Abstract
Latency reversal agents (LRAs), such as protein kinase C (PKC) agonists, constitute a promising strategy for exposing and eliminating the HIV-1 latent reservoir. PKC agonists activate NF-κB and induce deleterious pro-inflammatory cytokine production. Adjuvant pharmacological agents, such as ruxolitinib, a JAK inhibitor, have previously been combined with LRAs to reduce deleterious pro-inflammatory cytokine secretion without inhibiting HIV-1 reactivation in vitro. Histone deacetylase inhibitors (HDACi) are known to dampen pro-inflammatory cytokine secretion in the context of other diseases and synergize with LRAs to reactivate latent HIV-1. This study investigates whether a panel of epigenetic modifiers, including HDACi, could dampen PKC-induced pro-inflammatory cytokine secretion during latency reversal. We screened an epigenetic modifier library for compounds that reduced intracellular IL-6 production induced by the PKC agonist Ingenol-3,20-dibenzoate. We further tested the most promising epigenetic inhibitor class, HDACi, for their ability to reduce pro-inflammatory cytokines and reactivate latent HIV-1 ex vivo. We identified nine epigenetic modulators that reduced PKC-induced intracellular IL-6. In cells from aviremic individuals living with HIV-1, the HDAC1–3 inhibitor, suberohydroxamic acid (SBHA), reduced secretion of pro-inflammatory cytokines TNF-α, IL-5, IL-2r, and IL-17 but did not significantly reactivate latent HIV-1 when combined with Ingenol-3,20-dibenzoate. Combining SBHA and Ingenol-3,20-dibenzoate reduces deleterious cytokine production during latency reversal but does not induce significant viral reactivation in aviremic donor PBMCs. The ability of SBHA to reduce PKC-induced pro-inflammatory cytokines when combined with Ingenol-3,20-dibenzoate suggests SBHA can be used to reduced PKC induced pro-inflammatory cytokines but not to achieve latency reversal in the context of HIV-1.
Keywords: HIV-1, Latency Reversing Agent, Ingenol-3, 20-dibenzoate, suberohydroxamic acid, cytokine, HDACi
Graphical Abstract

1. Introduction
Antiretroviral therapy (ART) has improved the lives of individuals living with HIV-1 by durably blocking viral replication, reducing plasma viremia levels below the limit of detection (~20–50 copies/ml) [1–3], and allowing for reconstitution of the adaptive immune system. However, ART does not result in HIV-1 eradication due to the presence of a transcriptionally silent, long-lived latent viral reservoir [4–6]. The presence of this latent reservoir makes ART a lifelong necessity [7]. Additionally, not all patients have access to ART or are compliant with ART regimens due to high costs [8], side effects [9], and social stigma [10, 11]. Aviremic individuals taking ART are prone to low-level chronic immune activation and exhaustion [12], cardiovascular disease [13, 14], non-AIDS related cancers [15], and neurologic complications [16], underscoring the importance of strategies targeting the latent HIV-1 reservoir.
The “shock and kill” strategy [17] proposes that a latency reversing agent (LRA) be used to transcriptionally reactivate the latent reservoir followed by targeting infected cells with immune-mediated mechanisms [17, 18]. Immunological strategies include the use of broadly neutralizing antibodies (bNAbs), cytokines, vaccines, chimeric antigen receptors, and other immune-mediated mechanisms to enhance recognition of HIV-1 infected cells [19, 20]. While all strategies hold merit, enhanced targeting of latently infected cells via immune-mediated mechanisms would still be dependent on unmasking the latent reservoir.
Several mechanistic classes have emerged as potential LRAs including positive transcription elongation factor b (PTEFb) activators [21, 22], cytokines [23], histone deacetylase inhibitors (HDACi) [24–28], and protein kinase C (PKC) agonists [18, 28–30]. Spina et al. [29] demonstrated that PKC agonists are uniquely efficacious LRAs across multiple in vitro models of HIV-1 latency, whereas other well characterized LRAs such as HDACi had functionality only in select in vitro models. PKC agonists are thought to reactivate latent HIV-1 though activation of NF-κB and have been shown to additionally activate AP-1 and NFAT [31]. On the other hand, PKC agonists have undesired effects, including induction of T cell activation [32] and proinflammatory cytokine secretion. We previously demonstrated that latency reversal with the PKC agonist Ingenol-3,20-dibenzoate (IDB), used in combination with a second pharmacological agent, ruxolitinib (an FDA approved Janus Kinase [JAK] inhibitor), led to potent reactivation of latent HIV-1 in the absence of pro-inflammatory cytokine secretion [33]. Additionally, rapamycin, an inhibitor of mTOR, has been shown to reduce pro-inflammatory cytokine secretion induced upon reactivation of latent HIV-1 with CD3 and CD28 antibodies [34] and the BET inhibitor JQ-1 was recently shown to reduce PKC induced IL-8 and TNF-a secretion in PBMCs from cynomolgus macaques [35]. These studies indicate that latency reversal and proinflammatory cytokine secretion can be uncoupled. In the present study we expanded our search for epigenetic modifiers that could be used as an adjuvant therapy to reduce cytokine secretion induced by PKC agonists.
While ruxolitinib and rapamycin have been shown to reduce in vitro cytokine secretion in the context of HIV-1, HDACi have also been observed to inhibit pro-inflammatory cytokine secretion in vivo in the context of graft-versus-host disease [36] and rheumatoid arthritis [37] as well as in vitro, in the context of LPS stimulation of human peripheral blood mononuclear cells (PBMCs) [38]. Additionally, HDACi are well-known HIV-1 LRAs and it has been shown that they can have additive or synergistic activity with PKC agonists when reactivating latent HIV-1 [27, 39, 40]. Therefore, we hypothesized that HDACi and potentially other epigenetic modifiers could decrease PKC-induced pro-inflammatory cytokines secretion and be used as a combination therapy with PKC agonists to reactivate latent HIV-1. In this study, we performed a screen of epigenetic modifiers and report that suberohydroxamic acid (SBHA), an HDAC1- and 3-inhibitor, suppresses PKC-induced pro-inflammatory cytokines but does not significantly reactivate latent HIV-1.
