Abstract
Infection caused by pathogenic fungal species is one of the most challenging disease to be tackled today. The antifungal bacteria candidate can be found in terrestrial as well as aquatic ecosystems, with mangrove forests being one of them. The purpose of this study is to obtain candidate isolates of antifungal strains with a detection approach and gene mapping simulation of bioactive compounds producers and screening to determine qualitative antifungal activity. The research will be carried out by collecting sediment samples from the mangrove ecosystems of Karimunjawa and Mangkang sub-district of Semarang city, isolating and purifying bacteria with Humic Acid Vitamin Agar (HVA), International Streptomyces Project 1 (ISP 1) and Zobell (Marine Agar). added with antibiotics, qualitative antifungal ability screening of each isolate obtained, detection of the presence of PKS gene and NRPS using special primers using the Polymerase Chain Reaction (PCR) method, and molecular identification of each isolate by 16s rRNA sequencing method. Of the total 59 isolates produced from the sample isolation process, 31 isolates from Karimunjawa sediments and 8 isolates from Semarang sediments showed activity against test pathogenic bacteria, namely Candida albicans, Trichoderma sp., and Aspergillus niger. Detection of Biosynthethic Gene Cluster (BGC) showed that the genes encoding secondary metabolites (NRPS, PKS 1 and PKS 2) were detected in KI 2-2 isolates from Karimunjawa. NRPS were detected only in isolates SP 3-9, SH 3-4, KI 1-6, KI 2-2, KI 2-4. The secondary metabolite-encoding gene, PKS1, was detected in isolates SP 3-5, SP 3-8, KI 2-2. PKS II genes were detected only on isolates SP 2-4, SH 3-8, KI 1-6, KI 2-2, and KI 2-4. Isolate SP 3-5 was revealed as Pseudomonas aeruginosa (93.14%), isolate SP 2-4 was Zhouia amylolytica strain HN-181 (100%) and isolate SP 3-8 was P. aeruginosa strain QK -2 (100%).
Keywords: antifungal strains, mangrove, Pseudomonas aeruginosa, sediment, Zhouia amylolytica
1. Introduction
Mangroves have many marine organisms [1] and have an element content sources [2] and amino acid contents [3], antimicrobial potency [4], tannins and phosphorus [5] and antifungal secondary metabolites [6]. Infection caused by pathogenic fungal species is one of the most challenging disease to be tackled today. The increase in pathogenic fungi-induced infections are worsen with the rise in demand for antifungal substances which may cause harmful side effects, are no longer effective against new pathogenic strains, or even accelerate the rate of resistance to treatment [7]. Antifungal strains produce compounds that show promise in their application as treatments for infections caused by fungi, viruses, and bacteria as well as drugs in the therapy of various types of cancer. Bioactive compounds are generally a product of secondary metabolic processes, from which the term secondary metabolite is derived. Antibacterial compounds can be found naturally on land and water, one of which is in mangrove sediments [8].
The challenges that are often faced by researchers in an effort to obtain new drug alternatives are the rediscovery of known compounds and the significant investment of time and money [9]. Rapid developments in the fields of genomics, bioinformatics, metabolic engineering, and synthetic biology have opened up opportunities for the discovery of new alternative compounds that can be applied as antibiotics or as new types of drugs [10]. One of the methods used to search for candidate antifungal strains that have the potential to produce bioactive compounds is screening based on the presence and mapping of PKS (Polyketide Synthetase) and NRPS (Nonribosomal Peptide Synthetase) genes.
This study aims to obtain candidate isolates of bacterial strains with antifungal activity against pathogenic strains using a detection and simulation approach to map bioactive compounds-producing genes and screening for qualitative antifungal activity. The innovations and advantages in this research are: (1) Antibacterial strains isolated from mangrove sediments are known to produce good bioactive compounds; (2) Activation of silent genes uses several optimization experiments to produce new compounds with antifungal activity.
2. Materials and methods
2.1. Site and time research
The sediment samples were collected in May to July 2021 from two sites, namely Karimunjawa National Park, Jepara regency and Mangkang sub-district, Semarang city, Central Java. The reason for the sample collection in 2 different places is an area without factory pollution (Karimunjawa islands) and an area with many factories.
2.2. Procedure of research
2.2.1. Isolation of antifungal strains from mangrove sediment samples
The treatment of sediment samples until bacterial isolation was carried out with methodologies which refers to Davies-Bolorunduro et al. [11] with several modifications. Bacterial isolation was carried out using the spread plate method with multi stage dilutions by diluting 1 g of sediment sample in 9 mL of sterile seawater. Afterwards, this dilution was repeated by adding 1 mL of the first dilution into 9 mL of sterile seawater, and repeated up to three iterations. 50 µL of the dilution was taken and spread on the surface of three different media types, namely Peptone Yeast Agar (PYA), International Streptomyces Project 1 (ISP 1) [12], and Humic Acid Vitamin Agar (HVA) [13]. The antibiotics Nystatin (2.5 mg/L) and Nalidixic acid (60 mg/L) were introduced to the media in the preparation process. The results of the isolation were incubated at a temperature of 29–34 °C for 7–14 days.
