Abstract
Background:
The relationship between pain and stress is widely accepted, yet the underlying neuroendocrine mechanisms are poorly understood. Cortisol secretion during a stress response, may distract attention from a painful stimulus, inhibiting pain. However, when pain is the stressor, cortisol secretion may intensify the pain experience and condition a fear-based memory of pain. This study attempts to determine the relationship between acute pain, chronic pain, and cortisol in the traumatically injured population.
Study Design:
Secondary analyses of a prospective observational study with participants from a Midwestern Adult Level I Trauma Center post traumatic injury, with interview and serum cortisol taken at hospitalization (baseline) and 6 months after discharge, was completed using Ward’s Method hierarchical cluster analysis, Pearson’s correlations, and linear regressions.
Results:
Two major clusters were identified. The Chronic Pain (CP) group were those who had severe pain at discharge and continued to have severe pain as defined by Numeric Pain Score. The Resolved Pain (RP) group were those who had moderate pain at discharge and their pain improved or resolved. Pain score at discharge significantly, negatively correlated with baseline cortisol levels (r= −0.142, p=0.02). Minority status, single individuals, low cortisol at baseline, and greater psychological distress at baseline significantly increased the likelihood of developing chronic pain.
Conclusions:
Low cortisol and greater psychological stress, which are also associated with minority status and single individuals, contribute to chronic pain in the traumatically injured population. Trauma victims without an adequate cortisol response to acute injury and pain are at risk for development of chronic pain after injury.
Keywords: Cortisol, Acute pain, Chronic pain, injury
Introduction
Widely accepted is the relationship between pain and stress, yet the underlying neuroendocrine mechanisms are not well understood. In the brain, the amygdala is evoked by perceived threat to safety or well-being, this initiates a physiologic stress response and elicits the secretion of sympathetic catecholamines, as well as the neuroendocrine hormone cortisol, all of which promote actions involved in survival.1 Normally, cortisol secretion during a stress event may distract attention from a concurrent painful stimulus through anti-inflammatory effects, thereby allowing for survival actions to occur despite the existence of pain. This protection from acute pain supports life preserving maneuvers to evade threats, an adaptive measure for survival. When pain itself is the stressor, cortisol secretion may intensify its subjective experience, conditioning a fear-based memory of pain with the future goal of avoidance of pain.1 It is well known that fear avoidance contributes to the pain experience, and in fact amplifies the cycle of chronic pain and disability.2 What is originally an adaptive physiologic mechanism (cortisol secretion for stress response) then becomes maladaptive through an exaggeration of psychological response to acute pain (fear-based avoidance), intensifying the pain experience and impeding recovery.3 High initial pain in trauma patients increases the risk for the development of chronic pain after injury.4
We posit that higher cortisol response in a traumatic injury may contribute to the development of chronic pain over time by intensifying the initial pain experience through perceived higher pain scores. This study attempts to quantify the physiologic stress response in traumatic injury via cortisol measures, determine the relationship between cortisol levels and acute/chronic pain, and link the development of chronic pain to the physiologic stress response in patients with traumatic injury as measured by cortisol response.
Methods
This was a secondary analysis of a prospective longitudinal cohort study (Study on Trauma and Resilience, or STAR), in which the subjects were patients with traumatic injuries admitted to a single institution Midwestern Adult Level I Trauma Center. Participants were recruited after admission to the hospital and, after consent, underwent completion of a series of questionnaires as a part of a larger study on trauma and resilience. Initially eligible participants were identified using a trauma service census and individuals were excluded if they met any of the following exclusion criteria: 1) younger than 18 years of age, 2) traumatic brain injury resulting in greater than 30 minutes of peritraumatic amnesia, 3) injury that resulted in an inability to communicate, 4) Glasgow coma score (GCS) of less than 13 on admission, or 5) non-English speaking. Approval for these studies was provided by the IRB at this institution. Written informed consent was obtained from all participants prior to participation in the study.