2. Materials and Methods
Participants
Participants living with and without HIV-1 infection were recruited in accordance with active University of Utah Institutional Review Board (IRB) protocols 58246 and 67637 respectively. Participants living with HIV-1 infection were aviremic (plasma viral loads less than 50 HIV-1 RNA copies/mL) for a minimum of 6 months and prescribed an ART regimen initiated during chronic HIV-1 infection for a minimum of 12 months.
In vitro epigenetic inhibitor screening
PBMCs were isolated from healthy donors using a Lymphoprep density gradient (Cat# 07861, StemCell Technologies, USA) prior to being cultured in RPMI medium supplemented with 10% FBS and 5 U/mL penicillin/streptomycin overnight to remove monocytes via adherence. Non-adherent PBMCs were then cultured at a density of 1 × 105 cells/100μL in the presence of 100nM compounds from a Cayman Chemical Epigenetics Screening Library obtained from the University of Utah Drug Discovery Core Facility (Cayman Chemical Co., Cat# 11076, USA) or 100nM ruxolitinib (Cayman Chemical Co., Cat# 11609, USA), a control for cytokine inhibition, for 1.5 h at 37°C. Cells were then incubated in medium alone or the presence of 100nM IDB (Enzo Life Sciences, Cat# BML-PE186–001, USA), for 40 hours post-stimulant exposure. At 40 hours post-stimulant exposure, 0.067 μL/100μL BD GolgiStop™ Protein Transport Inhibitor (BD Biosciences, Cat# 554724, USA) was added to each sample to inhibit cytokine secretion. Cells were then fixed and stained at 48 hours prior to flow cytometry analysis.
Intracellular cytokine staining and flow cytometry
Cells were washed with 1x phosphate buffered saline (PBS) prior to staining the cells with 0.1μL/100 μL Fixable Viability Dye eFluor® 450 (Affymetrix eBioscience, Cat# 65–0863-14, USA) for 30 min at 4°C. Cells were then washed with 1x PBS prior to fixing with 100 μL BD Cytofix/Cytoperm™ Fixation and Permeabilization Solution (BD Biosciences, Cat# 554722, USA) for 30 min at 4°C. Once cells were fixed, they were washed with a perm/wash solution (1x PBS, 3% FBS, 0.1% Saponin, 0.05% Sodium Azide) prior to staining cells in 100 μL perm/wash with 0.5 μL APC anti-human IL-6 antibody (BioLegend®, Cat# 501112, USA) overnight at 4°C. Cells were finally washed in 1x PBS and re-suspended in PBS prior to flow cytometry [BD FACSCanto™ flow cytometer with FACSDiva™ acquisition software (Becton-Dickinson, Mountain View, CA, USA)] and analysis with FlowJo (TreeStar Inc, Ashland, OR, USA).
Selection of compounds
The percentage of IL-6-positive cells in IDB-alone-treated cells was compared to cells treated with epigenetic modifiers and IDB in order to calculate intracellular IL-6 fold-change. The top epigenetic modifiers that reduced intracellular IL-6 by four-fold or greater (‘hits’) were selected for testing in aviremic patient cells to determine whether these compounds would diminish PKC induced pro-inflammatory cytokines.
Ex vivo cell culture, qPCR, and cytokine measurements
Peripheral blood mononuclear cells (PBMCs) (Lymphoprep™, StemCell Technologies, Cat # 07861, USA) and resting CD4+ T cells (rCD4s) (EasySep™ Human Resting CD4+ T Cell Isolation Kit, StemCell Technologies, Cat # 17962, USA) were isolated from HIV-1 aviremic donors living with HIV-1. PBMCs were cultured at a density of 3 × 106/ml in RPMI supplemented with 10% FBS and 5U/mL penicillin/streptomycin. Two epigenetic modifiers (SBHA and panobinostat) that decreased intracellular IL-6 to the highest degree in the in vitro IL-6 screen were added at 75nM or 100nM concentrations in the presence of medium or IDB for 48 hours to evaluate ex vivo cytokine secretion. Cells cultured in medium alone were used as controls. Supernatant was collected and sent to ARUP Laboratories (USA) to measure ex vivo cytokine secretion via a commercially available quantitative multiplex bead assay to measure the following cytokines: interferon gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin (IL) 1 beta (IL-1β), IL-2, soluble IL-2 receptor (IL-2r), IL-4, IL-5, IL-6, IL-8, IL-10, IL-12, and IL-13.
Resting CD4s were cultured at a density of 5 × 106/mL in RPMI supplemented with 10% FBS and 5U/mL penicillin/streptomycin. Epigenetic modifiers (chidamide, apicidin, entinostat, suberohydroxamic acid (SBHA), pyroxamide, panobinostat, WDR5–0103, iniparib and CCG-100602) that reduced intracellular IL-6 were added at 50nM to rCD4s alone or in combination with IDB (45nM) for 48 hours. Medium or DMSO and Dynabead™ Human T-Activator αCD3/αCD28 (αCD3/αCD28) (Gibco™, Cat# 11132D, Ireland) stimulated cells were used as controls. At 48 hours post-stimulation, supernatant was collected and the rapid ex vivo evaluation of anti-latency assay (REVEAL) assay was performed as previously described [28] to quantify viral release.
