sampling and assay methodology can affect correlation among genes.
Our finding of a transient increase in PON2 might explain or contribute to the failure of the NET-PD clinical trial, as pioglitazone’s neuroprotection might have only lasted less than 3 weeks if PON2 is its predominant mechanism in this situation (11). Likewise, the success of pioglitazone in MPTP-treated monkeys could have been due to an effective activation of PON2 by pioglitazone and limitation of parkinsonian toxicity within 3 weeks of exposure to MPTP (9).
We previously found that one week treatment of pioglitazone in mice increases PON2 protein and Nfe2l2 mRNA in STR but not in HIP (13), whereas in the present study, PON2 expression was increased in all regions with DFC showing twice the amount of increase. Together these findings suggest that there are important species differences and a regional variations in pioglitazone effect. The regional pattern of pioglitazone’s effect may mirror the regional distribution of PON2 (1) and/or the PPARs, as they possess different tissue distribution and execute different biological functions(4). An additional factor to consider when evaluating in vivo responses to pioglitazone is the sex-dependent activity of PPARγ. For example, sexual dimorphic responses to pioglitazone have been reported in pain sensation in male and female mice: pioglitazone increased mechanical hypersensitivity (allodynia) only in female mice, which was blocked by testosterone and a PPARγ antagonist (15). It is possible that in female monkeys pioglitazone could have a sustained or a different effect on PON2 expression than in male monkeys. Indeed, PON2 appears to be regulated by estrogen as it has been found to be more highly expressed in female than in male rodent and nonhuman primate brains (1, 4), and in males, testosterone activation of PPARα indirectly suppresses PPARγ (14). Moreover, the NET-PD trial did not compare sex differences and the majority of each treatment cohort were males (68 and 74%) (11), so it is not yet known if pioglitazone could be an effective neuroprotective treatment in female PD patients.
In summary, PON2 is a promising neuroprotective target in PD and in other neurodegenerative diseases. Pioglitazone transiently increases PON2 expression in cortical and subcortical regions in normal nonhuman primates and future studies will address to what extent this is maintained in disease states or models. We have investigated the existence of homeostatic mechanisms that prevent long term increases in PON2 expression in the healthy monkey brain. Elucidating the regulatory mechanism(s) in place are critical for realizing PON2’s full potential as a neuroprotective target. Further studies are now warranted to investigate the intriguing link between pioglitazone-induced PON2 expression and protection against dopamine neuron loss, and whether increased PON2 expression can be sustained long-term by other PPARγ agonists.
Supplementary Material
Highlights.
Paraoxonase-2 (PON2) is an antioxidant enzyme and neuroprotective target
The diabetic drug pioglitazone (PIO) reduces the risk of Parkinson’s disease (PD)
Pioglitazone increased PON2 in multiple regions of primate brain after 1 week
After 3 weeks of pioglitazone treatment, PON2 expression had returned to baseline
Identifying the causes of PIO’s transient effect is key for advancing PD treatment
Funding
Supported by NIH grant AG048918. We also thank the Yale CTSA (UL1RR024139) and Yale School of Medicine for funding the 4000 Q-TRAP LC MS/MS system located within the Yale MS & Proteomics Resource of the WM Keck Foundation Biotechnology Resource Laboratory.
Abbreviations
- CSF
cerebrospinal fluid
- DFC
dorsolateral prefrontal cortex
- HIP
hippocampus
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- NET-PD
Neuroprotection Exploratory Trials of Parkinson’s Disease
- NHP
Nonhuman primate
- NFE2L2
Nuclear factor erythroid 2-related factor 2
- PON2
Paraoxonase-2
- PPARγ
peroxisome proliferator-activated receptor gamma
- SN
substantia nigra
- STR
striatum
- UCP2
uncoupling protein 2
Footnotes
Competing interests
The authors declare that they have no competing interests.
Declarations of interest: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Giordano G, Cole TB, Furlong CE, Costa LG. Paraoxonase 2 (PON2) in the mouse central nervous system: a neuroprotective role? Toxicol Appl Pharmacol. 2011;256(3):369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Devarajan A, Bourquard N, Hama S, Navab M, Grijalva VR, Morvardi S, et al. Paraoxonase 2 deficiency alters mitochondrial function and exacerbates the development of atherosclerosis. Antioxid Redox Signal. 2011;14(3):341–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parsanejad M, Bourquard N, Qu D, Zhang Y, Huang E, Rousseaux MW, et al. DJ-1 interacts with and regulates paraoxonase-2, an enzyme critical for neuronal survival in response to oxidative stress. PLoS One. 2014;9(9):e106601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jamwal S, Blackburn JK, Elsworth JD. PPARgamma/PGC1alpha signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol Ther. 2021;219:107705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garrick JM, Dao K, de Laat R, Elsworth J, Cole TB, Marsillach J, et al. Developmental expression of paraoxonase 2. Chem Biol Interact. 2016;259(Pt B):168–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrow BA, Roth RH, Redmond DE, Jr., Diano S, Elsworth JD. Susceptibility to a parkinsonian toxin varies during primate development. Exp Neurol. 2012;235(1):273–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morrow BA, Roth RH, Redmond DE, Elsworth JD. Impact of methamphetamine on dopamine neurons in primates is dependent on age: implications for development of Parkinson’s disease. Neuroscience. 2011;189:277–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadeghian M, Marinova-Mutafchieva L, Broom L, Davis JB, Virley D, Medhurst AD, et al. Full and partial peroxisome proliferation-activated receptor-gamma agonists, but not delta agonist, rescue of dopaminergic neurons in the 6-OHDA parkinsonian model is associated with inhibition of microglial activation and MMP expression. J Neuroimmunol. 2012;246(1–2):69–77. [DOI] [PubMed] [Google Scholar]
- 9.Swanson CR, Joers V, Bondarenko V, Brunner K, Simmons HA, Ziegler TE, et al. The PPAR-gamma agonist pioglitazone modulates inflammation and induces neuroprotection in parkinsonian monkeys. J Neuroinflammation. 2011;8:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melendez-Flores JD, Millan-Alanis JM, Gonzalez-Martinez A, Alvarez-Villalobos NA, Estrada-Bellmann I. Does glitazone treatment have a role on the prevention of Parkinson’s disease in adult diabetic population? A systematic review. Metab Brain Dis. 2020;35(7):1067–75. [DOI] [PubMed] [Google Scholar]
- 11.Investigators F-Z. Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 2015;14(8):795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shiner M, Fuhrman B, Aviram M. Macrophage paraoxonase 2 (PON2) expression is up-regulated by pomegranate juice phenolic anti-oxidants via PPAR gamma and AP-1 pathway activation. Atherosclerosis. 2007;195(2):313–21. [DOI] [PubMed] [Google Scholar]
- 13.Blackburn JK, Curry DW, Thomsen AN, Roth RH, Elsworth JD. Pioglitazone activates paraoxonase-2 in the brain: A novel neuroprotective mechanism. Exp Neurol. 2020;327:113234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aleshin S, Reiser G. Role of the peroxisome proliferator-activated receptors (PPAR)-alpha, beta/delta and gamma triad in regulation of reactive oxygen species signaling in brain. Biol Chem. 2013;394(12):1553–70. [DOI] [PubMed] [Google Scholar]
- 15.Sorge RE, Mapplebeck JC, Rosen S, Beggs S, Taves S, Alexander JK, et al. Different immune cells mediate mechanical pain hypersensitivity in male and female mice. Nat Neurosci. 2015;18(8):1081–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


