Abstract
Background:
Fetal alcohol spectrum disorders (FASD) are preventable adverse outcomes consequent to prenatal alcohol exposure. Supplemental choline confers neuroprotection to the alcohol-exposed offspring, but its actions outside the brain are unclear. We previously reported that prenatal exposure of mice to 4.5g/kg alcohol decreased placental weight in females only, but decreased body weight and liver-to-body weight ratio and increased brain-to-body weight ratio in both sexes. Here we test the hypotheses that a lower alcohol dose will elicit similar outcomes, and concurrent choline treatment will mitigate these outcomes.
Methods:
Pregnant C57BL/6J mice were gavaged with alcohol (3g/kg; Alc) or maltodextrin (MD) from embryonic day (E) 8.5–17.5. Some also received subcutaneous injection of 100mg/kg choline chloride (Alc+Cho, MD+Cho). Outcomes were evaluated on E17.5.
Results:
Alc dams had lower gestational weight gain than MD; this was normalized by choline. In males, Alc decreased placental weight whereas choline increased placental efficiency, and Alc+Cho (vs. MD) trended to further reduce placental weight and increase efficiency. Despite no significant alcohol effects on these measures, choline increased fetal body weight but not brain weight, thus reducing brain-to-body weight ratio in both sexes. This ratio was also lower in the Alc+Cho (vs. MD) fetuses. Alc reduced liver weight and liver-to-body weight ratio; choline did not improve these. Placental weight and efficiency correlated with litter size, whereas placental efficiency correlated with fetal morphometric measurements.
Conclusions:
Choline prevents the alcohol-reduced gestational weight gain and fetal body weight and corrects fetal brain sparing, consistent with clinical findings of improvements in alcohol-exposed children born to mothers receiving choline supplementation. Importantly, we show that choline enhances placental efficiency in the alcohol-exposed offspring but does not normalize fetal liver growth. Our findings support supplementing choline during pregnancy to mitigate the severity of FASD, and emphasize the need to examine choline’s actions in different organ systems.
Keywords: Placenta, fetal alcohol syndrome, intervention, sex-specific effects, fetal growth
INTRODUCTION
Fetal alcohol spectrum disorders (FASD) describe a constellation of preventable growth, physical, cognitive, and behavioral anomalies caused by prenatal alcohol exposure (PAE) (Hoyme et al., 2016). Although health efforts emphasize alcohol abstinence during pregnancy, 12% of pregnant women in the U.S. still drink alcohol and 4% report engaging in binge drinking in the past 30 days (Denny et al., 2019). One result is a high FASD prevalence ranging from 1–5% in the U.S. (May et al., 2018). Because of the lifelong implications of FASD, there is a critical need to identify effective interventions that mitigate the teratogenic impacts of alcohol. Nutrition is long recognized to be important for normal fetal development and is viewed as a safe therapeutic option for improving pregnancy outcomes and offspring health (Wakimoto et al., 2015). Many studies have examined the efficacy of various nutritional interventions for reducing the severity of FASD; however, to date the only one shown to have some efficacy in humans with FASD is choline, both in the presence and absence of micronutrient supplementation (Kable et al., 2015, Jacobson et al., 2018, Wozniak et al., 2015, Wozniak et al., 2020, Warton et al., 2021).
Choline is an essential nutrient that contributes to the synthesis of molecules involved in neurotransmission, cell signaling, membrane structure, and lipid transport, and it is a significant one-carbon donor for processes including nucleotide synthesis, DNA methylation, and control of gene expression. As such, choline has a critical role in fetal development and offspring long-term health (Caudill et al., 2020). To support rapid tissue growth and proper fetal development, pregnancy imposes a high choline demand, with the adequate intake set at 450 mg/day (Caudill et al., 2020). However, many pregnant women fail to meet this recommendation (Jensen et al., 2007, Wallace and Fulgoni, 2017). Inadequate choline intake is also documented in alcohol-drinking pregnant women (May et al., 2014, Carter et al., 2017), and their choline need may be even higher because of the alcohol-impaired absorption and metabolism of several nutrients interrelated with choline metabolism such as folate and methionine (Zeisel, 2011). Indeed, inadequate choline intake during rat pregnancy exacerbates alcohol’s teratogenic effects such that these offspring have lower birth weight, delayed eye opening, impaired motor coordination, and fewer successes in a hindlimb coordination task (Idrus et al., 2017).
Both clinical and preclinical studies conducted during the neonatal and postnatal period demonstrate protective effects of choline against select cognitive and neurobehavioral deficits consequent to PAE (Akison et al., 2018). In contrast, the effectiveness of prenatal choline supplementation is less studied. In rodents, prenatal choline supplementation normalizes the lower birth weight and brain weight, improves neuroanatomical structure, and prevents behavioral abnormalities including delayed righting reflex, negative geotaxis, and issues related to sensorimotor integration and motor functions in the alcohol-exposed offspring (Thomas et al., 2009, Bottom et al., 2020). Similarly, infants whose mothers drank alcohol during pregnancy and received choline and a multivitamin/mineral supplement during pregnancy have higher birth weight and perform better in a habituation-dishabituation test (Kable et al., 2015). Maternal choline supplementation also improves postnatal growth, performance on an eyeblink conditioning test, and visual recognition memory as well as increases brain volumes in infants with PAE (Jacobson et al., 2018, Warton et al., 2021).
Although these studies show promising benefits of choline with respect to the developing brain, it remains unclear if there are improvements in other organs that are also targets of PAE and whether these effects of prenatal choline supplementation are sex-dependent. To assess the importance of fetal sex in modulating FASD outcomes, we previously generated a high dose (4.5 g/kg), binge exposure FASD mouse model and reported that this alcohol regimen decreases placental weight only in females, but it significantly decreases body weight and liver-to-body weight ratio as well as increases brain-to-body and brain-to-liver weight ratios in both male and female fetuses, collectively indicating the presence of asymmetric growth reduction (Kwan et al., 2020). To determine if these outcomes are specific to the high alcohol dose, we repeated these analyses in our established intermediate dose, binge exposure FASD mouse model (Virdee et al., 2021). Furthermore, we conducted a prenatal choline intervention in this model, and examined its impacts on morphometric parameters of the alcohol-exposed fetuses. We hypothesized that this alcohol dose would elicit outcomes similar to those observed in the higher alcohol dose and that concurrent choline treatment will mitigate these alcohol-induced effects in fetal organs in a fetal sex-dependent manner, as suggested by our previous studies (Waddell and Mooney, 2017, Bearer et al., 2015, Kwan et al., 2020).
MATERIALS AND METHODS
Mouse generation and alcohol exposure
Five-week-old C57BL/6J (B6) mice were obtained from Jackson Laboratories (stock #: 000664; Bar Harbor, ME) and housed in an Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC) accredited facility with a 12/12 h light/dark cycle. All procedures complied with the guidelines for animal care established by the National Institutes of Health and with prior approval by the David H. Murdock Research Institute Institutional Animal Care and Use Committee (IACUC). Females were fed a nutritionally adequate, defined diet (AIN-93G; Catalog # TD.94045; Envigo Teklad, Madison, WI) (Reeves et al., 1993) from arrival through pregnancy. At age 8-week, females were bred overnight and the morning a sperm-positive plug was identified was designated embryonic day (E) 0.5. On E8.5 pregnant females were randomly assigned to one of four experimental groups: control (MD), alcohol-exposed (Alc), control + choline (MD+Cho), and alcohol-exposed + choline (Alc+Cho). Alcohol-exposed animals (Alc) received 3.0 g/kg BW alcohol via intragastric gavage once per day from E8.5 through E17.5. Control animals (MD) received a single gavage of maltodextrin per day over the same time frame. To match the alcohol calories (21 kcal/kg BW), the MD dose would be 5.25 g/kg BW, which is hyperosmotic when given as a single gavage and is harmful to the mouse. To minimize stress, we adjusted the MD controls to 80% of alcohol calories; this represents a difference of 0.1 kcal/d and 1.01% of total daily calories, within the variance of daily calorie intake. All dams were weighed on E0.5 and then daily from E8.5 to E17.5.
Choline administration
Choline-treated animals (Alc+Cho, MD+Cho) received the gavage as above plus a subcutaneous injection of 100 mg/kg choline chloride (#F6522120; Balchem, New Hampton, NY) once per day immediately after the gavage from E8.5 through E17.5; controls received an equal volume of saline. The AIN-93G diet contains 1 mg free choline/g diet. Dams in this study, on average, consume 3.2 g diet per day, thereby consuming 3.2 mg free choline per day. The choline injection provides 74.6 mg free choline/kg body weight. For a 32 g dam (the average weight at E17.5), this delivers 2.4 mg free choline per day, thus providing an additional 75% choline each day.
Blood alcohol concentration analysis
Blood alcohol concentration (BAC) was determined as described previously (Virdee et al., 2021, Kwan et al., 2020) at 15 minutes following the alcohol gavage, using the Analox GM7 Micro-Stat as per the manufacturer’s protocol.