Statistical Analysis
Significant changes in reactivation or the secretion of pro-inflammatory cytokines in cells treated with epigenetic modifiers in combination with IDB compared to IDB alone was calculated with GraphPad Prism Version 5.0f (GraphPad Software, Inc., San Diego CA, USA). Statistical significance was calculated using a non-parametric Wilcoxon matched-pairs signed rank test.
3. Results
HDACi have been previously described to inhibit cytokine secretion [36–38]. Therefore, we investigated the ability of HDACi and other epigenetic modifiers to reduce pro-inflammatory cytokine secretion upon latency reversal with a PKC agonist. We conducted a screen of 96 epigenetic modifiers (Cayman Chemical Co.) in primary CD4+ T cells to identify those that more efficiently quelled the induction of pro-inflammatory cytokines during latency reversal with ingenol-3,20-dibenzoate (IDB). We used IL-6 as a prototypical cytokine for the screen. Nine epigenetic modifiers were identified which reduced intracellular IL-6 levels by four-fold or greater in the context of IDB stimulation (Figure 1). These ‘hits’ include six HDACi (Chemical Abstracts Service (CAS) # 743420–02-2 (chidamide), CAS # 183506–66-3 (apicidin), CAS # 209783–80-2 (entinostat), CAS # 38937–66-5 (suberohydroxamic acid (SBHA)), CAS # 382180–17-8 (pyroxamide), CAS # 404950–80-7 (panobinostat)), one mixed lineage leukemia (MLL) inhibitor Cas # 890190–22-4 (WDR5–0103), and two compounds categorized as ‘miscellaneous’ as they did not have the same function as other epigenetic modifiers in the library. Out of the two compounds categorized as miscellaneous, compound Cas # 160003–66-7 (iniparib), has an unknown function and compound Cas # 1207113–88-9 (CCG-100602) is an inhibitor of Rho pathway-mediated signaling [41]. The positive control, ruxolitinib, also reduced intracellular IL-6 by greater than fourfold as previously shown [33]. As HDACi accounted for six out of nine ‘hits’, HDACi were selected for further validation ex vivo on cells from aviremic individuals living with HIV. We examined SBHA and panobinostat, two of the top HDACi to reduce intracellular IL-6, for their ability to reduce secretion of a broad panel of pro-inflammatory cytokines ex vivo (Figure 2).
Figure 1. Epigenetic modifiers screening identifying compounds that dampen cytokine production induced by Ingenol-3,20-dibenzoate.
Screening of 96 epigenetic modifiers for compounds that reduced intracellular IL-6 induced by IDB in healthy donor PBMCs (n=1) revealed nine epigenetic modifier ‘hits’ that reduce intracellular IL-6 by fourfold or greater (at or below dotted line) when compared to IDB treatment alone. Ruxolitinib was included as a positive control for reduction of intracellular IL-6 when combined with Ingenol-3,20-dibenzoate. Compounds are listed by CAS number and color coded according to categorized function. All nine ‘hits’ were selected for further testing.
Figure 2. A) SBHA reduces pro-inflammatory cytokine secretion induced by IDB ex vivo.
Solid lines represent the mean change in pro-inflammatory cytokine concentrations in the supernatant of PBMCs isolated from HIV-1 positive aviremic individuals (n = 8) and treated with SBHA (75nM or 100nM) and IDB (100nM) for 72 hours compared to IDB alone. The dotted line represents the baseline for the assay. *P value <0.05; **P value <0.01. B) Panobinostat has no significant effect on IDB induced pro-inflammatory cytokine production ex vivo. PBMCs from aviremic individual was treated with Panobinostat (100nM) and IDB (100nM). The mean change in pro-inflammatory cytokines is indicated by a solid line and dotted lines indicate the baseline for the assay.
Treatment of aviremic patient PBMCs with IDB significantly increased TNF-α (p = 0.0156), IFN-γ (p = 0.0312), IL-1β (p =0.0156), IL-5 (p = 0.0156), IL-8 (p = 0.0156), IL-13 (p = 0.0156), and IL-17 (p = 0.0156) (Figure 2A). The addition of 75nM or 100nM SBHA to 100nM IDB was found to significantly reduce secreted levels of TNF-α (p = 0.0078), IL-5 (p = 0.0156), IL-2r (p = 0.0078), and IL-17 (p = 0.0156) (n = 8) (Figure 2A) ex vivo. For IL-13 statistical significance was not achieved (p = 0.0547) upon treatment of cells with IDB and 75nM SBHA.
However, the addition of SBHA to IDB stimulation did not diminish the induction of IFN-γ and IL-8, which are significantly induced by IDB treatment. The effect of SBHA on IDB-induced IL-6 and IL-1β levels appeared inconsistent between donors ex vivo, with donors clustered into two distinct populations. Three donors (H027, H052, and H058) appeared to cluster above the mean IL-6 level when donor PBMCs were treated with IDB and SBHA at 75nM or 100nM. In the remaining 5 donors (H037, H055, H063, H065 and H068), IL-6 appeared to decrease to near the limit of detection, though this decrease was not statistically significant for the overall population (n = 8) when treated with either 75nM (p =0.0625) or 100nM (p = 0.1250) SBHA. Secreted IL-2, IL-4, IL-10, and IL-12 levels remaining unchanged upon treatment with IDB and the combination therapy. The addition of 100nM panobinostat to 100nM IDB did not significantly reduce secreted levels of any cytokines measured (Figure 2B; TNF-α (p = 0.2500), IFN-γ (p = 0.5000), IL-1β (p = 0.2500), IL-2 (p > 0.9999), IL-2r (p = 0.2500), IL-4 (p > 0.9999), IL-5 (p = 0.5000), IL-6 (0.5000), IL-8 (p > 0.9999), IL-10 (0.5000), IL-12 (no difference), IL-13 (p = 0.2500)) (Figure 3B). Secreted IL-17 was not measured.
