Abstract
Background
Female patients have been shown to experience worse clinical outcomes after acute myocardial infarction (AMI) compared with male patients. However, it is unclear what trend these differences followed over time.
Methods
Data from patients hospitalized with AMI between 2004 and 2015 in the National Inpatient Sample were retrospectively analyzed, stratified according to sex. Multivariable logistic regression analyses were performed to examine the adjusted odds ratios (aORs) of invasive management and in-hospital outcomes according to sex. The Mantel-Haenszel extension of the χ2 test was performed to examine the trend of management and in-hospital outcomes over the study period.
Results
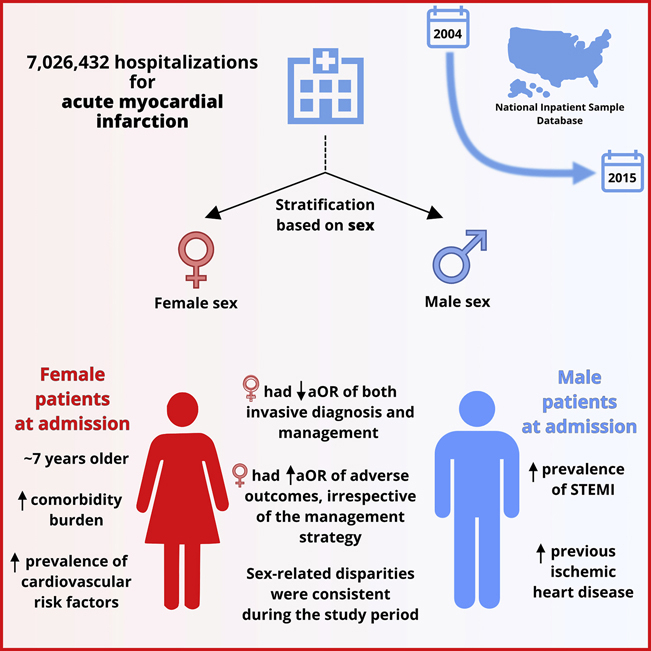
Of 7,026,432 AMI hospitalizations, 39.7% (n = 2,789,494) were women. Overall, women were older (median: 77 vs 70 years), with a higher prevalence of risk factors such as diabetes, hypertension, and depression. Women were less likely to receive coronary angiography (aOR, 0.92; 95% confidence interval [CI], 0.91-0.93) and percutaneous coronary intervention (aOR, 0.82; 95% CI, 0.81-0.83) compared with men. Odds of all-cause mortality were higher in women (aOR, 1.03; 95% CI, 1.02-1.04; P < 0.001) and these rates have not narrowed over time (2004 vs 2015: aOR, 1.07 [95% CI, 1.04-1.09] vs 1.11 [95% CI, 1.07-1.15), with similar observations recorded for major adverse cardiovascular and cerebrovascular events.
Conclusions
In this temporal analysis of AMI hospitalizations over 12 years, we showed lower receipt of invasive therapies and higher mortality rates in women, with no change in temporal trends. There needs to be a systematic and consistent effort toward exploring these disparities to identify strategies to mitigate them.
Graphical abstract
Résumé
Contexte
Il a été démontré que les femmes présentent de moins bons résultats cliniques après un infarctus aigu du myocarde (IAM) que les hommes. Cependant, la tendance de ces différences dans le temps n’est pas claire.
Méthodologie
Les données de la National Inpatient Sample sur les patients hospitalisés pour un IAM entre 2004 et 2015 ont été analysées rétrospectivement, stratifiées selon le sexe. Des analyses de régression logistique multidimensionnelles ont été effectuées pour examiner les rapports de cotes ajustés (RCA) de la prise en charge par un traitement invasif et des résultats obtenus en milieu hospitalier en fonction du sexe. Le test du χ2 étendu de Mantel-Haenszel a été effectué pour examiner la tendance de la prise en charge et des résultats en milieu hospitalier au cours de la période d’étude.
Résultats
Sur 7 026 432 patients hospitalisés pour un IAM, 39,7 % (n = 2 789 494) étaient des femmes. Dans l’ensemble, les femmes étaient plus âgées (âge médian : 77 vs 70 ans), avec une plus forte prévalence de facteurs de risque comme le diabète, l’hypertension et la dépression. Les femmes étaient moins susceptibles que les hommes de subir une coronarographie (RCA : 0,92; intervalle de confiance [IC] à 95 % : 0,91-0,93) et une intervention coronarienne percutanée (RCA : 0,82; IC à 95 % : 0,81-0,83). Les probabilités de mortalité toutes causes confondues étaient plus élevées chez les femmes (RCA : 1,03; IC à 95 % : 1,02-1,04; p < 0,001), et ces taux n’ont pas diminué avec le temps (2004 vs 2015 : RCA : 1,07 [IC à 95 % : 1,04-1,09] vs 1,11 [IC à 95 % : 1,07-1,15), des observations similaires étant consignées pour les événements cardiovasculaires et vasculaires cérébraux majeurs.
Conclusions
Dans cette analyse temporelle des hospitalisations pour IAM sur 12 ans, nous avons montré que les femmes subissaient moins de traitements invasifs et présentaient des taux de mortalité plus élevés, sans changement dans les tendances temporelles. Il faut un effort systématique et cohérent pour explorer ces disparités afin de cibler des stratégies pour les atténuer.
Acute myocardial infarction (AMI) is the most acute presentation of ischemic heart disease and the leading cause of mortality in men and women worldwide, accounting for 17.9 million deaths globally per year (31% of all deaths).1, 2, 3 Ischemic heart disease-related mortality has declined in recent years because of increased awareness of cardiovascular risk,4 advances in pharmacological therapy, coronary revascularization, and cardiovascular prevention.5 Notwithstanding, several studies have shown a higher incidence of adverse events in women after AMI,6,7 and have attributed worse outcomes in women to their lower rate of receipt of invasive management, in the form of coronary angiography (CA) or percutaneous coronary intervention (PCI).6 Furthermore, anatomical and biological factors could place women at a greater risk of mechanical and procedural complications after AMI, such as smaller-sized arteries and differences in plaque characteristics.8,9
Most of the evidence on sex differences in AMI management and outcomes to date is limited to data from highly selected cohorts (such as age younger than 55 years10,11 or randomized controlled trials), which might not be representative of real-world practice, certain geographical regions,12 specific syndromes (eg, ST-elevation myocardial infarction [STEMI] only),13 or are of relatively small sample sizes.14 However, it is unclear whether there have been temporal changes in sex-based differences and in particular whether disparities have narrowed, especially in light of the increasing recognition of sex disparities in recent years. Older studies from the national registry of myocardial infarction in the United States (1994-2006) suggested a narrowing in the differences in outcomes between sexes, with an overall improvement in mortality outcomes.15 In contrast, a national French registry analysis of 5000 STEMI patients hospitalized between 2006 and 2011 showed significant persistent sex-based differences in management and outcomes.16 Although these findings provide us with insight into sex differences in a STEMI population, it is unclear whether these differences are also observed across a broader spectrum of presentations. Moreover, their analysis was derived from a cohort from close to more than a decade ago, after which there have been many advances in AMI care and improvements in outcomes with the development of regional PCI, services.
