Abstract
Background
Postural orthostatic tachycardia syndrome (POTS) is a chronic form of orthostatic intolerance that primarily impacts female patients of childbearing age. The role of sex differences in POTS is not well understood. We sought to identify sex differences in diagnosis, symptoms, comorbidities, and treatments in female and male patients diagnosed with POTS.
Methods
A comprehensive survey was designed in partnership by Dysautonomia International (East Moriches, NY) and Vanderbilt University Medical Center (Nashville, TN). Patients were recruited through Dysautonomia International’s website and social media channels. The survey was delivered online through a secure research data capture database. Responses were analyzed according to biological sex. Continuous variables are presented as median (25th percentile-75th percentile), and categorical variables are presented as number and proportion of participants.
Results
A total of 8919 patients reported a physician diagnosis of POTS and were included in this analysis. The majority of respondents were female (93.7%). Female and male patients experienced misdiagnosis at similar rates (76.2% vs 74.9%, P = 0.5) and saw a similar number of doctors before diagnosis (5 [3-8] vs 5 [3-8], P = 0.9). Despite these similarities, diagnostic delay was longer for female, compared with male, patients (1.50 [0.25-5.25] years vs 0.92 [0.08-2.91] years, P < 0.001).
Conclusions
Despite the primarily female demographic of POTS patients, female patients experience more challenges with diagnosis than male patients. Increased awareness and recognition of POTS may help to reduce the diagnostic challenges in both female and male patients, and improve treatment and management for individuals living with this debilitating disorder.
Résumé
Contexte
Le syndrome de tachycardie orthostatique posturale (STOP) est une forme chronique d’intolérance orthostatique qui touche principalement les femmes en âge de procréer. L’incidence du sexe sur le STOP n’est pas bien comprise. Nous avons cherché à déterminer les différences entre les sexes en ce qui a trait au diagnostic, aux symptômes, aux comorbidités et aux traitements chez les patients féminins et masculins ayant reçu un diagnostic de STOP.
Méthodologie
Une enquête exhaustive a été conçue en partenariat par Dysautonomia International (East Moriches, NY, États-Unis) et le Vanderbilt University Medical Center (Nashville, TN, États-Unis). Les patients ont été recrutés par l’entremise du site Web de Dysautonomia International et des médias sociaux. L’enquête a été réalisée en ligne au moyen d’une base de données sécurisée de saisie de données de recherche. Les réponses ont été analysées en fonction du sexe biologique. Les variables continues sont présentées sous forme de médiane (25e percentile-75e percentile), et les variables nominales sont présentées sous forme de nombre et de proportion de participants.
Résultats
Au total, 8 919 patients ont signalé un diagnostic de STOP établi par un médecin et ont été inclus dans cette analyse. La majorité des répondants étaient des femmes (93,7 %). Les patients de sexe féminin et masculin ont été mal diagnostiqués dans des proportions similaires (76,2 % vs 74,9 %, p = 0,5) et ont vu un nombre similaire de médecins avant le diagnostic (5 [3-8] vs 5 [3-8], p = 0,9). Malgré ces similitudes, le retard de diagnostic était plus long chez les femmes que chez les hommes (1,50 [0,25-5,25] an contre 0,92 [0,08-2,91] an, p < 0,001).
Conclusions
Même si les patients atteints de STOP sont principalement des femmes, ces dernières ont plus de difficultés à recevoir un diagnostic que les hommes. Une meilleure connaissance et reconnaissance du STOP peut contribuer à réduire les problèmes liés au diagnostic chez les patients féminins et masculins, et à améliorer le traitement et la prise en charge des personnes atteintes de ce trouble débilitant.
Postural orthostatic tachycardia syndrome (POTS) is a chronic form of orthostatic intolerance that primarily affects female patients of childbearing age. More than 85% of POTS patients are female.1,2 Patients with POTS experience a heart rate increase of 30 beats per minute within 10 minutes of assuming an upright posture, in the absence of orthostatic hypotension ( > 20/10 mm Hg), and in association with chronic orthostatic symptoms.3 Patienfts with POTS experience many challenges, including an extreme diagnostic delay,2 an unsatisfactory quality of life,4 and significant economic and employment hardships.5 The Canadian Cardiovascular Society has recently published a position statement about POTS.3
Although the majority of POTS patients are female, the effects of biological sex on the patient experience, as well as sex differences in clinical presentation, are not well understood. In other cardiovascular conditions primarily viewed through a male lens, there are sex differences in presentation and access to care. For example, female patients with chest pain are less likely than male patients to be referred to a cardiologist,6 and female patients with myocardial infarction experience a longer delay from onset to treatment.7 It is not known if sex differences in POTS, a condition that primarily affects female patients, follow the same pattern as in other cardiovascular conditions. Using a large survey of POTS patients, we sought to explore whether there were sex differences in symptom presentation, comorbid conditions, or access to care in what is primarily a female disorder. We tested the hypotheses that female patients would experience more symptoms and face more healthcare barriers than male patients.
