Abstract
Introduction: India experienced 2 waves of COVID-19 pandemic caused by SARS-CoV-2 and reported the second highest caseload globally. Seroepidemiologic studies were done to track the course of the pandemic. We systematically reviewed and synthesized the seroprevalence of SARS-CoV-2 in the Indian population.
Methods: We included studies reporting seroprevalence of IgG antibodies against SARS-CoV-2 from March 1, 2020 to August 11, 2021 and excluded studies done only among patients with COVID-19 and vaccinated individuals. We searched published databases, preprint servers, and government documents using a combination of keywords and medical subheading (MeSH) terms of “Seroprevalence AND SARS-CoV-2 AND India”. We assessed risk of bias using the Newcastle-Ottawa scale, the appraisal tool for cross-sectional studies (AXIS), the Joanna Briggs Institute (JBI) critical appraisal tool, and WHO's statement on the Reporting of Seroepidemiological Studies for SARS-CoV-2 (ROSES-S). We calculated pooled seroprevalence along with 95% Confidence Intervals (CI) during the first (March 2020 to February 2021) and second wave (March to August 2021). We also estimated seroprevalence by selected demographic characteristics.
Results: We identified 3821 studies and included 53 studies with 905379 participants after excluding duplicates, screening of titles and abstracts and full-text screening. Of the 53, 20 studies were of good quality. Some of the reviewed studies did not report adequate information on study methods (sampling = 24% (13/53); laboratory = 83% [44/53]). Studies of ‘poor’ quality had more than one of the following issues: unjustified sample size, nonrepresentative sample, nonclassification of nonrespondents, results unadjusted for demographics and methods insufficiently explained to enable replication. Overall pooled seroprevalence was 20.7% in the first (95% CI = 16.1 to 25.3) and 69.2% (95% CI = 64.5 to 73.8) in the second wave. Seroprevalence did not differ by age in first wave, whereas in the second, it increased with age. Seroprevalence was slightly higher among women in the second wave. In both the waves, the estimate was higher in urban than in rural areas.
Conclusion: Seroprevalence increased by 3-fold between the 2 waves of the pandemic in India. Our review highlights the need for designing and reporting studies using standard protocols.
Keywords: Seroprevalence, Systematic review, Meta-analysis, COVID-19, SARS-CoV-2
Introduction
COVID-19 caused by SARS-CoV-2 virus has spread rapidly across the world since December 2019. The pandemic has overwhelmed the health systems of developed and developing nations alike (Chowdhury and Jomo, 2020, Clapham H. et al., 2020). Countries lacked the required ability to test, trace, treat, and isolate/quarantine the infected population. It is well established that true community burden would remain higher than the reported caseload owing to various reasons like asymptomatic infections, the differences in testing strategies by time and place, variable sensitivities of laboratory tests used for diagnosis, and other factors influencing the health-seeking behaviour of the population (M. V. Murhekar & Clapham, 2021). Hence, estimating the actual number of people previously infected with SARS-CoV-2 aids in understanding the extent of spread and burden. This, in turn, could guide public health strategies for controlling transmission and that of future policies (Lai et al., 2020a).
In this context, seroprevalence studies help estimate the proportion of the population that has evidence of SARS-CoV-2 specific antibodies in their blood during a given time period (Clapham et al., 2020), both owing to the infection and vaccination. WHO developed a standardized protocol for seroprevalence studies on COVID-19 (WHO, 2020) for systematic collection and rapid sharing of exposure data in a format that can be easily aggregated, tabulated, and analyzed across many different settings as well as to inform public health responses and policy decisions. As such, many SARS-CoV-2 seroprevalence studies have been carried out around the world (Alserehi et al., 2021, Poustchi et al., 2021, Venugopal et al., 2021, WHO, 2021). Although the results have varied considerably between studies and locations, they have consistently indicated that the true burden of previously infected is considerably higher than that of the reported number of confirmed cases (Xinhua et al., 2021).
Influenced by local epidemiologic factors, COVID-19 manifested as several local epidemics of different stages at any point in time. In the Indian context, the initial nationwide lockdown from March to April 2020 seemingly delayed the spread of infection. Subsequent to relaxations of lockdown, the incidence increased from April 2020 and peaked in September 2020 (MoHFW, 2021). Thereafter, the number of cases reported per day kept declining until mid-February 2021. In the meantime, COVID-19 vaccinations became available in India from January 2021 onwards, and the second wave of the COVID-19 pandemic in India during March to July 2021 was driven predominantly by the Delta variant of SARS-CoV-2 (INSACOG bulletin, 2021) because of its higher transmissibility (Cent Dis Control Prev, 2020).
In India, 3 nationwide seroprevalence studies had been conducted apart from a number of state, regional, and setting-specific studies (Murhekar et al., 2020; Murhekar et al., 2021a, Murhekar et al., 2021b). These were mostly commissioned by the public health authorities to guide the response. Though there is sufficient primary evidence, there are considerable variations in estimates. Hence, synthesized or pooled information may be useful across India, not just limited to cities or states, for guiding public health decisions. Further, interpretation of such estimates from seroprevalence studies should account for the limitations and uncertainties in the design, conduct, and analysis of these studies. If not considered, any flawed understanding could result in flawed decisions towards controlling the spread. Meta-research of such seroprevalence studies by systematically identifying methodologic lacunae and limitations in each of the primary studies could be a way forward in this regard.
Also, systematic reviews of global COVID-19 seroprevalence documented considerable heterogeneity of the studies and hence, an India-specific systematic review is warranted (Bobrovitz et al., 2021, Xinhua et al., 2021). Further, global dashboard of seroprevalence studies, SeroTracker, provides an automated country-wise pooled estimate, has duplicate entries of the same study from multiple sources, has unverified sources, and quality of data for many studies (SeroTracker 2020). We conducted a systematic review to summarize the seroprevalence of SARS-CoV-2 and quantitatively estimate the pooled seroprevalence of SARS-CoV-2 in the Indian population.
Methods
This study was registered with PROSPERO, CRD42021254997, and reported in compliance with Preferred Reporting Items for Systematic review and Meta-analyses Protocol (PRISMA-P) checklist (Page et al., 2021).
Location of studies: We included studies on seroprevalence of SARS-CoV-2 in humans conducted in India.
Repositories: We searched MEDLINE (PubMed), Embase, SCOPUS, Web of Science core collection, and 2 preprint servers (medRxiv and bioMxiv) (BioRxiv Org 2021; MedRxiv.Org, 2021) In addition to the repositories mentioned, government documents were also searched for in respective state government websites. When needed, subject experts were contacted to ensure the inclusion of potentially relevant studies that might have been missed while searching above mentioned repositories.
Period of searching: We searched the repositories for studies published between March 1, 2020, and August 10, 2021. The last search was performed on August 11, 2021.
Search terms and strategies: The search was done using a combination of keywords and MeSH terms for “Seroprevalence AND SARS-CoV-2 AND India” (Supplementary Table 1). These combinations of terms were used for searching studies in each of the repositories.
Study selection: All English-language observational studies, including cross-sectional, case-control, and cohort studies published between March 1, 2020 and August 11, 2021, were screened for title and abstract by 2 independent reviewers with the following inclusion and exclusion criteria: We included seroprevalence studies that reported the seroprevalence of SARS-CoV-2 Ig-G antibodies among the general population in India or in a specific well-defined population in India: healthcare workers, contacts of COVID-19 patients, and other well-defined cohorts, among all age groups. We excluded studies that reported seroprevalence exclusively among COVID-19 positive participants, participants vaccinated for COVID-19, and animals. Further, we did not include abstracts of congress meetings or conference proceedings, study protocols, commentaries, reviews, and case reports.