Therefore, in the present study we sought to examine the temporal trends in invasive management and in-hospital outcomes of both sexes over a period of 12 years in a national cohort of AMI hospitalizations in the United States.
Methods
Data source
The National Inpatient Sample (NIS) is a set of the largest publicly available all-payer longitudinal databases of hospital in-patient discharges in the United States. It is developed for the Agency for Healthcare Research and Quality (AHRQ), which administers the Healthcare Cost and Utilization Project.17,18 It contains anonymized retrospectively collected data on primary and secondary discharge diagnoses and procedures from more than 7 million hospitalizations annually. Therefore, it can be used for the national and regional estimation of hospital utilization, quality, and other related issues. The NIS data set was designed to approximate a 20% stratified sample of the US community hospitals, excluding rehabilitation and long-term acute care hospitals, and provides sampling weights to calculate national estimates that represent more than 95% of the US population. Previous validation studies have shown that it has better demographic capture compared with a large multistate electronic health record data set and that it is highly comparable with other related databases.19,20
Study design and population
All hospitalizations of adults (18 years of age and older) with a principal discharge diagnosis of AMI between January 2004 and September 2015 were included, stratified according to sex. International Classification of Diseases, Ninth Revision (ICD-9) and Clinical Classification Software codes were used to identify STEMI and non-STEMI (NSTEMI), patient comorbidities, procedures, and clinical outcomes (Supplemental Table S1). Additional comorbidities were identified using the existing 29 AHRQ Elixhauser comorbidity measures. Hospital-related factors including hospital bed size, region, and location/teaching status were analyzed to account for any hospital-level differences. The “Hospital bed size” variable refers to the number of short-term acute hospital beds and is specific to the hospital’s location and teaching status. Missing data represented 0.4% (n = 27,042) of the original data set and, therefore, such cases were excluded (flow diagram: Supplemental Fig. S1).
Outcomes
The primary outcome was in-hospital mortality. Secondary outcomes included major acute cardiovascular and cerebrovascular events (MACCE; composite of all-cause mortality, acute stroke/transient ischemic attack, and cardiac complications), all-cause bleeding, and acute stroke/transient ischemic attack. Cardiac complications included hemopericardium, cardiac tamponade, coronary dissection, and any pericardiocentesis procedure. The process outcome was the receipt of invasive management for AMI, in the form of CA and/or PCI.
Statistical analysis
Statistical Package for the Social Sciences (SPSS) statistical software version 25 (IBM Corp, Armonk, NY) was used for statistical data analysis. We assessed the normality of data distribution using the Kolmogorov-Smirnov test. Data were expressed as median (interquartile range) for continuous nonparametric data and as whole numbers (percentages) for categorical data. Quantitative nonparametric data were analyzed using the Mann-Whitney U test, whereas the χ2 test was used for the comparison of categorical variables between the study groups. All analyses were conducted with appropriate sampling weights provided by the AHRQ for each individual discharge.
Multivariable binomial logistic regression analyses were used to determine the adjusted odds ratios (aORs) and 95% confidence intervals (CIs) of invasive management and in-hospital adverse outcomes between sexes (Appendix 1).
Trend analysis with a Mantel-Haenszel extension of the χ2 test of trend (linear-by-linear association) was conducted to establish trends of invasive management and in-hospital adverse outcomes over 12 years.
Results
Patient characteristics
A total of 7,026,432 records of AMI hospitalizations between 2004 and 2015 were included, of which 2,789,494 (39.7%) were women. Overall, women were, on average, 7 years older than men (median, 77 vs 70 years). The 2 groups were comparable on characteristics such as household income class, hospital bed size, and weekend admission. STEMI presentation was more common in men compared with women (32.6% vs 24.3%, respectively; P < 0.001). The prevalence of conventional cardiovascular risk factors such as diabetes, stroke, hypertension, and chronic kidney disease was higher in women compared with men (36.7% vs 32.8%, 3.6% vs 2.7%, 69.8% vs 65.1%, and 17.7% vs 16.2%, respectively; P < 0.001 for each). In contrast, men had a higher prevalence of previous cardiovascular disease, as evidence by the higher prevalence of previous AMI, PCI, coronary artery bypass grafting, and angina (9.3% vs 7.5%, 11% vs 7.8%, 6.9% vs 4.9%, and 7.8% vs 5.3%, respectively; P < 0.001 for each). Smoking was less prevalent in female patients (21.8% vs 32.8%, P < 0.001), obesity was slightly more often prevalent (12.8% vs 11.5%, P < 0.001), but depression and hypothyroidism were substantially more prevalent in female patients (9.2% vs 4.7%, P < 0.001 and 16.5% vs 5.4%, P < 0.001, respectively; Table 1).
Table 1.