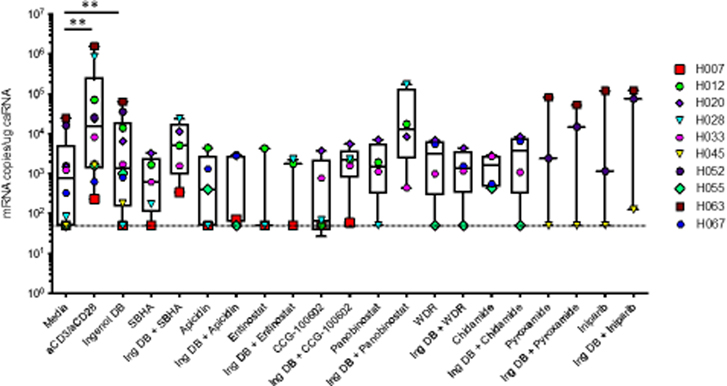
Figure 3. HIV-1 latency reversal induced by epigenetic modifiers alone or in combination with Ingenol-3,20-dibenzoate ex vivo.
Treatment of rCD4 cells isolated from HIV-1 positive aviremic individuals with Ingenol-3,20-dibenzoate significantly increased viral latency reactivation compared to media alone. The positive control αCD3/αCD28 resulted in viral reactivation in all donors. All nine epigenetic modifiers alone or in combination with Ingenol3,20-dibenzoate did not increase reactivation significantly compared to Ingenol-3,20-dibenzoate treatment alone. *P value <0.05; ** P value <0.01. The dotted line indicates the limit of detection of the assay (50 copies/ug cell associated RNA (caRNA)).
We then examined the ability of SBHA and panobinostat to reactivate latent HIV-1 when used in combination with IDB ex vivo in aviremic patient CD4+ T cells. While treatment with IDB or αCD3/αCD28 significantly induced reactivation of the latent reservoir (mean = 12,406 mRNA copies/mL, p value = 0.0078 and mean = 254,182 mRNA copies/mL, p value = 0.0020 respectively, n=10) when compared with media alone, treatment with IDB in combination with SBHA (p = 0.0625) or panobinostat (p = 0.2500) did not significantly reactivate latent HIV-1 (Figure 3). Upon testing the remaining epigenetic modifier ‘hits’ identified in our in vitro cytokine screen, none of the remaining epigenetic modifiers significantly reactivated latent HIV in patient samples when combined with IDB (apicidin (p = 0.1250), entinostat (p = 0.5000), CCG-100602 (p = 0.0625), WDR5–0103 (p = 0.5000), chidamide (p = 0.5000), pyroxamide (p = >0.9999), iniparib (p = 0.2500)) compared to media alone. Additionally, all nine epigenetic modifiers when used individually failed to reactivate latent HIV-1 to significant levels (Figure 3).
4. Discussion
In order to eliminate the latent HIV-1 reservoir, LRA therapies must be evaluated based on both their potency and deleterious side effects. PKC agonists are among the most potent LRAs that have emerged across multiple models of HIV-1 latency [29, 30]. However, PKC agonists are also known to induce T cell activation [32] and secretion of pro-inflammatory cytokines [33, 42]. Previous reports have demonstrated that LRAs can be combined with a second pharmacological agent to suppress deleterious pro-inflammatory cytokine secretion induced upon treatment with PKC agonists [33] and TCR stimulation [34] without reducing HIV-1 viral reactivation. While HDAC inhibitors such as suberanilohydroxamic acid (SAHA) [36, 38], Trichostatin A [37], and Nicotinamide [37] have been shown to reduce proinflammatory cytokine secretion in other disease contexts [36, 37], here we examined the ability of HDACi and other epigenetic modifiers to do so in the context of HIV-1.
We found that SBHA, an HDAC1–3 inhibitor, significantly reduced Ingenol-3,20-dibenzoate (IDB) induced secreted TNF-α, IL2-r, IL-5, and IL-17 in PBMCs from aviremic individuals living with HIV-1 ex vivo. While SBHA has been previously described to induce cell cycle arrest [43], apoptosis [43–45], cell differentiation [46], and has been shown to decrease Herpes simplex virus type 1 viral genome expression in vitro [47], SBHA has not been described to inhibit the production of pro-inflammatory cytokines. However, HDAC1–3 inhibition is a known mechanism of inhibiting the production of pro-inflammatory cytokines [48–51]. HDAC1- and HDAC3-independent knock-down have been previously shown to reduced LPS-induced IL-6 and TNF-α in murine microglia [48] and IL-1 pro-inflammatory cytokine induction in HEK293IL-1R and mouse embryonic fibroblasts [51]. Additionally, other HDAC1–3 inhibitors such as entinostat have been shown to reduce the production of pro-inflammatory cytokines IL1-β and IL-6 in a rat collagen-induced arthritis model [49] and inflammatory gene mRNA levels for IL-1β, IL-17, and IFN-γ in an experimental autoimmune neuritis rat model [50]. In this study we showed that entinostat reduced intracellular IL-6 production but to a lower degree than SBHA, panobinostat, and pyroxamide and was therefore not studied further. However, it does strengthen the case that HDAC1–3 inhibition is a candidate pathway for inhibiting pro-inflammatory cytokines induced by PKC stimulation during HIV-1 latency reversal, as we demonstrated by combining known HDAC1–3 inhibitor SBHA with IDB to reduce the production of PKC-induced TNF-α, IL-17, IL-2r, and IL-5 ex vivo. While SBHA reduced PKC-induced pro-inflammatory cytokines, panobinostat, a pan-HDAC inhibitor, did not significantly reduce pro-inflammatory cytokines ex vivo. Panobinostat has been previously shown to significantly reduce IL-6 in a Hodgkin’s Lymphoma phase II clinical trial [52] and IFN-γ secretion in a lymphocyte-target cell killing assay [53]. Other pan-HDACi such as TSA have been reported to reduce IL-6 [37] and TNF-α [37, 54] secretion in LPS-stimulated macrophages, although conflicting studies have shown that TSA has no effect on other pro-inflammatory genes such as IL-1β and IL-10 [54]. It is unclear why panobinostat did not reduce PKC-induced pro-inflammatory cytokine production ex vivo.