Participants:
Recruitment occurred Monday through Friday over a 19-month period using daily review of the trauma division inpatient census. Patients on the daily census were screened for the following inclusion criteria: experienced a traumatic event, which was defined as meeting criterion A for a diagnosis of Post-Traumatic Stress Disorder (PTSD) according to DSM-V; at least 18 years of age; English speaking; and able to provide written informed consent within seven days of admission. Cognition was also assessed through chart review for documented GCS of 13 or greater. Patients who did not have currently adequate cognitive capacity, defined as premorbid or injury-related condition with GCS of 13 or less (e.g. moderate or severe TBI); persons in police custody; and patients having active psychotic or self-harm symptoms were excluded. A stipend of a $35 gift card was given for participation at 6 months.
Approximately 3915 patients were admitted to the Trauma center, about 1618 were screened, and 278 patients were recruited to the original prospective study over the 19-month time period. Of the 278 participants recruited, n=168 returned for the six-month visit, reflecting a 60% retention rate comparable to other longitudinal investigations involving survivors of traumatic injury (e.g., 57%).5 Participants lost to follow-up (n=106) were younger (37.23±14.28; t(276)=2.53, p=0.011), but did not differ from participants who were retained in terms of gender (χ2(1)=0.69, p=0.408), race/ethnicity (χ2(3)=1.67, p=0.644), or mechanism of injury (χ2(9)=12.30, p=0.197). The mean ISS at the baseline assessment for the group was 10.35 and the mean ISS at the 6 months follow up was 10.27. These were not significantly different. Substance use was measured and 30% of patients used marijuana prior to their injury, 2.9% used cocaine, and 54.9% used alcohol. Of the baseline population, 16.5% had a history of chronic pain and 15.8% had a diagnosis of chronic pain at the time of hospitalization.
Measures:
Participant demographic and injury-related data were obtained prospectively within the primary study. These data included sex, age, race/ethnicity, insurance status, relationship status, Injury Severity Score (ISS), mechanism of injury (MOI), assaultive versus non-assaultive injury, and hospital length of stay (LOS). Pain was assessed via the Numeric Pain Score (NPS) at the time of discharge from the hospital. The Brief Pain Inventory was conducted at 6 months following the index traumatic event for evaluation of pain at that specific time point relative to injury. Non-fasting whole blood samples measuring cortisol were drawn at initial hospitalization and at the 6 month follow up. The average time of day in which the blood samples were obtained was 11:56am (SD=1.58 hours) at hospitalization and 12:17pm (SD=2.39 hours) at 6 months. The average time from Emergency Department admission to baseline blood draw was 2.6 days (SD=1.8) with the majority collected within 2 days of admission.
Since this was a secondary analysis of a data collected for the Study of Trauma and Resilience. The primary outcome in the original study was to evaluate the development of chronic PTSD which is diagnosed at 6 months after a traumatic injury. In pain literature, both 3 and 6-month time points are utilized as the designation for chronic pain. Therefore, the 6-month time point is supported as an appropriate time at which to measure the development of chronic pain.
Analysis:
Ward’s Method hierarchical cluster analysis was used to identify trajectories of the transition of pain over time from hospitalization to 6 months after the index traumatic injury. This was an exploratory analysis to determine similar groups of how pain changes over time after an injury. NPS greater than or equal to 4, moderate or severe pain at 6 months, was defined as chronic pain because this level of pain is more significant and life interfering pain than mild pain (NPS <4).
Two groups or pain clusters were identified, one with resolution of acute pain over time (Resolved Pain cluster) and one with transition into chronic pain (Chronic Pain cluster). Pearson correlations were then completed between cortisol levels and pain ratings. Linear regressions were then performed to predict the relationship between baseline demographic variables (i.e., sex, age, and minority status), baseline cortisol levels, baseline psychological distress (i.e., anxiety, depression, acute distress) and the 2-level pain trajectories to determine significant relationships. Cortisol levels were split along the median to create a binary variable (i.e., low Cortisol versus high Cortisol). Minority status was also transformed into a binary variable (i.e., white versus minority status). Black and African-Americans made up 43% of the population and Hispanic/Latinos made up only 8% of the total population. Whites made up 48% of the population. Therefore, Blacks/African Americans and Hispanics racial groups were combined.