Patient characteristics according to sex
| Characteristic | Sex |
P | |
|---|---|---|---|
| Female (39.7%) | Male (60.3%) | ||
| Number of weighted discharges | 2,789,494 | 4,236,938 | |
| Median age (IQR), years | 77 (66-85) | 70 (60-80) | < 0.001 |
| Age group, % | < 0.001 | ||
| 18-29 | 0.2 | 0.3 | |
| 30-49 | 7.6 | 13.5 | |
| 50-79 | 57.4 | 68.8 | |
| ≥ 80 | 34.8 | 17.4 | |
| STEMI, % | 24.3 | 32.6 | < 0.001 |
| Elective admission, % | 6.7 | 7.1 | < 0.001 |
| Weekend admission, % | 25.9 | 26.0 | 0.003 |
| Primary expected payer, % | < 0.001 | ||
| Medicare | 68.6 | 50.0 | |
| Medicaid | 6.3 | 5.9 | |
| Private insurance | 19.1 | 33.2 | |
| Self-pay | 4.0 | 6.8 | |
| No charge | 0.4 | 0.7 | |
| Other | 1.6 | 3.4 | |
| Median household income (percentile), % | < 0.001 | ||
| 0-25 | 30.3 | 27.7 | |
| 26-50 | 27.5 | 27.1 | |
| 51-75 | 23.2 | 24.2 | |
| 76-100 | 19.0 | 21.0 | |
| Cardiogenic shock, % | 4.7 | 5.1 | < 0.001 |
| Cardiac arrest, % | 1.5 | 1.7 | < 0.001 |
| Ventricular tachycardia, % | 2.0 | 2.9 | < 0.001 |
| Ventricular fibrillation, % | 1.9 | 3.2 | < 0.001 |
| Cardiac tamponade, % | 0.063 | 0.055 | < 0.001 |
| Hemopericardium, % | 0.027 | 0.023 | < 0.001 |
| Comorbidities, % | |||
| Atrial fibrillation | 18.4 | 15.5 | < 0.001 |
| Dyslipidaemia | 51.0 | 57.7 | < 0.001 |
| Thrombocytopenia | 2.1 | 2.6 | < 0.001 |
| Dementia | 2.5 | 1.2 | < 0.001 |
| Smoking history | 21.8 | 32.8 | < 0.001 |
| Previous AMI | 7.5 | 9.3 | < 0.001 |
| History of IHD | 70.2 | 81.2 | < 0.001 |
| Previous PCI | 7.8 | 11.0 | < 0.001 |
| Previous CABG | 4.9 | 6.9 | < 0.001 |
| Previous CVA | 3.6 | 2.7 | < 0.001 |
| Family history of CAD | 5.3 | 7.8 | < 0.001 |
| Deficiency anemias | 18.9 | 12.0 | < 0.001 |
| Chronic blood loss anemia | 1.4 | 0.9 | < 0.001 |
| Congestive heart failure | 1.1 | 0.7 | < 0.001 |
| Valvular disease | 0.329 | 0.198 | < 0.001 |
| Hypertension | 69.8 | 65.1 | < 0.001 |
| Peripheral vascular disorders | 11.5 | 10.6 | < 0.001 |
| Pulmonary circulation disorders | 0.137 | 0.082 | < 0.001 |
| Chronic pulmonary disease | 23.5 | 18.9 | < 0.001 |
| Coagulopathy | 3.9 | 4.7 | < 0.001 |
| Obesity | 12.8 | 11.5 | < 0.001 |
| Weight loss | 2.7 | 1.8 | < 0.001 |
| Diabetes mellitus, uncomplicated | 29.8 | 27.2 | < 0.001 |
| Diabetes mellitus with complications | 6.9 | 5.6 | < 0.001 |
| Hypothyroidism | 16.5 | 5.4 | < 0.001 |
| Drug abuse | 1.3 | 2.5 | < 0.001 |
| Alcohol abuse | 1.0 | 4.0 | < 0.001 |
| AIDS | 0.1 | 0.2 | < 0.001 |
| Depression | 9.2 | 4.7 | < 0.001 |
| Peptic ulcer disease excluding bleeding | 0.034 | 0.031 | 0.068 |
| Liver disease | 1.0 | 1.3 | < 0.001 |
| Chronic renal failure | 17.7 | 16.2 | < 0.001 |
| Other neurological disorders | 7.2 | 4.9 | < 0.001 |
| Paralysis | 1.9 | 1.4 | < 0.001 |
| Psychoses | 2.5 | 1.8 | < 0.001 |
| RA/collagen vascular diseases | 3.6 | 1.3 | < 0.001 |
| Solid tumour without metastasis | 1.2 | 1.6 | < 0.001 |
| Metastatic cancer | 0.891 | 0.853 | < 0.001 |
| Lymphoma | 0.467 | 0.508 | < 0.001 |
| Fluid and electrolyte disorders | 23.7 | 16.6 | < 0.001 |
| Bed size of hospital, % | < 0.001 | ||
| Small | 11.6 | 10.1 | |
| Medium | 25.4 | 24.5 | |
| Large | 65.4 | 63.0 | |
| Hospital Region, % | < 0.001 | ||
| Northeast | 19.9 | 18.6 | |
| Midwest | 23.9 | 23.2 | |
| South | 39.8 | 40.0 | |
| West | 16.4 | 18.2 | |
| Location/teaching status of hospital, % | < 0.001 | ||
| Rural | 11.5 | 9.4 | |
| Urban non-teaching | 41.6 | 40.8 | |
| Urban teaching | 47.0 | 49.8 | |
AMI, acute myocardial infarction; CABG, coronary artery bypass graft; CAD, coronary artery disease; CVA, cerebrovascular accident; IHD, ischemic heart disease; IQR, interquartile range; PCI, percutaneous coronary intervention; RA, rheumatoid arthritis; STEMI, ST-elevation myocardial infarction.
In-hospital management
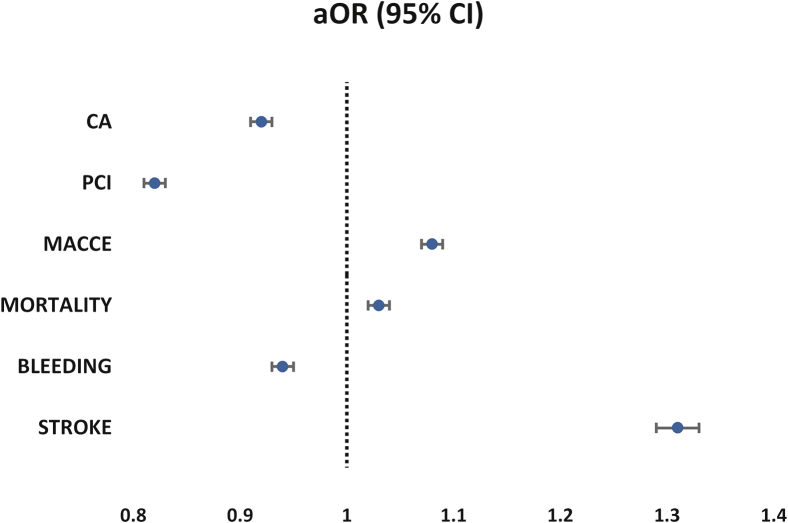
Overall, receipt of invasive therapies was higher in men than in women (CA, 70.1% vs 57.2% and PCI, 48.8% vs 34.8%, respectively; P < 0.001 for both) (Table 2, Supplemental Fig. S2). Women had significantly lower odds of receiving invasive therapy than men after adjustment for differences in baseline covariates (CA: aOR, 0.92 [95% CI, 0.91-0.93]; PCI: aOR, 0.82 [95% CI, 0.81-0.83]; P < 0.001 for both; Table 3, Fig. 1).