In addition to reducing PKC-induced pro-inflammatory cytokines, we aimed to identify an epigenetic modifier which could be used in combination with IDB without reducing latency reversal. HDACi such as SAHA [24, 55, 56] and panobinostat [57] have been previously shown to reactivate latent HIV-1in clinical trials. However, in vitro primary cell models of HIV-1 latency and ex vivo aviremic patient cell studies have produced conflicting results as to the ability of panobinostat and SAHA to reactivate latent HIV-1 [28, 58–60]. In this study, panobinostat did not significant reactivate latent HIV-1 in our ex vivo model, as has been seen previously [58]. SAHA was not evaluated as a LRA in this study as it did not significantly reduce PKC-induced intracellular IL-6 in our in vitro screen. While we identified that SBHA significantly reduces IDB induced pro-inflammatory cytokines TNF-α, IL-17, IL-2r, and IL-5 in this study, the combination prevented significant latency reversal, suggesting that combining SBHA with IDB would not be an efficient strategy to reactivate latent HIV-1. This raises the question if HIV-1 reactivation can be uncoupled from pro-inflammatory cytokine induction.
Combining LRAs with a second pharmacological agent has been previously examined as a strategy to reduce pro-inflammatory cytokine induction during HIV-1 latency reversal. We and others have shown that drug classes, including a Janus Kinase inhibitor [33], mTOR inhibitor [34], and BET inhibitor [35], can be combined with latency reversal agents to successfully reduce pro-inflammatory cytokine induction during HIV-1 reactivation ex vivo. Though we tested three BET inhibitors in our epigenetic inhibitor screen, including JQ-1, we did not see a significant reduction in intracellular IL-6, as was seen previously [35], and did not evaluate these compounds further. While this study did not identify a successful combination therapy, the success of these previous studies indicates that potent LRAs, such as PKC agonists, that can induce high levels of pro-inflammatory cytokines during HIV-1 latency reversal, may be suitable for the clinic when combined with a second pharmacological agent. However, this strategy has yet to be evaluated in the clinic.
Acknowledgements
The authors express their sincere gratitude to the study participants for their continued participation in this work and ongoing translational research. Additional thanks to Chelsea McCarty Allen, PhD, for her statistical analysis consultation and recommendations. This work was supported in part by the National Institute of Allergy and Infectious Diseases (NIAID) grants AI122377-01 and AI143567-02 (VP), NIAID Ruth L. Kirschstein National Research Service Award T32AI055434-11 (ETL), NIAID grant R21AI124823-02 (LRB), and the Doris Duke Charitable Foundation Clinical Scientist Development Award CSDA201612 (AMS). The research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR002538. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Competing interests
The authors declare no competing interests.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics approval
Heathy donors and HIV-1 positive aviremic individuals on ART were recruited for phlebotomy according to Institutional Review Board (IRB) protocols 67637 (approved May 31, 2017) and 58246 (approved Jan 4, 2017) at the University of Utah.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, Richman DD, Valentine FT, Jonas L, Meibohm A, Emini EA, Chodakewitz JA. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337(11):734–9. [DOI] [PubMed] [Google Scholar]
- 2.Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, Eron JJ Jr., Feinberg JE, Balfour HH Jr., Deyton LR, Chodakewitz JA, Fischl MA. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337(11):725–33. [DOI] [PubMed] [Google Scholar]
- 3.Perelson AS, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho DD. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature. 1997;387(6629):188–91. [DOI] [PubMed] [Google Scholar]
- 4.Chun TW, Stuyver L, Mizell SB, Ehler LA, Mican JA, Baseler M, Lloyd AL, Nowak MA, Fauci AS. Presence of an inducible HIV-1 latent reservoir during highly active antiretroviral therapy. Proceedings of the National Academy of Sciences. 1997;94(24):13193–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finzi D, Hermankova M, Pierson T, Carruth LM, Buck C, Chaisson RE, Quinn TC, Chadwick K, Margolick J, Brookmeyer R, Gallant J, Markowitz M, Ho DD, Richman DD, Siliciano RF. Identification of a reservoir for HIV-1 in patients on highly active antiretroviral therapy. Science (New York, NY). 1997;278(5341):1295–300. [DOI] [PubMed] [Google Scholar]
- 6.Wong JK, Hezareh M, Günthard HF, Havlir DV, Ignacio CC, Spina CA, Richman DD. Recovery of replication-competent HIV despite prolonged suppression of plasma viremia. Science (New York, NY). 1997;278(5341):1291–5. [DOI] [PubMed] [Google Scholar]
- 7.Torbett BE, Goodsell DS, Richman DD, editors. The Future of HIV-1 Therapeutics-Resistance is Futile. Cham: Springer International Publishing; 2015. [Google Scholar]