Materials and Methods
Survey design and delivery
A comprehensive survey was developed by Vanderbilt University Medical Center (Nashville, TN) in conjunction with Dysautonomia International (East Moriches, NY), a POTS patient advocacy group, in 2014. Dysautonomia International’s Patient Advisory Board participated in developing and testing this survey in an iterative process. This survey aimed to capture the experience of a diverse group of POTS patients, in contrast to previous research, which focused on physiological findings in small cohorts at specialized autonomic centres. The survey addressed several topics, including diagnostic journey, patient experience, symptoms, comorbidities, treatments, and the RAND-36 health-related quality-of-life measure (Santa Monica, CA).2,8 The survey was written in English and delivered online. Due to the length of time needed to complete this survey (45-90 minutes), patients had the option to save their responses and return to complete the survey at a later time. Survey information was stored in a secure Research Data Capture (REDCap)9 electronic database at Vanderbilt University. This study received ethical approval from both the Vanderbilt University Institutional Review Board (IRB#140303) and the University of Calgary Conjoint Health Research Ethics Board (CHREB; REB15-2922).
Study participants
The majority of POTS patients were recruited through Dysautonomia International’s website and social media channels. An open survey link was provided for participation in this study, and specific patients were not targeted for a response. There were no geographic restrictions on participation. Inclusion criteria were a self-reported physician diagnosis of POTS, access to an electronic device with an Internet connection, and the ability to read and understand English. Informed consent was provided electronically by patients (parent/guardian consent in addition to patient assent if the patient was under the age of 18 years) in order to complete the survey. Participants may have unintentionally or intentionally completed the survey more than once. Due to the comprehensive (and lengthy) nature of this survey, multiple responses from a single participant are considered unlikely.
Research survey data
Survey data were collected from July 2015 through April 2021. Deidentified data were exported from REDCap and imported into SPSS Statistics for Windows, version 26.0 (IBM Corp, Armonk, NY). Only patients who provided informed consent and reported a physician diagnosis of POTS were included in the analysis. Patients who answered “prefer not to say” to a specific question were excluded from the total respondents for that question.
We asked participants to tell us their assigned “gender at birth.” This was used as a surrogate for biological sex, as genetic testing was not performed.
Symptoms were grouped into composite symptom variables based on body system. For example, if a patient had one or more of nausea, vomiting, diarrhea, constipation, bloating, or stomach pain, they were categorized as “yes” for gastrointestinal symptoms. This process was also used to combine autoimmune disorders and neuropathies into a single composite variable. Analysis of the RAND-36 health-related quality-of-life data was limited to participants age ≥ 18 years, as this tool has been validated primarily in adults.
Statistical analysis
Continuous variables are reported as median (25th percentile, 75th percentile). Categorical variables are presented as frequencies and percentages. Mann-Whitney U tests were used to compare continuous variables for female vs male patients. Pearson’s χ2 tests were used to compare sex differences in diagnosis, symptoms, and treatments. Odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated to compare sex differences and comorbidities. A 2-sided P value of < 0.05 was considered statistically significant. Bonferroni correction for multiple comparisons was used for analysis of the RAND-36 health-related quality-of-life data.
Results
Participants
A total of 8919 individuals consented to participate in this survey and reported a physician diagnosis of POTS. The majority of patients were female (93.7%; n = 8355). More female than male patients identified their race as white (93.7% vs 89.8%, P < 0.001, and more female than male patients were 18 years of age or older (89.6% vs 73.5%, P < 0.001). Of participants who disclosed their geographic location (n = 6436), 86% were residing in the United States at the time of the survey.