Tissue collection
Dams were euthanized on E17.5 four hours after gavage and choline injection by overdose of isoflurane, and the uterine horn was removed and weighed. The uterine horn included all fetuses and placentas in situ, amniotic fluids and sacs and membranes, as well as resorptions if present; thus, its weight measurement represented the collective sum of the whole implantation unit. The number of surviving fetuses was recorded, and the placentas and fetuses were weighed. Comparison of the uterine horn weight data against individual placental and fetal weight data revealed if the alcohol-choline treatments affected the whole implantation unit or selectively impacted the fetus or placenta (or both) of one or both sexes. Fetal brain and liver were weighed, and tails were collected for assessment of fetal sex.
Genotyping for fetal sex
PCR for sex determination was done as previously described (Kwan et al., 2020). Briefly, DNA was isolated and PCR was performed using the Platinum Taq DNA Polymerase PCR kit (Catalog #10966–034, Invitrogen, Carlsbad, CA) as per the manufacturer’s instructions. Primers for the Sry gene were forward: 5′-TGGGACTGGTGACAATTGTC-3′, reverse: 5′-GAGTACAGGTGTGCAGCTCT-3′ (Integrated DNA Technologies, Coralville, IA). The bands at 402bp for the Sry amplicon were visualized using the BioRad Molecular Imager Gel Doc XR+ Imaging System (BioRad, Hercules, CA).
Statistical analysis
Data were first checked for outliers using the Grubbs’ Test (Grubbs, 1969), and no data outliers were identified. Maternal weight data were analyzed by Repeated Measures Analysis of Variance (ANOVA), and maternal total gestational weight gain and all the litter data were analyzed by 2-Way ANOVA. Prior to the analysis, these data were checked for normality assumption using the Shapiro-Wilk Test and equal sphericity assumption using the Mauchly’s Test of Sphericity or equal variance assumption using the Levene’s Test. The Repeated Measures ANOVA model included experimental groups, gestational day, and their interactions. The 2-Way ANOVA model included alcohol exposure, choline supplementation, and their interactions. Post hoc pairwise comparisons were performed with Benjamini-Hochberg correction to adjust for multiple testing.
Because our previous findings in the high dose, binge exposure FASD mouse model demonstrate a sex-dependent effect of alcohol and maternal nutrition (Kwan et al., 2020), the placental and fetal outcomes were analyzed separately for males and females using mixed linear model (MLM) to test our a priori hypothesis of sex-dependent responses to alcohol and choline. This conservative approach, which controls for data dependency and variability, included litter as an independent random effect factor, and alcohol exposure, choline supplementation, and their interactions as independent fixed effect factors. Litter size was also included in the model as a covariate when it was statistically significant (p < 0.05). We also performed the MLM analyses without stratification by fetal sex, and included fetal sex and its interactions with alcohol exposure and/or choline supplementation in the MLM model as independent fixed effect factors. These additional analyses indicated that there was a significant main effect of fetal sex and/or interaction effect with fetal sex for the placental and fetal outcomes (p < 0.05), supporting our a priori hypotheses of sex-dependent responses to alcohol and choline. Because our a priori hypothesis was that choline would modulate alcohol’s effects, we performed post hoc pairwise comparisons with Benjamini-Hochberg correction regardless of whether there was a significant alcohol-choline interaction in order to make biologically meaningful conclusions (Wei et al., 2012). For placental weight and efficiency (calculated as the ratio of fetal body weight to placental weight), fetal body weight, fetal liver weight, fetal brain-to-body weight and brain-to-liver weight ratios, the assumptions of normality and/or equal variance were not met, and natural-log transformation was performed prior to MLM analyses.
Because the magnitude and direction of the relationships among placental and fetal morphometric parameters can be indicative of possible pathologies and presence of compensatory responses for maintaining fetal growth and survival (Krombeen et al., 2019), and because our previous findings in the high dose, binge exposure FASD mouse model show sex-dependent compensatory responses to the adverse intrauterine environment (Kwan et al., 2020), we also performed correlation analyses in the current study to examine the relationships among the placenta and fetal organs in response to the lower alcohol dose and prenatal choline supplementation. These analyses were determined separately for males and females by Pearson correlation analyses, and Benjamini-Hochberg correction was used to adjust for multiple testing.
The ANOVA and MLM analyses were performed using SPSS, Version 26 (SPSS Inc, Chicago, IL). The correlation analyses were performed using the ggpubr package under the R software, Version 3.4.3. Maternal and litter data are presented as means ± SDs because these outcomes represented a single measure from each dam, whereas placental and fetal data are presented as means ± SEMs because these outcomes represented multiple measurements from each litter, which was the experimental unit in the MLM analyses (Altman and Bland, 2005). Statistical significance was defined as p < 0.05, and 0.05 ≤ p < 0.1 was considered to indicate trends.
RESULTS
Pregnancy outcomes
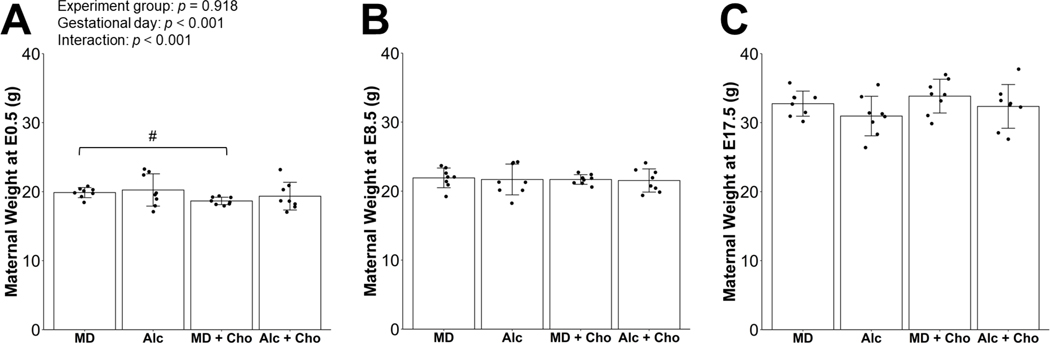
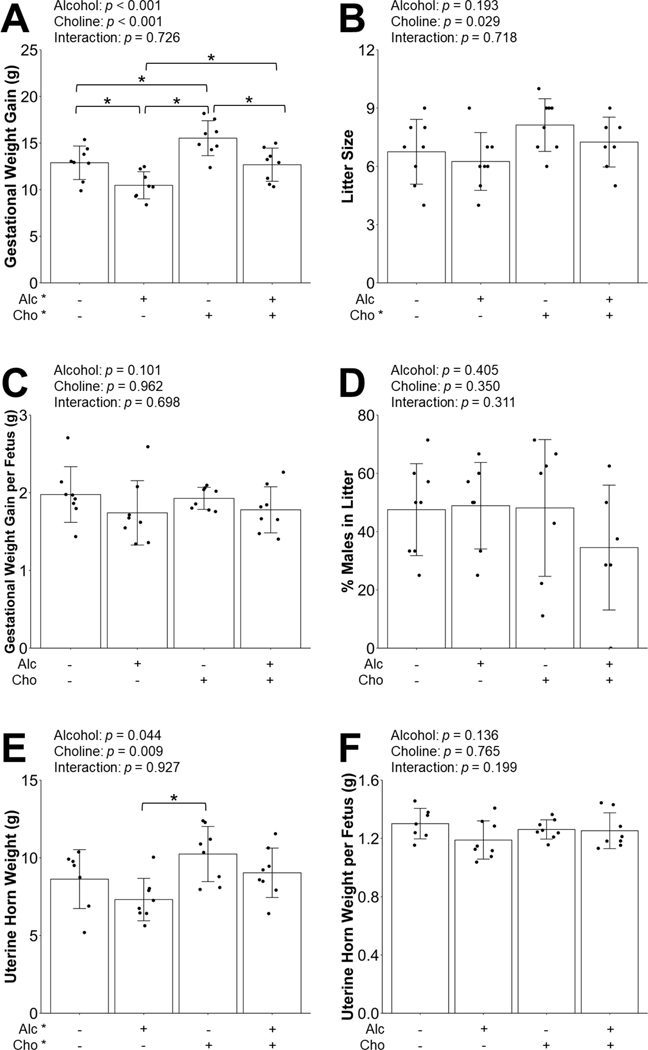
The mean BAC of mice receiving 3.0 g/kg BW alcohol and the choline supplementation was 196.9 ± 29.0 mg/dL at 15 minutes post-gavage. This BAC is not different from the BAC values obtained from the same mouse strain in prior studies (211 ± 14 mg/dL) (Virdee et al., 2021). There was a significant main effect of gestational day on maternal body weights (p < 0.001), but not experimental group, reflecting that dams in all groups gained weight from E0.5 to E17.5 (Figure 1). There was also a significant interactive effect of gestational day x experimental group (p < 0.001), which was likely driven by the differences in the body weight at E0.5 between the MD+Cho and MD dams (−6%, p = 0.072; Figure 1A). PAE significantly reduced whereas choline supplementation significantly increased gestational weight gain (Figure 2A). Alc dams had 19% lower gestational weight gain (p = 0.014) and MD+Cho dams had 20% higher gestational weight gain compared to MD dams (p = 0.010). The Alc+Cho dams also had higher gestational weight gain (+21%, p = 0.020) compared to the Alc dams and gained a similar amount of weight as the MD group, although less than the MD+Cho dams (−18%, p = 0.009).