Table 2.
Comparison of treatments and in-hospital adverse outcomes for the different sex groups
| Variable | Sex |
P | |
|---|---|---|---|
| Female (39.7%) | Male (60.3%) | ||
| Treatment, % | |||
| CA | 57.2 | 70.1 | < 0.001 |
| PCI | 34.8 | 48.8 | < 0.001 |
| CABG | 6.3 | 10.5 | < 0.001 |
| Thrombolysis | 1.1 | 1.5 | < 0.001 |
| Use of assist device or IABP | 3.7 | 5.7 | < 0.001 |
| Outcomes, % | |||
| MACCE | 8.5 | 6.1 | < 0.001 |
| All-cause mortality | 6.8 | 5.1 | < 0.001 |
| All-cause bleeding | 3.3 | 3.0 | < 0.001 |
| Cardiac complications | 0.084 | 0.074 | < 0.001 |
| Postprocedural hemorrhage | 0.7 | 0.7 | 0.418 |
| Stroke | 2.0 | 1.2 | < 0.001 |
| Median length of stay (IQR), days | 5 (3-8) | 4 (2-8) | < 0.001 |
| Median total charges (IQR), USD$ | 41,254 (20,718-78,877) | 50,151 (25,284- 95,125) | 0.003 |
CA, coronary angiography; CABG, coronary artery bypass graft; IABP, intra-aortic balloon pump; IQR, interquartile range; MACCE, major adverse cardiac and cerebrovascular events (composite of mortality, acute stroke/ transient ischemic attack and cardiac complications); PCI, percutaneous coronary intervention.
Table 3.
Adjusted odds of invasive management and in-hospital adverse outcomes in women∗
| Variable | Female sex, OR (95% CI) | P |
|---|---|---|
| Invasive management | ||
| CA | 0.92 (0.91-0.93) | < 0.001 |
| PCI | 0.82 (0.81-0.83) | < 0.001 |
| Outcomes | ||
| MACCE | 1.08 (1.07-1.09) | < 0.001 |
| All-cause mortality | 1.03 (1.02-1.04) | < 0.001 |
| All-cause bleeding | 0.94 (0.93-0.95) | < 0.001 |
| Cardiac complications | 1.12 (1.06-1.19) | < 0.001 |
| Stroke | 1.31 (1.29-1.33) | < 0.001 |
CA, coronary angiography; CI, confidence interval; MACCE, major adverse cardiac and cerebrovascular events (composite of mortality, acute stroke/transient ischemic attack and cardiac complications); OR, odds ratio; PCI, percutaneous coronary intervention.
Reference group is men.
Figure 1.
Adjusted odds ratios (aORs) of invasive management and in-hospital adverse outcomes in women (reference group is men). CA, coronary angiography; CI, confidence interval; MACCE, major adverse cardiac and cerebrovascular events (composite of mortality, acute stroke/transient ischemic attack and cardiac complications); PCI, percutaneous coronary intervention.
Similar findings were observed in the STEMI subgroup (Supplemental Table S2). Women with STEMI were less likely to receive CA (aOR, 0.89 [95% CI, 0.88-0.90; P < 0.001) or PCI (aOR, 0.85 [95% CI, 0.84-0.86]; P < 0.001; Supplemental Table S3).
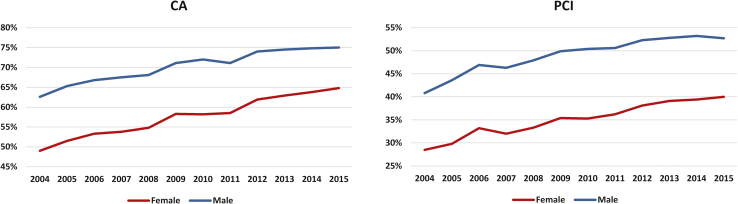
Furthermore, although there was a gradual increase in rates of receipt of invasive therapy for both sexes over the 12 years analyzed, women had persistently lower rates of CA over the years studied (Supplemental Table S4, Fig. 2). Even after adjustment for differences in baseline covariates women had persistently lower odds of receipt of CA (2004: aOR, 0.95 [95% CI, 0.93-0.96]; 2015: aOR, 0.94 [95% CI, 0.92-0.95]; P for trend < 0.001) and PCI (2004: 0.90 [95% CI, 0.89-0.92]; 2015: 0.83 [95% CI, 0.82-0.84]; P for trend < 0.001) over time (Supplemental Table S5, Supplemental Fig. S3).
Figure 2.
Receipt of invasive management according to sex from 2004 to September 31, 2015. P < 0.001 for all trends. CA, coronary angiography; PCI, percutaneous coronary intervention.
In-hospital clinical outcomes
Overall, crude in-hospital outcomes were worse in women than in men (mortality: 6.8% vs 5.1%; bleeding: 3.3% vs 3.0%; stroke: 2.0% vs 1.2%, respectively; P < 0.001 for all; Table 2). Similar findings were reported in the STEMI (Supplemental Table S2), as well as in the PCI subgroup in which women had higher rates of adverse outcomes than men (mortality: 3.8% vs 2.5%; bleeding: 3.2% vs 2.1%; stroke: 1.2% vs 0.6%, respectively; P < 0.001 for all; Supplemental Table S6).
The overall adjusted odds of MACCE and mortality were higher in women than in men (aOR, 1.08 [95% CI, 1.07-1.09]; aOR, 1.03 [95% CI, 1.02-1.04], respectively; P < 0.001 for all). Women had an increased risk of stroke compared with men (aOR, 1.31 [95% CI, 1.29-1.33]; P < 0.001). In contrast, the odds of bleeding were lower in women than in men (aOR, 0.94 [95% CI, 0.93-0.95]; P < 0.001; Table 3, Fig. 1). In the STEMI subgroup analysis, women were at increased odds of all complications (MACCE, mortality, bleeding, and stroke) (Supplemental Table S3).