Diagnostic journey
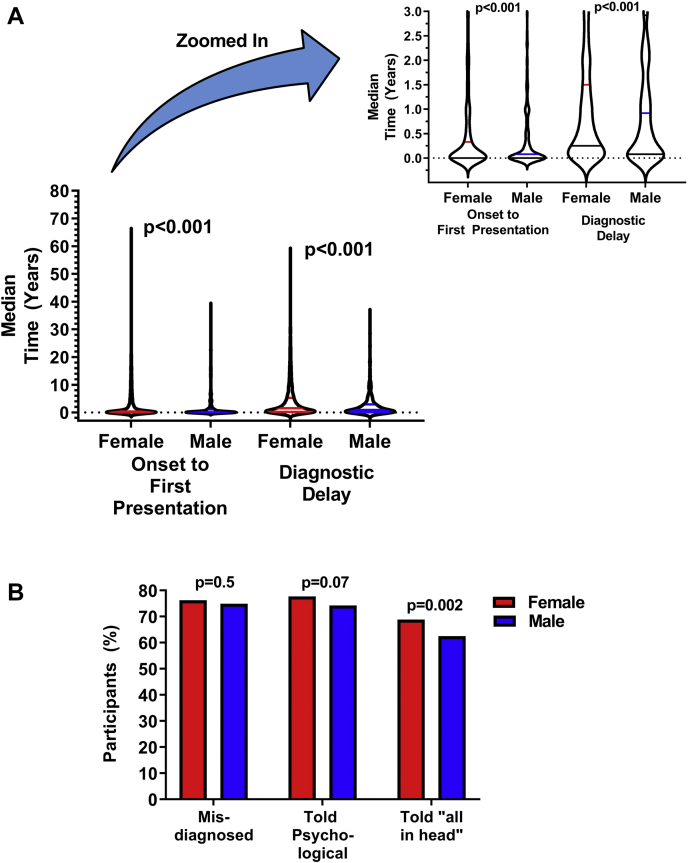
The median time from symptom onset to first physician presentation was longer in female than male patients (0.25 [0.00-3.00] years vs 0.08 [0.00-1.00] years, P < 0.001; Fig. 1A). Before a POTS diagnosis, female and male patients saw a similar number of doctors for their POTS symptoms (5 [3-8] vs 5 [3-8], P = 0.9). Female and male patients also experienced misdiagnosis at similar rates (76.2% vs 74.9%, P = 0.5; Fig. 1B). Despite the similar rates of misdiagnosis and the number of physicians seen, diagnostic delay was significantly longer in female than male patients (1.50 [0.25-5.25] years vs 0.92 [0.08-2.92] years, P < 0.001). The mean diagnostic delay (as opposed to the median delay) was almost 2 years longer in female than male patients (4.36 ± 0.08 vs 2.67 ± 0.08 years, P < 0.001).
Figure 1.
(A) Median time from postural orthostatic tachycardia syndrome (POTS) symptom onset to first physician presentation for POTS (left), and time from first physician presentation for POTS to POTS diagnosis (right) in female vs male patients. Time from onset to presentation and from presentation to diagnosis were both significantly longer in female patients. The inset is a zoomed-in version of the main figure, to better illustrate the median and interquartile range. (B) Challenges with diagnosis in female and male patients. A similar proportion of female and male patients were misdiagnosed and were told their illness was psychological. More female than male patients were told their illness was “all in their head.”
More female than male patients were diagnosed with POTS by a cardiologist or cardiac electrophysiologist, and fewer female than male patients were diagnosed with POTS by a neurologist. More female than male patients also saw one or more doctors who said they had “never heard of POTS” after diagnosis (69.7% vs 58.3%, P < 0.001).
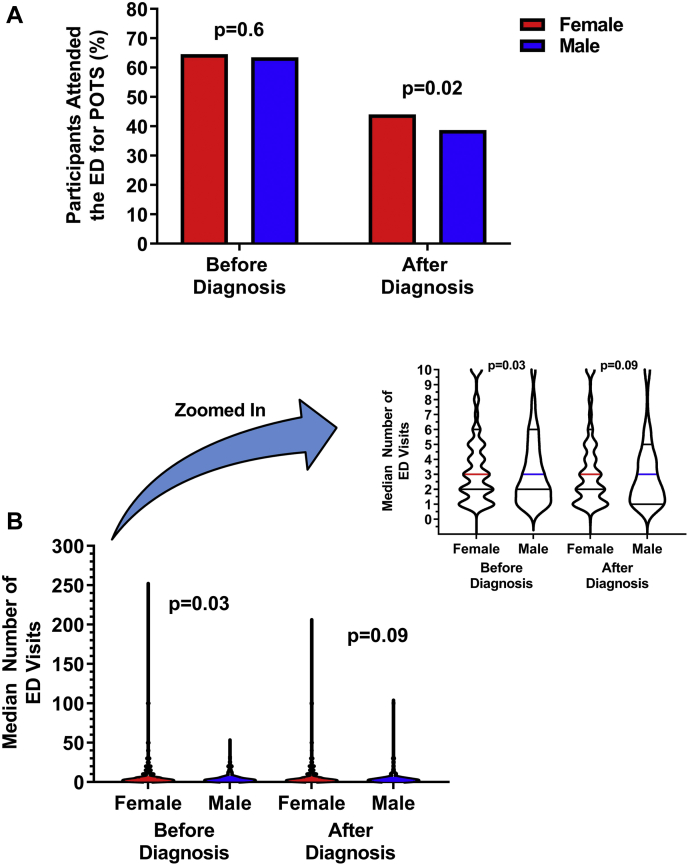
Before diagnosis, a similar proportion of female and male patients visited the emergency department at least once for POTS symptoms (64.6% vs 63.5%, P = 0.6; Fig. 2A), but female patients reported more emergency department visits than male patients (3 [2-6] vs 3 [2-6] visits, P < 0.03; Fig. 2B). The mean number (not median) of emergency department visits before diagnosis was 6.6 ± 0.2 for female patients and 4.9 ± 0.3 for male patients. After diagnosis, a larger proportion of female than male patients attended the emergency department at least once for POTS symptoms (44.0% vs 38.7%, P = 0.02), but female and male patients who did attend reported a similar number of visits (3 [1-5] vs 3 [1-5], P = 0.09).
Figure 2.