Figure 1:
Effect of prenatal alcohol exposure and choline supplementation on maternal body weight on embryonic day (E) 0.5 (A), on E8.5 (B), and on E17.5 (C). Data were analyzed using Repeated Measures ANOVA, and post hoc pairwise comparisons were performed with Benjamini-Hochberg correction to adjust for multiple testing. Values are means ± SDs. n=8 dams for MD, Alc, MD+Cho, and Alc+Cho groups. Each dot represents an individual dam. # denotes 0.05 ≤ p < 0.1 for the indicated comparison. Abbreviations: Alc, alcohol; Cho, choline; MD, maltodextrin.
Figure 2:
Effect of prenatal alcohol exposure and choline supplementation on gestational weight gain (A), litter size (B), gestational weight gain per fetus (C), percent males in litter (D), uterine horn weight (E), and uterine horn weight per fetus (F). The uterine horn included all fetuses and placentas in situ, amniotic fluids and sacs and membranes, as well as resorptions if they were present; thus, its weight measurement represented the collective sum of the whole implantation unit. Data were analyzed using two-way ANOVA, and post hoc pairwise comparisons were performed with Benjamini-Hochberg correction to adjust for multiple testing. Values are means ± SDs. For Figures 2A-2C and 2E-2F, n=8 litters for MD, Alc, MD+Cho, and Alc+Cho groups. For Figure 2D, the sex of four fetuses in four litters could not be determined accurately due to poor fetal DNA quality. As such, there were n=8 litters for MD, n=7 litters for Alc and MD+Cho, and n=6 litters for Alc+Cho groups for this outcome measure. Each dot represents an individual litter. Treatment group labeled with * has a significant main effect (p < 0.05). * within the graph denotes p < 0.05 for the indicated comparison. Abbreviations: Alc, alcohol; Cho, choline; MD, maltodextrin.
As reported previously (Virdee et al., 2021), this 3.0 g/kg alcohol regimen did not cause any litter loss. Choline supplementation, but not PAE, was found to increase litter size (Figure 2B). Neither PAE nor choline supplementation had a significant effect on gestational weight gain per fetus or percent of males in each litter (Figure 2C–D). Choline supplementation increased whereas PAE decreased uterine horn weight, which is likely related to their effects on litter size. This outcome was higher in MD+Cho than Alc (+40%, p = 0.006; Figure 2E) but did not differ among dams from other groups, nor were there any between-group differences in uterine horn weight per fetus (Figure 2F). Overall, these data suggest that this alcohol dose and choline supplementation did not adversely impact the whole implantation unit. Summary of the outcomes of these ANOVA analyses is provided in Table 1.
Table 1:
Summary of the outcomes from the Analysis of Variance (ANOVA) analyses of data shown in Figures 1–2.
| Independent Variables | F Statistics | p Values | Partial Eta Squared (η2) | |
|---|---|---|---|---|
|
| ||||
| Figure 1A–C: Maternal body weight, analyzed by Repeated Measures ANOVA | ||||
| Experimental group | F(3,21) = 0.166 | p = 0.918 | η2 = 0.023 | |
| Gestational day | F(2,14) = 1358.192 | p < 0.001 | η2 = 0.995 | |
| Interaction | F(6,42) = 5.467 | p < 0.001 | η2 = 0.439 | |
|
| ||||
| Figure 2A: Gestational weight gain, analyzed by 2-Way ANOVA | ||||
| Alcohol exposure | F(1,28) = 18.467 | p < 0.001 | η2 = 0.397 | |
| Choline supplementation | F(1,28) = 15.638 | p < 0.001 | η2 = 0.358 | |
| Interaction | F(1,28) = 0.125 | p = 0.726 | η2 = 0.004 | |
|
| ||||
| Figure 2B: Litter size, analyzed by 2-Way ANOVA | ||||
| Alcohol exposure | F(1,28) = 1.783 | p = 0.193 | η2 = 0.060 | |
| Choline supplementation | F(1,28) = 5.320 | p = 0.029 | η2 = 0.160 | |
| Interaction | F(1,28) = 0.133 | p = 0.718 | η2 = 0.005 | |
|
| ||||
| Figure 2C: Gestational weight gain per fetus, analyzed by 2-Way ANOVA | ||||
| Alcohol exposure | F(1,28) = 2.869 | p = 0.101 | η2 = 0.093 | |
| Choline supplementation | F(1,28) = 0.002 | p = 0.962 | η2 = 0.000 | |
| Interaction | F(1,28) = 0.153 | p = 0.698 | η2 = 0.005 | |
|
| ||||
| Figure 2D: Percent males in Litter, analyzed by 2-Way ANOVA | ||||
| Alcohol exposure | F(1,24) = 0.719 | p = 0.405 | η2 = 0.029 | |
| Choline supplementation | F(1,24) = 0.910 | p = 0.350 | η2 = 0.037 | |
| Interaction | F(1,24) = 1.071 | p = 0.311 | η2 = 0.043 | |
|
| ||||
| Figure 2E: Uterine horn weight, analyzed by 2-Way ANOVA | ||||
| Alcohol exposure | F(1,27) = 4.475 | p = 0.044 | η2 = 0.142 | |
| Choline supplementation | F(1,27) = 7.816 | p = 0.009 | η2 = 0.224 | |
| Interaction | F(1,27) = 0.008 | p = 0.927 | η2 = 0.000 | |
|
| ||||
| Figure 2F: Uterine horn weight per fetus, analyzed by 2-Way ANOVA | ||||
| Alcohol exposure | F(1,27) = 2.364 | p = 0.136 | η2 = 0.080 | |
| Choline supplementation | F(1,27) = 0.091 | p = 0.765 | η2 = 0.003 | |
| Interaction | F(1,27) = 1.731 | p = 0.199 | η2 = 0.060 | |
Placental outcomes
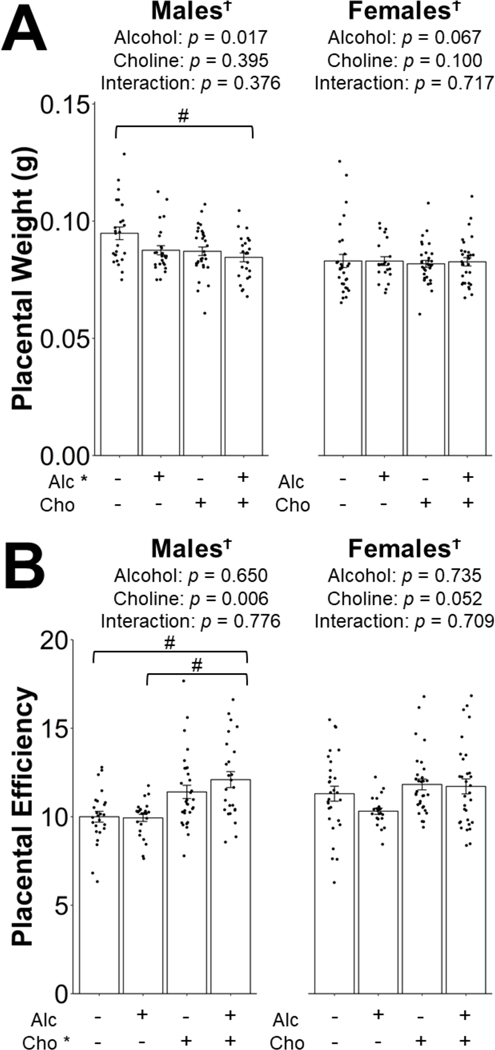
Summary of the MLM analyses on placental outcomes is provided in Table 2. In males, PAE, but not choline, decreased placental weight. This was higher for MD males than for any other group (Figure 3A); however, this only reached a statistical trend compared to Alc+Cho males (−11%, p = 0.078; Figure 3A). In contrast, choline supplementation, but not PAE, increased placental efficiency. This trended to be higher in Alc+Cho compared to MD (+21%, p = 0.096) and in Alc+Cho compared to Alc (+22%, p = 0.096; Figure 3B). In contrast, neither PAE nor choline supplementation had significant effects on female placental weight or efficiency (Figure 3A–B).