Screening and selection of studies: We used Rayyan software (Rayyan – Intelligent Systematic Review, 2021) for screening, deduplication, and selection of studies for the review. Two independent reviewers (N.J. and A.B.) screened titles and abstracts independently to include studies that met the eligibility criteria. Similarly, full texts of the screened studies were assessed in detail. A third reviewer (M.S.K.) resolved any conflicts between the 2 independent assessors. When full-text documents of studies were not available on any internet-based sources, we contacted the corresponding authors of the respective studies.
Data extraction: We extracted the following data from eligible studies: the author's name, publication date, study design, study period, sampling period, study population, study setting (rural or urban), demographics of study participants (age, gender, and occupation), frequency and type of exposures (travel history, contact history, and comorbidities), laboratory methodology for serologic confirmation of SARS-CoV-2, and predefined outcomes (i.e., the total number of participants, the number of participants provided single or paired sera, and the number of seropositive participants). From eligible studies, we extracted the data on the number of participants who provided specimens and the number of seropositive participants to calculate the pooled seroprevalence. From serial cross-sectional studies, we calculated the sum of the total number of participants who provided specimens and the total number of seropositive participants during the whole study period. For cohort studies (with paired serum), we extracted the data only from the first blood collection. Although many studies did adjust for various factors, we decided to use the unadjusted estimates in our analyses for ease of interpretation across different studies.
Risk of bias assessment: We assessed the risk of bias using multiple scales as we included studies with different study designs. We used the following scales and criteria to assess bias in the studies analyzed: the Newcastle-Ottawa scale (NOS), the Appraisal tool for Cross-Sectional Studies (AXIS) tool (Downess MJ et al, 2016.), the Joanna Briggs Institute (JBI) critical appraisal tool (Martin J., 2017), and WHO's Reporting Of Sero-Epidemiologic Studies—SARS-CoV-2 (ROSES-S) statement (Group WHOSTW, 2021). The Newcastle-Ottawa Scale (NOS) contains 3 domains that are graded on 10-point stars. Domain 1 evaluates the methodologic quality of each study (5 stars), domain 2 assesses the comparability of the study (2 stars), and domain 3 evaluates the outcome measure and related statistical analysis (3 stars). Based on the total score, the studies were categorized into 4 groups: unsatisfactory (0 to 4 stars), satisfactory (5 to 6 stars), good (7 to 8 stars), and very good studies (8 to 10 stars). AXIS tool, JBI scale, and WHO CONSISE-ROSES-S statement comprehensively assess study design (representativeness of study participants), laboratory analysis, steps taken for internal validation, and outcome adjustment (correction for demographics and/or test performance for external validation). Two independent reviewers assessed the quality of studies. In the case of NOS, the average score for that particular study was taken as the final score. For other scales, a third reviewer resolved any conflicts between the 2 independent assessors.
Effect measure: In the meta-analysis, we estimated the overall pooled seroprevalence of SARS-CoV-2 in India and that of stratum-specific estimates by pandemic waves, age, gender, residence, and study population.
Statistical analysis: We described characteristics of the identified studies like study population, study period, study setting, study design, laboratory methods, and statistical analysis employed. For all eligible studies included in the analysis, we calculated the pooled seroprevalence with 95% confidence interval (CI) using a random-effects model and the inverse variance weighting. We visually represented the prevalence estimates using the forest plot. To explore heterogeneity, we calculated the pooled seroprevalence by various subgroups such as by age and gender; type of study participants: general population, specific, well-defined population like healthcare workers, in-patients of hospitals admitted for reasons other than COVID-19, and contacts of patients with COVID-19; time period: based on the epicurve of COVID-19 for India, we defined the 2 periods as first wave (March 2020 to February 2021) and second wave (March to August 2021), 3 sub-periods in first wave: prepeak (March to August 2020), peak (September to October 2020),postpeak (November 2020 to February 2021); and study setting: rural and urban. Heterogeneity was assessed by the I2 statistics which measures the variation across studies due to heterogeneity rather than chance. I2 >50% was considered as substantial heterogeneity, and Cochrane p <0.05 was taken as a cut off for significant heterogeneity. All statistical analyses were done using STATA 16 (Stata 2019).
Results
Selection of studies
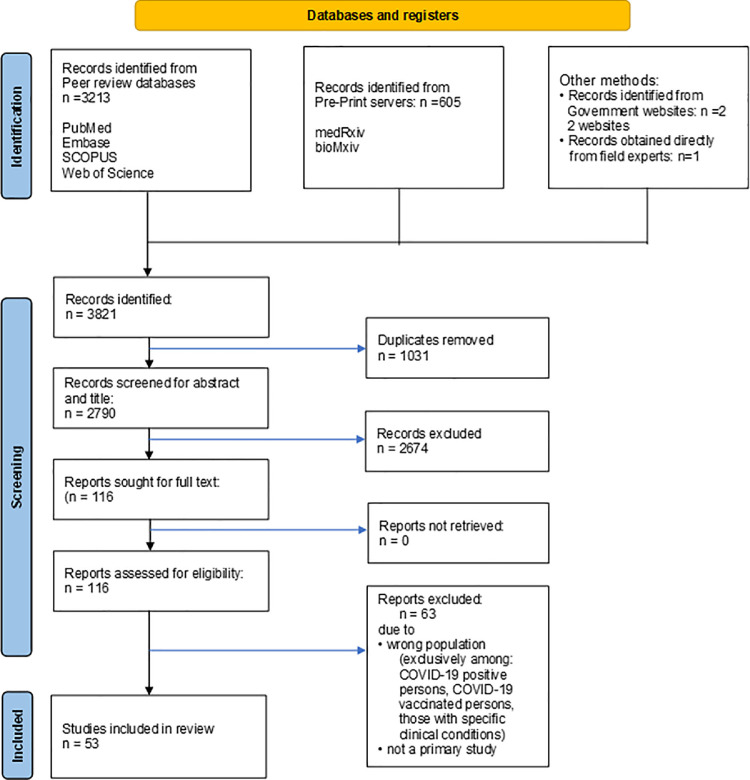
We identified a total of 3821 studies after systematically searching multiple data sources, with 3213 identified from peer-reviewed repositories, 605 from preprint servers, and 3 identified from government websites or contacting relevant health experts. After excluding 1031 duplicates and a further 2674 following the screening of titles and abstracts, 116 studies of SARS-CoV-2 serologic evidence were assessed for eligibility. Four full texts could not be retrieved online and were obtained directly from the study authors. Sixty-three studies were excluded, resulting in a total of 53 studies (Babu et al., 2021; George et al., 2021; Ghose et al., 2020; Ghosh et al., 2021; Goenka et al., 2020; Ramasamy S, 2021; Gupta et al., 2021; Inbaraj et al., 2021; V. Jain et al., 2021; Jessy et al., 2021; Joshi et al., 2021; Kar et al., 2021; Kerala, 2021; M. S. Khan et al., 2021; S. M. S. Khan et al., 2020; Kshatri, 2021; Kshatri et al., 2021; M. S. Kumar et al., 2021; N. Kumar et al., 2021; Laxmaiah et al., 2021, ICMR survey. Mint 2021; Madhusudan et al., 2021; Mahto et al., 2021; Makadia et al., 2021; Malani et al., 2021; Malani et al., 2021b; Mishra et al., 2021; Mohanan et al., 2021, MoHFW, 2021; M. Murhekar et al., 2020; M. V. Murhekar et al., 2021; M. V. Murhekar, et al., 2021; Naushin et al., 2021; Padma et al., 2021; Parai et al., 2021; Prakash et al., 2021a.; Prakash et al., 2021b; Prakash et al., 2021c; Prakash et al., 2021d; Am et al., 2021; Ray et al., 2020; Satpati et al., 2020; Selvaraju et al., 2021; Sen et al., 2021; Sharma et al., 2021; Sharma et al., 2021; Siddiqui S. et at., 2020; Singhal et al., 2020; Siva Ganesa Karthikeyan et al., 2021; Velumani et al., 2021; and Venkataraman et al., 2021) with 831,010 participants in the first wave (COVID-19 Serosurvey III Tamil Nadu, 2021; Misra et al., 2021; Selvaraju et al., 2021 and M. V. Murhekar, et al., 2021) with 74 369 participants in the second wave included in the systematic review and meta-analysis after full-text scrutiny (Figure 1 , Supplementary table 1).