In a subgroup analysis of all patients who underwent PCI, women were associated with increased odds of all complications, including MACCE (aOR, 1.27 [95% CI, 1.26-1.29]), mortality (aOR, 1.2 [95% CI, 1.18-1.22]), bleeding (aOR, 1.20 [95% CI, 1.20-1.24]), and stroke (aOR, 1.49 [95% CI, 1.45-1.53]; P < 0.001 for all; Table 4).
Table 4.
Adjusted odds of in-hospital adverse outcomes in females who underwent PCI∗
| Variables | OR (95% CI) | P |
|---|---|---|
| MACCE | 1.27 (1.26-1.29) | < 0.001 |
| All-cause mortality | 1.20 (1.18-1.22) | < 0.001 |
| All-cause bleeding | 1.22 (1.20-1.24) | < 0.001 |
| Stroke | 1.49 (1.45-1.53) | < 0.001 |
OR, odds ratios; CI, confidence interval; MACCE, major adverse cardiac and cerebrovascular events (composite of mortality, acute stroke/transient ischemic attack and cardiac complications).
Reference group is males.
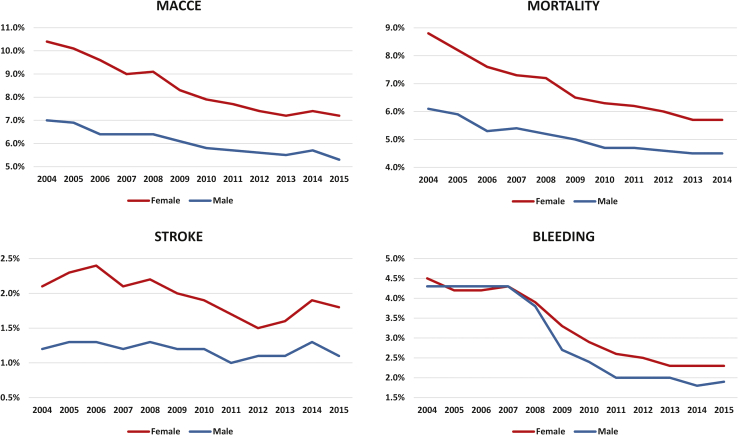
Over 12 years, there was a gradual decline in adverse event rates in both sexes (Fig. 3). However, in each year, the event rates of adverse outcomes were persistently higher in women compared with men (P for trend < 0.001’ Supplemental Table S4). Throughout the years studied, women were more likely to die, or have a major adverse cardiovascular event or stroke than men; except for major bleeding complications (P for trend < 0.001). Odds for major bleeding complications were similar in women compared with men initially, although from approximately 2009, the odds for bleeding were consistently higher in women compared with men with the highest values in 2014 (aOR, 1.2 [95% CI, 1.15-1.25]; P < 0.001; Supplemental Table S5, Supplemental Fig. S4). Similar trends were observed in the STEMI subgroup with consistently increased risk in women compared with men in almost all outcomes in the studied years except bleeding risk (P for trend < 0.001). Initially the trends of bleeding risk suggested lower odds in women. However, this pattern reversed in 2009, in which women had 35% (95% CI, 1.27- 1.44; P < 0.001) increased risk compared with men, after which the odds continued to be higher (Supplemental Table S7).
Figure 3.
In-hospital adverse outcomes according to sex from 2004 to September 31, 2015. P < 0.001 for all trends. MACCE, major adverse cardiac and cerebrovascular events (composite of mortality, acute stroke/transient ischemic attack and cardiac complications).
Discussion
To our knowledge, our study is the largest representative study of more than 7 million AMI admissions in the United States to report trends in sex-based differences in management and adverse outcomes over 12 years. Over the last decade, we witnessed improved awareness in sex-based cardiovascular risks, wider adoption of invasive management, and significant advances in the pharmacological management of acute coronary syndromes. Yet, we report no significant changes in disparities in AMI treatments and outcomes among the sexes. Women are still less likely to receive CA or PCI, and continue to have worse adverse outcomes compared with men. Even when women were offered PCI, their outcomes remained worse than men.
The 2014 American and the 2020 European society guidelines in the management of acute coronary syndromes do not differentiate between sexes, with no sex-specific differences in recommendations around the receipt of invasive management or medical therapies.21, 22, 23 Despite this, our analysis suggests that women are 10%-20% less likely to receive invasive therapies and more likely to have worse outcomes than men. The differences in management and outcomes between sexes have not narrowed over time. Furthermore, this analysis was adjusted for hospital-level factors (bed size, region, and location/teaching status) to alleviate any hospital-related effects.
Similar findings have been reported in older studies in which trends over a relatively short period were investigated. An analysis of 78,254 patients with AMI from the Get With The Guidelines (GWTG)-coronary artery disease (CAD) registry between 2001 and 2006 showed no difference in adjusted in-hospital mortality among the sexes in the overall AMI cohort (aOR, 1.04; 95% CI, 0.99-1.10) with women only at an increased risk of mortality only in the STEMI subgroup (aOR, 1.12; 95% CI, 1.02-1.23).24 In a more recent study, Stehli et al. reported on in-hospital and 30 days post-AMI outcomes of 13,451 patients between 2013 and 2016 from the Victorian Cardiac Outcomes registry and showed the worst outcomes in the STEMI subgroup in women (mortality: 8.4% vs 5.7%; bleeding: 3.5% vs 1.8%; P < 0.001 for both).25
Differences in outcomes between sexes could very well be related to the receipt and timing of invasive treatments. Women consistently have a higher risk profile at presentation compared with men, and are commonly older,26 have a greater comorbidity burden,27,28 and a higher prevalence of nonconventional risk factors,6,29 and are therefore less likely to be managed invasively. Similarly, sex bias or patient refusal of invasive cardiac procedures could mediate lower CA utilization in female patients.30,31 Even in patients at highest risk of ischemic complications, a contemporary study in 137,265 patients with NSTEMI has shown that women with high Global Registry of Acute Coronary Events (GRACE) scores were less likely to receive invasive management compared with men. Even when they received an invasive strategy, it was consistently delayed compared with men.32 In the STEMI subgroup, time to primary PCI was significantly greater in women than in men, irrespective of whether they had chest pain symptoms.33 Udell et al. evaluated differences in management in 104,817 STEMI patients from the GWTG-ACS database (2003-2008) with women 15% less likely to experience a door-to-balloon within 90 minutes than men even after adjustment for differences in baseline characteristics.34 Also, Leurent et al. observed significant differences in women compared with men among 5000 STEMI cases from the French registry in time from first medical contact to balloon inflation or thrombus aspiration (100 vs 94 minutes; P < 0.05), use of radial access (40% vs 51%; P < 0.001), death (9% vs 4%; P < 0.001), and even use of guideline-directed medical therapy at discharge.16 Interestingly, Setoguchi et al. reported no significant sex-related differences in the management of AMI with even lower mortality in women among patients aged 75 years or older.35 However, a smaller sample size (1625), a higher proportion of female patients (approximately 80%), and different time period (1999-2000) could play a role for differing findings.