Table 2:
| Independent Variables | F Statistics | p Values | β Estimate | F Statistics | p Values | β Estimate |
|---|---|---|---|---|---|---|
|
|
||||||
| Males | Females | |||||
|
| ||||||
| Figure 3A: Placental weight | ||||||
| Alcohol exposure | F = 7.078 | p = 0.017 | −0.080 | F = 3.425 | p = 0.067 | −0.036 |
| Choline supplement | F = 0.765 | p = 0.395 | −0.042 | F = 2.751 | p = 0.100 | 0.046 |
| Interaction | F = 0.830 | p = 0.376 | 0.041 | F = 0.132 | p = 0.717 | −0.016 |
|
| ||||||
| Figure 3B: Placental efficiency | ||||||
| Alcohol exposure | F = 0.211 | p = 0.650 | 0.009 | F = 0.117 | p = 0.735 | −0.039 |
| Choline supplement | F = 8.788 | p = 0.006 | 0.144 | F = 4.122 | p = 0.052 | 0.091 |
| Interaction | F = 0.083 | p = 0.776 | 0.031 | F = 0.142 | p = 0.709 | 0.041 |
|
| ||||||
| Figure 4A: Fetal body weight | ||||||
| Alcohol exposure | F = 0.319 | p = 0.577 | −0.064 | F = 0.670 | p = 0.420 | −0.067 |
| Choline supplement | F = 3.625 | p = 0.068 | 0.041 | F = 4.814 | p = 0.037 | 0.066 |
| Interaction | F = 0.894 | p = 0.353 | 0.081 | F = 0.491 | p = 0.489 | 0.062 |
|
| ||||||
| Figure 4B: Fetal brain weight | ||||||
| Alcohol exposure | F = 3.208 | p = 0.084 | −0.005 | F = 1.532 | p = 0.227 | −0.002 |
| Choline supplement | F = 1.105 | p = 0.302 | −0.003 | F = 0.936 | p = 0.342 | −0.001 |
| Interaction | F = 0.207 | p = 0.653 | 0.002 | F = 0.182 | p = 0.673 | −0.002 |
|
| ||||||
| Figure 4C: Fetal brain-to-body weight ratio | ||||||
| Alcohol exposure | F = 1.280 | p = 0.268 | −0.015 | F = 0.217 | p = 0.645 | 0.044 |
| Choline supplement | F = 14.189 | p = 0.001 | −0.108 | F = 22.356 | p < 0.001 | −0.083 |
| Interaction | F = 0.474 | p = 0.497 | −0.048 | F = 3.719 | p = 0.066 | −0.115 |
|
| ||||||
| Figure 4D: Fetal liver weight | ||||||
| Alcohol exposure | F = 15.480 | p = 0.001 | −0.193 | F = 9.862 | p = 0.004 | −0.217 |
| Choline supplement | F = 0.197 | p = 0.661 | 0.020 | F = 2.331 | p = 0.139 | 0.041 |
| Interaction | F = 0.001 | p = 0.973 | 0.003 | F = 0.611 | p = 0.441 | 0.087 |
|
| ||||||
| Figure 4E: Fetal liver-to-body weight ratio | ||||||
| Alcohol exposure | F = 17.926 | p < 0.001 | −0.006 | F = 9.516 | p = 0.005 | −0.007 |
| Choline supplement | F = 2.280 | p = 0.142 | −0.001 | F = 0.034 | p = 0.855 | −0.001 |
| Interaction | F = 1.200 | p = 0.282 | −0.004 | F = 0.020 | p = 0.888 | 0.001 |
|
| ||||||
| Figure 4F: Fetal brain-to-liver weight ratio | ||||||
| Alcohol exposure | F = 11.200 | p = 0.002 | 0.104 | F = 12.272 | p = 0.002 | 0.183 |
| Choline supplement | F = 3.589 | p = 0.069 | −0.096 | F = 14.140 | p = 0.001 | −0.071 |
| Interaction | F = 0.394 | p = 0.536 | 0.048 | F = 2.960 | p = 0.097 | −0.120 |
Figure 3:
Effect of prenatal alcohol exposure and choline supplementation on placental weight (A) and placental efficiency (B). Placental efficiency is calculated as the ratio of fetal body weight to placental weight. Data were analyzed separately for males and females using mixed linear model followed by post hoc Benjamini-Hochberg corrections to adjust for multiple comparisons. Values are means ± SEMs. n=8 litters for MD, Alc, MD+Cho, and Alc+Cho groups. Each dot represents an individual placenta. Treatment group labeled with * has a significant main effect (p < 0.05). † denotes a significant main effect of fetal sex or interaction with fetal sex (p < 0.05). # within the graph denotes 0.05 ≤ p < 0.1 for the indicated comparison. Abbreviations: Alc, alcohol; Cho, choline; MD, maltodextrin.
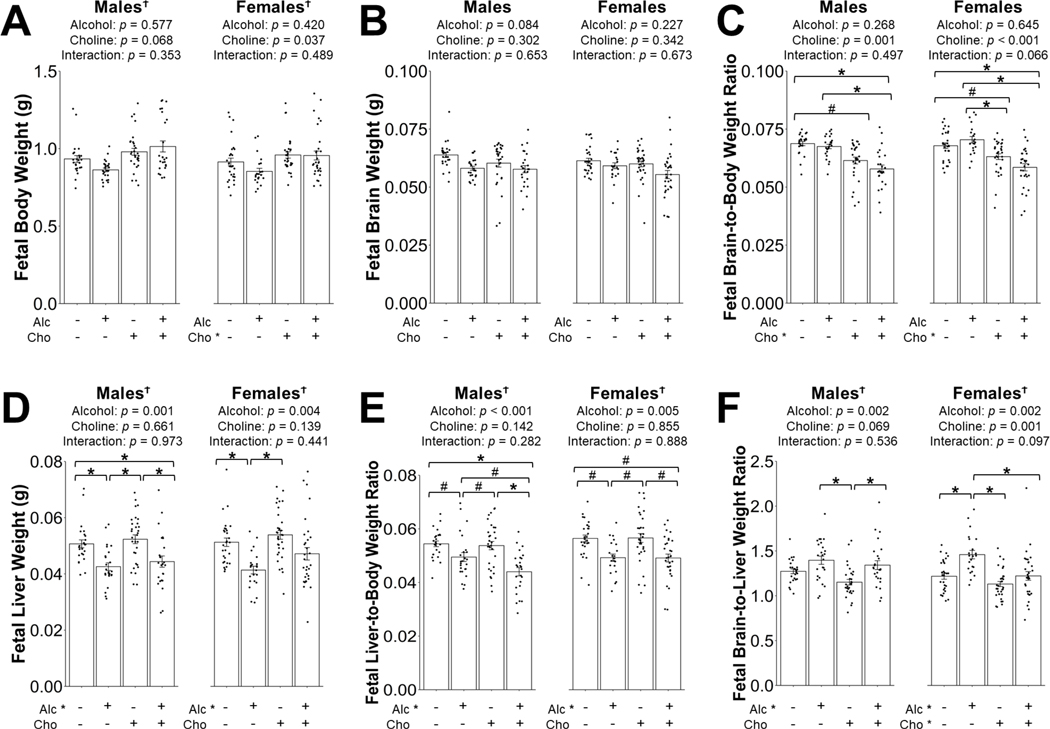
Fetal outcomes: Body and brain weights
Summary of the MLM analyses on all fetal outcomes is provided in Table 2. In males, PAE nor choline significantly affected the absolute body and brain weights (Figure 4A–B). Interestingly, choline supplementation decreased the male fetal brain-to-body weight ratio, and post hoc analyses indicated that this was reduced in both MD+Cho (−12% compared to MD, p = 0.070) and in Alc+Cho (−13% compared to Alc, p = 0.012; −16% compared to MD, p = 0.012; Figure 4C). No significant differences in the brain-to-body weight ratio were found between the Alc+Cho and MD+Cho males.
Figure 4:
Effect of prenatal alcohol exposure and choline supplementation on fetal body weight (A), fetal brain weight (B), fetal brain-to-body weight ratio (C), fetal liver weight (D), fetal liver-to-body weight ratio (E), and fetal brain-to-liver weight ratio (F). Data were analyzed separately for males and females using mixed linear model followed by post hoc Benjamini-Hochberg corrections to adjust for multiple comparisons. Values are means ± SEMs. n=8 litters for MD, Alc, MD+Cho, and Alc+Cho groups. Each dot represents an individual fetus. Treatment group labeled with * has a significant main effect (p < 0.05). † denotes a significant main effect of fetal sex or interaction with fetal sex (p < 0.05). * within the graph denotes p < 0.05 for the indicated comparison. # within the graph denotes 0.05 ≤ p < 0.1 for the indicated comparison. Abbreviations: Alc, alcohol; Cho, choline; MD, maltodextrin.
In females, a main effect of choline in increasing absolute body weight, but not absolute brain weight, was found (Figure 4A–B). As seen in males, choline supplementation also decreased the brain-to-body weight ratio in both MD+Cho (−7% compared to MD, p = 0.086) and Alc+Cho (−16% compared to Alc, p < 0.001; −13% compared to MD, p = 0.003; Figure 4C). No significant differences in the brain-to-body weight ratio were found between the Alc+Cho and MD+Cho females.
Fetal outcomes: Liver weights
Alcohol significantly decreased the absolute and relative fetal liver weights. Specifically, Alc males had 16% lower liver weight compared to the MD males, (p = 0.022; Figure 4D) and 9% lower liver-to-body weight ratio (p = 0.068; Figure 4E). Choline supplementation did not improve liver weight or liver-to-body weight ratios. Compared with MD+Cho, Alc+Cho males still had a lower liver weight (−15%, p = 0.022; Figure 4D) and liver-to-body weight ratio (−19%, p = 0.003; Figure 4E). Because the body weight was slightly higher in Alc+Cho than Alc males, the liver-to-body weight ratio was lower in Alc+Cho compared to Alc (−10%, p = 0.097; Figure 4E). Compared to MD, Alc+Cho showed a lower liver weight (−14%, p = 0.033; Figure 4D) and liver-to-body weight ratio (−19%, p < 0.001; Figure 4E).