2.2.2. Characterization and purification of bacteria
Bacterial characterization was carried out on the isolated samples. Purification of the bacterial isolates was then carried out according to the method described in Davies-Bolorunduro et al. [11], with several modifications. Bacterial samples that have been cultured on isolation media are grouped according to their morphology. Each isolate was cultured on new media by streak plate method, which was then re-incubated at a temperature of 29–34 °C for 1–5 weeks.
2.2.3. Antifungal activity screening
Screening of bacterial samples with antifungal activity was carried out using the agar plug method based on the work with several modifications [14]. The pathogenic species tested consisted of Candida albicans, Trichoderma sp., and Aspergillus niger which were collected from the Laboratory of the Faculty of Medicine, Universitas Diponegoro, Semarang. The bacterial sample culture that had been incubated for 2 weeks was cut into a cylindrical shape (about 7 mm in diameter), then attached to the surface of Potato Dextrose Agar (PDA) media that had been inoculated with the test pathogenic fungus. The test samples were incubated at a temperature of 29–34 °C Observations and measurements of the inhibition zone were carried out after 72 h of incubation.
2.2.4. Extraction of antibacterial strain DNA from the samples
The Chelex method was used in the extraction of antibacterial strain DNA from the samples [15]. 2–3 inoculation loop of bacterial colonies were taken and introduced in a mixture of 500 µL of 0.5% saponin solution (in Phosphate Buffer Saline) and 100 µL of ddH2O. Samples were soaked for 12–24 hours at 4 °C to lyse the cell walls of actinomycetes.
Samples that have been soaked using saponins were processed using a centrifugal machine at 9000 rpm for 15 minutes. The supernatant from the centrifuge was discarded, then 1 mL of PBS solution was added to the natant, then the mixture was vortexed until homogeneous. The homogeneous mixture of natant and PBS was then put back in the centrifugal machine for 10 minutes. The resulting supernatant was removed. 100 µL of ddH2O and 50 µL of Chelex 20% solution (vortex Chelex solution before use) were added to the remaining natant. The samples were then heated at 95 °C for 5 minutes, then vortexed, after which they were reheated at 95 °C for 5 minutes. The samples were re-centrifuged for 15 min, then the supernatant was transferred to fresh 1.5 mL microtubes and were ready to be used as a DNA template.
2.2.5. NRPS gene amplification
Detection of the NRPS gene by PCR method was achieved using primer pairs DKF (5′-AAGGCNGGCGSBGCSTAYSTGCC-3′) and MTR (5′-TTGGGBIKBCCGGTSGINCCSGAGGTG-3′) [15], 10 mM each, 0.5 L, which was mixed with 7.5 L Thermo Scientific2X Phire Plant Direct PCR Master Mix, 6 l ddH2O, and 0.5 L DNA template of each isolate. The amplification process was carried out in 40 cycles, as follows: initial denaturation stage (95 °C, 5 min), followed by 10 cycles of denaturation stages (95 °C, 1 min), annealing (60 °C, 30 sec), extension stage (72 °C, 1 min), then 30 cycles of denaturation (95 °C, 1 min), annealing (40 °C, 30 sec), extension stage (72 °C, 1 min), and final extension (72 °C, 10 min).
2.2.6. PKS-I and PKS-II gene amplification
Detection of the PKS-I gene was carried out by amplification of DNA Template as much as 1 l with primers MDPQQR f (5′-RTRGAYCCNCAGCAICG-3′) and HGTGT r (5′-VGTNCCNGTGCCRTG-3′) (El Samak et al., 2018), with concentrations 10 mM, 0.5 µL each, 7.5 µL Thermo Scientific2X Phire Plant Direct PCR Master Mix, and 6 µL ddH2O, and 0.5 µL DNA template from each actinomycetes isolate. The amplification process was carried out in 40 cycles, as follows: initial denaturation stage (95 °C, 5 min), followed by 10 cycles of denaturation stages (95 °C, 1 min), annealing (60 °C, 30 sec), extension stage (72, 1 min), then 30 cycles of denaturation (95 °C, 1 min), annealing (40 °C, 30 sec), extension stage (72 °C, 1 min), and final extension (72 °C, 10 min).
PKS-II gene amplification was carried out by mixing primer pairs PF6 (5′-TSGCSTGCTTGGAYGCSATC-3′) and PR6 (5′TGGAANCCGCCGAABCCGCT-3) (El Samak et al., 2018) with a concentration of 10 mM, 0.5 µL each, 0.5 µL of extracted DNA template, 7.5 µL of Thermo Scientific2X Phire Plant Direct PCR Master Mix, and 6 µL of ddH2O. PCR amplification was carried out in 30 cycles, as follows: initial denaturation (96 °C, 5 min), followed by denaturation (96 °C, 1 sec), annealing (58 °C, 1 min), extension (72 °C, 1.5 min), and the final extension (72 °C, 10 min).
2.2.7. 16S rRNA amplification of active isolates
Amplification of the 16S rRNA gene was carried out by mixing 1 µL of DNA Template, primer pair 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1429R (5′-GGTTACCTTGTTACGACTT-3′) [16] with a concentration of 10 mM, 1 µL each, 12.5 µL of Thermo Scientific2X Phire Plant Direct PCR Master Mix, and 9.5 µL of ddH2O. PCR was carried out in 40 cycles with the following steps: initial denaturation (98 °C, 5 min), denaturation (98. 5 sec), annealing (55 °C, 5 sec), extension (72 °C, 1 min), and final extension (72 °C, 1 min).