(A) Emergency department (ED) utilization for postural orthostatic tachycardia syndrome (POTS) symptoms before and after diagnosis. (B) Median number of ED visits for POTS before (left) and after (right) diagnosis. The inset is a zoomed-in version of the main figure, to better illustrate the median and interquartile range. Extreme outlier values (375 visits in 1 female patient before diagnosis, and 600 visits in 1 female patient after diagnosis) were excluded from this box plot.
When asked “When you were symptomatic, but BEFORE you were diagnosed with POTS, did any doctor tell you that you were suffering from a psychological or psychiatric problem?,” a similar proportion of male and female patients were told that they had a psychological disorder (Table 1). However, more female than male patients were told that their ”symptoms were ‘all in their head’ or something similar.” Before diagnosis, more female than male patients felt that “in their own opinion” they did have a psychological or psychiatric disorder (32.6% vs 22.6%, P < 0.001).
Table 1.
Diagnostic features reported by postural orthostatic tachycardia syndrome (POTS) patients
| Female patients |
Male patients |
P |
|||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Misdiagnosed | 5893 | 76.2 | 388 | 74.9 | 0.5 |
| Physical illness acknowledged but physician was unsure how to proceed | 5580 | 71.5 | 377 | 72.2 | 0.7 |
| Physician said it was a psychological disorder | 6074 | 77.7 | 389 | 74.2 | 0.07 |
| Physician said it was all in your head | 5370 | 68.8 | 328 | 62.5 | 0.002 |
| Suggested POTS to physician before physician suggested it | 2839 | 36.3 | 199 | 37.9 | 0.5 |
| Diagnosed by a cardiologist | 4668 | 56.6 | 288 | 41.7 | 0.02 |
| Diagnosed by a neurologist | 1491 | 18.1 | 127 | 22.8 | 0.005 |
After diagnosis, a similar proportion of female and male patients were told that they had a psychological disorder (38.0% vs 34.8%, P = 0.1), but more female than male patients continued to be told by a doctor that their illness was “all in their head” or something similar (26.8% vs 21.2%, P = 0.005), and more female than male patients were being treated for a psychological disorder at the time of the survey (35.7% vs 28.5%, P = 0.001).
After diagnosis, more female than male patients were told that their POTS was caused by a lack of exercise (26.5% vs 22.5%, P = 0.048) and that POTS could be cured by exercise (39.7% vs 35.1%, P = 0.04). However, after diagnosis, more female than male patients were also told that they should not exercise (14.3% vs 10.0%, P = 0.006).
POTS onset and symptoms
The age of initial POTS symptom onset was marginally older in male compared to female patients (17 [13-31] vs 17 [13-27] years, P = 0.04). The most common age of onset for both female and male patients was 14 years. More male than female patients experienced POTS onset within 3 months after a specific event (46.1% vs 40.9%, P = 0.02). The most common event preceding POTS onset was an infection (female patients: 42.1% vs male patients: 37.6%, P = 0.2). More male than female patients reported that a family member had also been diagnosed with POTS (15.3% vs 10.6%, P = 0.002).
Typical POTS symptoms, including lightheadedness, pre-syncope, chest pain, and palpitations, were more common in female than male patients. Additionally, a slightly larger proportion of female than male patients reported symptoms indicative of multi-system involvement, including gastrointestinal symptoms, allergic-like symptoms, and neurologic symptoms in the hands and feet. A summary of symptoms is shown in Table 2. The most common symptoms in female compared to male patients are shown in Supplemental Table S1, with a full summary of symptoms shown in Supplemental Table S2.
Table 2.
Symptoms
| Symptom | Overall |
Female patients |
Male patients |
P | |||
|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | ||
| Lightheadedness | 7382 | 98.7 | 6934 | 99.0 | 448 | 95.3 | < 0.001 |
| Headache | 7050 | 94.4 | 6638 | 94.9 | 412 | 87.8 | < 0.001 |
| Difficulty concentrating | 7035 | 94.4 | 6603 | 94.4 | 432 | 92.1 | 0.04 |
| Nausea | 6688 | 89.5 | 6331 | 90.4 | 357 | 76.0 | < 0.001 |
| Shortness of breath | 6574 | 88.1 | 6206 | 88.7 | 368 | 78.5 | < 0.001 |
| Palpitation | 6537 | 88.1 | 6171 | 88.7 | 366 | 79.4 | < 0.001 |
| Chest pain | 5857 | 78.6 | 5527 | 79.1 | 330 | 70.7 | < 0.001 |
| Tremulousness | 5715 | 76.8 | 5408 | 77.6 | 307 | 65.3 | < 0.001 |
| Blurred vision | 5574 | 74.8 | 5261 | 75.4 | 313 | 66.7 | < 0.001 |
| ≥ 1 GI symptoms | 7350 | 98.4 | 6905 | 98.6 | 445 | 94.7 | < 0.001 |
| Neurologic symptoms—foot | 6896 | 92.6 | 6516 | 93.4 | 380 | 81.7 | < 0.001 |
| Neurologic symptoms—hand | 6821 | 91.7 | 6473 | 92.2 | 391 | 83.9 | < 0.001 |
| Allergic-like symptoms | 5081 | 68.7 | 4789 | 69.1 | 292 | 62.4 | 0.003 |
GI, gastrointestinal.