Results
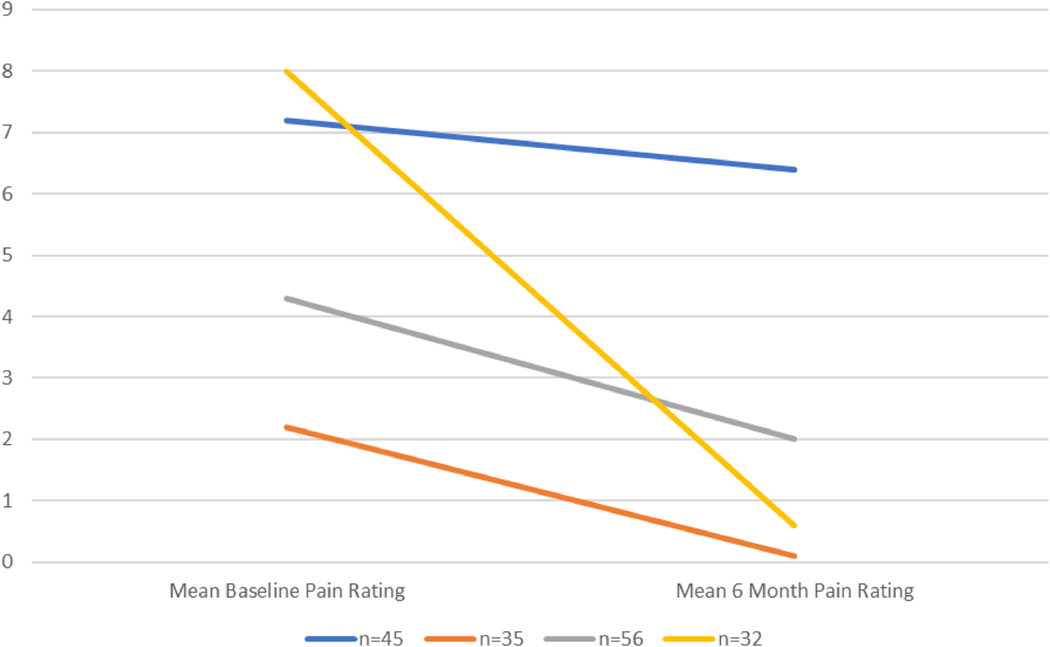
ISS did not correlate with baseline pain scores (r=−0.028, p=0.64) but did significantly correlate with 6-month pain scores (r=0.183, p=0.016). ISS did not correlate with baseline cortisol (r=0.118, p=0.051) but did correlate with 6-month cortisol (r=0.176, p=0.031). Initially, 4 groups were identified by the hierarchical cluster analysis. The groups were conceptualized as 1) chronic pain (NPS above 4 at both time points, with little recovery); 2) significant pain acutely though recovered (extremely elevated above NPS 4 at baseline, recovery to NPS less than 4 at 6 months), 3) mild pain acutely though recovered (slightly above NPS 4 at baseline, recovery to NPS less than 4 at 6 months), and 4) resilient (minimal pain at both time points) (Figure 1 and Table 1).
Figure 1.
Ward’s Method – hierarchical cluster analysis – 4 groups
Table 1 –
Ward’s Method – hierarchical cluster analysis – 4 groups.