2.2.8. DNA visualization, sequencing and sequence analysis
Electrophoresis, the application of electric current to DNA samples with agarose gel media, is an important step in the DNA visualization process. 1% agarose gel, 1% agarose powder dissolved in TAE buffer solution (Tris Acetate EDTA) mixed with GelRed dye for UV light visualization, was used in this study. The electrophoresis process was carried out at a voltage of 100 volts, with a current of 400 A for 30 min. The agarose gel was then transferred to a UV Transilluminator for visualization of the DNA bands.
The amplified samples were sequenced to determine the sequence of nucleotide bases using the Sanger Deoxy Method. Sequencing results were edited using MEGA 7.0 software, then data from 16s rDNA primers were matched with data from GenBank NCBI.
2.2.9. Mapping simulation of Biosynthetic Gene Cluster (BGC)
Biosynthetic Gene Cluster (BCG) mapping simulation was achieved by submitting the accession number of the whole genome sequence of the same bacterial species as the result of molecular identification of active isolates in AntiSMASH 6.0 (https://antismash.secondarymetabolites.org/).
3. Results
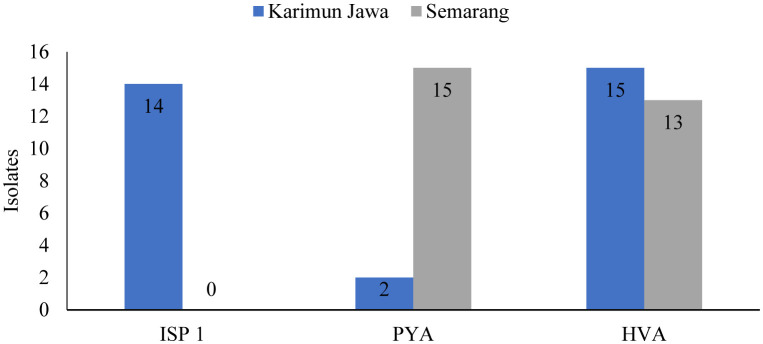
Bacterial isolation from Karimunjawa mangrove sediments using three different media types resulted in 31 isolates, of which there were 14 isolates on ISP 1 media, 2 isolates growing on PYA media, and 15 isolates growing on HVA media as shown in Table 1. Meanwhile, bacterial isolation from sediment samples from Mangkang, Semarang, showed that there were 28 isolates, where no isolates were found growing on ISP 1 media, 15 isolates were found to grow on PYA media, and 13 isolates were found to grow on HVA media as shown in the Table 2.
Table 1. Bacterial morphology of antifungal strain candidates from Karimunjawa.
| No | Isolate Code | Morphology | Elevation | Margin | Color |
| ISP 1 Medium | |||||
| 1 | KI 1-1 | Round with scalloped margin | Flat | Undulate | White |
| 2 | KI 1-2 | Round with scalloped margin | Convex | Undulate | Cream |
| 3 | KI 1-3 | Irregular | Flat | Undulate | Yellow |
| 4 | KI 1-4 | Round with raised margin | Crateriform | Undulate | Cream |
| 5 | KI 1-5 | Round with raised margin | Crateriform | Undulate | Cream |
| 6 | KI 1-6 | L-shaped | Raised | Undulate | White |
| 7 | KI 1-7 | Irregular | Umbonate | Undulate | Brown |
| 8 | KI 2-1 | Round | Flat | Entire | Brown |
| 9 | KI 2-2 | Round | Raised | Entire | Milk white |
| 10 | KI 2-3 | Round | Flat | Entire | Brown |
| 11 | KI 2-4 | Round | Flat | Entire | Brown |
| 12 | KI 2-5 | Round | Flat | Ciliate | Clear brown |
| 13 | KI 2-6 | Concentric | Raised | Entire | Dark brown |
| 14 | KI 3-1 | Round with raised margin | Crateriform | Ciliate | Brownish yellow |
|
| |||||
| PYA Medium | |||||
|
| |||||
| 15 | KP 1-1 | Round | Convex | Ciliate | Clear white |
| 16 | KP 2-1 | Thread-like | Flat | Entire | White |
|
| |||||
| HVA Medium | |||||
|
| |||||
| 17 | KH 1-1 | Filamentous | Flat | Branching | White |
| 18 | KH 1-2 | Round | Convex | Entire | Gray |
| 19 | KH 1-3 | Concentric | Flat | Entire | Gray |
| 20 | KH 2-1 | Round | Convex | Entire | Black |
| 21 | KH 2-2 | Round | Raised | Entire | Black |
| 22 | KH 2-3 | Round | Raised | Ciliate | Black |
| 23 | KH 2-4 | Round | Umbonate | Undulate | Black |
| 24 | KH 2-5 | Irregular | Raised | Undulate | White |
| 25 | KH 3-1 | Concentric | Crateriform | Undulate | White |
| 26 | KH 3-2 | Round | Umbonate | Ciliate | White |
| 27 | KH 3-3 | Round | Raised | Undulate | Brown |
| 28 | KH 3-4 | Round | Flat | Undulate | Black |
| 29 | KH 3-5 | Round | Convex | Entire | Reflective brown |
| 30 | KH 3-6 | Filamentous | Raised | Wooly | White |
| 31 | KH 3-7 | Round | Convex | Entire | Yellow |
Table 2. Bacterial morphology of antifungal strain candidates from Mangkang, Semarang.