Similar to their male littermates, alcohol significantly decreased the absolute and relative liver weights of the female fetuses. Alc females had a 20% lower liver weight than MD (p = 0.033; Figure 4D) and 13% lower liver-to-body weight ratio (p = 0.078; Figure 4E). Alc+Cho female liver weights did not differ from MD or MD+Cho (p = 0.31 and 0.17, respectively; Figure 4D). However, choline supplementation did not improve the effect of Alc on the fetal liver-to-body weight ratio (Alc+Cho vs. MD, −13%, p = 0.078; Alc+Cho vs. MD+Cho, −14%, p = 0.078; Figure 4E). Overall, these findings show that prenatal choline was unable to restore the alcohol-reduced liver weight.
Fetal brain-to-liver weight ratio was calculated to determine fetal growth quality, where a higher ratio indicates the presence of asymmetric growth. Alcohol significantly increased, whereas choline decreased, this ratio in both sexes. In males, this ratio was non-significantly higher in Alc and Alc+Cho (vs. MD, p = 0.12 and 0.39, respectively; Figure 4F), but was significantly higher in Alc+Cho males compared to MD+Cho males (+17%, p = 0.030; Figure 4F). In females, the brain-to-liver weight ratio was higher in the Alc animals compared to MD (+20%, p = 0.002; Figure 4F), and this alcohol effect was mitigated by choline supplementation (Alc+Cho vs. Alc, p = 0.002; Figure 4F). Overall, these data suggest that choline corrected the alcohol-induced asymmetric fetal growth in a sex-specific manner.
Correlation analyses
Irrespective of fetal sex, placental weight was inversely correlated with litter size (males: R = −0.41, p < 0.001; females: R = −0.40, p < 0.001) whereas placental efficiency was positively correlated with litter size (males: R = 0.29, p = 0.008; females: R = 0.21, p = 0.046). In addition, a positive correlation between litter size and liver-to-body weight ratio (R = 0.26, p = 0.024), as well as an inverse correlation between litter size and brain-to-liver weight ratio (R = −0.23, p = 0.035), were found only in the female fetuses. No other associations were detected (Table 3). Correlation analyses also showed a positive correlation between placental weight and brain weight (R = 0.25, p = 0.046) in the male fetuses. However, none of the female fetal morphometric measurements were associated with their placental weights (Table 4). Unlike placental weight, placental efficiency was associated with many fetal morphometric endpoints in a manner largely independent of fetal sex (Table 4). Specifically, it was positively associated with fetal body weight (males and females: R = 0.74, p < 0.001), brain weight (males: R = 0.20, p = 0.043; females: R = 0.36, p < 0.001), and liver weight (males: R = 0.29, p = 0.005; females: R = 0.44, p < 0.001), but inversely associated with brain-to-body weight ratio (males: R = −0.54, p < 0.001; females: R = −0.42, p < 0.001). Placental efficiency was also inversely associated with liver-to-body weight ratio in the males (R = −0.25, p = 0.011) while inversely correlated with brain-to-liver weight ratio in the females (R = −0.23, p = 0.017).
Table 3:
Correlation of the morphometric measurements of the placentas and fetuses with their litter size. a
| Males Only | Females Only | |||||
|---|---|---|---|---|---|---|
| R | P unadjusted | P BH-adjusted | R | P unadjusted | P BH-adjusted | |
|
| ||||||
| Placental weight | −0.41 | < 0.001 | < 0.001 * | −0.40 | < 0.001 | < 0.001 * |
| Placental efficiency b | 0.29 | 0.002 | 0.008 * | 0.21 | 0.023 | 0.046 * |
| Fetal body weight | 0.05 | 0.60 | 0.80 | −0.06 | 0.53 | 0.69 |
| Fetal brain weight | −0.15 | 0.11 | 0.18 | −0.05 | 0.60 | 0.69 |
| Fetal liver weight | 0.02 | 0.85 | 0.94 | 0.16 | 0.10 | 0.15 |
| Fetal brain-to-body weight | −0.2 | 0.041 | 0.11 | 0.009 | 0.92 | 0.92 |
| Fetal liver-to-body weight | −0.007 | 0.94 | 0.94 | 0.26 | 0.006 | 0.024 * |
| Fetal brain-to-liver weight | −0.16 | 0.09 | 0.18 | −0.23 | 0.013 | 0.035 * |
Litters from all 4 treatments were combined and included in the analyses. n=8 litters for MD, Alc, MD+Cho, and Alc+Cho groups. p values before and after multiple testing adjustment using Benjamini-Hochberg method are presented.
denotes statistical significance at p < 0.05.
Placental efficiency is calculated as the ratio of fetal body weight to placental weight.
Table 4:
Correlation of the morphometric measurements of the fetuses with their placental weight and efficiency. a,b
| Males Only | Correlation with Placental Weight | Correlation with Placental Efficiency | ||||
|---|---|---|---|---|---|---|
| R | P unadjusted | P BH-adjusted | R | P unadjusted | P BH-adjusted | |
|
| ||||||
| Fetal body weight | 0.08 | 0.40 | 0.48 | 0.74 | < 0.001 | < 0.001 * |
| Fetal brain weight | 0.25 | 0.008 | 0.046 * | 0.20 | 0.036 | 0.043 * |
| Fetal liver weight | 0.18 | 0.063 | 0.19 | 0.29 | 0.002 | 0.005 * |
| Fetal brain-to-body weight | 0.15 | 0.12 | 0.23 | −0.54 | < 0.001 | < 0.001 * |
| Fetal liver-to-body weight | 0.14 | 0.15 | 0.23 | −0.25 | 0.008 | 0.011 * |
| Fetal brain-to-liver weight | −0.05 | 0.64 | 0.64 | −0.16 | 0.10 | 0.10 |
|
| ||||||
| Females Only | Correlation with Placental Weight | Correlation with Placental Efficiency | ||||
| R | P unadjusted | P BH-adjusted | R | P unadjusted | P BH-adjusted | |
|
| ||||||
| Fetal body weight | 0.10 | 0.32 | 0.88 | 0.74 | < 0.001 | < 0.001 * |
| Fetal brain weight | 0.08 | 0.42 | 0.88 | 0.36 | < 0.001 | < 0.001 * |
| Fetal liver weight | 0.07 | 0.49 | 0.88 | 0.44 | < 0.001 | < 0.001 * |
| Fetal brain-to-body weight | −0.05 | 0.64 | 0.88 | −0.42 | < 0.001 | < 0.001 * |
| Fetal liver-to-body weight | 0.02 | 0.88 | 0.88 | −0.07 | 0.49 | 0.49 |
| Fetal brain-to-liver weight | −0.03 | 0.76 | 0.88 | −0.23 | 0.014 | 0.017 * |
Litters from all 4 treatments were combined and included in the analyses. n=8 litters for MD, Alc, MD+Cho, and Alc+Cho groups. p values before and after multiple testing adjustment using Benjamini-Hochberg method are presented.
denotes statistical significance at p < 0.05.
Placental efficiency is calculated as the ratio of fetal body weight to placental weight.
DISCUSSION
The most important finding of our study is identifying the placenta, an organ that is often overlooked in the FASD-choline literature, as another critical target of prenatal choline supplementation, which is already well-known to improve brain development and function. Specifically, we found that prenatal choline supplementation strongly increased placental efficiency. Furthermore, in males, choline concurrent with PAE trended to decrease the absolute placental weight but trended to increase the placental efficiency. Like the fetus, the placenta requires nutrients to support its growth and function. A smaller placenta requires less nutrients to sustain its size, thereby reducing competition with the fetus and sparing those nutrients for fetal use, and thus leading to a higher placental efficiency. Our findings suggest that choline may minimize a metabolic competition between the placenta and fetus by better allocating the available nutrients between them, so that the placenta can more efficiently support the fetus even under adverse intrauterine conditions like PAE.
We have previously reported that the placenta plays a central role in fetal responses to intrauterine stressors such as PAE and maternal protein insufficiency (Kwan et al., 2020). Our present study complements and extends these findings to demonstrate that the placenta is also involved in fetal responses to presumed positive intrauterine exposures such as choline intervention. However, whereas both placental weight and efficiency predict fetal growth in the presence of adverse stressors, only placental efficiency significantly correlates with various fetal morphometric outcomes in the presence of prenatal choline supplementation, underscoring the importance of assessing both outcomes.
Although placental efficiency, or the ratio of fetal weight to placental weight, is not a functional measure itself, it is associated with placental nutrient supply capacity (Hayward et al., 2016). It is influenced by placental vascular development and perfusion, and placental nutrient transport and metabolism; these processes are regulated, in part, by the placental epigenome (Winterhager and Gellhaus, 2017, Fowden et al., 2006). Alcohol is known to impair these placental processes (Marjonen et al., 2018, Subramanian et al., 2014, Gundogan et al., 2008, Falconer, 1990, Gundogan et al., 2015, Burd et al., 2007, Haycock and Ramsay, 2009, Loke et al., 2018). Whether choline is effective in reversing these alcohol-induced placental damages awaits further investigation. However, in healthy pregnant women (Jiang et al., 2012, Jiang et al., 2013), healthy pregnant mice (Kwan et al., 2017a, Kwan et al., 2017b, Kwan et al., 2018), and animal models of pregnancy disorders (King et al., 2017, King et al., 2019, Nam et al., 2017), maternal choline supplementation has been shown to improve these placental processes to enhance nutrient supply to the fetus and its organs, which contribute to better development. These additional in-depth analyses of the alcohol-exposed placentas are important because interpreting the placental efficiency ratio alone provides limited information and may lead to inaccurate conclusions as suggested in some scenarios of human pregnancy (Christians et al., 2018). Given that PAE dysregulates the availability of nutrients such as amino acids essential for neurotransmitter synthesis in the brain (Lunde-Young et al., 2018) and has lifelong adverse impacts on neurological outcomes (Lunde et al., 2016), our data suggest that improving placental efficiency in PAE pregnancy via prenatal choline intervention may support neurodevelopment, in part, by increasing the supply of nutrient substrates essential for critical processes such as synaptogenesis and neurotransmission.