Figure 1.
PRISMA flow chart of selection of studies.
Characteristics of included studies
Most studies were done exclusively in the Indian states of Tamil Nadu (n = 8), Maharashtra, Karnataka (n = 6 in each), and Odisha (n = 5) and by multiple stakeholders. Most of the studies were conducted exclusively in urban (n = 38) areas. Studies primarily focused on the general population (n = 28) or healthcare workers (n = 18) or both (n = 4).
Quality and Bias assessment
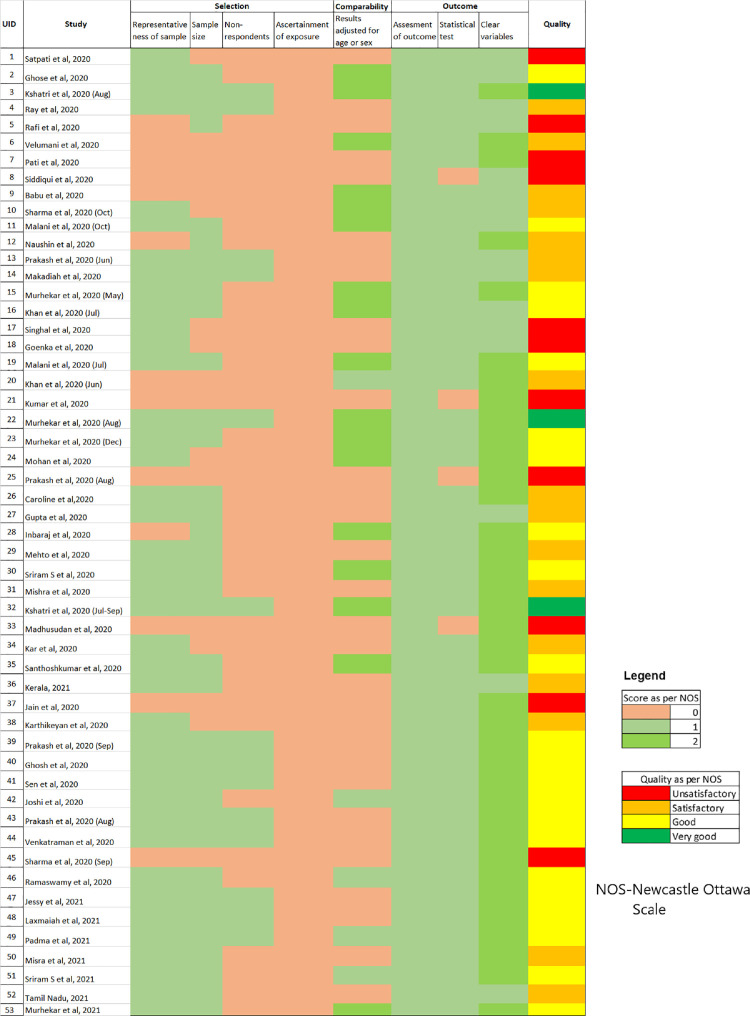
Most of the studies were of good quality (n = 21) on the NOS, with 17 of them categorized as “satisfactory” quality and only 4 were classified as “very good” quality. One-fifth of the studies (n = 11) were categorized as “unsatisfactory or poor” quality (Figure 2 ). Almost all of the high-quality (good and very good categories) seroprevalence studies identified were done in general population. There were very few high-quality studies among specific subpopulations like healthcare workers (n = 4) and none among close contacts of COVID-19. More than two-thirds of prevalence estimates (n = 35) were obtained from an appropriately sized sample and equal number of studies used nonrandom sampling (n = 33); whereas, one study used a nonrepresentative sampling frame (laboratory blood samples collected for thyroid profiling). (Supplementary table 2) In addition, the studies included in the analyses provided inadequate information about the demographic, health, and exposure variables. Importantly, the distinction of exposures reported (e.g., symptomatic and asymptomatic) in the studies relied on self-reported health status, which may not be reliable. Studies of “unsatisfactory or poor” quality, in particular, had multiple features of unjustified sample size, nonrepresentative sample, nonclassification of nonrespondents, results unadjusted for demographics, and methods insufficiently explained to enable replication.
Figure 2.
Assessment of the quality of studies using The Newcastle-Ottawa Scale (NOS).
Most studies used serologic tests with good sensitivity (>90%) and specificity (>95%), whereas 4 studies did not report test accuracy. Estimates of many of the general population-based seroprevalence studies (17 of 38) neither adjusted for the demographics of the study population nor for the diagnostic performance of the assay. Although the response rate of 19 studies raises concerns about nonresponse bias, only 6 studies took measures to address or characterize nonresponders. There were conflicts of interest in 16 studies that may have affected the authors’ interpretation of the results; of the 16, only one study acknowledged it. Information regarding ethics approval was missing in one of the studies.
Minimum standards of reporting
Seroprevalence studies did not provide adequate information on their methods. Some of the reviewed studies did not report adequate information on study methods (sampling = 24% [13/53]; laboratory = 83% [44/53]). Nearly half the studies did not mention the assumptions for sample size calculation. Although two-thirds (n = 33) of the studies justified sample size (Figure 2), 5 studies did not clearly define the study population, and 10 studies did not sufficiently describe the methods (including statistical methods) to enable them to be repeated. Six studies did not provide the inclusion or exclusion criteria.
Various diagnostic assays were used for the determination of seropositivity. None of the studies reviewed reported a rationale for their testing approach, and 4 of them did not state the sensitivity and specificity of the assays. Of the 53 studies, 25 did not describe the timing of the biologic sampling in relation to the disease epidemiology in the study population (the beginning, peak, and fall of virus transmission).
Laboratory methods were not reported as per reporting standards as well. None of the studies described positive and negative controls used, starting and end dilutions, and laboratory biosafety conditions. One-third (n = 17) did not describe the threshold or other parameters used to define “seropositivity”. A major proportion (n = 44) did not describe the specimen storage conditions, whereas two-fifth of the studies (n = 21) did not specify the antigen(s) used in the assay. Of all 53, only 2 studies described any known or potential immunologic cross-reactivity that may have biased the outcome measures.