| No | Isolate Code | Morphology | Elevation | Margin | Color |
| PYA Medium | |||||
|
| |||||
| 1 | SP 1-1 | Round | Growing to the inside | Entire | Brown |
| 2 | SP 2-1 | Round | Convex | Entire | Pink |
| 3 | SP 2-2 | Punctiform | Flat | Undulate | Clear yellow |
| 4 | SP 2-3 | Round | Flat | Undulate | White |
| 5 | SP 2-4 | Round | Flat | Entire | Yellow |
| 6 | SP 2-5 | Round | Flat | Entire | Brown |
| 7 | SP 3-1 | Irregular | Flat | Undulate | White |
| 8 | SP 3-2 | Round | Convex | Entire | Yellow |
| 9 | SP 3-3 | Round | Raised | Wooly | Black and white |
| 10 | SP 3-4 | Concentric | Raised | Entire | White |
| 11 | SP 3-5 | Round | Flat | Entire | Black |
| 12 | SP 3-6 | Irregular | Raised | Lobate | White |
| 13 | SP 3-7 | Round | Raised | Wooly | White |
| 14 | SP 3-8 | Round | Growing to the inside | Entire | Clear yellow |
| 15 | SP 3-9 | Punctiform | Flat | Lobate | Light gray |
|
| |||||
| HVA Medium | |||||
|
| |||||
| 16 | SH 1-1 | Round with radiating margin | Flat | Undulate | White and green |
| 17 | SH 1-2 | Round with raised margin | Flat | Ciliate | White |
| 18 | SH 1-3 | Irregular | Flat | Lobate | White and black |
| 19 | SH 1-4 | Irregular | Flat | Undulate | White and black |
| 20 | SH 2-1 | Round | Flat | Undulate | White |
| 21 | SH 3-1 | Round | Flat | Entire | Brown |
| 22 | SH 3-2 | Concentric | Flat | Ciliate | Brown |
| 23 | SH 3-3 | Round | Flat | Ciliate | White |
| 24 | SH 3-4 | Irregular | Raised | Undulate | Pink |
| 25 | SH 3-5 | Concentric | Flat | Irregular | White |
| 26 | SH 3-6 | Round with radiating margin | Raised | Undulate | White |
| 27 | SH 3-7 | Concentric | Flat | Branching | White and gray |
| 28 | SH 3-8 | Round | Raised | Entire | Gray |
Figure 1. Bacterial isolates of antifungal strain candidates based on collection site and culture media.
The results of the isolation of bacterial samples showed different shapes, elevations, edges and colors of each isolate. The shape of the bacteria found were round, curved, irregular, rounded with raised margins, round and concentric, and filamentous. Of the 59 isolates collected from Karimunjawa and Semarang coastal areas, the dominant bacterial shape was circular (43%). The coloration of bacterial isolates found varies greatly from white, cream, yellow, red, brown, gray and black. Colonies with white coloration were found in most samples (20%).
Table 3. Antifungal activity data of bacterial isolates.
| No | Isolate | Origin | Zone of Inhibition diameter against Pathogenic species (in cm) |
||
| C. albicans | Trichoderma sp. | A. niger | |||
| 1 | SP 2-4 | Mangkang, Semarang | - | - | 0.88 ± 0.11 |
| 2 | SP 3-5 | - | 1 ± 0.04 | 1.57 ± 0.08 | |
| 3 | SP 3-8 | 0.58 ± 0.03 | 1.31 ± 0.07 | 0.80 ± 0.06 | |
| 4 | SP 3-9 | 0.33 ± 0.01 | - | - | |
| 5 | SH 3-4 | - | 1.08 ± 0.03 | 1.31 ± 0.06 | |
| 6 | KI 1-6 | Karimun Jawa | - | - | 1.53 ± 0.18 |
| 7 | KI 2-2 | - | - | 0.82 ± 0.03 | |
| 8 | KI 2-4 | - | - | 1.26 ± 0.16 | |
Based on the number of isolates of bacteria from the Karimunjawa and Semarang mangrove sediments cultured in three different media, PYA media produced the most isolates from the Semarang sample (15 isolates), HVA media produced the most isolates from the Karimunjawa sample (15 isolates), whereas ISP media produced 14 isolates from Karimunjawa samples without resulting any from the Semarang samples. These results are presented in Table 1.
3.1. Antifungal activity
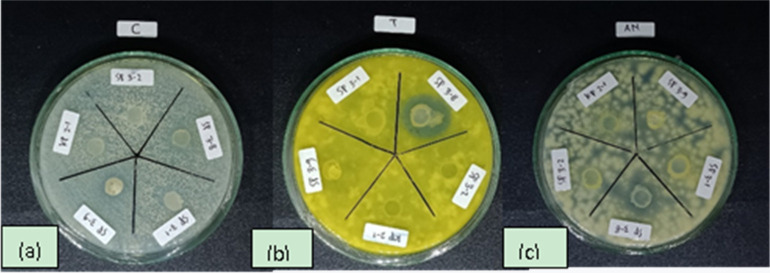
The screening of antifungal activities on bacterial isolates samples from all collection sites showed that there were 5 isolates from Semarang that were positive for the tested pathogenic species, namely isolates SP 2-4, SP 3-5, SP 3-8, SP 3-9 and SH 3-4. 3 isolates from Karimunjawa showed positive results against tested pathogenic species, namely Isolates KI 1-6, KI 2-2 and KI 2-4. Of all the positive results, only one isolate (SP 3-8) tested positive against C. albicans (0.58 ± 0.03), Trichoderma sp. (1.31 ± 0.07) (Figure 2) and A. niger (0.80 ± 0.06).