- 8.Long L, Fox M, Sanne I, Rosen S. The high cost of second-line antiretroviral therapy for HIV/AIDS in South Africa. AIDS. 2010;24(6):915–9. [DOI] [PubMed] [Google Scholar]
- 9.Saberi P, Neilands TB, Vittinghoff E, Johnson MO, Chesney M, Cohn SE. Barriers to antiretroviral therapy adherence and plasma HIV RNA suppression among AIDS clinical trials group study participants. AIDS Patient Care STDS. 2015;29(3):111–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rintamaki LS, Davis TC, Skripkauskas S, Bennett CL, Wolf MS. Social stigma concerns and HIV medication adherence. AIDS Patient Care STDS. 2006;20(5):359–68. [DOI] [PubMed] [Google Scholar]
- 11.Katz IT, Ryu AE, Onuegbu AG, Psaros C, Weiser SD, Bangsberg DR, Tsai AC. Impact of HIV-related stigma on treatment adherence: systematic review and meta-synthesis. J Int AIDS Soc. 2013;16(3 Suppl 2):18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.International ASSWGoHIVC, Deeks SG, Autran B, Berkhout B, Benkirane M, Cairns S, Chomont N, Chun TW, Churchill M, Di Mascio M, Katlama C, Lafeuillade A, Landay A, Lederman M, Lewin SR, Maldarelli F, Margolis D, Markowitz M, Martinez-Picado J, Mullins JI, Mellors J, Moreno S, O’Doherty U, Palmer S, Penicaud MC, Peterlin M, Poli G, Routy JP, Rouzioux C, Silvestri G, Stevenson M, Telenti A, Van Lint C, Verdin E, Woolfrey A, Zaia J, Barre-Sinoussi F. Towards an HIV cure: a global scientific strategy. Nat Rev Immunol. 2012;12(8):607–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Borges AH, Dubrow R, Silverberg MJ. Factors contributing to risk for cancer among HIV-infected individuals, and evidence that earlier combination antiretroviral therapy will alter this risk. Curr Opin HIV AIDS. 2014;9(1):34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.High KP, Brennan-Ing M, Clifford DB, Cohen MH, Currier J, Deeks SG, Deren S, Effros RB, Gebo K, Goronzy JJ, Justice AC, Landay A, Levin J, Miotti PG, Munk RJ, Nass H, Rinaldo CR Jr., Shlipak MG, Tracy R, Valcour V, Vance DE, Walston JD, Volberding P, HIV OARWGo, Aging. HIV and aging: state of knowledge and areas of critical need for research. A report to the NIH Office of AIDS Research by the HIV and Aging Working Group. J Acquir Immune Defic Syndr. 2012;60 Suppl 1:S1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gopal S, Achenbach CJ, Yanik EL, Dittmer DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elbirt D, Mahlab-Guri K, Bezalel-Rosenberg S, Gill H, Attali M, Asher I. HIV-associated neurocognitive disorders (HAND). Isr Med Assoc J. 2015;17(1):54–9. [PubMed] [Google Scholar]
- 17.Deeks SG. HIV: Shock and kill. Nature. 2012;487(7408):439–40. [DOI] [PubMed] [Google Scholar]
- 18.Spivak AM, Planelles V. HIV-1 Eradication: Early Trials (and Tribulations). Trends Mol Med. 2016;22(1):10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Burton DR, Hangartner L. Broadly Neutralizing Antibodies to HIV and Their Role in Vaccine Design. Annu Rev Immunol. 2016;34:635–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandergeeten C, Fromentin R, Chomont N. The role of cytokines in the establishment, persistence and eradication of the HIV reservoir. Cytokine Growth Factor Rev. 2012;23(4–5):143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartholomeeusen K, Xiang Y, Fujinaga K, Peterlin BM. Bromodomain and extra-terminal (BET) bromodomain inhibition activate transcription via transient release of positive transcription elongation factor b (P-TEFb) from 7SK small nuclear ribonucleoprotein. J Biol Chem. 2012;287(43):36609–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peng W, Hong Z, Chen X, Gao H, Dai Z, Zhao J, Liu W, Li D, Deng K. Thiostrepton Reactivates Latent HIV-1 through the p-TEFb and NF-kappaB Pathways Mediated by Heat Shock Response. Antimicrob Agents Chemother. 2020;64(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chun TW, Engel D, Mizell SB, Ehler LA, Fauci AS. Induction of HIV-1 replication in latently infected CD4+ T cells using a combination of cytokines. J Exp Med. 1998;188(1):83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Archin NM, Kirchherr JL, Sung JA, Clutton G, Sholtis K, Xu Y, Allard B, Stuelke E, Kashuba AD, Kuruc JD, Eron J, Gay CL, Goonetilleke N, Margolis DM. Interval dosing with the HDAC inhibitor vorinostat effectively reverses HIV latency. J Clin Invest. 2017;127(8):3126–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu G, Swanson M, Talla A, Graham D, Strizki J, Gorman D, Barnard RJ, Blair W, Sogaard OS, Tolstrup M, Ostergaard L, Rasmussen TA, Sekaly RP, Archin NM, Margolis DM, Hazuda DJ, Howell BJ. HDAC inhibition induces HIV-1 protein and enables immune-based clearance following latency reversal. JCI Insight. 2017;2(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Divsalar DN, Simoben CV, Schonhofer C, Richard K, Sippl W, Ntie-Kang F, Tietjen I. Novel Histone Deacetylase Inhibitors and HIV-1 Latency-Reversing Agents Identified by LargeScale Virtual Screening. Front Pharmacol. 2020;11:905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laird GM, Bullen CK, Rosenbloom DI, Martin AR, Hill AL, Durand CM, Siliciano JD, Siliciano RF. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest. 2015;125(5):1901–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spivak AM, Bosque A, Balch AH, Smyth D, Martins L, Planelles V. Ex Vivo Bioactivity and HIV-1 Latency Reversal by Ingenol Dibenzoate and Panobinostat in Resting CD4(+) T Cells from Aviremic Patients. Antimicrob Agents Chemother. 2015;59(10):5984–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spina CA, Anderson J, Archin NM, Bosque A, Chan J, Famiglietti M, Greene WC, Kashuba A, Lewin SR, Margolis DM, Mau M, Ruelas D, Saleh S, Shirakawa K, Siliciano RF, Singhania A, Soto PC, Terry VH, Verdin E, Woelk C, Wooden S, Xing S, Planelles V. An indepth comparison of latent HIV-1 reactivation in multiple cell model systems and resting CD4+T cells from aviremic patients. PLoS Pathog. 2013;9(12):e1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darcis G, Kula A, Bouchat S, Fujinaga K, Corazza F, Ait-Ammar A, Delacourt N, Melard A, Kabeya K, Vanhulle C, Van Driessche B, Gatot JS, Cherrier T, Pianowski LF, Gama L, Schwartz C, Vila J, Burny A, Clumeck N, Moutschen M, De Wit S, Peterlin BM, Rouzioux C, Rohr O, Van Lint C. An In-Depth Comparison of Latency-Reversing Agent Combinations in Various In Vitro and Ex Vivo HIV-1 Latency Models Identified Bryostatin-1+JQ1 and Ingenol-B+JQ1 to Potently Reactivate Viral Gene Expression. PLoS Pathog. 2015;11(7):e1005063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Genot EM, Parker PJ, Cantrell DA. Analysis of the role of protein kinase C-alpha, - epsilon, and -zeta in T cell activation. J Biol Chem. 1995;270(17):9833–9. [DOI] [PubMed] [Google Scholar]
- 32.Korin YD, Brooks DG, Brown S, Korotzer A, Zack JA. Effects of prostratin on T-cell activation and human immunodeficiency virus latency. J Virol. 2002;76(16):8118–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spivak AM, Larragoite ET, Coletti ML, Macedo AB, Martins LJ, Bosque A, Planelles V. Janus kinase inhibition suppresses PKC-induced cytokine release without affecting HIV-1 latency reversal ex vivo. Retrovirology. 2016;13(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin AR, Pollack RA, Capoferri A, Ambinder RF, Durand CM, Siliciano RF. Rapamycin-mediated mTOR inhibition uncouples HIV-1 latency reversal from cytokineassociated toxicity. J Clin Invest. 2017;127(2):651–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Washizaki A, Murata M, Seki Y, Kikumori M, Tang Y, Tan W, Wardani NP, Irie K, Akari H. The Novel PKC Activator 10-Methyl-Aplog-1 Combined with JQ1 Induced Strong and Synergistic HIV Reactivation with Tolerable Global T Cell Activation. Viruses. 2021;13(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reddy P, Maeda Y, Hotary K, Liu C, Reznikov LL, Dinarello CA, Ferrara JL. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc Natl Acad Sci U S A. 2004;101(11):3921–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grabiec AM, Krausz S, de Jager W, Burakowski T, Groot D, Sanders ME, Prakken BJ, Maslinski W, Eldering E, Tak PP, Reedquist KA. Histone deacetylase inhibitors suppress inflammatory activation of rheumatoid arthritis patient synovial macrophages and tissue. J Immunol. 2010;184(5):2718–28. [DOI] [PubMed] [Google Scholar]
- 38.Leoni F, Zaliani A, Bertolini G, Porro G, Pagani P, Pozzi P, Dona G, Fossati G, Sozzani S, Azam T, Bufler P, Fantuzzi G, Goncharov I, Kim SH, Pomerantz BJ, Reznikov LL, Siegmund B, Dinarello CA, Mascagni P. The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc Natl Acad Sci U S A. 2002;99(5):2995–3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez M, de Vinuesa AG, Sanchez-Duffhues G, Marquez N, Bellido ML, MunozFernandez MA, Moreno S, Castor TP, Calzado MA, Munoz E. Bryostatin-1 synergizes with histone deacetylase inhibitors to reactivate HIV-1 from latency. Curr HIV Res. 2010;8(6):418–29. [DOI] [PubMed] [Google Scholar]
- 40.Lu HK, Gray LR, Wightman F, Ellenberg P, Khoury G, Cheng WJ, Mota TM, Wesselingh S, Gorry PR, Cameron PU, Churchill MJ, Lewin SR. Ex vivo response to histone deacetylase (HDAC) inhibitors of the HIV long terminal repeat (LTR) derived from HIV-infected patients on antiretroviral therapy. PLoS One. 2014;9(11):e113341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evelyn CR, Wade SM, Wang Q, Wu M, Iniguez-Lluhi JA, Merajver SD, Neubig RR. CCG-1423: a small-molecule inhibitor of RhoA transcriptional signaling. Mol Cancer Ther. 2007;6(8):2249–60. [DOI] [PubMed] [Google Scholar]
- 42.Kulkosky J, Culnan DM, Roman J, Dornadula G, Schnell M, Boyd MR, Pomerantz RJ. Prostratin: activation of latent HIV-1 expression suggests a potential inductive adjuvant therapy for HAART. Blood. 2001;98(10):3006–15. [DOI] [PubMed] [Google Scholar]
- 43.You BR, Park WH. Suberoyl bishydroxamic acid inhibits the growth of A549 lung cancer cells via caspase-dependent apoptosis. Mol Cell Biochem. 2010;344(1–2):203–10. [DOI] [PubMed] [Google Scholar]
- 44.Ning L, Greenblatt DY, Kunnimalaiyaan M, Chen H. Suberoyl bis-hydroxamic acid activates Notch-1 signaling and induces apoptosis in medullary thyroid carcinoma cells. Oncologist. 2008;13(2):98–104. [DOI] [PubMed] [Google Scholar]