Comorbidities
More female than male patients reported one or more comorbidities (85.0% vs 70.9%, P < 0.001). More female than male patients reported comorbid diagnoses of Ehlers-Danlos syndrome (EDS), mast cell activation syndrome (MCAS), and autoimmune disorders. Rates of neuropathies were not different in female compared to male patients. A summary of the most common comorbidities is shown in Table 3, and a full summary is shown in Supplemental Table S3.
Table 3.
Comorbid disorders
| Comorbid disorder | Overall (N = 7307) |
Female patients (n = 6847) |
Male patients (n = 460) |
Odds ratio | 95% CI | |||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Migraine | 2901 | 39.7 | 2801 | 40.9 | 100 | 21.7 | 2.5 | 2.0–3.1 |
| Irritable bowel syndrome | 2185 | 29.9 | 2107 | 30.8 | 78 | 17.0 | 2.2 | 1.7–2.8 |
| Ehlers-Danlos syndrome | 1796 | 24.2 | 1724 | 25.2 | 72 | 15.7 | 1.8 | 1.4–2.3 |
| Asthma | 1540 | 21.1 | 1472 | 21.5 | 68 | 14.8 | 1.6 | 1.2–2.1 |
| Chronic fatigue syndrome | 1453 | 19.9 | 1387 | 20.3 | 66 | 14.3 | 1.5 | 1.2–2.0 |
| Fibromyalgia | 1367 | 18.7 | 1330 | 19.4 | 37 | 8.0 | 2.8 | 2.0–2.9 |
| Raynaud’s phenomenon | 1159 | 15.9 | 1133 | 16.5 | 26 | 5.7 | 3.3 | 2.2–4.9 |
| ≥ 1 neuropathy | 1143 | 15.6 | 1077 | 15.7 | 66 | 14.3 | 1.1 | 0.9–1.5 |
| ≥ 1 autoimmune disease | 1156 | 15.8 | 1124 | 16.4 | 32 | 7.0 | 2.6 | 1.8–2.8 |
| Gastroparesis | 966 | 13.2 | 942 | 13.8 | 24 | 5.2 | 2.9 | 1.9–4.4 |
| Vasovagal syncope | 831 | 11.4 | 804 | 11.7 | 27 | 5.9 | 2.1 | 1.4–3.2 |
| Inappropriate sinus tachycardia | 850 | 11.6 | 824 | 12.0 | 26 | 5.7 | 2.3 | 1.5–3.4 |
| Mast cell activation syndrome | 777 | 10.6 | 755 | 11.0 | 22 | 4.8 | 2.5 | 1.6–3.8 |
CI, confidence interval.
Treatments
At the time of taking the research survey, more female than male patients were taking beta-blockers (Supplemental Table S4A), selective-serotonin reuptake inhibitors, and benzodiazepines. Desmopressin use was higher in male than female patients. The use of other medication was not different between male and female patients. More female than male patients reported increasing their dietary salt intake (Supplemental Table S4B) and taking electrolyte supplements, but the use of salt supplements (salt tablets, salt sticks) was not different. More female than male patients also reported using compression garments.
Social and educational aspects
More female than male patients report losing at least one friend due to POTS (56.3% vs 48.0%, P < 0.001). A similar proportion of male and female patients have lost a serious relationship with a partner or spouse due to POTS (22.1% vs 21.0%, P = 0.06), but more female than male patients have lost relationships with other family members due to POTS (23.6% vs 18.7%, P = 0.016). In patients age 18 years or older, more female than male patients developed POTS before completing their formal education (58.2% vs 45.3%, P < 0.001). A similar proportion of female and male patients age 18 years or older missed school due to POTS (85.9% vs 90.6%, P = 0.09) or dropped out of school due to POTS (23.3% vs 28.4%, P = 0.1).
Quality of life
RAND-36 health-related quality-of-life scores were evaluated in female and male participants age 18 years of age or older. Female patients had lower RAND-36 physical health–related composite scores, male patients had lower mental health composite scores, and overall health-related composite scores were not different in male vs female patients. A summary of the RAND-36 health-related quality-of-life domains and composite scores is shown in Table 4.
Table 4.