Figure 2. Isolate (SP 3-8) tested positive against C. albicans (0.58 ± 0.03 mm) (a), Trichoderma sp. (1.31 ± 0.07 mm) (b) and A. niger (0.80 ± 0.06) (c).
Table 4. Encoding genes for secondary metabolites.
| No | Isolate | Origin | Encoding genes for secondary metabolites |
||
| NRPS | PKS |
||||
| PKS I | PKS II | ||||
| 1 | SP 2-4 | Mangkang, Semarang | - | - | ✓ |
| 2 | SP 3-5 | - | ✓ | - | |
| 3 | SP 3-8 | - | ✓ | ✓ | |
| 4 | SP 3-9 | ✓ | - | - | |
| 5 | SH 3-4 | ✓ | - | - | |
| 6 | KI 1-6 | Karimunjawa | ✓ | - | ✓ |
| 7 | KI 2-2 | ✓ | ✓ | ✓ | |
| 8 | KI 2-4 | ✓ | - | ✓ | |
3.2. Detection of biosynthetic Gene Cluster (BGC)
Detection of Biosynthethic Gene Cluster (BGC) showed that the genes encoding secondary metabolites (NRPS, PKS 1 and PKS 2) were detected in KI 2-2 isolates from Karimunjawa. NRPS genes were detected only in isolates SP 3-9, SH 3-4 (From Mangkang, Semarang), KI 1-6, KI 2-2, KI 2-4 (From Karimunjawa islands). The secondary metabolite-encoding gene, PKS1, was detected in isolates SP 3-5, SP 3-8 (From Mangkang, Semarang), KI 2-2 (From Karimunjawa islands). Whereas PKS-II genes were detected in isolates SP 2-4, SP 3-8 (From Mangkang, Semarang), KI 1-6, KI 2-2, and KI 2-4 (From Karimunjawa islands).
Based on the number of isolate reactions in screening antifungal activity, 3 sample isolates were selected from Semarang for the next stage of research; isolates SP 2-4, SP 3-5 and SP 3-8.
The PCR and electrophoresis processes that were applied to bacterial isolates from the samples produced several DNA bands on the media for the test as shown in Table 5.
Table 5. BLAST homology of isolates SP 2-4, SP 3-5 and SP 3-8.
| No | Isolate | Relative similarity | Query Cover | Percent Identify | E value | Acc Number |
| 1. | SP 2-4 | Z. amylolytica strain HN-181 16S ribosomal RNA gene | 100% | 100% | 0.0 | DG423481.1 |
| 2. | SP 3-5 | P. aeruginosa strain A-25 16S ribomosal RNA gene | 99% | 93.14% | 0.0 | MT573198.1 |
| 3. | SP 3-8 | P. aeruginosa strain QK-2 16S ribosomal RNA gene | 100% | 100% | 0.0 | MH746105.1 |
The high correlation value between isolates SP 3-5 with the sequences in the database (93.14%) indicated that this molecular identification (P. aeruginosa strain A-25 16S) should be a close match at the genus level. While isolates SP 2-4 (Z. amylolytica strain HN-181 16S) and isolate SP 3-8 (P. aeruginosa strain QK-2 16S) were exact matches with the sequences found in the database (100%).
Mapping simulations of Z. amylolytica (NZ_FPAG01000003) and P. aeruginosa (KV830163.1) genes showed that Z. amylolytica genes contain carotenoids (28%), as shown in Table 6. It was also revealed that P. aeruginosa genes contains L-2-amino-4-methoxy-trans-3-butenoic acid, as shown in Table 7.
Table 6. BGC mapping simulation results the Zhouia amylolytica sample.
| Region | Region Location (Nucleotides) | Type | Most Simillar Known Cluster | Similarity |
| Region 3.1 | 342857-363693 | Terpene | Carotenoid | 28% |
*Note: Genetic mapping simulation of Z. amylolytica (NZ_FPAG01000003).
Table 7. Mapping simulation results of the Biosynthetic Gene Cluster (BGC) of the P. aeruginosa sample.