- 45.Zhang XD, Gillespie SK, Borrow JM, Hersey P. The histone deacetylase inhibitor suberic bishydroxamate regulates the expression of multiple apoptotic mediators and induces mitochondria-dependent apoptosis of melanoma cells. Mol Cancer Ther. 2004;3(4):425–35. [PubMed] [Google Scholar]
- 46.Breslow R, Jursic B, Yan ZF, Friedman E, Leng L, Ngo L, Rifkind RA, Marks PA. Potent cytodifferentiating agents related to hexamethylenebisacetamide. Proc Natl Acad Sci U S A. 1991;88(13):5542–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shapira L, Ralph M, Tomer E, Cohen S, Kobiler O. Histone Deacetylase Inhibitors Reduce the Number of Herpes Simplex Virus-1 Genomes Initiating Expression in Individual Cells. Front Microbiol. 2016;7:1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Durham BS, Grigg R, Wood IC. Inhibition of histone deacetylase 1 or 2 reduces induced cytokine expression in microglia through a protein synthesis independent mechanism. J Neurochem. 2017;143(2):214–24. [DOI] [PubMed] [Google Scholar]
- 49.Lin HS, Hu CY, Chan HY, Liew YY, Huang HP, Lepescheux L, Bastianelli E, Baron R, Rawadi G, Clement-Lacroix P. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br J Pharmacol. 2007;150(7):862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang ZY, Zhang Z, Schluesener HJ. MS-275, an histone deacetylase inhibitor, reduces the inflammatory reaction in rat experimental autoimmune neuritis. Neuroscience. 2010;169(1):370–7. [DOI] [PubMed] [Google Scholar]
- 51.Ziesche E, Kettner-Buhrow D, Weber A, Wittwer T, Jurida L, Soelch J, Muller H, Newel D, Kronich P, Schneider H, Dittrich-Breiholz O, Bhaskara S, Hiebert SW, Hottiger MO, Li H, Burstein E, Schmitz ML, Kracht M. The coactivator role of histone deacetylase 3 in IL-1-signaling involves deacetylation of p65 NF-kappaB. Nucleic Acids Res. 2013;41(1):90–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oki Y, Buglio D, Zhang J, Ying Y, Zhou S, Sureda A, Ben-Yehuda D, Zinzani PL, Prince HM, Harrison SJ, Kirschbaum M, Johnston PB, Shen A, von Tresckow B, Younes A. Immune regulatory effects of panobinostat in patients with Hodgkin lymphoma through modulation of serum cytokine levels and T-cell PD1 expression. Blood Cancer J. 2014;4:e236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Klein JM, Henke A, Sauer M, Bessler M, Reiners KS, Engert A, Hansen HP, von Strandmann EP. The histone deacetylase inhibitor LBH589 (panobinostat) modulates the crosstalk of lymphocytes with Hodgkin lymphoma cell lines. PLoS One. 2013;8(11):e79502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Weiss U, Moller M, Husseini SA, Manderscheid C, Hausler J, Geisslinger G, Niederberger E. Inhibition of HDAC Enzymes Contributes to Differential Expression of ProInflammatory Proteins in the TLR-4 Signaling Cascade. Int J Mol Sci. 2020;21(23). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Archin NM, Liberty AL, Kashuba AD, Choudhary SK, Kuruc JD, Crooks AM, Parker DC, Anderson EM, Kearney MF, Strain MC, Richman DD, Hudgens MG, Bosch RJ, Coffin JM, Eron JJ, Hazuda DJ, Margolis DM. Administration of vorinostat disrupts HIV-1 latency in patients on antiretroviral therapy. Nature. 2012;487(7408):482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Elliott JH, Wightman F, Solomon A, Ghneim K, Ahlers J, Cameron MJ, Smith MZ, Spelman T, McMahon J, Velayudham P, Brown G, Roney J, Watson J, Prince MH, Hoy JF, Chomont N, Fromentin R, Procopio FA, Zeidan J, Palmer S, Odevall L, Johnstone RW, Martin BP, Sinclair E, Deeks SG, Hazuda DJ, Cameron PU, Sekaly RP, Lewin SR. Activation of HIV transcription with short-course vorinostat in HIV-infected patients on suppressive antiretroviral therapy. PLoS Pathog. 2014;10(10):e1004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rasmussen TA, Tolstrup M, Brinkmann CR, Olesen R, Erikstrup C, Solomon A, Winckelmann A, Palmer S, Dinarello C, Buzon M, Lichterfeld M, Lewin SR, Ostergaard L, Sogaard OS. Panobinostat, a histone deacetylase inhibitor, for latent-virus reactivation in HIV-infected patients on suppressive antiretroviral therapy: a phase 1/2, single group, clinical trial. Lancet HIV. 2014;1(1):e13–21. [DOI] [PubMed] [Google Scholar]
- 58.Bullen CK, Laird GM, Durand CM, Siliciano JD, Siliciano RF. New ex vivo approaches distinguish effective and ineffective single agents for reversing HIV-1 latency in vivo. Nat Med. 2014;20(4):425–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tsai P, Wu G, Baker CE, Thayer WO, Spagnuolo RA, Sanchez R, Barrett S, Howell B, Margolis D, Hazuda DJ, Archin NM, Garcia JV. In vivo analysis of the effect of panobinostat on cell-associated HIV RNA and DNA levels and latent HIV infection. Retrovirology. 2016;13(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wei DG, Chiang V, Fyne E, Balakrishnan M, Barnes T, Graupe M, Hesselgesser J, Irrinki A, Murry JP, Stepan G, Stray KM, Tsai A, Yu H, Spindler J, Kearney M, Spina CA, McMahon D, Lalezari J, Sloan D, Mellors J, Geleziunas R, Cihlar T. Histone deacetylase inhibitor romidepsin induces HIV expression in CD4 T cells from patients on suppressive antiretroviral therapy at concentrations achieved by clinical dosing. PLoS Pathog. 2014;10(4):e1004071. [DOI] [PMC free article] [PubMed] [Google Scholar]