Many studies failed to report the participant flow, number of participants at each stage, and reasons for nonresponse. Thirty-eight studies reported the number of participants at each stage of the study; a little over one-fourth (n = 16) used flow diagrams to illustrate the participant flow, and 11 gave reasons for nonparticipation at each stage. Yet, 11 of 29 relevant studies did not state how the nonindependence of data was managed. In addition, 19 studies did not discuss the limitations of the study.
Seroprevalence estimates by the pandemic waves
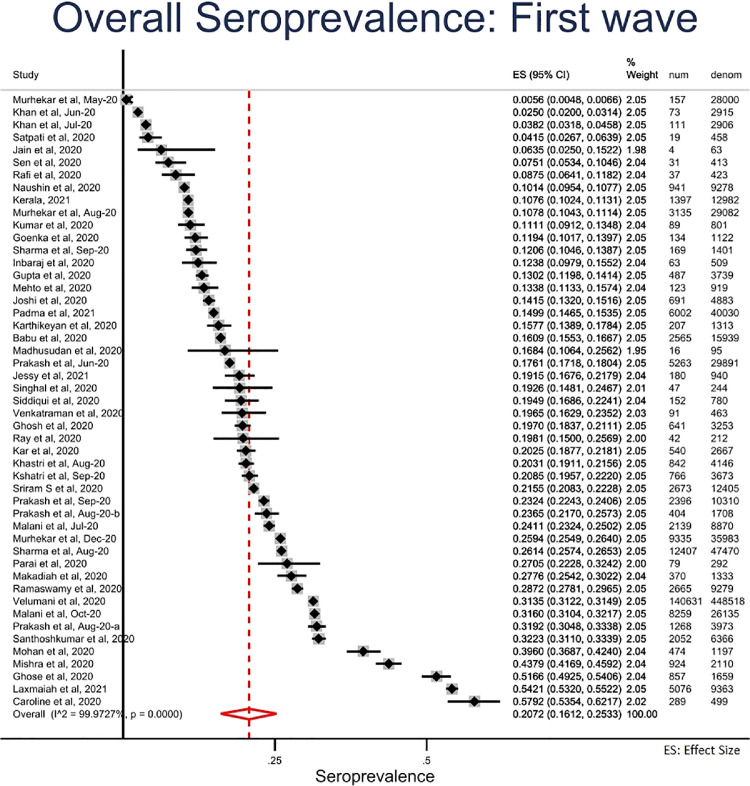
First wave (March 2020-February 2021): We estimated the pooled seroprevalence to be 20.7% (95% CI = 16.1% to 25.3%) in the first wave (Figure 3, Figure 3b a). Seroprevalence did not differ by age and gender. Seroprevalence was higher in the urban areas (21.6%; 95% CI = 16.8% to 26.4%) than in the rural areas (14.6%; 6.2% to 23.0%) (Table 1 ).
Figure 3.
Overall seroprevalence of SARS-CoV-2 infection during first wave (March 2020-February 2021), India.
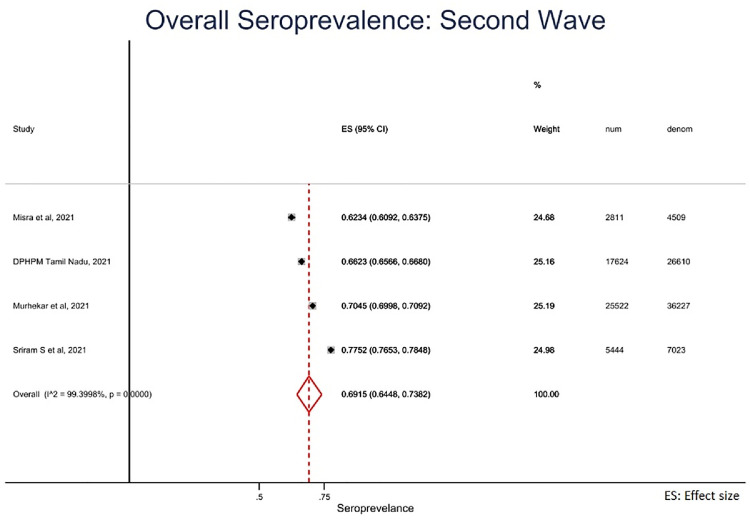
Figure 3b.
Overall seroprevalence of SARS-CoV-2 infection during Second wave (March 2021-August 2021), India.
Table 1.
Pooled overall seroprevalence against SARS-CoV-2 in India by selected socio-demographic characteristics, March 2020-August 2021.
| Characteristics | First wave (March 2020-February 2021) | Second wave (March 2021-August 2021) | |||||
|---|---|---|---|---|---|---|---|
| Number of studies (n) | Pooled Seroprevalence (%) | 95% CI | Number of studies (n) | Pooled Seroprevalence (%) | 95% CI | ||
| Overall | 49 | 20.7 | 16.1-25.3 | 4 | 69.2 | 64.5-73.8 | |
| Sex | Male | 42 | 20.3 | 16.6-23.9 | 2 | 67.4 | 66.7-68.1 |
| Female | 42 | 21.4 | 17.3-25.4 | 2 | 71.0 | 70.3-71.6 | |
| Age group | ≤20 | 20 | 20.8 | 15.8-25.8 | 2 | 63.1 | 52.8-73.5 |
| 21-40 | 34 | 20.4 | 16.5-24.4 | 2 | 77.2 | 62.5-91.9 | |
| 41-60 | 35 | 23.5 | 18.5-28.6 | 2 | 81.2 | 75.5-87.0 | |
| >60 | 33 | 20.1 | 15.6-24.6 | 2 | 78.5 | 73.4-83.5 | |
| Residence | Rural | 9 | 14.6 | 6.2-23.0 | 2 | 65.1 | 64.6-65.7 |
| Urban | 38 | 21.6 | 16.8-26.4 | 3 | 73.7 | 67.2-80.1 | |
The overall pooled seroprevalence among the general population was 22.7% (95% CI = 17.4% to 27.9%) and among healthcare workers was 16.9% (95% CI = 12.8% to 21.0%). Symptomatic participants had higher seroprevalence among both general population (41.6%, 95% CI = 29.9% to 53.4%) and healthcare workers (33.3%; 95% CI = 22.6% to 44.0%) than asymptomatic participants (general population = 23.4%; 95% CI = 16.5% to 30.3%; healthcare workers = 14.2%; 95% CI = 8.1% to 20.2%). The seroprevalence among the general population also varied across states, with a higher seroprevalence in the Southern (22.2%) compared with Northern region (12.0%) (Supplementary table 3).
Second wave (March-August 2021): We estimated pooled seroprevalence as 69.2% (95% CI = 64.5% to 73.8%) during the second wave (Figure 3b). In contrast to the first wave, seroprevalence was lowest in the age group less than 20 years (63.1%; 95% CI: 52.8% to 73.5%) and highest in age group between 41 and 60 years (81.2%; 95% CI: 75.5% to 87.0%). Similar to the first wave, seroprevalence was not different by gender. Seroprevalence in the urban areas (73.7%; 95% CI = 67.2% to 80.1%) was higher than in the rural areas (65.1%; 95%CI = 64.6% to 65.7%). (Table 1)
Discussion
We did a systematic review and meta-analysis of published reports or preprints of seroprevalence studies of SARS-CoV-2 done among different populations across India. Majority of these were cross-sectional studies conducted in urban settings and among the general population in few Indian States and were of satisfactory quality. Overall, one-fifth in the first wave and 2 in 3 participants in the second wave had evidence of SARS-CoV-2 infection. Seroprevalence did not differ by age in the first wave, which is consistent with epidemic transmission. Whereas higher seroprevalence observed in the older age groups in the second wave could be due to prioritization of the elderly for COVID-19 vaccination. High seroprevalence observed in urban areas compared with rural areas in both the waves could be due to high population density.