Our previous work examining prenatal co-exposure to alcohol and a maternal low protein diet also documents sex-dependent placental adaptation and prediction of fetal growth, with placental weight predicting fetal growth only in the females, whereas placental efficiency predicts fetal growth in a sex-independent manner (Kwan et al., 2020). We propose that this sexual dimorphic response may be related to the different developmental strategies employed by the females and males, where the males’ strategy is deemed to be riskier because they utilize the available nutrients to support their somatic growth, in contrast to the females’ strategy of investing these resources in their placental development and reserves to prepare for additional challenges in the future. Here we find that placental weight no longer predicts fetal growth in the presence of prenatal choline supplementation in either males or females. Instead, litter size modulates the effects on placental weight and efficiency, the latter of which then drives and predicts fetal growth in a sex-independent manner. It is possible that choline may create an intrauterine environment that “levels the playing field” for both the alcohol-exposed males and females. Under such circumstance, their developmental strategies become comparable, and their placentas no longer need to respond in a manner that requires them to choose between utilizing the resources for supporting growth or investing and preparing for future insults. As such, the sexual dimorphic placental response is no longer apparent under positive prenatal exposure of choline supplementation. Indeed, we argue that supplementing choline in PAE pregnancy may actually favor the developmental strategy of the males, who otherwise may not be able to survive and thrive because of placental failure. This perhaps explains the modulatory effects of litter size on placental outcomes and the more pronounced effects of prenatal choline supplementation in the alcohol-exposed males in comparison to their female littermates observed in our study.
Another important finding of our study is that choline’s effects differ between each alcohol-exposed fetal organ. Growth relationships among fetal organs are often presented as ratios of organ-body weight and organ-organ weight, which can indicate fetal growth quality and inform candidate mechanisms mediating abnormal fetal growth (Torres-Rovira et al., 2013). Our prior work shows that alcohol increases the brain-to-body weight ratio, which indicates the presence of “brain-sparing” (Kwan et al., 2020). The effects of PAE on brain sparing are influenced by factors such as alcohol doses, developmental window of alcohol exposure, species and strains, age of assessment, and maternal nutritional status (Xu et al., 2008, Maier et al., 1999, Breit et al., 2019). In addition to a higher brain-to-body weight ratio, we also found that alcohol increases the brain-to-liver weight ratio and lowers liver-to-body weight ratio (Kwan et al., 2020). When the placenta supplies insufficient oxygen and nutrients to the fetus, the fetus adapts by redistributing its blood circulation to preferentially supply these resources to the brain rather than liver. This increases the brain-to-body weight ratio, and limits liver growth to generate a lower liver-to-body weight ratio. The brain-to-liver weight ratio further informs whether the fetal weight reduction is symmetric or asymmetric, with a higher ratio indicating asymmetric growth and potential placental dysfunctions (Godfrey et al., 2012, Roza et al., 2008).
Fetuses whose mothers received choline supplementation, particularly the females, had higher body weight but not absolute brain weight. This indicates that choline did not increase fetal brain weight proportionally to fetal body weight, suggesting that choline may have improved the oxygen and nutrient supply such that these resources are no longer prioritized for the brain, but are now sufficient to also support somatic growth. This is reflected in the lower fetal brain-to-body weight ratio, suggesting that choline corrects the alcohol-induced brain-sparing phenotype. Because the presence of fetal brain-sparing can indicate a higher risk of behavioral problems in later life (Roza et al., 2008), we predict that the choline-supplemented offspring are less likely to have behavioral issues in postnatal life. Thus, our data are consistent with previous reports of the neuroprotective effects of choline, which is known to mitigate neuroanatomical abnormalities and cognitive and behavioral impairments induced by PAE (Thomas et al., 2009, Thomas et al., 2010, Kable et al., 2015, Idrus et al., 2017, Jacobson et al., 2018, Bottom et al., 2020, Warton et al., 2021).
Given that choline normalized “brain-sparing”, implying a normalized blood distribution among the fetal organs, we would expect that choline would also normalize fetal liver growth. However, we found that prenatal choline supplementation does not protect against the alcohol-induced reduction in fetal liver weight or fetal liver-to-body weight ratio nor does it improve the brain-to-liver weight ratio in males. The growth deficits in liver may impair its functions and potentially contribute to the metabolic syndrome of PAE observed in later life (Gårdebjer et al., 2018, Weeks et al., 2020, Lunde et al., 2016). Given choline’s well-established benefits in preventing liver dysfunction in non-alcohol-drinking adults (Zeisel and da Costa, 2009), and the ability of betaine (a choline derivative) to alleviate liver injury in alcohol-drinking animals (Barak et al., 1994, Ji and Kaplowitz, 2003), and our observation that prenatal choline corrects the alcohol-induced fetal brain-sparing phenotype, this surprising finding highlights the possibility that fetal liver may respond to choline differently from fetal brain.
The reasons for this differential response to choline between fetal brain and liver are unknown. Because of the high choline demand during PAE pregnancy (Caudill et al., 2020, Zeisel, 2011), it is possible that the dose of our choline supplement may still be insufficient for the alcohol-exposed fetuses, as this dose represents an additional 75% of their estimated daily choline intake (National Research Council, 1995). As such, the alcohol-exposed fetus may still prioritize the choline supply to support brain development. This argument is further supported by a recent study in a rat model of periconceptional alcohol exposure, which reports improved fetal liver growth in offspring whose mothers receive choline supplementation at 2X and 4X the recommended intake level (Steane et al., 2021). Choline uptake between fetal brain and liver may also differ because the ubiquitous choline transporter SLC44A1, which encodes the choline transporter-like protein 1 (CTL1), is highly abundant in the fetal brain but is expressed in lower amounts in fetal liver (Yuan et al., 2004). Additional research is warranted to determine if higher choline doses would protect both fetal brain and liver against alcohol’s toxicity in this FASD model and thereby improve the cognitive and metabolic health of individuals prenatally exposed to this insult.
In conclusion, our study demonstrates that choline given concurrently with PAE prevents body weight reduction and prevents brain-sparing in alcohol-exposed fetuses in a sex-independent fashion, and improves placental efficiency predominantly in alcohol-exposed males. Although molecular mechanisms mediating the changes by choline require further investigation, our findings highlight the necessity of studying the alcohol-choline interaction not just in the fetal brain but also in other fetal organs, especially the placenta which is known to program future offspring health (Thornburg et al., 2016) and has strong predictability for fetal morphometric markers as revealed by our correlation analyses. Our study also identifies several unique benefits of prenatal choline supplementation independent of PAE, which may be related to the suboptimal choline content in the AIN-93G diet (Klurfeld et al., 2021). This mirrors the human situation where the choline intake is similarly lower than optimal in many pregnant women (Jensen et al., 2007, Wallace and Fulgoni, 2017). Thus, our study also offers an opportunity to examine the benefits of choline supplementation in pregnancy without PAE. Given that pregnant women who drink alcohol during pregnancy (May et al., 2014, Carter et al., 2017) also do not consume sufficient choline to meet their higher need (Caudill et al., 2020, Zeisel, 2011), our study provides additional data to the growing FASD-choline literature supporting prenatal choline supplementation as a promising therapy to improve the health outcomes of individuals who are exposed to alcohol during gestation.
Acknowledgements
The authors thank Katie Walter for performing the sex genotyping of these fetuses, Alyson Selchick and Olivia Rivera for technical assistance, and Balchem for the kind donation of choline.
This work was supported by NIH/NIAAA awards R01AA024980 and R01AA022413 to SMM, R01AA011085 and R01AA022999 to SMS, F32AA027121 to STCK, and internal funding from the UNC Nutrition Research Institute.
Footnotes
The authors have no conflicts of interest to declare.