RAND-36 health-related quality-of-life domain scores for participants age ≥ 18 years
| Domain | Female patients | Male patients | P |
|---|---|---|---|
| Physical functioning | 35 (20–55) | 45 (25–70) | < 0.001 |
| Role limitations due to physical health | 0 (0–0) | 0 (0–0) | 0.8 |
| Role limitations due to emotional health | 67 (0–100) | 33 (0–100) | 0.001 |
| Energy and fatigue | 15 (5–30) | 20 (10–30) | 0.2 |
| Emotional well-being | 64 (48–76) | 56 (40–72) | < 0.001 |
| Social functioning | 38 (25–63) | 38 (13–50) | 0.03 |
| Pain | 45 (23–68) | 45 (33–70) | < 0.001 |
| General health | 25 (15–40) | 30 (20–45) | < 0.001 |
| Composite scores | |||
| Physical health | 32 (21–46) | 38 (24–51) | < 0.001 |
| Mental health | 53 (37–69) | 48 (33–65) | 0.001 |
| Overall health | 49 (36–63) | 49 (24–51) | 0.6 |
Median scores (25th-75th percentiles) are reported. After correction for multiple comparisons, a P value of 0.005 was considered significant, indicated by boldface.
RAND-36, short-form, 36-item health questionnaire (Santa Monica, CA).
Discussion
The majority of survey respondents meeting inclusion criteria for this study were female and white, consistent with our understanding of the POTS demographic.3,10,11 The reasons for the female predominance are not well understood but may be related to female sex hormones12 or baseline differences in autonomic function.13
Overall, both female and male patients with POTS experience challenges with diagnosis, multi-system symptoms, and comorbidities. There is a lack of awareness and recognition of POTS in the medical community, and this is reflected in the high rates of misdiagnosis and the prolonged diagnostic delay in some patients with POTS. Despite the primarily female demographic of this disorder, female patients with POTS are impacted more than male patients regarding challenges with diagnosis, symptom burden, rates of comorbidities, and quality of life. The reasons for this disparity are unknown, but it may be related to sex differences in the underlying pathophysiology of POTS, or diagnostic and treatment biases affecting female patients differently than male patients.
Diagnostic journey
Both female and male patients experienced a significant diagnostic delay, although the mean delay was almost 2 years longer in female than in male patients. Prolonged diagnostic delay is consistent with findings in a UK-based POTS cohort, in which the mean time from symptom onset to diagnosis ranged from 5 to 8 years.14 In a study of patients at the Mayo Clinic, the mean time from symptom onset to diagnosis was 4 years.1 The explanation for this discrepancy, despite male and female patients being misdiagnosed at similar rates, and seeing a similar number of doctors before diagnosis, may be related to a sex bias in diagnosis. Misdiagnosis with a psychological illness was common in both male and female patients, and has been reported in other POTS cohorts, including a UK-based survey of POTS patients in which 48% received an incorrect psychological diagnosis.15 Although both male and female patients were told their POTS symptoms might be psychological in nature, despite no increased rate of significant psychiatric illness in this population,16 female patients were more likely to be told their illness was “all in their head.” Another possible explanation for the sex difference is that female patients might have a more complex presentation, with increased comorbidities, which could contribute to the diagnostic delay. At this time, it is not known what features of POTS lead to this sex difference in diagnostic delay.
The time from symptom onset to physician presentation was also longer in female than male patients. It is possible that female patients are more likely to think these symptoms are “just normal” and downplay the severity of their symptoms. Alternatively, based on their prior interactions with the medical system, some female patients might feel that their doctor will not take their symptoms seriously.
After the diagnosis of POTS, the proportion of patients who were told their illness was “all in their head” decreased, but it was still significantly larger in female compared to male patients. More female than male patients were also being treated for a psychological disorder after diagnosis. This difference is consistent with data from the general population, which show that more women and girls than men and boys are diagnosed with depression and anxiety.17,18 In addition, more female patients visited the emergency department for POTS even after a POTS diagnosis, potentially indicating a gap in the medical management of POTS, or more severe symptoms in female patients.
POTS onset and symptoms
Although the most common (mode) age of onset in both female and male patients was 14 years, the median age of POTS onset was slightly higher in male than female patients. More male patients also reported onset of POTS after a specific event, reflecting the heterogeneity of pathophysiology in POTS. In a study of pediatric POTS patients, about one third reported POTS onset after an event, and more male than female patients reported POTS onset after infection.11 This result is different than our findings that a similar proportion of male and female patients reported POTS after infection, but this may reflect a difference between adult and pediatric populations.
Symptom burden was high in both female and male patients, consistent with findings in other studies,1,11 but a higher proportion of female patients reported symptoms, particularly multi-system symptoms. The reasons for this finding are not clear. The top 10 symptoms in female compared to male patients were the same, except for muscle pain reported commonly among female patients and trouble falling asleep reported commonly by male patients. In a UK-based survey of POTS patients, 90% of patients reported lightheadedness, consistent with our findings.15
Comorbidities
Comorbidities were common in both male and female patients, but a larger proportion of female patients reported one or more comorbidities. Common POTS comorbidities, including EDS, MCAS, and gastroparesis were more common in female patients. UK-based POTS surveys found that 18%14 and 51%15 of patients had comorbid diagnoses of EDS, and an evaluation of POTS patients found that 31% met the criteria for hypermobile EDS.19 In our cohort, 26% reported comorbid EDS, within the range of other studies. An association among POTS, EDS, and MCAS has also been reported,20 but the underlying pathophysiology linking these 3 disorders is not fully understood. Autoimmune disorders were also more common in female patients, and are generally more common among women and girls in the general population.21 A higher rate of comorbidities in the female patients may indicate a sex difference in the underlying pathophysiology of POTS.