| Mean baseline pain rating (SD) | Mean 6 mo pain rating (SD) | |
|---|---|---|
| n = 45 | 7.2 (2.1) | 6.4 (1.9) |
| n = 35 | 2.2 (1.3) | 0.1 (0.4) |
| n = 56 | 4.3 (1.5) | 2 (1.6) |
| n = 32 | 8 (1.0) | 0.6 (0.9) |
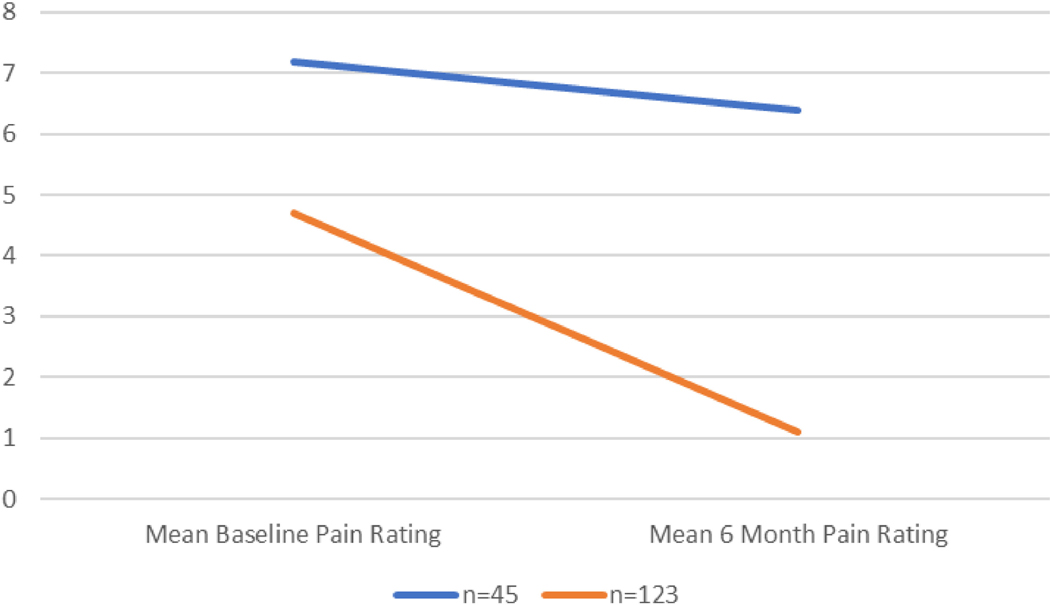
Those in the three groups with minimal pain levels at 6 months were collapsed into one cluster so to compare them with participants endorsing ongoing pain concerns at 6 months post injury, including the resilient patients (Figure 2 and Table 2). The Chronic Pain (CP) cluster were those participants who had severe pain at discharge and continued to have severe pain. The Resolved Pain (RP) cluster were those who had moderate pain at discharge and pain improved or resolved. Pain score at discharge significantly negatively correlated with baseline cortisol levels (r= −0.142, p=0.02). Minority status, single individuals, low cortisol at baseline, and greater psychological distress at baseline significantly increased the likelihood of developing chronic pain (Table 3). While pain score at discharge significantly negatively correlated with baseline cortisol levels, pain score at 6 months did not significantly correlate with cortisol at 6 months (r=−0.031, p=0.706).
Figure 2.
Mean Pain Ratings at Baseline and 6-month Follow Up, by cluster-2 Groups
Table 2.
Ward’s Method – hierarchical cluster analysis – 2 groups
| Mean baseline pain rating (SD) | Mean 6 mo pain rating (SD) | |
|---|---|---|
| n = 45 | 7.2 (2.1) | 6.4 (1.9) |
| n = 123 | 4.7 (2.5) | 1.1 (1.4) |
Table 3.