| Region | Region Location (Nucleotides) | Type | Most Simillar Known Cluster | Similarity |
| Region 1 | 96108-138540 | NRPS | L-2-amino-4-methoxy-trans-3-butenoic acid | 100% |
4. Discussion
Bacterial isolation was carried out from samples collected in sedimentary habitats in the mangrove ecosystem to obtain species that have antifungal potential. Different media were used in the isolation process. This method aims to maximize the number of bacterial isolates produced from the process. The results of bacterial isolation from mangrove sediments produced the most results in ISP 1 medium (14 isolates) from Karimunjawa sample, although the medium did not produce isolates from the Semarang sample. On the other hand, the PYA medium only resulted in 2 isolates from the Karimunjawa sample but managed to produce 15 isolates from the Semarang sample. HVA medium produced almost the same number of isolates between Karimunjawa samples (15 isolates) and mango samples (13 isolates). The difference in the number of isolates in Karimunjawa and Semarang with different media showed different results. A medium had an effect on the growth of isolates. A good growth medium is a medium that could provide a source of carbon and other minerals needed for growth and activities. Microorganisms need nutrients to support cell growth, the nutrients needed are carbon (C), nitrogen (N), phosphorus (P), sulfur (S), potassium (K), magnesium (Mg), calcium (Ca), sodium (Na) and iron (Fe), while the micronutrients needed are copper (Cu), manganese (Mn), zinc (Zn), nickel (Ni), molybdenum (Mo), and cobalt (Co) [17]. Minerals have an important role in enzyme reactions as cofactors in metabolic processes [18]. The combination of mineral mixtures also plays an important role in electrolyte and osmotic regulation in cells and small amounts. Minerals have an influence on cell growth and product formation. From the statements, it can be concluded that the minerals contained in the medium are also capable of supplying energy for the growth of isolates, so it is suspected that no isolates were found in the ISP 1 medium in Semarang because the media incidentally has not been able to supply isolate growth energy. The condition at the sampling point is abnormal condition because ISP 1 media should have a lot of isolates growing because ISP 1 is richer in nutrients [19].
Of the total 59 isolates produced from the sample isolation process, 31 isolates from Karimunjawa and 8 isolates from Semarang showed activity against test pathogenic bacteria, namely C. albicans, Trichoderma sp. and A. niger, which consisted of 3 Karimunjawa isolates and 5 Semarang isolates. Of the 8 isolates, there was only 1 isolate that was active against the three tested pathogenic bacteria; isolates SP 3-8.
Prior to identification of bacteria with potential as antifungals, NRPS, PKS 1 and PKS 2 were detected, namely to determine the presence or absence of PKS 1 or 2 genes. The results showed that the PKS samples SP 2-4, SP 3-5 and SP 3-8 meaning that the bacteria produced higher polyketide active compounds. Bacterial results by molecular method showed a 100% match with P. aeruginosa strain QK-2. While isolate SP 3-5 showed 93.14% similarity with P. aeruginosa. Based on the molecular identification of isolate SP 3-5, the isolate was believed to be close to the genus Pseudomonas. The results of the molecular identification of SP 2-4 proved that the isolate had 100% similarity with Z. amylolytica.
Pseudomonas sp. are commonly found in mangrove sediments and is known to possess antibacterial capability [20]. This species works in synergy with 3 other bacterial species, Flavobacterium sp., Acinetobacter sp., and B. subtilis, which collectively have the potential to combat the growth of pathogenic species, to the extent that they are proven to remove strong odor from waste. The consortium of bacteria also saw application in turning organic waste into compost [21].
The bacterium P. aeruginosa is known to be one of six pathogenic bacteria consisting of Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, P. aeruginosa, and Enterobacter spp., which are generally associated with antimicrobial resistance, and also known by the abbreviation ESCAPE [22]. This information supports that P. aeruginosa is a dangerous bacterial pathogen, and that the species can survive antibiotics. This is presumably because this type of bacteria has toxic secondary metabolites that can kill fungi.
Based on gene mapping simulations, it is known that this species has a compound L-2-amino-4-methoxy-trans-3-butenoic acid. l-2-Amino-4-methoxy-trans-3-butenoic acid (AMB) is a potent antibiotic and toxin produced by P. aeruginosa. P. aeruginosa gene contains L-2-amino-4-methoxy-trans-3-butenoic acid as shown in Table 7. P. aeruginosa toxin L-2-amino-4-methoxy-trans-3-butenoic acid (AMB) is a non-proteinogenic amino acid which is toxic for prokaryotes and eukaryotes [23]. This means that the potential of these bacteria is known to be toxic to microorganisms and other biota. It is proven by the research results shown in the Table 7, namely as an anti-fungal.
The results of research conducted by Pringgenies et al. [24] found that the supernatant resulted from the extraction of P. aeruginosa found in symbiosis with the gastropod Cerithidea sp. contains Pyocyanin (pyo) and Phenazin I-carboxyic (PCA) pigments, which falls into the category of phenazine pigments. The results of studies on pyocyanin showed that when pyocyanin biosynthesis was inhibited, a decrease in the pathogenicity of P. aeruginosa was observed in vitro. This suggests that pyocyanin is most responsible for the initial colonization of P. aeruginosa in vivo [25].
The bacterial sample has a striking green color, which comes from the pigment phenazine.
Phenazine is a natural pigment which plays a vital role as anti-cancer, anti-malaria, anti-tumor and antibiotic agent. It is these characteristics that are believed to complicate the recovery of patients suffering from P. aeruginosa infestation-induced infections, as stated in. P. aeruginosa has the ability to form biofilm [26]. Antibiotic treatment was unable to eradicate bacterial infections with this biofilm-forming ability because of the intrinsic tolerance and development of resistance caused by mutations. Biofilm tolerance to antibiotics is multifactorial, involving physical, physiological, and genetic determinants.
On the other hand, bacterial antibiotic resistance in biofilms is caused by mutations and driven by repeated exposure of bacteria to high levels of antibiotics. This is interesting information because in pathogenic bacteria there are compounds that have the potential to be anti-cancer. The role of biotechnology in the form of fractionation will obtain promising results in the pharmaceutical field in the future.