Treatments
Beta-blockers were used more commonly in female than in male patients. The overall usage of beta-blockers in this group (42%) was lower than that in a cohort from the Mayo Clinic, in which 77% of patients were using beta-blockers.1 This disparity could be due to differences in care at a specialist centre compared to clinical care in the general population. More female patients than male patients were diagnosed by a cardiologist, which may explain the increased beta-blocker use among female patients. Female patients reported more frequent use of nonpharmacologic treatments, including increased salt and electrolyte intake, as well as compression garments. This could result from the greater symptom burden in female patients, leading them to be more likely to engage in multiple treatment modalities.
Quality of life
Past research has demonstrated reduced quality of life in POTS patients compared to healthy individuals.4,22 Review of sex differences revealed that female patients had lower physical health–related quality-of-life composite scores, and male patients had lower physical health–related quality-of-life scores. Lower physical health–related quality of life in female patients may be reflective of their increased comorbidities and symptoms, or it may indicate a gap in the treatment of these patients. Lower mental health–related quality of life in male patients may be related to being the minority, as the disorder primarily impacts female patients. Male patients may feel more isolated and have more difficulty finding support.
Limitations
Sex is defined as a set of biological attributes, and gender is defined as socially constructed identities, roles, and behaviours.23 This survey asked participants about their biological sex only, not gender, and therefore, the role of gender differences in POTS could not be explicitly evaluated.
These research survey data are self-reported, and POTS patients could intentionally or unintentionally report inaccurate information, or complete the questionnaire more than once. This comprehensive research survey took some POTS patients up to 90 minutes to complete. It is unlikely that a patient would take the time to complete such a survey more than once. Patients were primarily recruited through online social media, which may have created a barrier to survey access for patients who do not use social media. As participants may have completed the survey several years after POTS diagnosis, it is possible that recall bias influenced participant responses, although the large impact of the illness on patient lives makes recollection more likely. There may also be selection bias, whereby patients who have had significant improvement in symptoms may be less engaged in the patient community and therefore less likely to participate than participants who have ongoing symptoms. The survey was also written in English, and patients were required to read English (or use a translator) to complete this survey, limiting its accessibility to non–English-speaking patients.
Finally, a larger proportion of male patients, compared with female patients, were under the age of 18 years. It is possible that some of the observed differences could be due to differences in age and not sex.
Future directions
This study has revealed significant challenges in the diagnosis of POTS, including diagnostic delays that are worse for female patients. Further research to understand these differences may help increase awareness and recognition of POTS. A future study including gender differences, in addition to sex differences, in POTS may also reveal a greater understanding of societal impact on POTS patients.
Conclusions
Female patients with POTS experience more symptoms, and a higher burden of comorbidities, than male patients. Despite their sicker phenotype, female patients experience a longer diagnostic delay than male patients. Increasing knowledge and awareness about POTS, and targeted physician education, may help reduce this diagnostic delay and improve care for all patients with POTS.
Acknowledgements
The authors acknowledge the many POTS patients who took the time to complete this comprehensive survey. The authors also acknowledge the Dysautonomia International Patient Advisory Board for their key role in the development of the survey.
Funding Sources
This work was supported in part by the National Heart, Lung and Blood Institute (NHLBI) of the National Institutes of Health (NIH) under award number P01 HL056693 and by the National Centre for Advancing Translational Sciences (NCATS) award UL1 TR000445. SRR receives research support from the Canadian Institutes of Health Research (CIHR; Ottawa, Ontario, Canada) grant MOP142426. Ms. Kate M. Bourne holds a CIHR (Ottawa, Ontario, Canada) Vanier Canada Graduate Scholarship.
Disclosures
C.A.S. is a consultant for Lundbeck NA, Ltd. I.B. is a consultant for Lundbeck NA, Ltd., and Theravance. R.S.S. is a Cardiac Arrhythmia Network of Canada (CANet; London, Ontario, Canada) Network Investigator. S.R.R. is a consultant for Lundbeck NA, Ltd., Medscape, Spire Learning, and the Academy for Continued Healthcare Learning, developing continuing medical education teaching materials about neurogenic orthostatic hypotension. S.R.R. is Chair, Data Safety and Monitoring Board for Arena Pharmaceuticals; on the Board of Directors of the American Autonomic Society and the Canadian Cardiovascular Society Academy, both without financial compensation; and on the Medical Advisory Board of Dysautonomia International and PoTS UK, both without financial compensation. All the other authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: Survey information was stored in a secure Research Data Capture (REDCap) electronic database at Vanderbilt University (Nashville, Tennessee, USA). This study received ethical approval from both the Vanderbilt University Institutional Review Board (IRB#140303; Nashville, Tennessee, USA) and the University of Calgary Conjoint Health Research Ethics Board (CHREB; REB15-2922; Calgary, Ontario, Canada).