Linear Regressions Predicting Pain Trajectory (as defined by cluster analysis)
| Demographics | p-value |
|---|---|
| Sex | 0.1713 |
| Age | 0.6656 |
| Ethnicity/Race | 0.0784 |
| Minority - binary | 0.0094 |
| Insurance | 0.6309 |
| Relationship | 0.0332 |
| Injury Characteristics | |
| ISS | 0.1357 |
| MOI_Intention | 0.2732 |
| Hospital Days | 0.7726 |
| Biomarkers at Baseline | |
| Cortisol - continuous | 0.0762 |
| Cortisol - binary | 0.0006 |
| Baseline Psych | |
| PCL-5 Total | 0.0334 |
| ITSS PTSD | 0.4102 |
| ITSS PTSD Risk | 0.8782 |
| ITSS DEP | 0.0459 |
| ITSS DEP Risk | 0.2043 |
| DASS Depressions Total | 0.0022 |
| DASS Anxiety Total | 0.0002 |
| DASS Stress Total | 0.0164 |
| ERQ Reappraisal | 0.4139 |
| ERQ Suppression | 0.0193 |
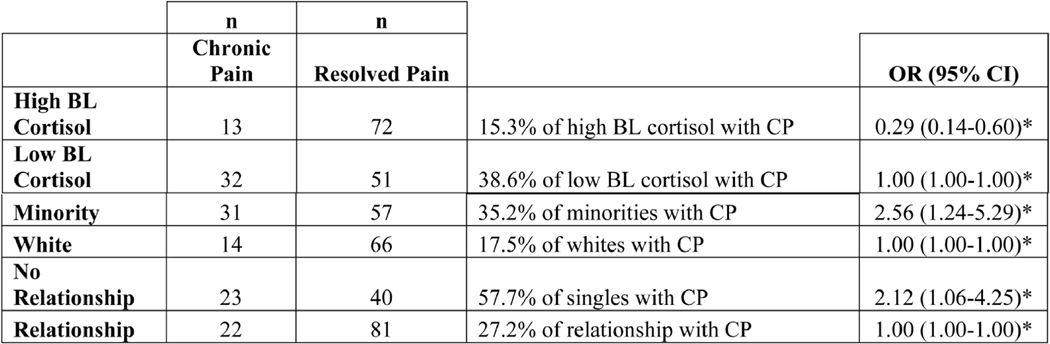
In the linear regression analysis with the outcome of the 2-level pain clusters, CP versus RP, several important variables predicted the development of chronic pain. These predictors included minority status, single individuals, greater psychological distress at hospitalization, use of suppression as a mode of emotion regulation. Interestingly, there was a significant difference in cortisol levels based upon race/ethnicity, with minorities having significantly lower cortisol levels at baseline [t(271)=−2.69, p=0.0076] and minorities more likely to be “Low Cortisol” at baseline compared to whites [χ2 (1, N=273) = 10.37, p=0.001]. Specifically, when looking at the likelihood of developing chronic pain, low cortisol at baseline had an odds ratio (OR) probability of 1, minority status had an OR of 2.56, and being single had an OR of 2.12 (Figure 3). However, ISS, hospital length of stay, and mechanism of injury did not significantly contribute to the development of chronic pain (Table 4, Table 5).
Figure 3.
Likelihood of Developing Chronic Pain
Table 4:
Demographics
| 6-month follow up (N = 168) | |
|---|---|
| Mean Age (SD; range) | 42.79 (16.54; 18.1–89.4) |
| Gender | 69.8% Male |
| MOI – Assaultive? | 71.5%% Non-assaultive injury |
| Insurance? | 84.8% Insured |
| Relationship status | 62.4% In a committed relationship |
| TBI | 98.8% Did not suffer mTBI |
| LOC | |
| No | 83.7% |
| Yes | 7.0% |
| Unknown | 9.3% |
| Mean ISS (SD; range) | 10.27 (6.13; 0–29) |
| Race | |
| Caucasian/White | 48% |
| African American/Black | 43% |
| Hispanic/Latino | 8% |
| American Indian/Alaskan Native | 1% |
| Highest Education Completed | |
| At least some college | 57% |
| High school graduate | 25% |
| Less than High school | 18% |
Table 5:
Mechanism of Injury
| 6-month follow up (N = 168) | |
|---|---|
| Mechanism of Injury | |
| Motor vehicle crash | 33% |
| Gun shot wound | 16% |
| Fall | 18% |
| Stab | 10% |
| Motorcycle crash | 8% |
| Other | |
| Crush/Recreational Injury | 6% |
| Assault | 2% |
| Other | 1% |
| Pedestrian Struck | 6% |
In simple univariate analysis, when evaluating the development of chronic pain in whites versus Blacks/African Americans, the development of chronic pain was significantly higher in Blacks/African Americans (Chi Square=7.7, p=0.005). Also, the mode of injury was separated into assaultive versus non-assaultive and there was no difference between the groups in the development of chronic pain (Chi square=2.4, p=0.11).
Discussion
A painful stimulus causes a stress response in individuals experiencing the pain, and cortisol secretion can regulate the sensation of pain, protecting the individual from some of the unpleasantness of the pain event. Individuals vary in their cortisol response to pain, both at initial injury and some months later. Variations in initial or baseline cortisol levels at the time of a pain event do affect the trajectory of a patient’s pain resolution; however, at 6 months from the injury cortisol levels did not correlate with development or absence of continued pain. Therefore, cortisol response at the time of hospitalization appears to be an important risk factor for the transition to chronic pain in the traumatically injured.