The majority of the seroprevalence studies were done in urban settings and in a limited number of Indian states by multiple stakeholders. This could have been necessitated by the significant burden of COVID-19 reported from urban settings during the first wave in contrast to the reported higher burden from small towns and rural areas during the second wave (Ranjan et al., 2021). Such results obtained by the multiple stakeholders remained unsynthesized. In addition, significant proportions of COVID-19 cases were reported from a wide variety of occupations apart from just the healthcare workers; for instance, tourism staff (Kaushal & Srivastava, 2021), retail workers, hospitality industry (Business Excellence and Management 2020), transport staff, security staff (Koh, 2020a), and construction workers (Koh, 2020b). Furthermore, SARS-CoV-2 infection and its impact are likely to be more among those from already disadvantaged ethnic and community groups (Raju et al., 2021). Thus, fewer studies in specific regions, populations, and exposure groups limits our understanding of SARS-CoV-2 transmission in the country. Moving forward, more appropriate sampling frames tailored to the highly uneven and inequitable distribution of COVID-19 (as determined by real-time–polymerase chain reaction) are needed to obtain more representative estimates of seroprevalence in different study settings and among a variety of exposed population groups.
Poor methodologic quality in certain studies identified in the current review is reflected in a similar global review (Bobrovitz N. et al., 2020, Galanis et al., 2021). The need for rapid assessment could have led to lack of methodologic rigor and hence, the lack of validity of the generated estimates. In addition, lack of standardization of the obtained estimates in these studies made it difficult to compare the estimates between the studies. Though such adjustments of estimates provide a small degree of correction with large samples, it cannot be a substitute for robust sampling method. It would be prudent to use standard serosurvey protocols (Kumar et al., 2020, WHO, 2021) for conducting studies of methodologic quality and for generating valid and somewhat comparable estimates.
The poor reporting of studies missed out on key information necessary for interpretation of study methods for potential selection and information bias. Sometimes, media reports (Hindustan Times, 2021; India News - Times of India, 2021; Livemint, 2021) were used for early and timely dissemination of seroprevalence study results well before they could be published in peer-reviewed literature. It is therefore essential that these media sources also provide the essential information needed for their readers to interpret their findings and give the readers the complete picture. Where standardization of estimates is not done, it would be ideal to report the test details such as type of test, company name, and test sensitivity and specificity to enable informed comparison of results. The use of WHO ROSES-S statement for reporting such studies would address reporting related issues.
The pooled estimates for the 2 waves from the current meta-analysis are different from that reported in SeroTracker (SeroTracker 2020). This could be owing to pooling of estimates from studies done during first and second waves in the SeroTracker and different inclusion/exclusion criteria. For instance, SeroTracker included studies among convalescent blood donors, whereas we did not. For subgroup analysis, although our categories were restricted to general population and specific special exposure groups, SeroTracker categorizes them into many overlapping categories (for instance: “household and community samples” and “multiple general populations”), which could have led to biased subgroup estimates.
According to the pooled seroprevalence estimate, more than two-thirds of the country's population had SARS-CoV-2 antibodies by August 2021, with variations by place and individual characteristics. This pooled seroprevalence is higher than that of the global or regional levels (Xinhua et al., 2021). This could be due to differences in the types, scale, intensity, and adherence to the public health strategies implemented across these settings (Lai et al., 2020b).
Limitations
Our study had several limitations. First, each of the included studies could have used varying thresholds for seropositivity. This made it difficult to predict direction of bias in the generated estimates. The pooled estimate could have been influenced by different serologic assays and methods with varying sensitivities and specificities employed in the included studies. Second, cross‐reactive immunity which could have led to the overestimation of SARS-CoV-2 seroprevalence cannot be ignored. Third, seroprevalence of antibodies could have been influenced by the vaccination coverage during the second wave. However, limited information provided in the studies did not allow us to distinguish between natural and vaccine-induced antibodies. Finally, the result of the subgroup analysis did not explain the source of heterogeneity. Hence, we did not do a meta-regression to further explore the heterogeneity.
Conclusion
To conclude, we systematically reviewed SARS-CoV-2 seroprevalence studies in India and identified that the overall quality of the reviewed studies was of “satisfactory” level. Our meta-analysis documented a 3-fold increase in seroprevalence between the 2 waves of infection. More than two-thirds of the India's population had IgG antibodies against SARS-CoV-2 by August 2021 and was higher than that of the global or regional levels. On the basis of the findings, we recommend that studies need to be designed based on standard protocols adapted to the study setting, including the use of validated standardized assays and reporting. Futhermore, central registries of seroprevalence initiatives might facilitate coordinated design and conduct of studies and facilitate evidence synthesis. Finally, we recommend serial seroprevalence studies following vaccine introduction to investigate the early serologic response to vaccination and track the population immunity against SARS-CoV-2.
Registration and protocol
This study was registered with PROSPERO, CRD42021254997 and reported in compliance with Preferred Reporting Items for Systematic review and Meta-analyses Protocol (PRISMA-P) checklist. The review protocol has not been published and is not available publicly.
Competing interests
Manoj V Murhekar, Tarun Bhatnagar, and Muthusamy Santhosh Kumar were part of the national COVID-19 serosurvey group and coordinated 4 rounds of serosurveys in India. These authors were not involved in the risk of bias assessment.
Support
Nonfinancial support for the review was provided by the ICMR-National Institute of Epidemiology.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.12.353.