References
- AKISON LK, KUO J, REID N, BOYD RN & MORITZ KM 2018. Effect of Choline Supplementation on Neurological, Cognitive, and Behavioral Outcomes in Offspring Arising from Alcohol Exposure During Development: A Quantitative Systematic Review of Clinical and Preclinical Studies. Alcohol Clin Exp Res, 42, 1591–1611. [DOI] [PubMed] [Google Scholar]
- ALTMAN DG & BLAND JM 2005. Standard deviations and standard errors. Bmj, 331, 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARAK AJ, BECKENHAUER HC & TUMA DJ 1994. S-adenosylmethionine generation and prevention of alcoholic fatty liver by betaine. Alcohol, 11, 501–3. [DOI] [PubMed] [Google Scholar]
- BEARER CF, WELLMANN KA, TANG N, HE M. & MOONEY SM 2015. Choline Ameliorates Deficits in Balance Caused by Acute Neonatal Ethanol Exposure. Cerebellum, 14, 413–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOTTOM RT, ABBOTT CW 3RD & HUFFMAN KJ 2020. Rescue of ethanol-induced FASD-like phenotypes via prenatal co-administration of choline. Neuropharmacology, 168, 107990. [DOI] [PubMed] [Google Scholar]
- BREIT KR, ZAMUDIO B. & THOMAS JD 2019. The effects of alcohol and cannabinoid exposure during the brain growth spurt on behavioral development in rats. Birth Defects Res, 111, 760–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURD L, ROBERTS D, OLSON M. & ODENDAAL H. 2007. Ethanol and the placenta: A review. J Matern Fetal Neonatal Med, 20, 361–75. [DOI] [PubMed] [Google Scholar]
- CARTER RC, SENEKAL M, DODGE NC, BECHARD LJ, MEINTJES EM, MOLTENO CD, DUGGAN CP, JACOBSON JL & JACOBSON SW 2017. Maternal Alcohol Use and Nutrition During Pregnancy: Diet and Anthropometry. Alcohol Clin Exp Res, 41, 2114–2127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAUDILL MA, OBEID R, DERBYSHIRE E, BERNHARD W, LAPID K, WALKER SJ & ZEISEL SH 2020. Building better babies: should choline supplementation be recommended for pregnant and lactating mothers? Literature overview and expert panel consensus. Eur Gyn Obstet, 2, 149–161. [Google Scholar]
- CHRISTIANS JK, GRYNSPAN D, GREENWOOD SL & DILWORTH MR 2018. The problem with using the birthweight:placental weight ratio as a measure of placental efficiency. Placenta, 68, 52–58. [DOI] [PubMed] [Google Scholar]
- DENNY CH, ACERO CS, NAIMI TS & KIM SY 2019. Consumption of alcohol beverages and binge drinking among pregnant women aged 18–44 years - United States, 2015–2017. MMWR Morb Mortal Wkly Rep, 68, 365–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FALCONER J. 1990. The effect of maternal ethanol infusion on placental blood flow and fetal glucose metabolism in sheep. Alcohol Alcohol, 25, 413–6. [PubMed] [Google Scholar]
- FOWDEN AL, SIBLEY C, REIK W. & CONSTANCIA M. 2006. Imprinted genes, placental development and fetal growth. Horm Res, 65 Suppl 3, 50–8. [DOI] [PubMed] [Google Scholar]
- GÅRDEBJER EM, CUFFE JSM, WARD LC, STEANE S, ANDERSON ST, DOREY ES, KALISCH-SMITH JI, PANTALEON M, CHONG S, YAMADA L, WLODEK ME, BIELEFELDT-OHMANN H. & MORITZ KM 2018. Effects of periconceptional maternal alcohol intake and a postnatal high-fat diet on obesity and liver disease in male and female rat offspring. Am J Physiol Endocrinol Metab, 315, E694–e704. [DOI] [PubMed] [Google Scholar]
- GODFREY KM, HAUGEN G, KISERUD T, INSKIP HM, COOPER C, HARVEY NCW, CROZIER SR, ROBINSON SM, DAVIES L, SOUTHAMPTON WOMEN’S SURVEY STUDY, G. & HANSON MA 2012. Fetal liver blood flow distribution: role in human developmental strategy to prioritize fat deposition versus brain development. PloS one, 7, e41759–e41759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRUBBS FE 1969. Procedures for Detecting Outlying Observations in Samples. Technometrics, 11, 1–21. [Google Scholar]
- GUNDOGAN F, ELWOOD G, LONGATO L, TONG M, FEIJOO A, CARLSON RI, WANDS JR & DE LA MONTE SM 2008. Impaired placentation in fetal alcohol syndrome. Placenta, 29, 148–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GUNDOGAN F, GILLIGAN J, QI W, CHEN E, NARAM R. & DE LA MONTE SM 2015. Dose effect of gestational ethanol exposure on placentation and fetal growth. Placenta, 36, 523–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAYCOCK PC & RAMSAY M. 2009. Exposure of mouse embryos to ethanol during preimplantation development: effect on DNA methylation in the h19 imprinting control region. Biol Reprod, 81, 618–27. [DOI] [PubMed] [Google Scholar]
- HAYWARD CE, LEAN S, SIBLEY CP, JONES RL, WAREING M, GREENWOOD SL & DILWORTH MR 2016. Placental adaptation: What can we learn from birthweight:placental weight ratio? Front Physiol, 7, 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOYME HE, KALBERG WO, ELLIOTT AJ, BLANKENSHIP J, BUCKLEY D, MARAIS AS, MANNING MA, ROBINSON LK, ADAM MP, ABDUL-RAHMAN O, JEWETT T, COLES CD, CHAMBERS C, JONES KL, ADNAMS CM, SHAH PE, RILEY EP, CHARNESS ME, WARREN KR & MAY PA 2016. Updated Clinical Guidelines for Diagnosing Fetal Alcohol Spectrum Disorders. Pediatrics, 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IDRUS NM, BREIT KR & THOMAS JD 2017. Dietary choline levels modify the effects of prenatal alcohol exposure in rats. Neurotoxicol Teratol, 59, 43–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON SW, CARTER RC, MOLTENO CD, STANTON ME, HERBERT JS, LINDINGER NM, LEWIS CE, DODGE NC, HOYME HE, ZEISEL SH, MEINTJES EM, DUGGAN CP & JACOBSON JL 2018. Efficacy of Maternal Choline Supplementation During Pregnancy in Mitigating Adverse Effects of Prenatal Alcohol Exposure on Growth and Cognitive Function: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Alcohol Clin Exp Res, 42, 1327–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JENSEN HH, BATRES-MARQUEZ SP, CARRIQUIRY A. & SCHALINSKE KL 2007. Choline in the diets of the US population: NHANES, 2003–2004 FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology 21, lb219. [Google Scholar]
- JI C. & KAPLOWITZ N. 2003. Betaine decreases hyperhomocysteinemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology, 124, 1488–99. [DOI] [PubMed] [Google Scholar]
- JIANG X, BAR HY, YAN J, JONES S, BRANNON PM, WEST AA, PERRY CA, GANTI A, PRESSMAN E, DEVAPATLA S, VERMEYLEN F, WELLS MT & CAUDILL MA 2013. A higher maternal choline intake among third-trimester pregnant women lowers placental and circulating concentrations of the antiangiogenic factor fms-like tyrosine kinase-1 (sFLT1). FASEB J, 27, 1245–53. [DOI] [PubMed] [Google Scholar]
- JIANG X, YAN J, WEST AA, PERRY CA, MALYSHEVA OV, DEVAPATLA S, PRESSMAN E, VERMEYLEN F. & CAUDILL MA 2012. Maternal choline intake alters the epigenetic state of fetal cortisol-regulating genes in humans. FASEB J, 26, 3563–74. [DOI] [PubMed] [Google Scholar]
- KABLE JA, COLES CD, KEEN CL, URIU-ADAMS JY, JONES KL, YEVTUSHOK L, KULIKOVSKY Y, WERTELECKI W, PEDERSEN TL & CHAMBERS CD 2015. The impact of micronutrient supplementation in alcohol-exposed pregnancies on information processing skills in Ukrainian infants. Alcohol, 49, 647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING JH, KWAN STC, YAN J, JIANG X, FOMIN VG, LEVINE SP, WEI E, ROBERSON MS & CAUDILL MA 2019. Maternal choline supplementation modulates placental markers of inflammation, angiogenesis, and apoptosis in a mouse model of placental insufficiency. Nutrients, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING JH, KWAN STC, YAN J, KLATT KC, JIANG X, ROBERSON MS & CAUDILL MA 2017. Maternal choline supplementation alters fetal growth patterns in a mouse model of placental insufficiency. Nutrients, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLURFELD DM, GREGORY JF & FIOROTTO ML 2021. Should the AIN-93 Rodent Diet Formulas be Revised? J Nutr, 151, 1380–1382. [DOI] [PubMed] [Google Scholar]