Previous studies have concluded that individuals showing the largest reactive cortisol response reported less pain during a stressful experience, supporting cortisol as a protective mechanism against pain.6 The individual acute stress response is likely to influence neurophysiological mechanisms underlying the perception of pain.6 In this study, it appears that higher cortisol response at hospitalization may be predictive of less pain at 6 months post trauma, similar to previous studies.
For the participants who had severe pain upon discharge and continued severe pain at 6 months, the factors that most strongly correlated with this trajectory were minority status, single status, low baseline or initial cortisol, and greater psychological distress at the time of injury. Another factor identified in participants who developed chronic pain (continued severe pain at 6 months post injury) was the use of suppression as a method of emotion regulation, identified by the above-mentioned questionnaires. Minority status, not having a relationship, and low baseline cortisol were the greatest factors in likelihood of developing chronic pain, while being white, involved in a relationship, and high baseline cortisol were slightly more protective.
High pain on admission to the hospital and on hospital discharge for trauma patients has been found to be an important predictor for the development of chronic pain at 4 months post injury.4,7 In fact, the probability for developing chronic pain with a discharge NPS of 0–2 is 0.06, NPS 2–4 is 0.29, NPS 4–6 is 0.35, NPS 6–8 is 0.50, and NPS 8–10 are 0.80.7 Therefore, high hospital acute pain predicts the development of chronic pain in the traumatically injured population. Similarly, in this secondary analysis of a prospective observational study, specifically in the pain cluster analysis, the 2 pain clusters (Resolved Pain and Chronic Pain) supported high discharge pain as a significant predictor of the development of chronic pain after injury. Generally held presumptions on pain have suggested that level of pain related to the injury and how the person reacts to the pain are the main determinants in risk for the development of chronic pain. It is also presumed that high pain equals high secretion of cortisol1. However, if these presumptions are true, we could also assume that severe continued pain should elicit a sustained high cortisol response. However, in this study we demonstrate that the cortisol response is actually low in those patients that develop chronic pain, not high as expected with the traditional cortisol/pain relationship premise.
One explanation for this altered, conflicting response could be that those with low cortisol at the time of injury have cortisol dysfunction caused by preinjury stress experiences. Entering into a traumatic injury experience with prior life circumstances that result in sustained, long term cortisol surges or activations (a known contributor to cortisol dysfunction) may then place patients in the category of low initial baseline cortisol response, which in this study has been linked to the development of chronic pain. It has been documented that prolonged cortisol secretion in response to a chronic stress, with recurrent reactivation and repeated surges of cortisol can result in cortisol dysfunction and chronic stress-induced hypocortisolism has been well documented and linked to pain somatization disorders.1,8
One of the most interesting findings in this study was that minority patients are more likely than white patients to have experienced low cortisol levels. Current theory suggests that ethnic/racial differences in cortisol response are related to experiences of chronic stress in general and experiences of discrimination more specifically, which are more prevalent among African American and Latino groups.9Flatter slopes of cortisol response have been linked to multiple emotional and physical health problems, including immune-related and metabolic conditions.10 African American adults have been found to exhibit a lower cortisol awakening response compared to White adults, an effect that is even more pronounced in low-socio-economic status African Americans.11 This puts minority patients are greater risk for the development of chronic pain after trauma and must be a priority for study in future trauma and pain research. In this study, when evaluating the development of chronic pain in whites versus Blacks/AAs, the development of chronic pain was significantly higher in Blacks/African Americans.