Appendix. Supplementary materials
References
- Alserehi HA, Alqunaibet AM, Al-Tawfiq JA, Alharbi NK, Alshukairi AN, Alanazi KH, et al. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn Microbiol Infect Dis. 2021;99 doi: 10.1016/j.diagmicrobio.2020.115273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- bioRxiv.org [internet] 2021. The preprint server for Biology. https://www.biorxiv.org/ [Google Scholar]
- Babu GR, Sundaresan R, Athreya S, Akhtar J, Pandey PK, Maroor PS, et al. The burden of active infection and anti-SARS-CoV-2 IgG antibodies in the general population: Results from a statewide survey in Karnataka. India. International Journal of Infectious Diseases. 2021;108:27–36. doi: 10.1101/2020.12.04.20243949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovitz N, Arora RK, Cao C, Boucher E, Liu M, Donnici C, et al. Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and meta-analysis. PLOS ONE. 2021;16 doi: 10.1371/journal.pone.0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovitz N, Arora RK, Yan T, Rahim H, Duarte N, Boucher E, et al. Lessons from a rapid systematic review of early SARS-CoV-2 serosurveys. MedRxiv 2020:2020.05.10.20097451. 2020 doi: 10.1101/2020.05.10.20097451. [DOI] [Google Scholar]
- CDC [internet]. Delta Variant: What We Know About the Science. Cent Dis Control Prev 2020. [cited 2021 Sep 28]. Available from https://www.cdc.gov/coronavirus/2019-ncov/variants/delta-variant.html
- Xinhua Chen, Chen Z, Azman AS, Deng X, Sun R, Zhao Z, et al. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. Lancet Glob Health. 2021;9:e598–e609. doi: 10.1016/S2214-109X(21)00026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury AZ, Jomo KS. Responding to the COVID-19 Pandemic in Developing Countries: Lessons from Selected Countries of the Global South. Development. 2020;63:162–171. doi: 10.1057/s41301-020-00256-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham H, Hay J, Routledge I, Takahashi S, Choisy M, Cummings D, et al. Seroepidemiologic Study Designs for Determining SARS-COV-2 Transmission and Immunity - Emerging Infectious Diseases journal - CDC 2020. 26:9. doi:10.3201/eid2609.201840. [DOI] [PMC free article] [PubMed]
- COVID-19 Serosurvey III Tamil Nadu, COVID-19 . Government of Tamil Nadu; 2021. [internet] COVID-19 India. http://covidindiaupdates.in/ [Google Scholar]
- Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS)BMJ Open. 2016;6 doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George CE, Inbaraj LR, Chandrasingh S, de Witte LP. High seroprevalence of COVID-19 infection in a large slum in South India; what does it tell us about managing a pandemic and beyond? Epidemiol Infect. 2021;149:e39. doi: 10.1017/S0950268821000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose A, Bhattacharya S, Karthikeyan A, Kudale A, Monteiro J, Joshi A, et al. Community prevalence of antibodies to SARS-CoV-2 and correlates of protective immunity in five localities in an Indian metropolitan city SUMMARY Background 2020. MedRxiv. 2020 doi: 10.1101/2020.11.17.20228155. 2020.11.17.20228155. [DOI] [Google Scholar]
- Ghosh S, Yadav AK, Rajmohan KS, Bhalla S, Sekhawat VS, Prashant J, et al. Seropositivity of severe acute respiratory syndrome coronavirus 2 infection among healthcare workers of the Armed Forces medical services, India: A multicentric study. Med J Armed Forces India. 2021;77:S359–S365. doi: 10.1016/j.mjafi.2021.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goenka M, Afzalpurkar S, Goenka U, Das SS, Mukherjee M, Jajodia S, et al. Seroprevalence of COVID-19 Amongst Health Care Workers in a Tertiary Care Hospital of a Metropolitan City from India. J Assoc Physicians India. 2020;68:14–19. doi: 10.2139/ssrn.3689618. [DOI] [PubMed] [Google Scholar]
- Group WHOSTW.ROSES-S. Statement from the World Health Organization on the reporting of seroepidemiologic studies for SARS-CoV-2. Influenza Other Respir Viruses. 2021;15:561–568. doi: 10.1111/irv.12870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Dwivedi T, Gajendra S, Sahoo B, Gupta SK, Vikas H, et al. Seroprevalence of antibodies to SARS-CoV-2 in healthcare workers & implications of infection control practice in India. INDIAN J MED RES. 2021:7. doi: 10.4103/ijmr.IJMR_3911_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindustan Times . 2021. [internet]. 6th Delhi sero survey shows 97% prevalence of antibodies. 2020. [cited. https://www.hindustantimes.com/india-news/6th-delhi-sero-survey-shows-97-prevalence-10163543569509.html. [Google Scholar]
- Inbaraj LR, George CE, Chandrasingh S. Seroprevalence of COVID-19 infection in a rural district of South India: A population-based seroepidemiological study. PLOS ONE. 2021;16 doi: 10.1371/journal.pone.0249247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INSACOG bulletin . 2021. [internet] Department of Biotechnology-India-18-06-2021. https://dbtindia.gov.in/sites/default/files/INSACOG%20%20BULLETIN%20-%2018-06-21%20for%20public%20release.pdf. [Google Scholar]
- Jain V, Gupta M, Grover M, Nare T, Srivastava S, Bhardwaj P, et al. COVID-19 seropositivity among non-medical frontline office staff from two cities in Rajasthan, India. J Fam Med Prim Care. 2021;10:2400. doi: 10.4103/jfmpc.jfmpc_2381_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessy SJ, Beegum MS, Genga S, Bindu G, Chintha S, Sasidharan S, et al. Prevalence of SARS CoV-2 infection among Health Care Workers of a hybrid tertiary COVID 19 hospital in Kerala. Infectious Diseases (except HIV/AIDS); 2021. doi: 10.1101/2021.07.19.21260792. [DOI]
- Joshi A, Shankar P, Chatterjee A, Singh J, Pakhare A, Yadav K, et al. Heterogeneous patterns of COVID-19 transmission in an Urban set up – sero-epidemiological survey data from Ujjain, Madhya Pradesh (a central Indian city) Data Brief. 2021;37 doi: 10.1016/j.dib.2021.107169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kar SS, Sarkar S, Murali S, Dhodapkar R, Joseph NM, Aggarwal R. Prevalence and Time Trend of SARS-CoV-2 Infection in Puducherry, India, August–October 2020. Emerg Infect Dis. 2021;27:4. doi: 10.3201/2702.204480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal V, Srivastava S. Hospitality and tourism industry amid COVID-19 pandemic: Perspectives on challenges and learnings from India. Int J Hosp Manag. 2021;92 doi: 10.1016/j.ijhm.2020.102707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerala . 2021. [internet] Kerala sero surveillance report. https://health.kerala.gov.in/pdf/Sero_Surveillance_Report_v7. [Google Scholar]
- Khan MS, Haq I, Qurieshi MA, Majid S, Bhat AA, Qazi TB, et al. SARS-CoV-2 Seroprevalence Among Healthcare Workers by Workplace Exposure Risk in Kashmir. India. J Hosp Med. 2021;16 doi: 10.12788/jhm.3609. [DOI] [PubMed] [Google Scholar]
- Khan SMS, Qurieshi MA, Haq I, Majid S, Bhat AA, Sahila Nabi, et al. Seroprevalence of SARS-CoV-2 specific IgG antibodies in District Srinagar, northern India – A cross-sectional study. PLOS ONE. 2020;15 doi: 10.1371/journal.pone.0239303. org/10.1371/journal.pone.0239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D. Occupational risks for COVID-19 infection. Occup Med. 2020;70:3–5. doi: 10.1093/occmed/kqaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh D. Migrant workers and COVID-19. Occup Environ Med. 2020;77:634–636. doi: 10.1136/oemed-2020-106626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshatri JS. Serological surveys to inform SARS-CoV-2 epidemic curve: a cross-sectional study from Odisha. India. Sci Rep. 2021:10. doi: 10.1038/s41598-021-89877-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kshatri JS, Bhattacharya D, Praharaj I, Mansingh A, Parai D, Kanungo S, et al. Seroprevalence of SARS-CoV-2 in Bhubaneswar, India: findings from three rounds of community surveys. Epidemiol Infect. 2021;149:e139. doi: 10.1017/S0950268821000972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MS, Bhatnagar T, Manickam P, Kumar VS, Rade K, Shah N, et al. National sero-surveillance to monitor the trend of SARS-CoV-2 infection transmission in India: Protocol for community-based surveillance. Indian J Med Res. 2020;151:419–423. doi: 10.4103/ijmr.IJMR_1818_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar MS, Thangaraj JWV, Saravanakumar V, Selvaraju S, Kumar CPG, Sabarinathan R, et al. Monitoring the trend of SARS-CoV-2 seroprevalence in Chennai, India, July and October 2020. Trans R Soc Trop Med Hyg. 2021 doi: 10.1093/trstmh/trab136. [DOI] [Google Scholar]
- Kumar N, Bhartiya S, Desai S, Mutha A, Beldar A, Singh T. Seroprevalence of Antibodies Against SARS-CoV-2 Among Health Care Workers in Mumbai, India. Asia Pac J Public Health. 2021;33:126–128. doi: 10.1177/1010539520977307. [DOI] [PubMed] [Google Scholar]
- Lai C-C, Wang J-H, Hsueh P-R. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review. Int J Infect Dis. 2020;101:314–322. doi: 10.1016/j.ijid.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai C-C, Wang J-H, Hsueh P-R. Population-based seroprevalence surveys of anti-SARS-CoV-2 antibody: An up-to-date review. Int J Infect Dis. 2020;101:314–322. doi: 10.1016/j.ijid.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxmaiah A, Rao NM, Arlappa N, Babu J, Kumar PU, Singh P, et al. SARS-CoV-2 seroprevalence in the city of Hyderabad, India in early 2021. Infectious Diseases (except HIV/AIDS) 2021 doi: 10.1101/2021.07.18.21260555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livemint [internet] Over 75% people in these states have covid antibodies: ICMR survey. Mint 2021. [cited 2021 Dec 2] Available from https://www.livemint.com/news/india/these-states-have-over-75-seropositivity-icmr-s-national-sero-survey-finds-11627468536788.html.