- KROMBEEN SK, BRIDGES WC, WILSON ME & WILMOTH TA 2019. Factors contributing to the variation in placental efficiency on days 70, 90, and 110 of gestation in gilts. J Anim Sci, 97, 359–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWAN STC, KING JH, GRENIER JK, YAN J, JIANG X, ROBERSON MS & CAUDILL MA 2018. Maternal choline supplementation during normal murine pregnancy alters the placental epigenome: Results of an exploratory study. Nutrients, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWAN STC, KING JH, YAN J, JIANG X, WEI E, FOMIN VG, ROBERSON MS & CAUDILL MA 2017a. Maternal choline supplementation during murine pregnancy modulates placental markers of inflammation, apoptosis and vascularization in a fetal sex-dependent manner. Placenta, 53, 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWAN STC, KING JH, YAN J, WANG Z, JIANG X, HUTZLER JS, KLEIN HR, BRENNA JT, ROBERSON MS & CAUDILL MA 2017b. Maternal choline supplementation modulates placental nutrient transport and metabolism in late gestation of mouse pregnancy. The Journal of Nutrition, 147, 2083–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KWAN STC, PRESSWOOD BH, HELFRICH KK, BAULCH JW, MOONEY SM & SMITH SM 2020. An interaction between fetal sex and placental weight and efficiency predicts intrauterine growth in response to maternal protein insufficiency and gestational exposure window in a mouse model of FASD. Biol Sex Differ, 11, 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOKE YJ, MUGGLI E, NGUYEN L, RYAN J, SAFFERY R, ELLIOTT EJ, HALLIDAY J. & CRAIG JM 2018. Time- and sex-dependent associations between prenatal alcohol exposure and placental global DNA methylation. Epigenomics, 10, 981–991. [DOI] [PubMed] [Google Scholar]
- LUNDE-YOUNG R, DAVIS-ANDERSON K, NAIK V, NEMEC M, WU G. & RAMADOSS J. 2018. Regional dysregulation of taurine and related amino acids in the fetal rat brain following gestational alcohol exposure. Alcohol (Fayetteville, N.Y.), 66, 27–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUNDE ER, WASHBURN SE, GOLDING MC, BAKE S, MIRANDA RC & RAMADOSS J. 2016. Alcohol-Induced Developmental Origins of Adult-Onset Diseases. Alcohol Clin Exp Res, 40, 1403–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAIER SE, MILLER JA & WEST JR 1999. Prenatal binge-like alcohol exposure in the rat results in region-specific deficits in brain growth. Neurotoxicol Teratol, 21, 285–91. [DOI] [PubMed] [Google Scholar]
- MARJONEN H, TOIVONEN M, LAHTI L. & KAMINEN-AHOLA N. 2018. Early prenatal alcohol exposure alters imprinted gene expression in placenta and embryo in a mouse model. PLoS One, 13, e0197461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY PA, CHAMBERS CD, KALBERG WO, ZELLNER J, FELDMAN H, BUCKLEY D, KOPALD D, HASKEN JM, XU R, HONERKAMP-SMITH G, TARAS H, MANNING MA, ROBINSON LK, ADAM MP, ABDUL-RAHMAN O, VAUX K, JEWETT T, ELLIOTT AJ, KABLE JA, AKSHOOMOFF N, FALK D, ARROYO JA, HERELD D, RILEY EP, CHARNESS ME, COLES CD, WARREN KR, JONES KL & HOYME HE 2018. Prevalence of fetal alcohol spectrum disorders in 4 US communities. Jama, 319, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAY PA, HAMRICK KJ, CORBIN KD, HASKEN JM, MARAIS AS, BROOKE LE, BLANKENSHIP J, HOYME HE & GOSSAGE JP 2014. Dietary intake, nutrition, and fetal alcohol spectrum disorders in the Western Cape Province of South Africa. Reprod Toxicol, 46, 31–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAM J, GREENWALD E, JACK-ROBERTS C, AJEEB TT, MALYSHEVA OV, CAUDILL MA, AXEN K, SAXENA A, SEMERNINA E, NANOBASHVILI K. & JIANG X. 2017. Choline prevents fetal overgrowth and normalizes placental fatty acid and glucose metabolism in a mouse model of maternal obesity. J Nutr Biochem, 49, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NATIONAL RESEARCH COUNCIL 1995. Nutrient requirements of laboratory animals,: fourth revised edition, 1995, Washington, DC, The National Academies Press. [PubMed] [Google Scholar]
- REEVES PG, NIELSEN FH & FAHEY GC JR. 1993. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr, 123, 1939–51. [DOI] [PubMed] [Google Scholar]
- ROZA SJ, STEEGERS EAP, VERBURG BO, JADDOE VWV, MOLL HA, HOFMAN A, VERHULST FC & TIEMEIER H. 2008. What is spared by fetal brain-sparing? Fetal circulatory redistribution and behavioral problems in the general population. American journal of epidemiology, 168, 1145–1152. [DOI] [PubMed] [Google Scholar]
- STEANE SE, FIELDING AM, KENT NL, ANDERSEN I, BROWNE DJ, TEJO EN, GÅRDEBJER EM, KALISCH-SMITH JI, SULLIVAN MA, MORITZ KM & AKISON LK 2021. Maternal choline supplementation in a rat model of periconceptional alcohol exposure: Impacts on the fetus and placenta. Alcohol Clin Exp Res. [DOI] [PubMed] [Google Scholar]
- SUBRAMANIAN K, NAIK VD, SATHISHKUMAR K, YALLAMPALLI C, SAADE GR, HANKINS GD & RAMADOSS J. 2014. Chronic binge alcohol exposure during pregnancy impairs rat maternal uterine vascular function. Alcohol Clin Exp Res, 38, 1832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS JD, ABOU EJ & DOMINGUEZ HD 2009. Prenatal choline supplementation mitigates the adverse effects of prenatal alcohol exposure on development in rats. Neurotoxicol Teratol, 31, 303–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS JD, IDRUS NM, MONK BR & DOMINGUEZ HD 2010. Prenatal choline supplementation mitigates behavioral alterations associated with prenatal alcohol exposure in rats. Birth Defects Res A Clin Mol Teratol, 88, 827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THORNBURG KL, KOLAHI K, PIERCE M, VALENT A, DRAKE R. & LOUEY S. 2016. Biological features of placental programming. Placenta, 48 Suppl 1, S47–s53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TORRES-ROVIRA L, TARRADE A, ASTIZ S, MOURIER E, PEREZ-SOLANA M, DE LA CRUZ P, GOMEZ-FIDALGO E, SANCHEZ-SANCHEZ R, CHAVATTE-PALMER P. & GONZALEZ-BULNES A. 2013. Sex and breed-dependent organ development and metabolic responses in foetuses from lean and obese/leptin resistant swine. PLoS One, 8, e66728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VIRDEE MS, SAINI N, KAY CD, NEILSON AP, KWAN STC, HELFRICH KK, MOONEY SM & SMITH SM 2021. An enriched biosignature of gut microbiota-dependent metabolites characterizes maternal plasma in a mouse model of fetal alcohol spectrum disorder. Sci Rep, 11, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL J. & MOONEY SM 2017. Choline and Working Memory Training Improve Cognitive Deficits Caused by Prenatal Exposure to Ethanol. Nutrients, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAKIMOTO P, AKABIKE A. & KING JC 2015. Maternal Nutrition and Pregnancy Outcome—A Look Back. Nutrition Today, 50, 221–229. [Google Scholar]
- WALLACE TC & FULGONI VL 2017. Usual Choline Intakes Are Associated with Egg and Protein Food Consumption in the United States. Nutrients, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARTON FL, MOLTENO CD, WARTON CM, WINTERMARK P, LINDINGER NM, DODGE NC, ZÖLLEI L, VAN DER KOUWE AJ, CARTER RC, JACOBSON JL, JACOBSON SW & MEINTJES EM 2021. Maternal choline supplementation mitigates alcohol exposure effects on neonatal brain volumes. Alcohol Clin Exp Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEEKS O, BOSSÉ GD, ODERBERG IM, AKLE S, HOUVRAS Y, WRIGHTON PJ, LABELLA K, IVERSEN I, TAVAKOLI S, ADATTO I, SCHWARTZ A, KLOOSTERMAN D, TSOMIDES A, CHARNESS ME, PETERSON RT, STEINHAUSER ML, FAZELI PK & GOESSLING W. 2020. Fetal alcohol spectrum disorder predisposes to metabolic abnormalities in adulthood. J Clin Invest, 130, 2252–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEI J, CARROLL RJ, HARDEN KK & WU G. 2012. Comparisons of treatment means when factors do not interact in two-factorial studies. Amino Acids, 42, 2031–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINTERHAGER E. & GELLHAUS A. 2017. Transplacental Nutrient Transport Mechanisms of Intrauterine Growth Restriction in Rodent Models and Humans. Front Physiol, 8, 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOZNIAK JR, FINK BA, FUGLESTAD AJ, ECKERLE JK, BOYS CJ, SANDNESS KE, RADKE JP, MILLER NC, LINDGREN C, BREARLEY AM, ZEISEL SH & GEORGIEFF MK 2020. Four-year follow-up of a randomized controlled trial of choline for neurodevelopment in fetal alcohol spectrum disorder. J Neurodev Disord, 12, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOZNIAK JR, FUGLESTAD AJ, ECKERLE JK, FINK BA, HOECKER HL, BOYS CJ, RADKE JP, KROUPINA MG, MILLER NC, BREARLEY AM, ZEISEL SH & GEORGIEFF MK 2015. Choline supplementation in children with fetal alcohol spectrum disorders: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr, 102, 1113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- XU Y, TANG Y. & LI Y. 2008. Effect of folic acid on prenatal alcohol-induced modification of brain proteome in mice. Br J Nutr, 99, 455–61. [DOI] [PubMed] [Google Scholar]
- YUAN Z, WAGNER L, POLOUMIENKO A. & BAKOVIC M. 2004. Identification and expression of a mouse muscle-specific CTL1 gene. Gene, 341, 305–12. [DOI] [PubMed] [Google Scholar]
- ZEISEL SH 2011. What choline metabolism can tell us about the underlying mechanisms of fetal alcohol spectrum disorders. Mol Neurobiol, 44, 185–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZEISEL SH & DA COSTA KA 2009. Choline: an essential nutrient for public health. Nutr Rev, 67, 615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]