Several limitations were significant in this study. Cortisol was measured at different times in patients. Cortisol has diurnal fluctuations and measurement at similar times during the day would be most appropriate for comparison. Future studies must take the time of day for measurement into consideration. It is possible that a more exact drawing of cortisol could change the outcomes of the study. In future studies, it would be recommended to control time of day and obtain cortisol measures at both time points-waking peak measures and late-night trough. If appropriately measured at the same time of day at each time point, a cortisol change score could be developed to determine how the cortisol response alters over time after trauma. This change score could provide interesting information on how the stress system evolves after physical trauma. Another limitation in this study was that this trauma population included all patients in the inclusion criteria. Therefore, this was a heterogenous group that included all mechanisms of injury with many different injury types. Focusing on one injury type or mechanism of injury may improve generalizability for specific injury patterns in future studies. Also, other preinjury stressors such as discrimination, neighborhood violence, lack of access to healthcare and healthy foods are known pre-injury stressors but were not measured in this study. Adding measures to evaluate these stressors in future studies can provide further insight into pre-injury stress and its relationship to cortisol response and the development of chronic pain.
Conclusions
To summarize, higher baseline pain scores were associated with low initial cortisol. Low cortisol and greater psychological stress, which also appear to be associated with minority status and single individuals, are associated with the development of chronic pain in the traumatically injured population. Trauma patients who do not have an adequate cortisol response to acute injury and pain are at risk for the development of chronic pain after injury. Further exploration is needed to determine the etiology of a blunted cortisol response and may be associated with preinjury stressors.
Acknowledgement
The authors report no proprietary or commercial interest in any product mentioned or concept discussed in this article.
Funding for this study was through a National Institute of Mental Health grant (5R21MH102838 −02 PI Name: deRoon-Cassini, Terri A.)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hannibal KE, Bishop MD. Chronic stress, cortisol dysfunction, and pain: a psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys ther. 2014. December 1;94(12):1816–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crombez G, Eccleston C, Van Damme S, Vlaeyen JW, Karoly P. Fear-avoidance model of chronic pain: the next generation. Clin J Pain. 2012. July 1;28(6):475–83. [DOI] [PubMed] [Google Scholar]
- 3.Lucchetti G, Oliveira AB, Mercante JP, Peres MF. Anxiety and fear-avoidance in musculoskeletal pain. Curr Pain Headache R. 2012. October 1;16(5):399–406. [DOI] [PubMed] [Google Scholar]
- 4.Trevino C, Harl F, deRoon-Cassini T, Brasel K, Litwack K. Predictors of chronic pain in traumatically injured hospitalized adult patients. J Trauma Nurs. 2014. March 1;21(2):50–6. [DOI] [PubMed] [Google Scholar]
- 5.Martin-Herz SP, Zatzick DF, McMahon RJ. (2012). Health-Related Quality of Life in Children and Adolescents Following Traumatic Injury: A Review. Clin Child Fam Psychol. 2012 15(3):192–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vachon-Presseau E, Martel MO, Roy M, Caron E, Albouy G, Marin MF, Plante I, Sullivan MJ, Lupien SJ, Rainville P. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci. 2013. April 17;33(16):6826–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trevino CM, deRoon-Cassini T, Szabo A, Brasel K. Acute longitudinal pain trajectories in the traumatically injured. SOJ Anesthesiol Pain Manag. 2015;2(3):1–7. [Google Scholar]
- 8.Ehlert U, Gaab J, Heinrichs M. Psychoneuroendocrinological contributions to the etiology of depression, posttraumatic stress disorder, and stress-related bodily disorders: the role of the hypothalamus–pituitary–adrenal axis. Biol Psychol. 2001. Aug 1;57(1–3):141–52. [DOI] [PubMed] [Google Scholar]
- 9.Lewis TT, Cogburn CD, Williams DR. Self-reported experiences of discrimination and health: scientific advances, ongoing controversies, and emerging issues. Annu Rev Clin Psycho. 2015. Mar 28;11:407–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adam EK, Quinn ME, Tavernier R, McQuillan MT, Dahlke KA, Gilbert KE. Diurnal cortisol slopes and mental and physical health outcomes: A systematic review and meta-analysis. Psychoneuroendocrino. 2017. Sep 1;83:25–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett GG, Merritt MM, Wolin KY. Ethnicity, education, and the cortisol response to awakening: a preliminary investigation. Ethnic Health. 2004. Nov 1;9(4):337–47. [DOI] [PubMed] [Google Scholar]