However, it is important to note that several other factors could play a role in the observed sex gap in invasive management. Women, compared with men, have higher rates of AMI with nonobstructive coronary arteries (plaque erosion, coronary spasm, microvascular dysfunction, and stress-induced myocardial infarction), spontaneous coronary artery dissection,36 as well as type II AMI.37 Johnston et al. reported on the sex prevalence of AMI in patients with nonobstructive CAD among 95,849 patients (2005-2010) from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR). The prevalence of nonobstructive CAD in the STEMI group was 7% (6% in men vs 10% in women), and 17% in the NSTEMI group (11% in men vs 28% in women).38
Interestingly, we observed that the odds of bleeding were approximately 10% lower in women compared with men initially; although around 2009, the risk changed with a consistently higher risk in the order of 10%-20% observed in women. Higher bleeding risk in women compared with men could be multifactorial, with factors such as differences in response to antiplatelets,39 certain medications,39,40 and thrombolytic therapy,41 smaller body habitus and artery sizes,42,43 as well as procedural factors (such as access site choice).44,45 Other studies have confirmed the increased risk of major bleeding in women; for example, Nanna et al. reported higher rates of bleeding complications in 3041 patients older than 75 years who were hospitalized for AMI from the Comprehensive Evaluation of Risk Factors in Older Patients with Acute Myocardial Infarction (SILVER-AMI) in women with STEMI (26.2% vs 15.6%; P < 0.001) but not NSTEMI (17.8% vs 15.7%; P = 0.21).46
An important finding in our study is that even when women are treated with PCI, their outcomes remain worse, and this did not change over time. Although this elevated risk in women could be multifactorial, some of the factors could be avoided where possible. For example, in a recent study, Daugherty et al. investigated sex and bleeding risk associated with the use of bleeding-avoiding strategies (BAS) of bivalirudin, radial artery access, and closure devices among patients who underwent PCI, from 2008 to 2011. Among > 185,000 women who underwent PCI, the bleeding rate was reduced by 50% (12.5% vs 6.2%; P < 0.01) if any BAS was used. This reduction in bleeding events in women was even more significant than that observed in men (6.2% vs 3.0%; P < 0.01). Overall, BAS were less likely to be used in women compared with men and fewer women had PCI using radial access compared with men.47,48 Additionally, superiority of potent purinergic receptor P2Y12 inhibitors against thrombotic events in PCIs has been shown in trials.49 However, studies suggested underuse in women. For example, the association between the use of different types of purinergic receptor P2Y12 inhibitors and outcomes post primary PCI, was examined in > 89,000 patients from the British Cardiovascular Intervention registry. Women were more likely to receive clopidogrel and less likely to receive more potent antiplatelet treatments.50 The worse outcomes observed after PCI in women do not appear to be related to lesion complexity. An observational retrospective study in which CAD complexities were compared in 29,265 AMI patients treated in the Netherlands showed that women had less extensive CAD, with higher rates of single-vessel disease compared with men (49.4% vs 46.9%; P < 0.001) and lower rates of multivessel disease (47.2% vs 50.8%; P < 0.001). Despite less complex disease in women, women younger than 70 years of age had higher rates of mortality (7.3% vs 5.6%; P < 0.001).51 Another prospective study evaluated sex-based differences in patients with 100,704 drug-eluting stents (DES) implanted between 2005 and 2009 in Germany. Women, compared with men, had lower rates of use of DES in those with NSTEMI (24.8% vs 27.3%; P < 0.0001) especially in women older than 75 years, although no significant differences use of DES were observed in the STEMI group.52
There are several limitations to the present study. First, the data collected reflect in-patient outcomes only; longer follow-ups of adverse events would provide a more complete understanding of sex differences in outcomes. Second, individual risk factors, details regarding coronary anatomy, and time frames such as door-to-balloon time were not available in the NIS; this could provide further insight as to whether there are any sex-related differences in the complexity of coronary lesions and procedure approaches as well as details of timing of the index procedure. Similarly, the study lacks granularity regarding the “coronary artery dissection” variable, which did not include only iatrogenic events. Furthermore, no data regarding medications, antithrombotic therapy, Killip class, left ventricular ejection fraction, and creatinine clearance is captured in the NIS. These data might reveal sex differences in the prescription of different antiplatelet regimens, utilization and optimization of guideline-directed medical therapy, and their effect on MACCE. Also, in this study we were not able to match specific cardiovascular pathophysiologic features of AMI with the outcomes, which could have an effect on prognosis. Likewise, in this study we present a sex-based analysis and we did not account for gender-related aspects, which could also have a role in management bias. Finally, because NIS is an administrative database, there is always a risk of reporting and coding errors that represent a potential bias as is the under-reporting of other comorbidities. Furthermore, the ICD-9 codes in the data set are validated for the purpose of cardiovascular research.53
In conclusion, our nationwide temporal analysis shows persistent differences in the management and outcomes of AMI among the sexes over a period of 12 years, with women less likely to receive invasive therapies, and more likely to experience adverse outcomes including mortality, major bleeding, and stroke. The gap between sexes has not narrowed over time. A sex-based approach to the management of AMI, taking in to account the clinical and biological differences previously described, could possibly eliminate the persistent disparity in outcomes in the near future.
Funding Sources
None.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This research adhered to the relevant ethical guidelines.
See page S26 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.06.012.