The mapping simulation of the Z. amylolytica gene (NZ_FPAG01000003) revealed that this species possesses carotenoids (28%). Carotenoids are known to contain antioxidants that can protect cells from free radical damage. Several species are known to possess or produce carotenoids. The bacteria Erythrobacter sp. strain KJ5 which is a symbiote organism of the coral A. nasuta, is known to have zeaxanthin and -carotene [27]. In addition, it is also known that these bacteria do not have bacteriochlorophyll, but have at least 16 types of carotenoids, including -carotene and zeaxanthin [28]. Another research on compounds in Erythrobacter sp. strain KJ5 found that this species produces sulphur containing carotenoids, caloxanthin sulfate and nostoxanthin sulfate, in abundance [29].
Z. amylolytica is a species that thrives in sedimentary ecosystems, does not form spores, and was first isolated in samples collected from the South China Sea [30]. This species is known to be applied as a solution to the sediment problem of saltwater aquaculture ponds with Malachite green (MG) contamination [31]. This demonstrates the potential of Z. amylolytica as an antifungal, which has a wide range of applications in the aquaculture industry and other bioindustries.
5. Conclusions
Of the total 59 isolates produced from the sample isolation process, 31 isolates from Karimunjawa sediments and 8 isolates from Semarang sediments showed activity against test pathogenic bacteria, namely C. albicans, Trichoderma sp., and A. niger. Detection of Biosynthethic Gene Cluster (BGC) showed that the genes encoding secondary metabolites (NRPS, PKS 1 and PKS 2) were detected in KI 2-2 isolates from Karimunjawa. NRPS were detected only in isolates SP 3-9, SH 3-4, KI 1-6, KI 2-2, KI 2-4. The secondary metabolite-encoding gene, PKS1, was detected in isolates SP 3-5, SP 3-8, KI 2-2. PKS II genes were detected only on isolates SP 2-4, SH 3-8, KI 1-6, KI 2-2, and KI 2-4. Isolate SP 3-5 was revealed as P. aeruginosa (93.14%), isolate SP 2-4 was Z. amylolytica strain HN-181 (100%) and isolate SP 3-8 was P. aeruginosa strain QK-2 (100%).
Acknowledgments
The authors would like to thanks the Institute of Research and Community Services. Diponegoro University, Semarang, Indonesia for funding support on contract no. 185-27/UN7.6.1/PP/2021 and Fisheries and Marine Science Faculty to support the laboratory facility.
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
Author's contribution: Delianis Pringgenies and Wilis Ari Setyati contributed to the acquisition of data, analysis, and interpretation. Delianis Pringgenies drafted and provided critical revision of the article. Wilis Ari Setyati wrote the paper and contributed to the conception and design of the study. Delianis Pringgenies provided the final approval of the version to publish.
References
- 1.Ariyanto D, Bengen DG, Prartono T, et al. Distribution and abundance of Cerithideopsilla djadjariensis (Martin 1899) (potamididae) on avicennia marina in Rembang, Central Java, Indonesia. Egypt J Aquat Biol Fish. 2020;24:323–332. [Google Scholar]
- 2.Ariyanto D, Gunawan H, Puspitasari D, et al. The differences of the elements content in Rhizophora mucronata leaves from Asahan Regency, North Sumatra, Indonesia. Polish J Nat Sci. 2019;34:481–491. [Google Scholar]
- 3.Ningsih SS, Ariyanto D, Puspitasari D, et al. The amino acid contents in mangrove Rhizophora mucronata leaves in Asahan, North Sumatra, Indonesia. E3S Web Conf. 2020;151:1–3. [Google Scholar]
- 4.Pringgenies D, Setyati WA, Djunaedi A, et al. Exploration of antimicrobial potency of mangrove symbiont against multi-drug resistant bacteria. J Ilm Perikan dan Kelaut. 2021;12:222–232. [Google Scholar]
- 5.Ariyanto D, Bengen DG, Prartono T, et al. Short Communication: The relationship between content of particular metabolites of fallen mangrove leaves and the rate at which the leaves decompose over time. Biodiversitas. 2018;19:700–705. [Google Scholar]
- 6.Shen N, Liang Z, Liu Q, et al. Antifungal secondary metabolites isolated from mangrove rhizosphere soil-derived penicillium fungi. J Ocean Univ China. 2020;19:717–721. [Google Scholar]
- 7.Da X, Nishiyama Y, Tie D, et al. Antifungal activity and mechanism of action of Ou-gon (Scutellaria root extract) components against pathogenic fungi. Sci Rep. 2019;9:1–12. doi: 10.1038/s41598-019-38916-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Azman AS, Othman I, Velu SS, et al. Mangrove rare actinobacteria: Taxonomy, natural compound, and discovery of bioactivity. Front Microbiol. 2015;6:1–15. doi: 10.3389/fmicb.2015.00856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sharma P, Thakur D. Antimicrobial biosynthetic potential and diversity of culturable soil actinobacteria from forest ecosystems of Northeast India. Sci Rep. 2020;10:1–18. doi: 10.1038/s41598-020-60968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sekurova ON, Schneider O, Zotchev SB. Novel bioactive natural products from bacteria via bioprospecting, genome mining and metabolic engineering. Microb Biotechnol. 2019;12:828–44. doi: 10.1111/1751-7915.13398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies-Bolorunduro OF, Adeleye IA, Akinleye MO, et al. Wang PG. Anticancer potential of metabolic compounds from marine actinomycetes isolated from Lagos Lagoon sediment. J Pharm Anal. 2019;9:201–208. doi: 10.1016/j.jpha.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Q, Chen X, Jiang Y, et al. Actinobacteria-Basics and Biotechnological Applications. New York: 2016. Morphological Identification of Actinobacteria, In: Dharumadurai Dhanasekaran, Yi Jiang; pp. 60–84. [Google Scholar]