See page S51 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.08.014.
Supplementary Material
References
- 1.Thieben M.J., Sandroni P., Sletten D.M., et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 2.Shaw B.H., Stiles L.E., Bourne K., et al. The face of postural tachycardia syndrome—insights from a large cross-sectional online community-based survey. J Intern Med. 2019;286:438–448. doi: 10.1111/joim.12895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raj S.R., Guzman J.C., Harvey P., et al. Canadian Cardiovascular Society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol. 2020;36:357–372. doi: 10.1016/j.cjca.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 4.Benrud-Larson L.M., Dewar M.S., Sandroni P., et al. Quality of life in patients with postural tachycardia syndrome. Mayo Clin Proc. 2002;77:531–537. doi: 10.4065/77.6.531. [DOI] [PubMed] [Google Scholar]
- 5.Bourne K.M., Chew D.S., Stiles L.E., et al. Postural orthostatic tachycardia syndrome is associated with significant employment and economic loss. J Intern Med. 2021;290:203–212. doi: 10.1111/joim.13245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerc Liaudat C., Vaucher P., De Francesco T., et al. Sex/gender bias in the management of chest pain in ambulatory care. Womens Health (Lond) 2018;14 doi: 10.1177/1745506518805641. 1745506518805641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bugiardini R., Ricci B., Cenko E., et al. Delayed care and mortality among women and men with myocardial infarction. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.005968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hays R.D., Morales L.S. The RAND-36 measure of health-related quality of life. Ann Med. 2001;33:350–357. doi: 10.3109/07853890109002089. [DOI] [PubMed] [Google Scholar]
- 9.Harris P.A., Taylor R., Thielke R., et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garland E.M., Raj S.R., Black B.K., Harris P.A., Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69:790–798. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 11.Boris J.R., Bernadzikowski T. Demographics of a large paediatric postural orthostatic tachycardia syndrome program. Cardiol Young. 2018;28:668–674. doi: 10.1017/S1047951117002888. [DOI] [PubMed] [Google Scholar]
- 12.Fu Q., VanGundy T.B., Shibata S., et al. Menstrual cycle affects renal-adrenal and hemodynamic responses during prolonged standing in the postural orthostatic tachycardia syndrome. Hypertension. 2010;56:82–90. doi: 10.1161/HYPERTENSIONAHA.110.151787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christou D.D., Jones P.P., Jordan J., et al. Women have lower tonic autonomic support of arterial blood pressure and less effective baroreflex buffering than men. Circulation. 2005;111:494–498. doi: 10.1161/01.CIR.0000153864.24034.A6. [DOI] [PubMed] [Google Scholar]
- 14.McDonald C., Koshi S., Busner L., Kavi L., Newton J.L. Postural tachycardia syndrome is associated with significant symptoms and functional impairment predominantly affecting young women: a UK perspective. BMJ Open. 2014;4 doi: 10.1136/bmjopen-2013-004127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kavi L.N.M., Low D.A., Opie M., et al. A profile of patients with postural tachycardia syndrome and their experience of healthcare in the UK. Brit J Cardiol. 2016;23:1–6. [Google Scholar]
- 16.Raj V., Haman K.L., Raj S.R., et al. Psychiatric profile and attention deficits in postural tachycardia syndrome. J Neurol Neurosurg Psychiatry. 2009;80:339–344. doi: 10.1136/jnnp.2008.144360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert P.R. Why is depression more prevalent in women? J Psychiatry Neurosci. 2015;40:219–221. doi: 10.1503/jpn.150205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McLean C.P., Asnaani A., Litz B.T., Hofmann S.G. Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller A.J., Stiles L.E., Sheehan T., et al. Prevalence of hypermobile Ehlers-Danlos syndrome in postural orthostatic tachycardia syndrome. Auton Neurosci. 2020;224:102637. doi: 10.1016/j.autneu.2020.102637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vadas P., Guzman J., McGillis L., Mittal N., Walsh S. Cosegregation of postural orthostatic tachycardia syndrome, hypermobile Ehlers-Danlos syndrome, and mast cell activation syndrome. Ann Allergy Asthma Immunol. 2020;125:719–720. doi: 10.1016/j.anai.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Ali Y.S., Daamen N., Jacob G., et al. Orthostatic intolerance: a disorder of young women. Obstet Gynecol Surv. 2000;55:251–259. doi: 10.1097/00006254-200004000-00025. [DOI] [PubMed] [Google Scholar]
- 22.Bagai K., Song Y., Ling J.F., et al. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011;7:204–210. [PMC free article] [PubMed] [Google Scholar]
- 23.Heidari S., Babor T.F., De Castro P., Tort S., Curno M. Sex and gender equity in research: rationale for the SAGER guidelines and recommended use. Res Integr Peer Rev. 2016;1:2. doi: 10.1186/s41073-016-0007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.