- Madhusudan M, Sankar J, Dhanalakshmi K, Putlibai S, Balasubramanian S. Seroprevalence to SARS-CoV-2 Among Healthcare Workers in an Exclusive Pediatric Hospital. Indian Pediatr. 2021;58:279–280. doi: 10.1007/s13312-021-2170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahto M, Banerjee A, Biswas B, Kumar S, Agarwal N, Singh PK. Seroprevalence of IgG against SARS-CoV-2 and its determinants among healthcare workers of a COVID-19 dedicated hospital of India. American journal of blood research. 2021;11(1):44. doi: 10.25259/IJMS_463_2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makadia JS, Gohel MG, Chakrabarti C, Pethani JD. Prevalence of Seropositivity to SARS-CoV-2 among Health Care Workers in Tertiary COVID-19 Hospital, Ahmedabad, Gujarat. J Clin Diagn Res. 2021 doi: 10.7860/JCDR/2021/46817.14599. [DOI] [Google Scholar]
- Malani A, Ramachandran S, Tandel V, Parasa R, Imad S, Sudharshini S, et al. SARS-CoV-2 Seroprevalence in Tamil Nadu in October-November 2020 2021a. MedRxiv. 2021 doi: 10.1101/2021.02.03.21250949. 2021.02.03.21250949. [DOI] [Google Scholar]
- Malani A, Shah D, Kang G, Lobo GN, Shastri J, Mohanan M, et al. Seroprevalence of SARS-CoV-2 in slums versus non-slums in Mumbai, India. Lancet Glob Health. 2021;9:e110–e111. doi: 10.1016/S2214-109X(20)30467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. Joanna Briggs Institute 2017 [internet] Critical Appraisal Checklist for Analytical Cross Sectional Studies. 2017 [cited 2021 Sep 14] Available from https://jbi.global/sites/default/files/2019-05/JBI_Critical_Appraisal-Checklist_for_Analytical_Cross_Sectional_Studies2017
- Mishra M, Chaudhry R, Rana F, Nag DS, Rai S. Serosurveillance of Health Care Workers in a COVID Hospital: Immune Response, and Its Longevity. Cureus. 2021;13(3) doi: 10.7759/cureus.14020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra P, Kant S, Guleria R, Rai SK. WHO Unity Seroprevalence study team of AIIMS. Serological prevalence of SARS-CoV-2 antibody among children and young age (between age 2-17 years) group in India: An interim result from a large multi-centric population-based seroepidemiological study. Epidemiology. 2021 doi: 10.1101/2021.06.15.21258880. MedRxiv 2021: 2021.06.15.21258880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohanan M, Malani A, Krishnan K, Acharya A. Prevalence of SARS-CoV-2 in Karnataka, India. JAMA. 2021;325:1001. doi: 10.1001/jama.2021.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MoHFW [internet] MoHFW | Home. 2021. [cited 2021 Jun 29] Available from https://www.mohfw.gov.in/
- Murhekar M, Bhatnagar T, Selvaraju S, Rade K, Saravanakumar V, Vivian Thangaraj J, et al. Prevalence of SARS-CoV-2 infection in India: Findings from the national serosurvey, May-June 2020. Indian J Med Res. 2020;152:48. doi: 10.4103/ijmr.IJMR_3290_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar Manoj V, Bhatnagar T, Selvaraju S, Saravanakumar V, Thangaraj JWV, Shah N, et al. SARS-CoV-2 antibody seroprevalence in India, August–September, 2020: findings from the second nationwide household serosurvey. Lancet Glob Health. 2021;9:e257–e266. doi: 10.1016/S2214-109X(20)30544-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar Manoj V, Bhatnagar T, Thangaraj JWV, Saravanakumar V, Kumar MS, Selvaraju S, et al. SARS-CoV-2 seroprevalence among the general population and healthcare workers in India, December 2020–January 2021. Int J Infect Dis. 2021;108:145–155. doi: 10.1016/j.ijid.2021.05.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar MV, Bhatnagar T, Thangaraj JWV, Saravanakumar V, Santhosh Kumar M, Selvaraju S, et al. Seroprevalence of IgG antibodies against SARS-CoV-2 among the general population and healthcare workers in India, June-July 2021: A population-based cross-sectional study [published online ahead of print, 2021 Dec 10] PLoS Med. 2021;18(12) doi: 10.1371/journal.pmed.1003877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murhekar MV, Clapham H. COVID-19 serosurveys for public health decision making. Lancet Glob Health. 2021;9:e559–e560. doi: 10.1016/S2214-109X(21)00057-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naushin S, Sardana V, Tech M, Ujjainiya R, Kutum R, Bhaskar AK, et al. Insights from a Pan India Sero-Epidemiological survey (Phenome-India Cohort) for SARS-CoV-2. Elife. 2021;10:e66537. doi: 10.7554/eLife.66537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padma MR, Dinesh P, Sundaresan R, Athreya S, Shiju S, Maroor PS, et al. Second round statewide survey for estimation of the burden of active infection and anti-SARS-CoV-2 IgG antibodies in the general population of Karnataka. India. International Journal of Infectious Diseases. 2021;108:27–36. doi: 10.1101/2021.08.10.21261842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parai D, Choudhary HR, Dash GC, Peter A, Saket D, Roy R, et al. Seroprevalence of IgG antibody against SARS-CoV-2 among health care workers of anaesthesia departments from various hospital settings in India. 2021 doi: 10.1101/2021.03.20.21253819. MedRxiv 2021: 2021.03.20.21253819. [DOI] [Google Scholar]
- Poustchi H, Darvishian M, Mohammadi Z, Shayanrad A, Delavari A, Bahadorimonfared A, et al. SARS-CoV-2 antibody seroprevalence in the general population and high-risk occupational groups across 18 cities in Iran: a population-based cross-sectional study. Lancet Infect Dis. 2021;21:473–481. doi: 10.1016/S1473-3099(20)30858-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O, Solanki B, Sheth J, Makwana G, Kadam M, Vyas S, et al. SARS-CoV2 IgG antibody: Seroprevalence among health care workers. Clin Epidemiol Glob Health. 2021;11 doi: 10.1016/j.cegh.2021.100766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O, Solanki B, Sheth J, Oza D, Kadam M, Vyas S, et al. Population-based seropositivity for IgG antibodies against SARS-CoV-2 in Ahmedabad city. J Fam Med Prim Care. 2021;10:2363. doi: 10.4103/jfmpc.jfmpc_2062_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O, Solanki B, Sheth JK, Joshi B, Kadam M, Vyas S, et al. Assessing seropositivity for IgG antibodies against SARS-CoV-2 in Ahmedabad city of India: a cross-sectional study. BMJ Open. 2021;11 doi: 10.1136/bmjopen-2020-044101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O, Solanki B, Sheth JK, Kadam M, Vyas S. Severe acute respiratory syndrome coronavirus 2 immunoglobulin G antibody: Seroprevalence among contacts of COVID-19 cases. Journal of Family Medicine and Primary Care. 2021;10(6):2363. doi: 10.4103/jfmpc.jfmpc_2062_20. [DOI] [PubMed] [Google Scholar]