- 13.Hayakawa M, Nonomura H. Humic acid-vitamin agar, a new medium for the selective isolation of soil actinomycetes. J Ferment Technol. 1987;65:501–509. [Google Scholar]
- 14.Messaoudi O, Wink J. Diversity of actinobacteria isolated from date palms rhizosphere and saline environments : isolation, identification and biological activity evaluation. Microorganisms. 2020;8:1853. doi: 10.3390/microorganisms8121853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radjasa OK, Martens T, Grossart HP, et al. Antibacterial property of a coral-associated bacterium Pseudoalteromonas luteoviolacea against shrimp pathogenic Vibrio harveyi (In Vitro Study) HAYATI J Biosci. 2005;12:77–81. [Google Scholar]
- 16.El Samak M, Solyman SM, Hanora A. Antimicrobial activity of bacteria isolated from Red Sea marine invertebrates. Biotechnol Reports. 2018;19:e00275. doi: 10.1016/j.btre.2018.e00275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prescott LM, Harley JP, Klein D. Microbiology. 4 Eds. New York: The McGraw-Hill Companies; 1999. p. 685. [Google Scholar]
- 18.Majewski M, Kozlowska A, Thoene M, et al. Overview of the role of vitamins and minerals on the kynurenine. J Physiol Pharmacol. 2016;67:3–19. [PubMed] [Google Scholar]
- 19.Anggelina AC, Pringgenies D, Setyati WA. Presence of biosynthetic gene clusters (NRPS/PKS) in actinomycetes of mangrove sediment in Semarang and Karimunjawa, Indonesia. Environ Nat Resour J. 2021;19:391–401. [Google Scholar]
- 20.Thatoi H, Behera BC, Mishra RR, et al. Biodiversity and biotechnological potential of microorganisms from mangrove ecosystems: A review. Ann Microbiol. 2013;63:1–19. [Google Scholar]
- 21.Pringgenies D, Widiyadmi R, Ariyanto D, et al. Organic compost production from bacterial consortium of mangrove leaf litter. J Pengelolaan Perair. 2019;2:29–26. [Google Scholar]
- 22.Moradali MF, Ghods S, Rehm BHA. Pseudomonas aeruginosa lifestyle: A paradigm for adaptation, survival, and persistence. Front Cell Infect Microbiol. 2017;7:1–29. doi: 10.3389/fcimb.2017.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murcia NR, Waridel P, Maspoli A, et al. The Pseudomonas aeruginosa antimetabolite L-2-amino-4-methoxy-trans-3-butenoic acid (AMB) is made from glutamate and two alanine residues via a thiotemplate-linked tripeptide precursor. Front Microbiol. 2015;6:1–13. doi: 10.3389/fmicb.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pringgenies D, Diponegoro U, Ridlo A, et al. Production of phenazine pigments from marine symbiotic bacteria in gastropod Cerithidea sp. with different Growth Media. Conf Int Converence Nat Sci 2011. 2011:153–162. [Google Scholar]
- 25.Ho Sui SJ, Lo R, Fernandes AR, et al. Raloxifene attenuates Pseudomonas aeruginosa pyocyanin production and virulence. Int J Antimicrob Agents. 2012;40:246–251. doi: 10.1016/j.ijantimicag.2012.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ciofu O, Tolker-Nielsen T. Tolerance and resistance of pseudomonas aeruginosabiofilms to antimicrobial agents-how P. aeruginosa Can escape antibiotics. Front Microbiol. 2019;10:1–15. doi: 10.3389/fmicb.2019.00913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Juliadiningtyas AD, Pringgenies D, Heriyanto, et al. Preliminary investigation of the carotenoid composition of Erythrobacter sp. Strain KJ5 by high-performance liquid chromatography and mass spectrometry. Philipp J Sci. 2018;147:91–98. [Google Scholar]
- 28.Kanesaki Y, Setiyono E, Pringgenies D, et al. Complete genome sequence of the marine bacterium Erythrobacter flavus Strain KJ5. Microbiol Resosrces Announc. 2019;8:1–2. doi: 10.1128/MRA.00140-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Setiyono E, Heriyanto, Pringgenies D, et al. Sulfur-containing carotenoids from a marine coral symbiont Erythrobacter flavus Strain KJ5. Mar Drugs. 2019;17:1–15. doi: 10.3390/md17060349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu ZP, Wang BJ, Dai X, et al. Zhouia amylolytica gen. nov., sp. nov., a novel member of the family Flavobacteriaceae isolated from sediment of the South China Sea. Int J Syst Evol Microbiol. 2006;56:2825–2829. doi: 10.1099/ijs.0.64587-0. [DOI] [PubMed] [Google Scholar]
- 31.Yang CW, Chao WL, Hsieh CY, et al. Biodegradation of sulfamethoxazole in milkfish (Chanos chanos) pond sediments. Appl Sci. 2019;9:1–16. [Google Scholar]