Supplementary Material
References
- 1.Stehli J., Duffy S.J., Burgess S., et al. Sex disparities in myocardial infarction: biology or bias? Heart Lung Circ. 2021;30:18–26. doi: 10.1016/j.hlc.2020.06.025. [DOI] [PubMed] [Google Scholar]
- 2.Nowbar A.N., Gitto M., Howard J.P., Francis D.P., Al-Lamee R. Mortality from ischemic heart disease. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.118.005375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Health Topics: Cardiovascular Diseases. https://www.who.int/health-topics/cardiovascular-diseases/#tab=tab_1 Available at:
- 4.Leifheit-Limson E.C., D’Onofrio G., Daneshvar M., et al. Sex differences in cardiac risk factors, perceived risk, and health care provider discussion of risk and risk modification among young patients with acute myocardial infarction: the VIRGO study. J Am Coll Cardiol. 2015;66:1949–1957. doi: 10.1016/j.jacc.2015.08.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asaria P., Elliott P., Douglass M., et al. Acute myocardial infarction hospital admissions and deaths in England: a national follow-back and follow-forward record-linkage study. Lancet Public Health. 2017;2:e191–e201. doi: 10.1016/S2468-2667(17)30032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chandrasekhar J., Gill A., Mehran R. Acute myocardial infarction in young women: current perspectives. Int J Womens Health. 2018;10:267–284. doi: 10.2147/IJWH.S107371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gupta A., Wang Y., Spertus J.A., et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64:337–345. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian J., Wang X., Tian J., Yu B. Gender differences in plaque characteristics of nonculprit lesions in patients with coronary artery disease. BMC Cardiovasc Dis. 2019;19:45. doi: 10.1186/s12872-019-1023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mohamed W., Mohamed M.O., Hirji S., et al. Trends in sex-based differences in outcomes following coronary artery bypass grafting in the United States between 2004 and 2015. Int J Cardiol. 2020;320:42–48. doi: 10.1016/j.ijcard.2020.07.039. [DOI] [PubMed] [Google Scholar]
- 10.Defilippis E.M., Collins B.L., Singh A., et al. Women who experience a myocardial infarction at a young age have worse outcomes compared with men: the Mass General Brigham YOUNG-MI registry. Eur Heart J. 2020;41:4127–4137. doi: 10.1093/eurheartj/ehaa662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bucholz E.M., Strait K.M., Dreyer R.P., et al. Editor’s choice-sex differences in young patients with acute myocardial infarction: a VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6:610–622. doi: 10.1177/2048872616661847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Choi J., Daskalopoulou S.S., Thanassoulis G., et al. Sex- and gender-related risk factor burden in patients with premature acute coronary syndrome. Can J Cardiol. 2014;30:109–117. doi: 10.1016/j.cjca.2013.07.674. [DOI] [PubMed] [Google Scholar]
- 13.Yu J., Mehran R., Grinfeld L., et al. Sex-based differences in bleeding and long term adverse events after percutaneous coronary intervention for acute myocardial infarction: three year results from the HORIZONS-AMI trial. Catheter Cardiovasc Interv. 2015;85:359–368. doi: 10.1002/ccd.25630. [DOI] [PubMed] [Google Scholar]
- 14.Anstey D.E., Li S., Thomas L., Wang T.Y., Wiviott S.D. Race and sex differences in management and outcomes of patients after ST-elevation and non-ST-elevation myocardial infarct: results from the NCDR. Clin Cardiol. 2016;39:585–595. doi: 10.1002/clc.22570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vaccarino V., Parsons L., Peterson E.D., et al. Sex differences in mortality after acute myocardial infarction. Arch Intern Med. 2009;169:1767–1774. doi: 10.1001/archinternmed.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leurent G., Garlantézec R., Auffret V., et al. Gender differences in presentation, management and inhospital outcome in patients with ST-segment elevation myocardial infarction: data from 5000 patients included in the ORBI prospective French regional registry. Arch Cardiovasc Dis. 2014;107:291–298. doi: 10.1016/j.acvd.2014.04.005. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed M.O., Volgman A.S., Contractor T., et al. Trends of sex differences in outcomes of cardiac electronic device implantations in the United States. Can J Cardiol. 2020;36:69–78. doi: 10.1016/j.cjca.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 18.AHRQ. Agency for Healthcare Research and Quality. NIS Overview. https://www.hcup-us.ahrq.gov/nisoverview.jsp Available at: [DOI] [PubMed]
- 19.Birman-Deych E., Waterman A.D., Yan Y., et al. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43:480–485. doi: 10.1097/01.mlr.0000160417.39497.a9. [DOI] [PubMed] [Google Scholar]
- 20.DeShazo J.P., Hoffman M.A. A comparison of a multistate inpatient EHR database to the HCUP Nationwide Inpatient Sample. BMC Health Serv Res. 2015;15:384. doi: 10.1186/s12913-015-1025-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta L.S., Beckie T.M., DeVon H.A., et al. Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133:916–947. doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 22.Collet J.P., Thiele H., Barbato E., et al. 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2020;42:1289–1367. doi: 10.1093/eurheartj/ehaa575. [DOI] [PubMed] [Google Scholar]
- 23.Neumann F.J., Sousa-Uva M., Ahlsson A., et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J. 2019;40:87–165. doi: 10.1093/eurheartj/ehy855. [DOI] [PubMed] [Google Scholar]
- 24.Jneid H., Fonarow G.C., Cannon C.P., et al. Sex differences in medical care and early death after acute myocardial infarction. Circulation. 2008;118:2803–2810. doi: 10.1161/CIRCULATIONAHA.108.789800. [DOI] [PubMed] [Google Scholar]
- 25.Stehli J., Martin C., Brennan A., et al. Sex differences persist in time to presentation, revascularization, and mortality in myocardial infarction treated with percutaneous coronary intervention. J Am Heart Assoc. 2019;8 doi: 10.1161/JAHA.119.012161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Millett E.R.C., Peters S.A.E., Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ. 2018;363:k4247. doi: 10.1136/bmj.k4247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kautzky-Willer A., Harreiter J., Pacini G. Sex and gender differences in risk, pathophysiology and complications of type 2 diabetes mellitus. Endocrine Rev. 2016;37:278–316. doi: 10.1210/er.2015-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mascarenhas-Melo F., Marado D., Palavra F., et al. Diabetes abrogates sex differences and aggravates cardiometabolic risk in postmenopausal women. Cardiovasc Diabetol. 2013;12:61. doi: 10.1186/1475-2840-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McEwen B.S., Gray J.D., Nasca C. 60 Years of neuroendocrinology: redefining neuroendocrinology: stress, sex and cognitive and emotional regulation. J Endocrinol. 2015;226:T67–T83. doi: 10.1530/JOE-15-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heidenreich P.A., Shlipak M.G., Geppert J., McClellan M. Racial and sex differences in refusal of coronary angiography. Am J Med. 2002;113:200–207. doi: 10.1016/s0002-9343(02)01221-4. [DOI] [PubMed] [Google Scholar]