- Am Rafi, Tomy J, Mm Lisa, Thomas R, Valsan C, Unnikrishnan U, Sj Innah, Kuttichira P. Serosurveillance of SARS-CoV-2 among the Healthcare Workers of a Tertiary Care Teaching Institution during the Post Lockdown Phase in Central Kerala. India. Journal of Clinical & Diagnostic Research. 2021;15(7) doi: 10.1101/2021.01.27.21250502. [DOI] [Google Scholar]
- Raju E, Dutta A, Ayeb-Karlsson S. COVID-19 in India: Who are we leaving behind? Prog Disaster Sci. 2021;10 doi: 10.1016/j.pdisas.2021.100163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy S, Athotra A, Tiwari R, Tomar SP, Kuraria R, Seth RJ, et al. Cross-sectional Study on Sero-Prevalence of SARS-CoV-2 Infection in Jabalpur, Madhya Pradesh, India. J Commun Dis. 2021;53:82–88. doi: 10.24321/0019.5138.202114. [DOI] [Google Scholar]
- Ranjan R, Sharma A, Verma MK. Characterization of the Second Wave of COVID-19 in India. MedRxiv. 2021 doi: 10.1101/2021.04.17.21255665. 2021.04.17.21255665. [DOI] [Google Scholar]
- Ray A, Singh K, Chattopadhyay S, Mehdi F, Batra G, Gupta A, et al. Seroprevalence of anti-SARS-CoV-2 IgG antibodies in hospitalized patients at a tertiary referral center in North India 2020. MedRxiv. 2020 doi: 10.1101/2020.08.22.20179937. 2020.08.22.20179937. [DOI] [Google Scholar]
- Rayyan . 2021. [internet] Intelligent Systematic Review. https://www.rayyan.ai/ [Google Scholar]
- Satpati PS, Sarangi SS, Gantait KS, Endow S, Mandal NC, Panchanan K, et al. Sero-surveillance (IgG) of SARS-CoV-2 among Asymptomatic General population of Paschim Medinipur. West Bengal, India MedRxiv. 2020 doi: 10.1101/2020.09.12.20193219. 2020.09.12.20193219. [DOI] [Google Scholar]
- Selvaraju S, Kumar MS, Thangaraj JWV, Bhatnagar T, Saravanakumar V, Kumar CPG, et al. Population-Based Serosurvey for Severe Acute Respiratory Syndrome Coronavirus 2 Transmission, Chennai, India. Emerg Infect Dis. 2021;27:586–589. doi: 10.3201/eid2702.203938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraju S, Kumar MS, Thangaraj JWV, Bhatnagar T, Saravanakumar V, Kumar CPG, et al. Prevalence of IgG antibodies against SARS-CoV-2 among the general population and healthcare workers in Chennai, July. 2021 doi: 10.1371/journal.pmed.1003877. personal communication. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen S, Lall M, Faujdar DS, Shergill SPS, Patrikar S, Kaushik SK, et al. Estimating the proportion and IgG antibody response to SARS-CoV-2 in individuals joining a central educational institute from different parts of India by Enzyme-linked immunosorbent assay (ELISA) based serology. Med J Armed Forces India. 2021;77:S366–S372. doi: 10.1016/j.mjafi.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SeroTracker . 2020. [internet] SeroTracker. https://serotracker.com/en/Explore. [Google Scholar]
- Sharma N, Sharma P, Basu S, Saxena S, Chawla R, Dushyant K, et al. The seroprevalence and trends of SARS-CoV-2 in Delhi, India: A repeated population-based seroepidemiological study. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2021 doi: 10.1093/trstmh/trab109. trab109. [DOI] [PubMed] [Google Scholar]
- Sharma P, Chawla R, Bakshi R, Saxena S, Basu S, Bharti PK, et al. Seroprevalence of antibodies to SARS-CoV-2 and predictors of seropositivity among employees of a teaching hospital in New Delhi. India. Osong Public Health Res Perspect. 2021;12:88–95. doi: 10.24171/j.phrp.2021.12.2.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddiqui S, Naushin S, Pradhan S, Misra A, Tyagi A, Looma M, et al. SARS-CoV-2 Antibody Seroprevalence and Stability in a Tertiary Care Hospital-Setting. SSRN Electron J 2020. doi: 10.2139/ssrn.3696827. [DOI]
- Singhal T, Shah S, Naik R, Kazi A, Thakkar P. Prevalence of COVID-19 Antibodies in Healthcare Workers at the Peak of the Pandemic in Mumbai, India: A Preliminary Study. Indian J Med Microbiol. 2020;38:461–463. doi: 10.4103/ijmm.IJMM_20_308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva Ganesa Karthikeyan R, Rameshkumar G, Gowri Priya C, Lalitha P, Devi R, Iswarya M, et al. Seroprevalence of SARS-CoV-2 specific IgG antibodies among eye care workers in South India. Indian J Med Microbiol. 2021 doi: 10.1016/j.ijmmb.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stata . 2019. [internet] Stata 16. https://www.stata.com/new-in-stata/ [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. 2021:372. doi: 10.1136/bmj.n71. Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Times of India . 2021. [internet] Two-third of population surveyed in 11 states have coronavirus antibodies: ICMR serosurvey. https://timesofindia.indiatimes.com/india/two-third-of-population-surveyed-in-11-states-have-coronavirus-antibodies-icmr-serosurvey/articleshow/84829729.cms. [Google Scholar]
- Velumani A, Nikam C, Suraweera W, Fu SH, Gelband H, Brown PE, et al. SARS-CoV-2 Seroprevalence in 12 Cities of India from July-December 2020. MedRxiv. 2021 2021.03.19.21253429. [Google Scholar]
- Venkataraman A, Balasubramanian S, Putilibai S, Lakshan Raj S, Amperayani S, Senthilnathan S, et al. Correlation of SARS-CoV-2 Serology and Clinical Phenotype Amongst Hospitalised Children in a Tertiary Children's Hospital in India. J Trop Pediatr. 2021;67 doi: 10.1093/tropej/fmab015. fmab015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal U, Jilani N, Rabah S, Shariff MA, Jawed M, Mendez Batres A, et al. SARS-CoV-2 seroprevalence among health care workers in a New York City hospital: A cross-sectional analysis during the COVID-19 pandemic. Int J Infect Dis IJID Off Publ Int Soc Infect Dis. 2021;102:63–69. doi: 10.1016/j.ijid.2020.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO [inernet] Population-based age-stratified seroepidemiological investigation protocol for coronavirus 2019 (COVID-19) infection. 2021. [cited 2021 Oct 27] Available from https://www.who.int/publications-detail-redirect/WHO-2019-nCoV-Seroepidemiology-2020.2
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.