- 31.Daugherty S.L., Blair I.V., Havranek E.P., et al. Implicit gender bias and the use of cardiovascular tests among cardiologists. J Am Heart Assoc. 2017;6 doi: 10.1161/JAHA.117.006872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashid M., Curzen N., Kinnaird T., et al. Baseline risk, timing of invasive strategy and guideline compliance in NSTEMI: nationwide analysis from MINAP. Int J Cardiol. 2020;301:7–13. doi: 10.1016/j.ijcard.2019.11.146. [DOI] [PubMed] [Google Scholar]
- 33.Canto J.G., Rogers W.J., Goldberg R.J., et al. Association of age and sex with myocardial infarction symptom presentation and in-hospital mortality. JAMA. 2012;307:813–822. doi: 10.1001/jama.2012.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Udell J.A., Fonarow G.C., Maddox T.M., et al. Sustained sex-based treatment differences in acute coronary syndrome care: insights from the American Heart Association Get With The Guidelines Coronary Artery Disease Registry. Clin Cardiol. 2018;41:758–768. doi: 10.1002/clc.22938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Setoguchi S., Solomon D.H., Levin R., Winkelmayer W.C. Gender differences in the management and prognosis of myocardial infarction among patients > or = 65 years of age. Am J Cardiol. 2008;101:1531–1536. doi: 10.1016/j.amjcard.2008.02.033. [DOI] [PubMed] [Google Scholar]
- 36.Pizzi C., Xhyheri B., Costa G.M., et al. Nonobstructive versus obstructive coronary artery disease in acute coronary syndrome: a meta-analysis. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lichtman J.H., Leifheit E.C., Safdar B., et al. Sex differences in the presentation and perception of symptoms among young patients with myocardial infarction: evidence from the VIRGO study (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) Circulation. 2018;137:781–790. doi: 10.1161/CIRCULATIONAHA.117.031650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johnston N., Jönelid B., Christersson C., et al. Effect of gender on patients with ST-elevation and non-ST-elevation myocardial infarction without obstructive coronary artery disease. Am J Cardiol. 2015;115:1661–1666. doi: 10.1016/j.amjcard.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Patti G., De Caterina R., Abbate R., et al. Platelet function and long-term antiplatelet therapy in women: is there a gender-specificity? A ‘state-of-the-art’ paper. Eur Heart J. 2014;35:2213–2223. doi: 10.1093/eurheartj/ehu279. [DOI] [PubMed] [Google Scholar]
- 40.Tamargo J., Rosano G., Walther T., et al. Gender differences in the effects of cardiovascular drugs. Eur Heart J Cardiovasc Pharmacother. 2017;3:163–182. doi: 10.1093/ehjcvp/pvw042. [DOI] [PubMed] [Google Scholar]
- 41.Weaver W.D., White H.D., Wilcox R.G., et al. Comparisons of characteristics and outcomes among women and men with acute myocardial infarction treated with thrombolytic therapy. GUSTO-I investigators. JAMA. 1996;275:777–782. [PubMed] [Google Scholar]
- 42.Wilmot K.A., O’Flaherty M., Capewell S., Ford E.S., Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: clinical perspective. Circulation. 2015;132:997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Taqueti V.R., Shaw L.J., Cook N.R., et al. Excess cardiovascular risk in women relative to men referred for coronary angiography is associated with severely impaired coronary flow reserve, not obstructive disease. Circulation. 2017;135:566–577. doi: 10.1161/CIRCULATIONAHA.116.023266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rymer J.A., Kaltenbach L.A., Kochar A., et al. Comparison of rates of bleeding and vascular complications before, during, and after trial enrollment in the SAFE-PCI, trial for women. Circ Cardiovasc Interv. 2019;12 doi: 10.1161/CIRCINTERVENTIONS.118.007086. [DOI] [PubMed] [Google Scholar]
- 45.Shah R., Khan B. The MATRIX trial. Lancet. 2019;393:1803. doi: 10.1016/S0140-6736(19)30042-X. [DOI] [PubMed] [Google Scholar]
- 46.Nanna M.G., Hajduk A.M., Krumholz H.M., et al. Sex-based differences in presentation, treatment, and complications among older adults hospitalized for acute myocardial infarction: the SILVER-AMI study. Circ Cardiovasc Qual Outcomes. 2019;12 doi: 10.1161/CIRCOUTCOMES.119.005691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Daugherty S.L., Kim S., Thompson L., et al. Gender and bleeding risk following percutaneous coronary interventions: a contemporary report from the NCDR. J Am Coll Cardiol. 2012;59:E1803. [Google Scholar]
- 48.Kwok CS, Kontopantelis E, Kunadian V, et al. Effect of access site, gender, and indication on clinical outcomes after percutaneous coronary intervention: insights from the British Cardiovascular Intervention Society (BCIS). Am Heart J 2015;170:164-172, 172.e161-5. [DOI] [PubMed]
- 49.Wallentin L., Becker R.C., Budaj A., et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. New Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 50.Olier I., Sirker A., Hildick-Smith D.J.R., et al. Association of different antiplatelet therapies with mortality after primary percutaneous coronary intervention. Heart. 2018;104:1683–1690. doi: 10.1136/heartjnl-2017-312366. [DOI] [PubMed] [Google Scholar]
- 51.Ten Haaf M.E., Bax M., Ten Berg J.M., et al. Sex differences in characteristics and outcome in acute coronary syndrome patients in the Netherlands. Neth Heart J. 2019;27:263–271. doi: 10.1007/s12471-019-1271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Russ M.A., Wackerl C., Zeymer U., et al. Gender based differences in drug eluting stent implantation - data from the German ALKK registry suggest underuse of DES in elderly women. BMC Cardiovasc Dis. 2017;17:68. doi: 10.1186/s12872-017-0500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cozzolino F., Montedori A., Abraha I., et al. A diagnostic accuracy study validating cardiovascular ICD-9-CM codes in healthcare administrative databases. The Umbria Data-Value Project. PLoS One. 2019;14 doi: 10.1371/journal.pone.0218919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.